Abstract
A portion of a cDNA encoding a 35-kDa antigen from Toxoplasma gondii was cloned into the CKS expression vector and expressed in Escherichia coli. By using the enzyme-linked immunosorbent assay (ELISA), the recombinant protein (rP35 antigen) was examined for reactivity with immunoglobulin G (IgG) antibodies in the sera of pregnant women. Of these women, 41 had a toxoplasma serologic profile suggestive of recently acquired T. gondii infection (Sabin-Feldman dye test [DT] titers from 1:256 to 1:32,000, positive IgM ELISA titers from 2.3 to 9.7, positive IgA ELISA from 1 to >28, and acute patterns in the differential agglutination [AC/HS] test) (group I), and 50 women had a toxoplasma serologic profile suggestive of infection acquired in the distant past (low DT titers from 1:16 to 1:512, negative IgM ELISA titers from 0 to 0.8, and chronic patterns in the AC/HS test) (group II). The classification of acute or chronic profile was based on the individual's clinical history as well as the combination of the results of the toxoplasma serological profile. An additional group (group III) was composed of sera from 50 women who were seronegative for T. gondii antibodies in the DT. The results revealed that whereas 85.3% of women in group I had IgG antibodies that reacted with the rP35 antigen, only 8% of women in group II had IgG antibodies that reacted with the same antigen. In immunoblots, the rP35 antigen was recognized by IgG antibodies in a pool of sera from individuals with a toxoplasma serologic profile compatible with acute infection but not in a pool of sera from individuals with a serologic profile characteristic of a chronic infection. These results reveal that IgG antibodies against the P35 antigen are produced during the acute stage of the infection but are uncommon in the latent or chronic phase of the infection. Thus, the rP35 antigen may be a useful serologic marker to differentiate between recently acquired infection and that acquired in the more distant past.
Detection of infection due to Toxoplasma gondii in humans is usually made by the demonstration of specific antibodies in serum (2). The presence of immunoglobulin G (IgG) antibodies in a single sample of serum is sufficient to establish that the patient has been infected but does not give an indication as to when the infection occurred. In the United States there is no systematic serologic screening program for pregnant women, whereas in countries such as France and Austria sera are obtained at regular intervals throughout gestation from women who are seronegative when first tested. In the United States, a decision regarding whether the woman was recently infected, thereby placing her fetus at risk, is often made from the results of a single sample of serum. It is critical in pregnant women to determine as accurately as possible if they acquired their infection just prior to or during gestation. For this reason, the presence of IgG antibodies in a pregnant woman often leads to additional serological testing to attempt to determine if the infection was acquired during pregnancy or in the distant past (15). Of the recommended additional serological tests, those that demonstrate the presence of IgM antibodies are most frequently used. However, since IgM antibodies may remain detectable for more than 1 year after the initial infection, demonstration of these antibodies cannot be used to prove recently acquired infection (8, 19, 20). Because accurate diagnosis of recently acquired infection in pregnant women is important for clinical management of both the mother and her fetus, we have continued to search for better diagnostic methods (15, 20).
In previous studies (11, 12), our group observed that a 35-kDa protein was detected in immunoblots of tachyzoite extracts probed with serum obtained from individuals shortly after they became infected with T. gondii. In these studies, we postulated that this antigen might prove useful for detection of the acute stage of the infection. To investigate this hypothesis, a gene in the GenBank sequence database for T. gondii putatively identified as “P35” was selected for cloning and expression in bacteria. The expressed recombinant protein, rP35, was evaluated for its capacity to detect antibodies present during the early phase of infection with T. gondii by using an enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Construction of P35 fusion proteins.
The DNA sequence of the gene encoding a 35-kDa antigen (P35) from T. gondii was obtained from the GenBank database (accession number A19564) and the Toxoplasma EST database (1). A full-length P35 cDNA fragment was prepared from RNA isolated from tachyzoites of the RH strain and converted to cDNA with Moloney murine leukemia virus reverse transcriptase as previously described (16). Total tachyzoite cDNA was used as the template for amplification of the P35 sequence by using a standard PCR amplification protocol with Taq polymerase and primers corresponding to the entire predicted open reading frame. The full-length P35 cDNA was cloned into the CMP–2-keto-3-deoxy octulosonic acid synthetase (CKS) expression vector pJO200 (3) to generate the construct designated pJO200-P35. To construct a shorter P35 fusion protein embedded within the CKS open reading frame, a short DNA fragment corresponding to nucleotides 91 to 495 was prepared by PCR. An upstream sense primer (P35U, 5′-GAGCAGAAGGCCTTATGAACGGTCCTTTGAGTTATCATCC-3′) was synthesized with the additional recognition sequence for the restriction enzyme StuI, and a downstream antisense primer (P35D, 5′-TTCGCTCACGCGTATGGTGAACTGCCGGTATCT-3′) was synthesized with the additional recognition sequence for the restriction enzyme MluI. Briefly, primers P35U and P35D were used in a PCR containing plasmid pJO200-P35 as the source of P35 template. The resulting PCR products were digested with MluI and StuI, and the 405-bp fragment was spliced into the StuI/MluI sites of pJO200. The resulting construct (pJO200-P35S) expresses 135 amino acids from P35 embedded in frame between CKS amino acids 1 to 171 upstream and 171 to 260 downstream.
Production and purification of CKS recombinant proteins.
For expression of recombinant CKS-P35 (rP35) or nonrecombinant CKS proteins, competent Escherichia coli JM101 (Stratagene, La Jolla, Calif.) was transformed with recombinant pJO200-P35S or nonrecombinant pJO200 plasmids, respectively. Bacterial cultures were grown in TB media (16) supplemented with 50 μg of ampicillin per ml and 20 mM glucose at 37°C overnight. Several 2-liter culture flasks containing 400 ml of TB medium supplemented with 50 μg of ampicillin per ml and 3 mM glucose were inoculated with 40 ml of the overnight culture. The cultures were grown at 37°C with vigorous shaking until the optical density at 600 nm (OD600) reached 0.8 to 1.0. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM, and growth was continued for 4 h at 37°C. The cells were pelleted at 14,000 × g. The pellets were rinsed with TE buffer (50 mM Tris-HCl, pH 8.5; 1 mM ethylenediaminetetraacetic acid). Pellets were transferred to a Dounce homogenizer and resuspended in 5 ml of lysis buffer (TE, 0.5% Triton X-100, 1 mg of lysozyme per ml) per g of pellet weight. After 30 min of incubation on ice, MgCl2 was added to 20 mM and DNase was added to 13 μg/ml. The suspension was stirred on ice for 30 min. Phenylmethylsulfonyl fluoride and aprotinin were added to 18 and 8 μg/ml, respectively. The lysates were centrifuged at 25,000 × g for 30 min. For CKS-P35, the supernatant was discarded, while for nonrecombinant pCKS the supernatant was saved. The nonrecombinant CKS supernatant (containing the majority of the CKS protein) was treated with 50% saturated ammonium sulfate to precipitate proteins and centrifuged at 25,000 × g, and the sediment was dissolved in phosphate-buffered saline, pH 7.2 (PBS), and dialyzed in 4 liters of PBS. To wash the insoluble proteins for the nonrecombinant CKS and rP35 preparations, the pellets were transferred to a Dounce tissue grinder and homogenized in 5 ml of TE (containing 5% Triton X-100) per g of pellet weight. The suspensions were centrifuged at 25,000 × g for 30 min, and the supernatants were discarded. The wash procedure was repeated three times: once with TE plus 1% sodium deoxycholate, once with TE plus 0.5 M NaCl, and finally with TE alone. The final insoluble pellets were solubilized in TE with 8 M urea and 1 mM dithiothreitol and stirred at room temperature overnight. The suspension was dialyzed against PBS. The dialyzed proteins were centrifuged at 14,000 × g to pellet the residual insoluble proteins, and the supernatant was filtered through a 0.2 μm (pore size) filter. For pCKS, the solubilized proteins were combined with the ammonium sulfate-precipitated protein fraction.
Immunoblot analysis.
T. gondii lysate antigens were prepared from tachyzoites of the RH strain. The parasites were harvested from the peritoneal cavity of Swiss-Webster mice as previously described (13). Reduced lysate was prepared by resuspension of tachyzoites in reducing sample buffer containing 0.5% sodium dodecyl sulfate, 25 mM Tris-HCl (pH 6.8), 170 mM β-mercaptoethanol, 8.4% glycerol, and 0.01% bromophenol blue. Nonrecombinant CKS and rP35 proteins were prepared in the same reducing sample buffer. All samples were boiled for 5 min. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 10% slab gels and transferred to nitrocellulose membrane. For immunoblot analyses with human sera, the membranes with reduced rP35 antigen, nonrecombinant CKS antigen, and T. gondii lysate antigen were incubated with sera that had been diluted 1:100 in PBS–0.05% Tween 20 (PBS-T) containing 5% nonfat dry milk (16). The conjugate used was horseradish peroxidase-conjugated goat anti-human IgG (Caltag Laboratories) at a previously determined optimal dilution of 1:3,000 in PBS-T containing 3% bovine serum albumin (BSA). The substrate, 3,3′-diaminobenzidine tetrahydrochloride (Sigma Chemicals), was used at a final concentration of 0.1 mg/ml in PBS. Control immunoblots performed to test for the reactivity of the conjugates to either rP35 antigen, nonrecombinant CKS antigen, or T. gondii lysate antigen did not reveal any bands.
Serum samples.
Sera were provided by the Toxoplasma Serology Laboratory of our Institute and had been stored frozen for no longer than 2 years. They were from 141 pregnant women and were divided into three groups based on their serologic test results as follows: group I was composed of sera from 41 women with a serologic profile consistent with a recently acquired T. gondii infection (acute profile), and group II was composed of sera from 50 women with a serologic profile consistent with chronic infection. The serological tests used to classify these sera were: the Sabin-Feldman dye test (DT), the double-sandwich IgM ELISA (IgM-ELISA), the double-sandwich IgA ELISA (IgA-ELISA), and the differential agglutination (AC/HS) test (7, 8, 20). The latter test compares the titers obtained with formalin-fixed tachyzoites (HS antigen) with those obtained with acetone- or methanol-fixed tachyzoites (AC antigen) to determine whether infection was acquired recently or in the more distant past (20). The DT, IgM-ELISA, IgA-ELISA, and AC/HS tests comprise the “toxoplasma serological profile” (8). Sera from women in group I had high DT titers (from 1:256 to 1:32,000), positive IgM-ELISA titers (from 2.3 to 9.7), positive IgA-ELISA titers (from 1 to >28), and acute patterns in the AC/HS test. Sera from women in group II had low DT titers (from 1:16 to 1:512), negative IgM-ELISA titers (from 0 to 0.8), and chronic patterns in the AC/HS test. The classification of acute or chronic profile was based on the individual's clinical history as well as the combination of the results of the toxoplasma serological profile (7, 8). An additional group (group III) was composed of sera from 50 women who were seronegative for T. gondii antibodies in the DT test. A pool of serum samples from five seronegative individuals, each of whom was negative when their sera were tested undiluted in the DT, was used as a negative control for the immunoblots and the ELISA. Serum from a patient with a recently acquired toxoplasmic lymphadenopathy was used as a positive control on each ELISA plate.
ELISA.
Each well of a microtiter plate (Nunc, Roskilde, Denmark) was coated with 0.1 ml of a 10-μg/ml dilution of rP35 fusion protein or nonrecombinant CKS in 0.05 M carbonate buffer (pH 9.6) or with carbonate buffer only at 4°C overnight. In preliminary experiments, 10 μg of rP35 antigen per ml was determined to be the optimal concentration with which to coat the wells of the ELISA plates. Consequently, the control nonrecombinant CKS antigen preparation was also used at 10 μg/ml to coat plates. After incubation at 4°C overnight, the plates were washed three times with PBS-T and postcoated with 200 μl per well of 3% BSA in PBS-T at 37°C for 2 h. The plates were then washed, and 100 μl of test or control serum diluted 1:50 in 1% BSA in PBS-T was applied to each well with rP35 antigen preparation, nonrecombinant CKS antigen preparation, or without antigen. Plates were incubated at 37°C for 1 h and washed, and then 100 μl of horseradish peroxidase-conjugated goat anti-human IgG at a dilution of 1:1,000 was added to each well. The plates were incubated at 37°C for 1 h and washed, and then 100 μl of phosphate-citrate buffer (50 mM citric acid, 100 mM sodium phosphate; pH 5.0) containing 0.3 mg of o-phenylenediamine (OPD; Sigma) per ml and 0.15% H2O2 was added to each well. For the substrate 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS), the ABTS kit was used according to the manufacturer's instructions (Kirkegaard and Perry, Gaithersburg, Md.). The OD490 values were measured with an automatic ELISA reader (Dynatech Laboratories, Chantilly, Va.) after 15 min of incubation at room temperature. Each sample was run in duplicate wells. Results were determined for each patient by taking the mean value of the absorbency readings of duplicate wells.
RESULTS
Reactivity of T. gondii IgG antibodies in immunoblots with rP35.
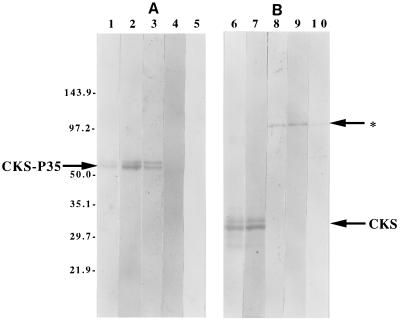
Three pools of sera, each consisting of four sera from groups I, II, or III, were studied in immunoblots of rP35 or CKS. IgG antibodies in the pooled sera from group I but not those in pooled sera of group II or III reacted strongly with the rP35 (Fig. 1A). IgG antibodies in each serum pool did not react with CKS but reacted weakly with other E. coli proteins of higher molecular weight present in the CKS preparation (Fig. 1B).
FIG. 1.
Immunoblots with rP35 protein (A) and immunoblots with the CKS control preparation (B). The rP35-CKS proteins had an approximate molecular size of 54 kDa (panel A, arrow). The CKS protein had an approximate molecular size of 34 kDa (panel B, arrow). In panel A, lane 1 was stained with amido black only and lane 2 shows reactivity of the rP35 with a monoclonal antibody to the CKS protein. Lanes 3, 4, and 5 show reactivity of the rP35 with pooled sera from individuals in group I, II, or III, respectively. Only antibodies in sera of group I individuals reacted with the rP35 protein. In panel B, lane 6 was stained with amido black and lane 7 with a monoclonal antibody to the CKS protein. Lanes 8, 9, and 10 show the reactivity of the CKS preparation with pooled sera from individuals in group I, II, or III, respectively. Antibodies in the sera of individuals in these groups did not react with the CKS protein, but weak cross-reactions occurred with other proteins in the CKS preparation (indicated with an arrow and a star).
Reactivity of T. gondii IgG antibodies in ELISA with rP35.
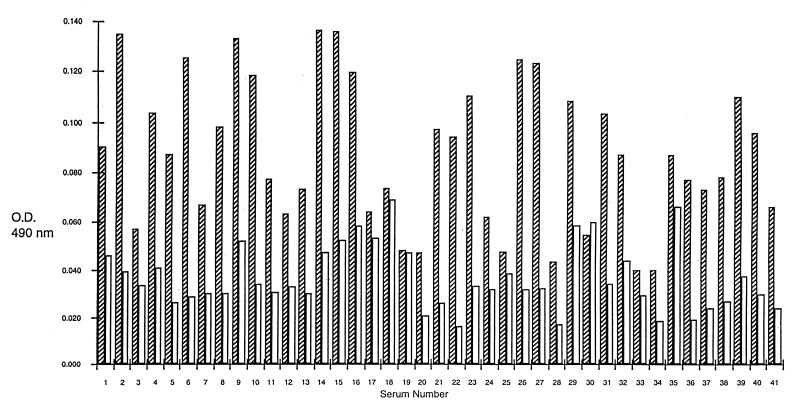
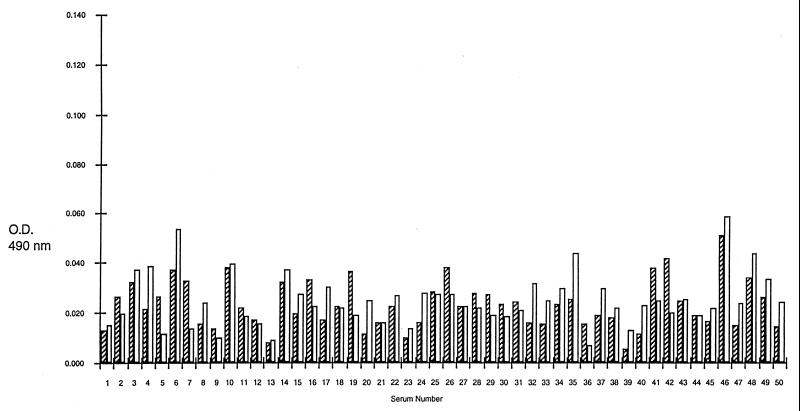
A total of 41 sera from group I and 50 from group II were examined individually in ELISA by using rP35 (rP35 ELISA) or the CKS protein preparations (control ELISA) in parallel. Of the 41 sera from group I, 40 (97.6%) had absorbency readings higher in the rP35 ELISA than in the control ELISA and 1 had absorbency readings higher in the control ELISA than in the rP35 ELISA (Fig. 2). In contrast, of the 50 sera from group II, 30 (60%) had readings in the control ELISA that were equal to or higher than in the rP35 ELISA and the remaining 20 (40%) had absorbency readings in the rP35 ELISA that were only slightly higher than the readings noted in the control ELISA (Fig. 3). The mean of the group I sera (0.0513 ± 0.0045 [the standard deviation]) was significantly (P = 0.0001) higher than the mean of the group II sera (0.0031 ± 0.0008 [the standard deviation]).
FIG. 2.
ELISA results in 41 sera from pregnant women with a serologic profile consistent with a recently acquired infection with T. gondii (group I). The hatched columns represent OD490 results with the rP35 preparation, and the clear columns are results with the CKS control preparation.
FIG. 3.
ELISA results of 50 sera from pregnant women with serologic profile consistent with a chronic infection (group II). The hatched columns represent OD490 results obtained with the rP35 preparation, and the clear columns represent results obtained with the CKS control preparation.
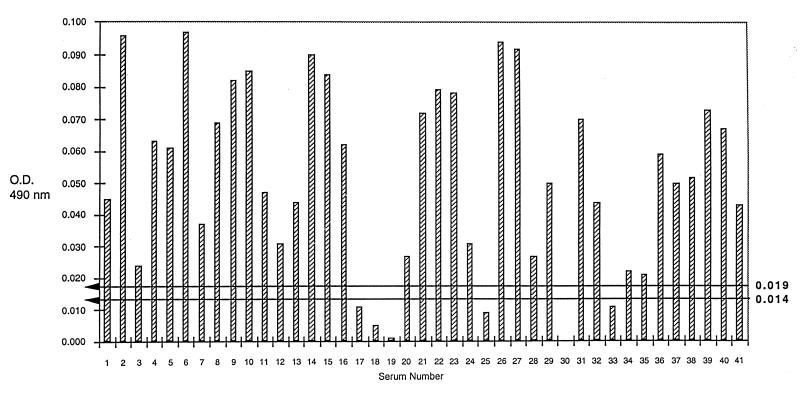
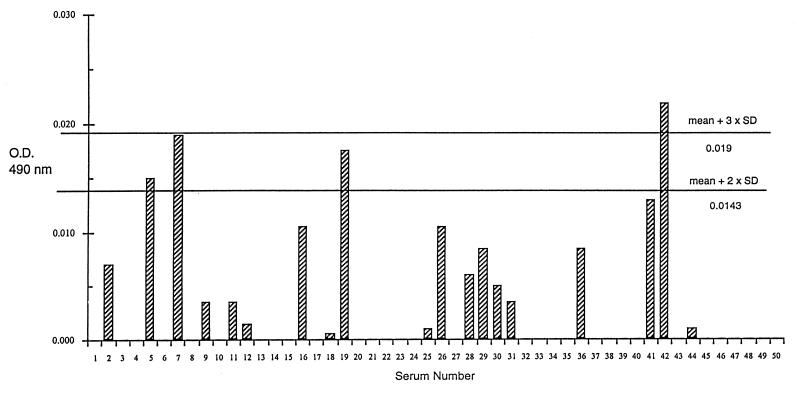
To determine whether the reactivity of IgG antibodies with rP35 could be used to differentiate group I from group II sera, the absorbency readings of the rP35 ELISA were normalized by subtraction of the readings from the control ELISA. Thereafter, a cutoff value was determined based on the mean plus two standard deviations of the normalized readings noted with group II sera. Using this cutoff value (i.e., 0.014), 35 (85.3%) of 41 group I sera had normalized readings higher than the cutoff value (Fig. 4). In contrast, only 4 (8%) of the 50 group II sera had normalized readings higher than the cutoff value (Fig. 5). When compared with interpretations made based on the toxoplasma serological profile results, the sensitivity of the rP35 ELISA for recently acquired infection was 85.3% and the specificity was 92%.
FIG. 4.
ELISA results with rP35 protein in the sera from 41 pregnant women with serologic profile consistent with recently acquired infection with T. gondii (group I) after subtraction of the results of the same sera with the CKS control preparation. The horizontal lines represent the cutoff values of 0.014 (mean plus two standard deviations) and 0.019 (mean plus three standard deviations) obtained from the results noted with sera of group II individuals as described in Results.
FIG. 5.
ELISA results with rP35 protein in the sera of 50 pregnant women with a serologic profile consistent with chronic infection (group II) after subtraction of the results of the same sera with the CKS control preparation. Only four sera had results higher than the cutoff value of 0.014, and only one had results higher than the cutoff value of 0.019.
Using a cutoff value (i.e., 0.019) based on the mean plus three standard deviations of the group II readings, 35 (85.3%) of 41 group I sera (Fig. 4) and only 1 (2%) of the 50 group II sera (Fig. 5) had normalized readings higher than the cutoff value.
Reproducibility of the rP35 ELISA and control ELISA was investigated in three experiments conducted in a 3-day interval by using five human sera with a serologic profile of recently acquired infection and five sera with serologic characteristics of a latent infection. By using one-way analysis of variance, no significant variation was observed among the means (P = 0.965) and no significant difference was observed among the standard deviations of the three tests (P = 0.958). Since the OD readings in the ELISA with OPD as the peroxidase substrate were relatively low for all specimens, a more sensitive substrate, ABTS, was used in parallel with 10 sera from each group (I, II, and III). In all specimens, the readings with ABTS were seven times higher than with OPD (data not shown). However, the readings were also increased in control wells incubated with the peroxidase conjugate without patient sera (data not shown).
DISCUSSION
Existing serologic tests for diagnosis of infection with T. gondii rely primarily on the use of live or chemically treated tachyzoites or whole extracts of tachyzoites. Whole parasite extracts are complex mixtures of antigens, each of which can influence the results of the test. One approach that addresses this problem is the use of recombinant antigens which might allow not only for more accurate standardization of tests but also have the potential to be used in the creation of new tests capable of differentiating recently acquired infections from those acquired in the more distant past.
One of the difficulties in using recombinant antigens expressed in E. coli for serodiagnosis in humans is the prevalence of antibodies against the fusion partner (in our case the CKS protein) or E. coli antigen contaminants in the partially purified recombinant protein preparation. Indeed, immunoblots that use pools of sera from women with an acute or chronic toxoplasma serological profile or seronegative women showed antibodies that reacted with E. coli proteins with a molecular weight higher than the CKS protein or the rP35 antigen. However, these immunoblots proved that IgG antibodies reactive specifically to rP35 exist in the sera of women with an acute serologic profile but not in those with a chronic serologic profile or in seronegative women. These observations resulted in our using a control ELISA with a crude E. coli extract that contained CKS protein run in parallel to ensure that an rP35 ELISA signal would be specific for the rP35 antigen preparation. Although subtraction of the control ELISA reading reduced the signal with the rP35 ELISA, it was done in all groups to the same relative degree and did not affect the pattern of the readings when the groups were compared. Similar differentiation between patients with an acute serologic profile and a chronic serologic profile was found when the readings with the P35 ELISA were used without subtraction of the control. However, the control ELISA served as a marker for serum samples from uninfected patients that might have a high background against E. coli proteins that resulted in rP35 ELISA readings above the cutoff value, thereby causing false-positive results. The background to E. coli antigens might be reduced and the specific detection of sera from T. gondii-infected individuals improved by expressing P35 as a fusion to a carrier protein that can be affinity purified. Although the ELISA readings with OPD as a substrate were low and could be increased by using ABTS as a substrate, the background with OPD is very low so the “signal to noise” ratio is high. Furthermore, since the readings with ABTS increased to the same relative degree in all specimens, the change to ABTS did not alter the number of specimens in group I that have readings above the cutoff value derived from group II.
The results of the present study with rP35 suggests that this antigen may be a serologic marker of recently acquired infection. The rP35 ELISA had a high sensitivity (85.3%) and a high specificity (92%) with the mean plus two standard deviations of the group II readings as the cutoff value. However, the specificity of the rP35 ELISA was improved (98%) without affecting the sensitivity (85.3%) by using a higher cutoff value (mean plus three standard deviations of the group II readings). The observation that the majority of pregnant women (85.3%) with an acute pattern in the toxoplasma serological profile were positive by the rP35 ELISA, while few women (8%) with a chronic serological profile were positive suggests that IgG antibodies to P35 are produced during the acute stage of the infection but infrequently persist in the latent or chronic infection.
Many reports have described the successful use of recombinant antigens for demonstration of antibodies to T. gondii in human sera (10, 14, 17, 18). In several reports the recombinant antigens were used to attempt to distinguish between acute and chronic infection. Tenter and Johnson (18) originally reported that 68% of sera from patients with acute toxoplasmosis and 14% of sera from individuals with chronic T. gondii infection had IgG antibodies that reacted with one or both of their recombinant H4 and H11 antigens in an ELISA. These same authors later reported an increase in sensitivity (81%) for sera from patients with acute toxoplasmosis by using a mixture of recombinant H4 and H11 antigens; however, these authors did not test sera from patients with chronic infection (5). Although Martin et al. (9) found that a high percentage of individuals infected with T. gondii had IgG antibodies that reacted with a recombinant Rop2 antigen, it was difficult to distinguish between individuals with acute and chronic infections (97.6% of patients with acute infection and 82.8% of individuals with chronic infection were positive by a Rop2 ELISA). Parmley et al. (10) demonstrated that 100% of sera from patients with acute toxoplasmosis (defined by using the same criteria as in the present study) had IgG antibodies that reacted with a recombinant surface antigen P22. Although most of the sera from individuals with chronic T. gondii infection (defined as above) had IgG antibodies that reacted with recombinant P22, the mean value from the acutely infected group was markedly higher than the mean from the chronically infected group. Furthermore, there was very little overlap in readings between the two groups, suggesting that a cutoff value could be determined that would exclude the majority of the chronically infected group without eliminating any from the acutely infected group. Using a recombinant form of the dense granule antigen Gra6, Redlich and Muller applied a cutoff value to their ELISA results and found that 89% of sera from patients with acute toxoplasmosis and only 0.4% of sera from individuals with chronic infection were positive for IgG antibodies (14). In the present study, the rP35 ELISA had a high sensitivity and high specificity which is similar to that reported with recombinant Gra6 (14) and significantly better than that reported with recombinant Rop2 (9). However, it is difficult to make comparisons between the results of each of these studies because the serological criteria for placing each sera into groups with serologic evidence of an acute or chronic infection varies between investigators. In most of these studies the presence of specific IgG and IgM antibodies was sufficient to place a serum sample in the group with acute infection, while the absence of specific IgM antibodies in patients with specific IgG antibodies was used to place specimens in the group with chronic infection. However, since the presence of IgM antibodies that persist into the chronic stage of infection has been demonstrated and is common (7), additional testing would be required in these sera in order to group them with sufficient accuracy. The only tests available at present for this are the AC/HS differential agglutination and avidity tests (6–8, 20). In the present study, sera were grouped based on the interpretation of the results of a much more extensive panel of serologic tests including IgA- and IgE-ELISA and the AC/HS test.
One concern with the present study is that sera from six of the pregnant women with an acute pattern in the toxoplasma serological profile were found to be negative by the rP35 ELISA. Like the avidity test (4, 6), the toxoplasma serological profile is an indicator of the approximate timing of the infection but definitively reveals if the infection was acquired in the more distant past (7, 8). Furthermore, the AC/HS method agrees more than 97% with the results of the avidity test (J. S. Remington, unpublished observation). A serological profile indicative of an acute infection, however, is not definitive for recently acquired infection, particularly when only a single serum sample is available. Among the indicators of an acute pattern in the toxoplasma serological profile are a positive IgM titer and an acute pattern in the AC/HS test (7, 8). However, both tests can remain positive for many months after initial infection. Therefore, it is possible that in the present study the sera of the six pregnant women who had an acute pattern in the toxoplasma serological profile but who had P35 ELISA readings below the cutoff had been obtained many months after seroconversion at a time when their P35-specific IgG antibodies had disappeared but their IgM and AC/HS titers remained positive. To address this question it is necessary to perform rP35 ELISA studies with sera from pregnant women drawn sequentially for many months after seroconversion. However, since sera sent to our laboratory are not from any systematic screening program, we infrequently obtain sequential sera drawn more than 3 weeks after the first serum is drawn. Thus, the sequential sera needed to address the question of the duration of the IgG response to P35 were not available for this study. With a high sensitivity and high specificity for the sera from recently acquired infection, the P35 antigen appears to be a potentially useful serologic marker in pregnant women to differentiate recently acquired infection from an infection acquired in the more distant past.
ACKNOWLEDGMENT
This work was supported by U.S. Public Health Service grant AI04717.
REFERENCES
- 1.Ajioka J W, Boothroyd J C, Brunk B P, Hehl A, Hillier L, Manger I D, Marra M, Overton G C, Roos D S, Wan K I, Waterston R, Sibley L D. Gene discovery by EST sequencing in Toxoplasma gondii reveals sequences restricted to the ApicomplexA. Genome Res. 1998;8:18–28. doi: 10.1101/gr.8.1.18. [DOI] [PubMed] [Google Scholar]
- 2.Beaman M H, McCabe R E, Wong S Y, Remington J S. Toxoplasma gondii. In: Mandel G L, Bennett J E, Dolin R, editors. Principles and practices of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2455–2475. [Google Scholar]
- 3.Bolling T J, Mandecki W. An Escherichia coli expression vector for high level production of heterologous proteins in fusion with CMP-KDO synthetase. BioTechniques. 1990;8:488–492. [PubMed] [Google Scholar]
- 4.Hedman K, Lappalainen M, Seppala I, Makela O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 5.Johnson A M, Roberts H, Tenter A M. Evaluation of a recombinant antigen ELISA for the diagnosis of acute toxoplasmosis and comparison with traditional antigen ELISAs. J Med Microbiol. 1992;37:404–409. doi: 10.1099/00222615-37-6-404. [DOI] [PubMed] [Google Scholar]
- 6.Lappalainen M, Kostella P, Koskiniemi M, Ammala P, Hiilesmaa V, Teramo K, Raivio K O, Remington J S, Hedman K. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J Infect Dis. 1993;167:691–697. doi: 10.1093/infdis/167.3.691. [DOI] [PubMed] [Google Scholar]
- 7.Liesenfeld O, Press C, Flanders R, Ramirez R, Remington J S. Study of Abbott Toxo IMx System for detection of immunoglobulin G and immunoglobulin M toxoplasma antibodies: value of confirmatory testing for diagnosis of acute toxoplasmosis. J Clin Microbiol. 1996;34:2526–2530. doi: 10.1128/jcm.34.10.2526-2530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liesenfeld O, Press C, Montoya J G, Gill R, Isaac-Renton J L, Hedman K, Remington J S. False-positive results of immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM Test. J Clin Microbiol. 1997;35:174–178. doi: 10.1128/jcm.35.1.174-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin V, Arcavi M, Santillan G, Amendoeira M R R, Neves E S, Griemberg G, Guarnera E, Garberi J C, Angel S O. Detection of human toxoplasma-specific immunoglobulins A, M, and G with recombinant Toxoplasma gondii ROP2 protein. Clin Diagn Lab Immunol. 1998;5:627–631. doi: 10.1128/cdli.5.5.627-631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmley S F, Sgarlato G D, Mark J, Prince J B, Remington J S. Expression, characterization and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J Clin Microbiol. 1992;30:1127–1133. doi: 10.1128/jcm.30.5.1127-1133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potasman I, Araujo F G, Desmonts G, Remington J S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986;154:650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- 12.Potasman I, Araujo F G, Thulliez P, Desmonts G, Remington J S. Toxoplasma gondii antigens recognized by sequential samples of serum obtained from congenitally infected infants. J Clin Microbiol. 1987;25:1926–1931. doi: 10.1128/jcm.25.10.1926-1931.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince J B, Auer K L, Huskinson J, Parmley S F, Araujo F G, Remington J S. Cloning, expression, and cDNA sequence of surface antigen P22 from Toxoplasma gondii. Mol Biochem Parasitol. 1990;43:97–106. doi: 10.1016/0166-6851(90)90134-8. [DOI] [PubMed] [Google Scholar]
- 14.Redlich A, Muller W A. Serodiagnosis of acute toxoplasmosis using a recombinant form of the dense granule antigen GRA6 in an enzyme-linked immunosorbent assay. Parasitol Res. 1998;84:700–706. doi: 10.1007/s004360050473. [DOI] [PubMed] [Google Scholar]
- 15.Remington J S, McLeod R, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: W. B. Saunders; 1995. pp. 140–267. [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Tenter A M, Johnson A M. Human antibody response to the nucleoside triphosphate hydrolase of T. gondii. J Immunoassay. 1990;11:579–590. doi: 10.1080/01971529008055051. [DOI] [PubMed] [Google Scholar]
- 18.Tenter A M, Johnson A M. Recognition of recombinant T. gondii antigens by human sera in an ELISA. Parasitol Res. 1991;77:197–203. doi: 10.1007/BF00930858. [DOI] [PubMed] [Google Scholar]
- 19.Wilson M, Remington J S, Clavet C, Varney G, Press C, Ware D The FDA Toxoplasmosis Ad Hoc Working Group. Evaluation of six commercial kits for the detection of human immunoglobulin M antibodies to Toxoplasma gondii. J Clin Microbiol. 1997;35:3112–3115. doi: 10.1128/jcm.35.12.3112-3115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong S Y, Remington J S. Toxoplasmosis in pregnancy. Clin Infect Dis. 1994;18:853–862. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]