Abstract
Background:
Antimicrobial resistance (AMR) in Escherichia coli is an alarming issue worldwide, including in the Gulf Cooperation Council (GCC) countries, yet the prevailing gene patterns have not recently been reviewed. This study was conducted to determine and report on the dominant E. coli antimicrobial resistant gene patterns in GCC countries.
Method:
A scoping review identified the predominant AMR genes in GCC countries: CTX M, TEM, SHV, NDM, OXA, and VIM genes. For the systematic review, two authors independently searched Scopus, PubMed, Google Scholar, Science Direct, and Web of Science for interventional, clinical, or observational studies on the chosen AMR-conferring genes in E. coli published from GCC countries between January 2013 and June 2019, when the last search was carried out. The search strategy followed the PRISMA guidelines. The risk of bias was assessed using a 6-item standardized checklist. Random-effects modeling was used for all analyses.
Results:
A total 32 studies were included in the final synthesis of evidence. Overall, CTX-M (53.8%) was the most prevalent gene in the region followed TEM (40.6%), NDM-1 (28.4%), OXA (24.3%), VIM (8.5%), and SHV (7.8%). Most included studies were from Saudi Arabia: CTX-M was again most common with a prevalence of 46.8% from 5442 isolates.
Conclusion:
The risk of bias analysis showed a mean quality score of 4.25 ± 0.75, indicating high-quality in studies included in this meta-analysis. This review found that CTX-M gene is the most common AMR-conferring gene in E. coli strains from most GCC countries.
Keywords: Antimicrobial resistance genes, CTX-M, Escherichia coli, Gulf Cooperation Council countries, NDM, OXA
INTRODUCTION
Infections caused by multiple antimicrobial-resistant (AMR) bacteria are a major therapeutic challenge in both hospital and community settings.[1] The Middle East region, which includes the Gulf Cooperation Council (GCC) countries, is not immune to this issue.[2] Studies from this region have identified potential clinical risks associated with extended-spectrum beta-lactamase (ESBL)-producing bacteria,[3,4] NDM-1-carrying resistant bacteria, and other multidrug-resistant strains.[5] This suggests a potential wide prevalence of antimicrobial resistance in this region.
In Saudi Arabia, antimicrobial resistance has been reported in Gram-negative bacteria isolated from both community and hospital settings,[3] including ESBL-producing Escherichia coli.[3,6] Bindayna et al.[7] reported that 51% of antibiotic-resistant E. coli isolates from this country produced both CTX-M and TEM enzymes, while another reported 22% of the resistant isolates containing both metallo-beta-lactamase (MBL) and ESBL.[8] The United Arab Emirates (UAE) has one of the highest rates of ESBL prevalence in the Arabian Gulf region. A study in 2018 reported the presence of blaCTX-M and blaTEM and blaSHV genes among E. coli isolates.[9] NDM, OXA-48 and VIM were also found in the Arabian Peninsula in E. coli.[10] A study in Kuwait demonstrated a high prevalence rate CTX-M among E. coli isolates. It also reported the presence of VIM and NDM-1.[11] Several studies from Oman have reported carbapenem resistance in E. coli is facilitated through NDM and OXA-48 carbapenemases.[12,13] The incidence of antibiotic-resistant bacteria is increasing rapidly in humans in Qatar.[14] For this reason, a hospital-based antibiotic-resistant bacteria surveillance system exists in this country to monitor the antimicrobial resistance from both outpatient and in-patient clinics.[14]
The genotype description of AMR determinants in bacteria plays a crucial role in understanding and controlling the drug resistance.[15] However, to the best of the authors’ knowledge, no review has analyzed the AMR gene patterns prevailing in GCC countries. In our scoping review, six AMR genes (CTX M, TEM, SHV, NDM, OXA, and VIM genes) were identified to be dominant in the GCC region. Subsequently, this systematic review was conducted to analyze the AMR patterns of these six genes in E. coli.
MATERIALS AND METHODS
The present study used the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) as a guideline for reporting the findings.[16]
Search strategy
An electronic search was conducted in Scopus, PubMed, Google Scholar, Science Direct, and Web of Science for articles published between January 2013 and June 2019. We conducted a literature search beginning from 2013 because a previous study has analyzed the prevalence of AMR genes in the GCC between 1999 and 2012. The search strategy included relevant keywords: “antimicrobial resistance” OR “antibiotic resistance” OR “drug-resistance” AND “Enterobacteriaceae” OR “Escherichia coli” AND “Middle East” OR “Gulf Co-operation Council (GCC)” OR “Saudi Arabia (KSA)” OR “Bahrain” OR “Kuwait” OR “Oman” OR “United Arab Emirates (UAE)” OR “Qatar” AND “resistant genes” OR “Extended-Spectrum Beta-Lactamase (ESBL)” OR “Metallo beta-lactamase (MBL)” OR “CTX- M” OR “NDM” OR “OXA” OR “TEM” OR “VIM” OR “SHV”.
Inclusion criteria
Interventional, clinical, and observational studies that analyzed the selected six AMR-conferring genes in E. coli clinical isolates from the GCC countries, and in which the resistant genes were detected by molecular methods, were included. A language filter was applied to only include articles published in English. The last search was carried out in June 2019.
Exclusion criteria
To eliminate factors that may incur potential quality or methodological issues, studies were excluded from if any of the following criteria were met:
Studies that were conducted on E. coli strains from environmental resources such as food, water, and air
Studies that reported secondary data
Studies on other AMR genes not considered for this study
Studies reporting resistance genes by phenotypic methods
Case reports, short communications, abstracts, review articles, letters, editorials, and studies not published in English
Unpublished/non-peer-reviewed data.
Main outcomes and measures
The principal outcome of this review was to report the prevalence of the selected six AMR genes in GCC countries. Two authors (KMB, RMJ) independently carried out a review of titles and abstracts based on the inclusion/exclusion criteria.
Data extraction
Two authors (KMB, HE) performed the initial data extraction in duplicates. Any discrepancies regarding study eligibility were discussed with the other authors to reach a consensus. To standardize the data extraction, the following variables were collected from each study: type of bacterial isolates, country, year, sample size, and type of antibiotic-resistant genes. Extracted data were entered into Microsoft Excel Sheet for analysis.
Statistical analysis and reporting
One author (HJ) performed the data analysis. A series of single-group meta-analyses was performed based on the sample size and event rate. Random-effects modeling was used for all analyses; therefore, it was assumed that there is not only one true effect size, rather, a distribution of true effect sizes. The authors sought to estimate the mean of this distribution of true effect sizes. Moderator analysis was performed on the variable country and was done using subgroup analyses. All statistical analyses were performed using the Comprehensive Meta-Analysis version 3.0 (Biostat, Englewood, NJ, USA).
Tau (τ2) and I2 statistics were used to assess the heterogeneity of the solicited studies within and between studies, respectively. Furthermore, the classical measure of heterogeneity is Cochran's Q, which is calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies, with the weights being those used in the pooling method. Q is distributed as a Chi-square statistic with k (number of studies) minus 1 degree of freedom.
Critical appraisal of studies (quality assessment)
Two reviewers independently assessed the methodological quality of studies using a standardized checklist consisting of the following six items: sample size, sampling technique, standardization of data collection, appropriateness of statistical analyses, quality of reporting results, and generalizability. The appraisal scores ranged from 0 to 6: Scores of 0–2 correspond to low quality, 3 and 4 to medium quality, and 5 and 6 to high quality. The quality score was set for each study by consensus of all the authors after discussion. We used the Newcastle-Ottawa Scale (NOS) as a guide for assessing the quality of nonrandomized studies in meta-analysis.[17]
RESULTS
Search strategy results
The search retrieved a total of 200 studies (42 studies in Scopus, 51 in PubMed, 38 in Science Direct, 24 in Web of Science, and 45 in Google Scholar). Of these, 114 were screened, the full text of 61 articles was assessed for eligibility, and finally, 32 studies were included for the qualitative synthesis [Figure 1].
Figure 1.
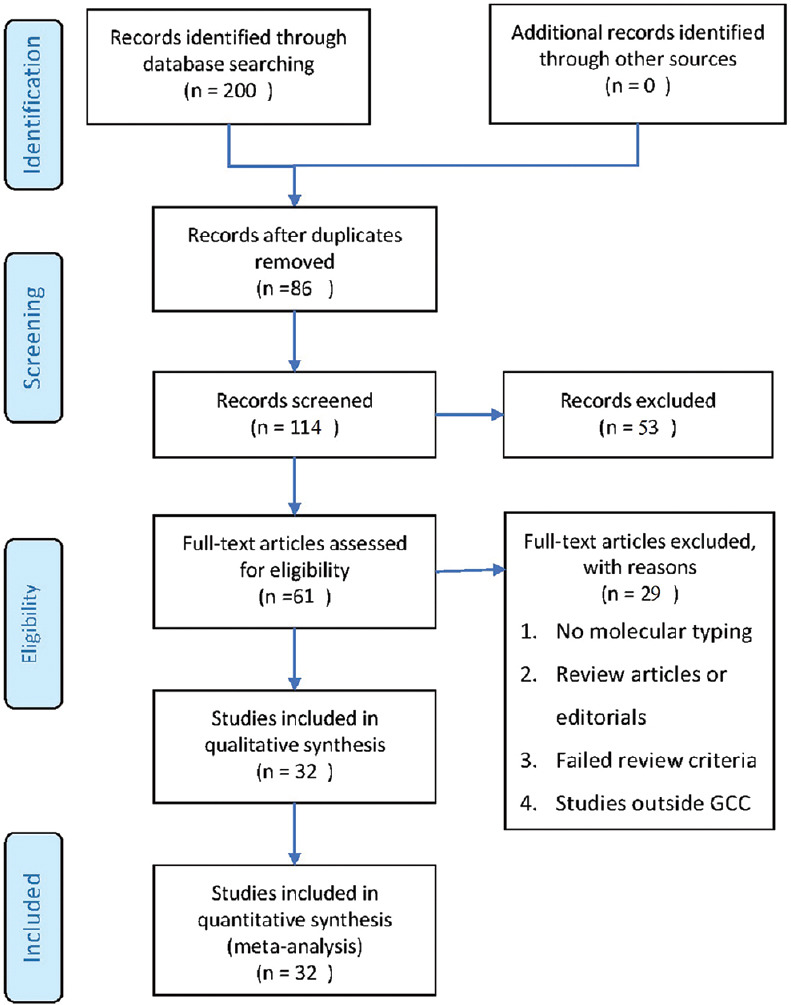
Stages of evaluation of the collected studies
Baseline characteristics and risk of bias
Study characteristics (i.e., authors, year, country, sample size, type of antibiotic-resistant genes) are summarized in Table 1. Critical appraisal of studies or quality assessment revealed that the mean quality score was 4.25 (±0.75) [Table 1].
Table 1.
Study characteristics
| Authors | Country | Sample size | CTX-M | TEM | NDM | OXA | VIM | SHV | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Eltai et al., 2018[18] | Qatar | 95 | 65 | 3 | 1 | 5 | |||
| Elhassan et al., 2016[19] | Saudi Arabia | 359 | 57 | 19 | 3 | 5 | |||
| Abd El Ghany et al., 2018[20] | Saudi Arabia | 10 | 9 | 9 | 6 | 5 | 4 | ||
| Jamal et al., 2013[5] | Kuwait | 3 | 2 | 1 | 3 | 2 | 4 | ||
| Jamal et al., 2016[21] | Kuwait | 4 | 3 | 4 | 0 | 4 | |||
| Dashti et al., 2014[22] | Kuwait | 83 | 34 | 2 | 1 | 4 | |||
| Alsultan et al., 2013[23] | Saudi Arabia | 60 | 0 | 44 | 20 | 4 | |||
| Al Sheikh et al., 2014[24] | Saudi Arabia | 50 | 3 | 26 | 1 | 4 | |||
| Zowawi et al., 2014[13] | Saudi Arabia | 266 | 7 | 1 | 1 | 5 | |||
| Al-agamy et al., 2014[25] | Saudi Arabia | 152 | 31 | 5 | |||||
| Hassan et al., 2013[26] | Saudi Arabia | 139 | 106 | 70 | 33 | 5 | |||
| Al-Mijalli, 2016[27] | Saudi Arabia | 75 | 22 | 15 | 27 | 11 | 4 | ||
| Marie et al., 2013[8] | Saudi Arabia | 3358 | 2698 | 2465 | 2338 | 297 | 1169 | 5 | |
| Hassan and Abdalhamid 2014[28] | Saudi Arabia | 251 | 81 | 0 | 5 | ||||
| Leangapichart et al., 2016[29] | Saudi Arabia | 10 | 1 | 9 | 1 | 4 | |||
| Sonnevend et al., 2015[10] | Arabian Peninsula | 28 | 9 | 9 | 1 | 4 | |||
| Alyamani et al., 2017[30] | Saudi Arabia | 58 | 27 | 22 | 28 | 2 | 4 | ||
| Abd El Ghany et al., 2018[20] | Saudi Arabia | 10 | 6 | 6 | 4 | 3 | |||
| Alzahrania et al., 2016[31] | Saudi Arabia | 14 | 3 | 14 | 3 | ||||
| Hassan et al., 2014[32] | Saudi Arabia | 15 | 9 | 3 | |||||
| Mashwal et al., 2017[33] | Saudi Arabia | 117 | 10 | 3 | 0 | 5 | |||
| Soliman et al., 2018[34] | Saudi Arabia | 46 | 28 | 2 | 3 | 4 | |||
| Ahmed et al., 2016[35] | Qatar | 629 | 29 | 13 | 3 | 5 | |||
| Alfaresi et al., 2018[9] | UAE | 39 | 39 | 39 | 2 | 15 | 4 | ||
| Shahid, 2014[36] | Bahrain | 75 | 70 | 4 | |||||
| Yasir et al., 2018[37] | Saudi Arabia | 211 | 201 | 177 | 14 | 11 | 5 | ||
| Alam MZ et al., 2016[38] | Saudi Arabia | 877 | 5 | ||||||
| Alqasim et al., 2018[39] | Saudi Arabia | 100 | 31 | 4 | 8 | 0 | 5 | ||
| Leangapichart et al., 2016[40] | Saudi Arabia | 18 | 17 | 5 | 4 | ||||
| Somily et al., 2015[41] | Saudi Arabia | 50 | 48 | 13 | 0 | 4 | |||
| Ahn et al., 2015[42] | UAE | 1 | 0 | 1 | 2 | ||||
| AlTamimi et al., 2017[43] | Saudi Arabia | 26 | 1 | 6 | 4 |
Synthesis of evidence
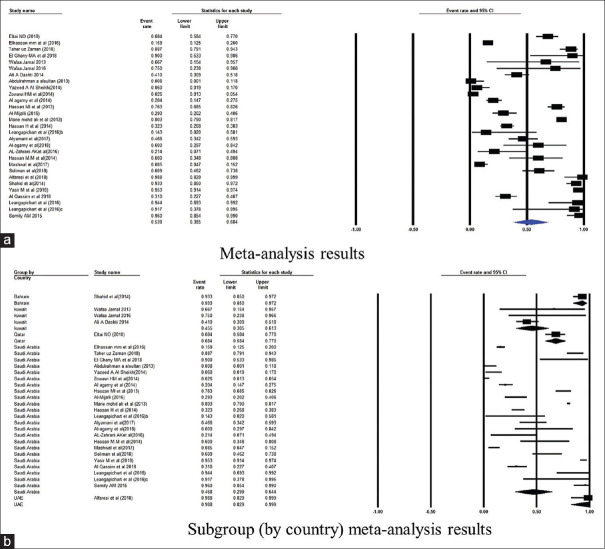
The initial analysis showed the following prevalence pattern of AMR genes in the GCC region. CTX-M (53.8%) appeared to be the most common AMR gene followed by TEM (40.6%), NDM-1 (28.4%), OXA (24.3%), VIM (8.5%), and SHV (7.8%), respectively. The overview of this result and detailed assessment of heterogeneity are presented in Table 2.
Table 2.
Prevalence of antimicrobial resistance genes
| Parameters | CTX M | TEM | NDM | OXA | VIM | SHV |
|---|---|---|---|---|---|---|
| Number of studies (K) | 29 | 23 | 8 | 10 | 5 | 20 |
| Number of isolates (n) | 6428 | 5600 | 3774 | 856 | 3454 | 5705 |
| Proportion (95% CI) | 53.8 (38.5-68.4) | 40.6 (25.8-57.4) | 28.4 (9.6-59) | 24.3 (11.3-44.7) | 8.5 (2.9-22.6) | 7.8 (4.1-14.3) |
| Q | 1262.909 | 706.709 | 130.882 | 147.876 | 10.866 | 298.658 |
| Df (Q) | 28 | 22 | 7 | 9 | 4 | 19 |
| P | 0.001 | 0.001 | 0.001 | 0.001 | 0.028 | 0.001 |
| I 2 | 97.783 | 96.887 | 94.652 | 93.914 | 63.186 | 93.638 |
| Tau2 | 2.483 | 2.256 | 2.979 | 1.988 | 0.914 | 1.743 |
CI: Confidence interval, df: Degree of freedom
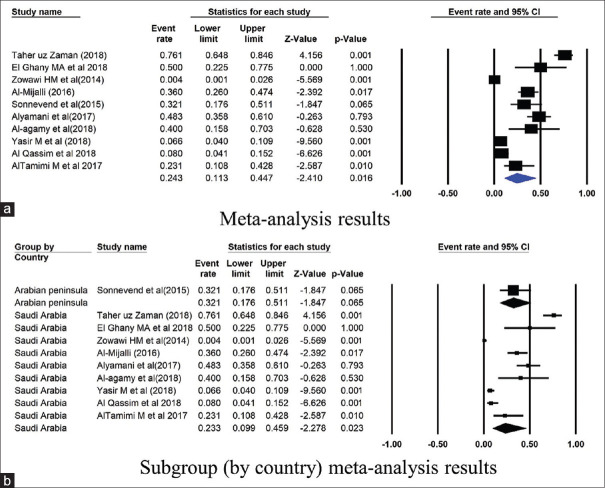
Further, the subgroup analysis was conducted based on the country of published studies. Figures 2–7 summarize this subgroup analysis. In Saudi Arabia, the most commonly found gene was CTX-M: its prevalence was 46.8% of 5442 isolates from 23 studies. Similarly, of the 90 isolates from the three studies from Kuwait, the prevalence of CTX-M was 45.5% [Figure 2]. There were very few studies from other GCC countries.
Figure 2.
Forest plots showing (a) meta-analysis results (b) subgroup (by country) meta-analysis results for CTX-M gene. CI, confidence interval. Horizontal lines stand for 95% CIs. The size of the squares shows the weight that individual study had in the meta-analysis
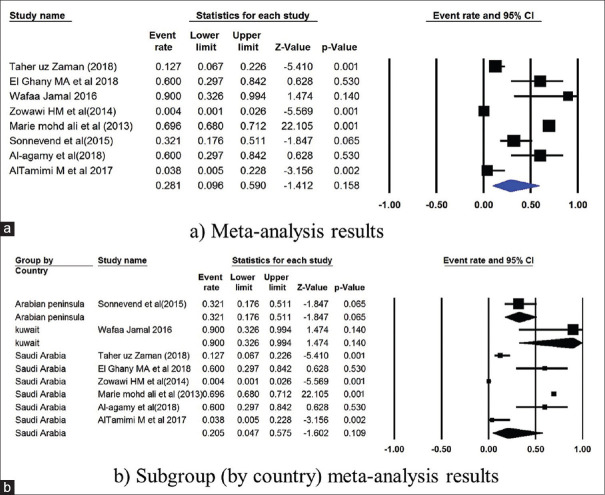
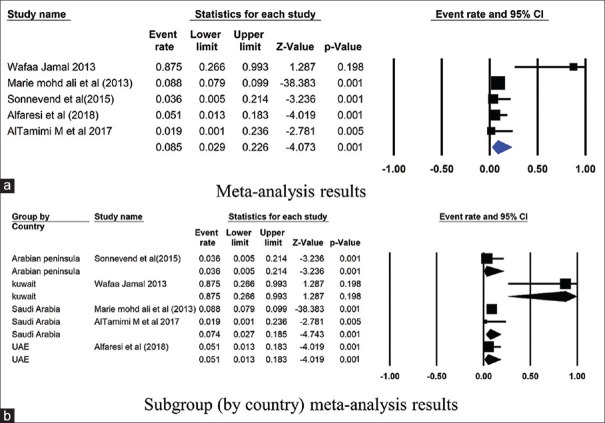
Figure 7.
Forest plots showing (a) meta-analysis results (b) subgroup (by country) meta-analysis results for SHV gene. CI, confidence interval. Horizontal lines stand for 95% CIs. The size of the squares shows the weight that individual study had in the meta-analysis
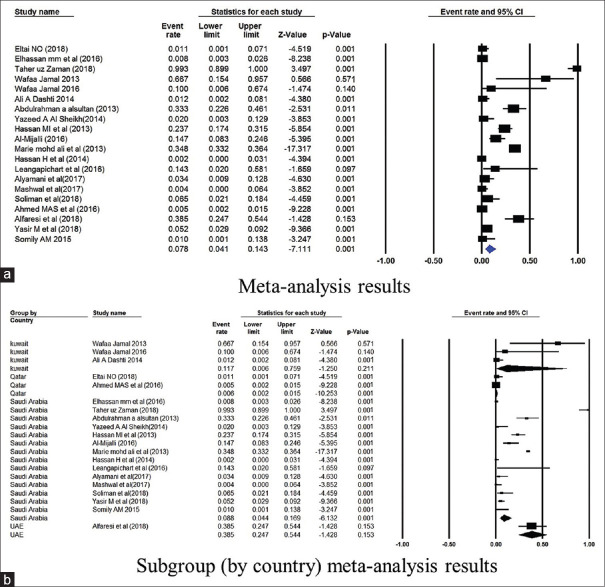
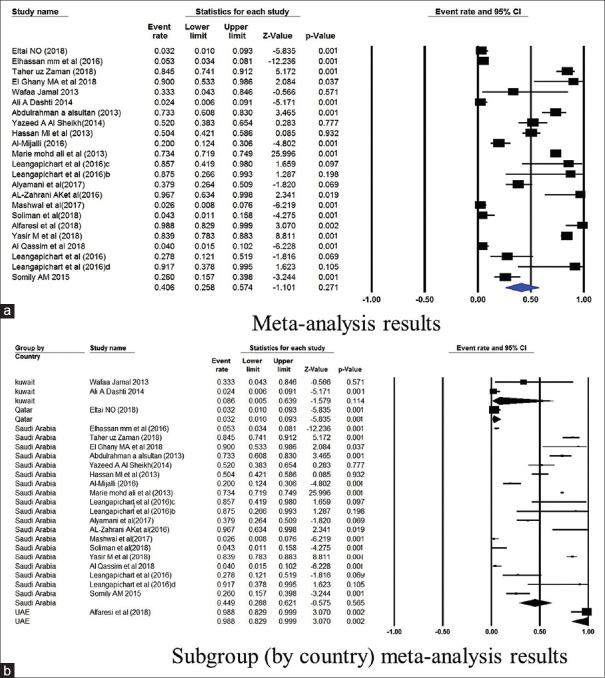
The prevalence of TEM gene in Saudi Arabia was 44.9% (4751 isolates from 19 studies) and 8.6% in Kuwait (86 isolates from 2 studies) [Figure 3]. The NDM gene was studied in 6 studies from Saudi Arabia, and had a mean point prevalence of 20.5% [Figure 4]. Saudi Arabia also reported studies on OXA gene (9 studies, 23.3% prevalence) and VIM gene (2 studies, 7.4%% prevalence) [Figures 5 and 6]. In terms of the SHV gene, 14 studies were from Saudi Arabia (4852 isolates), 3 from Kuwait (90 isolates), and 2 from Qatar (724 isolates) [Figure 7]. The prevalence of the SHV gene in Saudi Arabia, Kuwait, and Qatar was 8.8%, 11.7%, and 0.6%, respectively. Figure 8 demonstrates the presence of the six antimicrobial resistant genes in E. coli in each country.
Figure 3.
Forest plots showing (a) meta-analysis results (b) subgroup (by country) meta-analysis results for TEM gene. CI, confidence interval. Horizontal lines stand for 95% CIs. The size of the squares shows the weight that individual study had in the meta-analysis
Figure 4.
Forest plots showing (a) meta-analysis results (b) subgroup (by country) meta-analysis results for NDM gene. CI, confidence interval. Horizontal lines stand for 95% CIs. The size of the squares shows the weight that individual study had in the meta-analysis
Figure 5.
Forest plots showing (a) meta-analysis results (b) subgroup (by country) meta-analysis results for OXA gene. CI, confidence interval. Horizontal lines stand for 95% CIs. The size of the squares shows the weight that individual study had in the meta-analysis
Figure 6.
Forest plots showing (a) meta-analysis results (b) subgroup (by country) meta-analysis results for VIM gene. CI, confidence interval. Horizontal lines stand for 95% CIs. The size of the squares shows the weight that individual study had in the meta-analysis
Figure 8.
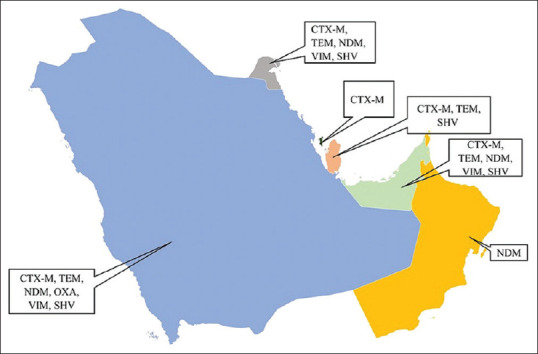
Antimicrobial resistant genes in E. coli in various GCC countries
DISCUSSION
Antibiotic-resistant bacteria, which are a serious threat to the treatment of bacterial infections, arise as a result of exposure to antibiotics in clinical and agricultural settings. Among the Gram-negative bacteria, E. coli has emerged as a serious health hazard over the past 20 years.[44] Regional monitoring is necessary for controlling the spread of antimicrobial resistance genes in E. coli. To our knowledge, our meta-analysis is the first to report the prevalence of the six AMR genes conducted in the GCC region.
The results presented here showed a predominance of CTX-M gene in the GCC countries. Infections with CTX-M-producing bacteria are of huge importance, as increasing rates of ESBL producers result in prescribing carbapenems, in turn resulting in the emergence and spread of untreatable carbapenem resistance.[45] Articles from Saudi Arabia showed a predominance of CTX-M gene types, which were in proportion to the studies published in Kuwait. This consistency between countries could be due to the misuse or overuse of identical class of antibiotics in humans and animals, type of population and frequent travel, increased global migration, contamination of the environment and the food chain with human and animal wastes.[46]
In recent years, CTX-M-type ESBLs is reported in Europe, North America and Latin America.[47,48,49] In fact, the spread of CTX-M gene is prominent worldwide, which is alarming. A study in China reported that CTX-M type accounted for 70% of ESBL-producing E. coli strains in the past 10 years.[50] Studies from Tunisia have reported an increase in the prevalence of CTX-M since 2000 in healthcare settings.[51] A study from south-eastern United States found CTX-M in 68% of the E. coli isolates.[52] A study from Southern Chile has reported the presence of CTX-M, TEM and SHV among E. coli isolates.[47] Recently, a study from Cuba reported 61.1% of CTX-M among E. coli clinical strains.[53] Countries such as India, China, Korea, Japan, and Taiwan have also reported predominance of CTX-M-type ESBLs.[54] Regular global monitoring of the CTX-M gene and the genotypes are important for early intervention in addressing the rise of resistant bacteria.[55]
The present analysis also showed the prevalence of TEM (40.6%), NDM (28.4%), OXA (24.3%), VIM (8.5%), and SHV (7.8%) in the GCC region. In Iran, one study reported that the frequency of SHV and TEM was 8.4% and 50%, respectively,[56] while another reported it as 92% and 70%, respectively, from strains isolated from bile specimens.[57] In addition, Kazemian et al. reported the presence of CTX-M in 21.5%, TEM in 16.9% and SHV in 16.9% of E. coli strains.[58] In India, the frequency of CTX-M among E. coli isolates was reported at 57.7%, TEM 30.1%, SHV 10.6%, and NDM-1 18.2%.[59] Recently, a study from Cuba reported 31.7% TEM and 0.7% NDM-1 among E. coli clinical strains.[53] A study in Turkey observed TEM and CTX-M in 72.72% and 22.72% of E. coli isolates.[60]
In Europe, the prevalence of carbapenem resistance is yet relatively low (0–1.6%). In a recent study by Sepp et al., where 10,780 clinical E. coli strains from Northern and Eastern Europe were screened, only one NDM-1-producing E. coli was found. The authors also reported that the most commonly observed carbapenemase was blaOXA-48.[61] Another study from Germany also found that the most common carbapenemase was OXA-48, and this has rapidly spread from Europe and the Middle East to every continent.[62] The major reservoir of NDM producers in the Asian continent is China and India, with an abundance of NDM-1 variants.[63] Low prevalence of NDM-1 gene has also been reported from Pakistan and the United Kingdom.[64] However, the spread of these isolates in the community necessitates an urgent call for resistance surveillance and molecular characterization of the resistant genes.[65]
Bell et al.[66] observed a strong relationship between antibiotic consumption and resistance in southern European countries. He stated the critical components of a strategy responsible to reduce bacterial resistance are the individual level prescribing and also the public policy addressing the problem at the national and the regional levels. Another factor contributing to the emergence and spread of resistance is self-medication.[67] Antibiotics consumption may not only produce resistance at the individual level but also spread resistance at the community, national, and regional levels.[66] Other factors contributing to antibiotic resistance are the presence of highly susceptible immunocompromised patients (e.g., cancer patients, or transplant recipients), fragile elderly patients, and inappropriate infection control measures in the hospital settings.[68]
The study has a limitation of lack of analysis of all the AMR genes in the GCC countries. Because of the low number of studies identifying resistance mechanisms in various GCC countries, the extent of the AMR resistance is not fully known.
CONCLUSION
This meta-analysis found that CTX-M gene is the most common AMR-conferring gene in E. coli strains from most GCC countries, generally followed by TEM, NDM, OXA, VIM, and SHV antimicrobial-resistance genes. These results indicate the need for more stringent and active measures across various platforms to raise awareness, stewardship, and surveillance to prevent and control further multidrug-resistant E. coli infections.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Peer review
This article was peer-reviewed by three independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mehrad B, Clark NM, Zhanel GG, Lynch JP., 3rd Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest. 2015;147:1413–21. doi: 10.1378/chest.14-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkhy HH, Assiri AM, Mousa HA, Al-Abri SS, Al-Katheeri H, Alansari H, et al. The strategic plan for combating antimicrobial resistance in Gulf Cooperation Council States. J Infect Public Health. 2016;9:375–85. doi: 10.1016/j.jiph.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Zowawi HM, Balkhy HH, Walsh TR, Paterson DL. β-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin Microbiol Rev. 2013;26:361–80. doi: 10.1128/CMR.00096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Zarouni M, Senok A, Rashid F, Al-Jesmi SM, Panigrahi D. Prevalence and antimicrobial susceptibility pattern of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the United Arab Emirates. Med Princ Pract. 2008;17:32–6. doi: 10.1159/000109587. [DOI] [PubMed] [Google Scholar]
- 5.Jamal W, Rotimi VO, Albert MJ, Khodakhast F, Nordmann P, Poirel L. High prevalence of VIM-4 and NDM-1 metallo-β-lactamase among carbapenem-resistant Enterobacteriaceae. J Med Microbiol. 2013;62:1239–44. doi: 10.1099/jmm.0.059915-0. [DOI] [PubMed] [Google Scholar]
- 6.Alghoribi MF, Gibreel TM, Farnham G, Al Johani SM, Balkhy HH, Upton M. Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. J Antimicrob Chemother. 2015;70:2757–62. doi: 10.1093/jac/dkv188. [DOI] [PubMed] [Google Scholar]
- 7.Bindayna K, Khanfar HS, Senok AC, Botta GA. Predominance of CTX-M genotype among extended spectrum beta lactamase isolates in a tertiary hospital in Saudi Arabia. Saudi Med J. 2010;31:859–63. [PubMed] [Google Scholar]
- 8.Marie MA, John J, Krishnappa LG, Gopalkrishnan S. Molecular characterization of the β-lactamases in Escherichia coli and Klebsiella pneumoniae from a tertiary care hospital in Riyadh, Saudi Arabia. Microbiol Immunol. 2013;57:805–10. doi: 10.1111/1348-0421.12104. [DOI] [PubMed] [Google Scholar]
- 9.Alfaresi M, Kim Sing G, Senok A. First report of blaCTX-M-28 in Enterobacteriaceae isolates in the United Arab Emirates. J Pathog. 2018;2018:1304793. doi: 10.1155/2018/1304793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonnevend Á, Ghazawi AA, Hashmey R, Jamal W, Rotimi VO, Shibl AM, et al. Characterization of carbapenem-resistant Enterobacteriaceae with high rate of autochthonous transmission in the Arabian Peninsula. PLoS One. 2015;10:e0131372. doi: 10.1371/journal.pone.0131372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taqi M, Jamal W, Rotimi V. The prevalence of extended-spectrum β-lactamase (ESBL) and carbapenem-resistant Enterobacteriaceae (CRE) isolates in positive blood cultures of patients in A teaching hospital in Kuwait over A 2-year period. Open Forum Infect Dis. 2017;4(Suppl 1):S564. [Google Scholar]
- 12.Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin Microbiol Infect. 2012;18:E144–8. doi: 10.1111/j.1469-0691.2012.03796.x. [DOI] [PubMed] [Google Scholar]
- 13.Zowawi HM, Sartor AL, Balkhy HH, Walsh TR, Al Johani SM, AlJindan RY, et al. Molecular characterization of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in the countries of the Gulf Cooperation Council: Dominance of OXA-48 and NDM producers. Antimicrob Agents Chemother. 2014;58:3085–90. doi: 10.1128/AAC.02050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltai NO, Yassine HM, Al Thani AA, Abu Madi MA, Ismail A, Ibrahim E, et al. Prevalence of antibiotic resistant Escherichia coli isolates from fecal samples of food handlers in Qatar. Antimicrob Resist Infect Control. 2018;7:78. doi: 10.1186/s13756-018-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelgader SA, Shi D, Chen M, Zhang L, Hejair HM, Muhammad U, et al. Antibiotics resistance genes screening and comparative genomics analysis of commensal Escherichia coli isolated from poultry farms between China and Sudan. Biomed Res Int. 2018;2018:5327450. doi: 10.1155/2018/5327450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. [Last accessed on 2020 Jan 26]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 18.Eltai NO, Al Thani AA, Al-Ansari K, Deshmukh AS, Wehedy E, Al-Hadidi SH, et al. Molecular characterization of extended spectrum β -lactamases Enterobacteriaceae causing lower urinary tract infection among pediatric population. Antimicrob Resist Infect Control. 2018;7:90. doi: 10.1186/s13756-018-0381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elhassan MM, Hemeg HA, Ahmed AA. Dissemination of CTX-M extended-spectrum β-lactamases (ESBLs) among Escherichia coli and Klebsiella pneumoniae in Al-Madenah Al-Monawwarah region, Saudi Arabia. Int J Clin Exp Med. 2016;9:11051–7. [Google Scholar]
- 20.Abd El Ghany M, Sharaf H, Al-Agamy MH, Shibl A, Hill-Cawthorne GA, Hong PY. Genomic characterization of NDM-1 and 5, and OXA-181 carbapenemases in uropathogenic Escherichia coli isolates from Riyadh, Saudi Arabia. PLoS One. 2018;13:e0201613. doi: 10.1371/journal.pone.0201613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamal WY, Albert MJ, Rotimi VO. High prevalence of New Delhi Metallo-β-lactamase-1 (NDM-1) producers among carbapenem-resistant Enterobacteriaceae in Kuwait. PLoS One. 2016;11:e0152638. doi: 10.1371/journal.pone.0152638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dashti AA, Vali L, El-Shazly S, Jadaon MM. The characterization and antibiotic resistance profiles of clinical Escherichia coli O25b-B2-ST131 isolates in Kuwait. BMC Microbiol. 2014;14:214. doi: 10.1186/s12866-014-0214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsultan AA, Aboulmagd E, Amin TT. ESBL-producing E. coli and K. pneumoniae in Al-Ahsa, Saudi Arabia: Antibiotic susceptibility and prevalence of blaSHV and blaTEM. J Infect Dev Ctries. 2013;7:1016–9. doi: 10.3855/jidc.3764. [DOI] [PubMed] [Google Scholar]
- 24.Al Sheikh YA, Marie MA, John J, Krishnappa LG, Dabwab KH. Prevalence of 16S rRNA methylase genes among beta-lactamase-producing Enterobacteriaceae clinical isolates in Saudi Arabia. Libyan J Med. 2014;9:24432. doi: 10.3402/ljm.v9.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Agamy MH, Shibl AM, Hafez MM, Al-Ahdal MN, Memish ZA, Khubnani H. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli in Riyadh: Emergence of CTX-M-15-producing E.coli ST131. Ann Clin Microbiol Antimicrob. 2014;13:4. doi: 10.1186/1476-0711-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan MI, Alkharsah KR, Alzahrani AJ, Obeid OE, Khamis AH, Diab A. Detection of extended spectrum beta-lactamases-producing isolates and effect of AmpC overlapping. J Infect Dev Ctries. 2013;7:618–29. doi: 10.3855/jidc.2919. [DOI] [PubMed] [Google Scholar]
- 27.Al-Mijalli SHS. Extended-spectrum β-lactamase enzymes (ESBLs) produced by Escherichia coli urinary pathogens at Riyadh, Saudi Arabia. J Antimicrob Agents. 2016;2:1–9. [Google Scholar]
- 28.Hassan H, Abdalhamid B. Molecular characterization of extended-spectrum beta-lactamase producing Enterobacteriaceae in a Saudi Arabian tertiary hospital. J Infect Dev Ctries. 2014;8:282–8. doi: 10.3855/jidc.3809. [DOI] [PubMed] [Google Scholar]
- 29.Leangapichart T, Gautret P, Brouqui P, Memish ZA, Raoult D, Rolain JM. Acquisition of mcr-1 plasmid-mediated colistin resistance in Escherichia coli and Klebsiella pneumoniae during Hajj 2013 and 2014. Antimicrob Agents Chemother. 2016;60:6998–9. doi: 10.1128/AAC.01486-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alyamani EJ, Khiyami AM, Booq RY, Majrashi MA, Bahwerth FS, Rechkina E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann Clin Microbiol Antimicrob. 2017;16:1. doi: 10.1186/s12941-016-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alzahrania AK, Farag MM, Abbadia SH, Hassan MM, Gaber A, Abdel-Moneima AS. Antibiotic resistance profile and random amplification typing of β-lactamase-producing Enterobacteriaceae from the local area of Al-Taif and nearby cities in Saudi Arabia. Asian Biomed. 2016;10:219–28. [Google Scholar]
- 32.Hassan MM, Gaber A, Attia AO, Baiuomy AR. Molecular characterization of antibiotic resistance genes in pathogenic bacteria isolated from patients in Taif Hospitals, KSA. Am J Phytomed Clin Ther. 2014;2:939–51. [Google Scholar]
- 33.Mashwal FA, El Safi SH, George SK, Adam AA, Jebakumar AZ. Incidence and molecular characterization of the extended spectrum beta lactamase-producing Escherichia coli isolated from urinary tract infections in Eastern Saudi Arabia. Saudi Med J. 2017;38:811–5. doi: 10.15537/smj.2017.8.18578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soliman M, Wahid JB, Refaat KM. Phenotyping and molecular characterization of extended-spectrum beta-lactamases among clinical isolates of gram-negative bacilli in Arar Tertiary Care Hospital, Saudi Arabia. J Commun Dis. 2018;50:22–7. [Google Scholar]
- 35.Ahmed MA, Bansal D, Acharya A, Elmi AA, Hamid JM, Ahmed AM, et al. Antimicrobial susceptibility and molecular epidemiology of extended-spectrum betalactamase- producing Enterobacteriaceae from Intensive Care Units at Hamad Medical Corporation, Qatar. Antimicrob Resist Infect Control. 2016;5:4. doi: 10.1186/s13756-016-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahid M. Prevalence of CTX M extended-spectrum beta-lactamases in clinical gram-negative bacteria. Bahrain Med Bull. 2014;36:228–231. [Google Scholar]
- 37.Yasir M, Ajlan AM, Shakil S, Jiman-Fatani AA, Almasaudi SB, Farman M, et al. Molecular characterization, antimicrobial resistance and clinico-bioinformatics approaches to address the problem of extended-spectrum β-lactamase-producing Escherichia coli in western Saudi Arabia. Sci Rep. 2018;8:14847. doi: 10.1038/s41598-018-33093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alam MZ, Alam Q, Jiman-Fatani AA, Shukri HA, Haque A. A surveillance study on the prevalence and antimicrobial resistance pattern among different groups of bacteria isolated from Western province of Saudi Arabia. Biomed Res. 2017;28:898–906. [Google Scholar]
- 39.Alqasim A, Abu Jaffal A, Alyousef AA. Prevalence of multidrug resistance and extended-spectrum β-lactamase carriage of clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Int J Microbiol. 2018;2018:3026851. doi: 10.1155/2018/3026851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leangapichart T, Dia NM, Olaitan AO, Gautret P, Brouqui P, Rolain JM. Acquisition of extended-spectrum β-lactamases by Escherichia coli and Klebsiella pneumoniae in gut microbiota of pilgrims during the Hajj Pilgrimage of 2013. Antimicrob Agents Chemother. 2016;60:3222–6. doi: 10.1128/AAC.02396-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somily AM, Arshad MZ, Garaween GA, Senok AC. Phenotypic and genotypic characterization of extended-spectrum b-lactamases producing Escherichia coli and Klebsiella pneumoniae in a tertiary care hospital in Riyadh, Saudi Arabia. Ann Saudi Med. 2015;35:435–9. doi: 10.5144/0256-4947.2015.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn C, Butt AA, Rivera JI, Yaqoob M, Hag S, Khalil A, et al. OXA-48-producing Enterobacteriaceae causing bacteremia, United Arab Emirates. Int J Infect Dis. 2015;30:36–7. doi: 10.1016/j.ijid.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.AlTamimi M, AlSalamah A, AlKhulaifi M, AlAjlan H. Comparison of phenotypic and PCR methods for detection of carbapenemases production by Enterobacteriaceae. Saudi J Biol Sci. 2017;24:155–61. doi: 10.1016/j.sjbs.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padmini N, Ajilda AAK, Sivakumar N, Selvakumar G. Extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae: Critical tools for antibiotic resistance pattern. J Basic Microbiol. 2017;57:460–70. doi: 10.1002/jobm.201700008. [DOI] [PubMed] [Google Scholar]
- 45.Geyer CN, Fowler RC, Johnson JR, Johnston B, Weissman SJ, Hawkey P, et al. Evaluation of CTX-M steady-state mRNA, mRNA half-life and protein production in various STs of Escherichia coli. J Antimicrob Chemother. 2016;71:607–16. doi: 10.1093/jac/dkv388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: A rundown of a global crisis. Infect Drug Resist. 2018;11:1645–58. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pavez M, Troncoso C, Osses I, Salazar R, Illesca V, Reydet P, et al. High prevalence of CTX-M-1 group in ESBL-producing Enterobacteriaceae infection in Intensive Care Units in southern Chile. Braz J Infect Dis. 2019;23:102–10. doi: 10.1016/j.bjid.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guiral E, Pons MJ, Vubil D, Marí-Almirall M, Sigaúque B, Soto SM, et al. Epidemiology and molecular characterization of multidrug-resistant Escherichia coli isolates harboring bla (CTX-M) group 1 extended-spectrum β-lactamases causing bacteremia and urinary tract infection in Manhiça, Mozambique. Infect Drug Resist. 2018;11:927–36. doi: 10.2147/IDR.S153601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merino I, Hernández-García M, Turrientes MC, Pérez-Viso B, López-Fresneña N, Diaz-Agero C, et al. Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J Antimicrob Chemother. 2018;73:2973–80. doi: 10.1093/jac/dky296. [DOI] [PubMed] [Google Scholar]
- 50.Xia S, Fan X, Huang Z, Xia L, Xiao M, Chen R, et al. Dominance of CTX-M-type extended-spectrum β-lactamase (ESBL)-producing Escherichia coli isolated from patients with community-onset and hospital-onset infection in China. PLoS One. 2014;9:e100707. doi: 10.1371/journal.pone.0100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zorgani A, Almagatef A, Sufya N, Bashein A, Tubbal A. Detection of CTX-M-15 among uropathogenic Escherichia coli isolated from five major hospitals in Tripoli, Libya. Oman Med J. 2017;32:322–7. doi: 10.5001/omj.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen LF, Freeman JT, Nicholson B, Keiger A, Lancaster S, Joyce M, et al. Widespread dissemination of CTX-M-15 genotype extended-spectrum-β-lactamase-producing Enterobacteriaceae among patients presenting to community hospitals in the southeastern United States. Antimicrob Agents Chemother. 2014;58:1200–2. doi: 10.1128/AAC.01099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quiñones D, Aung MS, Carmona Y, González MK, Pereda N, Hidalgo M, et al. High prevalence of CTX-M type extended-spectrum beta-lactamase genes and detection of NDM-1 carbapenemase gene in extraintestinal pathogenic Escherichia coli in Cuba. Pathogens. 2020;9:65. doi: 10.3390/pathogens9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Copur Cicek A, Saral A, Ozad Duzgun A, Yasar E, Cizmeci Z, Ozlem Balci P, et al. Nationwide study of Escherichia coli producing extended-spectrum β-lactamases TEM, SHV and CTX-M in Turkey. J Antibiot. 2013;66:647–50. doi: 10.1038/ja.2013.72. [DOI] [PubMed] [Google Scholar]
- 55.Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72:2145–55. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
- 56.Komijani M, Bouzari M, Rahimi F. Detection of TEM, SHV and CTX-M antibiotic resistance genes in Escherichia coli isolates from infected wounds. Med Lab J. 2017;11:30–5. [Google Scholar]
- 57.Fatemi SM, Doosti A, Tavakoli H, Moayednia R, Ghasemi-Dehkordi P, Kelidari B, et al. Antibiotic susceptibility patterns of isolated bacteria from bile fluids of patients with gallstone disease in Isfahan city. Arch Biol Sci. 2015;67:611–7. [Google Scholar]
- 58.Kazemian H, Heidari H, Ghanavati R, Ghafourian S, Yazdani F, Sadeghifard N, et al. Phenotypic and genotypic characterization of ESBL-, AmpC-, and carbapenemase-producing Klebsiella pneumoniae and Escherichia coli isolates. Med Princ Pract. 2019;28:547–51. doi: 10.1159/000500311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borah VV, Saikia KK, Chandra P, Hazarika NK, Chakravarty R. New Delhi metallo-β-lactamase and extended spectrum β-lactamases co-producing isolates are high in community-acquired urinary infections in Assam as detected by a novel multiplex polymerase chain reaction assay. Indian J Med Microbiol. 2016;34:173–82. doi: 10.4103/0255-0857.176853. [DOI] [PubMed] [Google Scholar]
- 60.Bali E, Açık L, Sultan N. Phenotypic and molecular characterization of SHV, TEM, CTX-M and extended-spectrum β-lactamase produced by Escherichia coli, Acinobacter baumannii and Klebsiella isolates in a Turkish hospital. Afr J Microbiol Res. 2010;4:650–4. [Google Scholar]
- 61.Sepp E, Andreson R, Balode A, Bilozor A, Brauer A, Egorova S, et al. Phenotypic and molecular epidemiology of ESBL-, AmpC-, and carbapenemase-producing Escherichia coli in Northern and Eastern Europe. Front Microbiol. 2019;10:2465. doi: 10.3389/fmicb.2019.02465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18:413–31. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 63.Khan AU, Maryam L, Zarrilli R. Structure, genetics and worldwide spread of New Delhi Metallo-β-lactamase (NDM): A threat to public health. BMC Microbiol. 2017;17:101. doi: 10.1186/s12866-017-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwal A, Srivastava J, Maheshwari U, Iftikhar M. Molecular characterization and antimicrobial susceptibility profile of New Delhi metallo-beta-lactamase-1-producing Escherichia coli among hospitalized patients. J Lab Physicians. 2018;10:149–54. doi: 10.4103/JLP.JLP_76_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abrar S, Ain NU, Liaqat H, Hussain S, Rasheed F, Riaz S. Distribution of blaCTX-M, blaTEM, blaSHV and blaOXA genes in extended-spectrum-β-lactamase-producing clinical isolates: A three-year multi-center study from Lahore, Pakistan. Antimicrob Resist Infect Control. 2019;8:80. doi: 10.1186/s13756-019-0536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dandachi I, Chaddad A, Hanna J, Matta J, Daoud Z. Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the Middle East using a one health approach. Front Microbiol. 2019;10:1941. doi: 10.3389/fmicb.2019.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog Glob Health. 2015;109:309–18. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.