Abstract
Over the past five years, several studies have reported deposition and retention of gadolinium in the brain after administration of gadolinium-based contrast agents (GBCAs) during radiological procedures. Patients with renal insufficiency cannot filter gadolinium efficiently; however, gadolinium is also retained in the brain of some adults and pediatrics with no renal impairment. In the literature, data is mostly available from retrospective magnetic resonance imaging (MRI) studies, where gadolinium deposition may be indirectly measured by evaluating changes in T1 signal intensity in the brain tissues, particularly in the deep gray matter such as the dentate nucleus and/or globus pallidus. Many pathological studies have reported a direct correlation between T1 signal changes and gadolinium deposition in human and animal autopsy specimens, which raised concerns on the use of GBCAs, particularly with linear chelators. The association between gadolinium accumulation and occurrence of physical and neurological side effects or neurotoxic damage has not yet been conclusively demonstrated. Studies have also observed that gadolinium is deposited in the extracranial tissues, such as the liver, skin, and bone, of patients with normal kidney function. This narrative review describes the effects of different types of GBCAs in relation to gadolinium deposition, evaluates current evidence on gadolinium deposition in various tissues of the human body, and summarizes the current recommendations regarding the use of GBCAs.
Keywords: Diagnostic imaging, gadolinium adverse effects, gadolinium deposition, gadolinium retention, gadolinium-based contrast agents (GBCAs), T1 hyperintensity
INTRODUCTION
Magnetic resonance imaging (MRI) uses strong magnetic fields to change the spin of atoms in human bodies.[1] The use of contrast-enhanced magnetic resonance imaging (CE-MRI) has become a necessary tool in diagnostics. Its use in pathological tissue diagnosis ranges from cancerous to inflammatory conditions. Worldwide, gadolinium-based contrast agents (GBCAs) have been frequently used for over three decades, and until recently, the assumption was that its use had limited risks.
In 2006, Marckmann et al. reported that some GBCAs may lead to nephrogenic systemic fibrosis (NSF) in patients with impaired renal function, wherein fibrotic changes may be seen in many tissues, predominately in the skin and muscle, causing contracture with time.[2] In general, the diagnosis of NSF is made on histopathology of a biopsy specimen from a clinically involved site, but it can progress rapidly and is sometimes fatal. The likelihood of developing NSF depends on the molecular type of GBCA, and it more common with linear chelator. The ACR Committee on Drugs and Contrast Media, the European Medicines Agency (EMA), and the U.S. Food and Drug Administration (FDA) have all classified GBCAs into three different groups based on the reported associations with NSF.[3,4,5] The ACR classification is given in Table 1. Group 1 GBCA shows the highest association with NSF (gadodiamide, gadopentetate, and gadoversetamide).[6] Therefore, it is essential that the renal glomerular filtration rate is determined before a CE-MRI is undertaken.
Table 1.
ACR manual classification of gadolinium-based agents relative to nephrogenic systemic fibrosis
| Group | Description | Generic and Trade names |
|---|---|---|
| Group I | Agents associated with the greatest number of NSF cases | Gadodiamide (Omniscan®) |
| Gadopentetate dimeglumine (Magnevist®) | ||
| Gadoversetamide (OptiMARK®) | ||
| Group II | Agents associated with few, if any, unconfounded cases of NSF | Gadobenate dimeglumine (MultiHance®) |
| Gadobutrol (Gadavist®) | ||
| Gadoterate acid (Dotarem®) | ||
| Gadoteridol (ProHance®) | ||
| Group III | Agents for which data remains limited regarding NSF risk, but for which few, if any unconfounded cases of NSF have been reported | Gadoxetate disodium (Eovist®) |
NSF – Nephrogenic systemic fibrosis; ACR – American College of Radiology
In 2013, a Japanese research group reported that signal intensity in the dentate nucleus (DN) and/or globus pallidus (GP) on unenhanced T1 weighted imaging (T1WI) may be because of the previously administrated GBCAs.[7] Consequently, additional studies confirmed the association between repeated administration of GBCAs and high T1 signal intensity in the DN and GP [Table 2]. However, most studies on this subject are retrospective MRI studies. Further, the studies have focused on understanding the pathomechanisms of GBCA accumulation in the brain, and the consequent possible risks. In addition, several studies have found that GBCAs can be deposited in other organs including the skin, bone, and liver, despite a normal renal function.[8,9,10] In line with these developments, regulatory actions on GBCAs have recently changed worldwide. Accordingly, this narrative review would assess gadolinium deposition from different GBCA's in various human tissues and discuss the current recommendations regarding the use of GBCAs.
Table 2.
Review of literature of studies showing association between repeated administration of GBCAs and high T1 signal intensity in the dentate nucleus and globus pallidus
| Study | Groups | Contrast agents | Remarks |
|---|---|---|---|
|
| |||
| Studies in adults | |||
| Kanda et al., 2014[7] | 19 patients underwent >6 CE-MRI examinations; 16 patients underwent more than 6 unenhanced examinations | Gadopentetate-dimeglumine gadodiamide | High SI in the DN and GP with direct relation to number of examinations |
| Errante et al., 2014[11] | 38 patients with MS underwent CE - >2 MRI scans; 37 patients with brain metastases underwent >2 CE-MRI scans | Gadodiamide | High SI in the DN and GP has a linear relationship with the CE-MRI in patients with MS and brain metastasis |
| Weberling et al., 2015[12] | 50 patients underwent more than 5 CE-MRI | Gadobenate-dimeglumine | High SI in the DN |
| Adin et al., 2015[13] | 184 patients with brain metastasis treated with brain irradiation underwent recurrent CE-MRI | Gadopentetate-dimeglumine | Repeated CE-MRI examinations shows persistent hyperintensity in the DN on unenhanced T1WI |
| Zhang et al., 2017[14] | 13 patients who had undergone >30 CE-MRIs | Gadodiamide gadopentetate- dimeglumine gadobenate | Increased SI on unenhanced T1WI at posterior thalamus, substantia nigra, red nucleus, cerebellar peduncle, colliculi, DN, and GP |
| Ramalho et al., 2016b[15] | Studied two different groups 18 patients with previous gadodiamide and current gadobenate dimeglumine exposure 44 patients with only gadobenate dimeglumine exposure | Gadodiamide gadobenate-dimeglumine | Patients with prior gadodiamide-exposed show greater T1 SI changed compared to those without previously gadodiamide-exposed |
| Kang et al., 2018[16] | 46 patients who had undergone >1 CE-MRIs | Gadobutrol | High SI in the DN and GP |
|
| |||
| Studies in pediatrics | |||
|
| |||
| Miller et al., 2015[17] | A pediatric patient who received 35 doses of linear GBCA in a 12-year period | Gadopentetate-dimeglumine | T1 hyperintensity at DN, GP, and posterior thalamus |
| Roberts and Holden, 2015[18] | A 13 years old girl with follow-up CE-MRI scan | Gadopentetate-dimeglumine | hyperintensity was noted within both the DN and GP bilaterally at follow-up |
| Roberts et al., 2016a[19] | 16 patients underwent>5 consecutive CE-MRI scans | Gadopentetate-dimeglumine | T1-hyperintensity at both DN is significantly correlated to the number of prior GBCA doses |
| Hu et al., 2016[20] | 21 patients received CE-MRI scans between 5 and 37 times; 21 controls of similar age without CE-MRI exposure | Gadopentetate-dimeglumine | Increased SI ratios in all 21 patients with GBCA exposure 18.6%±12.7% for the DN, and 12.4%±7.4% for the GP between the first and the most recent MRI scans |
| Flood et al., 2017[21] | 46 pediatric patients who had undergone >3 CE-MRI scans; 57 age-matched GBCA-control subjects | Gadopentetate-dimeglumine | SI in the pediatric brain increases on unenhanced T1-weighted MRI with repeated exposure to linear GBCA |
| Ryu et al., 2018[22] | 93 pediatric patients who had undergone >3 CE-MRI | Gadopentetate- gadoterate meglumine and gadoteridol | Reconfirmed that the signal intensity change is significantly and exclusively related to the use of linear agents |
|
| |||
| Autopsy studies in humans | |||
|
| |||
| McDonald et al., 2015[23] | 13 patients with more than 4 GBCA administrations before death; control group of 10 patients with no prior CE-MRI | Gadodiamide | Gadolinium CNS structures deposition was associated with GBCA administration and was independent of patients’ age, sex, renal function or interval between GBCAs exposure and death |
| Kanda et al., 2015a[24] | 5 patients received linear GBCAs before death; 5 patients with no history of GBCAs exposed before death | Gadopentetate-dimeglumine gadodiamide gadoteridol | Gadolinium was deposited in the brain, highest at DN and GP even in subjects without severe renal dysfunction |
| Murata et al., 2016[25] | 5 received gadoteridol; 2 received gadobutrol; 1 received gadobenate; 1 received gadoxetate; 9 controls | Gadoteridol gadobutrol gadobenate gadoxetate | Gadolinium was found with all agents in all brain areas sampled; highest levels in GP and DN |
| McDonald et al., 2017[26] | 5 patients underwent between 4-18 CE-MRI examinations compared to 10 patients with no history of GBCA exposure | Gadodiamide | The patient DN, pons, GP and thalamus, contained >0.1 µg of gadolinium per gram of tissue compared to those with no prior GBCAs exposure |
| McDonald et al., 2017[27] | 3 patients underwent 4, 8, 9 CE-MRI examinations respectively | Gadodiamide | The patient DN, pons, GP and thalamus, contained >0.1 µg of gadolinium per gram of tissue |
SI – Signal intensities; GBCAs – Gadolinium-based contrast agents; DN – Dentate nucleus; GP – Globus pallidus; MRI – Magnetic resonance imaging; CE-MRI – Contrast enhanced-MRI; MS – Multiple sclerosis; T1WI – T1 weighted imaging; CNS: Central nervous system
For the literature review, the author searched PubMed and websites of regulatory bodies such as US-FDA, UK Medicines and Healthcare products Regulatory Agency (MHRA), Pharmacovigilance Risk Assessment Committee of the European Medicines Agency PRAC-EMA, Japanese Pharmaceuticals and Medical Devices Agency, the Ministry of Food and Drug Safety in Korea, and Saudi Food and Drug Authority (SFDA). All articles that assessed the retention of gadolinium in any human tissue was assessed, irrespective of the study design and including case reports. Further, only articles published in English were considered, and gray literature (i.e., thesis, internal reports, non-peer reviewed journals, pharmaceutical industry journals) was not reviewed.[28,29,30,31]
GADOLINIUM: CHEMISTRY, STABILITY, AND BIODISTRIBUTION
Gadolinium, which is highly toxic to humans when used in its free form (Gd3+), is a rare earth element that has strong paramagnetic properties. As a result of its toxicity, it binds to a chelate molecule (aminopolycarboxylic acid ligands) in contrast agents. Contrast agents are divided into two binding forms, namely, linear and macrocyclic chelates, which bind with open and non-open chains, respectively. These are then subdivided into the ionic and non-ionic forms [Table 3]. Linear chelates are not as stable as the macrocyclic type. However, ionic linear chelates are more stable than the non-ionic linear chelates when incubated in human serum at 37°C and pH 7.4. As a result of its insolubility at physiological pH, free gadolinium is excreted very slowly. In addition, free gadolinium's ionic radius is close to that of calcium, thereby allowing it to compete with the various physiological processes of calcium. Consequently, free gadolinium can block some calcium-gated channels.[32,33,34,35,36]
Table 3.
Types of available GBCAs based on the type of ligand (linear or macrocyclic) and charge (ionic or non-ionic)
| Linear | Macrocyclic |
|---|---|
|
| |
| Ionic | |
| Gadopentetate dimeglumine (Magnevist®) | Gadoterate meglumine (Dotarem®) |
| Gadopentetate dimeglumine (Magnevision®) | Gadoterate meglumine (Clariscan®) |
| Gadoterate meglumine (Dotagraf®) | |
| Gadoterate meglumine (Dotagita®) | |
| Gadoterate meglumine (Cyclolux®) | |
| Gadobenate dimeglumine (MultiHance®) | |
| Gadoxetate disodium (Primovist®) | |
|
| |
| Nonionic | |
|
| |
| Gadodiamide (Omniscan®) | Gadoteridol (ProHance®) |
| Gadoversetamide (OptiMARK®) | Gadobutrol (Gadovist®) |
In vivo, the various endogenous cations (e.g., Fe3+, Mg2+, Cu2+, Zn2+, Ca2+) compete with Gd3+ ions for the ligand, whereas the endogenous anions (e.g., phosphate, carbonate, and hydroxide) compete for the Gd3+ ions. This competition may destabilize the gadolinium complex in biologic fluids and shift the dissociation equilibrium toward its free components, which rapidly bind to other agents. This exchange process is termed “transmetallation.”[36]
In addition, GBCAs can be divided into three categories according to biodistribution, namely, extracellular, combined intracellular-extracellular, and blood pool agents. When GBCAs are excreted by the kidney, 98% remain unchanged. Therefore, patients with poor renal function will have reduced GBCA excretion, leading to an accumulation of GBCAs in the body, which in turn increases the probability of dissociation and retention in the body. The biliary route is an important excretion pathway for combined intracellular–extracellular GBCAs in patients with poor renal function.[36]
GADOLINIUM DEPOSITION IN BRAIN AND ITS CLINICAL SIGNIFICANCE
Recurrent administration of GBCAs results in gadolinium deposition in patients with normal and abnormal renal function. The exact mechanism and clinical implications of the deposition of gadolinium are poorly understood. Possible theories about GBCAs deposition at brain include transmetallation, which results in the de-chelation of gadolinium, the administration of linear GBCAs results in a 15-times higher concentration of free gadolinium in the brain than macrocyclic GBCAs.[37,38,39] Another possible mechanism for gadolinium accumulation is a metal transporter-mediated accumulation of GBCA. A high concentration of gadolinium has been reported in the DN and GP,[40,41] with relatively high concentration of iron or calcium in these regions.[42] However, it has been suggested that GBCAs can traverse the blood–cerebrospinal fluid barrier, but the mechanism of this action remains unclear. Factors that might increase permeability of blood–brain barrier to GBCA such as cerebritis and meningitis need to be studied further.
A recent preclinical study revealed that GBCAs distribute along the glymphatic system (a so-called waste clearance system).[43] Naganawa et al.[44] reported one case with bright signal in the subarachnoid space and perivascular space on enhanced fluid-attenuated inversion recovery (FLAIR) lasts up to 4 hours after GBCA administration. This result suggests that intravenous GBCA can be transported through the glymphatic system and reach the brain even with in those with normal renal function.[40]
Many clinical conditions can cause high-signal intensities in the GP and DN on non-enhanced T1-weighted images (T1WI), including calcification, history of brain irradiation, Langerhans cell histiocytosis, multiple sclerosis, hepatic dysfunction, Wilson disease, total parental nutrition, neurofibromatosis type 1, manganese toxicity, Rendu–Osler–Weber disease, and hemodialysis.[45,46,47] In 2013, Kanda et al.[7] reported a possible association between T1 hyperintensities in the GP and DN on non-enhanced T1WI and gadolinium retention in patients with multiple exposures to GBCAs.
The majority of publications on this subject are retrospective MRI studies, in which gadolinium deposition is determined by assessing changes in the SI of T1 in brain tissue using the spin-echo (SE), turbo SE and gradient echo (MPRAGE) techniques, specifically in deep gray matter such as the DN and/or GP [Figure 1]. In one study, analysis of postmortem samples of the DN and GP from patients who had been administered gadodiamide (nonionic linear GBCA) revealed that it was primarily deposited in the capillary endothelium and neuronal interstitium and within the nucleus of the cell in only a couple of cases.[26] Zhang et al. describes the extent of signal hyperintensity on unenhanced T1-weighted brain MR images in 13 patients after >35 administrations of linear GBCA. T1 hyperintensities were observed not only in the DN (100%) and GP (100%) but also in the substantia nigra (100%), posterior thalamus (92%), 32D nucleus (77%), colliculi (77%), superior cerebellar peduncle (54%), caudate nucleus (31%), whole thalamus (23%), and putamen (15%).[14]
Figure 1.
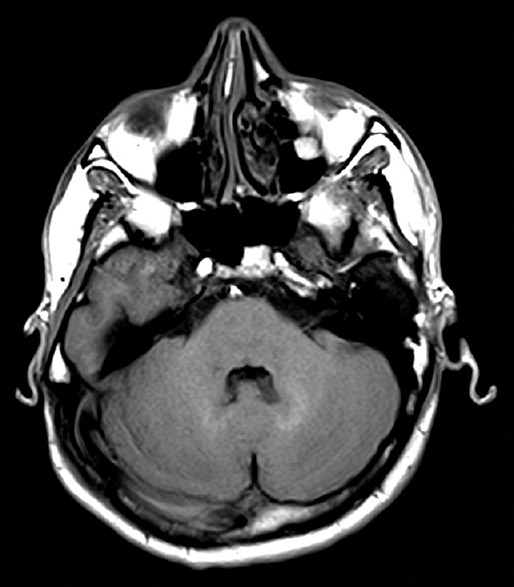
Bright signal intensity in T1 weighted images at dentate nucleus (DN) in relation to Pons
In 2017, Bauer et al.[48] conducted a retrospective pilot study that included 376 patients who underwent both contrast-enhanced MR and PET/CT imaging between 2004 and 2015. They found that the median SUVmax of the DN and GP was significantly lower in the group with exposure to gadolinium compared with the control group, with differences of 16% and 27%, respectively. Accordingly, the authors concluded that gadolinium deposition led to decreased FDG uptake due to decreased metabolism in the corresponding area. Unfortunately, they did not correlate these findings to clinical findings.
Subsequently, interest has grown in the medical community on whether clinical sequelae occurs when gadolinium deposition occurs. One of the largest studies conducted to date focused on the clinical manifestations of assumed gadolinium deposition in a total of 42 patients. These patients had reported symptoms beyond 3 months after administration of GBCA, such as central (n = 15, 36%) and peripheral pain (n = 26, 62%), headache (n = 28, 67%), bone pain (n = 26, 62%), skin changes (n = 22, 52%), and clouded mentation (n = 29, 69%).[49]
Gadolinium deposition primarily occurs in the DN and GP and if this results in neurological disorders, it will be characterized by movement disorders. However, it could also result in non-specific symptoms, including sensory symptoms and generalized bone and joint pain. To date, no studies have been conducted to determine whether peripheral nerve damage occurs as a result of the administration of gadolinium.[49]
GADOLINIUM DEPOSITION IN OTHER ORGANS
In addition to being deposited in the brain, GBCAs can be deposited in other organs including the skin, bone, and liver. For example, gadodiamide or gadoteridol could be deposited in the bones of patients undergoing total hip arthroplasty.[50] A study by Murata et al. reported that gadolinium is deposited in cortical bone at a much higher level than in brain tissue. This suggests that bone could act as a long-term storage site for gadolinium and infers a positive correlation between the two. This study proposed that the deposition of gadolinium in bone could be used as marker to estimate the level of gadolinium in the brain.[51] Another study by Roberts et al. reported a case of gadolinium accumulation in the skin of a patient with normal renal function who had been exposed to 61 cumulative doses of GBCAs.[52] A study by Maximova et al. reported the accumulation of gadolinium in the liver of pediatric patients who had normal hepatic and renal function but had an iron overload.[53]
CURRENT REGULATIONS REGARDING USE OF GBCAs
As a result of the risk of patients retaining gadolinium in the brain and other organs, PRAC-EMA made a recommendation to suspend the marketing of GBCAs that are based on linear chelators. However, the EMA highlighted that some linear GBCAs are still required for liver imaging, and thus authorized the marketing of Primovist (gadoxetate disodium) and MultiHance (gadobenate dimeglumine). The European Gadolinium Retention Evaluation Consortium recently announced that the potential risk of gadolinium to humans would be studied.[53]
In May 2017, the US-FDA determined that although GBCAs could be linked to gadolinium retention in the body, including the brain and other organs, no negative health impacts had been identified. Consequently, earlier FDA recommendations related to the use of GBCAs remain unchanged.[54]
In December 2017, the MHRA recommended the suspension of licenses for two linear agents, namely, gadodiamide and intravenous gadopentetic acid. It should be noted that authorization for the use of intra-articular gadopentetic acid remains. The licenses for gadobenate dimeglumine and gadoxetate were not suspended but were limited to imaging of the liver.[55] Furthermore, the MHRA recommended that the use of GBCAs should be limited to situations where it is essential to have the diagnostic information that would not be accessible through an unenhanced scan. In addition, it was recommended that the lowest effective dose possible should be used.[55]
In November 2017, the Japanese Pharmaceuticals and Medical Devices Agency requested specific changes to the GBCAs packages regarding precautions about gadolinium retention in the brain.[56] Similarly, in February and May 2018, the Ministry of Food and Drug Safety in Korea requested a mandatory revision of the precaution section in the GBCAs package to state that linear GBCAs can cause more gadolinium retention in brain compared to macrocyclic GBCAs. Therefore, linear GBCAs should be administered when macrocyclic GBCAs are not appropriate; for example, when a patient had previous allergic reactions to macrocyclic GBCAs or when other alternatives (e.g., gadoxetic acid disodium) are not available.[57,58]
In March 2018, the SFDA recommended that macrocyclic GBCAs should be the first choice of physicians when conducting contrast-enhanced MRI scans. The SFDA further recommended that repeated administration of linear GBCAs be limited to those situations where there is no other alternative.[59]
CONCLUSION
Linear GBCAs are less stable than macrocyclic GBCAs; therefore, they have a greater chance of brain deposition. The de-chelation of free gadolinium from unstable GBCAs can occur through transmetallation and active metal transporters in cell membranes. Current evidence suggests that GBCAs can access the brain through the blood–cerebrospinal fluid or the glymphatic system as an alternative access route. However, the understanding about the clinical implications of gadolinium retention, in the brain and other organs, remains poor. Therefore, additional studies are required to determine the exact damage resulting from the administration of gadolinium and the role other heavy metals reservoir of the human body play in the deposition of GBCAs. Findings from such studies may have potential to influence recommendations related to the use of GBCAs.
Peer review
This article was peer-reviewed by two independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mattson J, Simon M. Dean Book Co: Bar-Ilan University Press; 1996. The Pioneers of NMR and Magnetic Resonance in Medicine: The Story of MRI. [Google Scholar]
- 2.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, et al. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–62. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 3.American College of Radiology. Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. [Last accessed on 2019 Sep 20]. Version 10.3. Available from: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf .
- 4.European Medicines Agency. Questions and Answers on the Review of Gadolinium-Containing Contrast Agents. 2009. [Last accessed on 2018 May 26]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/gadolinium_31/WC500015635 .
- 5.Gadolinium-Based Contrast Agents & Nephrogenic Systemic Fibrosis FDA Briefing Document. Joint Meeting of the Cardiovascular and Renal Drugs and Drug Safety and Risk Management Advisory Committee. 2011. [Last accessed on 2018 May 26]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM190850.pdf .
- 6.Zhang B, Liang L, Chen W, Liang C, Zhang S. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS One. 2015;10:e0129720. doi: 10.1371/journal.pone.0129720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–41. doi: 10.1148/radiol.13131669. [DOI] [PubMed] [Google Scholar]
- 8.Lord ML, Chettle DR, Gräfe JL, Noseworthy MD, McNeill FE. Observed deposition of gadolinium in bone using a new noninvasive in vivo biomedical device: Results of a small pilot feasibility study. Radiology. 2018;287:96–103. doi: 10.1148/radiol.2017171161. [DOI] [PubMed] [Google Scholar]
- 9.White GW, Gibby WA, Tweedle MF. Comparison of Gd (DTPA-BMA) (Omniscan) versus Gd (HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41:272–8. doi: 10.1097/01.rli.0000186569.32408.95. [DOI] [PubMed] [Google Scholar]
- 10.Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, Ellen Campbell M, Hauschka PV, Hannigan RE. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics. 2009;1:479–88. doi: 10.1039/b905145g. [DOI] [PubMed] [Google Scholar]
- 11.Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014;49:685–90. doi: 10.1097/RLI.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 12.Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer HP. Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol. 2015;50:743–8. doi: 10.1097/RLI.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 13.Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM. Hyperintense dentate nuclei on T1-weighted MRI: Relation to repeat gadolinium administration. AJNR Am J Neuroradiol. 2015;36:1859–65. doi: 10.3174/ajnr.A4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Cao Y, Shih GL, Hecht EM, Prince MR. Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology. 2017;282:516–25. doi: 10.1148/radiol.2016152864. [DOI] [PubMed] [Google Scholar]
- 15.Ramalho J, Semelka RC, Alobaidy M, Ramalho M, Nunes RH, Castillo M. Signal intensity change on unenhanced T1-weighted images in dentate nucleus following gadobenate dimeglumine in patients with and without previous multiple administrations of gadodiamide. Eur Radiol. 2016;26:4080–8. doi: 10.1007/s00330-016-4269-7. [DOI] [PubMed] [Google Scholar]
- 16.Kang KM, Choi SH, Hwang M, Yun TJ, Kim JH, Sohn CH. T1 shortening in the globus pallidus after multiple administrations of gadobutrol: Assessment with a multidynamic multiecho sequence. Radiology. 2018;287:258–66. doi: 10.1148/radiol.2017162852. [DOI] [PubMed] [Google Scholar]
- 17.Miller JH, Hu HH, Pokorney A, Cornejo P, Towbin R. MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics. 2015;136:e1637–40. doi: 10.1542/peds.2015-2222. [DOI] [PubMed] [Google Scholar]
- 18.Roberts DR, Holden KR. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev. 2016;38:331–6. doi: 10.1016/j.braindev.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Roberts DR, Chatterjee AR, Yazdani M, Marebwa B, Brown T, Collins H, et al. Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol. 2016;37:2340–7. doi: 10.3174/ajnr.A4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu HH, Pokorney A, Towbin RB, Miller JH. Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: Evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol. 2016;46:1590–8. doi: 10.1007/s00247-016-3646-3. [DOI] [PubMed] [Google Scholar]
- 21.Flood TF, Stence NV, Maloney JA, Mirsky DM. Pediatric brain: Repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology. 2017;282:222–8. doi: 10.1148/radiol.2016160356. [DOI] [PubMed] [Google Scholar]
- 22.Ryu YJ, Choi YH, Cheon JE, Lee WJ, Park S, Park JE, et al. Pediatric brain: Gadolinium deposition in dentate nucleus and globus pallidus on unenhanced T1-weighted images is dependent on the type of contrast agent. Invest Radiol. 2018;53:246–55. doi: 10.1097/RLI.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 23.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:772–82. doi: 10.1148/radiol.15150025. [DOI] [PubMed] [Google Scholar]
- 24.Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276:228–32. doi: 10.1148/radiol.2015142690. [DOI] [PubMed] [Google Scholar]
- 25.Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: Preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51:447–53. doi: 10.1097/RLI.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 26.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Paolini MA, Murray DL, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR Imaging in adult patients without intracranial abnormalities. Radiology. 2017;285:546–54. doi: 10.1148/radiol.2017161595. [DOI] [PubMed] [Google Scholar]
- 27.McDonald JS, McDonald RJ, Jentoft ME, Paolini MA, Murray DL, Kallmes DF, et al. Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: A case-control study. JAMA Pediatr. 2017;171:705–7. doi: 10.1001/jamapediatrics.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasziou P, Irwig P, Bain C, Colditz G. Systematic Reviews in Health Care: A Practical Guide. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 29.Khan KS, Kunz R, Kleijnen J, Antes G. How to Review and Apply Findings of Health Care Research. London: RSM Press; 2003. Systematic Reviews to Support Evidence Based Medicine. [Google Scholar]
- 30.Mulrow C, Cook D, editors. Philadelphia: American Collage of Physiciens; 1998. Systematic Reviews: Synthesis of Best Evidence for Health Care Decision. [Google Scholar]
- 31.Glasziou P, Vandenbroucke JP, Chalmers I. Assessing the quality of research. BMJ. 2004;328:39–41. doi: 10.1136/bmj.328.7430.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M. MRI contrast agents: Basic chemistry and safety. J Magn Reson Imaging. 2012;36:1060–71. doi: 10.1002/jmri.23725. [DOI] [PubMed] [Google Scholar]
- 33.Runge VM. Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging. 2000;12:205–13. doi: 10.1002/1522-2586(200008)12:2<205::aid-jmri1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 34.Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009;30:1249–58. doi: 10.1002/jmri.21967. [DOI] [PubMed] [Google Scholar]
- 35.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–67. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann HJ. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43:817–28. doi: 10.1097/RLI.0b013e3181852171. [DOI] [PubMed] [Google Scholar]
- 37.Lohrke J, Frisk AL, Frenzel T, Schöckel L, Rosenbruch M, Jost G, et al. Histology and gadolinium distribution in the rodent brain after the administration of cumulative high doses of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol. 2017;52:324–33. doi: 10.1097/RLI.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idée JM, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: Difference between linear and macrocyclic agents. Invest Radiol. 2015;50:473–80. doi: 10.1097/RLI.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kartamihardja AA, Nakajima T, Kameo S, Koyama H, Tsushima Y. Impact of impaired renal function on gadolinium retention after administration of gadolinium-based contrast agents in a mouse model. Invest Radiol. 2016;51:655–60. doi: 10.1097/RLI.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 40.Kanda T, Nakai Y, Oba H, Toyoda K, Kitajima K, Furui S. Gadolinium deposition in the brain. Magn Reson Imaging. 2016;34:1346–50. doi: 10.1016/j.mri.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Kanda T, Oba H, Toyoda K, Kitajima K, Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol. 2016;34:3–9. doi: 10.1007/s11604-015-0503-5. [DOI] [PubMed] [Google Scholar]
- 42.Valdés Hernández Mdel C, Maconick LC, Tan EM, Wardlaw JM. Identification of mineral deposits in the brain on radiological images: A systematic review. Eur Radiol. 2012;22:2371–81. doi: 10.1007/s00330-012-2494-2. [DOI] [PubMed] [Google Scholar]
- 43.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based contrast enhancement of the perivascular spaces in the basal ganglia. Magn Reson Med Sci. 2017;16:61–5. doi: 10.2463/mrms.mp.2016-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai PH, Chen C, Liang HL, Pan HB. Hyperintense basal ganglia on T1-weighted MR imaging. AJR Am J Roentgenol. 1999;172:1109–15. doi: 10.2214/ajr.172.4.10587157. [DOI] [PubMed] [Google Scholar]
- 46.Oikonomou A, Chatzistefanou A, Zezos P, Mintzopoulou P, Vadikolias K, Prassopoulos P. Basal ganglia hyperintensity on T1-weighted MRI in Rendu-Osler-Weber disease. J Magn Reson Imaging. 2012;35:426–30. doi: 10.1002/jmri.22892. [DOI] [PubMed] [Google Scholar]
- 47.da Silva CJ, da Rocha AJ, Jeronymo S, Mendes MF, Milani FT, Maia AC, Jr, et al. A preliminary study revealing a new association in patients undergoing maintenance hemodialysis: Manganism symptoms and T1 hyperintense changes in the basal ganglia. AJNR Am J Neuroradiol. 2007;28:1474–9. doi: 10.3174/ajnr.A0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer K, Lathrum A, Raslan O, Kelly PV, Zhou Y, Hewing D, et al. Do gadolinium-based contrast agents affect 18F-FDG PET/CT uptake in the dentate nucleus and the globus pallidus? A pilot study. J Nucl Med Technol. 2017;45:30–3. doi: 10.2967/jnmt.116.180844. [DOI] [PubMed] [Google Scholar]
- 49.Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, et al. Gadolinium deposition disease: Initial description of a disease that has been around for a while. Magn Reson Imaging. 2016;34:1383–90. doi: 10.1016/j.mri.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 50.Gibby WA, Gibby KA, Gibby WA. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol. 2004;39:138–42. doi: 10.1097/01.rli.0000112789.57341.01. [DOI] [PubMed] [Google Scholar]
- 51.Roberts DR, Lindhorst SM, Welsh CT, Maravilla KR, Herring MN, Braun KA, et al. High levels of gadolinium deposition in the skin of a patient with normal renal function. Invest Radiol. 2016;51:280–9. doi: 10.1097/RLI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 52.Maximova N, Gregori M, Zennaro F, Sonzogni A, Simeone R, Zanon D. Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology. 2016;281:418–26. doi: 10.1148/radiol.2016152846. [DOI] [PubMed] [Google Scholar]
- 53.EMA's Final Opinion Confirms Restrictions on Use of Linear Gadolinium Agents in Body Scans; EMA/625317/2017. [Last accessed on 2020 Feb 15]. Available from: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/referral-procedures .
- 54.U.S. Food and Drug Administration. FDA Warns that Gadolinium Based Contrast Agents (GBCAs) are Retained in the Body; Requires New Class Warnings. 2017. [Last accessed on 2020 Feb 15]. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM589442 .
- 55.UK Medicines and Healthcare Products Regulatory Agency (MHRA) Update. 2017. [Last accessed on 2020 Feb 15]. Available from: https://www.gov.uk/drug-safetyupdate/gadolinium-containing-contrast-agents-removal-of-omniscan-and-iv-magnevist-restrictions-to-the-use-of-other-linear-agents#background-and-2007-european-review .
- 56.Pharmaceuticals and Medical Devices Agency. Report on the Investigation Results. Pharmaceuticals and Medical Devices Agency. 2017. November 11, [Last accessed on 2018 May 26]. Available from: http://www.pmda.go.jp/files/000221379.pdf .
- 57.Advance Notice on Mandatory Revision of the Precautions Section in the Package Insert of Gadolinium Agent. Ministry of Food and Drug Safety Web Site. 2018. February 27, [Last accessed on 2018 May 26]. Available from: http://www.mfds.go.kr/brd/m_74/view.do?seq=40761 .
- 58.Drug Safety Information-Gadolinium Agents. Ministry of Food and Drug Safety Decision Web Site. 2018. May 25, [Last accessed on 2018 May 26]. Available from: http://www.mfds.go.kr/brd/m_545/view.do?seq=268 .
- 59.Saudi Food and Drug Administration. [Last accessed on 2020 Feb 15]. Available from: https://www.sfda.gov.sa/ar/dr ug/about/sector_departments/national_pharmacovigilance_center/Documents/GadoterateGadoversetamideOptimarkDotarem.pdf .


