Abstract
In the current study, the antioxidant and anti-inflammatory potential of hydroethanolic extract of T. foenum-graecum seeds was evaluated. Phenolic profiling of T. foenum-graecum was conducted through high-performance liquid chromatography-photodiode array (HPLC-PDA) as well as through the mass spectrometry technique to characterize compounds responsible for bioactivity, which confirmed almost 18 compounds, 13 of which were quantified through a chromatographic assay. In vitro antioxidant analysis of the extract exhibited substantial antioxidant activities with the lowest IC50 value of both DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) inhibition assays. The extract was found to be non-toxic against human RBCs and murine macrophage RAW 264.7 cells. Moreover, the extract significantly (p < 0.001) reduced the lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNF-α), intrlukin-6 (IL-6), prostaglandin E2 (PGE2), and nitric oxide (NO) in RAW 264.7 cells in a concentration-dependent manner. The hydroethanolic extract of T. foenum-graecum exhibited considerable anti-inflammatory potential by decreasing the cellular infiltration to the inflammatory site in both carrageenan-induced peritonitis and an air pouch model of inflammation. Pretreatment with T. foenum-graecum extract caused significant improvement in antioxidants such as superoxide dismutase (SOD), CAT (catalase), malondialdehyde (MDA), and myeloperoxidase (MPO) against oxidative stress induced by carrageenan. Based on our results of in vivo and in vitro experimentation, we concluded that hydroethanolic extract of T. foenum-graecum is a potential source of phenolic compounds with antioxidant and anti-inflammatory potential.
Keywords: T. foenum-graecum, air pouch inflammation, antioxidants, oxidative stress markers, cellular infiltration, lipid peroxidation, peritonitis
1. Introduction
Inflammation is the protective response of the body to noxious stimuli, microbes, and chemicals or irritants [1]. It causes change in vascular permeability, blood flow alteration and increased migration of leucocytes to the inflammatory area, and results in pain, heat, redness, swelling and functional failure of the affected tissue [2]. Several pathological processes, including arthritis, diabetes, cancer, and other severe inflammatory conditions, are usually characterized by pain and inflammation [3]. Although several antioxidant, antinociceptive, and anti-inflammatory medicines are available, these drugs are arguably inaccessible, costly, less effective, and have multiple side effects [4]. Non-conventional medicines occupy a significant place in healthcare systems, as more than 80% of people worldwide depend on them for their daily healthcare requirements, particularly in Asia and Africa [5].
Trigonella foenum-graecum (Fenugreek) is a valuable medicinal plant belonging to the Fabaceae family [6]. Plant seeds are mostly used in Asian, African, and Mediterranean countries as major ingredients of daily diets and in domains such as cosmetics, fragrances, beverages, nutrition, medicine and industry [7]. The major pharmacological attributes of fenugreek are hypotensive, antioxidant, antiviral, anticarcinogenic, galactagogue, laxative, febrifuge, carminative, anticholesterolemic, antimicrobial, etc. [8,9]. Overproduction of nitrogen and reactive oxygen species, as well as insufficient quenching/stabilization in the body, causes oxidative stress, which destroys important biomolecules (nucleic acids, lipids, and proteins) [10]. Several human diseases, including cardiac disorders, diabetes, cancer, inflammation, and neurodegenerative diseases, are exacerbated or triggered by oxidative damage to these biological molecules [11]. Plant-derived natural products, particularly polyphenolics, and related antioxidant phytochemicals have a diverse range of bioactivities, including anti-inflammation, owing to their ability to quench and alleviate oxidative stress in biological systems, thus restoring health [12]. As a result, the latest research focus has switched towards plant-based natural products as one of the most encouraging sources of curative agents of inflammation and pain [13].
Plants are an abundant source of bioactive compounds, including phenolics, phenolic acids, simple phenolics, flavonoids, derivatives of hydroxycinnamic acid, and anthocyanins [14]. Based upon their physiological properties, including scavenging of free radicals, as well as antimutagenic, anti-inflammatory, and anti-carcinogenic effects, phenolic compounds of different classes continue to attract much scientific attention [15]. Previously, anti-inflammatory properties of T. foenum-graecum have been reported against the carrageenan-induced paw edema model of inflammation, but there is no report available against carrageenan-induced air pouch inflammation and carrageenan-induced peritonitis. The antioxidant effects of polyphenolics are mainly due to their redox potential, serving as hydrogen donors, potent reducing agents, metal chelators, along with singlet oxygen quenchers [16,17,18]. Several phenolics and flavonoids have been demonstrated to have significant effects on the functioning of the immune system, including inflammatory processes [19]. Quercetin, luteolin, hesperidin, and apigenin are flavonoids containing potential anti-inflammatory activities [20]. Therefore, the current work was planned to investigate the in vitro antioxidant profiling and anti-inflammatory potential of hydroethanolic extract of T. foenum-graecum as a potential source of a safe, effective, available, and cheap antioxidant and anti-inflammatory therapeutic agent. The plant was selected due to an ethnomedical record of its use in Ayurvedic medicine in managing oxidative-stress-linked illnesses such as diabetes, as well as hypolipidemic, antihypertensive, and reproductive disorders such as the treatment of hormonal disorders, inducing labor, increasing milk supply and decreasing menstrual pain.
2. Materials and Methods
2.1. Collection of Plant Material, Identification, and Extract Preparation
T. foenum-graecum seeds were collected from market and identified by botanists at the Department of Botany, University of Agriculture, Faisalabad, Pakistan. Seeds were washed, shade dried and grinded with electric mill. Bioactive compounds from T. foenum-graecum seed powder were extracted though solvent (ethanol: water mixture (7:3)) under constant shaking for 72 h. The process was repeated thrice. The sample mixture was collected, filtered, and concentrated on rotary evaporator. The dried seed extract was re-suspended in dimethyl sulfoxide (DMSO) to obtain different concentrations. The extracts were kept in refrigerator until used [21].
2.2. Chemical Fingerprinting of T. foenum-graecum Seed Extract
2.2.1. HPLC-PDA Analysis
The analytical and instrumental parameters, i.e., mobile phase composition, flow rate, and temperature, were optimized to achieve good separation among the phenolic profile of T. foenum-graecum extract by following the protocol of Hasany et al. [22]. The high-efficiency reverse-phase octadecyl column Spherisorb ODS-2 (Waters Corporation, Milford, MA, USA) bearing a particle size of 10 μm and dimensions (length × internal diameter) of 300 mm × 4.6 mm was used under gradient mode of elution on high-performance liquid chromatography equipped with photo diode array detector (HPLC-PDA) (waters alliance 2998). For the 0.5% acetic acid in water (A), when mixed with organic solvent methanol (B) in the sequence 80A:20B (0–3 min), 70A:30B (3–6 min), 65A:35B (6–9 min), and 55A:45B (10–20 min) and run in the mobile phase at 1 mL/min, the well-resolved peaks were observed within 40 min analysis time.
2.2.2. LC-MS/MS Analysis
UHPCL equipped with mass detector (QTOF-MS/MS) Agilent 6520 was used for the analysis of sample. UHPLC chromatographic conditions were used as mobile phase in gradient mode of elution: (A) 0.1% formic acid in water and (B) 0.1% formic acid in methanol and gradient flow with 10–20% A at 1–10 min, 20–30% A at 10.1-20 min, 30–50% A at 20.1–30 min, and 50–10% A at 30.1–40 min while flow rate was set at 0.5 mL/min. Column used was octadesylsilane waters (4.6 × 100 mm, 2.5 µm) at ambient temperature. Ionization source was ESI (electrospray ionization) source operating at both positive and negative ion modes. In the ionization source, pure nitrogen gas was used as collision as well as drying gas. The capillary temperature was adjusted to 350 °C and nebulizer pressure was set to 35 psi. Ion source parameters, including flow rate of drying gas was maintained to 10 L/min, while VCap, octapole RF peak voltages, fragmentor, and skimmer were maintained to 3500, 740, 150, and 65 V, respectively, and mass range was 150–1200 Da. MS/MS fragmentation was acquired at selected precursor ions of each peak [23,24].
2.3. Determination of Total Phenolic and Total Flavonoid Contents
Total phenolic contents in T. foenum-graecum seed extract were examined by the spectrophotometer method adopted by Liu et al. [25]. Briefly, T. foenum-graecum extract (20 µL) was mixed with Folin–Ciocalteu reagent (100 µL), distilled water (1.16 mL), and sodium carbonate solution (200 µL, 20%) and incubated for 30 min at 40 °C. Absorbances of reaction mixture were measured at 700 nm. TPCs in plant extract were calculated from calibration curve prepared by different concentration of gallic acid, and results are given as mg of gallic acid equivalents (GAE)/gram of plant dry weight. For TFC, plant extract (100 µL), deionized water (200 µL), sodium nitrite (250 µL, 5%), and aluminum chloride (250 µL, 10%) were mixed and reaction mixtures were incubated for 6 min. After incubation, sodium hydroxide solution (2.5 mL, 1 M) was added and incubated for another 15 min. The reaction mixture was then diluted with deionized water (2.5 mL) and read the absorbance at 500 nm. TFC in seed extracts were calculated from the calibration curve of catechin as CE (catechin equivalents)/microgram of dry weight of plant extract.
2.4. Measurement of Total Antioxidant Capacity (TAC)
For quantification of TAC, ammonium molybdate reagent (0.004 M ammonium molybdate, 0.028 M sodium phosphate, 0.6 M sulphuric acid) was prepared. T. foenum-graecum seed extract (0.1 mL) was mixed with 1 mL of ammonium molybdate reagent and 28 μL of sodium nitrite (5%). The reaction mixtures were heated in water bath at 95 °C for 90 min. The reaction mixtures were cooled and absorbances of reaction mixtures were noted at 765 nm against blank containing ammonium molybdate reagent only. TAC in plant extract was calculated in milligrams of ascorbic acid equivalents per gram of plant dry weight (mg AAEg−1DW) [26].
2.5. In Vitro Antioxidant Profiling of T. foenum-graecum Seed Extract
2.5.1. ABTS Cation Inhibition Assay
The ABTS cation radicals were generated by mixing stock solution of ABTS (7 mM, 5 mL) with potassium persulfate (148 mM, 88 µL), and diluted with sodium acetate buffer (pH 4.5, 20 mM) having optical density of 0.70 ± 0.02 at 734 nm. Plant extract (12 μL) was mixed with ABTS solution (188 μL) and kept for 30 min at RT. Reaction mixtures were noted at 734 nm [27].
2.5.2. DPPH Inhibition Assay
The DPPH free radicals were formed by mixing DPPH (8.87 mM) with methanol (99.9%). After which 10 µL of plant extract and 190 μL of DPPH solution was mixed and incubated at 30 °C for 15 min. Absorbances were noted at 520 nm [28].
2.6. In Vitro Cytotoxicity of T. foenum-graecum Seed Extract
2.6.1. Hemolytic Assay
Hemolytic activity of T. foenum-graecum seed extract was performed against human RBCs [29]. For this, fresh human blood (5 mL) was centrifuged for 5 min at 4000 rpm. Next, supernatant was drained, and cells were washed three times with phosphate buffer saline and 10% RBC suspension was prepared. T. foenum-graecum seed extract (20 µL) was mixed with 180 µL of RBC suspension in sterile tubes and mixed with agitation. The reaction mixtures were centrifuged (5000 rpm, 5 min) after incubation of 30 min at room temperature. Supernatant (100 µL) was mixed with PBS (900 µL). Triton-X (0.1%) and PBS were used as controls. Absorbances of reaction mixtures were noted at 576 nm.
2.6.2. Thrombolytic Activity
Thrombolytic activity of T. foenum-graecum seed extract was performed by clot lysis method [30]. The clot lysis (%) activity was measured against streptokinase by using the following formula:
| Thrombolytic activity (%) = (Wc1 − Wc2)/Wc1 × 100 |
Wc1 and Wc2 represent clot weights before and after lysis, respectively.
2.7. Cell Culture
The RAW 264.7 cells were provided by the Department of Cellular and Molecular Medicine, University of California, San Diego, CA, USA, and grown in DMEM (Dulbecco’s Modified Eagle Medium), 10% of FBS (fetal bovine serum) and streptomycin (100 μg/mL), and penicillin (100 units/mL) solution and incubated at 37 °C and 5% CO2 under humified conditions. Cells were counted by Trypan blue exclusion method [31].
2.7.1. Cytotoxicity Determination
For cytotoxicity determination of T. foenum-graecum seed extract, RAW 264.7 cells (2 × 105) were seeded in 96-well plate overnight at 37 °C under humified conditions at 5% CO2. The next day, different concentrations of T. foenum-graecum (100–1000 µL) were added and cells were again incubated at 37 °C for 72 h at 5% CO2. Doxorubicin was used as positive control. After incubation, 20 µL of MTT solution (5 mg/mL) was added into each well and further incubated for another 4 h. Then, microplate was centrifuged at 800× g for 20 min, supernatant was drained, DMSO (100 µL) was added to dissolve formazan crystals, and signal was recorded at 570 nm [32].
2.7.2. Enzyme-Linked Immunosorbent Assay (ELISA)
RAW 264.7 cells (2 × 105) were grown overnight in 96-well microplate and treated next day with different concentrations (50–300 µg/mL) of T. foenum-graecum extract and standard dexamethasone (10–60 µg/mL) and stimulated with LPS (1 µg/mL). Cells were incubated for 24 h at 5% CO2. After incubation, cells were centrifuged and supernatant was stored at −80 °C for TNF-α and IL-6 analysis [33,34].
2.7.3. Measurement of NO and PGE2
For measurement of NO and PGE2, RAW264.7 cells were (2 × 105) seeded in DMEM medium with 10% of FBS, streptomycin (100 μg/mL) and penicillin (100 units/mL) solution for 24 h, and next different concentrations of T. foenum-graecum (50–300 µg/mL) and standard dexamethasone (10–60 µg/mL) were added, and cells were stimulated with LPS (1 µg/mL) for 24 h at 5% CO2. After 24 h, plate was centrifuged, and supernatant was collected and analyzed for NO and PGE2 [35,36].
2.8. In Vivo Toxicological Evaluation of T. foenum-graecum Seed Extract
2.8.1. Ethical Considerations
In vivo animal study was approved by Institutional Animal Care and Use Committee (IACUC), University of Agriculture Faisalabad, Pakistan.
2.8.2. Animals
Healthy albino rats (150–200 g) were kept at 24 °C in polypropylene cages provided with softwood shavings as bedding material, with standard conditions and free access to standard rodent diet with water ad libitum. Prior to dosing, all rats were adapted to the laboratory setting for a time period of one week [37].
2.8.3. Acute and Subacute Toxicity Analysis
The rats were assigned into two groups, Group I labelled as placebo control receiving saline solution and Group II as the treatment group received dose of 2000 mg/kg BW T. foenum-graecum extract at single. The treatment was given only once on starting day of experimentation and rats were observed for behavioral motor and neuronal activities, including sleep, salivation, eye color, convulsions, lethargy, skin and fur appearance, tremors, and diarrhea. All activities were monitored closely and recorded at different time intervals. In the absence of symptoms of toxicity or mortality during acute toxicity period, subacute toxicity analysis was performed on new set of rats randomly assigned into four different groups and human equivalent doses (250, 500 and 1000 mg/kg BW, respectively) were given daily for 28 days. All experiments were carried out by using OECD (Organization of Economic Co-operation and Development) guidelines and rats were monitored in the same way as in acute toxicity. Animals were euthanized after 28 days, and blood samples, and parts of different organs were stored for hematological, biochemical, and histopathological profiling. The animals were properly disposed of in accordance with the established procedures [38].
2.9. Anti-Inflammatory Potential
2.9.1. Air Pouch Inflammation
Air pouch model of inflammation was used for assessment of in vivo anti-inflammatory potential of T. foenum-graecum seed extract [39]. Animals were randomly assigned into six groups. Air pouch on intracapsular region of rats was generated by injecting 5 mL of sterile air into dorsal side. An additional 3 mL of air was injected to air pouch after three days. After seven days of first injection, a carrageenan solution (0.5 mL, 1.5%) was given into the air cavity directly to execute inflammatory response. T. foenum-graecum seed extract treatment (100, 200 and 400 mg/kg) was also given along with carrageenan into the air cavity directly. Animals were sacrificed at different time points (6, 12, 24 h) and through cervical dislocation and pouch tissue were dissected precisely to collect the inflammatory exudate. Cellular infiltration in inflammatory exudate was measured to assess the anti-inflammatory response of plant extract. Morphological changes in the pouch tissues were also observed through histopathological examination.
2.9.2. Carrageenan-Induced Peritonitis
Animals (randomly assigned into six groups) were pretreated orally with T. foenum-graecum seed extract (100, 200, 400 mg/kg BW), saline solution (0.9%, placebo control) and dexamethasone (20 mg/kg BW, standard group) before 30 min of intraperitoneal injection of carrageenan. Then, animals were slaughtered after 4 h of carrageenan injection, and peritoneal cavities of animals were washed with normal saline solution. Cellular count was performed in peritoneal fluid by dissolving 20 µL of peritoneal fluid in Turk’s solution (0.38 mL). The collected peritoneal fluid was centrifuged at 10,000 rpm for 10 min and stored at −8 °C for analysis of oxidative stress and lipid per-oxidation parameters [40].
2.9.3. Measurement of Oxidative Stress Parameters and Lipid Peroxidation
Oxidative stress markers, including total oxidant status (TOS), total antioxidant status (TAS), and lipid peroxidation markers, i.e., myeloperoxidase (MPO) and malondialdehyde (MDA), along with superoxide dismutase (SOD) and catalase (CAT) level in air pouch, exudate and peritoneal fluid, were also measured [41,42,43,44,45].
2.10. Statistical Analysis
To determine statistical significance, one-way ANOVA was performed followed by multiple comparison tests through Tukey’s test. Obtained data were presented as mean ± standard deviation of the mean. IC50 values were also calculated using regression analysis. All analysis were performed using GraphPad Prism version 8 software (Graphpad Software Inc., San Diego, CA, USA) [46].
3. Results
3.1. Screening of Phytochemicals through HPLC-DAD
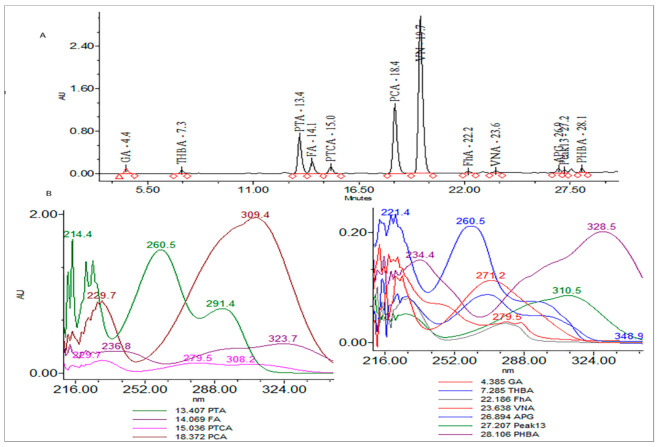
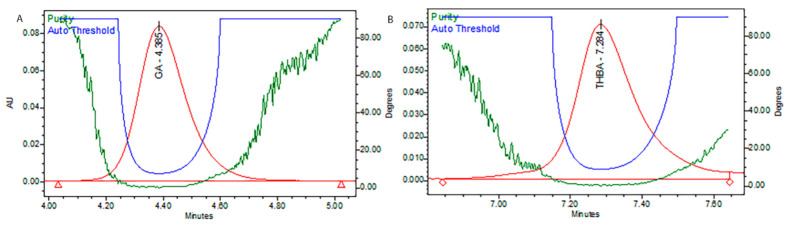
T. foenum extract was characterized for its bioactive constituents through HPLC-DAD using operating conditions previously discussed in the materials and method section (Figure 1). During the analysis, 13 compounds were identified in plant extract that were mostly phenolic acids and flavones. Peak 1 was identified as gallic acid having a response intensity of 0.17 AU (absorption unit), provided in Figure 1. When this peak was extracted for the PDA (photodiode array detector) spectrum, it showed lambda maximum (λmax nm) at 271.2 and 214.6 nm, which corresponds to a standard spectrum as well as the NIST library. The purity of the peak was also assured through the 3D spectrum as well as measuring the purity angle and purity threshold of the peak, which are given in Figure 2 as well as Table 1. The concentration of gallic acid was measured by comparing the area under the peak in comparison to the area under the peak of standard and determined 117.6 ± 1.5 mg/100 g DW. The other peaks that appeared in the chromatogram of the sample were investigated for their identification and quantification by comparison with the standards run as well as the NIST library, and the results are summarized in Table 1. The most abundant antioxidant and antimicrobial compound found was p-coumaric acid with a concentration of 256.7 ± 6.8 g/100 g of DE followed by ferulic acid 168.4 ± 1.8 g/100 g of DE. The results were in agreement with previous reports by [47,48], who reported the abundance of p-coumaric acid in plant extracts. The identification of phytochemicals indicates that antioxidant and other biological properties of extract could be due to the presence of these bioactive compounds. Almost all the phenolic acids identified have a hydroxyl group that could be responsible for the scavenging potential of these compounds [49]. Peaks of the chromatogram were identified through comparison with standards as well as the match index of the UV-visible spectrum of each peak using the NIST library. The identified compounds were presented in order of their elution on the reverse phase column.
Figure 1.
(A) Chromatogram of high-pressure liquid chromatography equipped with diode array detector (HPLC-DAD) at 280 nm and (B) extracted spectrum (200–400 nm) of each peak.
Figure 2.
Purity plots of representative peaks, (A) gallic acid, and (B) trihydroxybenzoic acid.
Table 1.
High-pressure liquid chromatography equipped with diode array detector (HPLC-DAD) summary results of each peak.
| Peak No. | Compound Name | Retention Time | ʎmax (nm) | Concentration mg/100 g DW |
|---|---|---|---|---|
| 1 | Gallic acid (GA) | 4.4 | 271.2, 214.6 | 117.6 ± 1.5 |
| 2 | Trihydroxybenzoic (TBHA) acid | 7.3 | 260.5, 221.4 | 112.6 ± 5.6 |
| 3 | Protochateuic acid (PTA) | 13.4 | 291.4, 260.5, 214.4 | 103.8 ± 2.4 |
| 4 | Ferulic acid (FA) | 14.1 | 323.7, 236.8 | 168.4 ± 1.8 |
| 5 | Protocatechualdehyde (PTCA) | 15.0 | 308.2, 279.5, 229.7 | 98.4 ± 2.3 |
| 6 | p-coumaric acid (PCA) | 18.4 | 309.4, 229.7 | 256.7 ± 6.8 |
| 7 | Vanillic acid (VN) | 19.7 | 279.5, 246.4 | 57.9 ± 2.4 |
| 8 | Protocatechualdehyde (PhA) | 22.2 | 280.6, 224.3 | 116.8 ± 1.9 |
| 9 | Vaniline (VNA) | 23.6 | 87.5 ± 2.4 | |
| 10 | Apigenic acid (APG) | 26.9 | 294.6, 261.7, 219.8 | 117.7 ± 3.6 |
| 13 | Syringic acid (peak 13) | 27.2 | 310.5, 222.5 | 95.6 ± 11.3 |
| 14 | p-hydroxybenzoic acid (PHBA) | 28.1 | 328.5, 234.4 | 113.7 ± 3.8 |
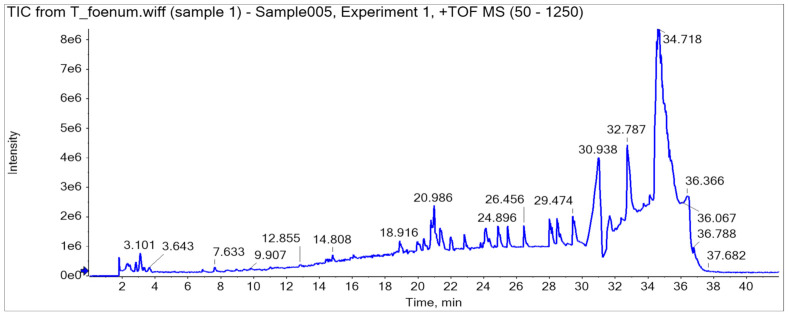
3.2. UHPLC-Q-TOF Chromatogram of T. foenum
HPLC analysis of plant extract led to the identification and quantification of 13 phenolics, and samples were further characterized through LC-MS/MS (Q-TOF) profile (a detailed description is provided in Table 2, Figure 3). A total ion current (TIC)-based chromatogram of plant extract of T. foenum is presented in Figure 2, and the precursor ion of each peak was further processed for fragmentation pattern (MS/MS) and compared with the NIST library as well as literature reported for the identification of phytochemicals present in the sample. The results are summarized in Table 2, which described each component with retention time in order of their elution order and fragmentation pattern. Although numerous peaks were recorded in the chromatogram, we reported almost 18 compounds, which were identified through the NIST library or literature. MS profiling of peak 1 (RT 2.34 min) produced a parent ion peak at [M − H]+ m/z 441.0840 Da and daughter ions 251.0365, 233.0297 and 124.9848 Da, which corresponds to catechin gallate, and its fragmentation pattern revealed it would be catechin 3-O-gallate. MS spectra are provided in Supplementary Materials Figure S1. The other peaks appeared in chromatogram were also processed for MS1 as well as MS2 at both positive and negative ion mode, and the results were compared with literature and are described in Table 2. Almost 18 compounds were confirmed through literature and their detailed description is provided in Supplementary Materials Figures S1–S17.
Table 2.
Ultra-high-pressure liquid chromatography equipped with electrospray ionization-quadrupole time-of-flight mass detector (UPLC-ESI-Q-TOF-MS/MS) characterization of phytochemicals of T. foenum.
| Serial# | Compound Detected | Retention Time (min) | MS | M − H (Predicted) | M − H (Found) | MS2 | Formula | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Catechin 3-O-gallate | 2.34 | 442.09 | 441.0827 | 441.0840 | 251.0365, 233.0297, 124.9848 |
C22H18O10 | [49,50] |
| 2 | 4-O-methylepigallocatechin | 11.20 | 320.0896 | 321.1569 | 321.1468 | 303.1364, 241.1007 | C16H16O7 | [50,51,52] |
| 3 | Sativanone | 12.84 | 300.1398 | 299.1425 | 299.1474 | 283.1462, 223.0883, 179.0974 | C17H16O5 | [50,52] |
| 4 | Gallic acid | 13.36 | 170.12 | 169.1345 | 169.1342 | 125.4622 | C7H6O5 | [50,52] |
| 5 | Kampferol | 18.34 | 286.23 | 285.1487 | 285.1425 | 245.1357 189.1109 |
C15H10O6 | [50,53] |
| 6 | Methylviolanone | 18.57 | 330.1103 | 329.1103 | 329.1539 | 311.1424, 279.1167 | C18H18O6 | [20,50] |
| 7 | homovanilic acid-hexoside | 19.23 | 344.138 | 343.1135 | 343.1762 | 279.1162, 255.1146, 181.0796, 163.0396 | C15H20O9 | [50,51] |
| 8 | 5-O-Feruloylquinic acid | 20.99 | 368.1568 | 367.1448 | 367.1648 | 337.1599, 293.1295, 191.0640 | C17H20O9 | [49,50,52] |
| 9 | Cyanidin | 25.17 | 288.2 | 287.2 | 287.1768 | 287.1768, 253.095, 167.1364, 149.1272 | C15H11O6 | [53,54] |
| 10 | Coumaroylquinic acid | 26.09 | 338.1002 | 337.1927 | 337.1900 | 265.1338, 173.1554 |
C16H18O8 | [51,52,53] |
| 11 | Quercetin | 27.15 | 302.236 | 301.1915 | 301.1950 | 295.1747 291.1551, 163.1428 |
C15H10O7 | [20,52] |
| 12 | Schisandrin C | 27.49 | 384.1573 | 385.1646 | 385.1393 | 339.1704, 137.0888, 123.0786 | C22H24O6 | [20,52] |
| 13 | p-coumaric acid O-hexoside | 27.95 | 326.1455 | 325.1529 | 325.1552 | 295.1080, 165.0856, 123.0763 | C15H18O8 | [20,52] |
| 14 | Hydroxytyrosol-4-o-glycoside | 29.29 | 316.2158 | 315.2085 | 315.2140 | 251.1190, 237.1404, 237.1042 177.1555 |
C14H20O8 | [50,55] |
| 15 | 3-Methoxysinensetin | 31.42 | 402.2315 | 403.2388 | 403.2755 | 399.2602, 383.2710, 199.0671 |
C21H22O8 | [52,54] |
| 16 | Pinoresinol | 31.74 | 358.1416 | 357.1343 | 357.2531 | 313.2297, | C20H22O6 | [52,54] |
| 17 | Glycitin | 32.45 | 446.3213 | 447.3286 | 447.3361 | 403.3065, 368.4185 | C22H22O10 | [20,52] |
| 18 | Dihydroferulic acid 4-O-glucuronide | 33.52 | 372.1156 | 371.1054 | 371.1674 | 291.1162, 266.9912, 73.0421 |
C6H20O10 | [50,55] |
Figure 3.
UHPLC-Q-TOF chromatogram of T. foenum.
3.3. Determination of TPC, TFC and In Vitro Antioxidant Activities
The results of the in vitro antioxidant profiling of T. foenum-graecum seed extract are presented in Table 3. The results reveal that T. foenum-graecum seed extract displayed excellent antioxidant potential with total phenolic contents of 454.93 ± 3.57 mg GAE/g, total flavonoid contents (TFC) of 135.04 ± 2.12 µg/CE and total antioxidant capacity (TAC) of 162.51 ± 3.81 per gram of dry plant extract. The extract represented a concentration-dependent activity of DPPH inhibition and ABTS scavenging assay with an IC50 value of 24.7 ± 2.70 and 15.8 ± 0.87 µg/mL, respectively, which is similar to standard.
Table 3.
In vitro antioxidant activities and total phenolic and flavonoid contents of T. foenum-graecum seed extract.
| Antioxidant Assay | T. foenum-graecum | Ascorbic Acid |
|---|---|---|
| Total phenolic contents (GAE mg/g) | 454.93 ± 3.57 | - |
| Total flavonoid contents (CE µg/g) | 135.04 ± 2.12 | - |
| Total antioxidant capacity (AAE mg/g) | 162.51 ± 3.81 | - |
| DPPH inhibition assay (IC50) (µg/mL) | 24.7 ± 2.70 a | 25.05 ± 1.45 a |
| ABTS cation inhibition (IC50) (µg/mL) | 15.8 ± 0.87 a | - |
Each value is mean ± SD of three replicates. Alphabets a shows significant difference at (p < 0.05). -, not determined; AAE, ascorbic acid equivalents; CE, catechin equivalents; GAE, gallic acid equivalents; (µg/mL), microgram per milliliter.
3.4. Toxicological Analysis of T. foenum-graecum Seed Extract
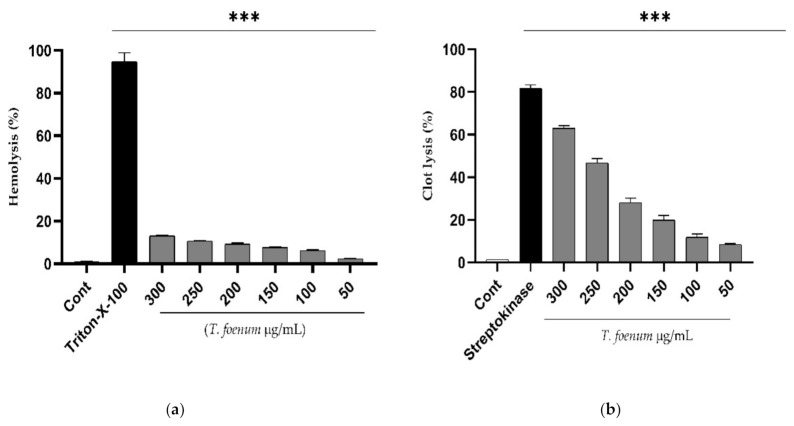
The results of hemolytic activity show that T. foenum-graecum seed extract has negligible toxicity in comparison to Triton-X (positive control), as shown in Figure 4. Further, the extract exhibits dose-dependent activity, and the hemolysis of erythrocytes increased with an increase in concentration of T. foenum-graecum seed extract. The concentration required for hemolysis of 50% RBCs (HC50 values) was estimated to be 2838 µg/mL, which is significantly (p < 0.001) different from HC50 of Triton-X-100 (64.98 µg/mL). Similarly, T. foenum-graecum extract showed a dose-dependent increase in clot lysis activity. Standard streptokinase (0.5 mL) and T. foenum-graecum extract at a high concentration (300 µg/mL) showed significant (p < 0.001) clot lysis activity of 81.82% and 63.01%, respectively comparing with the negative control (1.36%).
Figure 4.
Hemolytic (a) and thrombolytic activity (b) of different doses (50–300 µg/mL) of hydroethanolic extract of T. foenum-graecum seeds through hemolytic and thrombolytic assays. (***, significant at p < 0.001).
3.5. Cytotoxicity Determination
The results of cytotoxicity of T. foenum-graecum seed extract against RAW 264.7 cells are presented in Figure 5. Increased concentration of T. foenum-graecum seed extract has a negative impact on cell viability. The IC50 values for T. foenum-graecum seed extract and standard doxorubicin against RAW 264.7 cells were 1055 and 614.9 μg/mL, respectively. The results exhibit that plant extract showed low-to-moderate cytotoxicity in a concentration-dependent manner. Cell viability percentage was decreased with an increase in the concentration of plant extract. Cell viabilities in murine macrophages incubated with a different concentration (100–1000 µg/mL) of T. foenum-graecum seed extract were 91.53%, 87.64%, 76.79%, 73.67%, 71.06%, 68.25%, 62.47%, 56.87%, 55.60% and 47.78%, respectively. At concentrations (100–300 μg/mL), little cytotoxic effects were observed, and these concentrations were adopted further to examine the anti-inflammatory activity of T. foenum-graecum seed extract.
Figure 5.

Cytotoxic activity (cell viability) of hydroethanolic extract of T. foenum-graecum and doxorubicin (standard) against RAW 264.7 macrophage through MTT assay.
3.6. Effect of T. foenum-graecum Extract on TNF-α and IL-6
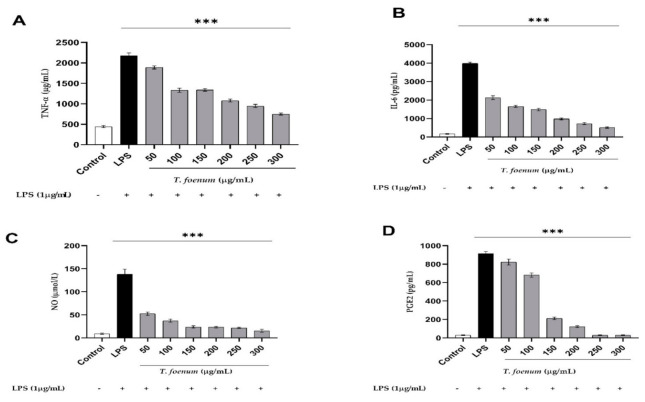
The tested concentration (50–300 μg/mL) of T. foenum-graecum seed extract showed a substantial decrease in the production of IL-6 (1889.92 ± 19.32, 1338.73 ± 12.14, 1330.42 ± 10.56, 1108.32 ± 20.87, 947.33 ± 16.45, 748.52 ± 9.45, respectively) (Figure 4a and TNF-α (2158.12 ± 31.87, 1715.21 ± 28.68, 1518.68 ± 26.98, 1045.01 ± 23.55, 741.33 ± 18.84, 522.99 ± 19.03, respectively) (Figure 4b) in comparison to LPS-stimulated macrophages (2177.83 ± 37.56 µg/mL for TNF-α and 3894.42 ± 49.73 pg/mL for IL-6), suggesting significant in vitro anti-inflammatory potential. Figure 4a,b, demonstrates the effect of T. foenum-graecum seed extract on the production of TNF-α and IL-6 in RAW 264.7 cells after stimulation with LPS at various concentrations. The results indicate that the plant extract inhibited both TNF-α and IL-6 production significantly (p < 0.001) at different concentrations of 50–300 μg/mL, with inhibition rates of 13.21%, 38.91%, 38.52%, 49.10%, 56.50%, 65.62% and 44.58%, 55.95%, 61.03%, 73.16%, 80.96%, 86.57%, respectively, with IC50 values of 192.7 µg/mL and 72.03 µg/mL.
3.7. NO and PGE2 Quantification
The results of NO and PGE2 inhibition by T. foenum-graecum seed extract are presented in Figure 6C,D. The findings show that both NO and PGE2 are significantly (p < 0.001) inhibited by T. foenum-graecum extract in culture of RAW 264.7 cells, reaching to the level of the control (without LPS) at higher concentrations of plant extract in a dose-dependent manner (Figure 6C,D). Moreover, a significant (p < 0.0001) increase in NO and PGE2 concentration was recorded after treatment with LPS, which was markedly (p < 0.001) restored to the normal level after treatment with standard and plant extract with IC50 values of 11.40 and 122.0 µg/mL.
Figure 6.
Anti-inflammatory profile of T. foenum-graecum seed extract through quantification of, TNF-α (A), IL-6 (B), NO (C) and PGE2 (D) measurement in RAW 264.7 cell’s culture medium after stimulation with LPS (1 µg/mL). +, treated with LPS, ***, significant at p < 0.001.
3.8. In Vivo Assays
3.8.1. Acute and Subacute Toxicity Analysis of T. foenum-graecum Seed Extract
The results from the acute toxicity analysis show that T. foenum-graecum seed extract did not show any harmful effects in the treated animals, as compared with the control group at a dosage of 2000 mg/kg BW. No morbidity or mortality was noticed in experimental animals during acute toxicity analysis. Consequently, the LD50 value of T. foenum-graecum seed extract examined was calculated to be >2000 mg/kg BW. During acute toxicity analysis, none of the animals exhibited any changes in behavior or symptoms related to the circulatory, respiratory, central, and autonomic nervous system (Table 4). As T. foenum-graecum extract treatment did not exhibit any adverse effects in the acute study, a human equivalent dose was selected for the long-term subacute toxicity study of 28 days. In subacute toxicity analysis, the effect of plant treatment on weight, relative organ weight, liver and kidney function indices, and hematological and histopathological parameters was evaluated. Table 5 shows the variation in body weight of rats after treatment with different doses of T. foenum-graecum seed extract. The body weight of animals treated with plant extract at different dosages (250, 500, and 1000 mg/kg) did not differ significantly (p > 0.05) from those in the control group. Similarly, in comparison to the control group, there was no significant (p > 0.05) difference in the absolute weights of the kidney and liver of treated animals (Table 6).
Table 4.
Sign and symptoms of acute oral toxicity in rats after treatment with T. foenum-graecum seeds extract.
| Observation | Control Group | Treatment Group |
|---|---|---|
| Behavior change | NO | NO |
| Salivation | NO | NO |
| Eye/skin appearance | NC | NC |
| Sleep | NO | NO |
| Diarrhea | NO | NO |
| Lethargy | NO | NO |
| Coma | NO | NO |
| Respiration/cardiac output | NO | NO |
| Mortality | NO | NO |
Table 5.
Effect of T. foenum-graecum seed extracts on body weights (gram) of male and female rats.
| Sex | Treatment | Time | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| Male | Control | 213.78 ± 3.39 | 223.54 ± 2.38 | 240.12 ± 5.79 | 272.13 ± 1.36 | 310.13 ± 1.05 |
| T. foenum-graecum | 210.14 ± 1.68 | 223.67 ± 2.30 | 242.27 ± 1.88 c | 265.55 ± 1.25 c | 295.53 ± 1.14 c | |
| Female | Control | 215.12 ± 1.05 | 230.59 ± 1.21 | 239.16 ± 2.68 | 290.19 ± 3.39 | 319.12 ± 5.81 |
| T. foenum-graecum | 212.1 ± 2.18 b | 211.66 ± 4.99 a | 233.12 ± 1.55 c | 250.41 ± 2.94 c | 269.64 ± 3.14 c | |
Each value is mean ± SD of three replicates; values with superscripts a, b and c are significant at 0.05, 0.01 and 0.001.
Table 6.
Relative weight of organs (g/g body weight) after treatment with T. foenum-graecum seed extract for 28 days.
| Sex | Organ | Control | T. foenum-graecum |
|---|---|---|---|
| Male | Liver | 2.46 ± 0.32 | 2.51 ± 0.47 |
| Kidney | 0.49 ± 0.03 | 0.49 ± 0.05 | |
| Female | Liver | 2.09 ± 0.16 | 2.31 ± 0.08 |
| Kidney | 0.49 ± 0.11 | 0.46 ± 0.30 |
Values are presented as mean ± SD.
3.8.2. Hematological and Biochemical Indices
The results of the subacute toxicity of T. foenum-graecum seed extract treatment on hematological parameters are presented in Table 7, and findings reveal that hematological parameters of treatment animals were not affected after treatment with plant extract for 28 days. Similarly, treatment of T. foenum-graecum seed extract in subacute toxicity did not cause any significant change in liver and kidney function indices (Table 8). No significant alteration in AST, ALT, ALP, γ-GT, total proteins, bilirubin, glucose, creatinine, total cholesterol, total glycerides, or blood urea nitrogen was detected in experimental animals in comparison to the control group. The little variation represented has no clinical significance, because values recorded are within the normal range of rats. Histopathological examinations of liver and kidney tissues did not exhibit any morphological alterations or abnormalities under the light microscope.
Table 7.
Effect on hematological parameters in male and female rats after treatment with T. foenum-graecum seed extract.
| Parameter | Male | Female | ||
|---|---|---|---|---|
| Control | T. foenum-graecum | Control | T. foenum-graecum | |
| Hemoglobin (g/dL) | 14.0 ± 1.40 | 14.0 ± 1.83 | 14.0 ± 1.5 | 15.0 ± 0.73 |
| Packed cell volume | 42 ± 1.05 | 43 ± 1.11 | 42 ± 1.68 | 46 ± 2.55 |
| Red Blood Cells (106/µL) | 7.7 ± 0.35 | 7.4 ± 0.53 | 6.68 ± 0.15 | 7.2 ± 0.19 |
| Mean corpuscular volume (fL) | 52 ± 1.13 | 54 ± 1.31 | 57 ± 2.62 | 59 ± 1.64 |
| Mean cell hemoglobin (pg) | 22 ± 2.54 | 21 ± 1.41 | 19.59 ± 2.23 | 19.91 ± 2.54 |
| Mean corpuscular hemoglobin concentration (g/L) | 35 ± 1.72 | 34 ± 1.03 | 36.01 ± 1.99 | 33.08 ± 1.88 |
| White blood cells (109/L) | 9.0 ± 0.31 | 8.9 ± 0.54 b | 7.90 ± 0.93 | 6.1 ± 0.26 b |
| Platelets (109/L) | 161 ± 2.43 c | 216 ± 3.14 c | 160 ± 11.46 | 225 ± 16.42 c |
| Neutrophils (%) | 10 ± 0.36 | 9 ± 0.52 | 10 ± 0.22 | 11 ± 0.41 |
| Lymphocytes (%) | 82 ± 2.74 | 85 ± 2.64 a | 82 ± 2.65 | 79 ± 2.95 a |
| Monocytes (%) | 6 ± 0.04 | 4 ± 0.53 b | 5 ± 0.62 | 7 ± 0.17 |
| Eosinophils (%) | 2 ± 0.08 | 2 ± 0.68 b | 3 ± 0.81 | 3 ± 0.19 |
Each value is mean ± SD of three replicates; values with superscripts a, b and c are significant at 0.05, 0.01 and 0.001.
Table 8.
Effect on biochemical parameters in male and female rats after treatment with T. foenum-graecum seed extract.
| Parameter | Male | Female | ||
|---|---|---|---|---|
| Control | T. foenum-graecum | Control | T. foenum-graecum | |
| Aspartate aminotransferase (AST) (U/L) | 34 ± 0.97 | 21 ± 2.04 c | 30 ± 1.66 | 21 ± 1.53 c |
| Alanine aminotransferase (ALT) (U/L) | 35 ± 1.23 | 24 ± 1.32 b | 33 ± 1.50 | 25 ± 1.71 a |
| Alkaline phosphatase (ALP) (U/L) | 449 ± 2.41 | 447 ± 1.78 c | 452 ± 2.72 | 442 ± 1.24 c |
| Gamma Glutamyl Transferase (γ-GT) (U/L) | 6.16 ± 0.56 | 5.70 ± 0.72 a | 6.71 ± 0.77 | 4.72 ± 0.15 c |
| Total Bilirubin (μmol/L) | 0.6 ± 0.03 | 0.6 ± 0.01 b | 0.6 ± 0.16 | 0.6 ± 0.16 |
| Direct Bilirubin (μmol/L) | 0.2 ± 0.04 | 0.3 ± 0.03 | 0.2 ± 0.04 | 0.2 ± 0.06 |
| Indirect Bilirubin (μmol/L) | 0.4 ± 0.02 | 0.3 ± 0.07 | 0.4 ± 0.05 | 0.4 ± 0.09 |
| Glucose(mmol/L) | 4.61 ± 1.56 | 4.93 ± 0.87 | 4.88 ± 1.00 | 4.72 ± 0.11 |
| Creatinine (μmol/L) | 60.27 ± 1.09 | 59.17 ± 1.90 | 61.16 ± 5.17 | 55.25 ± 2.26 |
| Total cholesterol (mmol/L) | 1.69 ± 0.32 | 1.47 ± 0.07 | 1.77 ± 0.52 | 1.41 ± 0.40 |
| Triglycerides (mmol/L) | 0.84 ± 0.37 | 0.80 ± 0.14 | 0.90 ± 0.15 | 0.84 ± 0.11 |
| Total protein (g/L) | 4.01 ± 0.59 | 3.91 ± 0.62 | 4.39 ± 0.45 | 4.28 ± 0.49 |
| Blood Urea Nitrogen (mmol/L) | 8.13 ± 0.37 | 7.08 ± 1.18 a | 9.11 ± 0.71 | 6.54 ± 1.16 a |
Each value is mean ± SD of three replicates; values with superscripts a, b and c are significant at 0.05, 0.01 and 0.001.
3.9. In Vivo Anti-Inflammatory Potential
3.9.1. Air Pouch Model of Inflammation
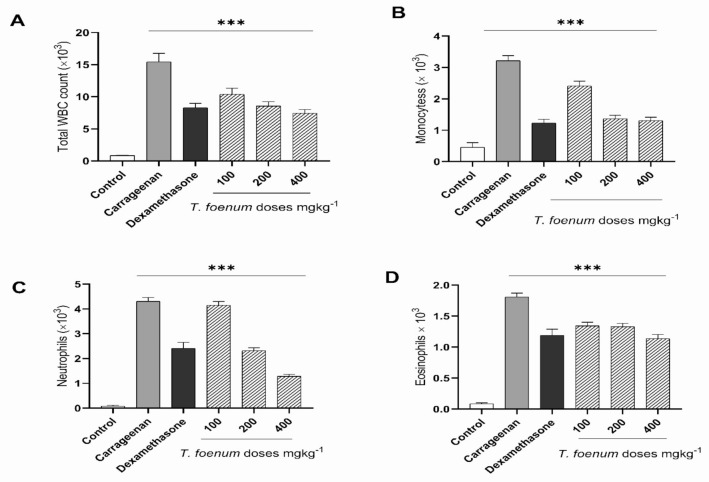
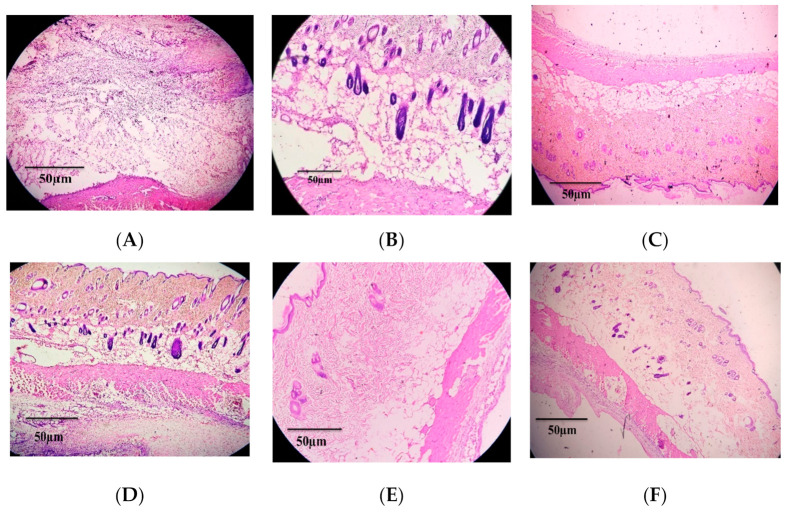
To evaluate the anti-inflammatory potential of T. foenum-graecum seed extract, inflammation of air pouch model was utilized, and inflammatory exudate was analyzed for different blood cells associated with inflammation (Figure 7). In inflammatory exudate of rats treated with carrageenan only, white blood cell (15 × 103 cells/mL) count was about 20-fold higher compared to the control group (0.83 × 103 cells/mL). Dexamethasone (10 mg/kg BW) treatment caused an almost 10-fold reduction (8.13 × 103 cells/mL) in WBC count. T. foenum-graecum seed extract suppressed the WBC population dose-dependently. For example, WBC count in the 200 mg/kg BW treatment group was equal to dexamethasone, and in the 400 mg/kg BW treatment group the effect was more significant than dexamethasone. As shown in Figure 5, injection of carrageenan caused a noticeable increase in monocytes as compared to the carrageenan control group. However, T. foenum-graecum extract by doses of 200 and 400 mg/kg BW led to significant (p < 0.001) reductions in monocytes (1.37 × 103 and 1.31 × 103, respectively) as compared to the carrageenan control (3.21 × 103). Moreover, T. foenum-graecum extract significantly reduced the carrageenan-induced proliferation of eosinophils and neutrophil in treatment groups, as compared to carrageenan-treated animals, in a dose-dependent manner, and this effect is comparable to dexamethasone (20 mg/kg BW), particularly at 400 mg/kg BW of T. foenum-graecum. Similarly, histopathological examination of air pouch tissue also exhibited a change in the thickness of the air pouch membrane and marked cellular influx to the inflammatory site with increased inflammatory response. The treatment with T. foenum-graecum extract and dexamethasone caused an increase in membrane thickness as compared to the carrageenan-treated group, in which the membrane was narrow and condensed and cellular influx was also reduced (Figure 8).
Figure 7.
Anti-inflammatory profile (cellular infiltration) (A) (Total WBCs), (B) (Monocytes), (C) (Neutrophils) and Eosinophils (D) of hydroethanolic extract of T. foenum-graecum against air pouch model of inflammation. Data are expressed as mean ± S.D. ***, significant at p < 0.001.
Figure 8.
Histopathological photographs of air pouch tissues after treatment with different concentrations of T. foenum-graecum extract at 100, 200, 400 mg/kg BW (A–C), carrageenan control (D), dexamethasone 20 mg/kg body weight (E) and normal control (F).
3.9.2. Carrageenan-Induced Peritonitis
Treatment of T. foenum-graecum seed extract before 30 min of intraperitoneal injection of carrageenan significantly (p < 0.001) reduced total WBC and neutrophil count in peritoneal exudate (Figure 9). Injection of carrageenan caused a 16-fold increase in WBC count (11.12 × 103) as compared to the control (0.66 × 103), and dexamethasone and T. foenum-graecum (200 and 400 mg/kg dose) decreased it to 6.97 × 103. Moreover, T. foenum-graecum extract significantly reduced the neutrophil count in peritoneal exudate of treated rats as compared to the control group.
Figure 9.

Anti-inflammatory effect A (Neutrophils) and B (Total WBCs) of hydroethanolic extract of T. foenum-graecum on carrageenan-induced peritonitis. Rats were pretreated orally with different concentrations of T. foenum-graecum extract and dexamethasone (20 mg/kg body weight). Data are expressed as mean ± SD. ***, significant at p < 0.001.
3.9.3. Effect of T. foenum-graecum on Antioxidant Enzymes and Stress Markers
The levels of enzymatic antioxidants, lipid peroxidation and oxidative stress markers in air pouch exudates and peritoneal fluid were significantly (p < 0.001) altered in the carrageenan-treated group as compared to the control group. However, treatment with dexamethasone and T. foenum-graecum significantly reversed the carrageenan-induced changes in these parameters. MPO and MDA levels in the carrageenan-treated group were significantly higher as compared to those of the control group (Table 9 and Table 10). Dexamethasone and T. foenum-graecum extract effectively reduced the MDA and MPO levels in the air pouch and peritoneal exudate, and the effect was dose dependent, with 400 mg/kg BW recorded as most effective dose. Similarly, carrageenan treatment increased the level of superoxide dismutase and catalase and hence TAS significantly (p < 0.001) at the inflammatory site, while dexamethasone significantly reduced the level of these enzymes, and this effect was similar to the T. foenum-graecum group at 400 mg/kg BW dose treatment group. Furthermore, total oxidant level (TOS) was also significantly higher among the carrageenan treatment group in comparison to that of the control group, which is significantly (p < 0.001) decreased in both dexamethasone and T. foenum-graecum treatment groups. The effect at higher doses of T. foenum-graecum (400 mg/kg) was similar to the dexamethasone-treated group.
Table 9.
Effects of different doses of T. foenum-graecum seed extract on oxidative stress and lipid peroxidation in air pouch exudate.
| Treatment | Dose (mg/kg) | Myeloperoxidase (U/mg Protein) |
Malondialdehyde (nmol/mg Protein) |
Catalase (µmol H2O2/µg of Protein) | Superoxide Dismutase (U/mg of Protein) |
Total Oxidant Status (µM H2O2 Eq/g) | Total Antioxidant Status (µM Trolox Eq/g) |
|---|---|---|---|---|---|---|---|
| Control | - | 364 ± 1.54 | 5.84 ± 4.61 | 321.37 ± 2.87 | 4.39 ± 0.28 | 1.98 ± 0.45 | 6.35 ± 0.28 |
| Carrageenan | - | 647 ± 1.46 c | 14.46 ± 1.67 c | 218.38 ± 4.53 | 2.39 ± 0.21 | 13.21 ± 0.48 c | 0.28 ± 0.05 |
| T. foenum-graecum | 100 | 273 ± 2.01 | 13.28 ± 1.72 | 328.48 ± 2.23 | 4.39 ± 0.38 b | 9.98 ± 1.16 | 6.39 ± 0.60 a |
| 200 | 214 ± 1.03 | 9.99 ± 1.44 | 438.38 ± 3.99 | 5.92 ± 0.83 | 7.95 ± 0.71 | 6.54 ± 0.75 | |
| 400 | 184 ± 2.04 | 7.98 ± 1.14 | 647.29 ± 8.31 c | 6.20 ± 0.21 c | 6.47 ± 1.39 | 8.84 ± 1.25 b | |
| Dexamethasone | 20 | 173 ± 1.27 a | 8.30 ± 1.87 a | 438.58 ± 11.60 b | 7.31 ± 0.33 | 0.27 ± 0.32 | 13.68 ±1.99 c |
Each value is mean ± SD of three replicates; values with superscripts a, b and c are significant at 0.05, 0.01 and 0.001.
Table 10.
Effects of different doses of T. foenum-graecum seed extract on oxidative stress and lipid peroxidation in carrageenan-induced peritonitis.
| Treatment | Dose (mg/kg) | Myeloperoxidase (U/mg Protein) |
Malondialdehyde (nmol/mg Protein) |
Catalase (µmol H2O2/µg of Protein) | Superoxide Dismutase (U/mg of Protein) |
Total Oxidant Status (µM H2O2 Eq/g) | Total Antioxidant Status (µM Trolox Eq/g) |
|---|---|---|---|---|---|---|---|
| Control | - | 34 ± 0.24 | 1.45 ± 0.01 | 212.07 ± 2.97 | 1.39 ± 0.08 | 0.85 ± 0.01 | 1.65 ± 0.28 |
| Carrageenan | - | 177 ± 3.06 | 4.86 ± 0.27 | 109.48 ± 0.43 | 0.89 ± 0.16 | 0.22 ± 0.08 | 0.61 ± 0.05 |
| T. foenum-graecum | 100 | 31.42 ± 0.61 | 4.38 ± 0.21 b | 108.08 ± 0.13 a | 1.12 ± 0.81 a | 0.48 ± 0.06 | 1.09 ± 0.20 |
| 200 | 29.09 ± 0.23 | 3.99 ± 0.14 | 148.58 ± 0.69 | 1.16 ± 0.38 | 0.61 ± 0.01 | 1.54 ± 0.19 | |
| 400 | 18.53 ± 0.41 | 2.98 ± 1.14 | 205.29 ± 1.61 c | 1.21 ± 0.19 b | 0.98 ± 0.04 a | 1.49 ± 0.12 | |
| Dexamethasone | 20 | 15.98 ± 108.27 c | 1.10 ± 0.07 c | 201.18 ± 1.46 | 1.46 ± 0.35 | 0.97 ± 0.02 | 1.88 ± 0.67 b |
Each value is mean ± SD of three replicates; values with superscripts a, b and c are significant at 0.05, 0.01 and 0.001. Eq: Equivalent.
4. Discussion
The in vitro and in vivo antioxidant and anti-inflammatory properties of the hydroethanolic extract of T. foenum-graecum were studied using different methods, and the overall findings have been corroborated. T. foenum-graecum is an old medicinal herb that dates back to Egyptian times and has significant antipyretic, anti-inflammatory and anti-nociceptic properties. In the current study, the phytochemical screening of T. foenum-graecum extract exhibited a considerable amount of phenolics and flavonoids and showed significant antioxidant activity. The results of our antioxidant and polyphenolic analysis are largely in agreement with Kenny et al. [56], who reported total phenolic contents (106.316 ± 0.377 mg GAE/g), FRAP (77.352 ± 0.627) and DPPH (35.338 ± 0.908) mg of Trolox equivalents per gram of Fenugreek ethyl acetate extract. Previously, Akbari et al. [57] also reported the ABTS and DPPH assays with IC50 values of 161.3 ± 2.21 and 172.6 ± 3.1 µg/mL. Similarly, TFC and TPC, as well as the fenugreek, were also reported (14.417 ± 0.23 mg QE/g and 38.97 ± 0.34 mg GAE, respectively).
Evaluating the toxicity profile of medicinal plants and plant-based products is typically a preliminary step for the screening of the therapeutic potential of plant-derived products [19,57]. Another approach for the evaluation of cytotoxicity is hemolytic assay, which is described by the subsequent release of hemoglobin after erythrocytes lysis [58]. The polyunsaturated fatty acids and hemoglobin mostly attack RBCs because of their redox-active oxygen transportation property [59]. Consequently, the oxidative process damages the proteins and lipids in the erythrocyte membrane during hemolysis [60]. This damage is associated with several other factors, including oxidative drugs, a high quantity of transition metals, radiation, insufficient antioxidant defense system and hemoglobinopathies [61]. Hemolysis of erythrocytes occurs when they are exposed to toxic natural or synthetic compounds. The HC50 of T. foenum-graecum extract is very high compared to Triton-X-100 and thus supports its application as a successful pharmaceutical drug in practice. The thrombolytic potential of T. foenum-graecum extract is an important finding, which may have implications in cardiovascular health, especially in atherothrombotic patients. These results are in agreement with Ktari et al. [62], which also reported cytotoxicity of T. foenum-graecum extract, and no hemolytic activity was reported against bovine RBCs.
Many of the classic experiments in the field of inflammation have been performed using murine macrophages [4,5]. The hydroethanolic extract of T. foenum-graecum demonstrated a noticeable inhibition of pro-inflammatory cytokines, NO and PGE2 after LPS stimulation. The activation of pro-inflammatory cytokines is among the most fundamental processes that occur during inflammatory pathways [63]. TNF-α is a potent pro-inflammatory cytokine produced by a variety of immunocompetent cells, such as neutrophils, dendritic cells, macrophages, and T helper cells, and is capable of attracting immune cells to the inflammatory site to initiate the inflammatory process [64]. The hydroethanolic extract of T. foenum-graecum (50–300 μg/mL) presented dose–response anti-inflammatory activity, since it showed greater TNF-α and IL-6 inhibition in the culture of RAW 264.7 cells after stimulation with LPS. TNF-α and IL-6 are measured as basic markers of pro-inflammatory processes produced by macrophages and have the ability to activate T cells [65]. As a result, inhibiting these cytokines is thought to be an efficient way to prevent and cure a variety of inflammatory disorders [66]. The hydroethanolic extract of T. foenum-graecum also inhibited NO and PGE2 production by RAW 264.7 cells and showed similar results to those of TNF-α and IL-6 in relation to the positive control dexamethasone, evidencing its antioxidant and anti-inflammatory action [67]. NO is a powerful radical that regulates the growth, function, and death of a variety of cell types involved in inflammatory and immunological responses. Excessive NO generation has been linked to the pathophysiology of oxidative damage and inflammation [68]. Previous inhibition of pro-inflammatory cytokines by T. foenum-graecum extract has also been reported in some studies [35]. The anti-inflammatory attributes of T. foenum-graecum may be due to the large number of polyphenolic substances that have been reported good anti-inflammatory agents in previous studies [19,20].
Oral administration of extracts is the most appropriate and economical method of drug delivery in animal models during toxicity analysis [69]. Moreover, acute oral toxicity analysis in the rat model can effectively predict human acute lethal dosages in clinical setups [70]. The body weight of animals is an important index for the determination of the toxicity of synthetic or natural compounds [71]. In the current study, there was no abnormal change in the weight of animals among the treatment group and control group up to a dose of 1000 mg/kg. Likewise, variation in organ weight is also a good indication for plant-induced abnormalities, which are commonly linked with treatment-related effects. There were no significant variations in the body or organ weight of animals after 28 days of treatment with hydroethanolic extracts of T. foenum-graecum seed extract. All of the animals showed normal weight gain, with no significant differences among control and treatment groups. The hematopoietic system in both animals and humans is extremely sensitive to toxic substances and acts as a key indicator of pathological and physiological condition [72]. In toxicological assessment, biochemical parameters are of prime significance due to their extreme sensitivity and ability to respond against changes induced by toxicological substances [29]. These biochemical markers have a significant role in the evaluation of toxicological changes induced by natural or chemical substances [73]. In the current study, none of the biochemical parameters showed significant (p > 0.05) changes from the untreated control group. Similarly, the histopathological examination of both liver and kidney tissues was also found to be morphologically normal. Previously, some studies also reported the acute and subacute toxicities of T. foenum-graecum extract, and no toxicological effects were reported on biochemical and hematological markers [74,75].
Carrageenan-induced inflammation in rats is a useful approach for testing natural products with potential anti-inflammatory activity and also for further elucidating their mechanism of action [76]. Carrageenan injection initiates an acute inflammatory response linked with hyperalgesia, usually classified by edema and increased response to mechanical and thermal stimuli [40]. The carrageenan-induced inflammation is linked with increased leukocytes migration, mainly neutrophils, and enhanced myeloperoxidase (MPO) activity [77]. Pro-inflammatory mediators such as MPO and NOx and proinflammatory cytokine levels (TNF-α and IL-6) can modulate the inflammatory response and can significantly alter the amplitude of leukocyte activation and migration [78]. The air pouch model is a useful in vivo model for studying localized inflammation with no systemic effects. The injection of air subcutaneously into the thoracic area induces morphological alterations in the pouch’s cellular lining that lasts several days [39]. This structural change develops in pouch lining, similar to that which occurs in the synovial cavity. Carrageenan injection directly into the air cavity produces an inflammatory response in patients with rheumatoid arthritis and several other chronic inflammatory diseases. Therefore, this model can be employed to screen anti-inflammatory compounds [79]. One of the major benefits of air pouch inflammation over carrageenan injection directly into the knee joint is the increased volume of pouch exudate, allowing for the measurement of several parameters from each animal [80,81]. Peritonitis induced by carrageenan is a well-established model of acute inflammation frequently used for testing novel anti-inflammatory drugs focusing on analysis or quantification of cellular migration, vascular permeability, and measurement of inflammatory parameters [82]. In the current study, both the air pouch and carrageenan-induced peritonitis showed significant infiltration of leukocytes, neutrophils, and monocytes to inflammatory exudates. The lining of the air pouch was intensely invaded with inflammatory cells. Neutrophil migration to the joints of rheumatoid arthritis patients causes the destruction of synovial tissue, cartilage structure and bones through the release of different proteases and harmful oxygen metabolites. The administration of T. foenum-graecum extract efficiently abrogated the cellular influx to the pouch exudates and reduced the morphological changes in the lining of the pouch tissues.
The production of free radicals at the inflammatory site is one of the major mechanisms of tissue damage produced by several inflammatory disorders [11,12]. Activated neutrophil infiltration to the inflammatory sites is a significant source of proinflammatory mediators and oxygen-derived free radicals, which induces inflammatory reactions [83]. It has been shown that, after the injection of carrageenan, the level of free radicals, and thus total oxidant status (TOS), rises in both the air pouch and peritoneal exudates [84]. These free radicals may target the plasma membrane causing malondialdehyde (MDA) to accumulate. MDA is a basic marker of oxidative stress. Myeloperoxidase is an enzyme found in leucocytes, involved in the formation of a wide range of reactive oxygen species [85]. MPO-derived oxidants have been shown to lead to tissue damage during inflammation [42,43]. Tissue damage related to oxidative stress can be reversed via the CAT and SOD enzyme. The activity of these enzymes controls the cytotoxic properties of toxic free radicals [41,44]. In this study, there were significant increases in catalase and SOD enzyme activity in both the air pouch and peritoneal exudate; hence, there was an increase in TAS level after treatment with T. foenum-graecum extract. Furthermore, there was a substantial decrease in MDA and MPO levels after treatment with T. foenum-graecum extract. Oxidative stress may, therefore, be inhibited by hydroethanolic extract of T. foenum-graecum. A decrease in MDA and MPO activity is linked to reduced inflammatory progression, and these effects could be attributed to polyphenols, quercetin, and gallic acid, among other compounds found in the plant extract.
5. Conclusions
In conclusion, hydroethanolic extract of T. foenum-graecum contains a significant number of polyphenolic compounds, which decreases cellular infiltration, lipid peroxidation, and the level of pro-inflammatory cytokines and inflammatory mediators. Pre-treatment of animals with T. foenum-graecum extract significantly improves tissue antioxidant status, which ameliorates oxidative stress and inflammatory processes induced by carrageenan. Moreover, the T. foenum-graecum extract was also characterized through ESI-Q-TOF MS/MS as well as HPLC-PDA to investigate the phenolic profile likely responsible for bioactivity. However, further studies are essential and need to be conducted to investigate the mechanisms of action, bioaccessibility, and bioavailability of these compounds for their proper nutritional and medicinal properties.
Acknowledgments
The authors are thankful to Christopher Glass, Professor, Cellular and Molecular Medicine, University of California, San Diego, USA, for providing RAW 264.7 cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11020364/s1, Figure S1: MS/MS spectrum of T. foenum at retention time 2.341 min; Figure S2: MS/MS spectrum of T. foenum at retention time 11.199 min; Figure S3: MS/MS spectrum of T. foenum at retention time 13.359 min; Figure S4: MS/MS spectrum of T. foenum at retention time 18.123 min; Figure S5: MS/MS spectrum of T. foenum at retention time 18.123 min; Figure S6: MS/MS spectrum of T. foenum at retention time 18.574 min; Figure S7: MS/MS spectrum of T. foenum at retention time 19.233 min; Figure S8: MS/MS spectrum of T. foenum at retention time 20.989 min; Figure S9: MS/MS spectrum of T. foenum at retention time 25.107 min; Figure S10: MS/MS spectrum of T. foenum at retention time 26.096 min; Figure S11: MS/MS spectrum of T. foenum at retention time 27.155 min; Figure S12: MS/MS spectrum of T. foenum at retention time 27.950 min; Figure S13: MS/MS spectrum of T. foenum at retention time 29.299 min; Figure S14: MS/MS spectrum of T. foenum at retention time 31.424 min; Figure S15: MS/MS spectrum of T. foenum at retention time 31.742 min; Figure S16: MS/MS spectrum of T. foenum at retention time 32.456 min; Figure S17: MS/MS spectrum of T. foenum at retention time 33.518 min.
Author Contributions
Conceptualization, M.S. and P.J.M.; data curation, H.F. and C.P.; formal analysis, M.R.; methodology, H.F. and C.P.; resources, P.J.M.; software, H.F.; supervision, M.S. and P.J.M.; writing—original draft, H.F.; writing—review and editing, M.A.P. and M.R.; HPLC and MS data analysis, R.A. All authors have read and agreed to the published version of the manuscript.
Funding
The current study was supported by the funding provided by HEC (Higher Education Commission), Pakistan, under the IRSIP (International Research Support Initiative Program).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of University of Agriculture, Faisalabad, Pakistan. No. 5496/ORIC, Dated 11-10-2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rauf A., Abu-Izneid T., Rashid U., Alhumaydhi F.A., Bawazeer S., Khalil A.A., Aljohani A.S.M., Abdallah E.M., Al-Tawaha A.R., Mabkhot Y.N., et al. Anti-Inflammatory, Antibacterial, Toxicological Profile, and in silico studies of Dimeric Naphthoquinones from Diospyros lotus. BioMed Res. Int. 2020;2020:7942549. doi: 10.1155/2020/7942549. [DOI] [Google Scholar]
- 2.Liu T., Zhang L., Joo D., Sun S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquardt P., Seide R., Vissiennon C., Schubert A., Birkemeyer C., Ahyi V., Fester K. Phytochemical Characterization and In vitro anti-Inflammatory, antioxidant and antimicrobial activity of Combretum collinum Fresen Leaves Extracts from Benin. Molecules. 2020;25:288. doi: 10.3390/molecules25020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djova S.V., Nyegue M.A., Messi A.N., Afagnigni A.D., Etoa F.X. Phytochemical Study of Aqueous Extract of Ochna Schweinfurthiana, F. Hoffm Powder Bark and Evaluation of Their Anti-Inflammatory, Cytotoxic, and Genotoxic Properties. Evid.-Based Complement. Altern. Med. 2019;2019:8908343. doi: 10.1155/2019/8908343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchete R., Oliveira S., Bagne L., Silva J.I.d.S., Valverde A.P., de Aro A.A., Figueira M.M., Fronza M., Bressam T.M., de Goes V.F.F., et al. Anti-Inflammatory and Antioxidant Properties of Alternanthera Brasiliana Improve Cutaneous Wound Healing in Rats. Inflammopharmacology. 2021;29:1443–1458. doi: 10.1007/s10787-021-00862-3. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt S.K., Javagal R.M., Nanjarajurs M.S., Eligar S.M. In vitro Anti-Inflammatory Property of a Quercetin-3-O-Diglucoside-7-O-Glucoside Characterized from Fresh Leaves of Trigonella foenum-graecum L. Int. J. Food Prop. 2021;24:1438–1452. doi: 10.1080/10942912.2021.1969946. [DOI] [Google Scholar]
- 7.Semalty M., Semalty A., Joshi G.P., Rawat M.S.M. Comparison of In vitro antioxidant activity of Trigonella foenum-graecum and T. corniculata Seeds. Res. J. Phytochem. 2009;3:63–67. doi: 10.3923/rjphyto.2009.63.67. [DOI] [Google Scholar]
- 8.Zameer S., Najmi A.K., Vohora D., Akhtar M. A review on therapeutic potentials of Trigonella foenum graecum (fenugreek) and its chemical constituents in neurological disorders: Complementary roles to its hypolipidemic, hypoglycemic, and antioxidant potential. Nutr. Neurosci. Int. J. Nutr. Diet Nerv. Syst. 2017;21:539–545. doi: 10.1080/1028415X.2017.1327200. [DOI] [PubMed] [Google Scholar]
- 9.Goyal S., Gupta N., Chatterjee S. Investigating Therapeutic Potential of Trigonella foenum-graecum L. as our defense mechanism against several human diseases. J. Toxicol. 2016;2016:1250387. doi: 10.1155/2016/1250387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gren A., Formicki G., Goc Z., Muchacka R., Knazicka Z., Lukac N., Massanyi P. Effects of Trigonella foenum graecum on the leukocytes in diabetes mice. J. Microbiol. Biotechnol. Food Sci. 2012;02:467–476. doi: 10.23880/doij-16000103. [DOI] [Google Scholar]
- 11.Yadav U.C.S., Baquer N.Z. Pharmacological Effects of Trigonella foenum-graecum L. in Health and Disease. Pharm. Biol. 2014;52:243–254. doi: 10.3109/13880209.2013.826247. [DOI] [PubMed] [Google Scholar]
- 12.Aryal B., Niraula P., Khadayat K., Adhikari B., Khatri Chhetri D., Sapkota B.K., Bhattarai B.R., Aryal N., Parajuli N. Antidiabetic, antimicrobial, and molecular profiling of selected medicinal plants. Evid.-Based Complement. Altern. Med. 2021;2021:5510099. doi: 10.1155/2021/5510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suresh P., Kavitha C.N., Babu S.M., Reddy V.P., Latha A.K. Effect of Ethanol Extract of Trigonella foenum graecum (Fenugreek) Seeds on Freund’s adjuvant-Induced arthritis in albino rats. Inflammation. 2012;35:1314–1321. doi: 10.1007/s10753-012-9444-7. [DOI] [PubMed] [Google Scholar]
- 14.Sidiq L.O., Segun P.A., Ogbole O.O. Total phenolic contents and antioxidant activity of nine medicinal plants used in Nigerian traditional medicine. Trop. J. Nat. Prod. Res. 2018;2:438–441. doi: 10.26538/tjnpr/v2i9.6. [DOI] [Google Scholar]
- 15.Mondal A., Maity T.K., Bishayee A. Analgesic and Anti-Inflammatory Activities of Quercetin-3-methoxy-4’-glucosyl-7-glucoside Isolated from Indian Medicinal Plant Melothria heterophylla. Medicines. 2019;27:59. doi: 10.3390/medicines6020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Garcia C.M., Ruiz-Ruiz J.C., Peraza-Echeverria L., Peraza-Sanchez S.R., Torres-Tapia L.W., Perez-Brito D., Tapia-Tussell R., Herrera-Chale F.G., Segura-Campos M.R., Quijano-Ramayo A., et al. Antioxidant, antihypertensive, anti-hyperglycemic, and antimicrobial activity of aqueous extracts from twelve native plants of the Yucatan Coast. PLoS ONE. 2019;14:e0213493. doi: 10.1371/journal.pone.0213493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian C., Zhang P., Yang C., Gao X., Wang H., Guo Y., Liu M. Extraction process, component analysis, and in vitro antioxidant, antibacterial, and anti-Inflammatory activities of total flavonoid extracts from Abutilon Theophrasti Medic. Leaves. Mediat. Inflamm. 2018;2018:3508506. doi: 10.1155/2018/3508506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manaswini N.K., Nazneen S., Shankar Rao G.B., Narender B., Vasudha B., Mohan M.R. Evaluation of Ocimum tenuiflorum and Syzygium aromaticum Phenolic Ethereal Oils for In-Vitro Anti-Inflammatory and Anti-Bacterial Activities. J. Drug Deliv. Ther. 2019;9:93–96. doi: 10.22270/jddt.v9i2.2383. [DOI] [Google Scholar]
- 19.Truong D.H., Nguyen D.H., Ta N.T.A., Bui A.V., Do T.H., Nguyen H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-Inflammatory activities of Severinia buxifolia. J. Food Qual. 2019;2019:8178294. doi: 10.1155/2019/8178294. [DOI] [Google Scholar]
- 20.Ali A., Bashmil Y.M., Cottrell J.J., Suleria H.A.R., Dunshea F.R. LC-MS/MS-QTOF Screening and Identification of Phenolic compounds from Australian grown herbs and their antioxidant potential. Antioxidants. 2021;10:1770. doi: 10.3390/antiox10111770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mostafa A.A., Al-Askara A.A., Almaary K.S., Dawoud T.M., Sholkamy E.N., Bakri M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018;25:361–366. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasany S.M., Huma R., Akram S., Ashraf R., Mushtaq M. Maceration-mediated liquid-liquid extraction and reverse-phase High-Performance Liquid Chromatography-Based Pragmatic Analysis of Silybins. J. Chromatogr. Sci. 2020;58:779–787. doi: 10.1093/chromsci/bmaa035. [DOI] [PubMed] [Google Scholar]
- 23.Lohvina H., Sandor M., Wink M. Effect of Ethanol Solvents on Total Phenolic Content and Antioxidant Properties of Seed Extracts of Fenugreek (Trigonella foenum-graecum L.) varieties and determination of phenolic Composition by HPLC-ESI-MS. Diversity. 2022;14:7. doi: 10.3390/d14010007. [DOI] [Google Scholar]
- 24.Zhang Y., Wang C., Yang F., Yang Z., Wang F., Sun G. UHPLC-ESI-Q-TOF-MS/MS analysis, antioxidant activity combined fingerprints for quality consistency evaluation of compound liquorice tablets. RSC Adv. 2018;8:27661–27673. doi: 10.1039/C8RA02431F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X., Jia J., Jing X., Li G. Antioxidant activities of extracts from Sarcocarp of Cotoneaster multiflorus. J. Chem. 2018;2018:4619768. doi: 10.1155/2018/4619768. [DOI] [Google Scholar]
- 26.Yusuf A.A., Lawal B., Abubakar A.N., Berinyuy E.B., Omonije Y.O., Umar S.I., Shebe M.N., Alhaji Y.M. In-vitro Antioxidants, antimicrobial and toxicological evaluation of Nigerian Zingiber officinale. Clin. Phytosci. 2018;4:2–9. doi: 10.1186/s40816-018-0070-2. [DOI] [Google Scholar]
- 27.Saleem A., Saleem M., Akhtar M.F. Antioxidant, anti-Inflammatory and antiarthritic potential of Moringa oleifera Lam: An Ethnomedicinal Plant of Moringaceae Family. S. Afr. J. Bot. 2020;128:246–256. doi: 10.1016/j.sajb.2019.11.023. [DOI] [Google Scholar]
- 28.Leite K.C.D.S., Garcia L.F., Lobón G.S., Thomaz D.V., Moreno E.K.G., De Carvalho M.F., Rocha M.L., Dos Santos W.T.P., Gil E.D.S. Antioxidant activity evaluation of dried herbal extracts: An electroanalytical approach. Rev. Bras. Farm. 2018;28:325–332. doi: 10.1016/j.bjp.2018.04.004. [DOI] [Google Scholar]
- 29.Mahfuz A., Salam F.B.A., Deepa K.N., Hasan A.H.M.N. Characterization of In-vitro antioxidant, cytotoxic, thrombolytic and membrane stabilizing potential of different extracts of Cheilanthes tenuifolia and Stigmasterol isolation from n-Hexane Extract. Clin. Phytosci. 2019;5:39. doi: 10.1186/s40816-019-0135-x. [DOI] [Google Scholar]
- 30.Ahmed F., Aktar F., Rashid M.A., Rahman M.S. Free radical scavenging, membrane stabilizing and thrombolytic potentials of the leaves of Quisqualis indica (L.) Bangladesh Pharm. J. 2021;24:99–104. doi: 10.3329/bpj.v24i2.54707. [DOI] [Google Scholar]
- 31.Kim J., Kim H., Choi H., Jo A., Kang H., Yun H., Im S., Choi C. Anti-Inflammatory effects of a Stauntonia hexaphylla fruit extract in Lipopolysaccharide-activated RAW-264.7 macrophages and rats by carrageenan-induced hind paw swelling. Nutrients. 2018;10:110. doi: 10.3390/nu10010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim Y., Park J.W., Kwon O.K., Lee J.W., Lee H.S., Lee S., Choi S., Li W., Jin H., Han S.B., et al. Anti-Inflammatory effects of a methanolic extract of Castanea Seguinii Dode in LPS-Induced RAW264.7 macrophage cells. Int. J. Mol. Med. 2018;41:391–398. doi: 10.3892/ijmm.2017.3223. [DOI] [PubMed] [Google Scholar]
- 33.Sandhiutami N.M.D., Moordiani M., Laksmitawati D.R., Fauziah N., Maesaroh M., Widowati W. In vitro assesment of anti-Inflammatory activities of Coumarin and Indonesian Cassia extract in RAW264.7 murine macrophage cell line. Iran. J. Basic Med. Sci. 2017;20:99–106. doi: 10.22038/ijbms.2017.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai B., Wei D., Zheng N.N., Chi Z.H., Xin N., Ma T.X., Zheng L.Y., Sumi R., Sun L. Coccomyxa Gloeobotrydiformis Polysaccharide Inhibits Lipopolysaccharide-Induced Inflammation in RAW 264.7 Macrophages. Cell. Physiol. Biochem. 2019;51:2523–2535. doi: 10.1159/000495922. [DOI] [PubMed] [Google Scholar]
- 35.Joshi D.V., Patil R.R., Naik S.R. Hydroalcohol extract of Trigonella foenum-graecum seed attenuates markers of inflammation and oxidative stress while improving exocrine function in diabetic rats. Pharm. Biol. 2014;53:201–211. doi: 10.3109/13880209.2014.913296. [DOI] [PubMed] [Google Scholar]
- 36.Lin C.Y., Wang W.H., Chen S.H., Chang Y.W., Hung L.C., Chen C.Y., Chen Y.H. Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. Int. J. Mol. Sci. 2017;18:2637. doi: 10.3390/ijms18122637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vysakh A., Jayesh K., Helen L.R., Jyothis M., Latha M.S. Acute oral toxicity and anti-Inflammatory evaluation of methanolic extract of Rotula aquatica roots in Wistar rats. J. Ayurveda Integr. Med. 2020;11:45–52. doi: 10.1016/j.jaim.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Modak D., Paul S., Sarkar S., Thakur S., Bhattacharjee S. Validating Potent anti-Inflammatory and anti-Rheumatoid Properties of Drynaria quercifolia rhizome methanolic extract through In vitro, in vivo, in silico and GC-MS-Based Profiling. BMC Complement. Med. Ther. 2021;21:89. doi: 10.1186/s12906-021-03265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arab H.H., El-Sawalhi M.M. Carvedilol alleviates adjuvant-Induced arthritis and subcutaneous air pouch edema: Modulation of oxidative stress and inflammatory mediators. Toxicol. Appl. Pharmacol. 2013;268:241–248. doi: 10.1016/j.taap.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Alves C.F., Alves V.B.F., de Assis I.P., Clemente-Napimoga J.T., Uber-Bucek E., Dal-Secco D., Cunha F.Q., Rehder V.L.G., Napimoga M.H. Anti-Inflammatory activity and possible mechanism of extract from Mikania laevigata in carrageenan-induced peritonitis. J. Pharm. Pharmacol. 2009;61:1097–1104. doi: 10.1211/jpp/61.08.0014. [DOI] [PubMed] [Google Scholar]
- 41.Haddadi R., Rashtiani R. Anti-Inflammatory and anti-hyperalgesic effects of Milnacipran in inflamed rats: Involvement of myeloperoxidase activity, cytokines and oxidative/nitrosative stress. Inflammopharmacology. 2020;28:903–913. doi: 10.1007/s10787-020-00726-2. [DOI] [PubMed] [Google Scholar]
- 42.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C.B., Rahu N. Oxidative stress and inflammation: What polyphenols can do for Us? Oxidative Med. Cell. Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nascimento C.P., Luz D.A., da Silva C.C.S., Malcher C.M.R., Fernandes L.M.P., Dalla Santa H.S., Gomes A.R.Q., Monteiro M.C., Ribeiro C.H.M.A., Fontes-Júnior E.A., et al. Ganoderma Lucidum Ameliorates Neurobehavioral Changes and Oxidative Stress Induced by Ethanol Binge Drinking. Oxidative Med. Cell. Longev. 2020;2020:2497845. doi: 10.1155/2020/2497845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta S.K., Kalaiselvan V., Srivastava S., Saxena R., Agrawal S.S. Trigonella foenum-graecum (Fenugreek) Protects against Selenite-Induced Oxidative Stress in Experimental Cataractogenesis. Biol. Trace Elem. Res. 2010;136:258–268. doi: 10.1007/s12011-009-8540-5. [DOI] [PubMed] [Google Scholar]
- 45.Zhishen J., Mengcheng T., Jianming W. The Determination of Flavonoid Contents in Mulberry and their Scavenging Effects on Superoxide Radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 46.Arruda C., Ribeiro V.P., Mejía J.A.A., Almeida M.O., Goulart M.O., Candido A.C.B.B., Dos Santos R.A., Magalhães L.G., Martins C.H.G., Bastos J.K. Green propolis: Cytotoxic and leishmanicidal activities of artepillin C, p-coumaric acid, and their degradation products. Rev. Bras. Farmacogn. 2020;30:169–176. doi: 10.1007/s43450-020-00043-3. [DOI] [Google Scholar]
- 47.El-Hamamsy S.M.A., El-Khamissi H.A.Z. Phytochemicals, Antioxidant Activity and Identification of Phenolic Compounds by HPLC of Pomegranate (Punica granatum L.) Peel Extracts. J. Agric. Chem. Biotechnol. 2020;11:79–84. doi: 10.21608/jacb.2020.95837. [DOI] [Google Scholar]
- 48.Saenjum C., Pattananandecha T., Nakagawa K. Detection of antioxidant phytochemicals isolated from Camellia japonica seeds using HPLC and EPR imaging. Antioxidants. 2020;9:493. doi: 10.3390/antiox9060493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monagas M., Suárez R., Gómez-Cordovés C., Bartolomé B. Simultaneous determination of nonanthocyanin phenolic compounds in red wines by HPLC-DAD/ESI-MS. Am. J. Enol. Vitic. 2005;56:139–147. [Google Scholar]
- 50.Chou O., Ali A., Subbiah V., Barrow C.J., Dunshea F.R., Suleria H.A. LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation. 2021;7:73. doi: 10.3390/fermentation7020073. [DOI] [Google Scholar]
- 51.Kivilompolo M., Obůrka V., Hyötyläinen T. Comparison of GC–MS and LC–MS methods for the analysis of antioxidant phenolic acids in herbs. Anal. Bioanal. Chem. 2007;388:881–887. doi: 10.1007/s00216-007-1298-8. [DOI] [PubMed] [Google Scholar]
- 52.Karar M.E., Kuhnert N. UPLC-ESI-Q-TOF-MS/MS characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaves, fruits and their herbal derived drops (Crataegutt Tropfen) J. Chem. Biol. Ther. 2015;1:2572-0406. [Google Scholar]
- 53.Olennikov D.N., Chirikova N.K., Vasilieva A.G., Fedorov I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants. 2020;9:526. doi: 10.3390/antiox9060526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeszka-Skowron M., Zgoła-Grześkowiak A., Frankowski R. Cistus incanus a promising herbal tea rich in bioactive compounds: LC–MS/MS determination of catechins, flavonols, phenolic acids and alkaloids—A comparison with Camellia sinensis, Rooibos and Hoan Ngoc herbal tea. J. Food Compos. Anal. 2018;74:71–81. doi: 10.1016/j.jfca.2018.09.003. [DOI] [Google Scholar]
- 55.Akbari S., Abdurahman N.H., Yunus R.M., Alara R.A., Abayomi O.O. Extraction, characterization and antioxidant activity of fenugreek (Trigonella-foenum graecum) seed oil. Mater. Sci. Energy Technol. 2019;2:349–355. doi: 10.1016/j.mset.2018.12.001. [DOI] [Google Scholar]
- 56.Kenny O., Smyth T.J., Hewage C.M., Brunton N.P. Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem. 2013;141:4295–4302. doi: 10.1016/j.foodchem.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 57.Farrell T., Poquet L., Dionisi F., Barron D., Williamson G. Characterization of hydroxycinnamic acid glucuronide and sulfate conjugates by HPLC–DAD–MS2: Enhancing chromatographic quantification and application in Caco-2 cell metabolism. J. Pharm. Biomed. Anal. 2011;55:1245–1254. doi: 10.1016/j.jpba.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 58.Tripathi U.N., Chandra D. Anti-Hyperglycemic and Anti-Oxidative Effect of Aqueous Extract of Momordica charantia Pulp and Trigonella foenum graecum Seed in Alloxan-Induced Diabetic Rats. Indian J. Biochem. Biophys. 2010;47:227–233. doi: 10.4161/oxim.2.5.9529. [DOI] [PubMed] [Google Scholar]
- 59.Owji H., Hemmati S., Heidari R., Hakimzadeh M. Effect of Alumina (Al2O3) Nanoparticles and Macroparticles on Trigonella foenum-graceum L. In vitro Cultures: Assessment of Growth Parameters and Oxidative Stress-Related Responses. 3 Biotech. 2019;9:419. doi: 10.1007/s13205-019-1954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kudumela R.G., McGaw L.J., Masoko P. Antibacterial Interactions, Anti-Inflammatory and Cytotoxic Effects of Four Medicinal Plant Species. BMC Complement. Altern. Med. 2018;18:199. doi: 10.1186/s12906-018-2264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar G., Karthik L., Rao K.V.B. Haemolytic Activity of Indian Medicinal Plants toward Human Erythrocytes: An In vitro Study. Elixir Appl. Bot. 2011;40:e5537. [Google Scholar]
- 62.Karim M.A., Islam M.A., Islam M.M., Rahman M.S., Sultana S., Biswas S., Hosen M.J., Mazumder K., Rahman M.M., Hasan M.N. Evaluation of Antioxidant, Anti-Hemolytic, Cytotoxic Effects and Anti-Bacterial Activity of Selected Mangrove Plants (Bruguiera Gymnorrhiza and Heritiera Littoralis) in Bangladesh. Clin. Phytosci. 2020;6:8. doi: 10.1186/s40816-020-0152-9. [DOI] [Google Scholar]
- 63.Ktari N., Trabelsi I., Bardaa S., Triki M., Bkhairia I., Salem R.B.S., Nasri M., Salah R.B. Antioxidant and hemolytic activities, and effects in rat cutaneous wound healing of a novel polysaccharide from fenugreek (Trigonella foenum-graecum) seeds. Int. J. Biol. Macromol. 2017;95:625–634. doi: 10.1016/j.ijbiomac.2016.11.091. [DOI] [PubMed] [Google Scholar]
- 64.Nautiyal C.S., Govindarajan R., Lavania M., Pushpangadan P. Novel Mechanism of Modulating Natural Antioxidants in Functional Foods: Involvement of Plant Growth Promoting Rhizobacteria NRRL B-30488. J. Agric. Food Chem. 2008;56:4474–4481. doi: 10.1021/jf073258i. [DOI] [PubMed] [Google Scholar]
- 65.Villa-De La Torre F., Kinscherf R., Bonaterra G., Arana-Argaez V.E., Méndez-González M., Cáceres-Farfán M., Borges-Argáez R. Anti-Inflammatory and Immunomodulatory Effects of Critonia Aromatisans Leaves: Downregulation of pro-Inflammatory Cytokines. J. Ethnopharmacol. 2016;190:174–182. doi: 10.1016/j.jep.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Patel N.K., Pulipaka S., Dubey S.P., Bhutani K.K. Pro-Inflammatory Cytokines and Nitric Oxide Inhibitory Constituents from Cassia Occidentalis Roots. Nat. Prod. Commun. 2014;9:661–664. doi: 10.1177/1934578X1400900519. [DOI] [PubMed] [Google Scholar]
- 67.Nworu C.S., Akah P.A., Okoye F.B.C., Toukam D.K., Udeh J., Esimone C.O. The Leaf Extract of Spondias Mombin, L. Displays an Anti-Inflammatory Effect and Suppresses Inducible Formation of Tumor Necrosis Factor-α and Nitric Oxide (NO) J. Immunotoxicol. 2011;8:10–16. doi: 10.3109/1547691X.2010.531406. [DOI] [PubMed] [Google Scholar]
- 68.Lin W.C., Deng J.S., Huang S.S., Wu S.H., Lin H.Y., Huang G.J. Evaluation of Antioxidant, Anti-Inflammatory, and Anti-Proliferative Activities of Ethanol Extracts from Different Varieties of Sanghuang Species. RSC Adv. 2017;7:7780–7788. doi: 10.1039/C6RA27198G. [DOI] [Google Scholar]
- 69.Dabitao D., Margolick J.B., Lopez J., Bream J.H. Multiplex Measurement of Proinflammatory Cytokines in Human Serum: Comparison of the Meso Scale Discovery Electrochemiluminescence Assay and the Cytometric Bead Array. J. Immunol. Methods. 2011;372:71–77. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sindhu G.S., Pushpan C.K., Nambisan B.A.H. Evaluation of anti-arthritic potential of Trigonella foenum graecum L. (Fenugreek) mucilage against rheumatoid arthritis. Prostaglandins Other Lipid Mediat. 2018;138:48–53. doi: 10.1016/j.prostaglandins.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Syam S., Bustamam A., Abdullah R., Sukari M.A., Hashim N.M., Mohan S., Looi C.Y., Wong W.F., Yahayu M.A., Abdelwahab S.I. β Mangostin Suppress LPS-Induced Inflammatory Response in RAW 264.7 Macrophages in vitro and Carrageenan-Induced Peritonitis in vivo. J. Ethnopharmacol. 2014;153:435–445. doi: 10.1016/j.jep.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 72.Naz R., Ayub H., Nawaz S., Islam Z.U., Yasmin T., Bano A., Wakeel A., Zia S., Roberts T.H. Antimicrobial Activity, Toxicity and Anti-Inflammatory Potential of Methanolic Extracts of Four Ethnomedicinal Plant Species from Punjab, Pakistan. BMC Complement. Altern. Med. 2017;17:302. doi: 10.1186/s12906-017-1815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loha M., Mulu A., Abay S.M., Ergete W., Geleta B. Acute and Subacute Toxicity of Methanol Extract of Syzygium Guineense Leaves on the Histology of the Liver and Kidney and Biochemical Compositions of Blood in Rats. Evid.-Based Complement. Altern. Med. 2019;2019:5702159. doi: 10.1155/2019/5702159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sholikhah E.N., Mustofa M., Nugrahaningsih D.A.A., Yuliani F.S., Purwono S., Sugiyono S., Widyarini S., Ngatidjan N., Jumina J., Santosa D., et al. Acute and Subchronic Oral Toxicity Study of Polyherbal Formulation Containing Allium sativum L., Terminalia bellirica (Gaertn.) Roxb., Curcuma Aeruginosa Roxb., and Amomum Compactum Sol. Ex. Maton in Rats. BioMed Res. Int. 2020;2020:8609364. doi: 10.1155/2020/8609364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Narasimhamurthy K., Viswanatha S., Ramesh B.S. Acute and subchronic toxicity assessment of debitterized fenugreek powder in the mouse and rat. Food Chem. Toxicol. 1999;37:831–838. doi: 10.1016/S0278-6915(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 76.Kandhare A.D., Bodhankar S.L., Mohan V., Thakurdesai P.A. Acute and repeated doses (28 days) oral toxicity study of glycosides based standardized fenugreek seed extract in laboratory mice. Regul. Toxicol. Pharmacol. 2015;72:323–334. doi: 10.1016/j.yrtph.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Moriasi G.A., Ireri A.M., Nelson E.M., Ngugi M.P. In vivo Anti-Inflammatory, Anti-Nociceptive, and in vitro Antioxidant Efficacy, and Acute Oral Toxicity Effects of the Aqueous and Methanolic Stem Bark Extracts of Lonchocarpus eriocalyx (Harms.) Heliyon. 2021;7:e07145. doi: 10.1016/j.heliyon.2021.e07145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa B., Colleoni M., Conti S., Parolaro D., Franke C., Elisa A. Oral Anti-Inflammatory Activity of Cannabidiol, a Non-Psychoactive Constituent of Cannabis, in Acute Carrageenan-Induced Inflammation in the Rat Paw. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2004;369:294–299. doi: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- 79.Yao L.J., Jalil J., Attiq A., Hui C.C., Zakaria N.A. The Medicinal Uses, Toxicities and Anti-Inflammatory Activity of Polyalthia Species (Annonaceae) J. Ethnopharmacol. 2019;229:303–325. doi: 10.1016/j.jep.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Spiller F., Alves M.K., Vieira S.M., Carvalho T.A., Leite C.E., Lunardelli A., Poloni J.A., Cunha F.Q., de Oliveira J.R. Anti-Inflammatory Effects of Red Pepper (Capsicum baccatum) on Carrageenan- and Antigen-Induced Inflammation. J. Pharm. Pharmacol. 2010;60:473–478. doi: 10.1211/jpp.60.4.0010. [DOI] [PubMed] [Google Scholar]
- 81.Nussbaum C., Klinke A., Adam M., Baldus S., Sperandio M. Myeloperoxidase: A Leukocyte-Derived Protagonist of Inflammation and Cardiovascular Disease. Antioxid. Redox Signal. 2013;18:692–713. doi: 10.1089/ars.2012.4783. [DOI] [PubMed] [Google Scholar]
- 82.Chniguir A., Zioud F., Marzaioli V., El-Benna J., Bachoual R. Syzygium aromaticum aqueous extract Inhibits Human Neutrophils Myeloperoxidase and Protects Mice from LPS-Induced Lung Inflammation. Pharm. Biol. 2019;57:56–64. doi: 10.1080/13880209.2018.1557697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silva A.O., Damaceno Alves A., de Almeida D.A.T., Balogun S.O., de Oliveira R.G., Aires Aguiar A., Soares I.M., Marson-Ascêncio P.G., Ascêncio S.D., de Oliveira Martins D.T. Evaluation of Anti-Inflammatory and Mechanism of Action of Extract of Macrosiphonia longiflora (Desf.) Müll. Arg. J. Ethnopharmacol. 2014;154:319–329. doi: 10.1016/j.jep.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 84.Basting R.T., Spindola H.M., de Oliveira Sousa I.M., Queiroz N.D., Trigo J.R., de Carvalho J.E., Foglio M.A. Pterodon Pubescens and Cordia Verbenacea Association Promotes a Synergistic Response in Antinociceptive Model and Improves the Anti-Inflammatory Results in Animal Models. Biomed. Pharmacother. 2019;112:108693. doi: 10.1016/j.biopha.2019.108693. [DOI] [PubMed] [Google Scholar]
- 85.Perez-Cano F.J., Castell M. Flavonoids, Inflammation and Immune System. Nutrients. 2016;8:659. doi: 10.3390/nu8100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and Supplementary Materials.