Abstract
People living with chronic hepatitis B virus (HBV) infection are at high risk of liver disease progression, which is positively associated with metabolic disorders, but inversely associated with dyslipidemia. Diet, including dietary antioxidants, is a lever of metabolic disorder management. In particular, elevated coffee consumption is associated with different metabolic outcomes in the general population. We aimed to test whether such associations occur in HBV-infected people. Based on cross-sectional data from the ANRS CO22 Hepather cohort, we performed logistic regression models with (i) dyslipidemia, (ii) hypertension, and (iii) diabetes as outcomes, and with demographic, clinical, and socio-behavioral (including coffee consumption) data as explanatory variables. Among 4746 HBV-infected patients, drinking ≥3 cups of coffee per day was associated with a higher risk of dyslipidemia (adjusted odds ratio [95% confidence interval] 1.49 [1.10–2.00], p = 0.009) and a lower risk of hypertension (0.64 [0.50–0.82], p = 0.001). It was not associated with diabetes. Elevated coffee consumption was associated with a higher risk of dyslipidemia and a lower risk of hypertension in HBV-infected patients, two effects expected to be associated with favorable clinical outcomes. Further studies should test whether such metabolic benefits translate into reduced mortality risk in this population.
Keywords: coffee, tea, hepatitis B, metabolic syndrome, dyslipidemia, hypertension, polyphenol
1. Introduction
Hepatitis B virus (HBV) infection affects hosts’ metabolic pathways. It is associated with lower levels of serum total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides (TG) [1,2]. Metabolic syndrome (MetS) is defined as having three or more of the following cardiovascular risk factors: central obesity, elevated TG, reduced HDL-cholesterol, elevated blood pressure, and elevated fasting glucose [3]. Accordingly, people infected with HBV have a lower prevalence of MetS [4,5]. More specifically, a strong inverse association was found between HBV infection and elevated TG, as well as a slight inverse association with both reduced HDL-cholesterol and elevated fasting glucose. No association was found with central obesity or elevated blood pressure [4].
However, MetS is itself a risk factor for hepatocellular carcinoma (HCC), non-HCC cancer, liver-related and all-cause mortality [5,6,7] in people infected with HBV, who are over 10 times more likely to die from liver cancer than people not infected [8]. Therefore, the relatively low prevalence of MetS in this population should not divert attention from its potential lethal effects.
The five MetS components do not appear to play the same role in the pathogenesis of liver disease in HBV-infected persons [5]. For example, total cholesterol was significantly lower in HBV patients who had advanced chronic liver disease than in those who did not [9]. Furthermore, hypercholesterolemia in HBV patients was independently associated with decreased liver cancer mortality, irrespective of statin use [10]. Moreover, low levels of triglycerides were associated with HCC in HBV-infected patients with type 2 diabetes [11,12]. Accordingly, low levels of cholesterol and triglycerides may be risk factors for liver disease in this population.
Modulating diet is one of the main strategies available for the prevention and treatment of MetS [13]. For instance, the Dietary Approaches to Stop Hypertension (DASH) diet is recognized as an effective dietary intervention to reduce blood pressure [14]. Furthermore, adherence to the Mediterranean diet was associated with lower TG and higher HDL-cholesterol [15]. These two dietary patterns are characterized by high amounts of antioxidants, which probably play a role in the observed protective effects [16,17]. While data are still inconclusive, dietary antioxidants may constitute protective factors against liver disease in HBV-infected patients [18,19,20]. Polyphenols comprise a large family of molecules bearing one or more phenolic rings. Most possess antioxidant (and anti-inflammatory) properties [21]. Coffee and tea constitute two main food sources of polyphenols [22,23,24]. In the general population, coffee consumption is associated with higher total cholesterol levels. Results for TG levels are less consistent [25,26,27]. Conversely, tea consumption seems to lower total cholesterol; again, results for TG levels are inconsistent [26,28]. With regard to hypertension, a blood pressure-lowering effect has been consistently documented for both beverages [29,30,31]. Moreover, coffee consumption seems to be more robustly associated with beneficial diabetes-related outcomes than tea [32,33,34]. To our knowledge, none of the above associations have been explored in people chronically infected with HBV. Managing lipid serum levels and blood pressure in HBV-infected patients—including through diet modulation—may be a lever to reduce the elevated risk of liver disease progression and HCC in this population for whom functional cure remains rare [8,35,36]. Accordingly, the present study aimed to test whether coffee consumption is associated with dyslipidemia, hypertension, and diabetes in HBV-infected patients.
2. Materials and Methods
2.1. Design and Participants
ANRS CO22 Hepather is a French, national, multicenter, prospective, observational cohort study of patients with hepatitis B or C virus infection (ClinicalTrials.gov registration number NCT01953458). It is promoted by the ANRS Emerging Infectious Diseases. Initiated in 2012, Hepather aims to increase knowledge of viral hepatitis [37]. Thirty-two hospital clinical centers, spread over French territory, are involved in data collection.
Sociodemographic, clinical, and biological data were collected at the cohort enrolment visit. Patients are followed on a yearly basis, and supplemental data are collected during visits related to particular events (e.g., HBV or hepatitis C-HCV-therapy initiation). Written informed consent was obtained from each patient before enrolment. The protocol was developed in accordance with the Declaration of Helsinki and French law for biomedical research, and was approved by the CPP Ile de France 3 ethics committee (Paris, France) and the French Regulatory Authority (ANSM).
2.2. Study Population
The main exclusion criterion in Hepather was HIV coinfection. For the present study, the study population comprised patients with chronic HBV virus infection at cohort enrolment (defined by positive HBsAg for at least six months). Patients with HCV or HDV coinfection were excluded from the study population, as were patients with no data on coffee consumption and those with unavailable data for the three outcomes (i.e., dyslipidemia, hypertension, and diabetes).
2.3. Data Collection
We used data collected during the cohort enrolment visit, where physicians completed a structured questionnaire during a face-to-face interview with their patients, and collected anthropometric measurements, as well as blood and urine samples.
The questionnaire collected clinical and sociodemographic data including gender, age, country of birth, time since HBV diagnosis, cannabis use, tobacco use, current and past alcohol use (number of standard drinks per day), current coffee consumption (number of cups per day), current tea consumption (number of cups per day), living with a partner (yes/no), average monthly household income, and educational level. Body height, weight, and waist circumference were measured. Data derived from enrolment blood samples included platelet count (platelets/L), aspartate aminotransferase (AST, IU/L), and alanine aminotransferase (ALT, IU/L) levels, and gamma glutamyltransferase (GGT, IU/L) level.
Information concerning the existence of medical comorbidities (and associated treatments) at enrolment, including on diabetes, hypertension, hypercholesterolemia and hypertriglyceridemia, was also gathered during the inclusion visit using an electronic case report form.
2.4. Outcomes
Three outcomes were selected for this study. The first, dyslipidemia, was defined as the presence of hypercholesterolemia or hypertriglyceridemia, or receiving treatments for this type of disorder. The second, hypertension, was defined as the presence of hypertension or receiving treatment for it. Finally, the third outcome, diabetes, was defined as the diagnosis of diabetes or associated treatment receipt.
2.5. Explanatory Variables
For coffee consumption, a three-category variable was created based on three thresholds: zero, one, and three or more cups per day. The one cup per day threshold was used to test for a potential dose response relationship between coffee consumption and the three study outcomes. The three cups or more per day threshold was chosen based on previous results showing the potential protective effect of this level of consumption on liver stiffness and mortality in patients likely to develop liver disease [38,39]. Given the expected dose-response effect of coffee consumption [29,40], we also tested the higher threshold of four cups per day when the three cups per day threshold was not significantly associated with the outcome.
For tea consumption, as the dose response is less clearly documented, two alternative thresholds were considered to characterize elevated tea consumption: one cup per day (i.e., daily consumption), and three or more cups per day. This led to two dichotomized variables.
Alcohol consumption was classified into four categories based on the threshold for unhealthy alcohol use (defined as >2 standard drinks per day for women and >3 standard drinks per day for men, in accordance with the French National Authority for Health [41]) as follows: abstinent (i) with or (ii) without a history of unhealthy alcohol use, (iii) current unhealthy alcohol use, and (iv) current moderate alcohol use (i.e., non-abstinent and healthy use). For both cannabis and tobacco use, participants were classified in the ‘current’, ‘former’, or ‘never’ user categories.
Poverty was defined as a standard of living lower than the 2015 French poverty threshold (1015 euros/month) [42]. Standard of living was calculated as disposable income divided by the number of consumption units in the household. Educational level was dichotomized into having an upper secondary school certificate or not.
Liver fibrosis was assessed using the FIB-4 index, which is a noninvasive marker of fibrosis calculated using age, AST level, ALT level, and platelet count, with the following formula: (age [years] × AST [IU/L])/(platelet count [109/L] × ALT [IU/L])1/2. The presence of advanced fibrosis was defined as an FIB-4 index > 3.25 [43,44].
2.6. Statistical Analyses
Study sample characteristics were compared between participants according to their coffee consumption (zero, one, and three or more cups per day). Chi-square and Kruskal–Wallis tests were used for categorical and continuous variables, respectively. Study sample characteristics were also compared between included patients and those patients excluded from the study population because of missing data.
Three separate logistic regression models were performed to test for an association between the use of the two studied polyphenol-rich beverages (i.e., coffee and tea) and the three study outcomes at cohort enrolment, after adjustment for other potential predictors. Associations were assessed by (adjusted) odds ratios ((a)ORs). Only variables with a liberal p-value < 0.20 in the univariable analyses were considered eligible for the multivariable model. The final multivariable model was built using a backward stepwise procedure, and the likelihood ratio test (p < 0.05) was used to define the variables to maintain in the final model.
For each outcome (i.e., dyslipidemia, hypertension, and diabetes), three multivariable models were run to better characterize the associations between the consumption variables and the outcome. In the first model (model 1), advanced liver fibrosis (FIB-4 index > 3.25) and the two metabolic disorders (i.e., outcomes) other than the outcome in question were not considered eligible as explanatory variables, as advanced liver fibrosis may directly cause diabetes [45,46], and metabolic disorders are likely to be co-occurring and to share a common pathogenesis pathway. In the second (model 2) and third (model 3) models, advanced liver fibrosis and metabolic disorders, respectively, were tested as potential explanatory variables.
To test for a dose response effect of coffee and tea, we also performed an alternative model to model 1 for each outcome, with hot beverage consumption considered as a continuous variable (i.e., cups per day). The other parameters were similar to those in model 1.
All analyses were performed with Stata software version 17.0 for Windows (StataCorp LP, College Station, TX, USA).
3. Results
3.1. Study Population Characteristics
The study population comprised 4746 participants (Figure 1).
Figure 1.
Flow chart of the study population (ANRS CO22 Hepather cohort). HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus.
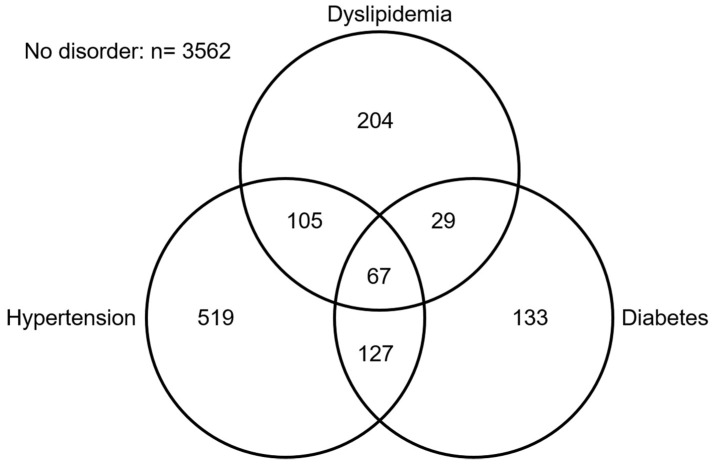
Participants’ characteristics according to their coffee consumption level are provided in Table 1. Most were men (63.3%), and the median age was 43 years (interquartile range [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]). Just over a quarter (26.8%) had elevated coffee consumption, while 39.1% had daily tea consumption. A quarter (24.9%) had at least one metabolic disorder, the most prevalent being hypertension (Figure 2). Characteristics of included vs. excluded participants are given in Supplementary Table S1.
Table 1.
Study sample characteristics according to coffee consumption (ANRS CO22 Hepather cohort, n = 4746).
| Study Population (N = 4746) N (%) |
Coffee Consumption N (%) |
||||
|---|---|---|---|---|---|
| Characteristics (% of missing) | 0 cup/day | 1–2 cups/day | ≥3 cups/day | p-value | |
| Gender | |||||
| Male | 3004 (63.3) | 973 (56.6) | 1086 (62.0) | 945 (74.2) | <10−3 |
| Female | 1742 (36.7) | 746 (43.4) | 667 (38.0) | 329 (25.8) | |
| Age (years) | |||||
| <40 | 1945 (41.0) | 910 (52.9) | 643 (36.7) | 392 (30.8) | <10−3 |
| 40–49 | 1157 (24.4) | 384 (22.3) | 406 (23.2) | 367 (28.8) | |
| 50–59 | 847 (17.8) | 231 (13.4) | 317 (18.1) | 299 (23.5) | |
| ≥60 | 797 (16.8) | 194 (11.3) | 387 (22.1) | 216 (17.0) | |
| Place of birth | |||||
| France | 1377 (29.0) | 387 (22.5) | 472 (26.9) | 518 (40.7) | <10−3 |
| Europe 1 | 497 (10.5) | 103 (6.0) | 208 (11.9) | 186 (14.6) | |
| North Africa | 412 (8.7) | 83 (4.8) | 199 (11.4) | 130 (10.2) | |
| Sub-Saharan Africa 2 | 1753 (36.9) | 921 (53.6) | 557 (31.8) | 275 (21.6) | |
| Asia | 707 (14.9) | 225 (13.1) | 317 (18.1) | 165 (13.0) | |
| Body mass index (kg/m2) 3 (0.9) | |||||
| <25 (under or normal weight) | 2378 (50.6) | 889 (52.2) | 881 (50.8) | 608 (48.1) | 0.259 |
| ≥25 and <30 (overweight) | 1631 (34.7) | 566 (33.2) | 601 (34.7) | 464 (36.7) | |
| ≥30 (obese) | 693 (14.7) | 248 (14.6) | 252 (14.5) | 193 (15.3) | |
| Living in a couple (0.1) | |||||
| No | 1654 (34.9) | 703 (41.0) | 563 (32.2) | 388 (30.5) | <10−3 |
| Yes | 3086 (65.1) | 1013 (59.0) | 1188 (67.8) | 885 (69.5) | |
| Coffee consumption | |||||
| None | 1719 (36.2) | / | / | / | |
| 1–2 cups/day | 1753 (36.9) | / | / | / | |
| ≥3 cups/day | 1274 (26.8) | / | / | / | |
| Tea consumption (0.3) | |||||
| Non-daily | 2881 (60.9) | 1001 (58.3) | 1019 (58.4) | 861 (67.8) | <10−3 |
| Daily | 1851 (39.1) | 717 (41.7) | 726 (41.6) | 408 (32.2) | |
| Tea consumption (0.3) | |||||
| <3 cups/day | 4240 (89.6) | 1521 (88.5) | 1586 (90.9) | 1133 (89.3) | 0.069 |
| ≥3 cups/day | 492 (10.4) | 197 (11.5) | 159 (9.1) | 136 (10.7) | |
| Cannabis use (0.7) | |||||
| Never | 4417 (93.7) | 1637 (95.8) | 1652 (94.9) | 1128 (89.3) | <10−3 |
| Former | 176 (3.7) | 38 (2.2) | 57 (3.3) | 81 (6.4) | |
| Current | 120 (2.5) | 34 (2.0) | 32 (1.8) | 54 (4.3) | |
| Tobacco use | |||||
| Never | 3072 (64.7) | 1369 (79.6) | 1140 (65.1) | 563 (44.2) | <10−3 |
| Former | 830 (17.5) | 181 (10.5) | 338 (19.3) | 311 (24.4) | |
| Current | 843 (17.8) | 169 (9.8) | 274 (15.6) | 400 (31.4) | |
| Alcohol use (0.4) | |||||
| Abstinent without history of unhealthy use | 2711 (57.3) | 1149 (67.2) | 983 (56.3) | 579 (45.5) | <10−3 |
| Moderate use | 1750 (37.0) | 484 (28.3) | 664 (38.0) | 602 (47.3) | |
| Current or past unhealthy use | 268 (5.7) | 77 (4.5) | 100 (5.7) | 91 (7.2) | |
| Living in poverty (3.0) | |||||
| No | 2389 (51.9) | 707 (42.5) | 922 (54.1) | 760 (61.4) | <10−3 |
| Yes | 2214 (48.1) | 955 (57.5) | 781 (45.9) | 478 (38.6) | |
| Educational level (1.4) | |||||
| <upper secondary school certificate | 2283 (48.8) | 803 (47.4) | 855 (49.5) | 625 (49.8) | 0.335 |
| ≥upper secondary school certificate | 2395 (51.2) | 892 (52.6) | 872 (50.5) | 631 (50.2) | |
| Time since HBV diagnosis—in years (3.3) | |||||
| Median [IQR] | 9.2 [3.9–17.0] | 7.6 [3.2–13.7] | 9.5 [4.3–17.7] | 11.2 [4.6–19.6] | <10−3 |
| Advanced liver fibrosis 4 (11.5) | |||||
| No | 4020 (95.7) | 1450 (95.5) | 1494 (94.8) | 1076 (97.2) | 0.010 |
| Yes | 181 (4.3) | 68 (4.5) | 82 (5.2) | 31 (2.8) | |
| Dyslipidemia | |||||
| No | 4341 (91.5) | 1626 (94.6) | 1588 (90.6) | 1127 (88.5) | <10−3 |
| Yes | 405 (8.5) | 93 (5.4) | 165 (9.4) | 147 (11.5) | |
| Hypertension | |||||
| No | 3928 (82.8) | 1449 (84.3) | 1404 (80.1) | 1075 (84.4) | 0.001 |
| Yes | 818 (17.2) | 270 (15.7) | 349 (19.9) | 199 (15.6) | |
| Diabetes | |||||
| No | 4390 (92.5) | 1604 (93.3) | 1602 (91.4) | 1184 (92.9) | 0.078 |
| Yes | 356 (7.5) | 115 (6.7) | 151 (8.6) | 90 (7.1) | |
1 The category ‘Europe’ included participants from the U.S. (n = 2), New Zealand (n = 1), and South America (n = 11). 2 The category ‘Sub-Saharan Africa’ included participants from Haiti (n = 44) and the Dominican Republic (n = 2). 3 World Health Organization categorization [47]. 4 Advanced liver fibrosis was defined as an FIB-4 score > 3.25 [43].
Figure 2.
Frequencies of metabolic disorders in the study population (ANRS CO22 Hepather cohort).
3.2. Factors Associated with Dyslipidemia
The results of univariable and multivariable logistic regressions for dyslipidemia are given in Table 2. Coffee consumption was associated with the outcome in all three multivariable models. Current elevated coffee consumption was associated with a higher likelihood of dyslipidemia in all three models (adjusted odds ratio (aOR) between 1.49 and 1.62), while moderate consumption only approached statistical significance. Other risk factors identified in all three models were older age and former or current tobacco use (Table 2). Daily tea consumption was associated with a lower likelihood of dyslipidemia in univariable analysis only.
Table 2.
Factors associated with dyslipidemia (ANRS CO22 Hepather cohort, n = 4746).
| Univariable Analysis | Multivariable Analysis (Model 1) 1 N = 4702 |
Multivariable Analysis (Model 2) 1 N = 4702 |
Multivariable Analysis (Model 3) 1 N = 4590 |
|||||
|---|---|---|---|---|---|---|---|---|
| OR [95% CI] | p-Value | aOR [95% CI] | p-Value | aOR [95% CI] | p-Value | aOR [95% CI] | p-Value | |
| Gender | ||||||||
| Male | 1 | |||||||
| Female | 0.61 [0.49–0.77] | <10−3 | ||||||
| Age (years) | <10−3 | <10−3 | <10−3 | <10−3 | ||||
| <40 | 1 | 1 | 1 | 1 | ||||
| 40–49 | 2.93 [2.01–4.27] | <10−3 | 2.51 [1.72–3.69] | <10−3 | 2.51 [1.72–3.69] | <10−3 | 2.24 [1.50–3.34] | <10−3 |
| 50–59 | 8.01 [5.65–11.34] | <10−3 | 6.40 [4.47–9.15] | <10−3 | 6.40 [4.47–9.15] | <10−3 | 4.65 [3.13–6.90] | <10−3 |
| ≥60 | 9.79 [6.93–13.82] | <10−3 | 7.72 [5.38–11.09] | <10−3 | 7.72 [5.38–11.09] | <10−3 | 4.43 [2.91–6.75] | <10−3 |
| Place of birth | <10−3 | |||||||
| France | 1 | |||||||
| Europe 2 | 0.93 [0.67–1.28] | 0.641 | ||||||
| North Africa | 0.70 [0.48–1.02] | 0.063 | ||||||
| Sub-Saharan Africa 3 | 0.36 [0.27–0.47] | <10−3 | ||||||
| Asia | 0.74 [0.55–1.00] | 0.050 | ||||||
| Body mass index (kg/m2) 4 | <10−3 | <10−3 | <10−3 | |||||
| <25 (under or normal weight) | 1 | 1 | 1 | |||||
| ≥25 and <30 (overweight) | 1.76 [1.39–2.22] | <10−3 | 1.48 [1.16–1.89] | 0.001 | 1.48 [1.16–1.89] | 0.001 | ||
| ≥30 (obese) | 2.30 [1.74–3.04] | <10−3 | 1.86 [1.39–2.48] | <10−3 | 1.86 [1.39–2.48] | <10−3 | ||
| Living in a couple | ||||||||
| No | 1 | |||||||
| Yes | 1.40 [1.12–1.76] | 0.003 | ||||||
| Coffee consumption | <10−3 | 0.033 | 0.033 | 0.009 | ||||
| None | 1 | 1 | 1 | 1 | ||||
| 1–2 cups/day | 1.82 [1.40–2.36] | <10−3 | 1.28 [0.97–1.70] | 0.083 | 1.28 [0.97–1.70] | 0.083 | 1.32 [0.99–1.75] | 0.061 |
| ≥3 cups/day | 2.28 [1.74–2.99] | <10−3 | 1.49 [1.10–2.00] | 0.009 | 1.49 [1.10–2.00] | 0.009 | 1.62 [1.19–2.20] | 0.002 |
| Tea consumption | ||||||||
| Non-daily | 1 | |||||||
| Daily | 0.80 [0.64–0.99] | 0.039 | ||||||
| Tea consumption | ||||||||
| <3 cups/day | 1 | |||||||
| ≥3 cups/day | 0.94 [0.67–1.32] | 0.720 | ||||||
| Cannabis use | 0.222 | |||||||
| Never | 1 | |||||||
| Former | 1.29 [0.79–2.11] | 0.302 | ||||||
| Current | 0.56 [0.25–1.29] | 0.173 | ||||||
| Tobacco use | <10−3 | <10−3 | <10−3 | <10−3 | ||||
| Never | 1 | 1 | 1 | 1 | ||||
| Former | 2.76 [2.18–3.49] | <10−3 | 1.63 [1.26–2.11] | <10−3 | 1.63 [1.26–2.11] | <10−3 | 1.67 [1.28–2.18] | <10−3 |
| Current | 1.45 [1.10–1.91] | 0.009 | 1.47 [1.08–1.98] | 0.013 | 1.47 [1.08–1.98] | 0.013 | 1.49 [1.09–2.04] | 0.013 |
| Alcohol use | <10−3 | |||||||
| Abstinent without history of unhealthy use | 1 | |||||||
| Moderate use | 1.59 [1.29–1.97] | <10−3 | ||||||
| Current or past unhealthy use | 2.12 [1.45–3.10] | <10−3 | ||||||
| Living in poverty | ||||||||
| No | 1 | |||||||
| Yes | 0.73 [0.59–0.90] | 0.003 | ||||||
| Educational level | ||||||||
| <upper secondary school certificate | 1 | |||||||
| ≥upper secondary school certificate | 0.61 [0.49–0.75] | <10−3 | ||||||
| Time since HBV diagnosis (years) | 1.03 [1.03–1.05] | <10−3 | 1.01 [1.00–1.02] | 0.011 | ||||
| Advanced liver fibrosis 5 | ||||||||
| No | 1 | - | - | |||||
| Yes | 1.14 [0.69–1.88] | 0.598 | - | - | ||||
| Diabetes | ||||||||
| No | 1 | - | - | 1 | ||||
| Yes | 4.88 [3.76–6.33] | <10−3 | - | - | 2.61 [1.94–3.52] | <10−3 | ||
| Hypertension | ||||||||
| No | 1 | - | - | 1 | ||||
| Yes | 4.22 [3.41–5.23] | <10−3 | - | - | 2.00 [1.55–2.59] | <10−3 | ||
1 In model 1, advanced liver fibrosis, diabetes, and hypertension were not considered eligible for multivariate analyses. In model 2, which was based on model 1, advanced liver fibrosis was considered eligible for multivariate analyses. In model 3, which was also based on model 1, both diabetes and hypertension were considered eligible for multivariate analyses. 2 The category ‘Europe’ included participants from the U.S. (n = 2), New Zealand (n = 1), and South America (n = 11). 3 The category ‘Sub-Saharan Africa’ included participants from Haiti (n = 44) and the Dominican Republic (n = 2). 4 World Health Organization categorization [47]. 5 Advanced liver fibrosis was defined as an FIB-4 score > 3.25 [43]. aOR, adjusted odds ratio; CI, confidence interval; HBV, hepatitis B virus.
When coffee and tea consumption were considered continuous variables, they were, respectively, positively (aOR 1.10, p < 0.001) and negatively (aOR 0.92, p = 0.029) associated with the outcome in univariable analyses. No such association was observed in the multivariable analysis (data not shown).
3.3. Factors Associated with Hypertension
The results of univariable and multivariable logistic regressions for hypertension are given in Table 3. Coffee consumption was associated with the outcome in all three multivariable models. Current elevated coffee consumption was associated with a lower likelihood of hypertension in all three models (adjusted odds ratio (aOR) between 0.64 and 0.66), while moderate consumption was not. Other risk factors identified in all three models were older age, being born in Sub-Saharan Africa, overweight, obesity, and current tobacco use (Table 3). Daily tea consumption was associated with a lower likelihood of hypertension in univariable analysis only.
Table 3.
Factors associated with hypertension (ANRS CO22 Hepather cohort, n = 4746).
| Univariable Analysis | Multivariable Analysis (Model 1) 1 N = 4702 |
Multivariable Analysis (Model 2) 1 N = 4702 |
Multivariable Analysis (Model 3) 1 N = 4590 |
|||||
|---|---|---|---|---|---|---|---|---|
| OR [95% CI] | p-Value | aOR [95% CI] | p-Value | aOR [95% CI] | p-Value | aOR [95% CI] | p-Value | |
| Gender | ||||||||
| Male | 1 | 1 | 1 | |||||
| Female | 0.75 [0.64–0.89] | 0.001 | 0.78 [0.63–0.95] | 0.014 | 0.79 [0.63–0.97] | 0.027 | ||
| Age (years) | <10−3 | <10−3 | <10−3 | <10−3 | ||||
| <40 | 1 | 1 | 1 | 1 | ||||
| 40–49 | 3.83 [2.84–5.17] | <10−3 | 4.02 [2.93–5.51] | <10−3 | 3.63 [2.60–5.06] | <10−3 | 3.69 [2.71–5.04] | <10−3 |
| 50–59 | 11.56 [8.71–15.35] | <10−3 | 13.02 [9.54–17.76] | <10−3 | 12.53 [9.02–17.39] | <10−3 | 10.58 [7.75–14.44] | <10−3 |
| ≥60 | 22.62 [17.11–29.91] | <10−3 | 26.58 [19.21–36.78] | <10−3 | 25.03 [17.75–35.30] | <10−3 | 21.62 [15.62–29.90] | <10−3 |
| Place of birth | <10−3 | <10−3 | <10−3 | <10−3 | ||||
| France | 1 | 1 | 1 | 1 | ||||
| Europe 2 | 0.71 [0.55–0.93] | 0.013 | 0.77 [0.57–1.05] | 0.103 | 0.74 [0.53–1.03] | 0.076 | 0.79 [0.58–1.07] | 0.126 |
| North Africa | 0.85 [0.64–1.11] | 0.231 | 0.93 [0.68–1.28] | 0.656 | 0.95 [0.68–1.31] | 0.740 | 0.85 [0.61–1.19] | 0.341 |
| Sub-Saharan Africa 3 | 0.57 [0.47–0.68] | <10−3 | 1.54 [1.20–1.98] | 0.001 | 1.55 [1.19–2.01] | 0.001 | 1.55 [1.20–1.99] | 0.001 |
| Asia | 0.54 [0.42–0.70] | <10−3 | 0.97 [0.73–1.30] | 0.861 | 1.02 [0.75–1.39] | 0.879 | 0.90 [0.68–1.21] | 0.491 |
| Body mass index (kg/m2) 4 | <10−3 | <10−3 | <10−3 | <10−3 | ||||
| <25 (under or normal weight) | 1 | 1 | 1 | 1 | ||||
| ≥25 and <30 (overweight) | 1.82 [1.52–2.17] | <10−3 | 1.56 [1.271.90] | <10−3 | 1.58 [1.27–1.95] | <10−3 | 1.44 [1.18–1.76] | <10−3 |
| ≥30 (obese) | 4.24 [3.47–5.19] | <10−3 | 3.97 [3.12–5.04] | <10−3 | 4.31 [3.36–5.55] | <10−3 | 3.25 [2.55–4.14] | <10−3 |
| Living in a couple | ||||||||
| No | 1 | |||||||
| Yes | 1.29 [1.10–1.52] | 0.002 | ||||||
| Coffee consumption | 0.001 | 0.001 | 0.004 | 0.003 | ||||
| None | 1 | 1 | 1 | 1 | ||||
| 1–2 cups/day | 1.33 [1.12–1.59] | 0.001 | 0.90 [0.73–1.11] | 0.338 | 0.91 [0.72–1.13] | 0.387 | 0.92 [0.74–1.14] | 0.425 |
| ≥3 cups/day | 0.99 [0.81–1.21] | 0.949 | 0.64 [0.50–0.82] | 0.001 | 0.65 [0.50–0.85] | 0.001 | 0.66 [0.51–0.85] | 0.001 |
| Tea consumption | ||||||||
| Non-daily | 1 | |||||||
| Daily | 0.77 [0.66–0.90] | 0.001 | ||||||
| Tea consumption | ||||||||
| <3 cups/day | 1 | |||||||
| ≥3 cups/day | 0.88 [0.68–1.14] | 0.329 | ||||||
| Cannabis use | 0.001 | |||||||
| Never | 1 | |||||||
| Former | 0.59 [0.37–0.95] | 0.028 | ||||||
| Current | 0.33 [0.16–0.68] | 0.002 | ||||||
| Tobacco use | <10−3 | 0.013 | 0.018 | 0.007 | ||||
| Never | 1 | 1 | 1 | 1 | ||||
| Former | 2.20 [1.84–2.63] | <10−3 | 1.19 [0.95–1.48] | 0.125 | 1.21 [0.96–1.52] | 0.111 | 1.26 [1.01–1.56] | 0.038 |
| Current | 0.63 [0.49–0.80] | <10−3 | 0.75 [0.57–1.00] | 0.047 | 0.76 [0.56–1.02] | 0.070 | 0.78 [0.59–1.03] | 0.083 |
| Alcohol use | <10−3 | |||||||
| Abstinent without history of unhealthy use | 1 | |||||||
| Moderate use | 1.26 [1.07–1.48] | 0.005 | ||||||
| Current or past unhealthy use | 2.42 [1.83–3.21] | <10−3 | ||||||
| Living in poverty | ||||||||
| No | 1 | |||||||
| Yes | 0.92 [0.78–1.07] | 0.258 | ||||||
| Educational level | ||||||||
| <upper secondary school certificate | 1 | 1 | 1 | |||||
| ≥upper secondary school certificate | 0.56 [0.48–0.65] | <10−3 | 0.81 [0.67–0.96] | 0.017 | 0.81 [0.67–0.98] | 0.031 | ||
| Time since HBV diagnosis—(years) | ||||||||
| Advanced liver fibrosis 5 | 1.03 [1.02–1.04] | <10−3 | ||||||
| No | 1 | 1 | ||||||
| Yes | 3.45 [2.54–4.70] | <10−3 | - | 1.50 [1.05–2.13] | 0.024 | - | ||
| Diabetes | - | - | ||||||
| No | 1 | - | - | 1 | ||||
| Yes | 7.23 [5.77–9.05] | <10−3 | - | - | 2.93 [2.23–3.84] | <10−3 | ||
| Hypertension | ||||||||
| No | 1 | - | - | 1 | ||||
| Yes | 4.22 [3.40–5.23] | <10−3 | - | - | 1.98 [1.52–2.57] | <10−3 | ||
1 In model 1, advanced liver fibrosis, diabetes, and hypertension were not considered eligible for multivariate analyses. Model 2 is based on model 1, but advanced liver fibrosis is considered eligible for multivariate analyses. Model 3 is based on model 1, but both diabetes and hypertension were considered eligible for multivariate analyses. 2 The category ‘Europe’ included participants from the U.S. (n = 2), New Zealand (n = 1), and South America (n = 11). 3 The category ‘Sub-Saharan Africa’ included participants from Haiti (n = 44) and the Dominican Republic (n = 2). 4 World Health Organization categorization [47]. 5 Advanced liver fibrosis was defined as an FIB-4 score > 3.25 [43]. aOR, adjusted odds ratio; CI, confidence interval; HBV, hepatitis B virus.
When coffee and tea consumption were considered as continuous variables, they were both negatively associated with the outcome in univariable analyses, but only coffee consumption remained in multivariable analysis (aOR 0.90 [95% confidence interval] [0.85; 0.95], p < 0.001) (data not shown).
3.4. Factors Associated with Diabetes
The results of univariable and multivariable logistic regressions for diabetes are given in Table 4. Coffee consumption was not associated with diabetes in multivariable analyses. Neither was it associated with the outcome when the threshold for elevated consumption was raised to four cups per day. Risk factors identified in all three multivariable models were older age, overweight and obesity, and living in poverty. Tea consumption was not associated with diabetes (Table 4).
Table 4.
Factors associated with diabetes (ANRS CO22 Hepather cohort, n = 4746).
| Univariable Analysis | Multivariable Analysis (Model 1) 1 N = 4702 |
Multivariable Analysis (Model 2) 1 N = 4702 |
Multivariable Analysis (Model 3) 1 N = 4590 |
|||||
|---|---|---|---|---|---|---|---|---|
| OR [95% CI] |
p- Value |
aOR [95% CI] | p-Value | aOR [95% CI] | p-Value | aOR [95% CI] | p-Value | |
| Gender | ||||||||
| Male | 1 | 1 | ||||||
| Female | 0.75 [0.59–0.94] | 0.014 | 0.75 [0.58–0.98] | 0.032 | ||||
| Age (years) | <10−3 | <10−3 | <10−3 | <10−3 | ||||
| <40 | 1 | 1 | 1 | 1 | ||||
| 40–49 | 2.74 [1.82–4.15] | <10−3 | 2.60 [1.68–4.02] | <10−3 | 2.59 [1.62–4.14] | <10−3 | 2.15 [1.38–3.35] | 0.001 |
| 50–59 | 7.49 [5.13–10.94] | <10−3 | 7.86 [5.22–11.83] | <10−3 | 7.79 [5.07–11.99] | <10−3 | 4.81 [3.10–7.44] | <10−3 |
| ≥60 | 11.44 [7.93–16.53] | <10−3 | 12.73 [8.34–19.43] | <10−3 | 11.71 [7.57–18.12] | <10−3 | 6.41 [4.04–10.17] | <10−3 |
| Place of birth | <10−3 | 0.048 | 0.016 | |||||
| France | 1 | 1 | 1 | |||||
| Europe 2 | 1.14 [0.78–1.64] | 0.501 | 1.20 [0.80–1.80] | 0.387 | 1.25 [0.82–1.92] | 0.299 | ||
| North Africa | 1.85 [1.31–2.61] | 0.001 | 1.67 [1.11–2.50] | 0.014 | 1.91 [1.27–2.87] | 0.002 | ||
| Sub-Saharan Africa 3 | 0.74 [0.56–0.98] | 0.037 | 1.39 [0.99–1.95] | 0.056 | 1.42 [1.00–2.02] | 0.051 | ||
| Asia | 0.91 [0.64–1.29] | 0.607 | 1.64 [1.11–2.42] | 0.013 | 1.67 [1.11–2.50] | 0.013 | ||
| Body mass index (kg/m2) 4 | <10−3 | <10−3 | <10−3 | <10−3 | ||||
| <25 (under or normal weight) | 1 | 1 | 1 | 1 | ||||
| ≥25 and <30 (overweight) | 2.87 [2.17–3.79] | <10−3 | 2.41 [1.78–3.25] | <10−3 | 2.14 [1.57–2.92] | <10−3 | 2.23 [1.64–3.04] | <10−3 |
| ≥30 (obese) | 6.06 [4.51–8.14] | <10−3 | 5.13 [3.67–7.17] | <10−3 | 5.02 [3.61–6.99] | <10−3 | 3.73 [2.63–5.27] | <10−3 |
| Living in a couple | ||||||||
| No | 1 | |||||||
| Yes | 0.94 [0.75–1.18] | 0.581 | ||||||
| Coffee consumption | 0.079 | |||||||
| None | 1 | |||||||
| 1–2 cups/day | 1.31 [1.02–1.69] | 0.034 | ||||||
| ≥ 3 cups/day | 1.06 [0.80–1.41] | 0.689 | ||||||
| Tea consumption | ||||||||
| Non-daily | 1 | |||||||
| Daily | 0.83 [0.67–1.05] | 0.117 | ||||||
| Tea consumption | ||||||||
| <3 cups/day | 1 | |||||||
| ≥3 cups/day | 0.72 [0.49–1.07] | 0.109 | ||||||
| Cannabis use | 0.080 | |||||||
| Never | 1 | |||||||
| Former | 0.71 [0.37–1.37] | 0.311 | ||||||
| Current | 0.30 [0.10–0.96] | 0.043 | ||||||
| Tobacco use | <10−3 | |||||||
| Never | 1 | |||||||
| Former | 1.79 [1.39–2.31] | <10−3 | ||||||
| Current | 0.82 [0.59–1.13] | 0.225 | ||||||
| Alcohol use | <10−3 | 0.024 | 0.011 | |||||
| Abstinent without history of unhealthy use | 1 | 1 | 1 | |||||
| Moderate use | 0.80 [0.63–1.02] | 0.076 | 0.75 [0.57–0.99] | 0.041 | 0.73 [0.55–0.97] | 0.030 | ||
| Current or past unhealthy use | 2.21 [1.54–3.17] | <10−3 | 1.29 [0.84–1.96] | 0.240 | 1.37 [0.89–2.10] | 0.152 | ||
| Living in poverty | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.32 [1.06–1.65] | 0.013 | 1.52 [1.17–1.98] | 0.002 | 1.73 [1.33–2.25] | <10−3 | 1.44 [1.10–1.88] | 0.008 |
| Educational level | ||||||||
| <upper secondary school certificate | 1 | |||||||
| ≥upper secondary school certificate | 0.53 [0.42–0.66] | <10−3 | ||||||
| Time since HBV diagnosis (years) | 1.00 [0.99–1.01] | 0.989 | ||||||
| Advanced liver fibrosis 5 | ||||||||
| No | 1 | - | 1 | - | ||||
| Yes | 3.60 [2.48–5.23] | <10−3 | - | 2.14 [1.40–3.28] | <10−3 | - | ||
| Diabetes | ||||||||
| No | 1 | - | - | 1 | ||||
| Yes | 7.23 [5.77–9.05] | <10−3 | - | - | 2.99 [2.28–3.92] | <10−3 | ||
| Hypertension | ||||||||
| No | 1 | - | - | 1 | ||||
| Yes | 4.88 [3.76–6.33] | <10−3 | - | - | 2.49 [1.84–3.38] | <10−3 | ||
1 In model 1, advanced liver fibrosis, diabetes, and hypertension were not considered eligible for multivariate analyses. Model 2 is based on model 1, but advanced liver fibrosis is considered eligible for multivariate analyses. Model 3 is based on model 1, but both diabetes and hypertension were considered eligible for multivariate analyses. 2 The category ‘Europe’ included participants from the U.S. (n = 2), New Zealand (n = 1), and South America (n = 11). 3 The category ‘Sub-Saharan Africa’ included participants from Haiti (n = 44) and the Dominican Republic (n = 2). 4 World Health Organization categorization [47]. 5 Advanced liver fibrosis was defined as an FIB-4 score > 3.25 [43]. aOR, adjusted odds ratio; CI, confidence interval; HBV, hepatitis B virus.
When considered as continuous variables, neither coffee nor tea consumption was associated with the outcome in univariable analyses (data not shown).
4. Discussion
In a large cohort of patients chronically infected with HBV, we found that elevated coffee consumption (≥3 cups per day) was associated with a higher risk of dyslipidemia, a lower risk of hypertension, and was not associated with diabetes. Tea consumption was not associated with any of these three outcomes.
These results were independent of BMI status, and remained valid after adjustment for advanced liver fibrosis and co-occurring metabolic disorders. FIB-4 may not be accurate in participants currently treated for HBV infection. Accordingly, results from model 2 should be cautiously interpreted. The dose response relationship that we observed for hypertension reflects the findings from previous studies [29,40,48]. The observed effects of coffee consumption on hypertension and dyslipidemia may partly mediate the hepatic benefits of coffee consumption previously highlighted in the HBV-infected population [49,50].
Our results for dyslipidemia and hypertension are consistent with those found in the general population [25,26,27,29,51]. In the general population, significant positive nonlinear associations between coffee consumption and the increase in total cholesterol, low-density lipoprotein cholesterol, and TG levels have been suggested, and coffee consumption may be associated with an elevated risk of dyslipidemia and cardiovascular diseases [51]. However, the anti-dyslipidemic properties of coffee consumption have also been documented, as have several putative-related mechanisms [52,53]. The possible lipid-raising effect of coffee might be partly imputable to cafestol and kahweol, two natural diterpenes that seem to have a modulatory effect on the low-density lipoprotein receptor [54,55], and may affect regulatory enzymes in the bile acid synthesis process and plasma lipid transfer protein levels [54]. Discrepancies between studies could be explained by an existing hypothesis that there is a differential lipid-raising effect between filtered and unfiltered coffee, the former having a weaker effect thanks to the filter’s capacity to retain cafestol. However, studies to date have provided no definitive evidence for such a differential effect [56,57,58].
In terms of HBV-infected patients, a population prone to liver cancer, this possible lipid-raising effect is of particular interest. HBV infection is associated with lower levels of serum total cholesterol, HDL cholesterol, and TG [1,2]. Furthermore, lower serum cholesterol is associated with more advanced stages of liver disease in this population [9,59], while higher serum cholesterol is associated with better liver cancer survival [10]. This inverse relationship between serum cholesterol and liver cancer has also been found in the general population [60]. Therefore, elevated levels of cholesterol and/or TG may be associated with better clinical outcomes in HBV-infected patients.
Just as for dyslipidemia, the literature shows contradictory results for whether or not long-term coffee consumption has a blood pressure-lowering effect [61,62]. Caffeine may increase blood pressure by raising catecholamine levels, leading, in turn, to vasoconstriction [63,64]. However, a recent randomized controlled trial investigating the effect of decaffeinated green coffee bean extract (800 mg per day over eight weeks) in people with MetS found a reduction in systolic blood pressure for the intervention group [65], suggesting that bioactive compounds other than caffeine were involved. It has been postulated that the hypotensive feature of coffee could be attributed to chlorogenic acids through a cortisol-lowering effect [66]. Moreover, coffee is a source of magnesium, potassium, manganese, and niacin [67,68,69], which may all participate in this blood pressure-lowering effect.
Finally, coffee, which is rich in phenolic compounds, is a significant source of dietary antioxidants [22,23,24], and has a higher antioxidant capacity than tea [70,71]. A higher plasma or dietary total antioxidant capacity is associated with lower likelihoods of dyslipidemia, hyperglycemia, and hypertension [72,73,74]. Accordingly, oxidative stress is an important underlying factor that leads to the development of metabolic disorders [75]. The lower antioxidant capacity, as well as the lower prevalence of high-level consumers in our study population, may explain the lack of association that we found between tea consumption and metabolic disorders. Indeed, the beneficial effects of tea consumption on metabolic syndrome reported in epidemiological studies or controlled trials generally appear when the level of consumption is at least 3 cups per day [76,77].
We found no association between coffee consumption and diabetes in our study population of persons with chronic HBV infection. No such association was found in a previous study that we conducted on HCV-infected persons in the same Hepather cohort [78]. Elsewhere, an inverse relationship has been found in the general population [32], and possible mechanisms are highlighted [32,79]. The protective effect of coffee intake on functional beta cell mass [79] may not be strong enough to significantly counteract the compromising effect of HBV infection [80]. Moreover, several clinical trials testing the effects of coffee (or coffee extracts) consumption on glucose metabolism yielded null results [65,81,82,83].
Tobacco use was identified as another behavioral and modifiable risk factor for metabolic disorders in the present study. Current and former tobacco use were both associated with a greater risk of dyslipidemia. This is consistent with data from the general population [84,85,86,87]. This dyslipidemia-inducing effect may be explained by the dysregulation of lipolysis in adipose tissue by nicotine [88]. The lack of any difference between current and former users may be related to a short duration of abstinence (data unavailable for our study).
Former tobacco use tended to be associated with a higher risk of hypertension, while current use tended to be associated with a lower risk in our models. The hypertensive effect of chronic tobacco use is known [89,90]. One possible explanation for the discrepancy found between former and current users is different exposure in terms of the duration and intensity of smoking, with former smokers possibly being previously long-term heavy smokers (data unavailable). The body weight-lowering effect of tobacco smoking [91] and the weight-gaining effect of quitting tobacco [92] may also explain the opposite tendencies which former and current use had on hypertension [93].
The main strengths of the present study are its large sample size (n = 4746) and the presence of demographic, clinical, and socio-behavioral variables. While data for well-known risk factors for metabolic disorders were not available (e.g., diet quality or physical activity), we may expect that adjustment for socio-demographic variables which are generally associated with those risk factors [94,95,96,97,98], at least partially captured such effects. Limitations must also be mentioned. Coffee and tea characteristics (roasting, caffeinated or not, filtered or not, green vs. black, etc.) may influence health effects [76,99,100,101], yet these data were not collected. Further research is needed to check whether the magnitude of the association between coffee or tea intake and metabolic disorders differs according to the type of coffee or tea consumed. Moreover, data on dietary habits such as adding sugar or milk were not collected; however, they may have been partially captured with country of birth, as the latter may reflect culture-driven habits. Using a food frequency questionnaire or a 24-h dietary recall would have improved the robustness of our conclusions. Finally, our study population was limited to people followed in hospital-based specialized services, and socio-behavioral data (including coffee consumption) were only collected during the cohort enrolment visit.
5. Conclusions
To conclude, drinking three or more cups of coffee per day was associated with a higher risk of dyslipidemia and a lower risk of hypertension in HBV-infected patients. These two effects are expected to be associated with favorable clinical outcomes. Further studies should explore associated mechanisms, and test whether these seemingly metabolic benefits translate into reduced mortality risks in HBV-infected patients.
This section is not mandatory, but can be added to the manuscript if the discussion is unusually long or complex.
Acknowledgments
We would like to thank the participating patients and clinicians at each site. We also thank INSERM-ANRS MIE for sponsoring, funding and conducting the ANRS CO22 Hepather cohort in collaboration with the French Association for the Study of the Liver (Association Française pour l’Etude du Foie: AFEF). Finally, our thanks to Jude Sweeney (Milan, Italy) for the English revision and copyediting of our manuscript. † ANRS/AFEF Hepather Study group Investigators: Laurent Alric, Delphine Bonnet, Virginie Payssan-Sicart, Chloe Pomes (CHU Purpan, Toulouse, France), Fabien Zoulim, Marianne Maynard, Roxane Bai, Lucie Hucault, François Bailly (Hospices Civils de Lyon, Lyon, France), François Raffi, Eric Billaud, David Boutoille, Maeva Lefebvre, Elisabeth André-Garnier (Hôpital Hôtel-Dieu, Nantes, France), Paul Cales, Isabelle Hubert, Adrien Lannes, Françoise Lunel, Jérôme Boursier (CHU Angers, Angers, France), Tarik Asselah, Nathalie Boyer, Nathalie Giuily, Corinne Castelnau, Giovanna Scoazec (Hôpital Beaujon, Clichy, France), Stanislas Pol, Hélène Fontaine, Emilie Rousseaud, Anaïs Vallet-Pichard, Philippe Sogni (Hôpital Cochin, Paris, France), Victor de Ledinghen, Juliette Foucher, Jean-Baptiste Hiriart, Jancell M’Bouyou, Marie Irlès-Depé (Hôpital Haut-Lévêque, Pessac, Bordeaux, France), Marc Bourlière, Si Nafa Si Ahmed, Valérie Oules (Hôpital Saint Joseph, Marseille, France), Albert Tran, Rodolphe Anty, Eve Gelsi, Régine Truchi (CHU de Nice, Nice, France), Dominique Thabut, Saloua Hammeche, Joseph Moussali (Hôpital de la Pitié Salptétrière, Paris, France), Xavier Causse, Barbara De Dieuleveult, Brahim Ouarani, Damien Labarrière (CHR La Source, Orléans, France), Nathalie Ganne, Véronique Grando-Lemaire, Pierre Nahon, Séverine Brulé, Betul ULKER (Hôpital Jean Verdier, Bondy, France), Dominique Guyader, Caroline Jezequel, Audrey Brener, Anne Laligant, Aline Rabot, Isabelle Renard (CHU Rennes, Rennes, France), François Habersetzer, Thomas F. Baumert, Michel Doffoel, Catherine Mutter, Pauline Simo-Noumbissie, Esma Razi (Hôpitaux Universitaires de Strasbourg, Strasbourg, France), Jean-Pierre Bronowicki, Hélène Barraud, Mouni Bensenane, Abdelbasset Nani, Sarah Hassani-Nani, Marie-Albertine Bernard (CHU de Nancy, Nancy, France), Georges-Philippe Pageaux, Dominique Larrey, Magda Meszaros (Hôpital Saint Eloi, Montpellier, France), Sophie Metivier, Christophe Bureau, Thibault Morales, Jean Marie Peron, Marie Angèle Robic (CHU Purpan, Toulouse, France), Thomas Decaens, Marine Faure, Bruno Froissart, Marie-Noelle Hilleret, Jean-Pierre Zarski (CHU de Grenoble, Grenoble, France), Ghassan Riachi, Odile Goria, Fatima Paris, Hélène Montialoux (CHU Charles Nicolle, Rouen, France), Vincent Leroy, Giuliana Amaddeo, Anne Varaut, Mélanie Simoes, Rachida Amzal (Hôpital Henri Mondor, Créteil, France), Olivier Chazouillières, Tony Andreani, Bénédicte Angoulevant, Azeline Chevance, Lawrence Serfaty (Hôpital Saint-Antoine, Paris, France), Didier Samuel, Teresa Antonini, Audrey Coilly, Jean-Charles Duclos Vallée, Mariagrazia Tateo (Hôpital Paul Brousse, Villejuif, France), Armand Abergel, Maud Reymond, Chanteranne Brigitte, Buchard Benjamin, Léon Muti (Hôpital Estaing, Clermont-Ferrand, France), Claire Geist, Guillaume Conroy, Raphaëlle Riffault (Centre Hospitalier Régional, Metz, France), Isabelle Rosa, Camille Barrault, Laurent Costes, Hervé Hagège (Centre Hospitalier Intercommunal, Créteil, France), Véronique Loustaud-Ratti, Paul Carrier, Maryline Debette-Gratien, (CHU Limoges, Limoges, France), Philippe Mathurin, Guillaume Lassailly, Elise Lemaitre, Valérie Canva, Sébastien Dharancy, Alexandre Louvet (CHRU Claude Huriez, Lille, France), Anne Minello, Marianne Latournerie, Marc Bardou, Thomas Mouillot (Dijon University Hospital, Dijon, France), Louis D’Alteroche, Didier Barbereau, Charlotte Nicolas, Laure Elkrief, Anaïs Jaillais (CHU Trousseau, 37044 Tours, France), Jérôme Gournay, Caroline Chevalier, Isabelle Archambeaud, Sarah Habes (CHU de Nantes, Nantes, France), Isabelle Portal (CHU Timone, Marseille, France), Moana Gelu-Simeon, Eric Saillard, Marie-Josée Lafrance, Lucie Catherine (CHU de Pointe-à-Pitre, Pointe-à-Pitre, Guadeloupe). Methodology and Coordinating Center: Fabrice Carrat (coordinator), Frederic Chau, Céline Dorival, Isabelle Goderel, Clovis Lusivika-Nzinga, Marc-Antoine Bellance, Jonathan Bellet, Priscilla Monfalet, Jessica Chane-Teng, Sephora Bijaoui, Grégory Pannetier, François Téoulé, Jérôme Nicol, Florian Sebal, Rafika Bekhti (Sorbonne University & INSERM U1136—IPLESP, Paris, France). Sponsors: Carole Cagnot, Anaïs Boston, Laura Nailler, Guillaume Le Meut (INSERM-ANRS-MIE, Paris, France), Alpha Diallo (Pharmacovigilance coordinator), Ventzislava Petrov-Sanchez (coordinator). Scientific Committee Voting members: Marc Bourlière (Hôpital St Joseph, Marseille), Jérôme Boursier (CHU Angers, Angers, France), Fabrice Carrat (Scientific Coordinator, Hôpital Saint-Antoine, Paris, France), Patrizia Carrieri (INSERM U912, Marseille, France), Elisabeth Delarocque-Astagneau (Inserm UMR1181, Paris), Victor De Ledinghen (Hôpital Haut-Lévêque, Pessac, Bordeaux, France), Céline Dorival (UPMC & INSERM U1136, Paris, France), Hélène Fontaine (Hôpital Cochin, Paris, France), Slim Fourati (Hôpital Henri Mondor, Créteil, France), Chantal Housset (Inserm UMR-S938 1 IFR65, Paris), Dominique Larrey (Hôpital Saint Eloi, Montpellier, France), Pierre Nahon (Hôpital Jean Verdier, Bondy, France), Georges-Philippe Pageaux (Hôpital Saint Eloi, Montpellier, France), Ventzislava Petrov-Sanchez (ANRS, Paris, France), Stanislas Pol (Principal Investigator, Hôpital Cochin, Paris, France), Mathias Bruyand (Agence Nationale de Santé Publique, Saint Maurice, France), Linda Wittkop (ISPED-INSERM U897, Bordeaux, France), Fabien Zoulim (Hospices Civils de Lyon, Lyon, France), Jessica Zucman-Rossi (Inserm U674/1162, Paris). Non-voting members: Marianne L’hennaff (ARCAT-TRT-5-CHV, France), Michèle Sizorn (SOS hépatites, France); one representative of INSERM-ANRS-MIE Pharmacovigilance team, Paris, France (Anaïs Boston, Alpha Diallo), Carole Cagnot (INSERM-ANRS-MIE, Paris, France), one member of Inserm Transfert, Paris, France (Alice Bousselet, Mireille Caralp), and one representative of each pharmaceutical company (MSD, Gilead, Abbvie).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11020379/s1, Table S1: Characteristics of included and excluded participants (ANRS CO22 Hepather cohort).
Author Contributions
T.B., C.P., P.C., M.B. (Morgane Bureau) and F.M.: Conceptualization, Methodology and Validation. V.D.B.: Data Curation. C.R.: Formal Analysis. S.P., F.C., H.F., M.B. (Marc Bourlière)., T.A., E.D.-A., D.L., J.-C.D.-V. and C.D.: Conceptualization, Investigation S.P., F.C., H.F., C.D. and V.P.-S.: Project Administration and Funding Acquisition. T.B. Writing—Original Draft Preparation. All authors: Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
The Hepather cohort is funded by INSERM-ANRS MIE (France REcherche Nord&sud Sida-vih Hepatites|Maladies Infectieuses Emergentes), ANR Equipex and Cohort (Agence Nationale de la Recherche), DGS (Direction Générale de la Santé) and MSD, Janssen, Gilead, Abbvie, BMS, Roche. These funding sources had no role in the writing of the manuscript, or in the decision to submit it for publication.
Institutional Review Board Statement
The protocol was developed in accordance with the Declaration of Helsinki and French law for biomedical research, and was approved by the CPP Ile de France 3 ethics committee (Paris, France) and the French Regulatory Authority (ANSM) (#2943 (30 December 2011)).
Informed Consent Statement
Written informed consent was obtained from each patient before enrolment.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang J., Ling N., Lei Y., Peng M., Hu P., Chen M. Multifaceted Interaction between Hepatitis B Virus Infection and Lipid Metabolism in Hepatocytes: A Potential Target of Antiviral Therapy for Chronic Hepatitis B. Front. Microbiol. 2021;12:484. doi: 10.3389/fmicb.2021.636897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan L.-B., Liao J., Han N., Zhou L.-Y., Wang X.-E., Wang Y.-J., Tang H. Association between Hepatitis B Virus Infection and Metabolic Syndrome in Southwest China: A Cross-sectional Study. Sci. Rep. 2020;10:6738. doi: 10.1038/s41598-020-62609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P.T., Loria C.M., Smith S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Li Y., Zhao Y., Wu J. Serum HBV surface antigen positivity is associated with low prevalence of metabolic syndrome: A meta-analysis. PLoS ONE. 2017;12:e0177713. doi: 10.1371/journal.pone.0177713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu M.-W., Lin C.-L., Liu C.-J., Yang S.-H., Tseng Y.-L., Wu C.-F. Influence of Metabolic Risk Factors on Risk of Hepatocellular Carcinoma and Liver-Related Death in Men with Chronic Hepatitis B: A Large Cohort Study. Gastroenterology. 2017;153:1006–1017.e5. doi: 10.1053/j.gastro.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Bin Lee Y., Moon H., Lee J., Cho E.J., Yu S.J., Kim Y.J., Zoulim F., Lee J., Yoon J. Association of Metabolic Risk Factors With Risks of Cancer and All-Cause Mortality in Patients with Chronic Hepatitis B. Hepatology. 2021;73:2266–2277. doi: 10.1002/hep.31612. [DOI] [PubMed] [Google Scholar]
- 7.Lam L., Fontaine H., Bourliere M., Lusivika-Nzinga C., Dorival C., Thabut D., Zoulim F., Habersetzer F., Asselah T., Duclos-Vallee J.-C., et al. Predictive factors for hepatocellular carcinoma in chronic hepatitis B using structural equation modeling: A prospective cohort study. Clin. Res. Hepatol. Gastroenterol. 2021;45:101713. doi: 10.1016/j.clinre.2021.101713. [DOI] [PubMed] [Google Scholar]
- 8.Si J., Yu C., Guo Y., Bian Z., Meng R., Yang L., Chen Y., Jin J., Liu J., Guo Z., et al. Chronic hepatitis B virus infection and total and cause-specific mortality: A prospective cohort study of 0.5 million people. BMJ Open. 2019;9:e027696. doi: 10.1136/bmjopen-2018-027696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger L.W., Forstner B., Schneglberger S., Muckenhuber M., Eigenbauer E., Scheiner B., Mandorfer M., Trauner M., Reiberger T. Patterns and prevalence of dyslipidemia in patients with different etiologies of chronic liver disease. Wien. Klin. Wochenschr. 2019;131:395–403. doi: 10.1007/s00508-019-01544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim G.-A., Shim J.-J., Lee J.S., Kim B.-H., Kim J.W., Oh C.H., Oh C.-M., Oh I.-H., Park S.-Y. Effect of Statin Use on Liver Cancer Mortality Considering Hypercholesterolemia and Obesity in Patients with Non-Cirrhotic Chronic Hepatitis B. Yonsei Med. J. 2019;60:1203–1208. doi: 10.3349/ymj.2019.60.12.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Ma R.C., So W.Y., Yu L.W., Kong A.P., Ko G.T., Xu G., Ozaki R., Tong P.C., Chan J.C. Low triglyceride and nonuse of statins is associated with cancer in type 2 diabetes mellitus: The Hong Kong Diabetes Registry. Cancer. 2011;117:862–871. doi: 10.1002/cncr.25455. [DOI] [PubMed] [Google Scholar]
- 12.Yang X., Wang Y., Luk A.O.Y., So W.Y., Ma R., Kong A.P.S., Xu G., Chan J.C.N. Enhancers and attenuators of risk associations of chronic hepatitis B virus infection with hepatocellular carcinoma in type 2 diabetes. Endocr.-Relat. Cancer. 2012;20:161–171. doi: 10.1530/ERC-12-0290. [DOI] [PubMed] [Google Scholar]
- 13.de la Iglesia R., Loria-Kohen V., Zulet M.A., Martinez J.A., Reglero G., Ramirez de Molina A. Dietary Strategies Implicated in the Prevention and Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2016;17:1877. doi: 10.3390/ijms17111877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippou C.D., Tsioufis C.P., Thomopoulos C.G., Mihas C.C., Dimitriadis K.S., Sotiropoulou L.I., Chrysochoou C.A., Nihoyannopoulos P.I., Tousoulis D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020;11:1150–1160. doi: 10.1093/advances/nmaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakaloudi D., Chrysoula L., Kotzakioulafi E., Theodoridis X., Chourdakis M. Impact of the Level of Adherence to Mediterranean Diet on the Parameters of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2021;13:1514. doi: 10.3390/nu13051514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhlaghi M. Dietary Approaches to Stop Hypertension (DASH): Potential mechanisms of action against risk factors of the metabolic syndrome. Nutr. Res. Rev. 2020;33:1–18. doi: 10.1017/S0954422419000155. [DOI] [PubMed] [Google Scholar]
- 17.Visioli F., Galli C. The role of antioxidants in the Mediterranean diet. Lipids. 2001;36:S49–S52. doi: 10.1007/s11745-001-0682-z. [DOI] [PubMed] [Google Scholar]
- 18.Bjelakovic G., Gluud L.L., Nikolova D., Bjelakovic M., Nagorni A., Gluud C. Antioxidant supplements for liver diseases. Cochrane Database Syst. Rev. 2011;3:CD007749. doi: 10.1002/14651858.CD007749.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha H.-L., Shin H.-J., Feitelson M.A., Yu D.-Y. Oxidative stress and antioxidants in hepatic pathogenesis. World J. Gastroenterol. 2010;16:6035–6043. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alavian S.M., Showraki A. Hepatitis B and its Relationship with Oxidative Stress. Hepat. Mon. 2016;16:e37973. doi: 10.5812/hepatmon.37973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva R.F.M., Pogačnik L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants. 2020;9:61. doi: 10.3390/antiox9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Bo C., Bernardi S., Marino M., Porrini M., Tucci M., Guglielmetti S., Cherubini A., Carrieri B., Kirkup B., Kroon P., et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients. 2019;11:1355. doi: 10.3390/nu11061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukushima Y., Tashiro T., Kumagai A., Ohyanagi H., Horiuchi T., Takizawa K., Sugihara N., Kishimoto Y., Taguchi C., Tani M., et al. Coffee and beverages are the major contributors to polyphenol consumption from food and beverages in Japanese middle-aged women. J. Nutr. Sci. 2014;3:e48. doi: 10.1017/jns.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nascimento-Souza M.A., de Paiva P.G., da Silva A., Duarte M.S.L., Ribeiro A.Q. Coffee and Tea Group Contribute the Most to the Dietary Total Antioxidant Capacity of Older Adults: A Population Study in a Medium-Sized Brazilian City. J. Am. Coll. Nutr. 2020;40:713–723. doi: 10.1080/07315724.2020.1823281. [DOI] [PubMed] [Google Scholar]
- 25.Cai L., Ma D., Zhang Y., Liu Z., Wang P. The effect of coffee consumption on serum lipids: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2012;66:872–877. doi: 10.1038/ejcn.2012.68. [DOI] [PubMed] [Google Scholar]
- 26.Cornelis M.C., van Dam R.M. Habitual Coffee and Tea Consumption and Cardiometabolic Biomarkers in the UK Biobank: The Role of Beverage Types and Genetic Variation. J. Nutr. 2020;150:2772–2788. doi: 10.1093/jn/nxaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nerurkar P., Gandhi K., Chen J. Correlations between Coffee Consumption and Metabolic Phenotypes, Plasma Folate, and Vitamin B12: NHANES 2003 to 2006. Nutrients. 2021;13:1348. doi: 10.3390/nu13041348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu R., Yang K., Li S., Dai M., Chen G. Effect of green tea consumption on blood lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2020;19:48. doi: 10.1186/s12937-020-00557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie C., Cui L., Zhu J., Wang K., Sun N., Sun C. Coffee consumption and risk of hypertension: A systematic review and dose–response meta-analysis of cohort studies. J. Hum. Hypertens. 2018;32:83–93. doi: 10.1038/s41371-017-0007-0. [DOI] [PubMed] [Google Scholar]
- 30.Peng X., Zhou R., Wang B., Yu X., Yang X., Liu K., Mi M. Effect of green tea consumption on blood pressure: A meta-analysis of 13 randomized controlled trials. Sci. Rep. 2014;4:6251. doi: 10.1038/srep06251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahdavi-Roshan M., Salari A., Ghorbani Z., Ashouri A. The effects of regular consumption of green or black tea beverage on blood pressure in those with elevated blood pressure or hypertension: A systematic review and meta-analysis. Complement. Ther. Med. 2020;51:102430. doi: 10.1016/j.ctim.2020.102430. [DOI] [PubMed] [Google Scholar]
- 32.Carlström M., Larsson S.C. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018;76:395–417. doi: 10.1093/nutrit/nuy014. [DOI] [PubMed] [Google Scholar]
- 33.Abe S.K., Inoue M. Green tea and cancer and cardiometabolic diseases: A review of the current epidemiological evidence. Eur. J. Clin. Nutr. 2021;75:865–876. doi: 10.1038/s41430-020-00710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jing Y., Han G., Hu Y., Bi Y., Li L., Zhu D. Tea Consumption and Risk of Type 2 Diabetes: A Meta-Analysis of Cohort Studies. J. Gen. Intern. Med. 2009;24:557–562. doi: 10.1007/s11606-009-0929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang L.S.Y., Covert E., Wilson E., Kottilil S. Chronic Hepatitis B Infection: A Review. JAMA. 2018;319:1802–1813. doi: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- 36.Montuclard C., Hamza S., Rollot F., Evrard P., Faivre J., Hillon P., Di Martino V., Minello A. Causes of death in people with chronic HBV infection: A population-based cohort study. J. Hepatol. 2015;62:1265–1271. doi: 10.1016/j.jhep.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Pol S., Bourliere M., Lucier S., Hezode C., Dorival C., Larrey D., Bronowicki J.-P., Ledinghen V.D., Zoulim F., Tran A., et al. Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1-mono-infected patients. J. Hepatol. 2017;66:39–47. doi: 10.1016/j.jhep.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Carrieri M.P., Protopopescu C., Marcellin F., Wittkop L., Lacombe K., Esterle L., Sogni P., Salmon-Ceron D. The impact of coffee consumption on fibrosis and steatosis in HIV-HCV co-infected patients. J. Hepatol. 2017;68:845–847. doi: 10.1016/j.jhep.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 39.Protopopescu C., Santos M., Sogni P., Marcellin F., Esterle L., Wittkop L., Rosenthal E., Morlat P., Roux P., de Araújo W., et al. Protective effect of cannabis and coffee consumption on HCV-related mortality in French HIV-HCV co-infected patients (ANRS CO13 HEPAVIH cohort) J. Hepatol. 2018;68:S142–S143. doi: 10.1016/S0168-8278(18)30501-4. [DOI] [Google Scholar]
- 40.Ding M., Bhupathiraju S.N., Chen M., van Dam R.M., Hu F.B. Caffeinated and Decaffeinated Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review and a Dose-Response Meta-analysis. Diabetes Care. 2014;37:569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haute Autorité de Santé (French National Authority for Health) Screening Tool for Early Detection and Brief Intervention (Outil D’aide au Repérage Précoce et à L’intervention Brève) 2014. [(accessed on 24 October 2019)]. Available online: https://www.has-sante.fr/jcms/c_1795221/fr/outil-d-aide-au-reperage-precoce-et-intervention-breve-alcool-cannabis-tabac-chez-l-adulte.
- 42.Institut National de la Statistique et des Etudes Economiques Définition—Pauvreté Monétaire/Seuil de Pauvreté/Seuil de Pauvreté/Insee. [(accessed on 10 December 2021)]; Available online: https://www.insee.fr/fr/metadonnees/definition/c1653.
- 43.Sterling R.K., Lissen E., Clumeck N., Sola R., Correa M.C., Montaner J., SSulkowski M., Torriani F.J., Dieterich D.T., Thomas D.L., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 44.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., Nalpas A., Dhalluin-Venier V., Fontaine H., Pol S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 45.García-Compeán D., González-González J.A., Lavalle-González F.J., González-Moreno E.I., Villarreal-Pérez J.Z., Maldonado-Garza H.J. Current Concepts in Diabetes Mellitus and Chronic Liver Disease: Clinical Outcomes, Hepatitis C Virus Association, and Therapy. Dig. Dis. Sci. 2016;61:371–380. doi: 10.1007/s10620-015-3907-2. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J., Shen Y., Cai H., Liu Y.-M., Qin G. Hepatitis B virus infection status and risk of type 2 diabetes mellitus: A meta-analysis. Hepatol. Res. 2015;45:1100–1109. doi: 10.1111/hepr.12481. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization Body Mass Index—BMI. [(accessed on 9 July 2019)];2019 Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- 48.Di Maso M., Boffetta P., Negri E., La Vecchia C., Bravi F. Caffeinated Coffee Consumption and Health Outcomes in the US Population: A Dose-Response Meta-Analysis and Estimation of Disease Cases and Deaths Avoided. Adv. Nutr. 2021;12:1160–1176. doi: 10.1093/advances/nmaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodge A., Lim S., Goh E., Wong O., Marsh P., Knight V., Sievert W., De Courten B. Coffee Intake Is Associated with a Lower Liver Stiffness in Patients with Non-Alcoholic Fatty Liver Disease, Hepatitis C, and Hepatitis B. Nutrients. 2017;9:56. doi: 10.3390/nu9010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C.L., Chang W.C., Yi C.H., Hung J.S., Liu T.T., Lei W.Y., Hsu C.S. Association of coffee consumption and liver fibrosis progression in patients with HBeAg-negative chronic hepatitis B: A 5-year population-based cohort study. J. Formos. Med. Assoc. 2019;118:628–635. doi: 10.1016/j.jfma.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Du Y., Lv Y., Zha W., Hong X., Luo Q. Effect of coffee consumption on dyslipidemia: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2020;30:2159–2170. doi: 10.1016/j.numecd.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Saeed M., Naveed M., BiBi J., Kamboh A.A., Phil L., Chao S. Potential nutraceutical and food additive properties and risks of coffee: A comprehensive overview. Crit. Rev. Food Sci. Nutr. 2019;59:3293–3319. doi: 10.1080/10408398.2018.1489368. [DOI] [PubMed] [Google Scholar]
- 53.Farias-Pereira R., Park C.-S., Park Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci. Biotechnol. 2019;28:1287–1296. doi: 10.1007/s10068-019-00662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren Y., Wang C., Xu J., Wang S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019;20:4238. doi: 10.3390/ijms20174238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urgert R., Der Wouw M.W.-V., Hovenier R., Meyboom S., Beynen A.C., Katan M.B. Diterpenes from coffee beans decrease serum levels of lipoprotein(a) in humans: Results from four randomised controlled trials. Eur. J. Clin. Nutr. 1997;51:431–436. doi: 10.1038/sj.ejcn.1600414. [DOI] [PubMed] [Google Scholar]
- 56.Urgert R., Katan M.B. THE cholesterol-raising factor from coffee beans. Annu. Rev. Nutr. 1997;17:305–324. doi: 10.1146/annurev.nutr.17.1.305. [DOI] [PubMed] [Google Scholar]
- 57.Rendón M.Y., Scholz M.B.D.S., Bragagnolo N. Physical characteristics of the paper filter and low cafestol content filter coffee brews. Food Res. Int. 2018;108:280–285. doi: 10.1016/j.foodres.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 58.Strandhagen E., Thelle D.S. Filtered coffee raises serum cholesterol: Results from a controlled study. Eur. J. Clin. Nutr. 2003;57:1164–1168. doi: 10.1038/sj.ejcn.1601668. [DOI] [PubMed] [Google Scholar]
- 59.Yan L.-T., Wang L.-L., Yao J., Yang Y.-T., Mao X.-R., Yue W., Mao Y.-W., Zhou W., Chen Q.-F., Chen Y., et al. Total bile acid-to-cholesterol ratio as a novel noninvasive marker for significant liver fibrosis and cirrhosis in patients with non-cholestatic chronic hepatitis B virus infection. Medicine. 2020;99:e19248. doi: 10.1097/MD.0000000000019248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao L., Deng C., Lin Z., Giovannucci E., Zhang X. Dietary Fats, Serum Cholesterol and Liver Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Studies. Cancers. 2021;13:1580. doi: 10.3390/cancers13071580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steffen M., Kuhle C., Hensrud D., Erwin P.J., Murad M.H. The effect of coffee consumption on blood pressure and the development of hypertension: A systematic review and meta-analysis. J. Hypertens. 2012;30:2245–2254. doi: 10.1097/HJH.0b013e3283588d73. [DOI] [PubMed] [Google Scholar]
- 62.Turnbull D., Rodricks J.V., Mariano G.F., Chowdhury F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017;89:165–185. doi: 10.1016/j.yrtph.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 63.Robertson D., Frölich J.C., Carr R.K., Watson J.T., Hollifield J.W., Shand D.G., Oates J.A. Effects of Caffeine on Plasma Renin Activity, Catecholamines and Blood Pressure. N. Engl. J. Med. 1978;298:181–186. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- 64.Echeverri D., Montes F.R., Cabrera M., Galán A., Prieto A. Caffeine’s Vascular Mechanisms of Action. Int. J. Vasc. Med. 2010;2010:834060. doi: 10.1155/2010/834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roshan H., Nikpayam O., Sedaghat M., Sohrab G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: A randomised clinical trial. Br. J. Nutr. 2018;119:250–258. doi: 10.1017/S0007114517003439. [DOI] [PubMed] [Google Scholar]
- 66.Revuelta-Iniesta R., Al-Dujaili E.A.S. Consumption of green coffee reduces blood pressure and body composition by influencing 11β-HSD1 enzyme activity in healthy individuals: A pilot crossover study using green and black coffee. BioMed Res. Int. 2014;2014:482704. doi: 10.1155/2014/482704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janda K., Jakubczyk K., Baranowska-Bosiacka I., Kapczuk P., Kochman J., Rębacz-Maron E., Gutowska I. Mineral Composition and Antioxidant Potential of Coffee Beverages Depending on the Brewing Method. Foods. 2020;9:121. doi: 10.3390/foods9020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olechno E., Puścion-Jakubik A., Socha K., Zujko M. Coffee Brews: Are They a Source of Macroelements in Human Nutrition? Foods. 2021;10:1328. doi: 10.3390/foods10061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kremer J.I., Gömpel K., Bakuradze T., Eisenbrand G., Richling E. Urinary Excretion of Niacin Metabolites in Humans after Coffee Consumption. Mol. Nutr. Food Res. 2018;62:e1700735. doi: 10.1002/mnfr.201700735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baeza G., Sarriá B., Bravo L., Mateos R. Polyphenol content, in vitro bioaccessibility and antioxidant capacity of widely consumed beverages. J. Sci. Food Agric. 2018;98:1397–1406. doi: 10.1002/jsfa.8607. [DOI] [PubMed] [Google Scholar]
- 71.Richelle M., Tavazzi I., Offord E. Comparison of the Antioxidant Activity of Commonly Consumed Polyphenolic Beverages (Coffee, Cocoa, and Tea) Prepared per Cup Serving. J. Agric. Food Chem. 2001;49:3438–3442. doi: 10.1021/jf0101410. [DOI] [PubMed] [Google Scholar]
- 72.Kim S.-A., Joung H., Shin S. Dietary pattern, dietary total antioxidant capacity, and dyslipidemia in Korean adults. Nutr. J. 2019;18:37. doi: 10.1186/s12937-019-0459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vichaibun V., Khananurak K., Sophonnithiprasert T. Comparative analysis of plasma total antioxidant capacity in patients with hyperglycemia and hyperglycemia plus dyslipidemia. Diabetes Metab. Syndr. Clin. Res. Rev. 2018;13:90–94. doi: 10.1016/j.dsx.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 74.Villaverde P., Lajous M., Macdonald C.-J., Fagherazzi G., Bonnet F., Boutron-Ruault M.-C. High dietary total antioxidant capacity is associated with a reduced risk of hypertension in French women. Nutr. J. 2019;18:31. doi: 10.1186/s12937-019-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rani V., Deep G., Singh R.K., Palle K., Yadav U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Liu W., Wan C., Huang Y., Li M. Effects of tea consumption on metabolic syndrome: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020;34:2857–2866. doi: 10.1002/ptr.6731. [DOI] [PubMed] [Google Scholar]
- 77.Grosso G., Stepaniak U., Micek A., Topor-Mądry R., Pikhart H., Szafraniec K., Pająk A. Association of daily coffee and tea consumption and metabolic syndrome: Results from the Polish arm of the HAPIEE study. Eur. J. Nutr. 2015;54:1129–1137. doi: 10.1007/s00394-014-0789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barré T., Nishimwe M.L., Protopopescu C., Marcellin F., Carrat F., Dorival C., Delarocque-Astagneau E., Larrey D., Bourlière M., Petrov-Sanchez V., et al. Cannabis use is associated with a lower risk of diabetes in chronic hepatitis C-infected patients (ANRS CO22 Hepather cohort) J. Viral Hepat. 2020;27:1473–1483. doi: 10.1111/jvh.13380. [DOI] [PubMed] [Google Scholar]
- 79.Kolb H., Martin S., Kempf K. Coffee and Lower Risk of Type 2 Diabetes: Arguments for a Causal Relationship. Nutrients. 2021;13:1144. doi: 10.3390/nu13041144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu D., Zhou L., Zhang X., Zeng Y., Bai L., Wu D., Tang H. Significantly Decreased Islet β Cell Function is Closely Associated with Hyperglycemia in Chronic Hepatitis B Patients. Int. J. Endocrinol. 2021;2021:1264707. doi: 10.1155/2021/1264707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kempf K., Herder C., Erlund I., Kolb H., Martin S., Carstensen M., Koenig W., Sundvall J., Bidel S., Kuha S., et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010;91:950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 82.Alperet D.J., Rebello S.A., Khoo E.Y.-H., Tay Z., Seah S.S.-Y., Tai B.-C., Tai E.S., Emady-Azar S., Chou C.J., Darimont C., et al. The effect of coffee consumption on insulin sensitivity and other biological risk factors for type 2 diabetes: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2019;111:448–458. doi: 10.1093/ajcn/nqz306. [DOI] [PubMed] [Google Scholar]
- 83.Morvaridi M., Rayyani E., Jaafari M., Khiabani A., Rahimlou M. The effect of green coffee extract supplementation on cardio metabolic risk factors: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2020;19:645–660. doi: 10.1007/s40200-020-00536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Craig W.Y., Palomaki G.E., Haddow J.E. Cigarette smoking and serum lipid and lipoprotein concentrations: An analysis of published data. BMJ. 1989;298:784–788. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim S.W., Kim H.J., Min K., Lee H., Lee S.-H., Kim S., Kim J.S., Oh B. The relationship between smoking cigarettes and metabolic syndrome: A cross-sectional study with non-single residents of Seoul under 40 years old. PLoS ONE. 2021;16:e0256257. doi: 10.1371/journal.pone.0256257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maeda K., Noguchi Y., Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: A meta-analysis. Prev. Med. 2003;37:283–290. doi: 10.1016/S0091-7435(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 87.Moradinazar M., Pasdar Y., Najafi F., Shahsavari S., Shakiba E., Hamzeh B., Fakhri N. Association between dyslipidemia and blood lipids concentration with smoking habits in the Kurdish population of Iran. BMC Public Health. 2020;20:673. doi: 10.1186/s12889-020-08809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Z., Wang D., Wang Y. Cigarette Smoking and Adipose Tissue: The Emerging Role in Progression of Atherosclerosis. Mediat. Inflamm. 2017;2017:3102737. doi: 10.1155/2017/3102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leone A. Does Smoking Act as a Friend or Enemy of Blood Pressure? Let Release Pandora’s Box. Cardiol. Res. Pract. 2011;2011:264894. doi: 10.4061/2011/264894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dikalov S., Itani H.A., Richmond B., Arslanbaeva L., Vergeade A., Rahman S.M.J., Boutaud O., Blackwell T., Massion P.P., Harrison D.G., et al. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am. J. Physiol. Circ. Physiol. 2019;316:H639–H646. doi: 10.1152/ajpheart.00595.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Audrain-McGovern J., Benowitz N.L. Cigarette Smoking, Nicotine, and Body Weight. Clin. Pharmacol. Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aubin H.-J., Farley A., Lycett D., Lahmek P., Aveyard P. Weight Gain in Smokers after Quitting Cigarettes: Meta-Analysis. [(accessed on 22 January 2020)];BMJ. 2012 345:e4439. doi: 10.1136/bmj.e4439. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3393785/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jayedi A., Rashidy-Pour A., Khorshidi M., Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: A systematic review and dose-response meta-analysis of more than 2.3 million participants. Obes. Rev. 2018;19:654–667. doi: 10.1111/obr.12656. [DOI] [PubMed] [Google Scholar]
- 94.Braveman P., Gottlieb L. The Social Determinants of Health: It’s Time to Consider the Causes of the Causes. Public Health Rep. 2014;129:19–31. doi: 10.1177/00333549141291S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Darmon N., Drewnowski A. Does social class predict diet quality? Am. J. Clin. Nutr. 2008;87:1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 96.Darmon N., Khlat M. An overview of the health status of migrants in France, in relation to their dietary practices. Public Health Nutr. 2001;4:163–172. doi: 10.1079/PHN200064. [DOI] [PubMed] [Google Scholar]
- 97.Schultz W.M., Kelli H.M., Lisko J.C., Varghese T., Shen J., Sandesara P., Quyyumi A.A., Taylor H.A., Gulati M., Harold J.G., et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith M., Hosking J., Woodward A., Witten K., Macmillan A., Field A., Baas P., Mackie H. Systematic literature review of built environment effects on physical activity and active transport—An update and new findings on health equity. Int. J. Behav. Nutr. Phys. Act. 2017;14:158. doi: 10.1186/s12966-017-0613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rendón M.Y., Scholz M.B.D.S., Bragagnolo N. Is cafestol retained on the paper filter in the preparation of filter coffee? Food Res. Int. 2017;100:798–803. doi: 10.1016/j.foodres.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 100.De Giuseppe R., Di Napoli I., Granata F., Mottolese A., Cena H. Caffeine and blood pressure: A critical review perspective. Nutr. Res. Rev. 2019;32:169–175. doi: 10.1017/S0954422419000015. [DOI] [PubMed] [Google Scholar]
- 101.Bobková A., Hudáček M., Jakabová S., Belej Ľ., Capcarová M., Čurlej J., Bobko M., Árvay J., Jakab I., Čapla J., et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J. Environ. Sci. Health Part B. 2020;55:495–500. doi: 10.1080/03601234.2020.1724660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.