Abstract
Simple Summary
Rice stripe virus is a disastrous viral disease that causes significant yield losses in rice production in South, Southeast, and East Asian countries. To decrease the use of chemical insecticides, genetic engineering has become a pivotal strategy to combat the virus. In this study, we constructed a dimeric artificial microRNA precursor expression vector that targets the viral MP gene based on the structure of the rice osa-MIR528 precursor. Marker-free transgenic plants successfully expressing the MP amiRNAs were obtained and were highly resistant to RSV infection. The novel rice germplasms generated are promising for RSV control.
Abstract
Rice stripe virus (RSV) causes one of the most serious viral diseases of rice. RNA interference is one of the most efficient ways to control viral disease. In this study, we constructed an amiRNA targeting the RSV MP gene (amiR MP) based on the backbone sequence of the osa-MIR528 precursor, and obtained marker-free transgenic rice plants constitutively expressing amiR MP by Agrobacterium tumefaciens-mediated transformation. A transient expression assay demonstrated that dimeric amiR MP could be effectively recognized and cleaved at the target MP gene in plants. Northern blot of miRNA indicated that amiR MP-mediated viral resistance could be stably inherited. The transgenic rice plants were highly resistant to RSV (73–90%). Our research provides novel rice germplasm for RSV control.
Keywords: rice stripe disease, amiRNAs, movement protein, marker-free transgenic rice
1. Introduction
Rice stripe disease, caused by rice stripe virus (RSV), is one of the most devastating viral diseases in rice production worldwide. The viral disease first appeared in Japan, and then emerged in over 20 provinces in China, including Jiangsu and Zhejiang. In the period 2002 to 2004, an outbreak of RSV caused devastating economic losses and affected about 80% of the rice cultivated in Jiangsu province [1,2]. RSV is classified in the genus Tenuivirus, and its genome has four single-stranded RNA genome segments. RNA1 encodes the RNA-dependent RNA polymerase (RdRp) on the complementary strand, while RNAs 2, 3 and 4 have an ambisense coding strategy [3]. RNA4 encodes a disease-specific protein (SP) in the virion sense and a movement protein (MP) in the complementary sense [4,5]. RSV is transmitted in a persistent, circulative–propagative manner by the planthopper Laodelphax striatellus, which makes it difficult for integrative management [6]. The deployment of resistant rice varieties is considered to be the most economic and efficient way to control the disease [7]. Traditional breeding for resistance is slow and inefficient and is challenged by the genetic linkage of agronomic traits with disease resistance genes [8].
In the last few decades, researchers have shown an increasing interest in antiviral genetic engineering in rice. In 1992, an attempt was made to engineer RSV-resistant rice by overexpressing the cp protein. The resulting transgenic plants showed partial RSV resistance but did not generate agriculturally useful lines [9]. In 2011, Takumi et al. designed seven specific siRNAs to target the dsRNAs of each gene in the RSV genome [10]. Rice plants expressing the siRNAs of pc1, CP and MP had the greatest virus resistance.
Among the various transgenic approaches to the creation of virus-resistant plants, one of the most effective involves the expression of artificial miRNA (amiRNA) targeting the virus [11,12]. MicroRNAs (miRNAs) are a class of endogenous, single-strand noncoding RNAs 18–25 nucleotides long, which play important roles in plant defense [13,14]. In 2006, Niu et al. designed new amiRNAs in Arabidopsis, which provided resistance to both turnip yellow mosaic virus (TYMV) and turnip mosaic virus (TuMV) [11]. Similarly, an amiRNA targeting cucumber green mottle mosaic virus (CGMMV) protected Nicotiana benthamiana from invading viruses and virusoids through RNA silencing mediated by the amiRNA [15]. Antiviral strategies using amiRNA have been successfully used to protect plants against plum pox virus (PPV), potato virus Y (PVY) and cotton leaf curl Burewala virus (CLCuBuV) [16,17,18], while transgenic rice expressing an amiRNA targeting the 3‘-UTR regions of the RSV CP gene had 54% resistance to RSV infection [19]. Compared with the siRNA antiviral strategy, the amiRNA approach overcomes the problem of self-silencing, as miRNAs are not associated with transgene silencing, but also decreases off-target effects [12,20].
In this study, we constructed an amiRNA targeting the RSV MP gene (amiR MP) and obtained marker-free transgenic rice expressing amiR MP. The transgenic plants were highly resistant to RSV and have provided a useful rice germplasm to control RSV.
2. Materials and Methods
2.1. Sequence and amiRNA Prediction
The amiRNA targeting the segment of the RSV MP gene was identified by the microRNA Target Finder software WMD (http://wmd3.weigelworld.org, accessed on 23 September 2021) and designated as amiR MP [21]. The entire sequence of osa-MIR528 was cloned from rice DNA by PCR amplification using primers primer-F1 and primer-R1 (Table S1). The predicted amiRNA sequence of RSV MP (TTTCTGAACTACATTAGTCGT) was used to replace the natural miR528/miR528* sequences by oligonucleotide-directed mutagenesis.
2.2. Transformation
The Agrobacterium tumefaciens strain EHA105 was transformed with the constructs expressing pCV amiR MP and full-length RSV MP by electroporation, and a final OD ratio of 2:1 was used for coinoculation [22]. N. benthamiana plants at 3 weeks old were infiltrated using pressure.
2.3. Production of T0 Generation Transgenic Plants
Rice variety Oryza sativa L. var. Zhegeng-88, a commercial rice variety grown in Zhejiang (https://www.ricedata.cn/variety/varis/609074.htm, accessed on 4 February 2022), was used as baseline to generate the transgenic plants. Plants were transformed using Agrobacterium as previously described [23] and regenerated on media containing kanamycin sulfate and hygromycin B. To identify transgenic plants, genomic DNA was isolated from leaf tissue by the CTAB method and was screened by PCR with the primers 35S and NOS (Table S1).
2.4. Detection of Foreign Gene Insertion Sites and Homozygotes
The T-DNA insertion sites of T1 transgenic lines were determined by thermal asymmetric interlaced polymerase chain reaction (TAIL-PCR) [24]. Primers were designed according to the sequence between the target fragments of LB and RB (Table S1).
2.5. Assessment of Resistance to RSV
Rice plants were inoculated using 10 viruliferous insects (second or third instar L. striatellus) per plant and kept in a cage containing 30 plants, as described previously [10]. The numbers of rice plants showing each of the different symptom types on their newly developing leaves were recorded at 4 weeks, as previously described [25].
2.6. RNA Analysis
Total RNA was isolated from samples using TRIzol reagent (Invitrogen, Carlsbad, USA) following the manufacturer’s protocol. For Northern blot analyses, 10 μg total RNA was heat-treated at 65 ℃ for 10 min in formamide buffer. Hybridization was performed as described previously [22]. The MP probes were amplified with primers NB-MP-F1/R1 and NB-MP-F2/R2 (Table S1) and labeled with digoxigenin (DIG).
The miRNA was extracted with an Easy Pure miRNA Kit (TransGen, Beijing, China) according to the manufacturer’s instructions. Wild-type (WT) rice was used as the negative control. For small RNA Northern blotting, approximately 1μg miRNAs were heat-treated at 65 ℃ for 10 min in formamide buffer. miRNAs were separated using a 12% polyacrylamide gel containing 7.5 M urea, transferred electrophoretically to Amersham HybondTM -N+ membranes (GE Healthcare, Hatfield, UK) using H2O buffer for 90 min, 0.3 A constant V, and fixed to the membrane by chemical crosslinking. The miRNA probe was commercially labeled with biotin (Sangon Biotech, Shanghai, China). Prehybridization and hybridization used ULTRAhyb®-Oligo Hybridization Buffer (Thermo Fisher Scientific, Waltham, MA, USA), and signal detection was performed with Chemiluminescent Nucleic Acid Detection Module (Thermo Scientific, Waltham, MA, USA) according the instruction manual [26].
2.7. Real-Time Quantitative PCR and Semiquantitative RT-PCR Analysis
The cDNA was synthesized according to the manufacturer’s protocol of TransScript® All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (One-Step gDNA Removal) Kit (TransGen, Beijing, China). The gene fragments were amplified using 2 x TOROGreen® HRM qPCR Master Mix (TOROIVD Technology, Shanghai, China) and the MP-specific primer for RT-qPCR analysis is listed in Table S1. The N. benthamiana Ubiquitin C (UBC) and O. sativa actin genes were used for internal reference control. The ABI QuantStudio5 Real-Time PCR System (Thermo Scientific, Waltham, USA) was used for the reactions and the results were calculated according to the ΔΔCT method. Semiquantitative RT-PCR was used to measure the expression of CP using the primers listed in Table S1.
2.8. Western Blotting
Total proteins for Western blot assay were extracted from leaf samples as described before [22]. Proteins were detected using anti-MP (1:5000) monoclonal primary antibodies and Horseradish Peroxidase-conjugated anti-rabbit secondary antibody (1:10,000; TransGen, Beijing, China). The antigen–antibody complexes were visualized using ECL buffer (Sigma-Aldrich, Saint Louis, MO, USA).
3. Results
3.1. Construction of the Vector Expressing amiRNA Targeting RSV MP mRNA (amiR MP)
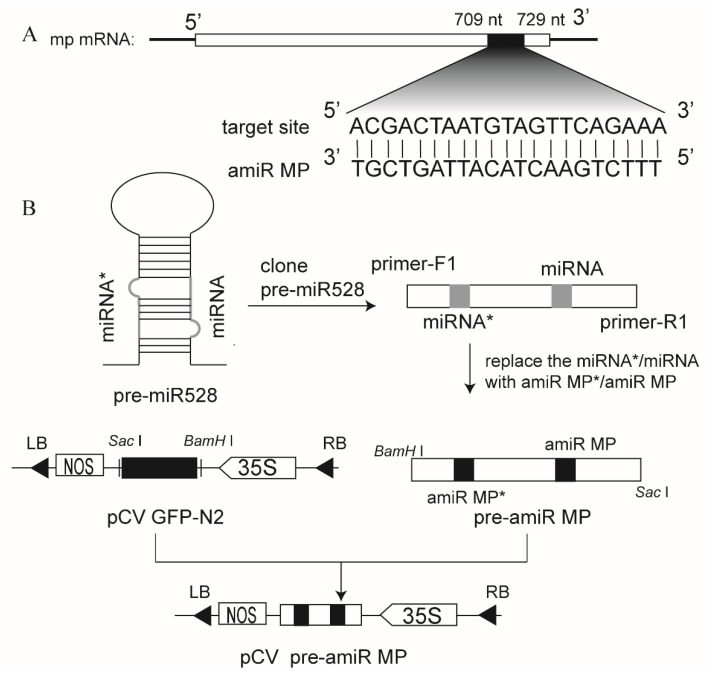
Artificial microRNAs of RSV MP (amiR MP) were predicted and chosen on the website (Figure 1A) and osa-MIR528 precursor was used as the backbone for expressing amiR MP, as described before [12,21]. The natural miR528/miR528* sequences were replaced by viral amiR MP/amiR MP* sequences using oligonucleotide-directed mutagenesis and a precursor of amiR MP (pre-amiR MP) was obtained, as shown in the schematic diagram (Figure 1B). The pre-amiRNA fragment was then inserted into the vector pCV GFP-N2 to obtain transient expression vectors pCV pre-amiR MP (Figure 1B).
Figure 1.
Schematic diagram showing the construction of the expression vector of amiR MP. (A) The target site of amiR MP. (B) Strategy for artificial miRNA construction. The natural miR528/miR528* sequences were replaced by viral amiR MP/amiR MP* sequences using oligonucleotide-directed mutagenesis and were cloned into the vector pCV GFP-N2. The 35S promoter and Nos-T nopaline synthetase terminator were used in this construction.
3.2. Expression of amiR MP Decreased Accumulation of MP mRNA in a Transient Expression Assay
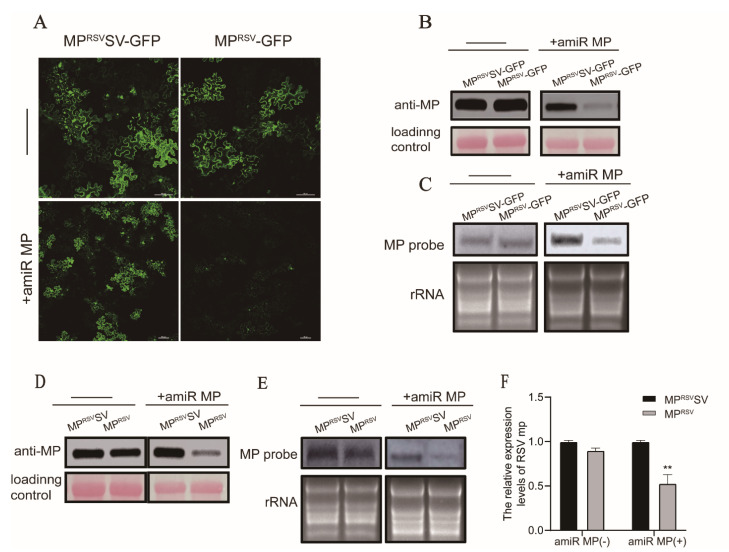
To test whether expression of MP amiRNA precisely cleaves the target mRNA MP, we performed a transient expression assay in N. benthamiana. The MPRSV fragment and a synonymous mutant named MPRSVSV, which could not be targeted by amiR MP, were inserted into the transient expression vector pCAMBIA1300. As shown in Figure 2A, the green fluorescence of the MPRSVSV-GFP and MPRSV-GFP was similar without transient expression of amiR MP. However, the intensity of MPRSV-GFP fluorescence was sharply decreased when coexpressed with amiR MP (Figure 2A). Western blot using an anti-MP antibody and Northern blot using an MP probe confirmed a significant reduction in MP accumulation in the infiltrated patch, but not with MPRSVSV (Figure 2B,C). In order to eliminate any effect of miRNA targeting GFP, unfused MPRSVSV and MPRSV were transiently expressed. Western blot results showed similar levels of MPRSV and MPRSVSV protein accumulation in the absence of amiR MP, but when amiR MP was coexpressed, the transcription and protein level of MPRSV was significantly lower than that of MPRSVSV (Figure 2D,E). RT-qPCR confirmed that transcripts of the MP gene in the patch coexpressing MPRSV and amiR MP were at about half the levels of those in the control MPRSVSV patch (Figure 2F). In summary, the results indicate that the amiRNA of MP effectively recognized and cleaved the target RNA in plants.
Figure 2.
Expression of amiR MP decreased accumulation of MP mRNA in a transient expression assay. (A) The green fluorescence of MPRSV-GFP and synonymous mutant MPRSVSV-GFP, with and without expression of amiR MP observed at 48 hpi. Bars, 100 μm. (B,C) Western blot and Northern blot analysis of GFP-fused target mRNA from coinoculated N. benthamiana leaves. Loading control and rRNA were used to show that equal amounts of total protein and RNA were loaded. (D,E) Western blot and Northern blot analysis of GFP-unfused target mRNA from coinoculated N. benthamiana leaves. Loading control and rRNA were used to show that equal amounts of total protein and RNA were loaded. (F) qRT-PCR analysis of unfused target mRNA from coinoculated N. benthamiana leaves. Three independent experiments were performed for analysis. Asterisks mark significant differences according to t-test; ** p value ≤ 0.01.
3.3. Generation of Marker-Free Transgenic Rice Expressing amiR MP
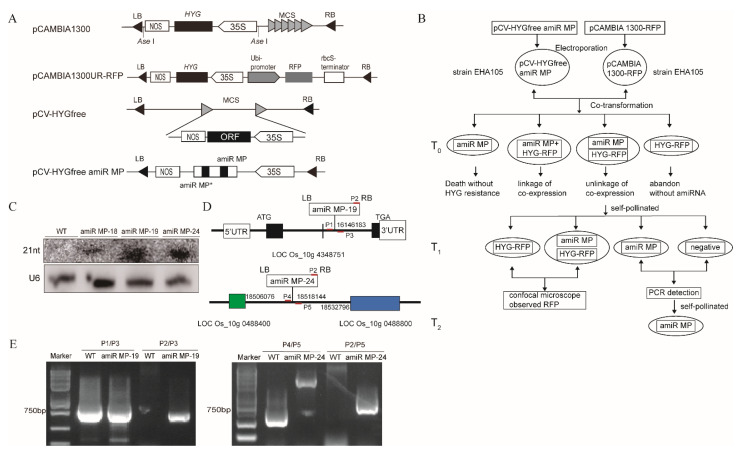
Marker-free transgenic rice plants expressing amiR MP were generated using the cotransformation strategy shown in the schematic diagram (Figure 3A,B). The marker vector pCAMBIA 1300UR-RFP uses pCAMBIA 1300 as a backbone and this contains the maize ubiquitin promoter (ubi-promoter) and rbcS terminator for expression of RFP [27] (Figure 3A). To construct the marker-free expression vector (PCV-HYG-free amiR MP), pCAMBIA1300 was modified by digesting with Ase I to remove the hygromycin B (HYG) resistance gene, but retaining the 35S promoter and a NOS terminator (pCV-HYG-free) (Figure 3A). The prepared rice calli were then subjected to Agrobacterium tumefaciens-mediated cotransformation with PCV-HYG-free amiR MP vector and marker vector pCAMBIA 1300UR-RFP. T0 generation plants were tested for HYG tolerance selection and PCR detection. Self-fertilization of T0 plants allowed the un-linked genes amiR MP and marker RFP to be separated in the T1 generation plants. A simple test for RFP expression eliminated the progeny containing the marker gene, and a combination of PCR testing of the remaining plants efficiently identified the marker-free transgenic plants. Seven lines of T2-generation marker-free transgenic plants were obtained by this procedure (Supplementary Figure S1).
Figure 3.
Generation of marker-free transgenic rice plants expressing amiR MP. (A) The construction of expression vectors pCAMBIA1300-RFP and pCV-HYGfree:amiR MP for cotransformation. The Ubi-P ubiquitin promoter and Nos-T nopaline synthetase terminator were used in this construction. (B) Schematic diagram showing the generation of marker-free transgenic rice expressing amiR MP. (C) Northern blot analysis of specific amiRNAs from transgenic plants amiR MP-18, amiR MP-19 and amiR MP-24. WT indicates wild-type plants. (D) Detection of insertion sites of amiR MP in transgenic lines by thermal asymmetric interlaced polymerase chain reaction (TAIL-PCR). ATG presents the starting codon, TGA presents termination codon, P1/P2/P3/P4/P5 the PCR primer sites. (E) Results of PCR reactions to identify the homozygous lines.
To investigate whether the transgenic plants generate amiRNA, we isolated small RNAs from transgenic plants, with WT plants as controls. In Northern blot assays of small RNAs, amiR MP was detected in transgenic plants (lines 18, 19 and 24), but not in the WT (Figure 3C), confirming that expression of amiR MP in transgenic plants was stably inherited.
To eliminate any deviation of miRNA function caused by insertion into a functional region of the genome, we identified the insertion sites of transgenic lines by TAIL-PCR. The amiR MP in line 19 was shown to be located in the gene intergenic spacer of LOC_Os10g4348751 of chromosome 10, and the amiR MP in line 24 between genes LOC_Os10g048840 and LOC_Os10g048800 (Figure 3D). The primers to identify homozygous plants were designed by using the sequences forward and backward of the insertion site and that of T-DNA. When amplified by PCR using the P2/P3 or P2/P5 primers, there was a band from transgenic plants lines 19 and 24 but not from the WT, indicating that the T-DNA had inserted into the rice genome. When amplification was conducted using the P1/P3 primers, the same band was identified in line 19 as in the WT showing line 19 to be heterozygous. Similarly, when amplification was performed using the P4/P5 primers, the band amplified from line 24 was different to that from the WT showing that this line is homozygous (Figure 3E).
3.4. Transgenic Rice Plants Were Highly Resistant to RSV
As shown in Tables S2–S5 and Figure 4A,B, the phenotypes of the transgenic and wild-type plants were similar. The expression of amiR MP in vivo had little effect on the growth phenotypes such as plant height, tiller number and rate of seed-setting.
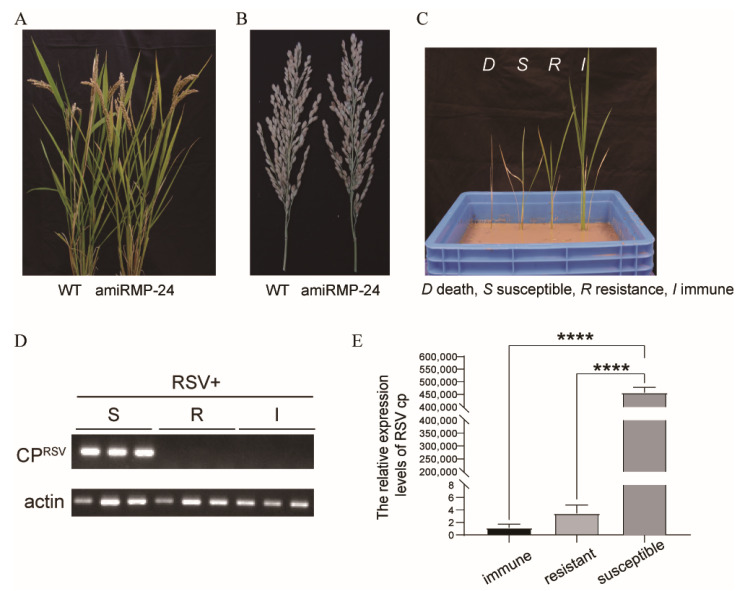
Figure 4.
Transgenic rice plants showed high resistance against RSV infection. (A) The phenotypes of T2-generation transgenic amiR MP-24 plants compared with WT (Wild-type, Zhegeng-88) controls. (B) Spikes of amiR MP-24 and WT plants. (C) Symptoms in transgenic plants at 28 dpi following inoculation with RSV. D death: represents dead plants, S susceptible: stunting with chlorotic stripes, R resistant: mild stunting without chlorotic stripes, I immune: no symptoms. (D) RT-PCR detection of the accumulation of CP in RSV-infected plants with different symptoms. (E) RT-qPCR detection of the accumulation of CP in RSV-infected plants with different symptoms. Asterisks mark significant differences according to t-test; **** p value ≤ 0.0001.
To examine if the designed amiRNAs targeting the RSV MP gene conferred resistance in the T2-generation transgenic plants, seven transgenic lines (30 plants in each line) were screened and inoculated with RSV by allowing viruliferous vector insects (L. striatellus) to feed on them. Wild-type plants were used as controls and the appearance of symptoms was observed daily. After 4 weeks, the symptoms of RSV were recorded on each plant as dead (D), susceptible (S), resistant (R), or immune (I) (Figure 4C). RT-PCR and RT-qPCR were used to detect the accumulation of RSV CP on the inoculated plants (Figure 4D,E). The transgenic plants showed varying degrees of RSV resistance (Table 1) and those with a resistance ratio > 85% (amiR MP-18, amiR MP-19 and amiR MP-24) were considered highly resistant.
Table 1.
Response of the T2-generation transgenic plants to inoculation with RSV. In total, 30 plants of each line were inoculated.
| Transgenic Line | Numbers of Plants with Different Resistance Levels | Resistance Ratio (%) a | Resistance Status b | |||
|---|---|---|---|---|---|---|
| I | R | S | D | |||
| amiR MP-7 | 16 | 6 | 6 | 2 | 73 | + |
| amiR MP-8 | 21 | 3 | 4 | 2 | 80 | + |
| amiR MP-10 | 19 | 1 | 8 | 2 | 67 | − |
| amiR MP-12 | 18 | 6 | 3 | 3 | 80 | + |
| amiR MP-18 | 18 | 8 | 3 | 1 | 87 | ++ |
| amiR MP-19 | 17 | 9 | 2 | 2 | 87 | ++ |
| amiR MP-24 | 26 | 1 | 2 | 1 | 90 | ++ |
| WT | 6 | 5 | 18 | 1 | 37 | − |
I immune, R resistant, S susceptible, D dead; a resistant plants include I and R plants; b highly resistant ++; resistant +; susceptible −.
4. Discussion
Rice production is always constrained by viruses, among which RSV is a devastating pathogen that causes significant yield losses in most Southeast Asian countries. The application of insecticides against the intermediate insect vectors is one of the most successful ways to prevent damage and yield loss from rice viruses, but the high cost and environment pollution are major burdens in rice production [28,29]. With the development of genetic engineering, RNA interference has become a pivotal strategy to combat these viruses [30]. Several studies have introduced siRNAs to target the virus genomes and have obtained transgenic rice resistant to viruses such as rice dwarf virus (RDV) [31,32], RSV [10] and rice black streaked dwarf virus (RBSDV) [33]. Unlike siRNA, miRNAs are single-strand noncoding RNA around 22 nucleotides long [34,35] and there is mounting evidence that they play a critical role in virus infection [36,37]. However, the amiRNA approach has rarely been used in rice to provide resistance to RSV. Here, by using amiRNA-mediated RNA silencing, we generated transgenic plants carrying a dimeric amiRNA precursor producing miRNA targeting the viral MP to resist RSV infection.
A previous study suggested that the variable levels of resistance to viral infection of plants that harbor RNAi constructs depended on the particular viral gene used as the target for RNAi, and that best resistance to RSV was obtained targeting the cp and mp sequences [10]. The T2-generation of cp target transgenic rice plants still exhibited immunity to RSV infection. However, unlike siRNA, Sun et al. obtained only 54.17% resistance efficiency in transgenic rice with amiRNA targeting RSV CP [19]. In this study, transgenic rice plant lines targeting the RSV MP exhibited a much higher level of resistance to RSV (93%), underlining the importance of selecting the best target sequence.
If there is to be large-scale commercial cultivation of such transgenic crops, plants without selection markers are desired. Such plants are currently usually produced by a cotransformation strategy. Some researchers construct a binary vector that carries two separate T-DNAs, one containing a selection gene and the other for expression of the desired gene [38,39,40]. However, this method is hampered by the difficulty in obtaining cotransformation vectors due to the lack of convenient restriction sites. Another method is to construct two vectors containing different T-DNAs and to transfer them to Agrobacterium separately [41,42]. However, as a cotransformation strategy, this involves a very heavy workload to screen large numbers of transgenic plants by a PCR assay. In 2005, Chen et al. constructed a double T-DNA vector containing the green fluorescent protein (GFP) gene to easily monitor the segregation of marker genes, thus facilitating screening of marker-free progeny [43]. Here, we constructed a marker vector carrying an RFP selection gene for the convenient observation of red fluorescence in rice leaves. Therefore, when we cotransformed with the RFP marker vector and amiR MP vector, we could observe red fluorescence quickly in T1-generation transgenic rice under a confocal microscope and use PCR testing to identify the marker-free transgenic plants conveniently. Among the cotransformed plants, we obtained seven lines containing two unlinked T-DNAs, enabling the segregation of transgenic rice in the subsequent generation.
Our marker-gene-free transgenic rice plants contain only the antiviral miRNA and exhibit 90% resistance to RSV infection. As a large number of virus-resistant transgenic crops have emerged, the biological safety of transgenic plants has raised concerns, including off-target effects [44,45]. However, since the transgenic plants express only a short sequence through amiRNA-mediated strategies, these potential risks are likely to be minimal [11]. These plants are a new germplasm resource for breeding rice resistant to RSV.
5. Conclusions
In this study, a construct of artificial microRNA of RSV MP based on the backbone sequence of the osa-MIR528 precursor was cotransformed with an RFP marker vector into rice to obtain marker-free transgenic plants. These transgenic plants, constitutively expressing amiR MP, had high resistance to RSV infection.
Acknowledgments
We thank J. Adams, Minehead, UK for help in correcting the English of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology11020332/s1, Figure S1: Selection of positive marker-free transgenic plants, Table S1: Primers used for analysis, Table S2: Plant height of homozygous transgenic plants amiR MP-24, Table S3: Tiller number of homozygous transgenic plants amiR MP-24, Table S4: seed-setting rate of homozygous transgenic plants amiR MP-24, Table S5: Grains per spike of homozygous transgenic plants amiR MP-24.
Author Contributions
Conceptualization, F.Y. and L.Z.; methodology, Q.Y.; software, L.Z. and X.A.; validation, L.Z., Q.Y. and X.A.; formal analysis, L.Z.; investigation, L.Z. and Q.Y.; resources, Q.Y. and F.Y.; data curation, L.Z.; writing—original draft preparation, L.Z.; writing—review and editing, Y.L.; visualization, Y.L.; supervision, F.Y.; project administration, J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of China (31772239), the National Program of Transgenic Science and Technology (2016ZX08001-002) and the K. C. Wong Magna Fund of Ningbo University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He M., Guan S.Y., He C.Q. Evolution of rice stripe virus. Mol. Phylogenet. Evol. 2017;109:343–350. doi: 10.1016/j.ympev.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Wei T.Y., Yang J.G., Liao F.L., Gao F.L., Lu L.M., Zhang X.T., Li F., Wu Z.J., Lin Q.Y., Xie L.H., et al. Genetic diversity and population structure of rice stripe virus in China. J. Gen. Virol. 2009;90:1025–1034. doi: 10.1099/vir.0.006858-0. [DOI] [PubMed] [Google Scholar]
- 3.Toriyama S., Takahashi M., Sano Y., Shimizu T., Ishihama A. Nucleotide sequence of RNA 1, the largest genomic segment of rice stripe virus, the prototype of the tenuiviruses. J. Gen. Virol. 1994;75:3569–3579. doi: 10.1099/0022-1317-75-12-3569. [DOI] [PubMed] [Google Scholar]
- 4.Xiong R., Wu J., Zhou Y., Zhou X. Identification of a movement protein of the tenuivirus rice stripe virus. J. Virol. 2008;82:12304–12311. doi: 10.1128/JVI.01696-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jian Y., Zhang F., Li J., Chen J.P., Zhang H.M., Sek-Man W. Integrative Analysis of the microRNAome and Transcriptome Illuminates the Response of Susceptible Rice Plants to Rice Stripe Virus. PLoS ONE. 2016;11:e0146946. doi: 10.1371/journal.pone.0146946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falk B.W., Tsai J.H. Biology and molecular biology of viruses in the genus Tenuivirus. Annu. Rev. Phytopathol. 1998;36:139–163. doi: 10.1146/annurev.phyto.36.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Lin H.A., Chen S.Y., Chang F.Y., Tung C.W., Chen Y.C., Shen W.C., Chen R.S., Wu C.W., Chung C.L. Genome-wide association study of rice genes and loci conferring resistance to Magnaporthe oryzae isolates from Taiwan. Bot. Stud. 2018;59:32–45. doi: 10.1186/s40529-018-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin S., Su Y., Gao S., Wu F., Hu T., Liu J., Li W., Wang D., Chen S., Jiang Y., et al. Deep Learning: Individual maize segmentation from terrestrial lidar data using faster R-CNN and regional growth algorithms. Front. Recent Dev. Plant Sci. 2018;9:866–875. doi: 10.3389/fpls.2018.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayakawa T., Zhu Y., Itoh K., Kimura Y., Izawa T., Shimamoto K., Toriyama S. Genetically engineered rice resistant to rice stripe virus, an insect-transmitted virus. J. Appl. Biol. Sci. 1992;89:9865–9869. doi: 10.1073/pnas.89.20.9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu T., Nakazono-Nagaoka E., Uehara-Ichiki T., Sasaya T., Omura T. Targeting specific genes for RNA interference is crucial to the development of strong resistance to rice stripe virus. Plant Biotechnol. J. 2011;9:503–512. doi: 10.1111/j.1467-7652.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 11.Niu Q.W., Lin S.S., Reyes J.L., Chen K.C., Wu H.W., Yeh S.D., Chua N.H. Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nat. Biotechnol. 2006;24:1420–1428. doi: 10.1038/nbt1255. [DOI] [PubMed] [Google Scholar]
- 12.Norman W., Hao C., Stephan O., Detlef W., Philippe H., Borevitz J.O. Highly specific gene silencing by artificial miRNAs in rice. PLoS ONE. 2008;3:e1829. doi: 10.1371/journal.pone.0001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S.R., Zhou J.J., Hu C.G., Wei C.L., Zhang J.Z. MicroRNA-mediated gene silencing in plant defense and viral counter-defense. Front. Microbiol. 2017;8:1–12. doi: 10.3389/fmicb.2017.01801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y.D., Gan Q.H., Chi X.Y., Qin S. Roles of microRNA in plant defense and virus offense interaction. Plant Cell Rep. 2008;27:1571–1579. doi: 10.1007/s00299-008-0584-z. [DOI] [PubMed] [Google Scholar]
- 15.Liang C.Q., Hao J., Li J., Barbara B., Laixin L. Artificial microRNA-mediated resistance to Cucumber Green Mottle Mosaic Virus in Nicotiana benthamiana. Planta. 2019;250:1591–1601. doi: 10.1007/s00425-019-03252-w. [DOI] [PubMed] [Google Scholar]
- 16.Simon-Mateo C., Garcia J.A. MicroRNA-guided processing impairs Plum pox virus replication, but the virus readily evolves to escape this silencing mechanism. J. Virol. 2006;80:2429–2436. doi: 10.1128/JVI.80.5.2429-2436.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang J., Song Y., Han Q., Zhu C., Wen F. The choice of target site is crucial in artificial miRNA-mediated virus resistance in transgenic Nicotiana tabacum. Physiol. Mol. Plant Pathol. 2011;76:2–8. [Google Scholar]
- 18.Ali I., Amin I., Briddon R.W., Mansoor S. Artificial microRNA-mediated resistance against the monopartite begomovirus Cotton leaf curl Burewala virus. Virol. J. 2013;10:231–238. doi: 10.1186/1743-422X-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L., Lin C., Du J., Song Y., Jiang M., Liu H., Zhou S., Wen F., Zhu C. Dimeric artificial microRNAs mediate high resistance to RSV and RBSDV in transgenic rice plants. Plant Cell Tissue Organ Cult. 2016;126:127–139. doi: 10.1007/s11240-016-0983-8. [DOI] [Google Scholar]
- 20.Molnar A., Bassett A., Thuenemann E., Schwach F., Karkare S., Ossowski S., Weigel D., Baulcombe D. Highly specific gene silencing by artificial micrornas in the unicellular alga Chlamydomonas reinhardtii. Plant J. 2009;58:165–174. doi: 10.1111/j.1365-313X.2008.03767.x. [DOI] [PubMed] [Google Scholar]
- 21.Yan F., Lu Y., Wu G., Peng J., Zheng H., Lin L., Chen J. A simplified method for constructing artificial microRNAs based on the osa-MIR528 precursor. J. Biotechnol. 2012;160:146–150. doi: 10.1016/j.jbiotec.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Chen B., Lin L., Lu Y., Peng J., Zheng H., Yang Q., Rao S., Wu G., Li J., Chen Z., et al. Ubiquitin-Like protein 5 interacts with the silencing suppressor p3 of rice stripe virus and mediates its degradation through the 26S proteasome pathway. PLoS Pathog. 2020;16:e1008780. doi: 10.1371/journal.ppat.1008780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toki S., Hara N., Ono K., Onodera H., Tagiri A., Oka S., Tanaka H. Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J. 2010;47:969–976. doi: 10.1111/j.1365-313X.2006.02836.x. [DOI] [PubMed] [Google Scholar]
- 24.Jia X., Lin X., Chen J. Linear and exponential TAIL-PCR: A method for efficient and quick amplification of flanking sequences adjacent to Tn5 transposon insertion sites. AMB Express. 2017;7:195–202. doi: 10.1186/s13568-017-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S., Jiang L., Yang J., Peng J., Lu Y., Zheng H., Lin L., Chen J., Yan F. Over-expression of Oryza sativa Xrn4 confers plant resistance to virus infection. Gene. 2018;639:44–51. doi: 10.1016/j.gene.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L., Lu Y., Zheng X., Yang X., Chen Y., Zhang T., Zhao X., Wang S., Zhao X., Song X., et al. The plant protein NbP3IP directs degradation of Rice stripe virus p3 silencing suppressor protein to limit virus infection through interaction with the autophagy-related protein NbATG8. New Phytol. 2021;229:1036–1051. doi: 10.1111/nph.16917. [DOI] [PubMed] [Google Scholar]
- 27.Dong R.X., Chen J., Wang X.M., Li J.S., Jie Z., Yang Y., Yu C.L., Cheng Y., Yan C.Q., Chen J.P. Agrobacterium -mediated transformation efficiency is altered in a novel rice bacterial blight resistance cultivar and is influenced by environmental temperature. Physiol. Mol. Plant Pathol. 2012;77:33–40. doi: 10.1016/j.pmpp.2011.11.004. [DOI] [Google Scholar]
- 28.Ban L., Zhang S., Huang Z., He Y., Peng Y., Gao C. Resistance monitoring and assessment of resistance risk to pymetrozine in Laodelphax striatellus (Hemiptera: Delphacidae) J. Econ. Entomol. 2012;105:2129–2135. doi: 10.1603/EC12213. [DOI] [PubMed] [Google Scholar]
- 29.He Y., Zhang J., Chen J., Shen J. Using synergists to detect multiple insecticide resistance in field populations of rice stem borer. Pestic. Biochem. Physiol. 2012;103:121–126. doi: 10.1016/j.pestbp.2012.04.008. [DOI] [Google Scholar]
- 30.Sasaya T., Nakazono-Nagaoka E., Saika H., Aoki H., Hiraguri A., Netsu O., Uehara-Ichiki T., Onuki M., Toki S., Saito K., et al. Transgenic strategies to confer resistance against viruses in rice plants. Front. Virol. 2014;4:1–11. doi: 10.3389/fmicb.2013.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han S., Wu Z., Yang H., Rong W., Yin Y., Xie L., Tien P. Ribozyme-mediated resistance to rice dwarf virus and the transgene silencing in the progeny of transgenic rice plants. Transgenic Res. 2000;9:195–203. doi: 10.1023/A:1008904230223. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu T., Yoshii M., Wei T., Hirochika H., Omura T. Silencing by RNAi of the gene for Pns12, a viroplasm matrix protein of Rice dwarf virus, results in strong resistance of transgenic rice plants to the virus. Plant Biotechnol. J. 2010;7:24–32. doi: 10.1111/j.1467-7652.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu T., Nakazono-Nagaoka E., Akita F., Uehara-Ichiki T., Omura T., Sasaya T. Immunity to Rice black streaked dwarf virus, a plant reovirus, can be achieved in rice plants by RNA silencing against the gene for the viroplasm component protein. Virus Res. 2011;160:400–403. doi: 10.1016/j.virusres.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Bartel D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 35.Mengistu A.A., Tenkegna T.A. The role of miRNA in plant-virus interaction: A review. Mol. Biol. Rep. 2021;48:2853–2861. doi: 10.1007/s11033-021-06290-4. [DOI] [PubMed] [Google Scholar]
- 36.Wang H., Jiao X., Kong X., Hamera S., Wu Y., Chen X., Fang R., Yan Y. A Signaling Cascade from miR444 to RDR1 in Rice Antiviral RNA Silencing Pathway. Plant Physiol. 2016;170:2365–2377. doi: 10.1104/pp.15.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhter Y., Khan J.A. Genome wide identification of cotton (Gossypium hirsutum)—Encoded microRNA targets against Cotton leaf curl Burewala virus. Gene. 2018;638:60–65. doi: 10.1016/j.gene.2017.09.061. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y., Lin S., Jiang M., Li K., Zhu C. Production of marker-free and RSV-resistant transgenic rice using a twin T-DNA system and RNAi. J. Biosci. 2013;38:573–581. doi: 10.1007/s12038-013-9349-0. [DOI] [PubMed] [Google Scholar]
- 39.Mccormac A.C., Fowler M.R., Chen D.F., Elliott M.C. Efficient co-transformation of Nicotiana tabacum by two independent T-DNAs, the effect of T-DNA size and implications for genetic separation. Transgenic Res. 2001;10:143–155. doi: 10.1023/A:1008909203852. [DOI] [PubMed] [Google Scholar]
- 40.Komari T., Hiei Y., Saito Y. Vectors carrying two separate t-DNAs for co- transformation of higher plants mediated by agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 1996;10:165–174. doi: 10.1046/j.1365-313X.1996.10010165.x. [DOI] [PubMed] [Google Scholar]
- 41.Poirier Y., Ventre G., Nawrath C. High-frequency linkage of co-expressing T-DNA in transgenic Arabidopsis thaliana transformed by vacuum-infiltration of Agrobacterium tumefaciens. Theor. Appl. Genet. 2000;100:487–493. doi: 10.1007/s001220050063. [DOI] [Google Scholar]
- 42.Daley M., Knauf V.C., Summerfelt K.R., Turner J.C. Co-transformation with one Agrobacterium tumefaciens strain containing two binary plasmids as a method for producing marker-free transgenic plants. Plant Cell Rep. 1998;17:489–496. doi: 10.1007/s002990050430. [DOI] [PubMed] [Google Scholar]
- 43.Chen S., Li X., Liu X., Xu H., Meng K., Xiao G., Wei X., Feng W., Zhu Z. Green fluorescent protein as a vital elimination marker to easily screen marker-free transgenic progeny derived from plants co-transformed with a double T-DNA binary vector system. Plant Cell Rep. 2005;23:625–631. doi: 10.1007/s00299-004-0853-4. [DOI] [PubMed] [Google Scholar]
- 44.Woodbury P.B., DiTommaso A., Thies J., Ryan M., Losey J. In: Effects of Transgenic Crops on the Environment, In Environmental Pest Management: Challenges for Agronomists, Ecologists, Economists and Policymakers. Coll M., Wajnberg E., editors. Wiley; Hoboken, NJ, USA: 2017. pp. 131–150. [Google Scholar]
- 45.He M., Zhang J., Shen L., Xu L., Luo W., Li D., Zhai N., Zhao J., Long Y., Pei X., et al. High-throughput sequencing analysis of microbial community diversity in response to indica and japonica bar-transgenic rice paddy soils. PLoS ONE. 2019;14:e0222191. doi: 10.1371/journal.pone.0222191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article or supplementary material.