Abstract
A growing number of diseases are linked to the misfolding of integral membrane proteins, and many of these proteins are targeted for ubiquitin-proteasome-dependent degradation. One such substrate is a mutant form of the Cystic Fibrosis Transmembrane Conductance Regulator (F508del-CFTR). Protein folding “correctors” that repair the F508del-CFTR folding defect have entered the clinic, but they are unlikely to protect the entire protein from degradation. To increase the pool of F508del-CFTR protein that is available for correction by existing treatments, we determined a structure-activity relationship to improve the efficacy and reduce the toxicity of an inhibitor of the E1 ubiquitin activating enzyme that facilitates F508del-CFTR maturation. A resulting lead compound lacked measurable toxicity and improved the ability of an FDA-approved corrector to augment F508del-CFTR folding, transport the protein to the plasma membrane, and maintain its activity. These data support a proof-of-concept that modest inhibition of substrate ubiquitination improves the activity of small molecule correctors to treat CF and potentially other protein conformational disorders.
Keywords: Cystic fibrosis, CFTR, ERAD, Ubiquitin, Protein folding, Protein degradation, E1 ubiquitin activating enzyme, Trikafta, Proteasome, Structure-activity relationship
Cystic fibrosis (CF) is the most common, inherited lethal disease in Caucasians, and the most common disease-associated mutation is the deletion of phenylalanine at position 508 in the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR normally functions as a chloride channel at the apical plasma membrane in several tissues and regulates salt and water balance.1 In contrast, in individuals who carry the F508del allele, the resulting F508del-CFTR protein inefficiently folds in the endoplasmic reticulum (ER)2 and is targeted for ubiquitination and proteasomal degradation via the ER associated degradation (ERAD) pathway.3,4 However, F508del-CFTR stability and even function are partially restored by incubating cell lines expressing the protein in the presence of chemical chaperones or at low temperature,5–7 a phenomenon that arises from the correction of thermodynamic and kinetic barriers to which the F508del-CFTR protein is normally subjected.8,9 These observations launched efforts to identify small molecule protein folding “correctors” that might similarly facilitate F508del-CFTR maturation. Ultimately, several folding correctors—as well as “potentiators” that augment channel activity at the plasma membrane—were isolated in high throughput chemical screens.10 Subsequent efforts improved the pharmacological properties of the compounds such that individuals who express F508del-CFTR—as well as other unstable but rarer CFTR alleles—are currently treated with a drug cocktail. This triple drug combination, known as Trikafta, includes both folding correctors as well as a potentiator.11–13
Although Trikafta significantly slows disease progress and ameliorates many of the symptoms exhibited by CF patients, the treatment fails to fully restore CFTR function.12,13 Thus, the F508del-CFTR protein is incompletely rescued and is almost certainly degraded to some extent in the ER. Moreover, there is some concern that the effects of the drugs in the Trikafta combination wane over time based on long-term administration of one component in the cocktail.14–16 In addition, the cost of Trikafta is exorbitant (>$280,000/year). Therefore, there remains a dire need to improve the existing drugs used to treat CF and to identify new targets and small molecules that augment the effects of Trikafta or function independently.
Because F508del-CFTR is targeted for degradation by the proteasome, early studies examined whether proteasome inhibitors might improve F508del-CFTR folding, protein trafficking to the plasma membrane, and function. Unfortunately, as a consequence of proteasome inhibition, the stabilized F508del-CFTR protein became highly poly-ubiquitinated, which triggered protein insolubility and thus resistance to correction.17,18 Based on these results as well as other data,19,20 we reasoned that a small molecular inhibitor of F508del-CFTR polyubiquitination might similarly stabilize the protein and increase the pool of F508del-CFTR that could still be repaired. Because proteasometargeted substrates are ubiquitinated via the sequential action of E1 ubiquitin-activating enzymes, E2 ubiquitin conjugating enzymes, and E3 ubiquitin ligases, any of these components might in principle be targeted to suppress F508del-CFTR ubiquitination. Indeed, a tool compound, PYR-41, inhibits the E1 ubiquitin activating enzyme., and at sub-critical doses, this compound enhances the ability of a F508del-CFTR corrector to stabilize the protein and promotes F508del-CFTR trafficking to and function at the plasma membrane.21
PYR-41 (Fig. 1) was isolated as a compound that stabilizes and thus activates p53, an outcome that was predicted to slow the growth of cancer cells.22 Further analysis indicated that the compound inhibited the E1 enzyme and, as anticipated, blocked TRAF6 and IκBα ubiquitination. However, PYR-41 is quite toxic, thus limiting its use and potentially confounding the interpretation of data on its ability to slow the degradation of F508del-CFTR.21
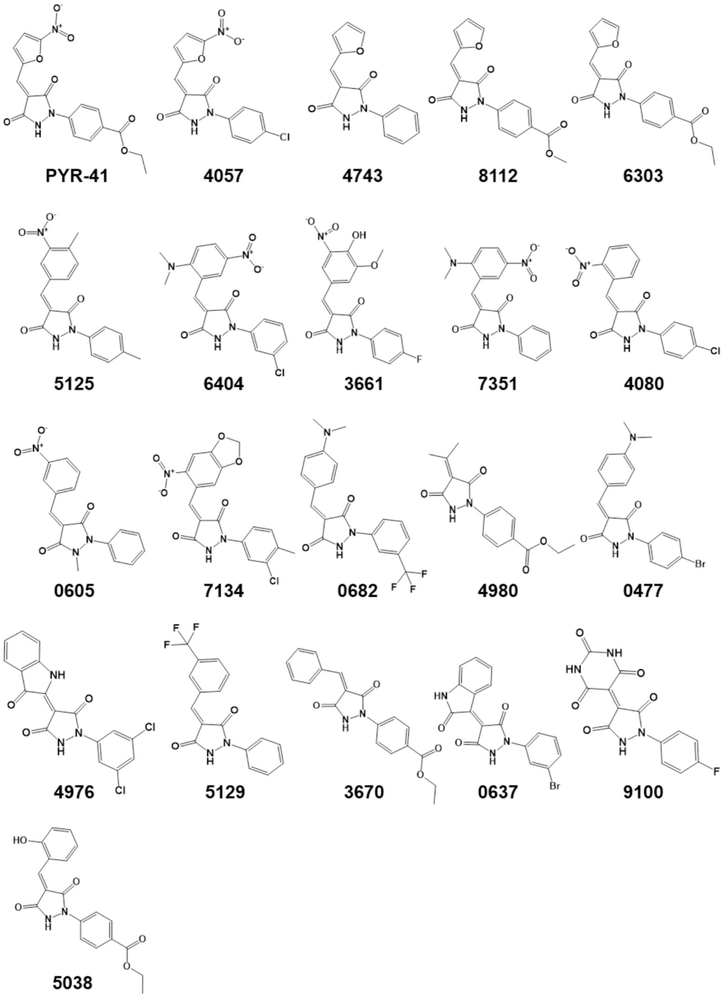
Fig. 1.
Structural analogs of PYR41, an inhibitor of the E1 ubiquitin activating enzyme, examined in this study. The 4-digit compound names are abbreviated from the Sigma Aldrich CPR compound library database identification numbers. Compounds 9731 and 0855 are identical to PYR-41 but from different suppliers, and are not shown. Please see Table S1 for further information.
To obtain less toxic and more potent inhibitors of F508del-CFTR ubiquitination, we sought to identify small molecule analogs and explore the SAR around PYR-41. Though several nitro- and furan-containing drugs are reported, furan derivatives and nitro-substituted heteroaromatics are documented in the literature as toxic fragments.23,24 Consequently, we explored different combinations of substitutions to reduce toxicity based on the 5-nitrofuran substitution on the pyrazolidine-3,5-dione (PD). To enhance the potency and efficacy of PYR-41, we also explored diverse aromatic substitutions in the 4-position of the PD core. Because it is unclear whether nitro substitution, furan heteroaromatics, or substitution on the 4-position of the PD core are critical contributors for pharmacophore activity, we took into consideration all possible combinations. During compound exploration, we preserved the 5-nitrofuran in 4057, 9731 and 0855 with varying substitutions on the PD core (Fig. 1). These compounds are close analogs of PYR-41 and are expected to preserve similar pharmacophoric features. In contrast, compounds 4743, 8112 and 6303 contained furans but without the nitro substitution, and compounds 5125, 6404, 3661, 7351, 4080, 0605 and 7134 all had nitro substituents on the phenyl ring system. In turn, 9100 and 5038 had unique substituents at the 4-position of the PD core, but resembled 3661 and PYR-41/6303/4980/3670, respectively. Finally, we selected compounds 0682, 4980, 0477, 4976, 5129, 3670 and 0637 which lacked both furan and nitro moieties.
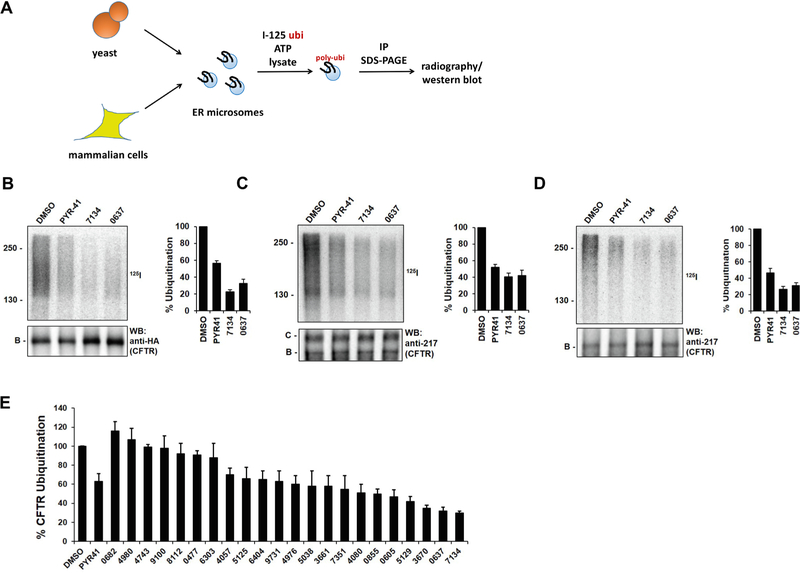
As a result of this analysis, 22 compounds were chosen (see Fig. 1 and below), and along with PYR-41 these compounds were obtained from commercial sources (see Table S1). Next, they were examined in an assay that directly monitors the covalent attachment of radiolabeled ubiquitin onto CFTR, or any CFTR mutant, that is embedded in ER-derived membranes (Fig. 2A).21,25 The membranes can be prepared from yeast or tissue culture (e.g., Human Embryonic Kidney-293; HEK293) cells that express a copy of CFTR that has been introduced via the transfection of an extra-chromosomal plasmid engineered for high level expression.25,26 In the presence of cell lysate and ATP, a polyubiquitin chain is added onto CFTR that can be visualized after CFTR is immunoprecipitated and analyzed by SDS-PAGE and radiography. Recent studies indicated, as anticipated, that F508del-CFTR is ubiquitinated to a significantly greater degree than CFTR in this assay, suggesting that the assay faithfully recapitulates the preferred selection of the mutant protein vs the wild-type protein for ERAD.25 These data are also consistent with the fact that a portion of wild-type CFTR is also targeted for ubiquitin-dependent degradation.2–4 In addition, the reaction is catalyzed by E3 ubiquitin ligases that deliver CFTR and F508del-CFTR for proteasome-dependent degradation.25,27,28.
Fig. 2.
PYR-41 and PYR-41 analogs inhibit CFTR polyubiquitination to varying degrees. (A) PYR-41 and structural analogs were examined in an in vitro ubiquitination assay using ER-membrane-embedded CFTR or F508del-CFTR isolated from yeast or HEK293 cells, an ATP regenerating system, 125I-ubiquitin, and yeast cell lysate, as published.25,29 Reactions with (B) yeast ER-derived membranes containing CFTR, (C) HEK293 cell membranes containing CFTR, and (D) HEK293 cell membranes containing F508del-CFTR were performed. Sample autoradiographs showing heterogeneous polyubiquitin chain profiles (125I, top) and western blots (WB, bottom) of the CFTR protein are shown. Molecular weight markers are presented to the left in the top panels and the migrations of the ER-resident B band and the mature C band are displayed below. Note that CFTR expressed in yeast is HA-tagged but antibody 217 was used to detect the untagged CFTR and F508del-CFTR proteins in microsomes prepared from HEK293 cells. (E) The relative amount of polyubiquitination in the presence of the indicated compounds was calculated as in part (B). Both 9731 and 0855 are identical to PYR-41 and, as anticipated, yielded a similar degree of inhibition in a blinded experiment. Compounds were added at a final concentration of 300 μM, and data represent the means of 3 independent experiments ± SD.
As anticipated, membranes from yeast or HEK293 cells (Fig. 2B and C, respectively) containing wild-type CFTR, or from HEK293 cells containing F508del-CFTR (Fig. 2D), supported the conjugation of heterogeneous polyubiquitin chains in the presence of DMSO. As hoped, compounds examined in this SAR analysis exhibited a range of inhibitory activities on the ubiquitination of both wild type and F508del-CFTR, with compounds 7134 and 0637 showing the greatest inhibitory effects (Fig. 2B–E). Notably, both 7134 and 0637 inhibited CFTR and F508del-CFTR ubiquitination to a greater degree than PYR-41. Assays to determine compound toxicity in HEK293 cells (Table 1) confirmed that PYR-41 was toxic (IC50 = 22 μM) and indicated that 0637 was marginally toxic (IC50 = 98 μM). In contrast, 7134 lacked toxicity at any concentration tested (up to 100 μM). As controls for this blinded experiment, compounds 9731 and 0855 were also included. These compounds are identical to PYR-41 but were purchased independently so that different commercial sources/lot numbers could be examined, and as expected both were as efficacious and toxic (P > 0.05) as PYR-41.
Table 1.
The effects of the compounds on HEK293 cell viability were examined using a commercial CellTiter-Glo assay in order to determine IC50 values, as published.30 Data represent the means of 3 independent experiments. PYR41 is shown for comparison. Note that 9731 is identical to PYR-41 and, as anticipated, yielded a similar IC50 in a blinded experiment. 0855, in this assay, appeared to be somewhat more toxic, but the P value was > 0.05.
| Compound | IC50 (μM) |
|---|---|
|
| |
| DMSO (vehicle) | >100 |
| PYR-41 | 22 |
| 0682 | >100 |
| 4980 | >100 |
| 4743 | >100 |
| 9100 | >100 |
| 8112 | >100 |
| 0477 | >100 |
| 6303 | >100 |
| 4057 | 12 |
| 5125 | >100 |
| 6404 | >100 |
| 9731 | 21 |
| 4976 | 90 |
| 5038 | >100 |
| 3661 | >100 |
| 7351 | >100 |
| 4080 | >100 |
| 0855 | 9.0 |
| 0605 | 86 |
| 5129 | 78 |
| 3670 | >100 |
| 0637 | 98 |
| 7134 | >100 |
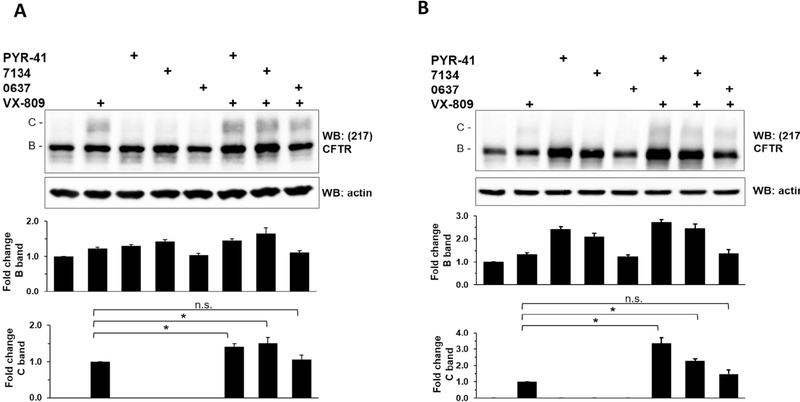
Because 7134 lacked toxicity and most effectively reduced the extent of CFTR and F508del-CFTR polyubiquitination in vitro, we measured the activity of this compound in cell-based assays that monitor CFTR stability and folding-dependent transport from the ER to the Golgi. PYR-41 and 0637 were examined in parallel. First, the ability of the compounds to stabilize and support the conversion of CFTR from the ER-resident “B band” to the Golgi-associated “C band” was monitored in HEK293 cells transfected with a plasmid encoding F508del-CFTR (Fig. 3A). DMSO was used as a vehicle control. In the absence of added compound, only the B band was evident, but when cells were treated with the FDA-approved corrector VX-80931 (Lumacaftor), a small amount of band C was evident. When VX-809 was combined with PYR-41, an increase in band B—representing stabilization of F508del-CFTR and protection from ERAD—as well as band C was apparent, consistent with published data.21 Similarly, when VX-809 was combined with 7134, a statistically significant increase in both bands was again apparent. To confirm these results, we next measured the activities of the same compounds in Cystic Fibrosis Bronchial Epithelial (CFBE) cells, which were derived from a CF patient homozygous for the F508del-CFTR mutation (Fig. 3B). In this cell line, VX-809 and 7134 increased the amount of band B as well as the magnitude of VX-809-dependent maturation to band C compared to VX-809 alone. The reason that 0637 was inactive in HEK293 or CFBE cells is unclear, but might relate to its metabolic profile, or to its marginal toxicity (Table 1).
Fig. 3.
7134 increases VX809-dependent maturation of F508del-CFTR. (A) HEK293 transiently expressing F508del-CFTR and (B) CFBE cells were treated with VX-809, PYR-41, 7134, and/or 0637 for 24 h, all at a final concentration of 10 μM. The cells were then lysed, and CFTR protein was analyzed by western blot as published.30 Actin served as a loading control. The levels of band B and band C CFTR were quantified from 4 independent experiments in HEK293 cells and 3 independent experiments in CFBE cells. Data were standardized to the amount of band B in the vehicle-only (DMSO) control and to the band C in the VX-809-treated cells, and represent the means ± SD. * p < 0.05.
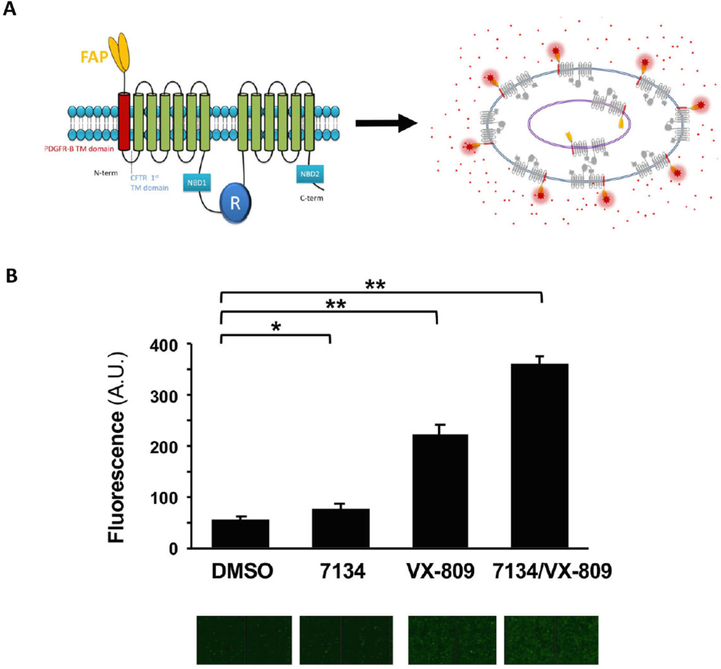
To verify these results using an alternate method, we made use of a cell line that expresses a fluorogen activating protein (FAP) fused to F508del-CFTR (Fig. 4A).32 When bound to FAP, the fluorescence of a malachite green derivative rises.33 Because the F508del-CFTR-FAP fusion was created so the FAP motif is exposed to the cell exterior only if F508del-CFTR traffics to the plasma membrane (Fig. 4A), and because the malachite green derivative is cell-impermeable, the fluorescent yield provides a direct and sensitive read-out of CFTR residence at the plasma membrane.32 Indeed, since the stable cells are less proficient at trafficking F508del-CFTR to the plasma membrane, the assay is an ideal confirmatory system to detect cell surface F508del-CFTR-FAP. As shown in Fig. 4B, administration of DMSO led to background cell surface fluorescence (quantified in top panels, with a raw image of malachite green-generated fluorescence shown below). While 7134 failed to increase cell surface residence, VX-809 led to a small but significant increase in F508del-CFTR at the surface. However, consistent with the western blot data in Fig. 3, the magnitude of the VX-809 effect rose further in the presence of 7134.
Fig. 4.
Compound 7134 increases the ability of VX-809 to facilitate the transport of an F508del-CFTR-fluorogen activating protein (FAP) fusion construct to the plasma membrane. (A) A model of the F508del-CFTR fusion protein. (B) Top, the relative amounts of malachite green fluorescence bound to the extracellular FAP in an F508del-CFTR-FAP fusion protein were determined as described32,34 and were calculated from 7 independent replicates. Final concentrations of 7134 and VX-809 were 10 μM. Mean ± SD values for each condition are shown; * p = 0.005, ** p < 0.0001. Bottom, raw representative images of cells in a 96-well dish from each condition are shown. The panel in part A was reproduced with permission.32
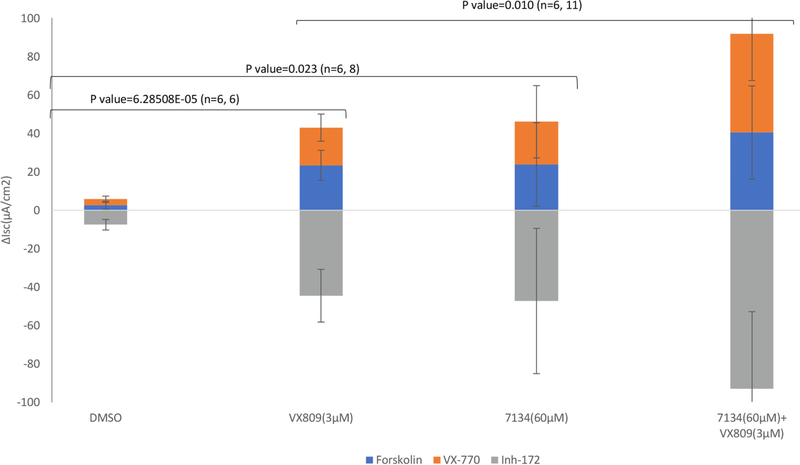
We previously reported that PYR-41 improves F508del-CFTR chloride current—and thus activity—in the presence of another F508del-CFTR corrector.21 Therefore, we explored whether 7134 could similarly facilitate not only F508del-CFTR trafficking but also activity at the plasma membrane when combined with VX-809 (Lumacaftor). We first confirmed that VX-809 improved CFTR-dependent current. As shown in Fig. 5, the activity was forskolin- (i.e., PKA-) dependent and was enhanced by VX-770 (Kalydeco), an FDA-approved CFTR potentiator35 (blue and orange bars, respectively). In contrast, a CFTR channel inhibitor, Inh-172,36 abolished activity (grey bars). Next, we examined 7134-dependent activation of F508del-CFTR. Although this compound exhibited minimal effects on the conversion of band B to band C when added to HEK293 or CFBE cells (Fig. 3), there was a modest increase in forskolin-dependent current in the presence of 7134 that was activated further by VX-770. This may arise from the ability of 7134 to support F508del-CFTR maturation in Fischer Rat Thyroid (FRT) cells, from the ability of band C below the detection level to support channel activity, and/or from the observation that band B/active CFTR can be detected at the plasma membrane in some cells.37 Regardless, consistent with the ability of 7134 to further stabilize and augment the maturation of F508del-CFTR in two other cell lines in the presence of VX-809 (Fig. 3 and 4), 7134 also increased VX-809-dependent channel activity in FRT cells. Combined with the data presented above, these results indicate that non-toxic inhibitors of CFTR ubiquitination can be obtained, and that when combined with a drug that promotes CFTR folding, increased restoration of channel transport and activity at the plasma membrane are evident.
Fig. 5.
Increased levels of active F508del-CFTR are produced with a combination of compound 7134 and VX-809. FRT cells expressing F508del-CFTR were plated at a final concentration of 110,000 cells/well and incubated with the indicated compounds or DMSO for 24 h. Short-circuit currents (Isc) were measured as described21 such that apical chloride levels were lower relative to the basolateral surface. Amiloride was also present to inhibit the epithelial sodium channel.38 The final concentrations of VX-809 and 7134 were 3 μM and 60 μM, respectively, which yielded maximal activity (data not shown). To confirm CFTR-specific current, cells were treated sequentially with forskolin (5 μM), VX-770 (5 μM), and CFTR Inh-172 (10 μM) where indicated. The number of replicates and p values are shown.
Overall, we set out to identify PYR-41 derivatives that could similarly inhibit F508del-CFTR polyubiquitination, enhance F508del-CFTR maturation in the presence of a folding corrector, and improve the activity of the corrected F508del-CFTR protein at the plasma membrane. Instead of using tool compounds that corrected the F508del-CFTR folding defect, an FDA-approved corrector (VX-809) was employed in this study. By identifying and characterizing a range of chemicals based on the parent compound, PYR-41, we were not only able to identify active compounds, but also those that were significantly less toxic. These studies confirm that modest inhibition of F508del-CFTR polyubiquitination improves the effects of protein folding correctors, a phenomenon that may be applicable to other protein conformational diseases for which folding correctors are being sought.39,40 Our work also provides a commercially available, expanded, and less toxic repertoire of inhibitors of protein ubiquitination to the research community.
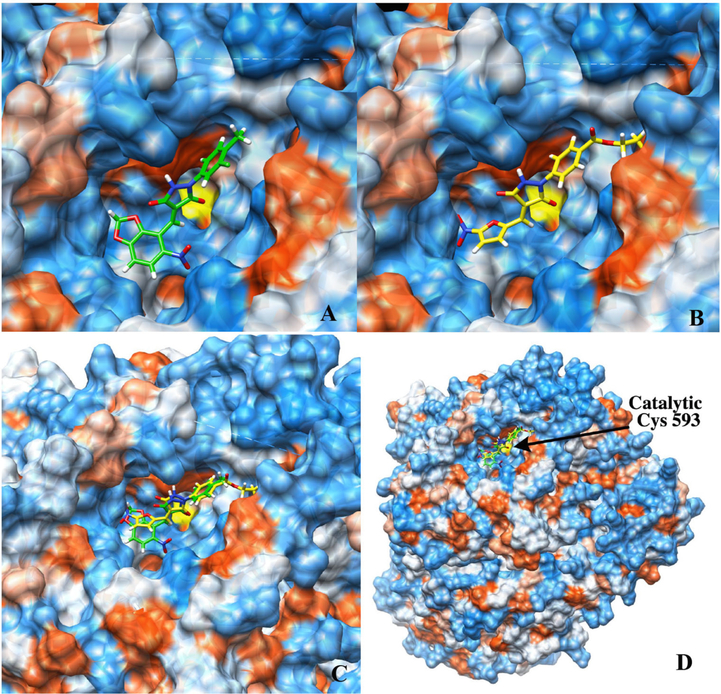
Nevertheless, it is possible that the newly identified lead compound, 7134, might in principle act on another component of the proteostasis pathway (i.e., independent of E1 ubiquitin activating enzyme). In fact, it is even possible that 7134 acts directly on CFTR, since F508del-CFTR-generated currents were heightened in the presence of 7134 (Fig. 5). While these possibilities are not trivial to exclude, we sought to build confidence in the fact that Pyr-41 and 7134 act at the same site within the E1 enzyme. To this end, we performed modeling studies with each compound and used the UCSF Chimera program41 to dock PYR-41 and 7134 within the Uba1 ubiquitin activating enzyme from S. pombe. Notably, this structure captured an active complex that included both the E1, an E2, and conjugated ubiquitin.42 As shown in Fig. 6, the molecules bound to a distinct pocket near the active site Cys, Cys-593 (also see Table S2 for key residues contacted by each molecule). Notably, both compounds formed extensive hydrogen bond networks within this pocket, often with the same residues (e.g., His-84, Asn-597, and Asn-681; Table S2). While future studies are needed to experimentally confirm these predicted binding sites, our in silico data strongly suggest that PYR-41 and 7134 exhibit the same mechanism of action. These data will also prove valuable to further refine the SAR for this emerging class of E1 inhibitors.
Fig. 6.
PYR-41 and 7134 bind similar sites in an E1 ubiquitin activating enzyme. (A) Localization of analog 7134 in a pocket near the active site Cys residue (Chimera docking score of −7.2). (B) Localization of PYR-41 in the same small molecule binding pocket (Chimera docking score of −7.3). (C) (D) Overlay of docked poses of 7134 and PYR-41. The position of the catalytic Cys (Cys-593) is indicated in (D). Molecular graphics and analyses were performed with UCSF Chimera developed by the Resource for Biocomputing, Visualization, and Information at the University of California, San Francisco. Protein target Ube1 (PDB ID: 5KNL) was truncated to chains A, B, and C. The binding site was determined based on overall accessibility of the ligand to the protein. Structures of PYR-41 and 7134 were minimized using the RHF-3–21G(*) basis set prior to docking, then docked to the protein within a 22 Å cube centered at −17.9410, −69.6827, and −19.7044 near Cys-593. Minimization of the best docked score for each was performed using 1000 steepest decent steps and 100 gradient steps within Chimera to obtain the final docking pose.
It is important to emphasize that the mechanism of action of E1 ubiquitin activating enzymes is complex, requiring several distinct activities and undergoing major structural rearrangements during the catalytic cycle.43 First, upon binding ATP and ubiquitin, the enzyme liberates pyrophosphate and then adds an AMP moiety onto the C-terminus of a bound ubiquitin molecule. Second, the ubiquitin-AMP conjugate is attacked by an active site Cys (which is depicted in Fig. 6), thereby forming an E1-ubiquitin intermediate. Third, after the enzyme undergoes a structural re-orientation, the ubiquitin is transferred onto the active site of an E2 enzyme, which has bound and engaged a distal site on the E1. Fourth, this final “transthioesterification” also requires subsequent major conformational alterations. And fifth, the E1 binds a second ubiquitin, which is thought to effect a conformational change. As a result of the complex nature of this reaction cycle, PYR-41 and 7134 might alter any or more than one of these steps.
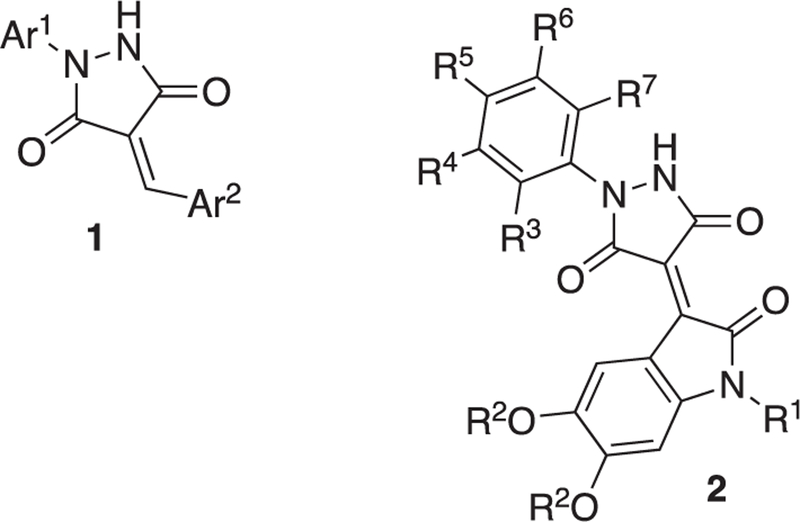
Finally, based on our data, several conclusions about an emerging SAR can be made. First, the compound with a nitro-furan that is quite similar to PYR-41, 4057, as well as 9731 and 0855, which are identical to PYR-41, were toxic, whereas those with the furan alone (6303, 8112, 4743) were not toxic. Indeed, the 5-nitrofuran moiety has been associated with significant off-target effects in cells and in vivo.44 It is therefore not surprising that these compounds show considerable (<22 μM) toxicity. The moderate toxicity observed for 4976, 0637, 5129, and 0605 does not follow a uniform structural pattern, but might be loosely associated with an electron-deficient Ar2 substituent (Fig. 7), which, of course, increases the electrophilicity of the 4-methylidenepyrazolidine-3,5-dione core structure. We also note—with a single exception (9100)—the pharmacophore in this series is composed of a 4-arylidene-1-arylpyrazolidine-3,5-dione (1). While not considered a scaffold known for toxic metabolites or aggregators, this moiety is flagged as potentially promiscuous in screening campaigns by machine learning modules.45 Furthermore, it is interesting to note that 7134 and 3670, which are not toxic and significantly inhibited F508del-CFTR ubiquitination, have relatively more electron-rich Ar2 substituents. Instead, the active but slightly toxic compound, 0637, has a 2-indolinone residue attached in the 3-position, whereas the closely related 4976 has a 3-indolinone attached at the 2-position of the heterocycle and shows decreased activity at equivalent toxicity. Taken together, an opportunity for a future round of SAR studies would be to further optimize substituent effects on the 2-indolinone in 0637, namely attaching electron-donating groups to the aromatic ring, for example R2 = Me, Et, or –CH2-, as shown in compound 2, and substituents on nitrogen (R2 = Me, Et, c-C3H7). In addition, the Ar1 substituent frequently bears halogen and ester functionalities, but neither one of these groups is clearly correlated with significant toxicity or rescue activity, suggesting an additional opportunity for further SAR investigations. Thus, in analog 2, R2 through R7 could be alternated from H through F, Cl, CN, Me, to MeO groups to fine-tune biological properties. In other future studies, it will also be critical to identify compounds that are more potent and lack the potential to become covalently attached to their target via a Michael addition. Michael acceptors are also metabolically unstable in cells.46 Overall, these studies set the stage for further work to improve the ability of Trikafta to repair F508del-CFTR by co-administration of a ubiquitination inhibitor. In addition to the fact that Trikafta fails to fully restore F508del-CFTR folding,12,13 some CF patients fail to benefit from this drug combination and associated toxicities can limit its use.47 Thus, tempering F508del-CFTR degradation via the use of a ubiquitination inhibitior is expected to improve Trikafta, potentially allowing for the administration of lower doses and greater efficacy.
Fig. 7.
Scaffolds and structural features for further development of an SAR.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants GM131732 and DK079307 and grant BRODSK18G0 from the Cystic Fibrosis Foundation to J.L.B., by National Institutes of Health grant HL139876 to E.J.S., and by National Institutes of Health grant DK068196 and grant FRIZZE13XX0 from the Cystic Fibrosis Foundation to R.A.F. We also thank John Mathias and Alexandra Taylor for technical assistance and support.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmcl.2021.128243.
References
- 1.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79: S215–255. [DOI] [PubMed] [Google Scholar]
- 2.Cheng SH, Gregory RJ, Marshall J, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63: 827–834. [DOI] [PubMed] [Google Scholar]
- 3.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. [DOI] [PubMed] [Google Scholar]
- 4.Jensen TJ, Loo MA, Pind S, Williams DB, Goldberg AL, Riordan JR. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. [DOI] [PubMed] [Google Scholar]
- 5.Sato S, Ward CL, Krouse ME, Wine JJ, Kopito RR. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem. 1996;271: 635–638. [DOI] [PubMed] [Google Scholar]
- 6.Brown CR, Hong-Brown LQ, Biwersi J, Verkman AS, Welch WJ. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones. 1996;1:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. [DOI] [PubMed] [Google Scholar]
- 8.Hoelen H, Kleizen B, Schmidt A, et al. The primary folding defect and rescue of DeltaF508 CFTR emerge during translation of the mutant domain. PLoS ONE. 2010;5, e15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du K, Sharma M, Lukacs GL. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol. 2005;12:17–25. [DOI] [PubMed] [Google Scholar]
- 10.Lukacs GL, Verkman AS. CFTR: folding, misfolding and correcting the DeltaF508 conformational defect. Trends Mol Med. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins FS. Realizing the dream of molecularly targeted therapies for cystic fibrosis. N Engl J Med. 2019;381:1863–1865. [DOI] [PubMed] [Google Scholar]
- 12.Middleton PG, Mall MA, Drevinek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381:1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisert KBPC Heltshe SL, Cooke G, Jorth P, Grogan B, Launspach J, Gallagher C, Welsh M, Accurso FJ, Hoffman L, McKone EF, Singh P. Long-term follow-up of CF patients with G551D mutations and chronic Pseudomonas infections treated wth Ivacaftor. Pediatr Pulmonol. 2016;51:S299. [Google Scholar]
- 15.Volkova N, Moy K, Evans J, et al. Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J Cyst Fibros. 2020; 19:68–79. [DOI] [PubMed] [Google Scholar]
- 16.Hisert KB, Heltshe SL, Pope C, et al. Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function Reduces Airway Bacteria and Inflammation in People with Cystic Fibrosis and Chronic Lung Infections. Am J Respir Crit Care Med. 2017;195:1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelman MS, Kannegaard ES, Kopito RR. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:11709–11714. [DOI] [PubMed] [Google Scholar]
- 18.Gelman MS, Kopito RR. Rescuing protein conformation: prospects for pharmacological therapy in cystic fibrosis. J Clin Invest. 2002;110:1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grove DE, Fan CY, Ren HY, Cyr DM. The endoplasmic reticulum-associated Hsp40 DNAJB12 and Hsc70 cooperate to facilitate RMA1 E3-dependent degradation of nascent CFTRDeltaF508. Mol Biol Cell. 2011;22:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grove DE, Rosser MF, Ren HY, Naren AP, Cyr DM. Mechanisms for rescue of correctable folding defects in CFTRDelta F508. Mol Biol Cell. 2009;20:4059–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung WJ, Goeckeler-Fried JL, Havasi V, et al. Increasing the endoplasmic reticulum pool of the F508del allele of the cystic fibrosis transmembrane conductance regulator leads to greater folding correction by small molecule therapeutics. PLoS One. 2016; 11, e0163615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Kitagaki J, Dai RM, et al. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007;67:9472–9481. [DOI] [PubMed] [Google Scholar]
- 23.Nepali K, Lee HY, Liou JP. Nitro-group-containing drugs. J Med Chem. 2019;62: 2851–2893. [DOI] [PubMed] [Google Scholar]
- 24.Dang NL, Hughes TB, Miller GP, Swamidass SJ. Computational approach to structural alerts: furans, phenols, nitroaromatics, and thiophenes. Chem Res Toxicol. 2017;30:1046–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estabrooks SK, Brodsky JL. Ubiquitination of disease-causing CFTR variants in a microsome-based assay. Anal Biochem. 2020;604, 113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatsukasa K, Huyer G, Michaelis S, Brodsky JL. Dissecting the ER-associated degradation of a misfolded polytopic membrane protein. Cell. 2008;132:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estabrooks S, Brodsky JL. Regulation of CFTR biogenesis by the proteostatic network and pharmacological modulators. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Younger JM, Chen L, Ren HY, et al. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126: 571–582. [DOI] [PubMed] [Google Scholar]
- 29.Nakatsukasa K, Brodsky JL. in vitro reconstitution of the selection, ubiquitination, and membrane extraction of a polytopic ERAD substrate. Methods Mol Biol. 2010; 619:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang AN, Liang M, Dominguez-Meijide A, et al. Synthesis and evaluation of esterified Hsp70 agonists in cellular models of protein aggregation and folding. Bioorg Med Chem. 2019;27:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. PNAS. 2011;108: 18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen MB, Hu J, Frizzell RA, Watkins SC. Simple image-based no-wash method for quantitative detection of surface expressed CFTR. Methods. 2016;96:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szent-Gyorgyi C, Schmidt BF, Creeger Y, et al. Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nat Biotechnol. 2008;26:235–240. [DOI] [PubMed] [Google Scholar]
- 34.Holleran JP, Glover ML, Peters KW, et al. Pharmacological rescue of the mutant cystic fibrosis transmembrane conductance regulator (CFTR) detected by use of a novel fluorescence platform. Mol Med. 2012;18:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Goor F, Straley KS, Cao D, et al. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–1130. [DOI] [PubMed] [Google Scholar]
- 36.Ma T, Vetrivel L, Yang H, et al. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem. 2002;277:37235–37241. [DOI] [PubMed] [Google Scholar]
- 37.Yoo JS, Moyer BD, Bannykh S, Yoo HM, Riordan JR, Balch WE. Non-conventional trafficking of the cystic fibrosis transmembrane conductance regulator through the early secretory pathway. J Biol Chem. 2002;277:11401–11409. [DOI] [PubMed] [Google Scholar]
- 38.Qadri YJ, Rooj AK, Fuller CM. ENaCs and ASICs as therapeutic targets. Am J Physiol Cell Physiol. 2012;302:C943–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong DS, Kelly JW. Chemical and/or biological therapeutic strategies to ameliorate protein misfolding diseases. Curr Opin Cell Biol. 2011;23:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng EC, Pettersen EF, Couch GS, Huang CC, Ferrin TE. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinf. 2006;7:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv Z, Rickman KA, Yuan L, et al. S. pombe Uba1-Ubc15 structure reveals a novel regulatory mechanism of ubiquitin E2 activity. Mol Cell. 2017;65:699–714 e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 2009;10:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Ishizaki H, Spitzer M, et al. ALDH2 mediates 5-nitrofuran activity in multiple species. Chem Biol. 2012;19:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stork C, Chen Y, Sicho M, Kirchmair J. Hit Dexter 2.0: machine-learning models for the prediction of frequent hitters. J Chem Inf Model. 2019;59:1030–1043. [DOI] [PubMed] [Google Scholar]
- 46.Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. [DOI] [PubMed] [Google Scholar]
- 47.Griese M, Costa S, Linnemann RW, et al. Safety and efficacy of elexacaftor/tezacaftor/ivacaftor for 24 weeks or longer in people with cystic fibrosis and one or more f508del alleles: interim results of an open-label phase 3 clinical trial. Am J Respir Crit Care Med. 2021;203:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.