Abstract
Background:
Rheumatic heart valve disease (RHVD) is a leading cause of cardiovascular death in low-middle-income countries and affects predominantly women. However, the underlying mechanisms of valvular damage remain scant, and regulators of sex predisposition are unknown.
Methods:
Proteomics analysis of human heart valves (Non-Diseased Aortic Valves/NDAV; Non-Diseased Mitral Valves/NDMV; Rheumatic Aortic Valve Disease/RAVD; Rheumatic Mitral Valve Disease/RMVD; n=30), followed by system biology analysis, identified prothymosin alpha (ProTα) as a protein associated with RHVD. Histology, multiparameter flow cytometry and ELISA confirmed the expression of ProTα. In vitro experiments using peripheral mononuclear cells and valvular interstitial cells (VICs) were performed using multiparameter flow cytometry and qPCR. In silico analysis of the RHVD and S. pyogenes proteomes were employed to identify mimic epitopes.
Results:
A comparison of NDMV and NDAV proteomes established the baseline differences between non-diseased aortic and mitral valves. Thirteen unique proteins were enriched in NDMV. Comparison of NDMV vs. RMVD and NDAV vs. RAVD identified 213 proteins enriched in rheumatic valves. The expression of the 13 NDMV-enriched proteins was evaluated across the 213 proteins enriched in diseased valves, resulting in the discovery of ProTα common to RMVD and RAVD. ProTα plasma levels were significantly higher in RHVD than healthy individuals. Immunoreactive ProTα colocalized with CD8+ T-cells in RHVD. Expression of ProTα and estrogen receptor alpha (ERα) correlated strongly in circulating CD8+ T-cells from RHVD patients. Recombinant ProTα induced expression of lytic proteins: perforin and granzyme-B, by CD8+ T-cells as well as higher ERα expression. In addition, recombinant ProTα increased levels of HLA-I in VICs. Treatment of CD8+ T-cells with specific ERα antagonist reduced cytotoxic potential promoted by ProTα. In silico analysis of RHVD and S. pyogenes proteomes revealed cross-reactivity between human type 1 collagen epitope and bacterial collagen-like protein, which induced CD8+ T-cells activation in vitro.
Conclusions:
ProTα-dependent CD8+ T-cell cytotoxicity was associated with ERα activity, implicating ProTα as a potential regulator of sex predisposition in RHVD. Moreover, ProTα facilitated recognition of type 1 collagen cross-reactive epitopes by CD8 T-cells, suggesting mechanisms provoking autoimmunity.
Keywords: Rheumatic heart valve disease, prothymosin alpha, autoimmunity, CD8+ T-cells, cytotoxicity
Introduction
Acute rheumatic fever (ARF) is triggered by pharyngeal Streptococcus pyogenes infection. Subsequently to ARF episodes, predisposed individuals can develop permanent damage of cardiac valves, characteristic of rheumatic heart disease.1 In resource-poor settings around the world, rheumatic heart valve disease (RHVD) is the leading cause of cardiovascular death in children and young adults.1,2 Because studies on RHVD are scarce, little progress has been made in this field, and no effective drug therapies are thus available,2,3 which have driven our research investigations. Although both aortic and mitral valves may be affected in RHVD, mitral valve damage is generally predominant.4
While ARF affects multiple organs depicted by transient inflammatory changes, RHVD is characterized by long-term valvular damage.5 The current notion of how S. pyogenes triggers a host-directed autoimmune response is based on molecular mimicry, the presence of common epitopes in the pathogen and the human host.6,7 However, this hypothesis does not explain why the host immune response specifically affects valvular tissue while sparing the myocardium.8 The natural history of disease suggests that immune components drive the generation and release of autoantigens in the tissue lesion, thereby boosting the immune response. Collagen may serve as an autoantigen in both ARF and RHVD, which could partially explain both the systemic and heart valves-specific manifestations.7,8 Recent reports demonstrate cardiac myosin as one of the major proteins in heart, which cross reacts with the group A carbohydrate or streptococcal M protein antigens.9 As is the case in many other autoimmune diseases, RHVD has higher prevalence in women.10 Sex hormones can affect the immune response by modulating gene expression through estrogen receptor alpha (ERα) stimulation.11 A direct role for estradiol in the development of autoimmune T-cell responses has been previously described.4,12 Although most of the studies were performed in CD4+ T-cells, it has become evident that CD8+ T-cells also play major roles as effectors of autoimmunity.13,14
In the present study, we identified prothymosin alpha (ProTα) as a key molecule contributing to RHVD immunopathogenesis using global proteomics, network analysis and comprehensive ex vivo and in vitro validation studies. This protein increases the expression of cytotoxic molecules by CD8+ T-cells associated with higher estrogen receptor alpha (ERα) activity, implicating a role of ProTα in sex predisposition in RHVD. In addition, our in silico analysis revealed molecular mimicry between human type 1 collagen and S. pyogenes collagen-like surface protein, which can be recognized by host CD8+ T-cells and thus, drive a host-directed autoimmune response against the heart valves.
Patients and Methods
The authors declare that all supporting data and detailed methods are available withing the article and its online Supplementary files.
Human heart valves global proteomics and selection of a protein associated with RHVD
A total of 30 human aortic and mitral valve leaflets were obtained from failing transplant hearts (Non-Diseased Aortic Valves/NDAV, n=6; Non-Diseased Mitral Valves/NDMV, n=6) and from replacement surgeries for RHVD (Rheumatic Aortic Valve Disease/RAVD, n=4; Rheumatic Mitral Valve Disease/RMVD, n=9) (Universidade Federal de Minas Gerais CAAE protocol #:32715214.9.0000.5149). Our patient cohort showed a predominance of RHVD in women (Supplementary Table I), and therefore our specimens were derived predominantly from female cases. Human aortic valve leaflets undergoing replacement due to Calcific Aortic Valve Disease (CAVD, n=5) were included as diseased controls (Brigham and Women’s Hospital IRB protocol #:2011P001703). All subjects gave informed consent for publication and sample collection. Samples were prepared for label-free global proteomics using liquid chromatography–tandem mass spectrometry performed on the Orbitrap Fusion Lumos (Online Data Supplement). Differentially expressed proteins in RHVD were identified, and prediction of their role in disease pathophysiology was explored using computational analysis. Network centrality score and enriched pathways analysis allowed the identification of ProTα as a protein associated with RHVD, to be further validated by ex vivo and in vitro approaches.
Ex vivo and in vitro assays
ProTα expression was confirmed ex vivo and its impact on leukocyte function was evaluated in vitro. Valvular tissue, peripheral mononuclear cells, and plasma samples from RHVD patients were analyzed using histology, multiparameter flow cytometry and ELISA, respectively. In vitro gain- and loss-of-function experiments were performed on peripheral mononuclear cells by multiparameter flow cytometry and on VICs by qPCR (Online Data Supplement).
In silico analysis for prediction of cross-reactive CD8 T-cell epitopes
Sequential in silico analysis was used to identify potential cross-reactive CD8 T-cell epitopes. First, multiple protein sequence alignment was used to verify the similarities between human type 1 collagen protein and S. pyogenes (serotype M1) collagen-like surface protein. Sequences of 9 homologous amino acids regions between human and S. pyogenes proteins were then used to predict CD8 T-cells epitopes. Subsequent analysis evaluated the ability of the peptides to bind to human leukocyte antigen class I (HLA-I). Additionally, we used a predictor tool (Online Data Supplement) to predict the relative ability of a peptide/HLA complex to elicit an immune response. Finally, immunogenic epitopes were selected for peptide docking to compare the binding pattern of human peptide and its bacterial homologue (Online Data Supplement).
Statistical Analysis
Differential proteome abundances (Online Data Supplement) were calculated using a two-group comparison by adjusting individual p values from every pairwise comparison (NDAV vs. NDMV; NDAV vs. RAVD; NDMV vs. RMVD and NDAV vs. CAVD). False discovery rate (FDR) was calculated using Percolator provided by Proteome Discoverer (PD) Package (version 2.2, Thermo Scientific) and peptides were filtered based on a 1.0% FDR. q values < 0.1 were considered statistically significant. Enriched pathways analysis using the differentially expressed proteins in the omics datasets were considered statistically significant when adjusted p values were ≤0.05. Hypergeometric tests were used to compute the p values and FDR correction was done for multiple testing. For ex vivo data, two-group comparisons between non-diseased and disease valves (NDAV vs. RAVD and NDMV vs. RMVD) were made using an unpaired t-test. For in vitro data, comparisons were made using two-way ANOVA followed by Tukey’s test. Normality distribution was assessed in each data set using Shapiro-Wilk and Kolmogorov-Smirnov tests. Correlation analyzes were performed using Pearson’s coefficient. P ≤ 0.05 were considered statistically significant. Group variances were verified using F test. For in silico data, epitopes were considered immunogenic when “Class I immunogenicity score” was >0.
Results
Unbiased proteomics and network analysis reveal ProTα as a novel protein associated with RHVD
We first compared NDAV and NDMV proteomes to identify proteins that could underpin the mitral valve’s predisposition to RHVD. Thirteen proteins were found enriched in NDMV (A6NLG9, FHL2, B4DI63, LOX, NDUFV2, ABI3BP, RAN, B2R9V7, CKM, DPT, MB, Q5U0B9 and PTMA/ProTα; q<0.1) (Figure 1A, left panel). To verify if these 13 NDMV-enriched proteins could be associated with RHVD progression, we evaluated their abundances across diseased valve (RAVD, RMVD and CAVD) proteomes. Comparisons of NDAV vs. RAVD and NDMV vs. RMVD proteomes showed 64 and 167 altered proteins (q<0.1) in RAVD and RMVD, respectively (Figure 1A, middle panels). Of the 13 proteins enriched in NDMV over NDAV, we found that 4 proteins (LOX, B4DI63, DPT and PTMA/ProTα) were also significantly enriched in RAVD, while another 4 proteins (DPT, LOX, FLH2 and PTMA/ProTα) were enriched in RMVD (q<0.1) (Figure 1A, middle panels). Collectively, a total of 213 proteins were altered in RHVD as depicted in the Venn diagram (Figure 1B). We also compared NDAV and CAVD proteomes in order to select targets specifically associated with RHVD. 397 proteins were altered by CAVD-associated stenosis (q<0.1); these included 4 proteins (LOX, FLH2, Q5U0B9 and CKM), which were also found among 13 NDMV-enriched proteins (Figure 1A, right panel).
Figure 1: Whole tissue proteomics and network analysis of non-diseased, rheumatic, and calcific human heart valves.
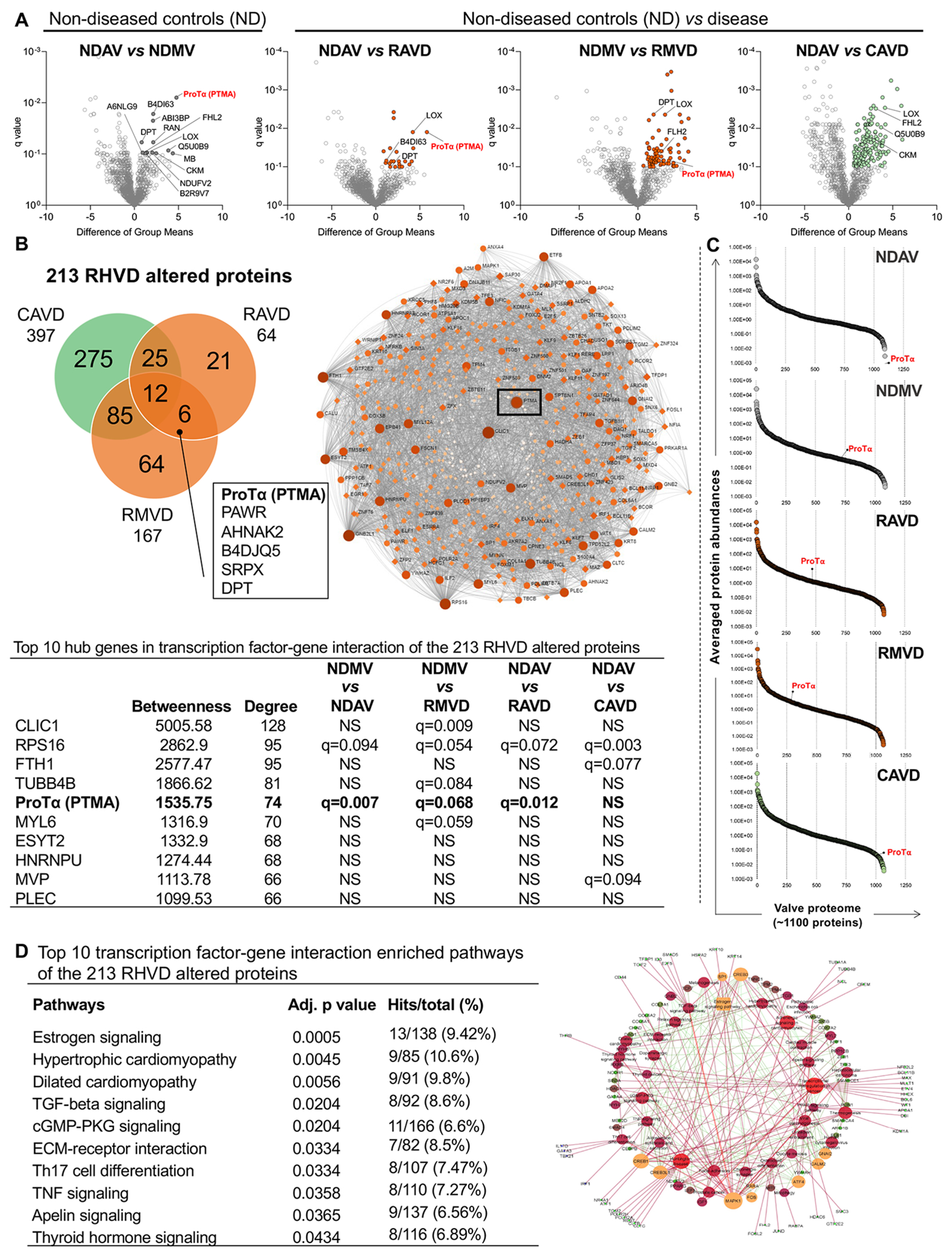
A, Volcano plots comparing ‘difference of group means’ of protein abundances in the valvular tissue proteome per ‘q value’. Group comparisons are indicated for each volcano plot. Significantly up-regulated genes are highlighted (q<0.1). B, Venn diagram showing the intersection of altered proteins from rheumatic and calcific heart valves proteomes. Proteins at the intersection of each pair of group comparison are shown. Box shows the common 6 proteins altered in both rheumatic aortic and mitral proteomes. C, Percentile ranking plots of averaged protein abundances of the valvular tissue proteomes for each group. ProTα location is mapped in each percentile rank plot. Transcription-factor network analysis shows that ProTα as a hub gene in RHVD altered proteome (q<0.1). D, Top 10 transcription-factor pathway analysis overrepresented in RHVD altered proteome (q<0.1). Abbreviations: NDAV, non-diseased aortic valves (n=6); NDMV, non-diseased mitral valves (n=6), RAVD, rheumatic aortic valve disease (n=4), RMVD, rheumatic mitral valve disease (n=9); CAVD, calcific aortic valve disease (n=5). Significantly differentially enriched proteins were calculated using a two-group comparison by adjusting individual p values from every pairwise comparison for significance (q<0.1). Enriched pathways were considered statistically significant when adjusted p values were ≤0.05.
DPT and ProTα were enriched in both RAVD and RMVD and associated with RHVD as they were not enriched in CAVD. Transcription factor-gene interaction networks showed ProTα as the gene with fifth-highest degree of centrality among the 213 RHVD altered proteins, based on the parameters of “betweenness” and “degree” (Figure 1C). CLIC1, FTH1 and TUBB4B while showing higher centrality, did not display differences in our initial comparison between NDAV and NDMV. Despite being differentially expressed in RHVD and having high centrality parameters, RPS16 was also found altered in CAVD, and therefore, not specifically associated with RHVD (Figure 1B). Percentile rank plots in which each protein of the heart valve proteomes (~1100 proteins) vs. their averaged abundances were used to map ProTα in the different groups. ProTα showed “upward mobility”, moving from the first to the second percentile ranks between NDAV and NDMV. In RAVD and RMVD, ProTα moved to the fourth percentile rank (Figure 1C). In NAVD and CAVD, ProTα showed very low abundance (Figure 1C). ProTα, PAWR, AHNAK2, B4DJQ5, SRPX and DPT were common proteins altered in both RAVD and RMVD. Pathway analysis identified hypertrophic cardiomyopathy, inflammation and cell signaling pathways highly represented among RHVD altered proteins. The estrogen signaling pathway, however, was the one with highest representation among RHVD altered proteins (Figure 1D). Together, our data identified ProTα as a key protein associated with RHVD.
Ex vivo analysis of heart valves and peripheral blood mononuclear cells revealed association between ProTα and CD8+ T-cells in RHVD
Histological analysis demonstrated that immunoreactive ProTα was highly expressed in both RAVD (p=0.033) and RMVD (p=0.016) as compared to NDAV and NDMV, corroborating the proteomic findings (Figure 2A, left panel). The RMVD inset shows that ProTα+ cells are localized in areas rich in inflammatory infiltrates (Figure 2A, left panel), which display increased frequency of CD4+ and CD8+ as compared to NDAV and increased frequency of CD68+ cells as compared to NDAV and NDMV (Online-only Data Supplement, Figure IA). In rheumatic tissue, quadruple-labeled immunofluorescence showed co-localization of ProTα+ with inflammatory cells (CD45+), but not with alpha-smooth muscle actin-positive (αSMA+) cells (Figure 2A). In addition, valvular fibroblasts (vimentin+) lacked ProTα expression (Online-only Data Supplement, Figure IB. ProTα+ cells correlated positively with leaflet thickness in RMVD (r2=0.310, p=0.016) (Figure 2A, top right panel). RAVD and RMVD leaflet thickness was significantly higher as compared to the non-diseased valves (Online-only Data Supplement, Figure IC).
Figure 2: Ex vivo analysis of ProTα expression in heart valves and peripheral blood mononuclear cells in non-diseased and RHVD patients.
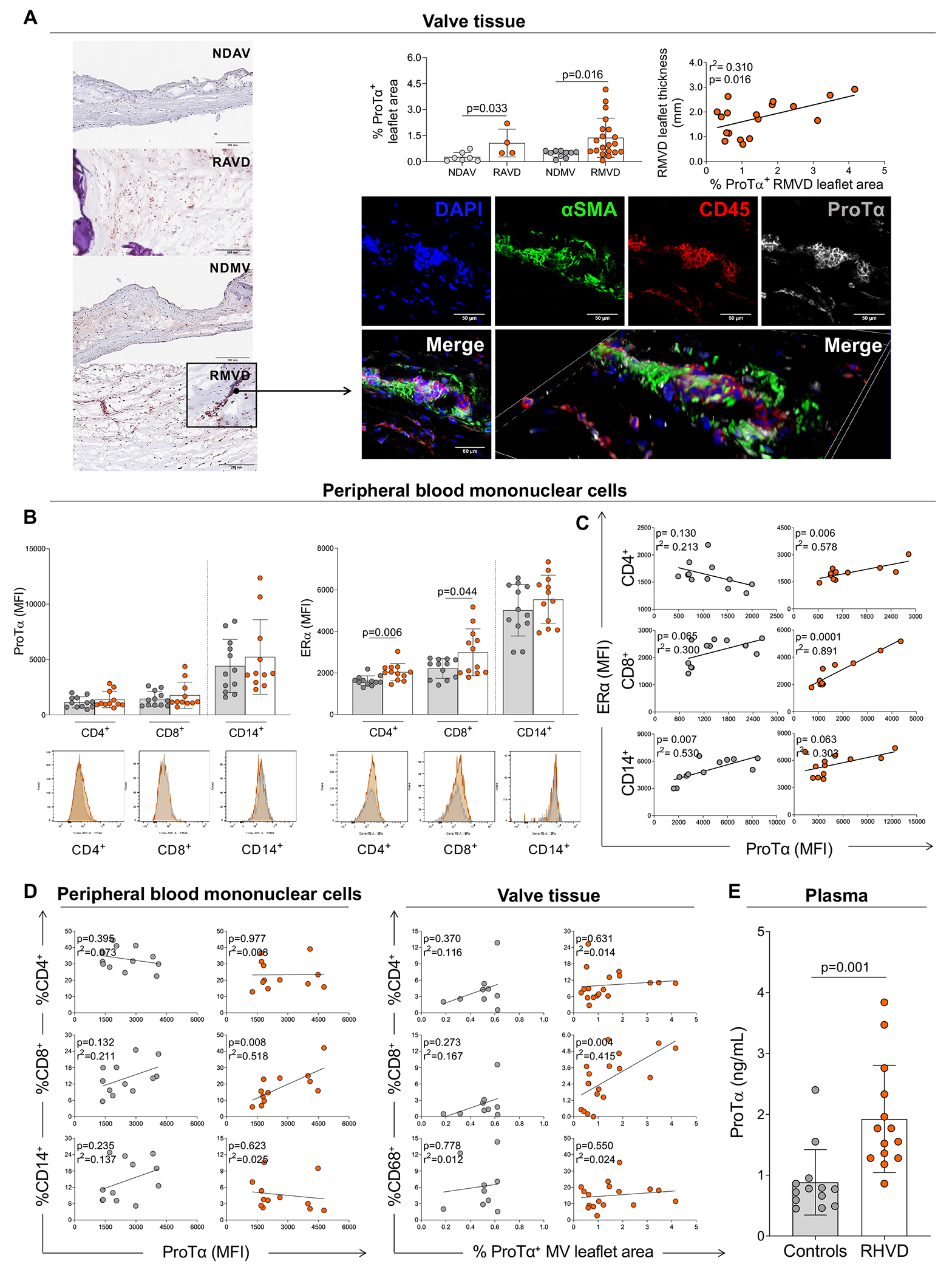
A, Histological evaluation of ProTα expression by immunohistochemistry staining in non-diseased aortic and mitral valves (NDAV, n=7 and NDMV, n=10) and rheumatic aortic and mitral valve disease (RAVD, n=4 and RMVD n=20). Quantification is reported as percentage of ProTα-positive area (% ProTα+ leaflet area) of the total leaflet area. Scale bars = 200 μm. Correlation analysis between RMVD leaflet thickness and ProTα+ RMVD leaflet area. Representative direct immunofluorescence staining for αSMA, CD45, and ProTα in RMVD. Scale bars = 50 μm. B, Median of intensity of fluorescence (MFI) of ProTα and estrogen receptor alpha (ERα) in healthy donors (gray dots) and rheumatic heart valve disease (RHVD) patients (orange dots) by different cell populations (CD4+, CD8+, and CD14+) in peripheral blood mononuclear cells. Representative histograms are show for each marker. C, Correlation analysis between MFI of ERα and ProTα by different cell populations (CD4+, CD8+, and CD14+) in peripheral blood mononuclear cells. D, Correlation analysis between percentage of CD4+, CD8+, and CD14+/CD68+ (valvular tissue/peripheral blood mononuclear cells) and ProTα. E, Plasma levels of ProTα in on health donors and RHVD patients. Bar graphs show the mean of values in each group and standard deviation. Two-group comparisons between non-diseased valves and disease valves were made using an unpaired t-test. Statistical significance is indicated in each graph.
Given the co-localization of ProTα with mononuclear cells in tissue and the previously proposed association of ProTα with the ERα transcriptional activity15, the expression of ProTα and ERα in peripheral blood mononuclear cells derived from healthy donors and RHVD patients was evaluated by multiparametric flow cytometry. ProTα expression did not differ in any of the three cell populations analyzed, including CD4+, CD8+ and CD14+ cells (Figure 2B). On the other hand, both CD4+ (p=0.004) and CD8+ (p=0.044) T-cells showed higher median intensity of fluorescence (MFI) of ERα in RHVD patients as compared to healthy controls. No differences in ERα expression in CD14+ cells were found (Figure 2B). ERα expression by CD4+ (r2=0.578, p=0.006) and CD8+ (r2=0.891, p=0.0001) T-cells and ProTα expression associated positively in RHVD patients but not in healthy donors (Figure 2C). ProTα expression was correlated with the frequency of circulating (r2=0.518, p=0.008) and valvular tissue (r2=0.415, p=0.004) inflammatory mononuclear cells only in CD8+ T-cells, but not in CD4+ or CD14+ T-cells (Figure 2D). Healthy donors had no association between the frequency of mononuclear inflammatory cells (CD4+, CD8+ or CD14+) and ProTα (Figure 2D). ProTα plasma levels were 2-fold higher in RHVD patients when compared to healthy donors (p=0.001) (Figure 2E).
Recombinant ProTα increases the expression of cytotoxic molecules and collagen binding ability by CD8+ T-cells accompanied by higher expression of ERα
Peripheral mononuclear cells were cultured to assess the effects of recombinant ProTα on T-cell function and ERα expression by flow cytometry. Cells were stimulated with estradiol (E2) alone or in combination with ProTα to verify the interference of sex hormones with the expression of the evaluated markers. CD8+ T-cells exert killing function by releasing cytotoxic granules containing granzyme-B (GzB) and perforin (Perf) or through the binding of the target cell membrane with the Fas ligand (FasL). First, we determined the frequency of CD8+ T-cells expressing these important functional molecules, as well as the effect of ProTα in the expression of such molecules. Gating strategies for flow cytometry analysis are shown in Figure 3A. ProTα lead to an increase in frequency of CD8+ T-cells expressing granzyme-B (%CD8+GzB+, p=0.017) and perforin (%CD8+Perf+, p=0.018) as compared to the non-stimulated cultures (CTRL) (Figure 3B). Because granzyme-B and perforin act synergistically in the cytotoxic pathway, we also evaluated the co-expression of these molecules in CD8+ T-cells (%CD8+GzB+Perf+) (Figure 3B). ProTα alone (p=0.015) and in combination with E2 (p=0.013) increased the frequency of CD8+GzB+Perf+ cells when compared to the CTRL (Figure 3B).
Figure 3: In vitro analysis of the effects of recombinant ProTα on CD8+ T-cells.
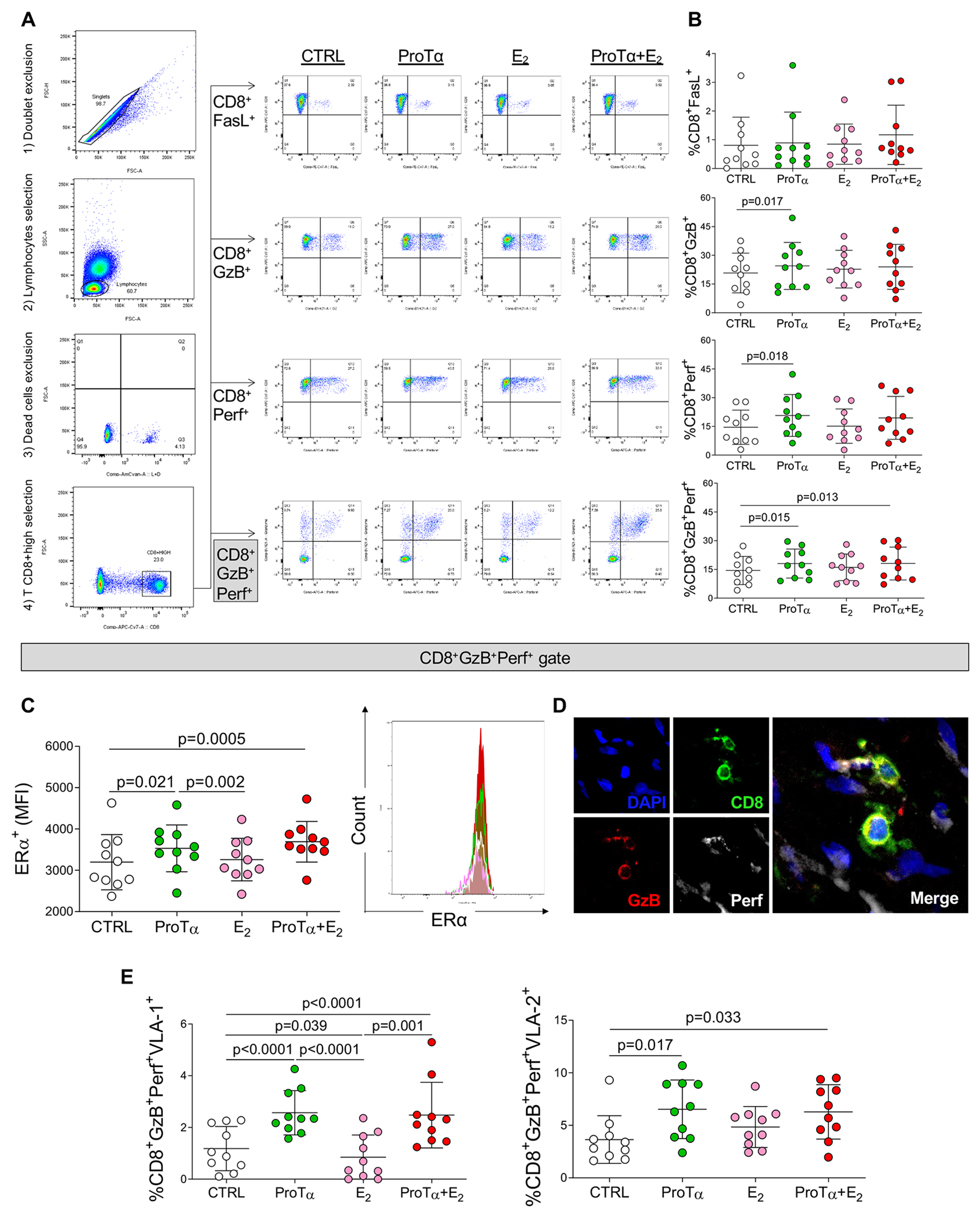
A, Representative gating strategy scatter plots to evaluate markers of cytotoxicity in CD8+ T-cells. After cell doublets exclusion, total lymphocytes region was gated based on cell size (forward scatter/FSC) and cell complexity (side scatter/SSC). Dead cells were excluded using a cell viability dye. CD8+ high cell population was gated to further analyze the markers of cytotoxicity: FasL, granzyme-B and perforin. Control cultures (non-stimulated, CTRL) are represented by white dots, ProTα stimulated cultures are represented by green dots, estradiol stimulated cultures (E2) are represented by pink dots, cultures stimulated by both ProTα and E2 (ProTα+E2) are represented by red dots. B, Frequency of CD8+ cells expressing FasL (CD8+FasL+), granzyme-B (CD8+GzB+), perforin (CD8+Perf+) and (CD8+GzB+Perf+). C, Mean intensity of fluorescence (MFI) of estrogen receptor alpha (ERα) gated in CD8+GzB+Perf+ cells. Representative histogram showing the MFI of ERα in the different culture conditions. D, Representative direct immunofluorescence staining for CD8, GzB and Perf in RHVD valvular lesion. Scale bars = 50 μm. E, Frequency of VLA-1 (CD8+GzB+Perf+VLA-1+) and VLA-2 (CD8+GzB+Perf+VLA-2+) gated in CD8+GzB+Perf+. Bar graphs show the mean of values in each group (n=10) and standard deviation. Comparisons among non-stimulated and stimulated cell cultures were made using two-way ANOVA followed by Tukey’s test. Statistical significance is indicated in each graph.
ERα MFI in CD8+GzB+Perf+ cells was higher in ProTα stimulated cultures (p=0.021), and even higher in the combination with E2 (p=0.0005), suggesting a greater activity of this receptor when both stimuli were combined (Figure 3C). ProTα-stimulated cultures (p=0.002) also showed higher ERα expression than E2-stimulated cultures (Figure 3C). Differences in ERα expression among the culture conditions can be better visualized in the representative histogram (Figure 3C, right panel). Figure 3D shows infiltrating CD8+ T-cells co-expressing granzyme-B and perforin in RHVD valvular lesion. Effects of ProTα on induction of expression of cytotoxic molecules by CD8+ T-cells were dose-dependent (Online-only Data Supplement, Figure II). Expression of the transcription factors associated with a pro-inflammatory T-cell phenotype (T-bet and RORγT) and regulatory phenotype (FOXP3) were not altered in CD4+ or CD8+ in any of the evaluated conditions (Online-only Data Supplement, Figure III).
To further understand whether ProTα influences CD8+ T-cells ability of interaction/recruitment to the valvular tissue, we evaluated the expression of collagen-binding alpha1beta1 (VLA-1) and alpha2beta1 (VLA-2) by circulating CD8+GzB+Perf+ cells. ProTα alone or in combination with E2 increased the frequency of both CD8+GzB+Perf+VLA-1+ cells (p<0.0001 and p=0.0001, respectively) and CD8+GzB+Perf+VLA-2+ cells (p=0.017 and p=0.033, respectively) (Figure 3E). ProTα-stimulated cultures also showed higher frequency of CD8+GzB+Perf+VLA-1+ cells than E2 stimulated cultures (p<0.0001). While the frequency of CD8+GzB+Perf+VLA-2+ was not altered with E2 stimuli, the frequency of CD8+GzB+Perf+VLA-1+ was decreased (p=0.039) (Figure 3E), suggesting that VLA-2 has higher pathological implication in RHVD. Together, these findings indicate that ProTα increased the expression of pathogenic responses of CD8+ T-cells through induction of cytotoxicity and collagen binding molecule VLA-2, which predominantly interacts with collagen type 1 (COL1) and laminin. Moreover, these effects were accompanied with increased ERα expression.
Human COL1A1 and COL1A2 and S. pyogenes collagen-like surface protein reveal mimic epitopes that can be recognized by CD8+ T-cells
Collagen is involved in a series of autoimmune disorders as also shown in RHVD8. Our proteomic analysis identified 17 members of collagen family in the human heart valve proteome (Figure 4A). Among them, 9 types of collagens differ in abundance between non-diseased and diseased valves (NDAV vs. RAVD and NDMV vs. RMVD) (Figure 4B). COL1A1, COL1A2, COL3A1, COL5A2 and CTHRC1 showed higher abundance in both RAVD and RMVD, as compared to NDAV and NDMV. Four out of 6 type of collagens (COL6A1, COL6A2, COL6A3, COL6A3) were lower in abundance in both RAVD and RMVD as compared to NDAV and NDMV. COL1A1 and COL1A2 were the most abundant types in these specimens (Figure 4B). Furthermore, RMVD had even higher COL1A1 (q=0.085) and COL1A2 (q=0.076) abundances as compared to NDMV, which can contribute to epitope spreading, a key phenomenon in progression of autoimmune diseases. The S. pyogenes (serotype M1) proteome available in the UniProt database (ID: UP000000750) contains a collagen-like surface protein among the bacterial membrane peptides (Figure 4C). We then investigated whether collagen peptide sequences are involved in RHVD molecular mimicry. This analysis was performed based on the identification of homologue epitope regions between human COL1A1 and COL1A2 and the S. pyogenes collagen-like surface protein. A total of 6 homologue peptide regions were detected (Figure 4C). Within each of these regions, we identified sequences of 9 peptides (9-mer) with higher binding ability to human leukocyte antigen class I (HLA-I) (highlighted in grey), which present antigens to CD8+ T-cells (Figure 4C). Capacity to proper binding HLA-I was measured by percentile rank score on different HLA alleles. We further performed an analysis based on the ability of each identified 9-mer to elicit an immune response (class I immunogenicity score >0). Epitopes 1, 2, 4 and 5 showed class I immunogenic scores > 0 (Figure 4C). Epitopes 1 and 2 were derived from COL1A1 sequences and can bind to HLA-A*03:01 alleles with percentile rank of 0.80 and 0.95, respectively. Epitopes 4 and 5 were derived from COL1A2 sequences with percentile rank scores of 0.81 and 0.84, respectively. We then assessed the immunogenicity of the corresponding 9-mer on S. pyogenes sequences; only the epitopes 2 and 5 showed class I immunogenic scores > 0.
Figure 4: Collagen expression in RHVD proteomes and identification of mimic epitopes with S. pyogenes “collagen-like surface protein”.
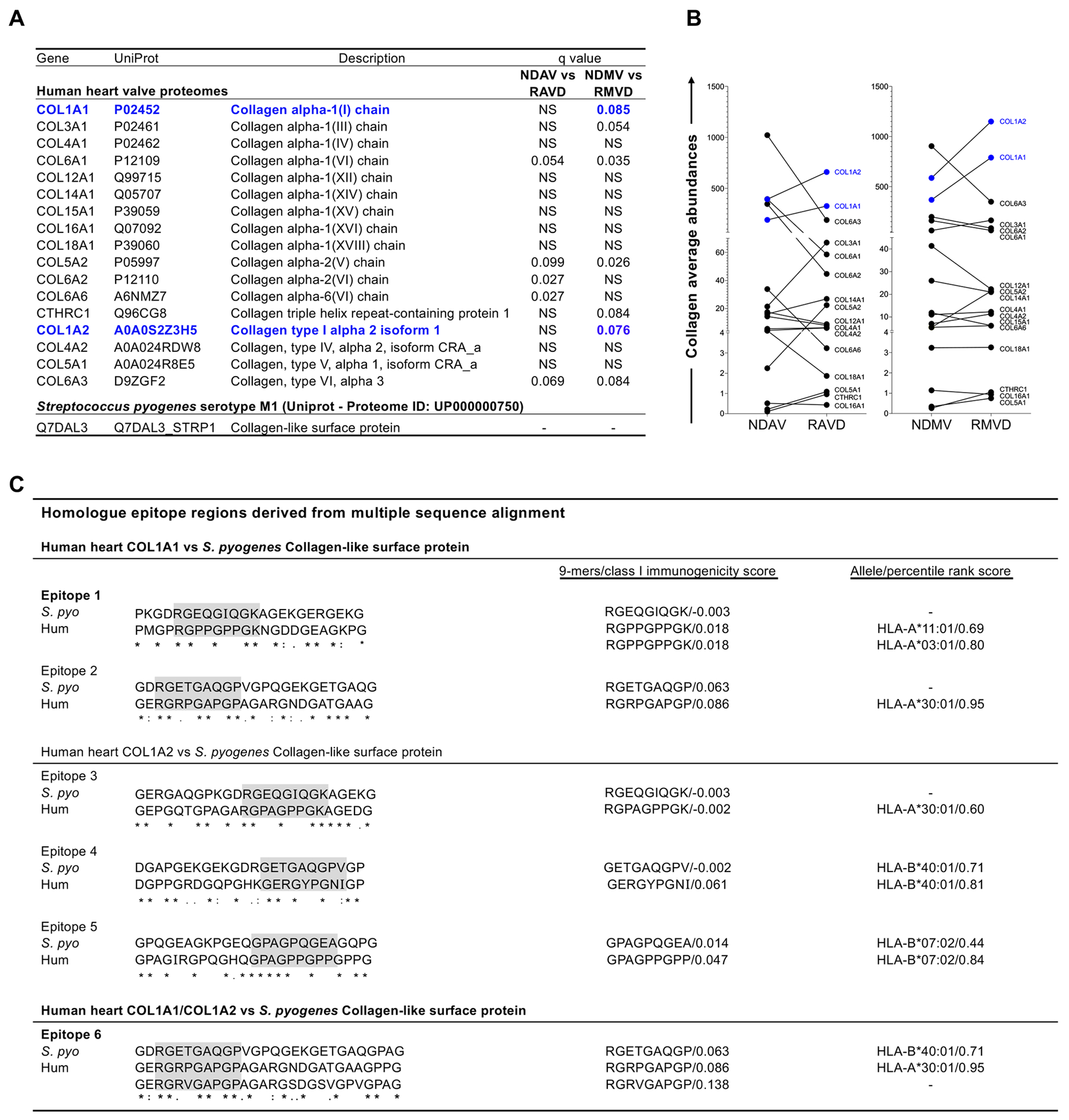
A, Types of collagens found in human heart valve proteomes and collagen-like surface protein found in S. pyogenes (serotype M1) proteome available in UniProt database. Statistical differences (q<0.1) between abundances of human collagen in non-diseased valves and RHVD valves (NDAV versus RAVD and NDMV versus RMVD) are shown. B, Graphs display the collagen average abundances between NDAV and RAVD and between NDMV and RMVD. Type 1 collagens (COL1A1 and COL1A2) are highlighted in blue. Significantly differentially enriched proteins were calculated using a two-group comparison by adjusting individual p values from every pairwise comparison for significance (q<0.1). C, Homologue epitope regions between human COL1A1 and COL1A2 and S. pyogenes “collagen-like surface protein” found in as derived by multiple sequence alignment. Identical amino acids are marked with an asterisk, highly conserved positions with a colon, and homologue positions with a period. Sequences of nine amino acids (9-mer) with higher ability of binding to human leukocyte antigen class I (HLA-I) are highlighted in grey. “Class I immunogenicity score” and “percentile rank score” are defined by the peptide ability to elicit immune response and capacity of properly bind in the HLA-I respectively.
Binding analysis of selected type 1 collagen mimic epitopes shows high binding efficiency to HLA-I groove
Lymphocyte activation is initiated by the interaction between peptides presented on HLA and T-cells. Mimic peptides can trigger autoimmune responses based on similar binding patterns on the antigen presenting molecules. Therefore, we analyzed the similarity in binding pattern between human and S. pyogenes sequences for 4 identified cross-reactive epitopes with respective HLA-I (HLA-A and HLA-B) (Figure 5A). Molecular docking analysis resulted in similar binding patterns of the bacterial collagen peptide and its homologous region in the human collagen protein. HPEPDock was used to input the pdb files of HLA-I and identified mimic peptides; this returned the top 100 docking models based on minimized docking energy scores. The top-scored models of minimized docking energy were analyzed and visualized for their molecular binding with the HLA-I receptors respective to mimic peptides on Chimera tool (Figure 5A). The obtained results determined that epitope 4 has the strongest binding energy score of −214.522 kJ/mol for human collagen peptide and epitope 1 has the strongest binding energy score of −191.517 kJ/mol for S. pyogenes collagen peptide, respectively.
Figure 5: Peptide docking of type 1 collagen mimic epitopes and effects of recombinant ProTα on HLA-I expression by VICs.
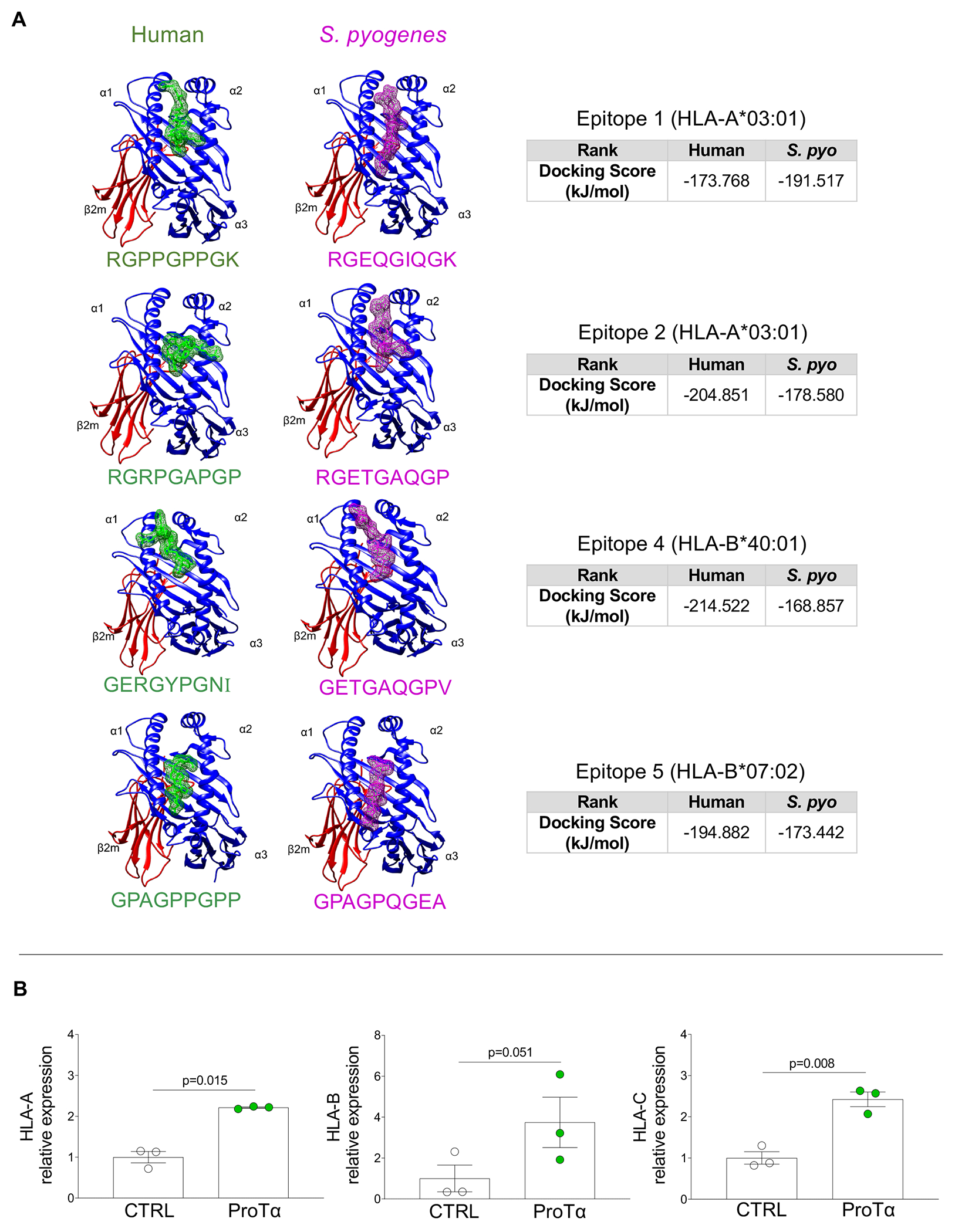
A, Peptide docking of four identified human collagen type 1 mimic epitopes (green) and homologous region on S. pyogenes (magenta) sequences showing their interaction on HLA-I groove. Peptides are positioned between α1 and α2 HLA-I domains. Binding energy scores are shown for each comparison pair. B, mRNA expression levels of HLA-A, B and by VICs non-stimulated (CTRL, white dots, n=3) and stimulated with recombinant ProTα for 24 hours (green, n=3). Bar graphs show the mean gene expression relative to the CTRL culture with error bars representing standard deviation. Two-group comparisons between non-stimulated and stimulated cell cultures were made using a paired t-test. Statistical significance is indicated in each graph.
ProTα increases HLA-I mRNA alleles in human valvular interstitial cells
Fibroblasts can be efficient antigen-presenting cells and therefore serve as immune regulators in inflammation.16,17 We thus evaluated the direct effects of ProTα on the expression of different HLAs on valvular interstitial cells (VICs). The mRNA levels of HLA-A (2.2-fold higher, p=0.015), HLA-B (3.7-fold-higher, p=0.051) and HLA-C (2.4-fold-higher, p=0.008) (Figure 5B) were increased in VICs exposed to recombinant ProTα as compared to the non-stimulated cultures, indicating that those cells can present antigens, and be potential targets of CD8+ T-cells, leading to valve damage.
A highly selective ERα antagonist decreases the ProTα-induced expression of cytotoxic molecules by CD8+ T-cells in vitro
Our data have shown a strong association between ERα expression and ProTα in CD8+ T-cells from RHVD patients. Moreover, treatment of peripheral mononuclear cells with recombinant ProTα induced the expression of cytotoxic molecules granzyme-B and perforin by CD8+ T-cells. In addition, recombinant ProTα increased the ERα expression in CD8+GzB+Perf+ cells. To test the hypothesis that ERα activity influences CD8+ T-cell cytotoxic potential, we treated purified CD8+ T-cells with 4 uM of highly selective ERα antagonist (1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxyphenol]-1H-pyrazole dihydrochloride/MPP). We then reintroduced these cells in the CD8 depleted mononuclear cell suspension, which was stimulated with recombinant ProTα. Gating strategies for flow cytometry analysis were the same as presented in Figure 3A.
As was the case in our initial in vitro experiments, recombinant ProTα was able to increase the frequency of expression of the lytic molecules by CD8+ T-cells (CD8+GzB+, p=0.028; CD8+Perf+, p=0.0002; CD8+GzB+Perf+, p=0.0002) in cell cultures without MPP (−) as compared to non-treated with ProTα cultures (CTRL) (Figure 6A). MPP reduced the frequency of CD8+GzB+ (p=0.003), CD8+Perf+ (p=0.029) and CD8+GzB+Perf+ (p=0.026) (Figure 6A). We then evaluated the ERα expression by CD8+GzB+Perf+ as well as VLA-2 expression. VLA-2 was also modulated by recombinant ProTα (p=0.045). MPP reduced the expression of ERα in CD8+GzB+Perf+ in ProTα stimulated cultures (p=0.008) (Figure 6B). MPP did not alter VLA-2 expression by CD8+GzB+Perf+. These results indicate that ERα influences CD8+ T-cell cytotoxicity but has no association with type 1 collagen binding.
Figure 6: Effects of a highly selective ERα antagonist on CD8 T-cells cytotoxicity and VLA-2 expression induced by ProTα.
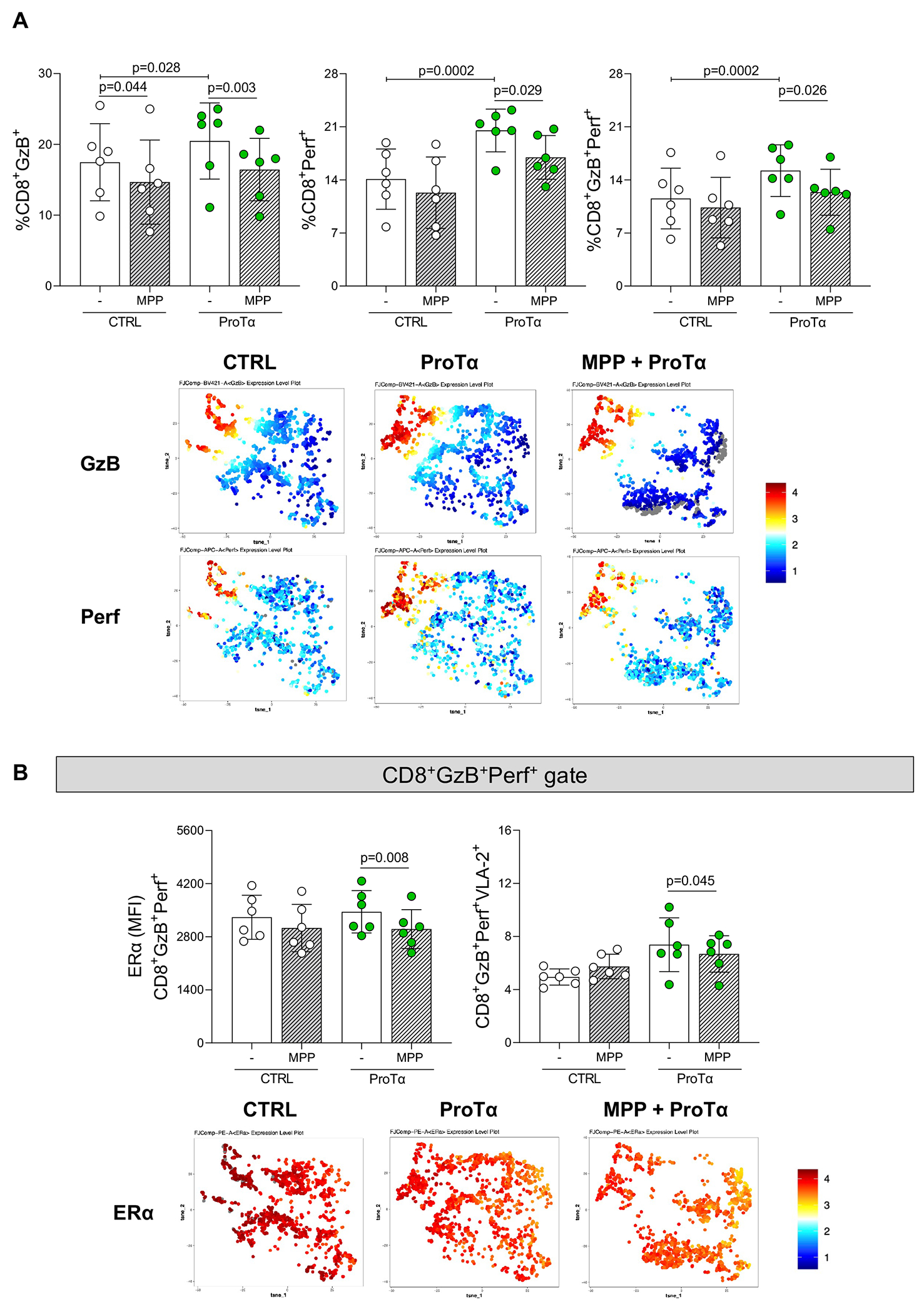
A, Comparison of the frequency CD8 T-cells expressing the cytotoxicity markers granzyme-B and perforin (%CD8+GzB+,%CD8+Perf+ and %CD8+GzB+Perf+) in peripheral blood mononuclear cells unstimulated (CTRL, white dots) and ProTα stimulated (green dots) in the absence (−) or in the presence of 4 uM (thick crosshatched) of 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP), a highly selective ERα antagonist. Representative tSNE of GzB and Perf expression on CTRL, ProTα stimulated (ProTα), and ProTα stimulated pre-treated with 4 uM of MPP (MPP 4 uM + ProTα). Colors correspond to phonograph-guided clustering. B, Comparison of the median of fluorescence intensity of ERα and frequency of VLA-2 in CD8+GzB+Perf+ T-cells. Representative tSNE of ERα ex pression on CTRL, ProTα stimulated (ProTα), and ProTα stimulated pre-treated with 4 uM of MPP (MPP 4 uM + ProTα). Colors correspond to phonograph-guided clustering. Bar graphs show the mean of values in each group (n=6) and standard deviation. Comparisons between non-stimulated (CTRL) and stimulated (ProTα) cell cultures and between non-MPP treated and MPP-treated were made using a two-way ANOVA followed by Tukey’s test. . Statistical significance is indicated in each graph.
Mimic type 1 collagen epitope RGRPGAPGP elicits CD8 T-cell activation in vitro
PBMCs from RHVD patients were stimulated with syntetic type 1 collagen epitopes (1, 2 and 4) predicted as imunogenic in our in silico analysis, to test their ability to induce CD8 T-cell activation. Anti-CD28 antibodies (2μg/mL) were added to cell cultures to provide co-stimulatory signal, required for antigen-specific T-cell activation. Epitope 2 (RGRPGAPGP) at the concentration of 20μM incresed the frequency of CD8 T-cell co-expressing granzyme B and perforin (CD8+GzB+Perf+, p=0.049) and CD69 (CD8+CD69+, p=0.017), an early activation T-cell marker (Figure 7A). Epitopes 1 and 4 also showed a slight increase of the evaluated makers, albeit not significant (Figure 7A). Moreover, multi-labeled immunofluorescence using customized antibodies against the epitope 2 demonstrated co-localization of CD8 T-cells (red) with type 1 collagen peptides (green; Figure 7B). CD8 T-cells interaction with epitope 2 can be vizualized as yellow signal in the Figure 7B. Taken together these experiments support our notion that type 1 collagen epitope 2 (RGRPGAPGP) is cross-reactive and could target autoimmune response of CD8 T-cells.
Figure 7: In vitro analysis of type 1 collagen mimic epitopes on CD8+ T-cell activation.
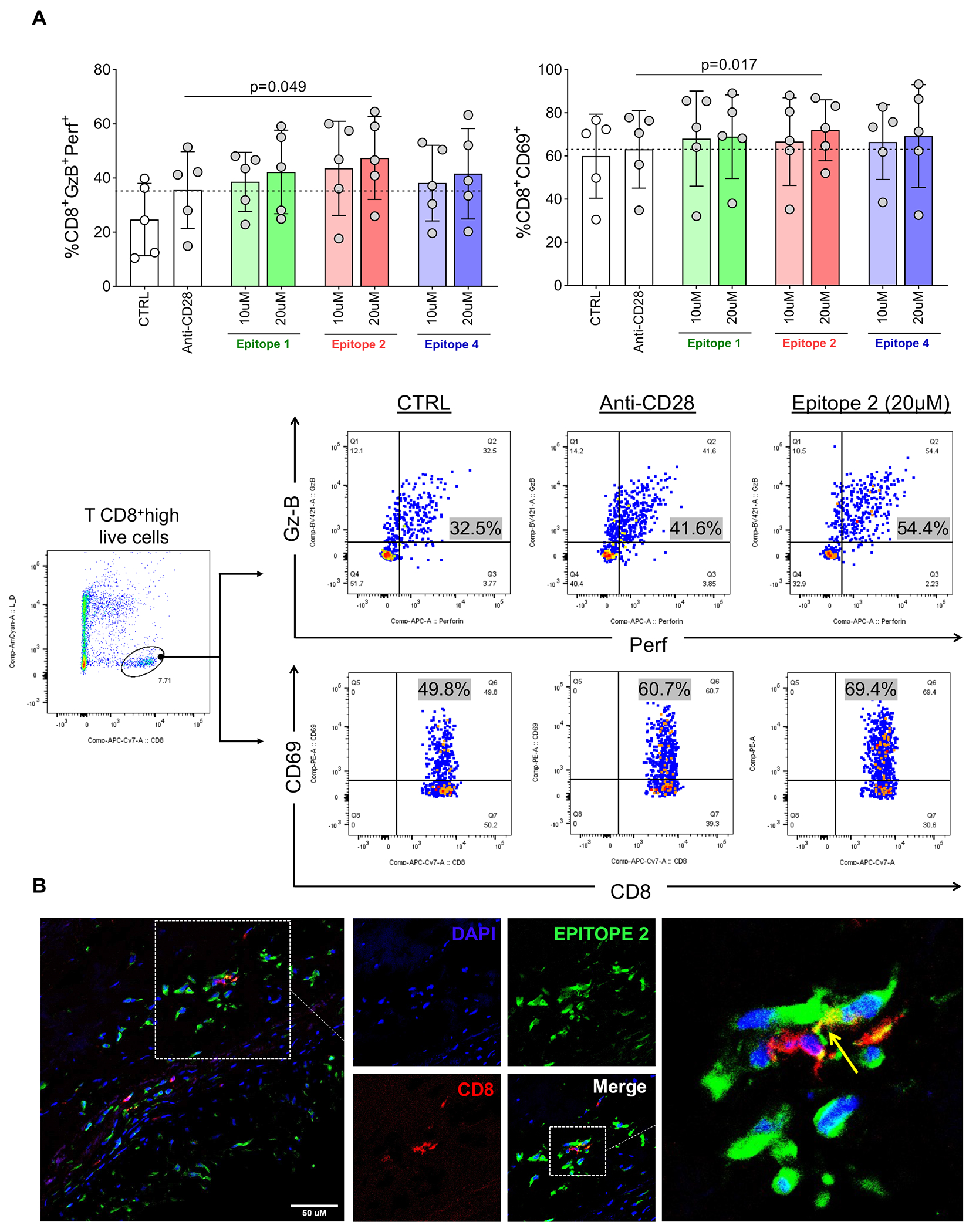
A, upper panel; Frequency of CD8+ cells coexpressing granzyme-B and perforin (CD8+GzB+Perf+, left) and CD69 (CD8+CD69+, right). Control cultures with media only (non-stimulated, CTRL) or stimulated with anti-CD28 only are represented by white and grey dots respectively. Cultures stimulated with 10μM and 20 μM of synthetic type 1 collagen mimic epitopes 1, 2 and 4 in combination with anti-CD28 are represented by green, red and blue bars, and grey dots respectively. Lower panel; Representative scatter plots showing the frequency of CD8+GzB+Perf+ and CD8+CD69+ in CTRL, anti-CD28 only and Epitope 2 (20 μM) plus anti-CD28 culture conditions. Bar graphs show the mean of values in each group (n=5) and standard deviation. Comparisons among non-stimulated and stimulated cell cultures were made using two-way ANOVA followed by Tukey’s test. Statistical significance is indicated in each graph. B, Representative direct immunofluorescence staining for CD8 and Epitope 2 in RHVD valvular lesion. Scale bar = 50 μm.
Collectively, our data show autocrine effects of ProTα on CD8+ T-cells by increasing the expression of tissue damaging lytic molecules (granzyme-B and perforin). ProTα further increases the expression of type 1 collagen binding (VLA-2) on CD8+ T-cells contributing to its interaction with COL1A1- and COL1A2-rich valvular tissue. Moreover, mimic epitopes between human COL1A1 and S. pyogenes can be recognized by CD8+ T-cells through HLA-I. In addition, paracrine function of ProTα was demonstrated through the increased expression of HLA-A, B and C on VICs, which may contribute to chronic inflammation and autoimmunity in RHVD (Figure 8).
Figure 8: The role of ProTα in RHVD.
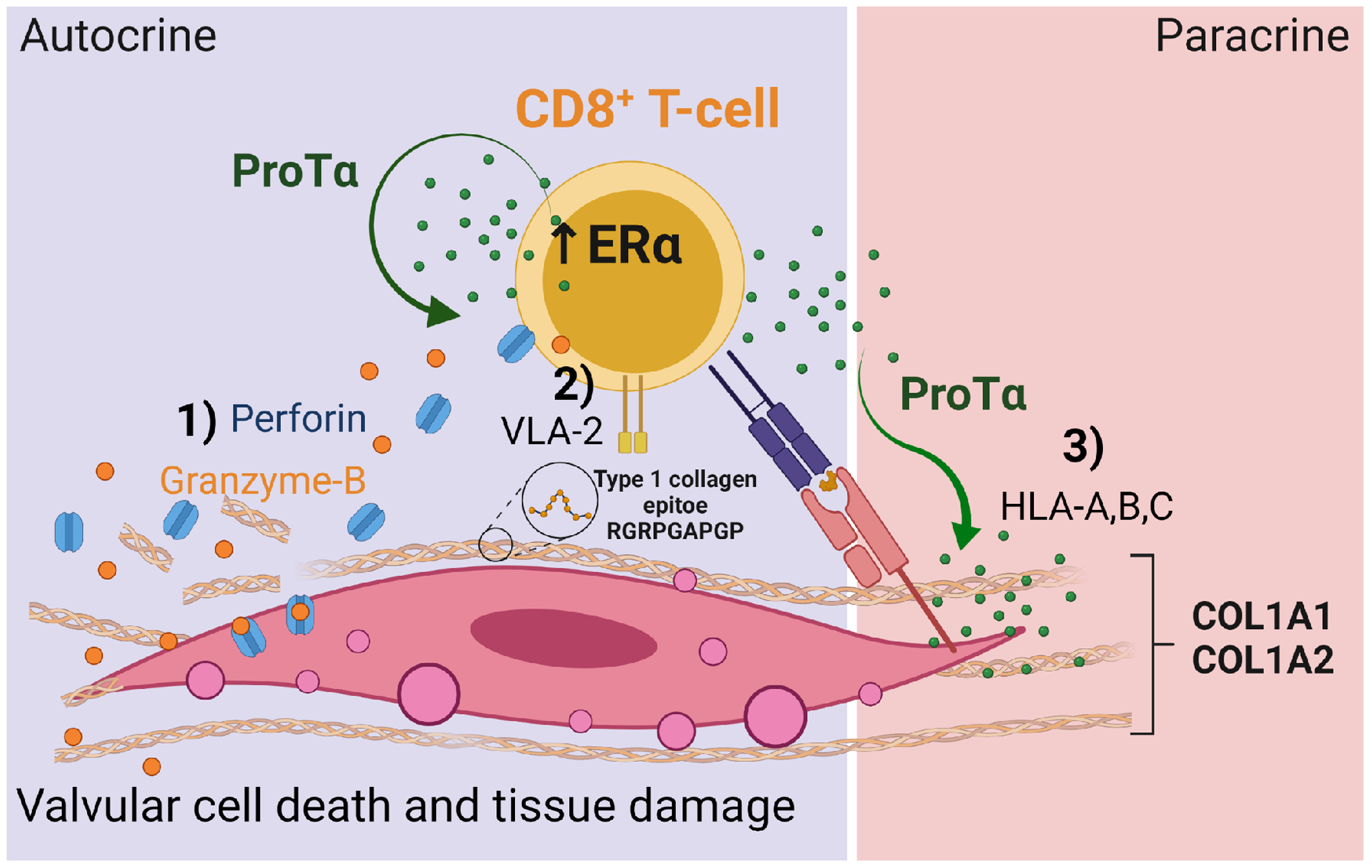
1) The paracrine effects of ProTα on CD8+ T-cells characterized by induction of expression of the lytic proteins granzyme-B and perforin and type 1 collagen receptor (VLA-2). 2) CD8+ T-cells recognize type 1 collagen mimic epitopes in valvular tissue. 3) The paracrine effects of ProTα on VICs characterized by induction of expression of the antigen presenting molecules HLA-A, B and C.
Discussion
RHVD causes irreversible damage to the heart valves, mainly mitral valves, while sparring myocardium and other organs during ARF. It is thus likely that valvular tissue composition/environment contribute to the RHVD progression. We, therefore, first compared NDAV and NDMV proteomes to identify proteins associated with mitral valve predisposition to RHVD. We discovered ProTα as a highly present protein in NDMV, which showed even higher abundance in RAVD and RMVD proteomes, suggesting its involvement in RHVD development. ProTα is a ubiquitously expressed polypeptide that can simultaneously exert pleiotropic intracellular and extracellular effects.18 Whitin cells, ProTα increases gene transcription,19,20 while acting as a biological response modifier mediating immune responses in a cytokine-like form, when it is secreted.21
Our data demonstrated that the aberrant expression of ProTα in RHVD is associated with the frequent inflammatory infiltrates in the valvular lesions. One of the hypotheses behind predisposition of the mitral valve to RHVD implicates hemodynamic factors. The mitral valve has a larger surface area as compared to aortic valve, which may result in slower blood flow velocity through the mitral leaflets and could favor the process of diapedesis.22,23 Active chronic inflammation in RHVD was evidenced in our proteomics data. TGF-β and TNF signaling as well as Th17 cell differentiation are among the most common pathways found in RHVD and have been extensively associated with pathologic processes, including fibrosis and autoimmunity.24,25 Therefore, higher content of immune cells in valvular interstitium carrying ProTα may predispose to lesion formation in mitral valve. It has been shown that ProTα levels are increased in cardiac tissue from myocardium infarction in response to ischemia.26 The authors have demonstrated that ProTα can reduce cardiomyocyte death, suggesting a cardioprotective role of this protein in myocardium.26 Thus, it is possible that heart failure also contributes to the aberrant ProTα expression in RHVD. Moreover, this can explain, in part, why the disease progresses in valves while sparring myocardium. Immune components, however, were not evaluated in this study. 26
No differences in the ProTα expression by different leukocyte subpopulations from RHVD patients as compared to healthy individuals was observed. It can be explained by the fact that ProTα is a secreted protein, hindering detection of the expression levels among cell populations. However, we detected increased plasma levels in RHVD patients as compared to healthy individuals. Taken together, our data suggest that ProTα can be explored as a RHVD biomarker.
While ProTα has immunoenhancing activity and has been associated with immune-related diseases,18 the molecular mechanisms underlying the effects of ProTα remain incompletely elucidated and have never been explored in cardiac valves. It was already shown that ProTα acts as an adjuvant upstream of lymphocyte stimulation and can promote the expansion of antigen-reactive effector cells.21 ProTα is thus viewed as a candidate adjuvant for cancer immunotherapy since it can help to boost immune responses.21 On the other hand, in autoimmune diseases such as in RHVD, it may contribute to the worsening of the dysfunctional immune response. Moreover, the evidence suggests that ProTα can increase ERα transcriptional activity in breast cancer cells, which motivated us to explore ERα activity in leucocytes from RHVD patients.15 Estradiol increases ProTα mRNA and protein expression.27 All immune cells highly express ERα and its activity has been a key interest in several studies investigating autoimmunity.28,29 Nearly 75% of all autoimmune disorders have sex-linked susceptibility.30 More than any other risk factor discovered, being women confers the greatest risk of developing autoimmune diseases. After stimulation, ERα-expressing T-cells, proliferated more and expressed more pro-inflammatory cytokines than T-cells lacking this receptor.4 Estrogen increases the inflammatory response and testosterone dampens it.31,32
Circulating CD4+ and CD8+ T-cells obtained from RHVD patients showed higher expression of ERα than healthy individuals. In addition, the ERα expression by CD4+ and CD8+ T-cells positively correlated with ProTα expression in RHVD patients but not in healthy individuals. Only CD8+ T-cells, however, correlated positively with ProTα expression in both blood and valve tissue. ERα expression from female CD8+ T-cells remains constant through life, pre- and post-menopause.30 A recent study on COVID-19 showed high accumulation of CD8+ T-memory cells expressing ProTα in patients with severe disease.33 Recombinant ProTα increased cytotoxic molecules by CD8+ T-cells, which was accompanied by increased ERα expression and VLA-2, a type 1 collagen binding. Thus, in addition to ProTα increasing the secretion of lytic molecules (granzyme-B and perforin) by CD8+ T-cells, it also increased the ability to interact with the valvular tissue favoring the immunologic synapse at the effector/target cell junction, culminating in tissue damage. VLA-2 expression by CD8+ T-cells may also contribute to a cell recruitment into heart valves. Granzyme-B release by CD8+ T-cells also has the potential to create autoimmune responses by cleaving tissue proteins, thereby contributing to the creation/exposition of epitopes.34 Destruction of valvular tissue integrity can fuel the immune responses and contribute to the initiation and propagation of the lesions. Epitope spreading has generally been invoked in the progression and chronicity of autoimmune diseases, and therefore, is considered a disease-regulator.34 Studies of experimental models have revealed a window of therapeutic opportunity in the face of disease-propagating epitopes.35,36 While it was already demonstrated that estrogen can increase secretion of granzyme-B,32 we found that blocking ERα on CD8+ T-cells by a highly selective antagonist decreased expression of cytotoxic molecules induced by ProTα, implicating a role of this protein in RHVD sex predisposition. The involvement of CD8+ T-cells in the pathogenesis of organ-specific autoimmune diseases has been not previously recognized. Genetic susceptibility and resistance to autoimmune diseases are widely associated with polymorphisms of HLA class II genes, and little evidence for an independent role for the closely linked HLA class I genes have been demonstrated. However, in recent years it has become increasingly evident that CD8+ T-cells play a key role in the pathogenesis of autoimmune diseases, rendering these cells as an attractive target for therapeutic intervention.37
Collagen-like surface protein is one of the major surface adhesins among group A Streptococcus.38 While the role of this protein is well estalished in virulence and invasion,39 less is known about its implication on breakdown of type 1 collagen immunotolerance and its capacity to trigger autoimmunity in RHVD. Some antigens, as might be the case of collagen, do not induce immune tolerance or activation in normal conditions, but can become targets for autoimmune attack.40 The antibacterial immune response against S. pyogenes begins in the pharyngeal epithelium by cells from innate immune system. These cells recognize and process bacterial antigens and present them to B lymphocytes, culminating in the production of immunoglobulins and activation of T-cells.4 Activated T-cells can recognize host epitopes that resemble bacterial structures evoking autoimmune responses, termed molecular mimicry. Mechanisms accounting for infection-triggered autoimmunity also include release of self-antigens from the damaged tissue, which enhance antigen presentation/recognition.40 Proteolytic molecules such as granzyme B and perforin resealed from activated of CD8+ T-cells may contribute to disruption of valvular endothelium and ECM, favoring access of the immune components to valve interstitium. Thus, in contrast to dominant antigenic determinants, subdominant cryptic antigens, as is the case of collagen can trigger self-specific immune responses.
Among our findings, we identified an immunogenic cross-reactive epitope (RGRPGAPGP, epitope 2) recognized by CD8 T-cells. This recognition can be facilitated by the ProTα stimulation through the induction of VLA-2 expression by CD8+ T-cells. Intriguingly, the epitope 2 showed the highest immunogenicity and better HLA-A*30:01 binding scores among all mimic epitopes identified in the in silico analysis. Collagen-like surface protein family was shown to be highly conserved among different bacterial strains and may participate in the colonization/binding of S. pyogenes to receptors on the host cells.41 Type 1 collagen mimic epitope 2 showed high binding efficiency to HLA-I groove and ability to elicit immune responses, and therefore, can be recognized by CD8+ T-cells according our in silico and in vitro experiments. The presence of collagen-reactive autoantibodies in the serum of ARF patients was shown to be associated to the development of RHVD.7,42 A recent study showed increased anti-human type 1 collagen serum from ARF patients compared to the controls.42 No correlation between antibodies against human type 1 collagen and S. pyogenes collagen-like surface protein serum levels was found.42 However, recognition of epitopes or immune effector functions triggered by those in host immune cells were not evaluated.
Previous studies described the antigen-presenting capacity of fibroblasts.16,43 We also evaluated the direct effects of ProTα on fibroblast-like VICs, and found that this protein can increase the expression of HLA-A, B and C. Thus, VICs are not passive bystanders but active participants in the development and maintenance of chronic inflammation and autoimmune responses in RHVD.16,44 We propose that ProTα has key role in inducing CD8+ T-cell mediated tissue pathology, in which VICs are a clear potential target.
RHVD occurs in resource-poor settings around the world where life-saving valve replacement surgeries are unrealistic therapeutical options to the majority of RHVD patients. Although antibiotics like penicillin are effective against S. pyogenes infection, improper medical care such as poor patient compliance, overcrowding, poverty, and repeated exposure to the bacteria, leads to ARF and RHVD. The development of a vaccine is the ideal strategy to control RHVD burden. Unlike vaccines for most infectious diseases, which elicit antibody responses and are intended to be preventative, a vaccine for an autoimmune disease must be therapeutic, resolving or controlling the ongoing T-cells self-reactive responses. Some current therapies for autoimmune diseases are based on the use of non-specific agents, which suppress systemically the function of many immune effector cells. However, indiscriminate immunosuppression often leads to serious, sometimes life-threatening side effects. Targeting disease-specific pathogenic T-cells is a promising way to develop immunotherapies to treat RHVD as immune response can be potentially modulated without triggering autoimmunity. Our study identified new mechanisms for the pathogenesis of RHVD that implicate ProTα as a contributor to CD8+ T-cells cytotoxicity associated with ERα activity, suggesting a role of ProTα in sex predisposition of RHVD. In addition, we found that ProTα facilitates CD8+ T-cell recognition of human type 1 collagen, which shows molecular mimicry with S. pyogenes and capability to elicit immune responses in those cells. As such, a better understanding of mechanisms for ProTα and estrogen sensitivity to control the CD8 T-cell function may offer new insights into potential strategies for treatment of rheumatic heart valve disease.
Supplementary Material
Clinical perspective.
What is new?
Prothymosin alpha was discovered using proteomics and network analysis and associated with immunopathogenesis of RHVD.
Prothymosin alpha increased estrogen receptor alpha activity and was implicated in RHVD sex predisposition.
Human type 1 collagen and S. pyogenes collagen-like surface functional immunogenic cross-reactive epitopes are recognized by CD8 T-cells.
What are the clinical implications?
RHVD occurs predominantly in women in resource-poor settings around the world where high complexity surgical intervention is unavailable therapeutical option.
Understanding the prothymosin alpha and estrogen sensitivity mechanisms to control the CD8 T-cell function may provide insigthts into treatment of RHVD.
Acknowledgments:
We acknowledge Eugenia Shvartz for her excellent histological assistance.
Sources of Funding:
This study is supported by the NIH grants R01 HL136431 and R01 HL147095 (to E.A.) and R01 HL141917 (to E.A. and R.L.).
Nonstandard Abbreviations and Acronyms
- ARF
acute rheumatic fever
- AV
aortic valve
- COL1
collagen type 1
- E2
estradiol
- ERα
estrogen receptor alpha
- FasL
Fas ligand
- Foxp3
Forkhead Box P3
- GzB
Granzyme B
- HLA
human leukocyte antigen
- MHC-I
major histocompatibility complex class I
- ND
non-diseased
- MPP
1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- Perf
Perforin
- RHVD
rheumatic heart valve disease
- RORγt
Retinoid-Related Orphan Receptor γt
- T-bet
T-box expressed in T cells
- VLA-1
collagen-binding receptors α1β1
- VLA-2
collagen-binding receptors α2β1
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, Sable C, Steer A, Wilson N, Wyber R et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passos LSA, Nunes MCP, Zilla P, Yacoub MH and Aikawa E. Raising awareness for rheumatic mitral valve disease. Glob Cardiol Sci Pract. 2020;2020:e202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Passos LSA, Nunes MCP and Aikawa E. Rheumatic Heart Valve Disease Pathophysiology and Underlying Mechanisms. Front Cardiovasc Med. 2020;7:612716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammad I, Starskaia I, Nagy T, Guo J, Yatkin E, Vaananen K, Watford WT and Chen Z. Estrogen receptor alpha contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal. 2018;11. [DOI] [PubMed] [Google Scholar]
- 5.Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carapetis J, Remenyi B, Taubert KA, Bolger AF, Beerman L et al. American Heart Association Committee on Rheumatic Fever E and Kawasaki Disease of the Council on Cardiovascular Disease in the Y. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806–1018. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham MW. Molecular Mimicry, Autoimmunity, and Infection: The Cross-Reactive Antigens of Group A Streptococci and their Sequelae. Microbiol Spectr. 2019;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinkla K, Rohde M, Jansen WT, Kaplan EL, Chhatwal GS and Talay SR. Rheumatic fever-associated Streptococcus pyogenes isolates aggregate collagen. J Clin Invest. 2003;111:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tandon R, Sharma M, Chandrashekhar Y, Kotb M, Yacoub MH and Narula J. Revisiting the pathogenesis of rheumatic fever and carditis. Nat Rev Cardiol. 2013;10:171–177. [DOI] [PubMed] [Google Scholar]
- 9.Dudding BA and Ayoub EM. Persistence of streptococcal group A antibody in patients with rheumatic valvular disease. J Exp Med. 1968;128:1081–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angum F, Khan T, Kaler J, Siddiqui L and Hussain A. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus. 2020;12:e8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taneja V Sex Hormones Determine Immune Response. Front Immunol. 2018;9:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol. 2018;9:2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na SY, Cao Y, Toben C, Nitschke L, Stadelmann C, Gold R, Schimpl A and Hunig T. Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain. 2008;131:2353–2365. [DOI] [PubMed] [Google Scholar]
- 14.Liblau RS, Wong FS, Mars LT and Santamaria P. Autoreactive CD8 T cells in organ-specific autoimmunity: emerging targets for therapeutic intervention. Immunity. 2002;17:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Martini PG, Delage-Mourroux R, Kraichely DM and Katzenellenbogen BS. Prothymosin alpha selectively enhances estrogen receptor transcriptional activity by interacting with a repressor of estrogen receptor activity. Mol Cell Biol. 2000;20:6224–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boots AM, Wimmers-Bertens AJ and Rijnders AW. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology. 1994;82:268–274. [PMC free article] [PubMed] [Google Scholar]
- 17.Ilangumaran S, Finan D, La Rose J, Raine J, Silverstein A, De Sepulveda P and Rottapel R. A positive regulatory role for suppressor of cytokine signaling 1 in IFN-gamma-induced MHC class II expression in fibroblasts. J Immunol. 2002;169:5010–5020. [DOI] [PubMed] [Google Scholar]
- 18.Samara P, Ioannou K and Tsitsilonis OE. Prothymosin Alpha and Immune Responses: Are We Close to Potential Clinical Applications? Vitam Horm. 2016;102:179–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karetsou Z, Kretsovali A, Murphy C, Tsolas O and Papamarcaki T. Prothymosin alpha interacts with the CREB-binding protein and potentiates transcription. EMBO Rep. 2002;3:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karetsou Z, Sandaltzopoulos R, Frangou-Lazaridis M, Lai CY, Tsolas O, Becker PB and Papamarcaki T. Prothymosin alpha modulates the interaction of histone H1 with chromatin. Nucleic Acids Res. 1998;26:3111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samara P, Karachaliou CE, Ioannou K, Papaioannou NE, Voutsas IF, Zikos C, Pirmettis I, Papadopoulos M, Kalbacher H, Livaniou et al. Prothymosin Alpha: An Alarmin and More. Curr Med Chem. 2017;24:1747–1760. [DOI] [PubMed] [Google Scholar]
- 22.Stewart WJ, Jiang L, Mich R, Pandian N, Guerrero JL and Weyman AE. Variable effects of changes in flow rate through the aortic, pulmonary and mitral valves on valve area and flow velocity: impact on quantitative Doppler flow calculations. J Am Coll Cardiol. 1985;6:653–662. [DOI] [PubMed] [Google Scholar]
- 23.Yeh YT, Serrano R, Francois J, Chiu JJ, Li YJ, Del Alamo JC, Chien S and Lasheras JC. Three-dimensional forces exerted by leukocytes and vascular endothelial cells dynamically facilitate diapedesis. Proc Natl Acad Sci U S A. 2018;115:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh RP, Hasan S, Sharma S, Nagra S, Yamaguchi DT, Wong DT, Hahn BH and Hossain A. Th17 cells in inflammation and autoimmunity. Autoimmun Rev. 2014;13:1174–1181. [DOI] [PubMed] [Google Scholar]
- 25.Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR and Yang SH. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int J Mol Sci. 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannavo A, Rengo G, Liccardo D, Pironti G, Scimia MC, Scudiero L, De Lucia C, Ferrone M, Leosco D, Zambrano N et al. Prothymosin alpha protects cardiomyocytes against ischemia-induced apoptosis via preservation of Akt activation. Apoptosis. 2013;18:1252–1261. [DOI] [PubMed] [Google Scholar]
- 27.Bianco NR and Montano MM. Regulation of prothymosin alpha by estrogen receptor alpha: molecular mechanisms and relevance in estrogen-mediated breast cell growth. Oncogene. 2002;21:5233–5244. [DOI] [PubMed] [Google Scholar]
- 28.Corradetti C, Jog NR, Cesaroni M, Madaio M and Caricchio R. Estrogen Receptor alpha Signaling Exacerbates Immune-Mediated Nephropathies through Alteration of Metabolic Activity. J Immunol. 2018;200:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham M and Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40:66–73. [DOI] [PubMed] [Google Scholar]
- 30.Phiel KL, Henderson RA, Adelman SJ and Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. [DOI] [PubMed] [Google Scholar]
- 31.Navarro FC and Watkins SK. Estrogen Stimulation Differentially Impacts Human Male and Female Antigen-Specific T Cell Anti-Tumor Function and Polyfunctionality. Gender and the Genome. 2017;1:1–13. [Google Scholar]
- 32.Navarro FC, Herrnreiter C, Nowak L and Watkins SK. Estrogen Regulation of T-Cell Function and Its Impact on the Tumor Microenvironment. Gender and the Genome. 2018;2:81–91. [Google Scholar]
- 33.Yu K, He J, Wu Y, Xie B, Liu X, Wei B, Zhou H, Lin B, Zuo Z, Wen W et al. Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res. 2020;30:814–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darrah E and Rosen A. Granzyme B cleavage of autoantigens in autoimmunity. Cell Death Differ. 2010;17:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan LS, Vanderlugt CJ, Hashimoto T, Nishikawa T, Zone JJ, Black MM, Wojnarowska F, Stevens SR, Chen M, Fairley JA et al. Epitope spreading: lessons from autoimmune skin diseases. J Invest Dermatol. 1998;110:103–109. [DOI] [PubMed] [Google Scholar]
- 36.Vanderlugt CL and Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. [DOI] [PubMed] [Google Scholar]
- 37.Clement M, Ladell K, Ekeruche-Makinde J, Miles JJ, Edwards ES, Dolton G, Williams T, Schauenburg AJ, Cole DK, Lauder SN et al. Anti-CD8 antibodies can trigger CD8+ T cell effector function in the absence of TCR engagement and improve peptide-MHCI tetramer staining. J Immunol. 2011;187:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachert BA, Choi SJ, LaSala PR, Harper TI, McNitt DH, Boehm DT, Caswell CC, Ciborowski P, Keene DR, Flores AR et al. Unique Footprint in the scl1.3 Locus Affects Adhesion and Biofilm Formation of the Invasive M3-Type Group A Streptococcus. Front Cell Infect Microbiol. 2016;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukomski S, Bachert BA, Squeglia F and Berisio R. Collagen-like proteins of pathogenic streptococci. Mol Microbiol. 2017;103:919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ercolini AM and Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen M, Eden A and Bjorck L. SclA, a novel collagen-like surface protein of Streptococcus pyogenes. Infect Immun. 2000;68:6370–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilapitiya DH, Harris PWR, Hanson-Manful P, McGregor R, Kowalczyk R, Raynes JM, Carlton LH, Dobson RCJ, Baker MG, Brimble M et al. Antibody responses to collagen peptides and streptococcal collagen-like 1 proteins in acute rheumatic fever patients. Pathog Dis. 2021;79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohyama H, Nishimura F, Meguro M, Takashiba S, Murayama Y and Matsushita S. Counter-antigen presentation: fibroblasts produce cytokines by signalling through HLA class II molecules without inducing T-cell proliferation. Cytokine. 2002;17:175–181. [DOI] [PubMed] [Google Scholar]
- 44.Zou Y, Bresnahan W, Taylor RT and Stastny P. Effect of human cytomegalovirus on expression of MHC class I-related chains A. J Immunol. 2005;174:3098–3104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


