Abstract
The commercially available diagnostic tests for syphilis are mostly based on the use of extracted antigens of Treponema pallidum. Pronounced cross-reactivities with other spirochete antigens are often reported. The aim of this study was to validate a novel multiparametric assay (the assay performed with the kit) INNO-LIA Syphilis for the confirmation of syphilis antibodies in a set of 840 documented human serum samples. All serum samples were previously tested at the French World Health Organization reference center for venereal diseases (Institute Alfred Fournier, Paris, France), with a consensus result provided for each sample. The study was conducted in two phases, with each phase involving a validation set (500 well-documented serum samples) and an exploratory set (340 serum samples) of serum samples, respectively. By measuring the sensitivity and specificity, we compared the result of the new assay with the consensus result on the basis of the results of a variable number of classical serological methods and clinical information when available. A sensitivity of 99.6% (95% confidence internal [CI], 98.5 to 99.9%) and a specificity of 99.5% (95% CI, 98.1 to 99.9%) were found for the new line immunoassay. Six of seven samples with indeterminate results by classical serology tested positive with the INNO-LIA Syphilis kit. This single multiparametric assay provides reliable confirmatory diagnostic information that must currently be obtained by the performance and interpretation of results of a combination of serological assays.
Syphilis is an infection most often transmitted by sexual contact. The causative agent is the human pathogen Treponema pallidum subsp. pallidum (9, 13). Serodiagnosis can be performed by two types of methods: (i) nontreponemal assays, such as the Venereal Disease Research Laboratory (VDRL) and rapid plasma reagin assays, and (ii) treponemal assays, such as fluorescent treponemal antibody-absorption (FTA-ABS), the microhemagglutination assay for antibody to T. pallidum (MHA-TP) or the T. pallidum hemagglutination assay (TPHA), and various enzyme-linked immunosorbent assays (ELISAs), which are typically more sensitive and yield more objective results than agglutination and fluorescence assays (7). All serological techniques have been shown to be cross-reactive with antibodies directed to other treponema species. False-positive reactivities can arise when conditions such as Lyme borreliosis, autoimmune disease, and human immunodeficiency virus (HIV) infection are present (2, 5, 10, 11, 15). Therefore, the use of only one type of test is insufficient for an accurate diagnosis (16). Moreover, the development of new sensitive assays such as ELISAs points to the questionable empirical threshold values that have been used in agglutination assays (i.e., for TPHA, 1:80) (8, 12, 17).
Although insensitive and of poor specificity, the nontreponemal antibody titers have been correlated with disease activity (1, 11). By contrast, treponema-specific antibody titers correlate poorly with disease activity and cannot be used to assess disease stage. Syphilis disease stage was previously evaluated by the T. pallidum immobilization assay (TPI), but it is rarely used today because it is difficult to perform, time-consuming, and, therefore, very expensive. Anti-T. pallidum titration by FTA-ABS has replaced TPI in many laboratories. However, the interpretation remains subjective and FTA-ABS is not suitable for large-scale testing situations (5, 14). Although Western blotting methods have been suggested as confirmatory assays, they include nonrelevant proteins, thereby inducing indeterminate and false-positive reactivity patterns (6). Furthermore, the reported sensitivity of about 94% (4) has resulted in their limited use.
The present study was designed to validate the new INNO-LIA Syphilis kit as a confirmatory assay for T. pallidum antibodies. We evaluated the assay in a two-phase study. First, we used well-characterized sera to assess sensitivity and specificity. In a second phase, we evaluated the performance with a somewhat less well documented collection of samples and studied any samples with discrepant results in detail. The specificity of the assay was also challenged by testing serum samples from patients with biological conditions, such as autoimmunity and pregnancy, who often test false-positive by assays for T. pallidum. Overall sensitivity and specificity were calculated with reference to consensus diagnostic assay results.
MATERIALS AND METHODS
Study design and sera.
This study involved a total of 840 human serum specimens tested by the French National Reference Center for Venereal Diseases (Institute Alfred Fournier, Paris, France). The samples had been collected over the past 5 years either for a first screening or for confirmatory testing following a reactivity in a first screening test. All samples were stored at −20°C prior to testing. In the first phase of the study, a unique collection of samples (all tested by TPI) was included (n = 500); of these, 276 were clearly negative (TPHA negative at 1:80 dilution, VDRL negative, and TPI negative) and 224 were clearly positive (TPHA positive at a dilution greater than or equal to 1:80 and TPI positive). The second phase involved the remaining 340 serum samples. In addition to serum samples, 13 cerebrospinal fluid (CSF) samples obtained from patients with clinically documented syphilis at different stages were also evaluated; this collection was tested by classical methods with mixed results, including equivocal serologic results for syphilis. Furthermore, sera from patients with the most common biological conditions that result in false-positive reactivities by serologic assays for syphilis were also investigated by various assays such as assays for anticardiolipin antibodies (n = 8), antinuclear antibodies (n = 10), and rheumatoid factors (n = 11). In addition, Lyme serology-positive sera (n = 12, Epstein-Barr virus capsid antigen immunoglobulin M (IgM)-positive sera (n = 8), sera from pregnant women (n = 10), and isolated sera with VDRL reactivities (n = 31) were tested. The Lyme serology-positive samples were screened by an in-house immunofluorescence technique with Borrelia burgdorferi B31; the results for six samples were further confirmed by Borrelia Western Blot IgG (Gull Laboratories, Bois d'Arcy, France).
Screening assays.
The VDRL assay used in this study uses a colloidal suspension of cholesterol-lecithin sensitized with beef heart-extracted cardiolipin (Sypal, Diagast, Loos, France) as a nontreponemal antibody detection system. In addition, treponema-specific antibodies were detected in an initial screening with sensitized sheep erythrocytes (RBCs; MHA-TP; Ames-Bayer, Puteaux, France). Repeat testing, in addition to the previous assay, if needed, was performed by using avian RBCs (LD Serokit TPHA, Labor Diagnostika GmbH, Heiden, Germany). Finally, a screening ELISA was used for further investigations; we used a kit with treponema recombinant antigens (ICE Syphilis; Murex Diagnostics B. V., Aalst, Belgium). All test procedures and interpretation of the results were performed according to the manufacturer's instructions.
Fluorescence methods (FTA-ABS).
Samples reactive by a screening method are usually tested by a fluorescent T. pallidum assay with cultured treponema organisms (TrepoSpot IF; BioMérieux, Marcy l'Etoile, France). Specific antibodies are detected with a fluorescein-labeled anti-human immunoglobulin after the absorption of nonspecific antibodies on T. phagedenis extracts (5). If specific fluorescence can be perceived at a sample dilution of 1:100, the sample is considered positive.
IgM detection methods.
Two methods have been used to determine IgM in human sera. A commercial assay (FTA-M; Sanofi Diagnostics Pasteur, Marne-la-Coquette, France) and an in-house IgM solid-phase hemadsorption assay (IgM-SPHA) with T. pallidum-sensitized RBCs were used.
TPI.
TPI was performed at the Institute Alfred Fournier by an in-house procedure, as follows. A 1/10 dilution of human serum was incubated with a suspension of freshly prepared treponema in the presence of complement proteins for 18 h at 37°C. A control procedure with inactivated complement proteins was also performed for each test. TPI determines the ability of antibody to immobilize live T. pallidum (Nichols strain), as visualized under a dark-field microscope and expressed as a percentage (number of immobilized spirochetes/total count). A sample is considered positive by TPI if at least 70% of Treponema are immobilized compared to the rate of immobilization for the corresponding control. The sample is considered negative if the value by TPI is less than 30% of that for the control, and TPI values between 30 and 70% of that for the control are considered inconclusive. For some TPI-positive samples, titration is obtained at the end dilution that shows immobilization of at least 50% of the T. pallidum spirochetes.
Consensus results by classical tests.
For the 340 less well documented samples, we established a consensus serological diagnostic for all samples on the basis of the available results of the classical assays (TPHA, FTA-ABS, and TPI). In some cases, if enough serum was available, repeat testing, in addition to a screening enzyme immunoassay (ICE Syphilis), was performed. In such cases, the global information was considered to define the consensus results. Since VDRL is often negative for patients with true late infections (who are positive by other techniques), VDRL data were not used to determine the consensus results. These consensus results were obtained as follows. A sample was considered positive if all available results were positive; a sample was considered negative if all available results were negative. When discrepant results were shown, the most predominant result was considered the consensus result; if discrepant results were present in equal numbers, no consensus could be reached and the result for the sample was therefore considered to be equivocal.
INNO-LIA Syphilis kit.
The INNO-LIA Syphilis kit uses recombinant antigens and synthetic peptides derived from T. pallidum (Nichols strain) membrane proteins. Briefly, the antigens used consisted of three immunodominant proteins (TpN47, TpN17, and TpN15) expressed as full-size proteins in Escherichia coli and one synthetic peptide (TmpA) derived from transmembrane protein A. In brief, the TmpA antigen was synthesized on a solid phase by using Tentagel S resin (Rapp Polymere GmbH, Tübingen, Germany) by standard 9-fluorenylmethoxycarbonyl amino acid chemistry. After completion of peptide synthesis, the peptide was cleaved from the resin and was then purified by reverse-phase high-performance liquid chromatography. The primary amino acid sequence of this antigen was optimized and included an N-terminal biotin residue to allow peptide immobilization on the nylon strips by using streptavidin binding properties.
The full-size T. pallidum genes expressing TpN47, TpN17, and TpN15 (accession nos. M88769, M74825, and M30941, respectively) were isolated from T. pallidum Nichols by PCR with specific primers based on the data bank sequences. Appropriate restriction enzyme sites were incorporated into these primers. The resulting sequences were cloned in the pBluescript SK(+) vector (Stratagene, La Jolla, Calif.) and were verified by DNA sequence analysis. The coding sequences were subsequently inserted in an E. coli expression vector and were transformed into the expression strain E. coli MC1061(pAcI). Both TpN47 and TpN15 were expressed as N-terminal fusion proteins with the first 25 amino acids of mouse tumor necrosis factor followed by a purification tag. The TpN17 protein was expressed as an N-terminal fusion with a purification tag only.
All proteins were purified by a similar procedure. The frozen cell pellets (from a 15-liter fermentation) were lysed in 6 M guanidine hydrochloride (GnHCl)–50 mM phosphate (pH 7.2) overnight at 4°C. The extracts were clarified by centrifugation after a freeze-thaw cycle of 2 h at −70°C. The supernatants were loaded in the presence of 20 mM imidazole on a chelating Sepharose FF column previously equilibrated with NiCl2 and loading buffer (6 M GnHCl, 50 mM phosphate, 20 mM imidazole [pH 7.2]). After loading of the proteins, the column was washed with wash buffer containing 50 mM imidazole. The fusion protein was eluted from the column with elution buffer (6 M GnHCl, 50 mM phosphate, 200 mM imidazole [pH 7.2]). After immobilized metal-affinity chromatography, TpN15 and TpN17 were desalted on a Sephadex G25 column with a 2 M urea buffer, while TpN47 was desalted with a 6 M urea buffer. Sodium dodecyl sulfate-polyacrylamide electrophoresis and Western blotting were used to estimate the purities of the recombinant proteins.
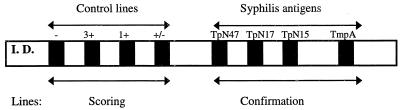
In addition to the syphilis antigens, control lines are used for a semiquantitative evaluation of the results as well as for the verification of sample addition and reagents. A schematic layout of the INNO-LIA strip is shown in Fig. 1. The assay procedure can be summarized as follows. Serum or plasma samples were diluted 1:100 and were incubated at room temperature (20°C) overnight, followed by three washing steps with washing buffer before the addition of a goat anti-human IgG (heavy and light chains) conjugated to alkaline phosphatase. Three washing steps were again performed, followed by the addition of a chromogen. Color development was then stopped with an appropriate stop solution. The use of color-coded reagents makes the different steps of the assay clearly distinct. In a visual reading protocol, after color development, each line was compared to the control lines, and the intensities were scored as follows: 0, no line or a line less intense than the +/− cutoff line; 0.5, a line as intense as the +/− cutoff line; 1, a line with an intensity between that of the cutoff line and equal to that of the 1+ control line; 2, a line with an intensity between that of the 1+ control line and that of the 3+ control line; 3, a line with an intensity equal to that of the 3+ control line; 4, a line with an intensity greater than that of the 3+ control line.
FIG. 1.
Layout of INNO-LIA Syphilis kit strip.
The scoring of the results obtained with the INNO-LIA Syphilis kit was done blinded without knowledge of the consensus test results. Samples were scored independently by two people (investigator A.E. and technician L.V.). Weakly reactive samples were scored 100% concordantly.
The interpretation algorithm of the INNO-LIA Syphilis kit was initially optimized for visual reading with an independent set of negative and positive samples (unpublished data). A sample is considered T. pallidum antibody negative if no band or an isolated band with a maximum intensity equal to 0.5 is present. If multiple bands with a minimum intensity equal to 0.5 are visible, the sample is considered T. pallidum antibody positive. Finally, a sample is considered indeterminate if a single band is visible with a minimum intensity equal to 1. Several representative INNO-LIA Syphilis kit reactivity patterns are shown in Fig. 2.
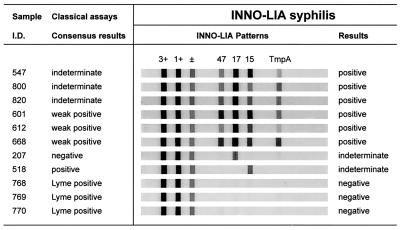
FIG. 2.
Representative patterns of reactivity with INNO-LIA Syphilis kit. Control lines are indicated (3+, 1+, and ±); antigen lines are those for the TpN47, TpN17, TpN15, and TmpA antigens, respectively. Sera correspond to the following (from top to bottom): six positive samples with indeterminate (samples 547, 800, and 820) or weakly positive (samples 601, 612, and 668) consensus results, two samples with indeterminate results with one negative (sample 207) and one positive (sample 518) consensus result; and three negative samples (samples 768, 769, and 770) with positive serology for Lyme borreliosis.
Statistics.
StatMate software (version 1.01; GraphPad, San Diego, Calif.) was used for the calculation of 95% confidence intervals (CIs) for proportions.
RESULTS
INNO-LIA Syphilis kit performance.
Among the 500 samples (224 positive and 276 negative samples) studied in the first phase, only 1 sample with a discrepant result was found, yielding a sensitivity of 100% (95% CI, 98.4 to 100%) and a specificity of 99.64% (95% CI, 98.0 to 99.9%) for the INNO-LIA Syphilis kit. The sample with a discrepant result had a negative serology but was scored as indeterminate by the INNO-LIA Syphilis kit. Unfortunately, no follow-up or further testing could be performed with this specimen. In the second phase of the study, among the remaining 340 serum samples with somewhat less well documented serologies, 8 samples had discrepant results, and 5 of these had equivocal serologies by classical testing. None of the Lyme borrelia-positive sera reacted in tests with the INNO-LIA Syphilis kit (the results for three such samples are shown in Fig. 2).
Comparison of INNO-LIA Syphilis kit results with the consensus results.
For the determination of the overall assay performance, we combined the samples studied in the two phases and calculated sensitivity and specificity. A summary of the results obtained with the INNO-LIA Syphilis kit testing compared to the consensus data obtained from classical tests is shown in Table 1. Sensitivity was evaluated for a total of 464 serum samples that were considered positive by a consensus of multiple tests (TPHA, FTA-ABS, and TPI) and clinical information. The INNO-LIA Syphilis kit confirmed the results for 462 of 464 samples positive for T. pallidum antibodies, resulting in a sensitivity of 99.6%. Table 2 presents the detailed results available for each sample with discrepant results. Briefly, two samples (samples 518 and 599) considered consensus positive were indeterminate with the INNO-LIA Syphilis kit. Both samples were doubtful by TPHA, positive by FTA-ABS (low titers) and ICE Syphilis EIA, but negative by VDRL. Only one sample was tested by TPI, and it had a negative result. None of the consensus-positive samples was found to be negative with the INNO-LIA Syphilis kit. For evaluating the specificity, 369 of 371 negative serum samples were correctly identified by INNO-LIA Syphilis kit, thereby yielding a specificity of 99.5%. Due to reactivity of a single band, two samples had indeterminate results with the INNO-LIA Syphilis kit. One sample (sample 207) was negative by four techniques but was not tested by the ICE Syphilis EIA; the other sample (sample 666) was negative by VDRL, TPHA, and TPI but positive by FTA-ABS and the ICE Syphilis EIA. None of the samples from this population were false positive. Both overall sensitivity and specificity fit within 95% CIs.
TABLE 1.
Comparison of INNO-LIA Syphilis kit results and classical testing consensus results
| Consensus result | No. of samples with the following INNO-LIA Syphilis kit result:
|
|||
|---|---|---|---|---|
| Negative | Indeterminate | Positive | Total | |
| Negative | 369 | 2a | 0 | 371 |
| Equivocal | 0 | 0 | 5b | 5 |
| Positive | 0 | 2c | 462 | 464 |
| Total | 369 | 4 | 467 | 840 |
One sample (sample 666) was positive by FTA-ABS and ICE Syphilis EIA and negative by agglutination assays.
The five samples were positive by at least one of the classical assays.
Doubtful or negative by agglutination assays (samples 518 and 599); consensus positive on the basis of the FTA-ABS and the ICE Syphilis EIA results.
TABLE 2.
Detailed results for sera from Table 1 with discrepant results
| Sample no. | Results by classical techniquesa
|
Consensus result | INNO-LIA Syphilis kit intensity
|
INNO-LIA Syphilis kit result | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPHA | VDRL | FTA-ABS | TPI (%) | ICE (S/CO) | TpN47 | TpN17 | TpN15 | TmpA | |||
| 523 | D80 | Neg | Neg | NT | 2.4 | Equivocal | 1 | 2 | 1 | 0 | Positive |
| 547 | D80 | Neg | Neg | NT | 4.1 | Equivocal | 0 | 1 | 2 | 0 | Positive |
| 800 | Neg | 2 | 100 | NT | 1.8 | Equivocal | 0.5 | 3 | 0.5 | 1 | Positive |
| 811 | D80 | 2 | Neg | NT | NT | Equivocal | 0.5 | 4 | 1 | 0 | Positive |
| 820 | Neg | 16 | 100 | NT | 2.2 | Equivocal | 0.5 | 3 | 1 | 0 | Positive |
| 666 | Neg | Neg | 100 | 10 | 4.4 | Negative | 0 | 4 | 0 | 0 | Indeterminate |
| 207 | Neg | Neg | Neg | Neg | NT | Negative | 0 | 1 | 0 | 0 | Indeterminate |
| 518 | D80 | Neg | 100 | NT | 3.2 | Positive | 0 | 0 | 1 | 0 | Indeterminate |
| 599 | D80 | 1 | 200 | 30 | 2.6 | Positive | 0 | 3 | 0 | 0 | Indeterminate |
TPHA, VDRL, and FTA-ABS results are expressed in titers. Neg, negative result, D80, a doubtful reading at a dilution of 1:80; NT, not tested; S/CO, value for the sample/cutoff value.
Analysis of discrepant results.
Discrepancies between the consensus result and the INNO-LIA Syphilis kit result were again analyzed in the context of their global information. In addition, due to subjective reading of the results obtained by agglutination techniques, if clear-cut positive or clear-cut negative results were not demonstrated at the tested dilution (1:80), a doubtful TPHA result was indicated as D80 (Table 2).
Serum and CSF samples with clinical information.
Thirteen CSF specimens obtained from 10 syphilitic patients were also evaluated in this study. Of these, 11 samples were classified as positive by the consensus result as well as with the INNO-LIA Syphilis kit (100% sensitivity). The two remaining CSF samples were considered equivocal by the consensus result (doubtful by TPHA, negative by FTA-ABS, and positive by the ICE Syphilis EIA); with the INNO-LIA Syphilis kit, one sample (sample 699) was positive and one sample (sample 701) was indeterminate (Table 3).
TABLE 3.
INNO-LIA Syphilis kit results for sera with clinical condition documentation
| Sample no. | Date, sample | Clinical conditiona | TPHA titer | VDRL titer | IgM resultb | FTA-ABS titer | TPI titer | INNO-LIA Syphilis band intensity
|
INNO-LIA Syphilis kit result | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TpN47 | TpN17 | TpN15 | TmpA | |||||||||
| 692 | 04 Feb 1997, blood | Primary syphilis | 10,240 | 32 | Posc | 1,600 | 4 | 4 | 4 | 4 | Positive | |
| 19 Feb 1997, blood | First injection of penicillin | 5,120 | 16 | Pos | 1,600 | 3 | 4 | 4 | 2 | Positive | ||
| 26 Feb 1997, blood | Second injection of penicillin | 5,120 | 16 | Pos | 800 | 3 | 4 | 4 | 4 | Positive | ||
| 26 Mar 1997, blood | Third injection of penicillin | 5,120 | 16 | Pos | 800 | 3 | 4 | 4 | 3 | Positive | ||
| 11 June 1997, blood | 5,120 | 16 | Negd | 800 | 4 | 4 | 4 | 2 | Positive | |||
| 693 | 07 Jul 1997, blood | Neurosyphilis | 20,480 | 64 | Pos | NTe | 1,600 | 3 | 4 | 4 | 4 | Positive |
| 11 Aug 1997, blood | Treatment | 20,480 | 64 | Pos | 6,400 | 1,600 | 4 | 4 | 4 | 4 | Positive | |
| 11 Aug 1997, CSF | NT | 16 | Neg | 800 | 800 | 3 | 4 | 4 | 4 | Positive | ||
| 05 Mar 1998, blood | Treatment | 20,480 | 16 | NT | 400 | 2 | 4 | 4 | 4 | Positive | ||
| 05 Mar 1998, CSF | 2,560 | Neg | NT | 25 | 3 | 4 | 4 | 4 | Positive | |||
| 694 | 22 Jan 1997, blood | Neurosyphilis | 5,120 | 8 | 1,600 | 4 | 4 | 4 | 4 | Positive | ||
| 22 Jan 1997, CSF | 80 | Neg | 100 | 1 | 3 | 0 | 0 | Positive | ||||
| 23 Apr 1997, blood | Treatment | 5,120 | 8 | 1,600 | 3 | 4 | 4 | 3 | Positive | |||
| 23 Apr 1997, CSF | 80 | Neg | NT | 1 | 4 | 0 | 0 | Positive | ||||
| 695 | 14 Dec 1996, blood | Neurosyphilis | NT | 64 | 6,400 | 3 | 4 | 4 | 4 | Positive | ||
| 14 Dec 1996, CSF | NT | 8 | 1,600 | 3 | 3 | 3 | 3 | Positive | ||||
| 18 Dec 1996, blood | Treatment | 20,480 | 8 | 1,600 | 100 | 3 | 4 | 4 | 4 | Positive | ||
| 18 Dec 1996, CSF | 5,120 | Neg | 400 | 50 | 3 | 4 | 4 | 4 | Positive | |||
| 696 | 16 Oct 1996, blood | Neurosyphilis | NT | 512 | 6,400 | 400 | 3 | 4 | 4 | 4 | Positive | |
| 16 Oct 1996, CSF | NT | NT | 1,600 | 100 | 3 | 4 | 4 | 4 | Positive | |||
| 697 | 12 Feb 1997, blood | Tertiary syphilis treatment | 10,240 | 64 | Pos | 1,600 | 3 | 4 | 4 | 3 | Positive | |
| 07 Jul 1997, blood | Treatment | 10,240 | 16 | Pos | 800 | 3 | 4 | 4 | 3 | Positive | ||
| 03 Sep 1998, blood | 5,120 | 16 | Pos | 800 | 3 | 4 | 4 | 3 | Positive | |||
| 699 | 24 Mar 1998: blood | Primary syphilis | 640 | 64 | Pos | 800 | 3 | 4 | 4 | 2 | Positive | |
| 05 May 1998, blood | Evolving primary syphilis | 5,120 | 128 | NT | 3 | 4 | 4 | 3 | Positive | |||
| 05 May 1998, CSF | D80 | Neg | Neg | 1 | 3 | 0.5 | 0 | Positive | ||||
| 04 Jun 1998, blood | Treated syphilis | 1280 | 32 | Neg | NT | 3 | 4 | 4 | 3 | Positive | ||
| 701 | 28 Aug 1998, blood | Congenital syphilis | 5,120 | NT | Pos | NT | 1 | 4 | 3 | 3 | Positive | |
| 28 Aug 1998, CSF | D80 | Neg | Neg | Neg | 0 | 3 | 0 | 0 | Indeterminate | |||
| 703 | 23 Jun 1998, blood | Neurosyphilis with HIV positivity | 20,480 | 2,048 | NT | 3 | 4 | 4 | 4 | Positive | ||
| 23 Jun 1998, CSF | 1,280 | 1 | NT | 3 | 4 | 3 | 3 | Positive | ||||
| 736 | Unknown, blood | Neurosyphilis with HIV positivity | 40,560 | 512 | Pos | NT | 3 | 4 | 4 | 3 | Positive | |
| Unknown, CSF | 5,120 | 4 | NT | 3 | 4 | 4 | 3 | Positive | ||||
Clinical data were from various hospitals in France or from the sexually transmitted disease clinic of the Institute Alfred Fournier.
IgM determination by FTA-ABS and/or IgM-SPHA.
Pos, positive result.
Neg, negative result.
NT, not tested.
DISCUSSION
In the course of the 20th century, syphilis serology testing has significantly contributed to limiting the spread of this venereal disease (15). Furthermore, disease activity has been linked to the results of multiple tests: TPHA-positive and VDRL-negative results are thought to be indicative of latent syphilis, while TPHA-positive and VDRL-positive results are usually indicative of infectious syphilis (1). To establish a conclusive diagnosis, TPI was often used to resolve conflicting results. This complex and expensive methodology has been progressively abandoned and substituted by other strategies for confirmation. For instance, several laboratories perform either TPHA and VDRL titration by serial dilution or TPHA and FTA-ABS titration for confirmation of syphilis (7).
The INNO-LIA Syphilis kit used in this study is a user-friendly methodology that makes use of color-coded reagents and simple interpretation criteria. It shows a high degree of performance as a multiparametric confirmation method. The assay validated in this study has been optimized to confirm the presence of specific antibodies to T. pallidum antigens in human sera and plasma. Nevertheless, testing of a limited series of CSF samples was also possible, and it was suggested that the kit had a higher degree of sensitivity with these samples than agglutination assays do. Since IgM molecules (agglutinins) do not cross the blood-brain barrier, detection of treponemal antibodies was somewhat less sensitive by agglutination techniques (TPHA, VDRL) than by other assays. This was clearly illustrated with the available paired samples from patients (serum and CSF samples) (Table 3). The high degree of sensitivity of the INNO-LIA Syphilis kit allowed the detection of low levels of IgG, like in the CSF of patient 699 (Table 3). Classical testing schemes classified five samples as equivocal due to discrepancies among the different assays (Table 2). Interestingly, these samples were clearly positive with the INNO-LIA Syphilis kit. This can reflect an improved sensitivity on the basis of the use of recombinant antigens versus nontreponemal antigens (in VDRL) (3), extracted treponemal antigens (in TPHA), or whole treponema organisms (in FTA-ABS) (14). The two samples with consensus-negative and INNO-LIA Syphilis kit-indeterminate results may also reflect the high degree of sensitivity of the new assay. On the other hand, since the specificity of the INNO-LIA Syphilis kit has been demonstrated with a large collection of negative samples (data partially shown in the present study) as well as a collection of difficult samples usually prone to false-positive reactivities (Table 4), the two consensus-positive samples that were indeterminate with the INNO-LIA Syphilis kit may illustrate the specificity of the INNO-LIA Syphilis kit. One of these samples (sample 599) had a doubtful result by agglutination techniques, was positive by FTA-ABS and the ICE Syphilis EIA, but was negative by TPI. This can also be due to a differential interference of antibodies to other treponemes that have no clinical significance (5).
TABLE 4.
INNO-LIA Syphilis kit results for sera containing antibodies for other infectious or autoimmune diseases
| Positive fora: | No. of specimens tested | INNO-LIA Syphilis kit results |
|---|---|---|
| Cardiolipin antibodies | 8 | Negative |
| Pregnancy | 10 | Negative |
| Rheumatoid factors | 11 | Negative |
| B. burgdorferi | 12 | Negative |
| Antinuclear antibodies | 10 | Negative |
| Epstein-Barr virus IgM | 8 | Negative |
| VDRL (biological false positive) | 31 | Negative |
Tested by immunofluorescence, EIA, or agglutination techniques at the Institute Alfred Fournier.
In conclusion, the INNO-LIA Syphilis kit can reliably replace various strategies for the confirmation of syphilis infections, which currently requires the performance of several assays. A new prospective study will allow us to determine a possible algorithm for disease staging by this multiparametric assay.
ACKNOWLEDGMENTS
A. Ebel thanks Innogenetics for providing the INNO-LIA Syphilis kits free of charge.
Fred Shapiro is acknowledged for critical review and editorial contributions.
REFERENCES
- 1.Aberle Grasse J, Orton S L, Notari IV E, Layug L P, Cable R G, Badon S, Popovsky M A, Grindon A J, Lenes B A, Williams A E. Predictive value of past and current screening tests for syphilis in blood donors: changing from a rapid plasma reagin test to an automated specific treponemal test for screening. Transfusion. 1999;39:206–211. doi: 10.1046/j.1537-2995.1999.39299154737.x. [DOI] [PubMed] [Google Scholar]
- 2.Backhouse J L, Hudson B J. Evaluation of immunoglobulin G enzyme immunoassay for serodiagnosis of yaws. J Clin Microbiol. 1995;33:1875–1878. doi: 10.1128/jcm.33.7.1875-1878.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkowitz K, Baxi L, Fox H E. False negative syphilis screening: the prozone phenomenon, non-immune hydrops and diagnosis of syphilis during pregnancy. Am J Obstet Gynecol. 1990;163:975–977. doi: 10.1016/0002-9378(90)91107-n. [DOI] [PubMed] [Google Scholar]
- 4.Byrne R E, Laske S, Bell M, Larson D, Phillips J, Todd J. Evaluation of a Treponema pallidum Western immunoblot assay as a confirmatory test for syphilis. J Clin Microbiol. 1992;30:115–122. doi: 10.1128/jcm.30.1.115-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson B, Hanson H S, Wasserman J, Brauner A. Evaluation of the fluorescent treponemal antibody-absorption (FTA-ABS) test specificity. Acta Dermatol Venereol. 1991;71:306–311. [PubMed] [Google Scholar]
- 6.Fujimura K, Ise N, Ueno E, Hori T, Fujii N, Okada M. Reactivity of recombinant Treponema pallidum (r-Tp) antigens with anti-Tp antibodies in human syphilitic sera evaluated by ELISA. J Clin Lab Anal. 1997;11:315–322. doi: 10.1002/(SICI)1098-2825(1997)11:6<315::AID-JCLA1>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen S A, Steiner B M, Rudolph A H. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1–21. doi: 10.1128/cmr.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefevre J C, Bertrand M A, Bauriaud R. Evaluation of the Captia-Enzyme Immunoassays for detection of immunoglobulins G and M to Treponema pallidum in syphilis. J Clin Microbiol. 1990;28:1704–1707. doi: 10.1128/jcm.28.8.1704-1707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukehart S A, Holmes K K. Spirochetal diseases. In: Fauci A S, Braunwald E, Isselbacher K J, et al., editors. Harrison's principles of internal medicine. 14th ed. New York, N.Y: McGraw-Hill; 1998. pp. 1023–1033. [Google Scholar]
- 10.Nandwani R, Evans D T P. Are you sure it's syphilis? A review of false positive serology. Int J STD AIDS. 1995;6:241–248. doi: 10.1177/095646249500600404. [DOI] [PubMed] [Google Scholar]
- 11.Rusnak J M, Butzin C, MacGlason D, Blatt S P. False-positive rapid plasma reagin tests in human immunodeficiency virus infection and relationship to anticardiolipin antibody and serum immunoglobulin levels. J Infect Dis. 1994;169:1356–1359. doi: 10.1093/infdis/169.6.1356. [DOI] [PubMed] [Google Scholar]
- 12.Silleti R P. Comparison of CAPTIA Syphilis G enzyme immunoassay with rapid plasma reagin test for detection of syphilis. J Clin Microbiol. 1995;33:1829–1831. doi: 10.1128/jcm.33.7.1829-1831.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh A E, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187–209. doi: 10.1128/cmr.12.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sordillo E M, Hoehl B, Belch J. False-negative fluorescent treponemal tests and confirmation of syphilis infection. J Infect Dis. 1998;178:294–295. doi: 10.1086/515609. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. HIV/AIDS and sexually transmitted diseases, WHO policy and strategic orientations. Report WHO/ASD/96.2. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 16.Young H. Syphilis: serology. Dermatol Clin. 1998;16:691–698. doi: 10.1016/s0733-8635(05)70034-6. [DOI] [PubMed] [Google Scholar]
- 17.Zrein M, Maure I, Boursier F, Soufflet L. Recombinant antigen-based enzyme immunoassay for screening of Treponema pallidum antibodies in blood bank routine. J Clin Microbiol. 1995;33:525–527. doi: 10.1128/jcm.33.3.525-527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]