Abstract
Non-invasive focused ultrasound stimulation (FUS) is a non-ionising neuromodulatory technique that employs acoustic energy to acutely and reversibly modulate brain activity of deep-brain structures. It is currently being investigated as a potential novel treatment for Parkinson’s disease (PD). This scoping review was carried out to map available evidence pertaining to the provision of FUS as a PD neuromodulatory tool. In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension for Scoping Reviews, a search was applied to Ovid MEDLINE, Embase, Web of Science and Cochrane Central Register of Controlled Trials on 13 January 2022, with no limits applied. In total, 11 studies were included: 8 were from China and 1 each from Belgium, South Korea and Taiwan. All 11 studies were preclinical (6 in vivo, 2 in vitro, 2 mix of in vivo and in vitro and 1 in silico). The preclinical evidence indicates that FUS is safe and has beneficial neuromodulatory effects on motor behaviour in PD. FUS appears to have a therapeutic role in influencing the disease processes of PD, and therefore holds great promise as an attractive and powerful neuromodulatory tool for PD. Though these initial studies are encouraging, further study to understand the underlying cellular and molecular mechanisms is required before FUS can be routinely used in PD.
Keywords: neuroscience, neuromodulation, Parkinson’s disease, scoping review, ultrasound
1. Introduction
Parkinson’s disease (PD) is a common and progressive neurodegenerative condition, characterised by the degeneration and death of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and reduced dopamine biosynthesis from surviving neurons [1]. As the disease advances, PD patients can experience motor manifestations typically starting with tremor, progressing to bradykinesia and typical “cogwheel” rigidity, postural instability and gait disorders [2]. These manifestations significantly impact the activities of daily living and health-related quality of life of these patients [2,3,4]. With the global increase in life expectancy and an ageing population, both the incidence and prevalence of PD have also been rising [5,6,7], steadily becoming a major public health issue [8].
Deep brain stimulation (DBS) of either the subthalamic nucleus (STN) or internal globus pallidus (GPi) is a well-recognised and established neurosurgical procedure approved for advanced PD refractory to medication. Many hypotheses have been proposed for the mechanisms by which DBS generates improvements in motor symptoms, but prevailing theories have focused on stimulation-induced disruption of pathological brain circuit activity [9,10], which occur at the ionic, protein, cellular and network levels [11]. Although it is effective and in aggregate it is a safe approach [12,13,14,15], just like any surgical procedure, DBS can be a double-edged sword; it is not free of complications and may even lead to more harm to the patient [16,17,18]. A consequence of this is strict patient criteria, and thus the number of people that can actually benefit from this treatment is relatively low. Accordingly, the demand for non-invasive alternatives to open stereotactic procedures is significant.
Currently, non-invasive but irreversible neuromodulatory ablative approaches such as stereotactic radiosurgery [19], and even magnetic resonance guided high-intensity focused ultrasound (MRgFUS) for subthalatomy or pallidotomy, are clinically employed for movement disorders [20,21,22,23,24] but are not without complications.
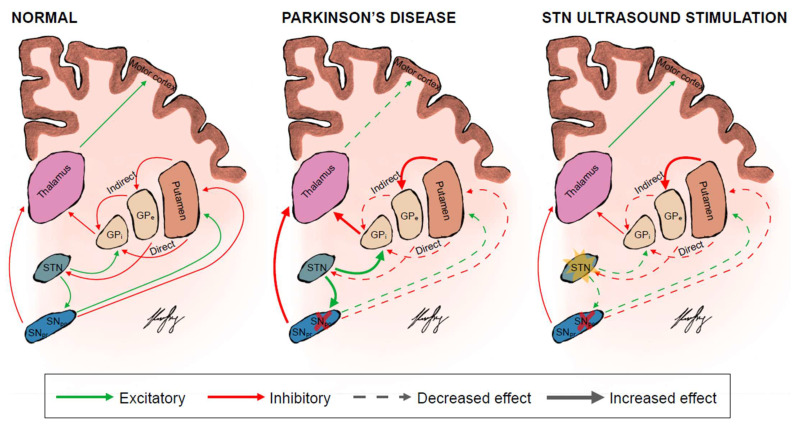
Transcranial magnetic stimulation (TMS) and transcranial current stimulation (TCS) have emerged as the cornerstone of non-invasive modulation of neural activity in particular regions of the brain [25,26,27,28]. Although promising, both TMS and TCS are limited by their broad radius of action [29]. This lack of spatial focality is particularly pronounced within the context of neuromodulating deep brain pathways such as the striato-pallido-thalamic network or deep structures including the STN and GPi, which are relevant to PD pathophysiology (Figure 1) [30,31]. TMS and TCS are generally constrained to targeting superficial cortical regions, as their efficacy declines exponentially with depth [30,31]. In contrast, focused ultrasound stimulation (FUS) is a non-invasive and non-ionising technique that employs acoustic energy and can acutely and reversibly modulate deep brain structures with sharp spatial resolution and non-invasive deep penetration [32,33,34]. FUS therefore holds promise as a powerful neuromodulatory tool for PD.
Figure 1.
The basal ganglia circuits in a normal person, PD patient and when they are theoretically modulated by focus ultrasound stimulation of the STN. The direct pathway facilitates while the indirect pathway inhibits movement. The colours represent the excitatory (green) and inhibitory (red) neuronal pathways. The thickness and dottedness of the lines between the regions represent the strength of signalling. The red cross over the SNpc represents degeneration and death of dopaminergic neurons in the SNpc and reduced dopamine biosynthesis from surviving neurons. The yellow star over the STN represents stimulation of the target STN. GPi = globus pallidus internus; GPe = globus pallidus externus; SNpc = substanstia nigra pars compacta; SNpr = substanstia nigra pars reticulata; STN = subthalamic nucleus.
In order to explore the potential of FUS as a neuromodulatory tool in PD, we undertook a scoping review to profile the existing literature. To our knowledge, this is the first scoping review of its kind. The overall aims of the scoping review were to collate, map, assess and describe the existing evidence base relating to this topic, in a formal, systematic and transparent way [35]. It is intended that the findings of this review can be used to identify potential gaps in knowledge and contribute to further development of research relating to the field of FUS and PD.
2. Materials and Methods
This scoping review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension for Scoping Reviews (PRISMA-ScR) [36]. Unlike systematic reviews, scoping reviews do not need to have a protocol registered [35]. A scoping review was chosen over a systematic review as emerging evidence relating to the provision of FUS in PD has not been comprehensively reviewed and large variation in reporting exists between studies [35,37,38].
2.1. Search Strategy
A search string was developed to identify original studies investigating the role of reversible low-intensity FUS (LIFUS) as a neuromodulatory tool in PD. Current clinically available ablative high-intensity FUS were beyond the scope of this review. The search terms comprised synonyms of two key concepts, namely ultrasound neuromodulation, and Parkinson’s disease. The search was applied to the following four electronic databases: Ovid MEDLINE, Embase, Web of Science and Cochrane Central Register of Controlled Trials (CENTRAL). Searches were performed for each database on 13 January 2022. No limits were applied (Supplementary Table S1).
2.2. Study Selection and Reliability
Articles were selected for inclusion in the review if they were published in a peer-reviewed journal in any language, and addressed the role of FUS as a neuromodulatory tool in PD. All titles and abstracts were screened independently by two reviewers (KSL and BC) against a set of pre-defined eligibility criteria. Potentially eligible studies were selected for full-text analysis. To ensure literature saturation, the reference lists of the included studies were scanned. A summary of the inclusion and exclusion criteria is presented in Supplementary Table S2. Disagreements were resolved by consensus or appeal to a third senior reviewer (DJW). Agreement among the reviewers on study inclusion was evaluated using Cohen’s kappa [39].
2.3. Data Extraction
A proforma was developed to conduct systematic data extraction with fields relating to (i) study design, (ii) country, (iii) subject models/participants, (iv) intervention assessed, (v) comparators against which FUS interventions are compared, (vi) data collection method and outcome measures and (vii) main findings. Two reviewers independently (KSL and BC) charted data from each eligible article. Any disagreements were resolved through discussion between the two reviewers or further adjudication by a third reviewer (DJW) until consistency was achieved.
2.4. Outcomes
Outcome variables were not predefined in this study, due to its exploratory rather than hypothesis-led nature.
2.5. Synthesis of Results
A narrative synthesis of data, with descriptive analyses where appropriate, was undertaken to enable the analysis of the relationships within and between studies, as well as assessing gaps in the literature. An analytical framework of quantitative and thematic approach was used to collate various themes that emerged from the existing data. The articles were also coded according to the categories identified in the data charting stage. Discrepancies in coding and synthesis of final frequency statistics were adjudicated by discussion.
3. Results
3.1. Characteristics of Inlcuded Studies
Number of articles screened and selected for inclusion are shown in Figure 2. Using the designated search terms, a total of 373 unique articles were identified and 11 were included in the final dataset [20,21,22,40,41,42,43,44,45,46,47,48,49,50]. Reliability of study selection between observers was substantial at both the title and abstract screening stage (Cohen’s κ = 0.94) and the full-text review stage (Cohen’s κ = 1.00) [39]. Among these 11 articles, 8 were from China and 1 each from Belgium, South Korea and Taiwan. All 11 studies were preclinical [40,41,42,43,44,45,46,47,48,49,50], including 1 computational in silico study [44]. The majority of the primary studies (6 of 11 (54.5%)) included were published from 2020 onwards, demonstrating the rapid pace at which the field is growing. Table 1 summarises the details and findings of all included studies.
Figure 2.
PRISMA flow diagram for studies included and excluded from the scoping review.
Table 1.
Characteristics and summary of findings from the included preclinical studies.
| Authors and Year | Country | Study Design | Sample Size, Types of Subjects/Participants | Intervention (Control) | Outcome Measures/Indicators | Main Findings Relating to FUS in PD |
|---|---|---|---|---|---|---|
| Chen X et al., 2021 | China | In vivo and in vitro | N = 5 per group. MPTP-induced C57BL/6 PD mice models. MPP+-induced N2a cells | LIFUS In vivo:
|
In vivo measures:
|
Efficacy:
|
| Dong Y et al., 2021 | China | In vivo | N = 20. 6-OHDA-induced Sprague-Dawley hemi-PD rat models | LIFUS
|
|
|
| Karmacharya MB et al., 2017 | Korea | In vitro | MPP+-induced PC12 cell PD models | Low-intensity ultrasound
|
|
|
| Sung CY. et al., 2021 | Taiwan | In vivo | N = 20. 6-OHDA-induced Sprague–Dawley PD rat models | LIFUS
|
|
|
| Tarnaud T et al., 2019 | Belgium | Computational modelling study | A computational model for ultrasonic stimulation of the STN is created by combining the Otsuka model with the bilayer sonophore model | LIFUS
|
|
|
| Wang Z et al., 2020 | China | In vivo | N = 11. MPTP-induced C57BL/6 PD mice models | LIFUS
|
|
|
| Xu T et al., 2020 | China | In vivo and in vitro | N = 12 per group. MPTP-induced C57BL/6 PD mice models and PC12 cells | LIFUS
|
In vivo measures: Locomotor behavior
|
Efficacy
|
| Yuan Y et al. 2020 | China | In vivo | N = 8 per group. MPTP-induced C57BL/6 PD mice models | LIFUS
|
|
|
| Zhao L et al., 2017 | China | In vitro | PC12 cells exposed to MPP+-induced neurotoxicity | Low-intensity ultrasound
|
|
|
| Zhou H et al., 2019a | China | In vivo | N = 8. MPTP induced C57BL/6 PD mice models | LIFUS
|
|
Efficacy
|
| Zhou H et al., 2019b | China | In vivo | N = 8. MPTP-induced C57BL/6 PD mice models | LIFUS
|
|
Efficacy
|
6-OHDA = 6-hydroxydopamine; BDNF = Brain-derived neurotrophic factor; DBS = Deep brain stimulation; FA = Fractional anisotropy; GDNF = Glial cell line-derived neurotrophic factor; GPi = Globus pallidus internus; HE = Haematoxylin and eosin; ISPPA = Spatial peak and pulse-average intensity; ISPTA = Spatial peak and temporal average intensity; LDH = Lactate dehydrogenase; LFP = Local field potential; LIFUS = Low-intensity focused ultrasound; MPP+ = 1-methyl-4-phenylpyridinium; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PD = Parkinson’s disease; ROS = Reactive oxygen species; SNpc = Substantia nigra pars compacta; STN = Subthalamic nucleus; TH = Tyrosine hydroxylase; TUNEL = Terminal deoxynucleotidyl transferase dUTP nick end labelling.
3.2. Outcome Measures
An extensive range of neuromodulatory outcomes were identified (Table 1). These outcome measures were considered and categorised into two main areas: those relevant to in vitro models and those relevant to in vivo models. The most frequently investigated outcome variable employed in in vitro studies were improvement in cell viability/reduction in apoptosis (n = 3), suppression of 1-methyl-4-phenylpyridinium ion (MPP+)-induced reactive oxygen species (ROS) generation (n = 3), attenuation of MPP+-induced suppression of mitochondrial complex I activity (n = 2), suppression of MPP+-induced expression of casein kinase 2 (CK2) that mediates ROS-dependent α-synuclein aggregation (n = 1) and finally dopamine release (n = 1). For the in vivo studies, the most frequently studied outcome variable to evaluate the effectiveness of FUS was locomotor behaviour (n = 6) assessed using a battery of tests such as the rotarod and pole and open field forced swimming tests. The next common measures to ascertain therapeutic effects included the proportion of tyrosine hydroxylase (TH) enzyme (n = 5) and glial cell line-derived neurotrophic factor (GDNF)-positive neurons in the SNpc (n = 2). Suppression of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced cell apoptosis/reduction of antioxidant enzyme activity (n = 2) and neuronal activity using c-Fos as a surrogate was also investigated (n = 2). Other investigated outcomes included the reduction in iron staining in the SNpc (n = 1), proportion of brain-derived neurotrophic factor (BDNF)-positive neurons in the SNpc (n = 1), dopamine content in the SNpc (n = 1) and fractional anisotropy (FA) and T2* values via magnetic resonance imaging (MRI) scanning (n = 1) and local field potentials in the motor cortex (n = 1). Safety outcomes included the lack of haemorrhage and morphological changes on brain sections (n = 4), typically ascertained by haematoxylin and eosin (HE) and Nissl staining.
3.3. Trends and Findings from In Vitro Preclinical Studies
Differentiated pheochromocytoma (PC12) and N2a cells exposed to MPP+-induced neuronal toxicity were the most commonly studied models of PD in vitro. Karmacharya showed that in MPP+ treated PC12 cells, low-intensity ultrasound attenuated mitochondrial ROS production and reversed the inhibition of mitochondrial complex I activity by MPP+ [42]. When cells treated with 400 or 800 μM MPP+ were stimulated with 30, 50 and 100 mW/cm2 ultrasound for 10 min, the mitochondrial ROS production was decreased as compared to non-stimulated group as demonstrated by a reduction in the MitoSOX Red intensity, which was found to be stimulation intensity dependent [42].
Mitochondrial integrity is tightly regulated by the balance between pro-apoptotic Bax and anti-apoptotic Bcl-2 [51,52,53,54,55,56,57,58]. In the context of MPTP and MPP+ neurotoxic damage, Bax downregulation attenuates DA neuron apoptosis [1,53,59], whereas Bcl-2 upregulation has neuroprotective effects against the depletion of striatal dopamine [53,59,60]. Zhao and colleagues showed that low-intensity ultrasound pre-treatment increased the Bcl-2/Bax ratio, prevented Cytochrome C release and suppressed cleaved-caspase 3 activity that would all be present after MPP+ exposure [48]. Additionally, low-intensity ultrasound attenuated MPP+-induced ROS accumulation and oxidative stress in PC12 cells by modulating the expression of antioxidant proteins haem oxygenase-1 (HO-1) and thioredoxin-1 (Trx-1), and improved mitochondrial membrane potential in both PC12 cells [48] and in N2a cells [40].
Low-intensity ultrasound improved cell viability in both PC12 and N2a cells after being treated with MPP+ as assessed by lactate dehydrogenase (LDH), MTT and TUNEL assays [42,48]. Pre-treatment with low-intensity ultrasound suppressed the MPP+-induced increase in the level of caspase-9 cleavage products [48], suggesting that low-intensity ultrasound can modulate the mitochondrial apoptotic pathway, but not endoplasmic reticulum stress-induced apoptotic pathway. Importantly, low-intensity ultrasound decreased MPP+-induced α-synuclein aggregation, levels of phosphorylated α-synuclein and expression of CK2 [42], which constitutively phosphorylates α-synuclein at S129 [61,62].
These findings suggest that low-intensity ultrasound can help to maintain integrity and function of challenged mitochondria. Together, this indicates that low-intensity ultrasound inhibited MPP+-induced mitochondrial dysfunction and apoptosis.
3.4. Trends and Findings from In Vivo Preclinical Studies
With a steady increase in the number of publications relating to research of FUS in PD, contemporary animal models were most often mouse and rat species. No primate, dog or pig species were used during the study period. Induction of a PD phenotype was typically by intraperitoneal injection of MPTP in C57BL/6 mice or by intracranial injection of 6-hydroxydopamine (6-OHDA) in Sprague–Dawley rats.
In the study by Zhou et al., they observed that LIFUS (30 min daily, for 12 days) targeting either the STN or GPi, upregulated Bcl-2, downregulated Bax and inhibited increments in protein levels of both Cytochrome C and cleaved-caspase 3, thereby reversing the changes of MPTP exposure in C57BL/6 mice [49]. These findings are in agreement with the in vitro findings by Zhao et al. in PC12 cells [48].
TH is the rate-limiting enzyme responsible for catalysing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), which is eventually transformed to dopamine [63]. Multiple studies showed that LIFUS ameliorated the reduction of TH in the SNpc and striatum induced by MPTP or 6-OHDA administration as detected by immunohistochemical analyses, demonstrating the restorative effects of LIFUS on the nigrostriatal pathway [40,41,43,46]. Importantly, Xu et al. showed that this rise in TH levels was accompanied by an increase in striatal dopamine levels as measured by isocratic elution and electrochemical detection [46].
In 6-OHDA-induced Sprague–Dawley hemi-lesioned-PD rat models, LIFUS to the striatum increased the levels of GDNF in the SNpc but not in the striatum [41,43]. GDNF has potent neuroprotective and neurorestorative effects particularly, but not exclusively, on dopaminergic neurons in animal models of PD [64]. The mechanism is still unclear, but a plausible explanation for the rise in GDNF levels with low-intensity transcranial ultrasound stimulation (LITUS) could be that LITUS disrupted the BBB acutely and promoted exogenous GDNF to enter the brain [65,66,67,68], or that LITUS resulted in glial activation, and the increase in astroglia promoted increased endogenous GDNF. Importantly, LIFUS of either the STN or GPi restored motor behaviour and coordination damaged by MPTP or 6-OHDA, as assessed by the rotarod and pole tests, in both PD mice [40,47,49,50] and rat models [43]. This treatment effect continued to improve with increased LIFUS duration [40,47,49,50].
To evaluate the safety of LIFUS in vivo, all studies had employed the use of HE staining to assess the presence of haemorrhage or tissue damage and Nissl staining to visualise neurons in brain sections [40,46,48,49,50]. All studies reported the absence of haemorrhage, or cytotoxic damage with normal neuronal density throughout the brain [40,46,48,49,50].
4. Discussion
4.1. Summary of Findings
This work represents the first attempt to comprehensively review the available preclinical and clinical evidence pertaining to the function of FUS as a non-invasive and reversible treatment for PD worldwide, during established irreversible neuromodulatory stereotactive procedures, such as DBS, and ablative approaches, such as stereotactic radiosurgery and MRgFUS.
Our review demonstrates preclinical evidence of the safety and efficacy of FUS neuromodulation, but also highlights the paucity of in vitro evidence relating to its mechanisms of action.
4.2. Implications of Findings: Association and Causality
A causal relationship between FUS and neuromodulation of PD is tenable. Based on the Bradford Hill criteria [69], this may be supported by the strength and dose-dependent association reported in in vitro and in vivo preclinical studies [20,21,22,40,41,42,43,44,45,46,47,48,49,50,70,71,72,73,74]. The literature has consistently proven that FUS is capable of eliciting various behavioural responses in various PD models [40,43,47,49,50,72,73,74,75], inducing protein expression changes in GDNF [41,43], TH [40,41,43,46] and dopamine levels [46] in the SNpc, which are implicated in the disease pathology of PD. Indeed, the treatment effect continued to improve with increased LIFUS duration, even suggesting a dose–response relationship [40,47,49,50].
These phenomena are reinforced further by the biological plausibility of a mechanical bioeffect [76]. Although FUS is known to have a thermal effect, with the parameters used for neuromodulation [77] this is unlikely to play a major role. Generally, low-intensity protocols are employed (<20 W/cm2) for the purpose of neuromodulation, which is within Food and Drug Administration limits [70,73,78]. At such intensities, the accompanying temperature rise is deemed too miniscule (<0.1 °C) to potentiate neuromodulation [79], or even cause tangible thermal damage [70,72]. Studies have alternatively suggested that the most probable mechanism of FUS action is through acoustic radiation force (ARF) generation, which promotes neuronal membrane strain, consequently modulating its capacitance and its embedded mechanosensitive channel proteins [80,81,82]. Consistent with this notion, particular channels appear to be directly modulated by FUS include voltage-gated sodium, potassium and calcium channels [70,83], and channels of the Piezo family [84]. Additionally, there are speculations that LITUS resulted in glial activation, and the increase in astroglia promoted increased endogenous GDNF [65,66,67,68,85,86]. There is compelling evidence that the neuromodulatory effects elicited by LIFUS in vivo are mediated additionally by non-neuronal mechanisms such as glia-neuron interaction in the mammalian brain. Astrocytes, with their long peripheral processes, have intimate spatial relationships with presynaptic and postsynaptic synapses of neighbouring neurons (also known as the tripartite synapse) [87,88,89]. Dysregulation of the astrocyte–dopaminergic neuron interaction has a role in the pathophysiology of PD [90]. It is also well-established that the Ca2+-dependent release of glutamate from astrocytes stimulate neuronal NMDA receptors (NMDARs), modulating their synaptic activity [91,92,93]. Oh and colleagues reported that this FUS-induced neuromodulation is initiated by the opening of TRPA1 Ca2+ channels in astrocytes, which are pressure sensitive. This astrocytic influx of Ca2+ entry prompts the release of gliotransmitters such as glutamate through Best1 channels, which eventually activates the NMDAR of neurons in the vicinity to elicit action potential firing [91]. Interestingly, Blackmore et al. corroborated these findings in a recent study that showed FUS restored long-term potentiation and memory in senescent mice. Specifically, they demonstrated that FUS significantly raised levels of TRPA1 levels in synaptosomal hippocampal fractions compared with sham treatments [94]. This increase, reflected by the changes observed for NMDAR subunits in these fractions, suggests that ultrasound-mediated astrocytic glutamate release is a likely mechanism by which FUS led to the observed improvements in senescent mice, which may also explain the improvements in locomotor behaviour as seen in PD models [94].
4.3. Implication on the Direction of Future Research
FUS holds promise as a powerful neuromodulatory tool for PD and is hence attractive, and studies have investigated this in a multitude of ways. Consistent reporting of the neuromodulatory effects of FUS can be facilitated by an agreed minimum set of indicators to be reported, for example, changes in the expression of neuroprotective proteins for in vitro studies. Consistent reporting of these outcomes across future studies will enhance the validity of evidence syntheses [95]. By virtue of the fact that preclinical studies such as those we have highlighted rely on models of PD pathology that might not completely reflect disease mechanisms in humans, it is particularly important that future studies assessing the neuromodulatory effects of FUS for PD meet criteria spanning across the cellular, molecular and behavioural levels. This will help build confidence in the novel intervention being of relevance to the human disease state. Additionally, preclinical animal models typically include a behavioural component to assess the motor deficits characteristic of PD. These usually include locomotor deficits evident in various motor behaviour and coordination assays. There is a general consensus that the animal models offer compelling similarity in functional deficits to those found in humans [96]. Therefore, an important outcome measure for any future intervention studies would likely include demonstration of improvements in these key movement metrics. Table 2 outlines a proposed checklist for the outcome measures to directly assess the neuromodulatory effects of FUS for PD as a starting point. This checklist is intentionally generic, representing a minimum set of critically important outcomes to report in all studies evaluating the introduction and evaluation of FUS neuromodulation relevant to PD and should not restrict investigators in their reporting of additional relevant outcomes. In future, this could be further refined by a Delphi consensus of various stakeholders—scientists, electrophysiologists, neurologists and neurosurgeons.
Table 2.
A proposed checklist for the outcome measures to directly assess the neuromodulatory effects of FUS for PD.
| Outcome Measures/Indicators |
|---|
| Preclinical—in vitro |
Changes in expression at the gene, RNA and protein level
|
Changes in mitochondrial integrity
|
| Preclinical—in vivo |
Changes in expression at the gene, RNA and protein level
|
Electrophysiological and synaptic properties
|
Behavioral outcomes
|
| Clinical |
Functional outcomes
|
FA = Fractional anisotropy; GDNF = Glial cell line-derived neurotrophic factor; MDS-UPDRS = Movement Disorder Society version of the United Parkinson’s Disease Rating Scale; MRI = Magnetic resonance imaging; PD = Parkinson’s disease; RNA = Ribonucleic acid; ROS = Reactive oxygen species; SNpc = Substantia nigra pars compacta; STN = Subthalamic nucleus; TH = Tyrosine hydroxylase; UDysRS = Unified dyskinesia rating scale; UPDRS = Unified Parkinson’s disease rating scale; US = United States.
This review additionally identified a current lack of in vitro studies that investigate the mechanistic changes induced by FUS. A significant caveat is that many of the in vivo models that assess the behavioural effects of FUS are confounded by the use of anaesthesia in their experimental design, which is unavoidable due to ethical reasons [45,46,97]. Kawaguchi et al. demonstrated that the typically used isoflurane dampens the motor-evoked potentials induced [98]. Of greater significance, several potential mechanisms of how FUS modulates membrane excitability coincide with those of the anaesthetic drugs [80]. As these studies employ the use of anaesthesia, the authors cannot exclude the possibility that the effects of FUS are altered under these conditions due to residual confounders [99]. Therefore, more in vitro studies such as those identified in this scoping review are recommended for more precise characterisation of the cellular mechanisms underpinning FUS. However, current in vitro studies, although captivating, are limited by the use of the PC12 cell culture to model PD. This is a pheochromocytoma cell line and does not exhibit the electrophysiological properties of post-mitotic midbrain dopaminergic neurons (mDANs). One way to circumvent this issue would be through the use of human-induced pluripotent stem cell line-derived mDANs [100], which enables a more accurate recapitulation of sporadic PD pathogenesis in vitro [101].
Chronic stimulation of deep brain structures using low-intensity FUS as a direct replacement for traditional electrode-based DBS removes the invasive procedure required for DBS but would impose the wearing of some form of targeted transducer device. However, given that certain FUS parameters seem to have effects lasting beyond the duration of stimulation, in both in vitro [102], and in in vivo PD models [49], we speculate on the possibility of finding a regular treatment protocol allowing for FUS to be given to the patient, which would induce a long-term therapeutic effect.
It is important to note that whilst the studies we identify specified no damaging effects of FUS, there remains the possibility, as with any neurostimulation tool, of functional adverse events. Whilst more work is therefore required to definitively establish this, it is nonetheless encouraging that, in a recent review of a number of human FUS studies, no evidence of lasting functional side effects or major adverse events was reported [103].
4.4. Strengths and Limitations
The findings are derived from a thorough search of three electronic databases. By including results from all study designs (preclinical and clinical), a comprehensive review of research evidence was achieved.
Only 11 studies qualified for inclusion in this review. Conference abstracts and other grey literature were excluded which may entrench publication bias or the ‘file-drawer problem’ [104]. However, during the screening process, conference abstracts rarely evinced sufficient details that would have been beneficial to this review. Nevertheless, we were still able to collate results in a structured manner and draw conclusions that represent a starting point for more robust future studies. We identified specific gaps in the existing literature, which are outlined in the ‘Implications of Findings and Direction of Future Research’ Section above.
5. Conclusions
This scoping review provides a starting point to better understand the research surrounding FUS in PD around the world. Preclinical evidence indicates that FUS is safe and has beneficial neuromodulatory effects on motor behaviour in PD. However, there is a current lack of mechanistic understanding for these beneficial effects of FUS in PD, either in vivo or in vitro; hence, there is need for further studies with more appropriate cellular models and clear reporting of our suggested outcome measures. FUS seems to have a role in influencing the disease process of PD, and therefore holds great promise as an attractive and powerful neuromodulatory tool for PD.
Acknowledgments
A special thank you to Kenneth Kek Wee Lee (Papa), Lena Lim (Mama) and Chio Tee Koh (Ahma) back home for their unwavering support and belief in me, without which, I would not be able to achieve my educational goals. Love truly overcomes all obstacles (different time zones and distance).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci12020289/s1, Table S1: Search strategies across the four electronic databases as of 13 January 2022, Table S2: Inclusion and exclusion criteria used to assess eligibility of studies.
Author Contributions
Conceptualisation, K.S.L.; methodology, K.S.L., O.C.-L. and D.J.W.; validation, K.S.L., O.C.-L. and D.J.W.; formal analysis, K.S.L.; investigation, K.S.L.; resources, K.S.L., O.C.-L. and D.J.W.; data curation, K.S.L., B.C., T.G.J.S. and A.G; writing—original draft preparation, K.S.L.; writing—review and editing, K.S.L., B.C., T.G.J.S., A.G., O.C.-L. and D.J.W.; visualisation, K.S.L., O.C.-L. and D.J.W.; supervision, O.C.-L. and D.J.W.; project administration, K.S.L., O.C.-L. and D.J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by grant MR/N0137941/1 for the GW4 BIOMED MRC DTP, awarded to the Universities of Bath, Bristol, Cardiff and Exeter from the Medical Research Council (MRC)/UKRI, and Bristol Research into Alzheimer’s and Care for the Elderly (BRACE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Please find available data in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vila M., Jackson-Lewis V., Vukosavic S., Djaldetti R., Liberatore G., Offen D., Korsmeyer S.J., Przedborski S. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2001;98:2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 3.Kadastik-Eerme L., Rosenthal M., Paju T., Muldmaa M., Taba P. Health-related quality of life in Parkinson’s disease: A cross-sectional study focusing on non-motor symptoms. Health Qual. Life Outcomes. 2015;13:83. doi: 10.1186/s12955-015-0281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carod-Artal F.J., Vargas A.P., Martinez-Martin P. Determinants of quality of life in Brazilian patients with Parkinson’s disease. Mov. Disord. 2007;22:1408–1415. doi: 10.1002/mds.21408. [DOI] [PubMed] [Google Scholar]
- 5.Hindle J.V. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing. 2010;39:156–161. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch L., Jette N., Frolkis A., Steeves T., Pringsheim T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2016;46:292–300. doi: 10.1159/000445751. [DOI] [PubMed] [Google Scholar]
- 7.Pringsheim T., Jette N., Frolkis A., Steeves T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 8.Yang W., Hamilton J.L., Kopil C., Beck J.C., Tanner C.M., Albin R.L., Ray Dorsey E., Dahodwala N., Cintina I., Hogan P., et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinson’s Dis. 2020;6:15. doi: 10.1038/s41531-020-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozano A.M., Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Lozano A.M., Lipsman N., Bergman H., Brown P., Chabardes S., Chang J.W., Matthews K., McIntyre C.C., Schlaepfer T.E., Schulder M., et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019;15:148–160. doi: 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntyre C.C., Anderson R.W. Deep brain stimulation mechanisms: The control of network activity via neurochemistry modulation. J. Neurochem. 2016;139((Suppl. S1)):338–345. doi: 10.1111/jnc.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie C.L., Shao B., Chen J., Zhou Y., Lin S.Y., Wang W.W. Effects of neurostimulation for advanced Parkinson’s disease patients on motor symptoms: A multiple-treatments meta-analysas of randomized controlled trials. Sci. Rep. 2016;6:25285. doi: 10.1038/srep25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St George R.J., Nutt J.G., Burchiel K.J., Horak F.B. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010;75:1292–1299. doi: 10.1212/WNL.0b013e3181f61329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams A., Gill S., Varma T., Jenkinson C., Quinn N., Mitchell R., Scott R., Ives N., Rick C., Daniels J., et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): A randomised, open-label trial. Lancet Neurol. 2010;9:581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver F.M., Follett K., Stern M., Hur K., Harris C., Marks W.J., Rothlind J., Sagher O., Reda D., Moy C.S., et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: A randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zrinzo L., Foltynie T., Limousin P., Hariz M.I. Reducing hemorrhagic complications in functional neurosurgery: A large case series and systematic literature review. J. Neurosurg. 2012;116:84–94. doi: 10.3171/2011.8.JNS101407. [DOI] [PubMed] [Google Scholar]
- 17.Xiaowu H., Xiufeng J., Xiaoping Z., Bin H., Laixing W., Yiqun C., Jinchuan L., Aiguo J., Jianmin L. Risks of intracranial hemorrhage in patients with Parkinson’s disease receiving deep brain stimulation and ablation. Parkinsonism Relat. Disord. 2010;16:96–100. doi: 10.1016/j.parkreldis.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Jitkritsadakul O., Bhidayasiri R., Kalia S.K., Hodaie M., Lozano A.M., Fasano A. Systematic review of hardware-related complications of Deep Brain Stimulation: Do new indications pose an increased risk? Brain Stimul. 2017;10:967–976. doi: 10.1016/j.brs.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Friehs G.M., Park M.C., Goldman M.A., Zerris V.A., Norén G., Sampath P. Stereotactic radiosurgery for functional disorders. Neurosurg. Focus. 2007;23:E3. doi: 10.3171/FOC-07/12/E3. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg H.M., Krishna V., Elias W.J., Cosgrove G.R., Gandhi D., Aldrich C.E., Fishman P.S. MR-guided focused ultrasound pallidotomy for Parkinson’s disease: Safety and feasibility. J. Neurosurg. 2020;135:792–798. doi: 10.3171/2020.6.JNS192773. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Fernández R., Máñez-Miró J.U., Rodríguez-Rojas R., Del Álamo M., Shah B.B., Hernández-Fernández F., Pineda-Pardo J.A., Monje M.H.G., Fernández-Rodríguez B., Sperling S.A., et al. Randomized Trial of Focused Ultrasound Subthalamotomy for Parkinson’s Disease. N. Engl. J. Med. 2020;383:2501–2513. doi: 10.1056/NEJMoa2016311. [DOI] [PubMed] [Google Scholar]
- 22.Jung N.Y., Park C.K., Kim M., Lee P.H., Sohn Y.H., Chang J.W. The efficacy and limits of magnetic resonance-guided focused ultrasound pallidotomy for Parkinson’s disease: A Phase I clinical trial. J. Neurosurg. 2018;130:1853–1861. doi: 10.3171/2018.2.JNS172514. [DOI] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov Safety and Initial Effectiveness of Transcranial MR Guided Focused Ultrasound for the Treatment of Parkinson’s Disease (TDPD) [(accessed on 31 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04002596.
- 24.ClinicalTrials.gov Parkinson’s Disease (PD) Treated with Focused Ultrasound Subthalamotomy at an Early Stage (EarlyFocus) [(accessed on 31 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04692116.
- 25.Cho S.S., Strafella A.P. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brys M., Fox M.D., Agarwal S., Biagioni M., Dacpano G., Kumar P., Pirraglia E., Chen R., Wu A., Fernandez H., et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial. Neurology. 2016;87:1907–1915. doi: 10.1212/WNL.0000000000003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ba M., Kong M., Guan L., Yi M., Zhang H. Repetitive transcranial magnetic stimulation (rTMS) improves behavioral and biochemical deficits in levodopa-induced dyskinetic rats model. Oncotarget. 2016;7:58802–58812. doi: 10.18632/oncotarget.11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefaucheur J.P., Antal A., Ayache S.S., Benninger D.H., Brunelin J., Cogiamanian F., Cotelli M., De Ridder D., Ferrucci R., Langguth B., et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin. Neurophysiol. 2017;128:56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 29.Fini M., Tyler W.J. Transcranial focused ultrasound: A new tool for non-invasive neuromodulation. Int. Rev. Psychiatry. 2017;29:168–177. doi: 10.1080/09540261.2017.1302924. [DOI] [PubMed] [Google Scholar]
- 30.Goetz S.M., Deng Z.D. The development and modelling of devices and paradigms for transcranial magnetic stimulation. Int. Rev. Psychiatry. 2017;29:115–145. doi: 10.1080/09540261.2017.1305949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonmassar G., Lee S.W., Freeman D.K., Polasek M., Fried S.I., Gale J.T. Microscopic magnetic stimulation of neural tissue. Nat. Commun. 2012;3:921. doi: 10.1038/ncomms1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien W.D. Ultrasound-biophysics mechanisms. Prog. Biophys. Mol. Biol. 2007;93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller J., Legon W., Opitz A., Sato T.F., Tyler W.J. Transcranial focused ultrasound modulates intrinsic and evoked EEG dynamics. Brain Stimul. 2014;7:900–908. doi: 10.1016/j.brs.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Legon W., Sato T.F., Opitz A., Mueller J., Barbour A., Williams A., Tyler W.J. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 2014;17:322–329. doi: 10.1038/nn.3620. [DOI] [PubMed] [Google Scholar]
- 35.Munn Z., Peters M.D.J., Stern C., Tufanaru C., McArthur A., Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 37.Lee K.S., Zhang J.J.Y., Nga V.D.W., Ng C.H., Tai B.C., Higgins J., Syn N. Tenets for the Proper Conduct and Use of Meta-Analyses: A Practical Guide for Neurosurgeons. World Neurosurg. 2022 doi: 10.1016/j.wneu.2021.09.034. in press. [DOI] [PubMed] [Google Scholar]
- 38.Lee K.S., Zhang J.J.Y., Alamri A., Chari A. Neurosurgery Education in the Medical School Curriculum: A Scoping Review. World Neurosurg. 2020;144:e631–e642. doi: 10.1016/j.wneu.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960;20:37–47. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 40.Chen X., Wang D., Zhang L., Yao H., Zhu H., Zhao N., Peng X., Yang K. Neuroprotective Effect of Low-Intensity Pulsed Ultrasound on the Mouse MPTP/MPP. Ultrasound Med. Biol. 2021;47:2321–2330. doi: 10.1016/j.ultrasmedbio.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 41.Dong Y., Liu D., Zhao Y., Yuan Y., Wang W., Wu S., Liang X., Wang Z., Liu L. Assessment of Neuroprotective Effects of Low-Intensity Transcranial Ultrasound Stimulation in a Parkinson’s Disease Rat Model by Fractional Anisotropy and Relaxation Time T2. Front. Neurosci. 2021;15:590354. doi: 10.3389/fnins.2021.590354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karmacharya M.B., Hada B., Park S.R., Choi B.H. Low-Intensity Ultrasound Decreases α-Synuclein Aggregation via Attenuation of Mitochondrial Reactive Oxygen Species in MPP(+)-Treated PC12 Cells. Mol. Neurobiol. 2017;54:6235–6244. doi: 10.1007/s12035-016-0104-z. [DOI] [PubMed] [Google Scholar]
- 43.Sung C.Y., Chiang P.K., Tsai C.W., Yang F.Y. Low-Intensity Pulsed Ultrasound Enhances Neurotrophic Factors and Alleviates Neuroinflammation in a Rat Model of Parkinson’s Disease. Cereb. Cortex. 2021 doi: 10.1093/cercor/bhab201. [DOI] [PubMed] [Google Scholar]
- 44.Tarnaud T., Joseph W., Martens L., Tanghe E. Computational Modeling of Ultrasonic Subthalamic Nucleus Stimulation. IEEE Trans. Biomed. Eng. 2019;66:1155–1164. doi: 10.1109/TBME.2018.2869042. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z., Yan J., Wang X., Yuan Y., Li X. Transcranial Ultrasound Stimulation Directly Influences the Cortical Excitability of the Motor Cortex in Parkinsonian Mice. Mov. Disord. 2020;35:693–698. doi: 10.1002/mds.27952. [DOI] [PubMed] [Google Scholar]
- 46.Xu T., Lu X., Peng D., Wang G., Chen C., Liu W., Wu W., Mason T.J. Ultrasonic stimulation of the brain to enhance the release of dopamine—A potential novel treatment for Parkinson’s disease. Ultrason. Sonochem. 2020;63:104955. doi: 10.1016/j.ultsonch.2019.104955. [DOI] [PubMed] [Google Scholar]
- 47.Yuan Y., Zhao Z., Wang Z., Wang X., Yan J., Li X. The Effect of Low-Intensity Transcranial Ultrasound Stimulation on Behavior in a Mouse Model of Parkinson’s Disease Induced by MPTP. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28:1017–1021. doi: 10.1109/TNSRE.2020.2978865. [DOI] [PubMed] [Google Scholar]
- 48.Zhao L., Feng Y., Shi A., Zhang L., Guo S., Wan M. Neuroprotective Effect of Low-Intensity Pulsed Ultrasound against MPP. Ultrasound Med. Biol. 2017;43:1986–1999. doi: 10.1016/j.ultrasmedbio.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Zhou H., Niu L., Meng L., Lin Z., Zou J., Xia X., Huang X., Zhou W., Bian T., Zheng H. Noninvasive Ultrasound Deep Brain Stimulation for the Treatment of Parkinson’s Disease Model Mouse. Research. 2019;2019:1748489. doi: 10.34133/2019/1748489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou H., Niu L., Xia X., Lin Z., Liu X., Su M., Guo R., Meng L., Zheng H. Wearable Ultrasound Improves Motor Function in an MPTP Mouse Model of Parkinson’s Disease. IEEE Trans. Biomed. Eng. 2019;66:3006–3013. doi: 10.1109/TBME.2019.2899631. [DOI] [PubMed] [Google Scholar]
- 51.Gross A., McDonnell J.M., Korsmeyer S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 52.Cory S., Adams J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 53.Sas K., Robotka H., Toldi J., Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 54.Rekha K.R., Selvakumar G.P. Gene expression regulation of Bcl2, Bax and cytochrome-C by geraniol on chronic MPTP/probenecid induced C57BL/6 mice model of Parkinson’s disease. Chem. Biol. Interact. 2014;217:57–66. doi: 10.1016/j.cbi.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Robinson E.J., Aguiar S.P., Kouwenhoven W.M., Starmans D.S., von Oerthel L., Smidt M.P., van der Heide L.P. Survival of midbrain dopamine neurons depends on the Bcl2 factor Mcl1. Cell Death Discov. 2018;4:107. doi: 10.1038/s41420-018-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kluck R.M., Bossy-Wetzel E., Green D.R., Newmeyer D.D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 57.Castro-Caldas M., Carvalho A.N., Rodrigues E., Henderson C.J., Wolf C.R., Rodrigues C.M., Gama M.J. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Mol. Neurobiol. 2012;46:475–486. doi: 10.1007/s12035-012-8295-4. [DOI] [PubMed] [Google Scholar]
- 58.Choi S., Oh J.Y., Kim S.J. Ginsenoside Rh2 induces Bcl-2 family proteins-mediated apoptosis in vitro and in xenografts in vivo models. J. Cell Biochem. 2011;112:330–340. doi: 10.1002/jcb.22932. [DOI] [PubMed] [Google Scholar]
- 59.Offen D., Beart P.M., Cheung N.S., Pascoe C.J., Hochman A., Gorodin S., Melamed E., Bernard R., Bernard O. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine neurotoxicity. Proc. Natl. Acad. Sci. USA. 1998;95:5789–5794. doi: 10.1073/pnas.95.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang L., Matthews R.T., Schulz J.B., Klockgether T., Liao A.W., Martinou J.C., Penney J.B., Hyman B.T., Beal M.F. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J. Neurosci. 1998;18:8145–8152. doi: 10.1523/JNEUROSCI.18-20-08145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okochi M., Walter J., Koyama A., Nakajo S., Baba M., Iwatsubo T., Meijer L., Kahle P.J., Haass C. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J. Biol. Chem. 2000;275:390–397. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- 62.Simón-Sánchez J., Schulte C., Bras J.M., Sharma M., Gibbs J.R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S.W., Hernandez D.G., et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devine M.J., Ryten M., Vodicka P., Thomson A.J., Burdon T., Houlden H., Cavaleri F., Nagano M., Drummond N.J., Taanman J.W., et al. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat. Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin L.F., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 65.Fan C.H., Ting C.Y., Lin C.Y., Chan H.L., Chang Y.C., Chen Y.Y., Liu H.L., Yeh C.K. Noninvasive, Targeted, and Non-Viral Ultrasound-Mediated GDNF-Plasmid Delivery for Treatment of Parkinson’s Disease. Sci. Rep. 2016;6:19579. doi: 10.1038/srep19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pouliopoulos A.N., Kwon N., Jensen G., Meaney A., Niimi Y., Burgess M.T., Ji R., McLuckie A.J., Munoz F.A., Kamimura H.A.S., et al. Safety evaluation of a clinical focused ultrasound system for neuronavigation guided blood-brain barrier opening in non-human primates. Sci. Rep. 2021;11:15043. doi: 10.1038/s41598-021-94188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen K.T., Wei K.C., Liu H.L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019;10:86. doi: 10.3389/fphar.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carpentier A., Canney M., Vignot A., Reina V., Beccaria K., Horodyckid C., Karachi C., Leclercq D., Lafon C., Chapelon J.Y., et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016;8:343re342. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 69.Fedak K.M., Bernal A., Capshaw Z.A., Gross S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes. Epidemiol. 2015;12:14. doi: 10.1186/s12982-015-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyler W.J., Tufail Y., Finsterwald M., Tauchmann M.L., Olson E.J., Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tufail Y., Yoshihiro A., Pati S., Li M.M., Tyler W.J. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 2011;6:1453–1470. doi: 10.1038/nprot.2011.371. [DOI] [PubMed] [Google Scholar]
- 72.Kubanek J., Shukla P., Das A., Baccus S.A., Goodman M.B. Ultrasound Elicits Behavioral Responses through Mechanical Effects on Neurons and Ion Channels in a Simple Nervous System. J. Neurosci. 2018;38:3081–3091. doi: 10.1523/JNEUROSCI.1458-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tufail Y., Matyushov A., Baldwin N., Tauchmann M.L., Georges J., Yoshihiro A., Tillery S.I., Tyler W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Kamimura H.A., Wang S., Chen H., Wang Q., Aurup C., Acosta C., Carneiro A.A., Konofagou E.E. Focused ultrasound neuromodulation of cortical and subcortical brain structures using 1.9 MHz. Med. Phys. 2016;43:5730. doi: 10.1118/1.4963208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pouget P., Frey S., Ahnine H., Attali D., Claron J., Constans C., Aubry J.F., Arcizet F. Neuronavigated Repetitive Transcranial Ultrasound Stimulation Induces Long-Lasting and Reversible Effects on Oculomotor Performance in Non-human Primates. Front. Physiol. 2020;11:1042. doi: 10.3389/fphys.2020.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoo S., Mittelstein D.R., Hurt R., Lacroix J., Shapiro M.G. Focused ultrasound excites neurons via mechanosensitive calcium accumulation and ion channel amplification. Nat Commun. 2022;13:493. doi: 10.1038/s41467-022-28040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krasovitski B., Frenkel V., Shoham S., Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA. 2011;108:3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoo S.S., Bystritsky A., Lee J.H., Zhang Y., Fischer K., Min B.K., McDannold N.J., Pascual-Leone A., Jolesz F.A. Focused ultrasound modulates region-specific brain activity. Neuroimage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kubanek J. Neuromodulation with transcranial focused ultrasound. Neurosurg. Focus. 2018;44:E14. doi: 10.3171/2017.11.FOCUS17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jerusalem A., Al-Rekabi Z., Chen H., Ercole A., Malboubi M., Tamayo-Elizalde M., Verhagen L., Contera S. Electrophysiological-mechanical coupling in the neuronal membrane and its role in ultrasound neuromodulation and general anaesthesia. Acta Biomater. 2019;97:116–140. doi: 10.1016/j.actbio.2019.07.041. [DOI] [PubMed] [Google Scholar]
- 81.Prieto M.L., Ömer O., Khuri-Yakub B.T., Maduke M.C. Dynamic response of model lipid membranes to ultrasonic radiation force. PLoS ONE. 2013;8:e77115. doi: 10.1371/journal.pone.0077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Renzhiglova E., Ivantsiv V., Xu Y. Difference frequency magneto-acousto-electrical tomography (DF-MAET): Application of ultrasound-induced radiation force to imaging electrical current density. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010;57:2391–2402. doi: 10.1109/TUFFC.2010.1707. [DOI] [PubMed] [Google Scholar]
- 83.Kubanek J., Shi J., Marsh J., Chen D., Deng C., Cui J. Ultrasound modulates ion channel currents. Sci. Rep. 2016;6:24170. doi: 10.1038/srep24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prieto M.L., Firouzi K., Khuri-Yakub B.T., Maduke M. Activation of Piezo1 but Not Na. Ultrasound Med. Biol. 2018;44:1217–1232. doi: 10.1016/j.ultrasmedbio.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang F., Shi Y., Lu L., Liu L., Cai Y., Zheng H., Liu X., Yan F., Zou C., Sun C., et al. Targeted delivery of GDNF through the blood-brain barrier by MRI-guided focused ultrasound. PLoS ONE. 2012;7:e52925. doi: 10.1371/journal.pone.0052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang Q., Deng J., Wang F., Chen S., Liu Y., Wang Z., Cheng Y. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp. Neurol. 2012;233:350–356. doi: 10.1016/j.expneurol.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 87.Allen N.J., Barres B.A. Neuroscience: Glia—More than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 88.De Luca C., Colangelo A.M., Virtuoso A., Alberghina L., Papa M. Neurons, Glia, Extracellular Matrix and Neurovascular Unit: A Systems Biology Approach to the Complexity of Synaptic Plasticity in Health and Disease. Int. J. Mol. Sci. 2020;21:1539. doi: 10.3390/ijms21041539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grosche J., Matyash V., Möller T., Verkhratsky A., Reichenbach A., Kettenmann H. Microdomains for neuron-glia interaction: Parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 90.Tremblay M.E., Cookson M.R., Civiero L. Glial phagocytic clearance in Parkinson’s disease. Mol. Neurodegener. 2019;14:16. doi: 10.1186/s13024-019-0314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oh S.J., Lee J.M., Kim H.B., Lee J., Han S., Bae J.Y., Hong G.S., Koh W., Kwon J., Hwang E.S., et al. Ultrasonic Neuromodulation via Astrocytic TRPA1. Curr. Biol. 2020;30:948. doi: 10.1016/j.cub.2020.02.042. [DOI] [PubMed] [Google Scholar]
- 92.Lee C.J., Mannaioni G., Yuan H., Woo D.H., Gingrich M.B., Traynelis S.F. Astrocytic control of synaptic NMDA receptors. J. Physiol. 2007;581:1057–1081. doi: 10.1113/jphysiol.2007.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clasadonte J., Dong J., Hines D.J., Haydon P.G. Astrocyte control of synaptic NMDA receptors contributes to the progressive development of temporal lobe epilepsy. Proc. Natl. Acad. Sci. USA. 2013;110:17540–17545. doi: 10.1073/pnas.1311967110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blackmore D.G., Turpin F., Palliyaguru T., Evans H.T., Chicoteau A., Lee W., Pelekanos M., Nguyen N., Song J., Sullivan R.K.P., et al. Low-intensity ultrasound restores long-term potentiation and memory in senescent mice through pleiotropic mechanisms including NMDAR signaling. Mol. Psychiatry. 2021 doi: 10.1038/s41380-021-01129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee K.S., Young A., King H., Jenkins A.T.A., Davies A. Variation in Definitions of Burn Wound Infection Limits the Validity of Systematic Review Findings in Burn Care: A Systematic Review of Reviews. Burns. 2021. in press . [DOI] [PubMed]
- 96.Björklund A., Cenci-Nilsson A. Recent Advances in Parkinson’s Disease. Volume 252 Elsevier; Amsterdam, The Netherlands: 2020. [Google Scholar]
- 97.Folloni D., Verhagen L., Mars R.B., Fouragnan E., Constans C., Aubry J.F., Rushworth M.F.S., Sallet J. Manipulation of Subcortical and Deep Cortical Activity in the Primate Brain Using Transcranial Focused Ultrasound Stimulation. Neuron. 2019;101:1109–1116. doi: 10.1016/j.neuron.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawaguchi M., Shimizu K., Furuya H., Sakamoto T., Ohnishi H., Karasawa J. Effect of isoflurane on motor-evoked potentials induced by direct electrical stimulation of the exposed motor cortex with single, double, and triple stimuli in rats. Anesthesiology. 1996;85:1176–1183. doi: 10.1097/00000542-199611000-00027. [DOI] [PubMed] [Google Scholar]
- 99.Han S., Kim M., Kim H., Shin H., Youn I. Ketamine Inhibits Ultrasound Stimulation-Induced Neuromodulation by Blocking Cortical Neuron Activity. Ultrasound Med. Biol. 2018;44:635–646. doi: 10.1016/j.ultrasmedbio.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 101.Soldner F., Hockemeyer D., Beard C., Gao Q., Bell G.W., Cook E.G., Hargus G., Blak A., Cooper O., Mitalipova M., et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clennell B., Steward T.G.J., Elley M., Shin E., Weston M., Drinkwater B.W., Whitcomb D.J. Transient ultrasound stimulation has lasting effects on neuronal excitability. Brain Stimul. 2021;14:217–225. doi: 10.1016/j.brs.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Legon W., Adams S., Bansal P., Patel P.D., Hobbs L., Ai L., Mueller J.K., Meekins G., Gillick B.T. A retrospective qualitative report of symptoms and safety from transcranial focused ultrasound for neuromodulation in humans. Sci. Rep. 2020;10:5573. doi: 10.1038/s41598-020-62265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simonsohn U., Nelson L.D., Simmons J.P. P-curve: A key to the file-drawer. J. Exp. Psychol. Gen. 2014;143:534–547. doi: 10.1037/a0033242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Please find available data in the Supplementary Materials.