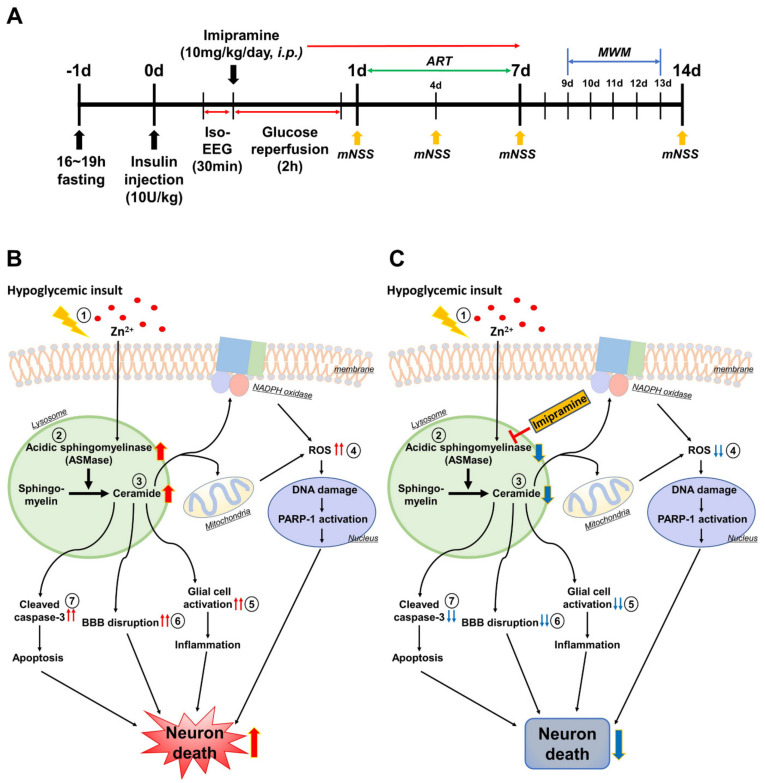
Figure 8.
Possible association of ASMase, ceramide, and neuronal death under hypoglycemia/glucose reperfusion conditions. This schematic illustration explains the effects of imipramine on the process of the hypoglycemia-induced neuronal death mechanism. (A) Experimental timeline. (B) Hypoglycemia-induced neuronal death mechanism: (1) after hypoglycemia, excessive zinc release and translocation from pre-synapse to post-synapse; (2) the excessive zinc activates ASMase abnormally; (3) the activated ASMase decomposes sphingomyelin into ceramide; (4) the increased ceramide acts on the mitochondria and NADPH oxidase, increasing ROS production; (5) the increased ceramide activates glial cells, causing inflammation; (6) the increased ceramide causes blood–brain barrier disruption; (7) the increased ceramide increases capase-3, resulting in apoptosis. When these conditions dominate, neuronal death is more likely to occur severely. (C) The effects of imipramine on hypoglycemia-induced neuronal death. The administration of imipramine can inhibit excessive ASMase activation and then ceramide production. Therefore, there is a possibility that imipramine can prevent neuronal death after hypoglycemia.