Abstract
Optimal scoring system for clinical prognostic factors in patients with unresectable hepatocellular carcinoma (HCC) is currently uncertain. We aimed to develop and externally validate an easy to use tool, particularly for this population, and named it the “unresectable hepatocellular carcinoma prognostic index” (UHPI). We evaluated the data of patients with treatment‐naive unresectable HCC who were diagnosed in the training center from 2010 to 2019 (n = 209). A simple prognostic model was developed by assigning points for each covariate in proportion to the beta coefficients in the Cox multivariable model. Predictive performance and distinction ability of the UHPI were further evaluated in an independent European validation cohort (n = 147) and compared with 11 other available models. A simple scoring system was derived, assigning 0.5/1/2 scores for six independent covariates including, the Child‐Pugh score, Eastern Cooperative Oncology Group performance status, maximum tumor size, vascular invasion or extrahepatic metastasis, lymph node involvement, and alpha‐fetoprotein. The UHPI score, ranging from 0 to 6, showed superior performance in prognosis prediction and outperformed 11 other staging or prognostic models, giving the highest homogeneity (c‐index, 6‐month and 1‐year area under the receiver operator characteristic curves), lowest Akaike information criterion, and –2 log‐likelihood ratio values. The UHPI score allocated well the risk of patients with unresectable HCC for mortality within the first year, using two cut‐off values (low‐risk, <0.5; intermediate‐risk, 0.5‐2; high‐risk, >2). Conclusion: The UHPI score can predict prognosis better than other systems in subjects with unresectable HCC and can be used in clinical practice or trials to estimate the 6‐month and 1‐year survival probabilities for this group.
A simple scoring system was derived, assigning 0.5 / 1 / 2 scores for six independent co‐variates including, the Child‐Pugh class, Eastern Cooperative Oncology Group performance status, maximum tumor size, vascular invasion or extrahepatic metastasis, lymph node involvement, and alpha fetoprotein.
UHPI score, ranging from 0 to 6, showed superior performance in prognosis prediction and outperformed 11 other staging or prognostic models in unresectable hepatocellular carcinoma patients.
UHPI‐stratified three risk categories can be used in clinical practice to assess 6th‐month and 1st‐year survival probabilities, and in clinical trials to estimate the potential candidates for more aggressive treatment approaches.

Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- AFP
alpha‐fetoprotein
- AIC
Akaike information criteria
- ALBI
albumin‐bilirubin
- AUROC
area under the receiver operator characteristic
- BCLC
Barcelona Clinic Liver Cancer
- BSC
best supportive care
- CHB
chronic hepatitis B
- CHC
chronic hepatitis C
- CI
confidence interval
- c‐index
concordance index
- CLIP
Cancer of the Liver Italian Program
- CPS
Child‐Pugh score
- CSPH
clinically significant portal hypertension
- CUPI
Chinese University prognostic index
- EASL
European Association for the Study of Liver Diseases
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- HKLC
Hong‐Kong Liver Cancer
- IQR
interquartile range
- ITA.LI.CA
Italian Liver Cancer
- MELD
Model for End‐Stage Liver Disease
- MELD‐Na
Model for End‐Stage Liver Disease‐Sodium
- MESH
Model to Estimate Survival in Patients With HCC
- MESIAH
Model to Estimate Survival in Ambulatory Patients With HCC
- NASH
nonalcoholic steatohepatitis
- NIACE
nodular numbers, tumor infiltration, alpha‐fetoprotein level, Child‐Pugh score, and Eastern Cooperative Oncology Group
- OS
overall survival
- ROC
receiver operating characteristic
- TACE
transarterial chemoembolization
- TARE
transarterial radioembolization
- TNM8
Tumor Node Metastasis version 8
- UHPI
unresectable hepatocellular carcinoma prognostic index
Hepatocellular carcinoma (HCC) is the seventh most common cancer and the second leading cause of cancer‐related deaths globally.( 1 ) Prognosis of HCC is complex and multifactorial. Unlike other solid malignancies, prognosis depends not only on tumor burden but also three other key factors: hepatic synthetic function, overall health status of the patient, and type of treatment. Staging of HCC is the crucial step for determining management strategy and thereby prognosis. To date, several clinical staging systems of HCC have been proposed. These include Barcelona Clinic Liver Cancer (BCLC), American Joint Committee on Cancer Tumor Node Metastasis version 8 (AJCC‐TNM8), Okuda, and the most recent Hong‐Kong Liver Cancer (HKLC).( 2 , 3 , 4 , 5 ) Due to the lack of ability to predict the expected prognosis in these staging systems, several prognostic scoring models have been developed to cover this insufficiency. Most prominent prognostic scoring models were the Chinese University prognostic index (CUPI), Cancer of the Liver Italian Program (CLIP), Japanese Integrated Staging (JIS), Tokyo Score, and the more recent Model to Estimate Survival in Ambulatory Patients With HCC (MESIAH), Model to Estimate Survival in Patients With HCC (MESH), and Italian Liver Cancer (ITA.LI.CA) prognostic scores.( 6 , 7 , 8 , 9 , 10 , 11 , 12 ) BCLC and HKLC are primarily staging systems that were designed to guide treatment decisions. The other systems targeted prognosis prediction that does not give guidance for treatment.
HCC encompasses heterogeneous subgroups that show differences in tumor burden and liver functions and is associated with wide differences in applied treatment modalities and survival outcomes. When HCC is caught in an early stage and treated with curative intent, the patient is expected to have a prolonged survival compared to HCCs not suitable to curative treatment modalities, so‐called unresectable HCC. However, treatment of HCCs beyond the curative options is not specific, and the outcome is usually unpredictable. Existing staging systems possess the paucity of being constructed from cohorts treated with various types of treatment modalities and using the statistically significant variables from patients with both early stage and unresectable HCC. In this regard, conventional staging systems may not be inclusive of literal prognostic parameters and representative of their exact powers when applied to patients with unresectable HCC. A prognostic model for advanced‐stage HCC according to the BCLC system (BCLC stage C) was developed in 2016 based on nodular numbers, tumor infiltration, alpha‐fetoprotein level (AFP), Child‐Pugh score (CPS), and Eastern Cooperative Oncology Group (ECOG) score (NIACE) but was only investigated in those with extrahepatic spread and did not cover all unresectable HCCs.( 13 ) The optimal prognostic system to refine the patients with HCC who are not candidates for curative therapy options is currently uncertain. A specific validated model established especially for this population is urgently needed.
In the present article, we aimed to derive a novel prognostic index for patients with HCC exceeding the curability border and named it the “unresectable HCC prognostic index” (UHPI). We then aimed to externally validate the UHPI in an independent European cohort. Another objective was to compare the UHPI with other conventional staging or prognostic scoring systems to determine whether it is the most suitable system for this group of patients.
Materials and Methods
Patient Selection and Study Design
We reviewed the database of consecutive patients with HCC who were treatment naive in the Gastroenterology and Hepatology Unit at Marmara University, School of Medicine Hospital, in a 9‐year period (February 2010‐March 2019). Baseline demographic, clinical, laboratory, and radiologic data were collected and evaluated. HCC was either diagnosed by typical radiologic appearance and/or histologically according to European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) guidelines.( 14 , 15 ) Lymph node involvement, vascular invasion, and metastasis were determined radiologically. Patients with insufficient entry or follow‐up data and uncertain HCC diagnosis were excluded. Clinically significant portal hypertension (CSPH) was defined as the presence of esophagogastric varices or thrombocytopenia with splenomegaly because hepatic venous pressure gradient measurement was not feasible for performing on this patient population.
Treatment decisions were guided at the multidisciplinary HCC council of our hospital with the attendance of an experienced hepatologist, medical oncologist, interventional radiologist, nuclear medicine physician, and liver‐transplant surgeon in line with the EASL and AASLD HCC clinical practice guidelines. All patients with adequate liver function and radiologically resectable tumors were initially evaluated for surgical resection. Patients within the Milan criteria (one tumor ≤5 cm; or three or fewer tumors with each tumor ≤3 cm) and having adequate performance status were offered liver transplantation.( 16 ) If they were not amenable or unwilling to undergo surgical approaches, they were offered transarterial chemoembolization (TACE), transarterial radioembolization (TARE), or local ablative procedure depending on the size, number, and position of tumoral lesions. Systemic therapy was considered when the patient was not suitable for any curative treatment and/or locoregional treatment modality. Patients with overt liver failure or poor performance status at the time of presentation were not given any anticancer therapy and followed by best supportive care (BSC).
Unresectable HCC was defined as a liver tumor limited to the liver but beyond Milan criteria and inadequate liver function and/or evidence of vascular or distant metastasis and/or poor patient performance (ECOG ≥2), making them unsuitable for curative therapies. Patients with HCC who underwent at least one curative treatment modality, including surgical resection, liver transplantation, and/or local ablative therapies, were excluded from the analysis. After the exclusion, 209 patients treated with either noncurative options (TACE, TARE, and/or systemic therapies) or followed with BSC were enrolled as the training cohort.
Calculation of Other Scoring Systems and Overall Survival
Twelve baseline scores (BCLC, TNM, CUPI, CLIP, JIS, Tokyo, Okuda, HKLC, MESH, MESIAH, NIACE, and ITA.LI.CA) were noted for each patient using the collected clinical, radiologic, and laboratory data. Patients were not included if they had any missing data relative to the 12 classifications. Overall survival (OS) time was calculated from the date of initial HCC diagnosis in our unit until the date of death or the last follow‐up, and survival was censored on March 1, 2020.
Development of the UHPI
The prognostic index was developed by considering all patient‐related (age, sex, body mass index, comorbidities, cigarette and alcohol consumption), liver‐related (etiology, laboratory, and complications of cirrhosis; indicators of liver disease severity, including CPS, Model for End‐Stage Liver Disease [MELD], and MELD‐Na score; albumin‐bilirubin [ALBI] grade; and tumor‐related (maximum tumor size, number of lesions, up to seven and up to 11 criteria, lymph node involvement, vascular invasion, and extrahepatic metastasis) candidate prognostic factors. Only variables that are commonly assessed in clinical practice were included in the model as potential parameters to enable comparison between different institutions. Cut‐off values were determined based on the most widely accepted thresholds or the Youden index identified by receiver operating characteristic (ROC) curves.
As indicators of liver disease severity (CPS, MELD, MELD‐Na, and ALBI) and intrahepatic tumor burden (tumor size and the number of lesions, up to seven and up to 11 criteria) share common parameters, only the variables with the most significant individual prognostic value, giving the highest individual hazard ratio in the univariate regression analysis were included in the multivariate model (Supporting Table S1). For indicators of liver disease severity, the parameters that are not involved in the one with the highest prognostic significance were included in the multivariate model separately to prevent any loss of predictive performance.
A scoring system was initially derived by assigning exact points for each covariate in proportion to the beta coefficients in the final multivariable model. To improve clinical practicality, coefficients from the final model were standardized by dividing the smallest coefficient and then rounding to allow simple calculation of the new index. We then performed sensitivity analysis to verify that the discriminatory power lost in this simplification process was negligible. Finally, the UHPI score was divided into three categories to obtain low‐risk, intermediate‐risk, and high‐risk 6‐month and 1‐year survival probabilities. Taking advantage of the significant shifts in the median OS time with increasing UHPI scores, the cutoffs for three‐group risk stratification were determined.
Validation of the UHPI
We validated the UHPI score in an external cohort of patients with unresectable HCC, defined by the same criteria, from the Hepatology Unit, University Hospital of Pisa, Italy. Similarly, treatment decisions were given by a multidisciplinary board with the attendance of the same specialties in the validation center, and both centers had identical treatment strategies throughout the study period. Same selection and exclusion criteria were applied to form the validation cohort. The UHPI score was checked for external validity in the Pisa cohort using the prespecified cut‐off values for categorical variables. We calculated 11 other staging models or prognostic scores, except the CUPI score due to lack of data on symptomatic presentation status, using the complete variables obtained from each patient. The performance of the UHPI was compared with other staging or prognostic systems in the validation cohort as well by applying the same analytic tools.
Ethical Considerations
The study protocol was approved by the local research ethical review board of the Marmara University, School of Medicine (Approval date July 24, 2020; Approval No. 09.2020.860). The study was done in accordance with the principles of the Helsinki Declaration. Informed consent was not required as this was a retrospective evaluation of the collected data.
Statistical Analysis
Continuous data were expressed as mean ± SD or medians with interquartile ranges (IQRs), while categorical variables were presented as absolute numbers with percentages. To assess potential prognostic factors, we performed the log‐rank test and Cox regression analysis in univariate analysis for categorical and noncategorical variables, respectively. Variables with P < 0.05 in univariate analysis were included in a Cox proportional regression model with a forward selection method to identify independent predictors of OS. Finally, variables with P < 0.05 were weighted using beta coefficients from the final multivariate model to derivate a prognostic scoring system.
We compared the prognosis prediction accuracy between the scoring systems using several methods to identify homogeneity, discriminatory ability, and monotonicity of gradients. Discriminatory capacity and goodness of fit for survival prediction of the UHPI score were tested and compared with other models using the concordance index (c‐index), Akaike information criteria (AIC), Wald test, and –2 log‐likelihood ratio derived from the Cox regression model.( 17 ) C‐index estimates the proportion of correct predictions, and a higher c‐index value indicates a better prognostic score. Results of the c‐index varied from 0.5 (no discrimination) to 1 (perfect discrimination). A c‐index value higher than 0.8 indicates an excellent model, while 0.7 to 0.8 is considered decent. The smaller AIC and –2 log‐likelihood ratio with higher Wald test values indicate better performance of the model. To evaluate the predictive accuracy for survival at 6 months and 1 year, we performed time‐dependent area under the receiver operating characteristic (AUROC) curves for each system. The median OS times were estimated using the Kaplan‐Meier method. All statistical analyses were conducted using SPSS version 20.0 (IBM, Armonk, NY) and R Open Source Software version 4.0.3.
Results
Baseline Characteristics of the Training Cohort
Baseline characteristics of the training (n = 209) and validation (n = 147) cohorts are shown in Table 1. The majority of the subjects were men (77%), and the median age was 64 years. The most common etiology of HCC was chronic hepatitis B (CHB) virus (57.4%) in the training cohort, followed by nonalcoholic steatohepatitis (NASH) and chronic hepatitis C (CHC) virus. The median CPS was 6.( 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ) The median maximum tumor size was 70 mm, and approximately half (52.6%) had a single lesion. Overall, 64.6% received active treatment. The median OS time was 9.2 (IQR, 1.0‐106.7) months, and 167 (79.9%) patients died in the study period. Six‐month and 1‐year survival rates were 63.6% (n = 133) and 42.6% (n = 89), respectively.
TABLE 1.
Demographic, Laboratory, Tumor, and Treatment Characteristics of the Training and Validation Cohort
| Training Cohort (n = 209) | Validation Cohort (n = 147) | P Value | |
|---|---|---|---|
| Age, years | 64 (20‐89) | 70 (38‐90) | <0.001 |
| Male sex | 165 (78.9) | 113 (76.9) | 0.64 |
| Cirrhosis | 183 (87.6) | 147 (100) | <0.001 |
| Etiology | |||
| CHB | 120 (57.4) | 21 (14.3) | |
| NASH | 45 (21.5) | 29 (19.7) | |
| CHC | 35 (16.7) | 70 (47.6) | <0.001 |
| Alcoholic | 6 (2.9) | 24 (16.3) | |
| CHB+CHC | 2 (1.0) | 1 (0.7) | |
| Autoimmune/PBC | 1 (0.5) | 1 (0.7) | |
| CHC+alcohol | — | 1 (0.7) | |
| ECOG performance status | |||
| 0 | 123 (58.9) | 104 (70.7) | |
| 1 | 45 (21.5) | 30 (20.4) | |
| 2 | 31 (14.8) | 10 (6.8) | 0.05 |
| 3 | 7 (3.3) | 3 (2.0) | |
| 4 | 3 (1.4) | — | |
| Laboratory Values | |||
| AST | 63 (13‐782) | 67 (14‐486) | 0.83 |
| ALT | 44 (7‐727) | 50 (5‐386) | 0.25 |
| ALP | 151 (42‐661) | 143 (22‐1,348) | 0.48 |
| GGT | 123 (22‐2,018) | 129 (18‐1,059) | 0.8 |
| Total bilirubin | 1.2 (0.3‐12.8) | 1.1 (0.3‐7.6) | 0.31 |
| Albumin | 3.6 (1.7‐6.4) | 3.7 (2.4‐4.9) | 0.09 |
| Creatinine | 0.8 (0.3‐6.6) | 0.8 (0.5‐2.0) | 0.06 |
| INR | 1.2 (0.8‐3.9) | 1.15 (0.95‐2.83) | 0.017 |
| Sodium | 137 (121‐148) | 139 (124‐144) | <0.001 |
| Platelet count, ×103 | 156 (38‐838) | 140 (40‐626) | 0.03 |
| AFP | 92.0 (1.4‐371,458.0) | 36.9 (1.4‐114,963.0) | 0.43 |
| CPS | 6 (5‐13) | 6 (5‐13) | 0.04 |
| Child‐Pugh class | |||
| A | 117 (56.0) | 105 (71.4) | |
| B | 77 (36.8) | 16 (10.9) | <0.001 |
| C | 15 (7.2) | 26 (17.7) | |
| MELD score | 9 (6‐20) | 10 (6‐27) | 0.004 |
| MELD‐Na score | 14 (9‐30) | 11 (5‐26) | <0.001 |
| ALBI score | –2.20 (–4.50 to –0.14) | –2.26 (–3.64 to –0.92) | 0.09 |
| ALBI grade | |||
| A1 | 52 (24.9) | 47 (32.0) | |
| A2 | 122 (58.4) | 81 (55.1) | 0.27 |
| A3 | 35 (16.7) | 19 (12.9) | |
| Ascites | 92 (44.0) | 52 (35.4) | 0.12 |
| Hepatic encephalopathy | 7 (3.3) | 13 (8.8) | 0.03 |
| CSPH | 127 (60.8) | 113 (76.9) | 0.001 |
| Maximum tumor size, mm | 70 (11‐200) | 57 (15‐160) | <0.001 |
| Number of tumoral lesions | |||
| 1 | 110 (52.6) | 34 (23.1) | |
| 2 | 25 (12.0) | 28 (19.0) | <0.001 |
| 3 | 17 (8.1) | 27 (18.4) | |
| >3 | 57 (27.3) | 58 (39.5) | |
| In up to 7 criteria | 56 (26.8) | 58 (39.5) | 0.01 |
| In up to 11 criteria | 115 (55.0) | 114 (77.6) | <0.001 |
| Lymph node involvement | 57 (27.3) | 32 (21.8) | 0.23 |
| Vascular invasion | 67 32.1) | 74 (50.3) | 0.001 |
| Portal vein | –55 (26.3) | –66 (44.9) | |
| Hepatic vein | –7 (3.4) | –4 (2.7) | |
| Inferior vena cava | –5 (2.4) | –4 (2.7) | |
| Extrahepatic metastasis | 16 (7.7) | 11 (7.5) | 0.95 |
| Treatment option | |||
| TACE | 95 (45.5) | 24 (16.3) | |
| Sorafenib | 25 (11.9) | 24 (16.3) | |
| TARE | 12 (5.7) | 13 (8.8) | <0.001 |
| TACE+TARE | 2 (1.0) | — | |
| TACE+Sorafenib | 1 (0.5) | 1 (0.7) | |
| TARE+Sorafenib | — | 3 (2.1) | |
| Best supportive care | 74 (35.4) | 82 (55.8) |
Unless otherwise indicated, values show median (IQR) or number (%).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; INR, international normalized ratio; IQR, interquartile range; NS, not significant; PBC, primary biliary cholangitis.
Baseline Characteristics of the Validation Cohort
Patients in the validation cohort were significantly older (median age, 70 years) than the training cohort, but sex proportions were similar. The most common etiology was CHC (47.6%), followed by NASH and CHB. The median CPS was 6 (IQR, 5‐13). The validation cohort had a significantly smaller median tumor size (57 mm), and nearly a quarter (23.6%) of them had a single lesion. Overall, 44.2% of the validation cohort received active treatment. The median OS time was 12.9 (IQR, 1.0‐104.2) months, and 133 (90.5%) patients died in the study period. Six‐month and 1‐year survival rates were 80.3% (n = 118) and 53.1% (n = 78), respectively. Application of staging and prognostic models to subjects in training and the validation cohort are presented in Supporting Table S2.
Derivation of the UHPI Model
Univariate and multivariate Cox proportional hazards regression analysis in the training cohort are presented in Table 2. The Cox proportional regression model confirmed that increasing CPS, ECOG score ≥2, maximum tumor size >8 cm, vascular invasion or extrahepatic metastasis, lymph node involvement, and AFP >500 ng/mL were independent predictors of worse survival outcomes. The UHPI model was generated according to independent predictors identified by Cox multivariate analysis (Table 3). The newly constructed UHPI model was able to predict survival outcomes better than the 12 previous staging or prognostic systems by showing the highest c‐statistic (0.82), 6‐month (0.84; IQR, 0.79‐0.90) and 1‐year (0.825; IQR, 0.771‐0.88) AUROC values, and Wald test, with the lowest AIC and –2 log‐likelihood ratio (Supporting Table S3 ).
TABLE 2.
Univariate and Multivariate Analysis with Potential Prognostic Factors in the Training Cohort
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | Beta Coefficient | HR | 95% CI | P Value | |
| Age, years | 0.99 | 0.94‐1.01 | 0.23 | ||||
| Male sex | 0.88 | 0.63‐1.22 | 0.44 | ||||
| Etiology (viral vs. nonviral) | 1.14 | 0..81‐1.60 | 0.44 | ||||
| Child‐Pugh class | |||||||
| A | <0.001 | <0.001 | |||||
| B | 2.25 | 1.64‐3.09 | <0.001 | 0.83 | 2.30 | 1.66‐3.20 | <0.001 |
| C | 5.12 | 2.93‐9.21 | <0.001 | 1.87 | 6.53 | 3.55‐12.00 | <0.001 |
| ECOG performance status (0‐1 vs. 2‐4) | 1.98 | 1.38‐2.86 | <0.001 | 0.84 | 2.32 | 1.55‐3.45 | <0.001 |
| CSPH | 1.28 | 0.94‐1.73 | 0.11 | ||||
| Maximum tumor size, (>8 cm vs. ≤8 cm) | 2.07 | 1.53‐2.79 | <0.001 | 0.70 | 2.02 | 1.45‐2.82 | <0.001 |
| Number of tumoral lesions | |||||||
| 1 | 0.02 | ||||||
| 2‐3 | 0.95 | 0.64‐1.40 | 0.78 | 0.25 | 1.28 | 0.85‐1.92 | 0.24 |
| >3 | 1.57 | 1.11‐2.23 | 0.01 | 0.27 | 1.30 | 0.90‐1.90 | 0.16 |
| Lymph node involvement | 1.59 | 1.15‐2.20 | 0.005 | 0.45 | 1.56 | 1.12‐2.19 | 0.009 |
| Vascular invasion or metastasis | 1.96 | 1.44‐2.66 | <0.001 | 0.66 | 1.95 | 1.39‐2.72 | <0.001 |
| AFP (>500 vs. ≤500 ng/mL) | 2.29 | 1.68‐3.10 | <0.001 | 0.58 | 1.79 | 1.29‐2.47 | <0.001 |
| Platelet count (<140 vs. ≤140, ×103) | 1.05 | 0.77‐1.41 | 0.77 | ||||
| ALT (<40 vs. ≥40 IU/L) | 1.35 | 0.99‐1.83 | 0.05 | 0.04 | 1.04 | 0.75‐1.44 | 0.81 |
| ALP (<200 vs. ≥200 IU/L) | 1.85 | 1.35‐2.53 | <0.001 | 0.28 | 1.32 | 0.92‐1.89 | 0.13 |
| GGT (<48 vs. ≥48 IU/L) | 1.19 | 0.80‐1.78 | 0.38 | ||||
| INR (<1.2 vs. ≥1.2) | 1.28 | 0.90‐1.80 | 0.16 | ||||
| Creatinine (≤1.1 vs. >1.1 mg/dL) | 1.10 | 0.74‐1.62 | 0.64 | ||||
| Sodium (<135 vs. ≥135 mEq/L) | 1.21 | 0.86‐1.69 | 0.27 | ||||
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; GGT, gamma‐glutamyltransferase; INR, international normalized ratio; NS, not significant.
TABLE 3.
Unresectable HCC Prognostic Index
| Variable | Point |
|---|---|
| Child‐Pugh class | |
| A | 0 |
| B | 1 |
| C | 2 |
| ECOG performance status | |
| 0‐1 | 0 |
| 2‐4 | 1 |
| Maximum tumor size | |
| ≤8 cm | 0 |
| >8 cm | 1 |
| Vascular invasion or extrahepatic metastasis | 1 |
| Lymph node involvement | 0.5 |
| AFP | |
| <500 ng/mL | 0 |
| ≥500 ng/mL | 0.5 |
Validation of the UHPI Model
The UHPI showed an excellent performance in the validation cohort by giving the highest c‐statistic (0.80), 6‐month (0.83; IQR, 0.75‐0.90) and 1‐year (0.85; IQR, 0.78‐0.91) AUROC value, and Wald test, with the lowest AIC and –2 log‐likelihood ratio, which registered that the UHPI was better than other systems (Supporting Table S3).
Clinical Utility of the UHPI Score
Overall survival distributions according to UHPI scores in the training and validation cohorts are given in Table 4. The UHPI score was divided into three categories to obtain low risk (<0.5), intermediate risk (0.5‐2), and high risk (>2) for 6‐month and 1‐year mortality in the training cohort according to the median OS time >24, 10‐24, and <10 months, respectively. In the training cohort, UHPI low risk (n = 27, 12.9%) showed an OS rate of 100% at 6 months and 96.3% 1 year, whereas the 6‐month and 1‐year OS rates were 78.3% and 50.9% for UHPI intermediate risk (n = 106, 50.7%) and 30.3% and 11.8% for UHPI high risk (n = 76, 36.4%), respectively.
TABLE 4.
OS Distributions According to UHPI Score in the Training and Validation Cohorts
| Training Cohort (n = 209) | Median (IQR) OS, Months | Validation Cohort (n = 147) | Median (IQR) OS, Months |
|---|---|---|---|
| UHPI‐0 (n = 27, 12.9%) | 45.3 (33.9‐56.6) | UHPI‐0 (n = 30, 20.4%) | 40.9 (18.9‐62.9) |
| UHPI‐0.5 (n = 12, 5.7%) | 16.9 (0.1‐37.0) | UHPI‐0.5 (n = 4, 2.7%) | 15.6 (0.1‐59.1) |
| UHPI‐1 (n = 42, 20.1%) | 13.5 (9.3‐17.8) | UHPI‐1 (n = 37, 25.2%) | 17.5 (8.3‐26.7) |
| UHPI‐1.5 (n = 25, 12.0% | 10.8 (7.7‐13.8) | UHPI‐1.5 (n = 17, 11.6%) | 13.1 (10.9‐15.3) |
| UHPI‐2 (n = 27, 12.9%) | 11.2 (6.8‐15.5) | UHPI‐2 (n = 17, 11.6%) | 9.4 (5.9‐12.8) |
| UHPI‐2.5 (n = 23, 11.0%) | 4.8 (2.6‐7.1) | UHPI‐2.5 (n = 15, 10.2%) | 9.8 (5.8‐13.9) |
| UHPI‐3 (n = 20, 9.6%) | 6.1 (0.4‐11.8) | UHPI‐3 (n = 11, 7.5%) | 7.1 (4.7‐9.5) |
| UHPI‐3.5 (n = 13, 6.2%) | 2.3 (1.6‐2.9) | UHPI‐3.5 (n = 7, 4.8%) | 4.8 (2.2‐7.4) |
| UHPI‐4 (n = 13, 6.2%) | 2.2 (1.1‐3.4) | UHPI‐4 (n = 3, 2.0%) | 3.8 (1.3‐6.2) |
| UHPI‐4.5 (n = 7, 3.3%) | 1.5 (0.7‐2.2) | UHPI‐4.5 (n = 3, 2.0%) | 3.9 (1.1‐6.7) |
| UHPI‐5.5 | ‐ | UHPI‐5.5 (n = 1, 0.7%) | 2.6 |
| UHPI‐6 | ‐ | UHPI‐6 (n = 2, 1.4) | 3.3 |
In the validation cohort, each risk group had compatible median survival time and prespecified OS cutoffs as the training cohort (Table 4). In the validation cohort, UHPI low risk (n = 30, 20.4%) showed OS rates of 100% at 6 months and 93.3% at 1 year, whereas 6‐month and 1‐year OS rates were 86.7% and 58.7% for UHPI intermediate‐risk (n = 75, 51%) and 54.8% and 14.3% for UHPI high‐risk (n = 42, 28.6%) groups, respectively (Fig. 1). The rates of patient follow‐up with BSC was significantly higher in the UHPI high‐risk group in both training (52.6%) and validation (78.6%) cohorts (Supporting Table S4).
FIG. 1.
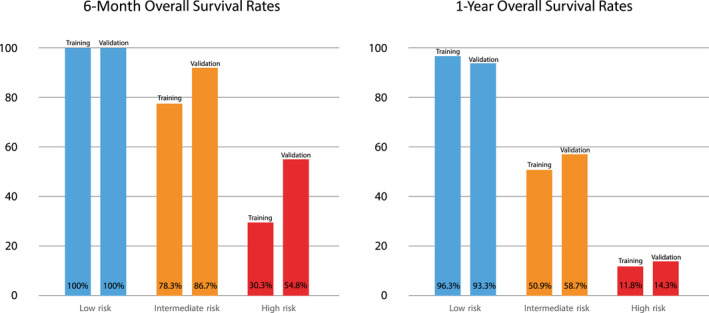
Survival rates within year 1 for risk groups according to the UHPI score in the training and validation cohort.
Subgroup Analysis of the UHPI Model According to Treatment Status
Patients who received active treatment had a higher OS than the BSC group both in training (active treatment, 12.8 months; 95% confidence interval [CI], 10.5‐15.2 months) versus BSC (active treatment 4.0 months; 95% CI, 2.0‐6.0 months; P < 0.001) and validation (active treatment, 27.9 months; 95% CI, 20.3‐35.4 months) versus BSC (active treatment, 8.7 months; 95% CI, 7.1‐10.4 months; P < 0.001) cohorts. As the decision to implement therapy or follow‐up with BSC is highly affected by the parameters used in the UHPI model, we did not include treatment status as an extra parameter to the multivariable model. Instead, we performed subgroup analysis according to treatment status as active treatment or BSC to reveal the efficacy of the UHPI model in different therapeutic approaches. Survival curves were significantly different among the three UHPI strata in training and validation sets for patients who received active treatment and were followed up with BSC (all log‐rank P < 0.001; Fig. 2).
FIG. 2.
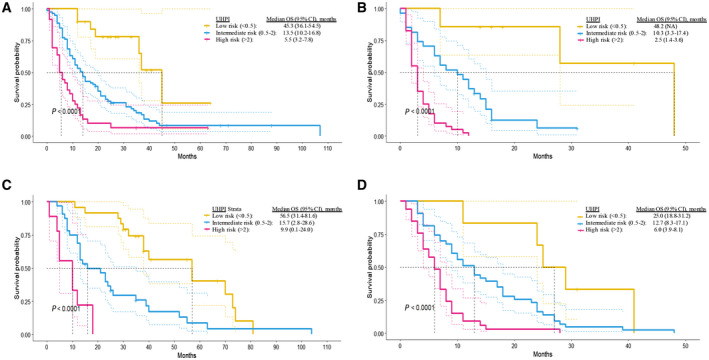
Subgroup analysis of the UHPI model according to treatment status. (A) Kaplan‐Meier curve of stratified survival in the training set that received active therapy. (B) Kaplan‐Meier curve of stratified survival in the training set that received BSC. (C) Kaplan‐Meier curve of stratified survival in the validation set that received active therapy. (D) Kaplan‐Meier curve of stratified survival in the validation set that received BSC.
Discussion
We developed and externally validated an easy to calculate scoring system to predict the prognosis of patients with HCC who cannot be treated with curative intent. The novel UHPI score comprises six routinely assessed parameters: CPS, ECOG performance status, maximum tumor size, vascular invasion or extrahepatic metastasis, lymph node involvement, and AFP. Patients get scores ranging from 0 to 6 and grouped into low, intermediate, and high risk according to their 1‐year OS rates. The UHPI can determine the survival outcome of unresectable patients with HCC better than major conventional models. We externally validated the UHPI score in an independent European cohort to assess its robustness and applicability in populations with different characteristics. The UHPI showed significant superior performance in classification of survival probabilities in both training and validation cohorts. For use in clinical practice, the UHPI low‐risk group had 100% 6‐month and >90% 1‐year OS probability, the intermediate‐risk group had 75%‐90% 6‐month and 50%‐60% 1‐year OS probability, and the high‐risk group had 30%‐60% 6‐month and <20% 1‐year survival probability. This new scoring model may provide valuable information, especially for 1‐year survival prediction.
The UHPI model accommodates rational variables, acknowledged as prognostic factors in the HCC literature. All components have been used in previous models but with different expressions. Most scoring systems use tumor size (except NIACE) and number of tumoral lesions (except Okuda) as factors in their model. Our cohort included only unresectable HCCs, and nodularity of HCC was not found to be an independent predictor of survival and therefore not included in the UHPI. Similarly, in a Chinese cohort with only unresectable HCCs, tumor size was demonstrated as an independent predictor of OS but not the number of tumoral lesions.( 18 ) The CPS is either used in its original form or by its covariates, including albumin, bilirubin, and/or ascites in most staging systems, except the TNM model, which uses only tumoral features. The Child‐Pugh stage has the most significant impact on survival outcomes, which explains the worst performance of the TNM system in survival prediction. The ECOG performance score is another well‐established parameter used in scoring systems and is very successful at representing the general health condition of the patient.( 19 ) ECOG performance (the best performance score being 0 and the worst 4) is used in several scoring systems with different categorization, including BCLC (0 vs. 1‐4), HKLC (0‐1 vs. 2‐4), ITA.LI.CA (0 vs. 1 vs. 2 vs. 3‐4), MESH (0‐1 vs. 2‐4), and NIACE (0 vs. 1‐4). We used ECOG categories in line with HKLC and MESH because an ECOG performance score of 1 is not an obstacle for any intervention or treatment in clinical practice. Vascular invasion with distant organ metastasis is also a commonly used parameter in HCC staging models as it is one of the main drivers of treatment decision and thereby prognosis. We did not separate the type of vascular invasion in the analysis as only a limited number of patients with extrahepatic vascular invasion were in the training cohort; consequently, the prognostic significance of the vascular invasion in our analysis is mainly representative of intrahepatic vascular invasion. We included vascular invasion and extrahepatic organ metastasis in the same category as used in HKLC, ITA.LI.CA, and MESH score, as the management strategies are similar. Lymph node involvement, a relatively weaker variable than others used in our model, has also been used in the TNM and BCLC models.
The prognostic and diagnostic value of AFP is well known in patients with HCC.( 20 , 21 ) Several prognostic AFP cut‐off values have been proposed as prognostic markers in HCC staging models, including MESH (cut‐off value, 20 ng/mL), NIACE (200 ng/mL), CLIP (400 ng/mL), CUPI (500 ng/mL), and ITA.LI.CA (1,000 ng/mL). In line with the CUPI score, we used the AFP cut‐off value of 500 ng/mL, which was determined by using the Youden index.
We selected the patients who were unresectable, as described in the EASL and AASLD HCC guidelines.( 22 , 23 ) This HCC subgroup is still clinically very heterogeneous. Some patients with unresectable HCC can initially be characterized by older age, poor performance, or altered liver function, making them unsuitable to any treatment option, while others may undergo locoregional treatment or systemic treatment for palliation. Local ablative therapies, including radiofrequency, microwave, or percutaneous ethanol ablation, are considered curative options for tumors smaller than 3 cm as they are almost equally effective as surgery in this group. However, a few reports have proposed that local ablative therapies might act as a curative option for even larger tumors.( 24 , 25 , 26 ) We did not include those who underwent any local ablative treatments to this analysis to preclude any potential bias of their unknown curative impact, although they are generally known as palliative modalities for unresectable HCCs.
The prognostic distinction of unresectable HCC is of great importance as it is currently the focus of clinical trials in HCC. In the last 2 decades, numerous randomized controlled clinical trials targeted improving the OS of unresectable HCCs.( 27 , 28 , 29 , 30 , 31 , 32 ) TACE is globally the most commonly used primary treatment modality in unresectable HCC.( 33 , 34 ) Briefly, an unresectable HCC is traditionally first considered for TACE, which has wide availability, and treated with other options if TACE is not convenient.( 35 ) However, with the recent advances in immunotherapy combined with targeted molecular therapies,( 36 ) the treatment algorithms might be about to change in the near future for unresectable HCCs. The UHPI risk assessment score is a promising tool for use in further clinical trials targeting unresectable HCC due to its superior prognostic risk stratification to predict 1‐year survival and ease in calculating during busy daily clinical practice. The UHPI also demonstrated validity in a different geographic region and ethnic population.
The UHPI risk score is derived based on a relatively small data set of predominantly patients with a history of CHB. Although we acknowledge that treatment modality and its efficacy may have a confounding impact on prognosis, we were not able to perform further subgroup analyses regarding the treatment status or etiology of underlying liver disease due to the small sample size. The UHPI model was validated in an independent European cohort, which consists of a Caucasian population with significantly different demographic characteristics, including the etiology of HCC mainly based on CHC. Therefore, we can report the excellent performance of the UHPI risk score, and the model works regardless of differences in demographics, underlying liver diseases, and treatment status. Considering the development cohorts in previous models, including patients with HCC treated with curative palliative approaches or followed with BSC, the UHPI risk score has a more homogeneous group of patients. Moreover, the higher rates of UHPI high‐risk patients who were followed up with BSC in our patient population compared to others is not surprising. We interpret this finding as a reflection of real‐life decisions rather than a potential bias of the study. Finally, we were not able to compare the UHPI score with another scoring model derived particularly for a similar patient population as ours, namely the Advanced Liver Cancer Prognostic System (ALCPS), in both training and validation cohorts due to the lack of several parameters in our data, including the type of symptom.( 37 ) The ALCPS was based on 11 prognostic factors and is not used in clinical practice owing to its complicated and impractical structure. In general, these limitations require further investigations in prospective larger cohorts. The UHPI model is not constructed to guide treatment decisions. The staging and treatment algorithms provided in BCLC, and HKLC in some Asian regions, are well endorsed, but they are both outperformed in distinguishing prognosis by other models in several comparative studies.( 18 , 38 , 39 , 40 ) We also generally acknowledge BCLC treatment algorithms in our centers with personalization in some circumstances in accordance with the EASL and AASLD guidelines. Yet, we developed the UHPI risk score to serve as a better prognostic model for survival in patients with heterogeneous unresectable HCC.
In conclusion, the present study derived and validated a novel prognostic risk scoring system for patients with unresectable HCC by using routinely evaluated parameters. The UHPI score can predict prognosis strongly and better than most of the accepted models in subjects with unresectable HCC. Furthermore, the three risk categories stratified in UHPI can be used in clinical practice to assess 6‐month and 1‐year survival probabilities and in clinical trials to estimate the potential candidates for more aggressive treatment approaches.
Supporting information
Table S1
Table S2
Table S3
Table S4
Acknowledgment
We thank the European Association for the Study of Liver Diseases Mentorship Program for providing the opportunity for collaboration between two centers.
Potential conflict of interest: Dr. Brunetto advises and is on the speakers’ bureau for AbbVie, Gilead, and Eisai‐MSD. The other authors have nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329‐338. [DOI] [PubMed] [Google Scholar]
- 3. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population‐based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93‐99. [DOI] [PubMed] [Google Scholar]
- 4. Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918‐928. [DOI] [PubMed] [Google Scholar]
- 5. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691‐1700.e1693. [DOI] [PubMed] [Google Scholar]
- 6. Leung TWT, Tang AMY, Zee B, Lau WY, Lai PBS, Leung KL, et al. Construction of the Chinese University prognostic index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer 2002;94:1760‐1769. [DOI] [PubMed] [Google Scholar]
- 7. Manghisi G, Elba S, Mossa A, Giorgio A, Aloisio V, Perrotta A, et al. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751‐755. [DOI] [PubMed] [Google Scholar]
- 8. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 2003;38:207‐215. [DOI] [PubMed] [Google Scholar]
- 9. Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut 2005;54:419‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang JD, Kim WR, Park KW, Chaiteerakij R, Kim B, Sanderson SO, et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology 2012;56:614‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu P‐H, Hsu C‐Y, Hsia C‐Y, Lee Y‐H, Huang Y‐H, Su C‐W, et al. Proposal and validation of a new model to estimate survival for hepatocellular carcinoma patients. Eur J Cancer 2016;63:25‐33. [DOI] [PubMed] [Google Scholar]
- 12. Farinati F, Vitale A, Spolverato G, Pawlik TM, Huo T‐L, Lee Y‐H, et al.; ITA.LI.CA Study Group . Development and validation of a new prognostic system for patients with hepatocellular carcinoma. PLoS Med 2016;13:e1002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adhoute X, Pénaranda G, Raoul JL, Blanc JF, Edeline J, Conroy G, et al. Prognosis of advanced hepatocellular carcinoma: a new stratification of Barcelona Clinic Liver Cancer stage C: results from a French multicenter study. Eur J Gastroenterol Hepatol 2016;28:433‐440. [DOI] [PubMed] [Google Scholar]
- 14. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma. Hepatology 2005;42:1208‐1236. [DOI] [PubMed] [Google Scholar]
- 15. European Association for the Study of the Liver; European Organisation For Research and Treatment of Cancer . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908‐943. Erratum in: J Hepatol 2012;56:1430. [DOI] [PubMed] [Google Scholar]
- 16. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693‐699. [DOI] [PubMed] [Google Scholar]
- 17. Brentnall AR, Cuzick J. Use of the concordance index for predictors of censored survival data. Stat Methods Med Res 2018;27:2359‐2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J‐F, Shu Z‐J, Xie C‐Y, Li QI, Jin X‐H, Gu W, et al. Prognosis of unresectable hepatocellular carcinoma: comparison of seven staging systems (TNM, Okuda, BCLC, CLIP, CUPI, JIS, CIS) in a Chinese cohort. PLoS One 2014;9:e88182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649‐655. [PubMed] [Google Scholar]
- 20. Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan D, et al. The threshold of alpha‐fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: a systematic review and meta‐analysis. PLoS One 2020;15:e0228857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and significance of alpha‐fetoprotein in hepatocellular carcinoma. Liver Int 2019;39:2214‐2229. [DOI] [PubMed] [Google Scholar]
- 22. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182‐236. Erratum in: J Hepatol 2019;70:817. [DOI] [PubMed] [Google Scholar]
- 23. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723‐750. [DOI] [PubMed] [Google Scholar]
- 24. Yin X‐Y, Xie X‐Y, Lu M‐D, Xu H‐X, Xu Z‐F, Kuang M, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long‐term outcome and prognostic factors. Cancer 2009;115:1914‐1923. [DOI] [PubMed] [Google Scholar]
- 25. Xu HX, Xie XY, Lu MD, Chen JW, Yin XY, Xu ZF, et al. Ultrasound‐guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol 2004;59:53‐61. [DOI] [PubMed] [Google Scholar]
- 26. Zhang NN, Lu W, Cheng XJ, Liu JY, Zhou YH, Li F. High‐powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol 2015;70:1237‐1243. [DOI] [PubMed] [Google Scholar]
- 27. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J‐F, et al. SHARP Investigators Study Group . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378‐390. [DOI] [PubMed] [Google Scholar]
- 28. Cheng A‐L, Kang Y‐K, Chen Z, Tsao C‐J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol 2009;10:25‐34. [DOI] [PubMed] [Google Scholar]
- 29. Kudo M, Finn RS, Qin S, Han K‐H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet 2018;391:1163‐1173. [DOI] [PubMed] [Google Scholar]
- 30. Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo‐controlled, double‐blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:565‐575. Erratum in: Lancet Gastroenterol Hepatol 2017;2:e6. [DOI] [PubMed] [Google Scholar]
- 31. Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux G‐P, et al.; SARAH Trial Group . Efficacy and safety of selective internal radiotherapy with yttrium‐90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open‐label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624‐1636. [DOI] [PubMed] [Google Scholar]
- 32. Abdel‐Rahman O, Elsayed Z. Yttrium‐90 microsphere radioembolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev 2020;11:CD011313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155‐2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takayasu K, Arii S, Ikai I, Kudo M, Matsuyama Y, Kojiro M, et al.; Liver Cancer Study Group of Japan . Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. Am J Roentgenol 2010;194:830‐837. [DOI] [PubMed] [Google Scholar]
- 35. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37:429‐442. [DOI] [PubMed] [Google Scholar]
- 36. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T‐Y, et al. IMbrave150 Investigators . Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894‐1905. [DOI] [PubMed] [Google Scholar]
- 37. Yau T, Yao TJ, Chan P, Ng K, Fan ST, Poon RT. A new prognostic score system in patients with advanced hepatocellular carcinoma not amendable to locoregional therapy: implication for patient selection in systemic therapy trials. Cancer 2008;113:2742‐2751. [DOI] [PubMed] [Google Scholar]
- 38. op den Winkel M, Nagel D, Sappl J, op den Winkel P, Lamerz R, Zech CJ, et al. Prognosis of patients with hepatocellular carcinoma. Validation and ranking of established staging‐systems in a large western HCC‐cohort. PLoS One 2012;7:e45066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Collette S, Bonnetain F, Paoletti X, Doffoel M, Bouché O, Raoul JL, et al. Prognosis of advanced hepatocellular carcinoma: comparison of three staging systems in two French clinical trials. Ann Oncol 2008;19:1117‐1126. [DOI] [PubMed] [Google Scholar]
- 40. Liu P‐H, Hsu C‐Y, Hsia C‐Y, Lee Y‐H, Su C‐W, Huang Y‐H, et al. Prognosis of hepatocellular carcinoma: assessment of eleven staging systems. J Hepatol 2016;64:601‐608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4


