Abstract
Low‐level alcohol consumption is associated with reduced cardiovascular disease (CVD) in the general population. It is unclear whether this association is seen in patients with nonalcoholic fatty liver disease (NAFLD) who have an increased risk of CVD. We examined the association between alcohol consumption and CVD‐related outcomes in subjects with NAFLD from a general population cohort. Subjects participating in the 1994‐1995 Busselton Health survey underwent clinical and biochemical assessment. NAFLD was identified using the Fatty Liver Index of >60, and alcohol consumption quantified using a validated questionnaire. CVD hospitalizations and death during the ensuing 20 years were ascertained using the Western Australian data linkage system. A total of 659 of 4,843 patients were diagnosed with NAFLD. The average standard drinks per week was 8.0 for men and 4.0 for women. Men consuming 8‐21 drinks per week had a 38% (hazard ratio [HR] 0.62, 95% confidence interval [CI] 0.43‐0.90) lower risk of CVD hospitalization as compared with men consuming 1‐7 drinks per week. With both men and women combined, consumption of 8‐21 drinks per week was associated with a 32% (HR 0.68, 95% CI 0.49‐0.93) reduction in CVD hospitalization in minimally adjusted and 29% (HR 0.71, 95% CI 0.51‐0.99) in fully adjusted models. No protective association was observed with binge drinking. There was no association between alcohol consumption and CVD death. Conclusion: Low to moderate alcohol consumption is associated with fewer CVD hospitalizations but not CVD death in subjects with NAFLD.
Abbreviations
- ALT
alanine aminotransferase
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- FLI
Fatty Liver Index
- GGT
gamma‐glutamyltransferase
- HDL
high‐density lipoprotein
- HR
hazard ratio
- ICD‐9
International Classification of Diseases, Ninth Revision
- ICD‐10
International Classification of Diseases, 10th Revision
- NAFLD
nonalcoholic fatty liver disease
- SBP
systolic blood pressure
Nonalcoholic fatty liver disease (NAFLD) incorporates a spectrum of disease ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), leading to hepatic fibrosis and potentially cirrhosis. Due to the worldwide increase in the prevalence of obesity, NAFLD has become the most common liver disorder in the Western and Eastern Industrialized countries, with a global prevalence of approximately 25%.( 1 ) NAFLD has a strong association with insulin resistance, and is therefore closely linked to type 2 diabetes mellitus and the metabolic syndrome. The latter is defined by a group of metabolic abnormalities with central adiposity, hypertension, dyslipidemia, and insulin resistance as its main components; almost 90% of patients with NAFLD have at least one of the characteristic features of metabolic syndrome, and about one third have the complete diagnosis,( 2 ) leading to the suggestion that NAFLD can be described as the hepatic manifestation of the metabolic syndrome.( 3 )
Components of metabolic syndrome are well‐demonstrated risk factors for cardiovascular disease (CVD),( 4 ) and, notably, an increasing body of evidence demonstrates that CVD is the leading cause of mortality in patients with NAFLD.( 5 ) NAFLD itself may be associated with a high risk of incident CVD that is independent of the traditional risk factors and main components of the metabolic syndrome.( 6 , 7 , 8 ) The magnitude of risk parallels the severity of underlying NAFLD, such that in patients with NASH or fibrosis, incident CVD disease is approximately 2.5‐fold higher.( 6 , 9 ) This is likely related to disturbance in glucose and lipid metabolism, and may have association with a systemic inflammatory environment precipitated in part by molecules and cytokines secreted from liver.( 8 ) The strong association between NAFLD and CVD necessitates a careful evaluation of risk factors and consideration of interventions to reduce the overall risk of CVD outcomes in all patients with NAFLD.
Alcohol consumption represents a modifiable factor for CVD; several meta‐analyses of observational studies have demonstrated that low levels of alcohol use are associated with a lower risk of CVD.( 10 , 11 ) This may be due to possible impact of alcohol on increasing levels of protective lipoprotein levels or reducing inflammatory and pro‐coagulant factors.( 12 ) Whether these observations extend to patients with NAFLD is controversial. In patients with NAFLD, some cross‐sectional studies have shown low to moderate alcohol consumption to be associated with less fibrosis and have a protective effect on histological severity of liver disease.( 13 , 14 , 15 ) However, whether low‐level alcohol consumption reduces CVD in subjects with NAFLD is unknown. With this in mind, we aimed to examine for associations between different levels of alcohol consumption on incident CVD events and CVD mortality in a well‐characterized population‐based cohort of patients with NAFLD with a significant length of follow‐up.
Our specific aims were to examine (1) the association between different levels of alcohol consumption below a cumulative total of 210 g per week, on incident CVD events and CVD mortality; (2) the association between patterns of alcohol consumption (binge versus nonbinge) on incident CVD events and mortality; and (3) the association between different types of alcohol beverages (wine, beer, spirits) on incident CVD events and mortality.
Patients and Methods
Study Population and Participants
The population of Busselton, a coastal area in the southwest of Western Australia, has been regularly surveyed since the Busselton Health Study was established in 1966.( 16 ) More than 90% of this population consists of individuals with Anglo‐Celtic ancestry. A follow‐up health survey of survivors from the previous surveys was conducted in 1994‐1995, as has been described previously.( 17 ) A total of 4,843 participated in the survey and provided a blood sample.
Baseline Measurements and Cardiovascular Outcomes
Participants in the 1994‐1995 survey completed a comprehensive health and lifestyle questionnaire, underwent various measurements and tests, and provided a fasting blood sample. Information on alcohol intake, smoking, diabetes, and use of medications was obtained by a standardized questionnaire. Participants were asked to report how many standard drinks of each type of alcohol (beer, wine, spirits) they consumed on each day last week. Diagrams were provided in relation to what constituted a standard drink (10 g of alcohol) for beer, wine and spirits. Participants were classified as never, former, or current smokers. Standardized protocols were used by trained assessors to obtain anthropometric measures, which included measurements of height, weight, and waist circumference. Body mass index was defined as weight (kilograms) divided by height (meters) squared. Blood pressure (systolic and diastolic) was recorded using a mercury sphygmomanometer after 5 minutes rest in a sitting position. Blood samples were obtained from the participants after an overnight fast at time of survey, serum separated and stored at 70°C. Blood samples obtained at the time of the survey were used in this analysis. These include serum alanine aminotransferase (ALT), gamma‐glutamyltransferase (GGT), total and high‐density lipoprotein (HDL) cholesterol, and triglycerides, and the laboratory methods have been described previously.( 18 )
Identification of Participants With NAFLD
Subjects with a Fatty Liver Index (FLI) score of >60 at baseline and a total standard drink intake of ≤ 21/week were classified as having NAFLD and were included. The FLI is a diagnostic algorithm of fatty liver based on waist circumference, body mass index (BMI), GGT and serum triglyceride levels, and is validated as an accurate predictor of fatty liver in population‐based studies.( 19 , 20 ) Patients were excluded if they had serological evidence of hepatitis B or C infection, C282Y/C282Y HFE genotype, drank >21 standard drinks per week, or if their alcohol consumption was unknown. In 1998 HFE genotyping was performed on DNA extracted from Guthrie cards.( 18 ) In 2005 the stored sera of subjects with ALT > 40 IU/L were evaluated for markers of liver disease, including hepatitis B and C serology.( 21 ) Overall, there were a total of 659 people (384 men and 275 women) who met these criteria and had the required baseline measures (outlined in Fig. 1).
FIG. 1.
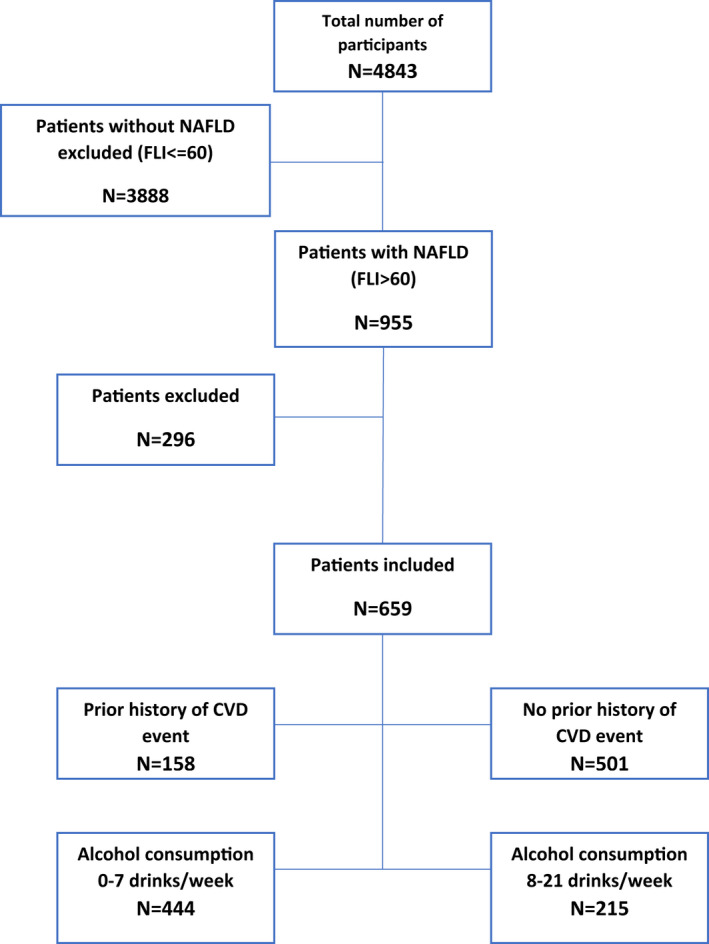
Summary of inclusion and exclusion criteria.
Evaluation of Cardiovascular Outcomes
Using record linkage to the hospital admissions and deaths for the period January 1, 1980, to June 30, 2014, hospital and death records of the survey participants were evaluated. International Classification of Diseases, Ninth Revision (ICD‐9) codes were used up to June 30, 1999, and ICD‐10 codes for subsequent events. History of CVD at baseline in 1994‐1995 was defined as any CVD hospital admission (ICD‐9 390‐459) during the 15 years before the survey (i.e., 1980 to 1994‐1995). Cardiovascular outcome events during the 20‐year follow‐up from survey attendance in 1994‐1995 to June 30, 2014, were obtained from hospital and death records during this period. Two outcome events were analyzed. Time to death from CVD (ICD‐9 401‐414, and 420‐449; ICD‐10 I10‐I25, I30‐I79, and G45) and time to first fatal or nonfatal cardiovascular event defined as a hospital admission with a principal diagnosis of coronary heart disease (ICD‐9 410‐414; ICD‐10 I20‐25), stroke (ICD‐9 430‐437; ICD‐10 I60‐68 and G45), heart failure (ICD‐9 428; ICD‐10 I50), peripheral arterial disease (ICD‐9 440‐448; ICD‐10 I70‐79), or death from CVD. This study was approved by the Human Research Ethics Committee of the Department of Health of Western Australia (Project No. 2011/60).
Power and Statistical Analyses
With the number of CVD events determined during follow‐up, the analysis had 80% power to detect a hazard ratio (HR) of 1.05 per standard drink in separate analyses of men and women and 80% power to detect a HR of 1.03 in combined analysis of men and women. With the number of CVD deaths determined on follow‐up, the analysis had 80% power to detect a HR of 1.08 per standard drink in separate analyses of men and women and 80% power to detect a HR of 1.05 in combined analysis of men and women.
Variables with skewed distributions were log‐transformed for use in regression models, and descriptive results for these variables are presented in both untransformed and transformed scales. The association between alcohol consumption variables and cardiovascular outcomes in participants with NAFLD were examined using Cox proportional hazards regression modeling. Six alcohol consumption variables were considered: total standard drinks of beer last week, total standard drinks of wine last week, total standard drinks of spirits last week, total standard drinks last week, alcohol type (none, beer only, wine only, spirits only, and mixed), and maximum standard drinks on any one day last week. Binge drinking was defined as four or more drinks on any one day. The estimated HRs with 95% confidence intervals (CIs) are reported for each alcohol variable after two levels of adjustment for potential confounders. Model A adjusted for age and CVD history, and model B is further adjusted for BMI and Framingham risk score variables (cholesterol, HDL cholesterol, smoking, diabetes, systolic blood pressure (SBP), and hypertension treatment). Analyses were conducted for men and women separately as well as combined, and for the full cohort and the subcohort of participants with no history of CVD at baseline (CVD‐free cohort). Combined analyses of men and women also adjusted for sex, and models for the CVD‐free cohort did not adjust for history of CVD. Models for alcohol type and maximum drinks on any one day were also adjusted for total drinks. A 95% CI for a HR that does not include the value 1 is significant at the 5% level (i.e., P < 0.05). Tests of the interaction between alcohol variable and sex were conducted to confirm whether the effect of alcohol was significantly different in men and women. A further interaction test was performed to assess whether the impact of alcohol consumption significantly differed in those with higher (>35 IU/L in men and >25 IU/L in women) and lower ALT levels at baseline. Statistical analyses were performed using SAS 9.4.
Results
Baseline Characteristics of Study Cohort
The baseline characteristics (in 1994‐1995) of the NAFLD cohort (384 men and 275 women, average age of 57 years) are given in Table 1. By selection, all NAFLD subjects had an FLI score > 60, and the average was 79 in both men and women. Overall, 11% had diabetes, 31% were on hypertension treatment, and mean BMI was 30.1 kg/m2 in men and 33.5 kg/m2 in women. A total of 24% of the cohort had a prior history of hospitalization for CVD.
TABLE 1.
Characteristics of NAFLD Cohort (n = 659) at Baseline and Number of Outcome Events Overall and by Gender
| Characteristic | Men (n = 384) | Women (n = 275) | All (n = 659) |
|---|---|---|---|
| Age (years) | 56.2 ± 13.9 | 57.8 ± 14.1 | 56.9 ± 14.0 |
| History of CVD | 92 (24.0) | 66 (24.0) | 158 (24.0) |
| Smoking status | |||
| Never | 137 (35.7) | 153 (55.6) | 290 (44.0) |
| Former | 197 (51.3) | 90 (32.7) | 287 (43.6) |
| Current | 50 (13.0) | 32 (11.6) | 82 (12.4) |
| Diabetes | 39 (10.2) | 35 (12.7) | 74 (11.2) |
| Hypertension treatment | 94 (24.5) | 109 (39.6) | 203 (30.8) |
| SBP (mm Hg) | 132 ± 15 | 133 ± 18 | 132 ± 17 |
| BMI (kg/m2) | 30.1 ± 2.7 | 33.5 ± 3.7 | 31.5 ± 3.6 |
| Waist (cm) | 104 ± 8 | 101 ± 9 | 103 ± 8 |
| Total cholesterol (mmol/L) | 5.84 ± 0.99 | 6.16 ± 1.07 | 5.97 ± 1.03 |
| HDL cholesterol (mmol/L) | 1.05 ± 0.26 | 1.29 ± 0.32 | 1.15 ± 0.31 |
| Triglycerides (mmol/L) | 2.24 ± 1.47 | 1.96 ± 0.99 | 2.13 ± 1.30 |
| Log triglycerides | 0.67 ± 0.49 | 0.57 ± 0.46 | 0.63 ± 0.48 |
| GGT (U/L) | 44.8 ± 34.4 | 37.1 ± 28.2 | 41.6 ± 32.2 |
| Log GGT | 3.64 ± 0.52 | 3.42 ± 0.58 | 3.55 ± 0.56 |
| FLI score | 78.6 ± 11.0 | 78.8 ± 11.2 | 78.7 ± 11.1 |
| ALT (IU/L) | 35.8 ± 21.4 | 26.3 ± 15.1 | 31.8 ± 19.6 |
| Higher ALT (men > 35, women > 25) | 133 (34.6) | 96 (34.9) | 229 (34.7) |
| CVD death | 47 (12.2) | 37 (13.5) | 84 (12.7) |
| CVD event | 128 (33.3) | 95 (34.5) | 223 (33.8) |
Data are shown as mean ± SD or number (%).
The average total standard alcohol drinks per week was 8.0 in men (5.1 beer, 2.1 wine, 0.9 spirits) and 4.0 in women (0.9 beer, 2.6 wine, 0.5 spirits), as indicated in Table 2. Only 6% of men and 20% of women were nondrinkers, and more than 50% of the cohort consumed 1‐7 standard drinks per week. Binge drinking (defined as four or more drinks on any one day) was reported by 29% of the men and 10% of the women. A total of 51% of the men were exclusive beer drinkers, and 50% of women were exclusive wine drinkers.
TABLE 2.
Quantity, Type and Pattern of Baseline Alcohol Consumption in NAFLD Cohort
| Characteristic | Men (n = 384) | Women (n = 275) | All (n = 659) |
|---|---|---|---|
| Alcohol consumption* | |||
| Beer | 5.1 ± 5.6 | 0.9 ± 2.7 | 3.3 ± 5.1 |
| Wine | 2.1 ± 4.0 | 2.6 ± 3.9 | 2.3 ± 3.9 |
| Spirits | 0.9 ± 2.7 | 0.5 ± 1.7 | 0.7 ± 2.4 |
| Total | 8.0 ± 6.4 | 4.0 ± 4.7 | 6.3 ± 6.1 |
| Total | |||
| None | 21 (5.5) | 55 (20.0) | 76 (11.5) |
| 1‐7 | 192 (50.0) | 176 (64.0) | 368 (55.8) |
| 8‐14 | 98 (25.5) | 31 (11.3) | 129 (19.6) |
| 15‐21 | 73 (19.0) | 13 (4.7) | 86 (13.1) |
| Max on any one day | 2.7 (2.3) | 1.5 (1.6) | 2.2 (2.1) |
| Max on any one day | |||
| 0‐3 | 273 (71.1) | 249 (90.5) | 522 (79.2) |
| ≥ 4 | 111 (28.9) | 26 (9.5) | 137 (20.8) |
Standard drinks last week.
Number of CVD Deaths and Events
Overall, there were 84 CVD deaths (47 in men, 37 in women), and 223 participants (128 men, 95 women) had a CVD event during the 20‐year period of follow‐up (Table 1).
Alcohol Consumption and Risk of Future CVD Events
Table 3 lists the HRs for alcohol consumption variables in relation to risk of CVD event in the NAFLD cohort. Modest alcohol consumption (8‐21 drinks per week) was associated with a 32% reduction in risk of future CVD events following adjustment for age, sex, and history of prior CVD. Modest alcohol consumption remained significantly associated with a lower risk of future CVD events with additional adjustment for BMI, smoking, diabetes, serum cholesterol, HDL cholesterol, SBP, and hypertension treatment.
TABLE 3.
Adjusted HR for Alcohol Consumption in Relation to Risk of Future CVD Events in the NAFLD Cohort
| Model A | Model B | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Total standard drinks per week of: | ||
| Beer | 0.974 (0.944, 1.005) | 0.977 (0.946, 1.009) |
| Wine | 1.007 (0.976, 1.038) | 1.008 (0.977, 1.041) |
| Spirits | 0.980 (0.927, 1.035) | 0.975 (0.922, 1.031) |
| Total | 0.983 (0.959, 1.007) | 0.984 (0.959, 1.009) |
| Total | ||
| None | 0.777 (0.510, 1.182) | 0.768 (0.493, 1.199) |
| 1‐7 (ref) | 1.000 | 1.000 |
| 8‐21 | 0.677 (0.491, 0.932) | 0.713 (0.512, 0.993) |
| Max on any one day | ||
| 0‐3 | 1.000 | 1.000 |
| ≥ 4 | 0.848 (0.544, 1.322) | 0.864 (0.553, 1.348) |
For beer, wine, spirits and total, HR is per additional drink last week. For max on any one day, the HR is adjusted for total drinks. Model A is adjusted for age, sex, and CVD history. Model B is adjusted for age, sex, BMI, CVD history, cholesterol, HDL, smoking, diabetes, SBP, and hypertension treatment.
When the analysis was stratified by sex, men who consumed 8‐21 drinks per week were found to have a 38% lower risk of CVD event as compared with men consuming 1‐7 drinks per week (HR 0.624, 95% CI 0.434‐0.898), as outlined in Supporting Table S1. This HR was no longer significant in the fully adjusted model B. Among women, modest alcohol consumption was associated with a HR of 0.85 of future CVD events (Supporting Table S1); however, this was not significant. No interactions between sex and type, pattern, or quantity of alcohol were noted (data not shown). Neither binge‐type drinking patterns, nor type of alcohol consumed, were associated with future CVD events. The impact of modest alcohol consumption on future risk of CVD was not significantly different in those with higher (>35 IU/L in men and >25 IU/L in women) as compared with lower ALT levels (P = 0.78).
Alcohol and Risk of CVD Death
Table 4 lists the HRs for alcohol consumption in relation to risk of CVD death in the full NAFLD cohort. None of the alcohol consumption variables showed a significant (P < 0.05) relationship with risk of CVD death in the full cohort in the minimally adjusted (model A) or fully adjusted (model B) models. Also, the sex*alcohol interaction was not significant, indicating no difference in the alcohol‐to‐CVD death relationship in men and women.
TABLE 4.
Adjusted HR for Alcohol Consumption in Relation to Risk of CVD Death in the Full NAFLD Cohort
| Model A | Model B | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Total standard drinks per week of: | ||
| Beer | 0.999 (0.950, 1.050) | 1.016 (0.965, 1.070) |
| Wine | 1.014 (0.963, 1.068) | 1.029 (0.976, 1.084) |
| Spirits | 0.939 (0.846, 1.044) | 0.909 (0.815, 1.015) |
| Total | 0.994 (0.952, 1.037) | 1.005 (0.961, 1.051) |
| Total | ||
| 0‐7 | 1.000 | 1.000 |
| 8‐21 | 0.706 (0.406, 1.228) | 0.813 (0.456, 1.448) |
| Max on any one day | ||
| 0‐3 | 1.000 | 1.000 |
| ≥ 4 | 0.961 (0.448, 2.064) | 1.096 (0.501, 2.399) |
For beer, wine, spirits and total, HR is per additional drink last week. For max on any one day, the HR is adjusted for total drinks. Model A is adjusted for age, sex, and CVD history. Model B is adjusted for age, sex, BMI, CVD history, cholesterol, HDL, smoking, diabetes, SBP, and hypertension treatment.
Discussion
Death from cardiovascular disease remains the principal cause of mortality in patients with NAFLD; thus, determination of lifestyle factors that modify CVD risk is important, with the potential to significantly improve patient outcomes. Among our NAFLD cohort of 659 patients followed for 20 years, we found that alcohol consumption of 8‐21 drinks per week was associated with 32% reduction in CVD events in minimally adjusted and 30% in fully adjusted models. Men who consumed 8‐21 drinks per week at baseline had a 38% lower risk of CVD event, as compared with men consuming 1‐7 drinks per week when adjusted for age and underlying CVD history but not in the fully adjusted models. These findings suggest a protective effect of moderate alcohol consumption in patients with NAFLD who by definition are not heavy alcohol consumers. Of note, consumption in a binge‐type fashion was not associated with reduced CVD risk, suggesting low and regular consumption is needed for cardio‐protection. We did not find specific associations with types of alcohol; however, this may be due to limited statistical power.
These findings are consistent with the previously conducted meta‐analyses, which reported that low‐level alcohol consumption was associated with a reduction in coronary heart disease outcomes in the general population, whereas a significantly increased risk of vascular outcomes was observed in those consuming more than 30‐60 g per day.( 10 , 11 , 22 ) This threshold is noteworthy for being the cutoff to define NAFLD, such that patients with NAFLD will not reach these thresholds. Cross‐sectional studies examining the association between alcohol consumption and markers of CVD risk in patients with NAFLD have been conflicting, with increased risk, no effect, and reduced risk being reported.( 23 , 24 , 25 ) VanWagner et al. reported in 570 patients with NAFLD that longitudinal alcohol consumption was associated with a lower prevalence of diabetes, lower hemoglobin A1c, and less use of anti‐hypertensive medications but no difference in coronary artery calcification or echocardiographic abnormalities.( 24 ) Further follow‐up of this relatively young cohort (mean age 50 years) will be required to determine the association between alcohol and clinical cardiovascular outcomes. Notably, these studies differed in their diagnostic strategies for NAFLD and used surrogate markers of CVD rather than clinical events and mortality related to CVD.
The exact pathogenesis of the potential beneficial effects of low to moderate alcohol on CVD events is uncertain. Rimm et al. in their meta‐analysis of 42 studies demonstrated changes in the HDL cholesterol, serum apolipoprotein, serum triglycerides, plasma fibrinogen, tissue‐type plasminogen activator, and plasminogen levels with alcohol dose of 30 g per day.( 26 ) Subsequent studies have found similar results with increased HDL and adiponectin levels, with the latter associated with lower risk of diabetes and CVD.( 27 ) Alcoholic beverages also have antioxidant activity due to phenolic compounds and flavonoids that inhibit synthesis of oxidized form of LDL, which is atherogenic.( 28 , 29 ) However it is important to emphasize that higher levels of alcohol are hazardous, with intake of greater than 30 g per day associated with increased mortality risk in patients with fatty liver, particularly in the presence of the metabolic syndrome.( 30 )
The strengths of our study include that it stems from a large population‐based cohort from the real world, focusing objective CVD morbidity and mortality endpoints over a 20‐year follow‐up. The assessment of alcohol consumption was obtained using comprehensive and standardized health and lifestyle questionnaires. We not only studied the impact of overall consumption but also the type and pattern of alcohol consumption, although the latter were not associated with the outcomes.
Limitations include the lack of population diversity, as more than 90% of the study population consisted of individuals with Anglo‐Celtic ancestry and does not include more diverse ethnic groups to provide more generalized application. Another potential limitation could be the use of FLI for diagnosis of NAFLD. Nonetheless, it has been validated in Western populations with excellent accuracy area under the receiver operating characteristic curve of 0.83 for determining presence of steatosis in comparison with histopathological diagnosis.( 31 )
However, the main question is how to apply this finding to our clinical practice and convey it as a public health message as a risk modification measure to reduce CVD. The cardiovascular benefits of moderate alcohol consumption should be weighed against any potential adverse events due to underlying comorbidities, as the impact of alcohol may be variable in different age groups. Similarly, the risk of injury from long‐term alcohol may be significant in one group, whereas the CVD may cause major disease burden in the other group. These factors require carefully conducted studies involving different population subgroups to identify those at high risk of mortality or morbidity from CVD and are likely to have significant risk reduction, outweighing any potential harm from alcohol consumption. The upper safe limit of the alcohol consumption is unknown, as are its overall benefits in reducing overall mortality due to lack of randomized controlled trials. Furthermore, vulnerable patients may be at risk of keeping themselves with in safe range of consumption with risk of ending up losing any beneficial effects.
In conclusion, our study demonstrates that low to moderate alcohol consumption of a total of 8‐21 standards drinks of alcohol is independently associated with fewer CVD events in subjects with NAFLD. Patients with NAFLD with inherent high risk of cardiovascular mortality due to increased prevalence of underlying metabolic syndrome may benefit from low to moderate consumption to reduce morbidity from CVD events. The integration of these findings into clinical practice requires careful selection of patients, in whom reduction CVD risk would outweigh any potential adverse events.
Supporting information
Supplementary Material
Acknowledgment
The authors thank the Busselton Population Medical Research Institute for permission to access Busselton Health Study data, and the Busselton population for their long‐standing support and participation in the Busselton Health Study. The authors wish to thank the staff at the Western Australian Data Linkage Branch and the Hospital Morbidity Data Collection for facilitating access to the linked data on hospital admissions and deaths and the State and Territory Registries of Births, Deaths and Marriages, the State and Territory Coroners, Victorian Department of Justice and Community Safety, and the National Coronial Information System, for enabling cause‐of‐death unit record file data to be used for this publication.
Supported by the internal investigator fund at the University of Western Australia.
Potential conflict of interest: Nothing to report.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917‐923. [DOI] [PubMed] [Google Scholar]
- 3. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844‐1850. [DOI] [PubMed] [Google Scholar]
- 4. Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 5. Adams LA, Lymp JF, St. Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 6. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 7. Meyersohn NM, Mayrhofer T, Corey KE, Bittner DO, Staziaki PV, Szilveszter B, et al. Association of hepatic steatosis with major adverse cardiovascular events, independent of coronary artery disease. Clin Gastroenterol Hepatol 2021;19:1480‐1488.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams LA, Anstee QM, Tilg H, Targher G. Non‐alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138‐1153. [DOI] [PubMed] [Google Scholar]
- 9. Baratta F, Pastori D, Angelico F, Balla A, Paganini AM, Cocomello N, et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol 2020;18:2324‐31.e4. [DOI] [PubMed] [Google Scholar]
- 10. Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, et al. Risk thresholds for alcohol consumption: combined analysis of individual‐participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018;391:1513‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta‐analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández‐Solà J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol 2015;12:576‐587. [DOI] [PubMed] [Google Scholar]
- 13. Kwon HK, Greenson JK, Conjeevaram HS. Effect of lifetime alcohol consumption on the histological severity of non‐alcoholic fatty liver disease. Liver International 2014;34:129‐135. [DOI] [PubMed] [Google Scholar]
- 14. Hagström H, Nasr P, Ekstedt M, Kechagias S, Önnerhag K, Nilsson E, et al. Low to moderate lifetime alcohol consumption is associated with less advanced stages of fibrosis in non‐alcoholic fatty liver disease. Scand J Gastroenterol 2017;52:159‐165. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell T, Jeffrey GP, de Boer B, MacQuillan G, Garas G, Ching H, et al. Type and pattern of alcohol consumption is associated with liver fibrosis in patients with non‐alcoholic fatty liver disease. Am J Gastroenterol 2018;113:1484‐1493. [DOI] [PubMed] [Google Scholar]
- 16. Knuiman MW, Jamrozik K, Welborn TA, Bulsara MK, Divitini ML, Whittall DE. Age and secular trends in risk factors for cardiovascular disease in Busselton. Aust J Public Health 1995;19:375‐382. [DOI] [PubMed] [Google Scholar]
- 17. Knuiman MW, Hung J, Divitini ML, Davis TM, Beilby JP. Utility of the metabolic syndrome and its components in the prediction of incident cardiovascular disease: a prospective cohort study. Eur J Cardiovasc Prev Rehabil 2009;16:235‐241. [DOI] [PubMed] [Google Scholar]
- 18. Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A population‐based study of the clinical expression of the hemochromatosis gene. N Engl J Med 1999;341:718‐724. [DOI] [PubMed] [Google Scholar]
- 19. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee‐Moradie F, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H‐magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin‐resistant individuals. Eur J Endocrinol 2014;171:561‐569. [DOI] [PubMed] [Google Scholar]
- 21. Olynyk JK, Knuiman MW, Divitini ML, Bartholomew HC, Cullen DJ, Powell LW. Effects of HFE gene mutations and alcohol on iron status, liver biochemistry and morbidity. J Gastroenterol Hepatol 2005;20:1435‐1441. [DOI] [PubMed] [Google Scholar]
- 22. Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta‐analysis. Addiction 2000;95:1505‐1523. [DOI] [PubMed] [Google Scholar]
- 23. Sinn DH, Gwak G‐Y, Cho J, Son HJ, Paik Y‐H, Choi MS, et al. Modest alcohol consumption and carotid plaques or carotid artery stenosis in men with non‐alcoholic fatty liver disease. Atherosclerosis 2014;234:270‐275. [DOI] [PubMed] [Google Scholar]
- 24. VanWagner LB, Ning H, Allen NB, Ajmera V, Lewis CE, Carr JJ, et al. Alcohol use and cardiovascular disease risk in patients with nonalcoholic fatty liver disease. Gastroenterology 2017;153:1260‐1272.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kashiwagi K, Yamaguchi A, Shiba S, Taniki N, Inoue N, Takaishi H, et al. Moderate alcohol consumption is not associated with subclinical cardiovascular damage but with hepatic fibrosis in non‐alcoholic fatty liver disease. Alcohol 2020;89:1‐7. [DOI] [PubMed] [Google Scholar]
- 26. Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta‐analysis of effects on lipids and haemostatic factors. BMJ 1999;319:1523‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta‐analysis of interventional studies. BMJ 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999;100:1050‐1055. [DOI] [PubMed] [Google Scholar]
- 29. Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low‐density lipoprotein by phenolic substances in red wine. Lancet 1993;341:454‐457. [DOI] [PubMed] [Google Scholar]
- 30. Younossi ZM, Stepanova M, Ong J, Yilmaz Y, Duseja A, Eguchi Y, et al. Effects of alcohol consumption and metabolic syndrome on mortality in patients with nonalcoholic and alcohol‐related fatty liver disease. Clin Gastroenterol Hepatol 2019;17:1625‐1633.e1. [DOI] [PubMed] [Google Scholar]
- 31. Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2014;40:1209‐1222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


