Abstract
Optimizing hearing in patients with a unilateral cochlear implant (CI) and contralateral acoustic hearing is a challenge. Evolutionary algorithms (EA) can explore a large set of potential solutions in a stochastic manner to approach the optimum of a minimization problem. The objective of this study was to develop and evaluate an EA-based protocol to modify the default frequency settings of a MAP (fMAP) of the CI in patients with bimodal hearing. Methods: This monocentric prospective study included 27 adult CI users (with post-lingual deafness and contralateral functional hearing). A fitting program based on EA was developed to approach the best fMAP. Generated fMAPs were tested by speech recognition (word recognition score, WRS) in noise and free-field-like conditions. By combining these first fMAPs and adding some random changes, a total of 13 fMAPs over 3 generations were produced. Participants were evaluated before and 45 to 60 days after the fitting by WRS in noise and questionnaires on global sound quality and music perception in bimodal binaural conditions. Results: WRS in noise improved with the EA-based fitting in comparison to the default fMAP (41.67 ± 9.70% versus 64.63 ± 16.34%, respectively, p = 0.0001, signed-rank test). The global sound quality and music perception were also improved, as judged by ratings on questionnaires and scales. Finally, most patients chose to keep the new fitting definitively. Conclusions: By modifying the default fMAPs, the EA improved the speech discrimination in noise and the sound quality in bimodal binaural conditions.
Keywords: cochlear implant, binaural hearing, speech discrimination in noise, quality of life, evolutionary algorithm, fitting
1. Introduction
Stereophony is based on combining information in the brain from the two ears. The brain makes use of many different cues to determine the 3D characteristics of an auditory landscape [1]. Their complete combination is required for stereophony to be achieved, but access to only some bilateral cues may still generate substantial benefits.
Advantages of binaural stimulation, as opposed to monaural hearing, are (1) redundancy (summation effect) which enhances the signal detection; (2) localization of the sound-source (in the horizontal plane) based on inter-aural time differences (ITD) and level differences (ILD); and (3) improved speech discrimination in noise when signal and noise are spatially separated (squelch effect).
Moreover, with binaural hearing, the head-shadow increases the signal-to-noise ratio at the farthest ear from the noise (as the head attenuates the noise), while this ratio decreases at the nearest ear to the noise source.
Bimodal binaural hearing refers to the use of a cochlear implant (CI) in one ear in combination with a functional acoustic hearing with or without a hearing aid (HA) on the contralateral side. This association provides adults and children with improved speech perception in quiet and in noise, better music perception, auditory comfort, higher sound quality, enhanced sound localization, and as a result, a better quality of life in comparison to unilateral CI [2,3,4,5]. The improvements are related to the integration of the electric hearing, offering auditory information in a relatively broad frequency range (between 0.07 and 8.5 kHz depending on the brand), and the contralateral acoustic input offering the acoustic fine-structure cues. In addition, bimodal hearing reduces the head-shadow effect due to single-sided deafness (SSD) and restores the binaural squelch and summation effects to some extent [3,6].
However, there is great variability in the integration process; while some bimodal users show substantial benefit, others receive little or no advantage.
This variability could be due to the characteristics of the individual listeners (neural survival, current spread, duration of deafness, lack of cortical plasticity), to different processing times between CI and contralateral HA or NH ear [7], to frequency mismatch between the CI and the contralateral HA or NH ear [8], or differences between automatic gain control (AGC) of the CI and the HA [9]. The latter three parameters could be rectifiable via signal processing and/or mapping [10].
Some patients experience even bimodal interference and report better hearing with one of the ears [11,12,13,14,15]. The possibility of this interference is further supported by the observation that in patients with bimodal binaural hearing, the deactivation of apical CI electrodes coding for frequencies perceived by the aided contralateral ear produces a more natural and less metallic sound without reducing the word discrimination in quiet and in noise [16]. In this case, binaural interactions were apparently improved by suppressing the temporal and/or frequency mismatch in the low frequencies at the cost of reduced cochlear implant performance.
Alternatively, binaural interactions could be improved through frequency band adjustments without electrode deactivation [10], or through adjustment of the temporal processing between the CI and the contralateral HA or ear [7,17].
The facility to obtain the bimodal integration appears to influence the auditory outcome [18]. Apart from patient-related factors, such as deprivation duration or a number of functional channels in the implanted ear, device-dependent factors, such as the asynchronous CI and acoustic inputs, different sound preprocessing strategies in the CI and the HA, or loudness and pitch mismatches can affect the speed and the quality of this integration [18].
Based on these findings, an improvement of the spatial CI coding, which relies on the cochlear tonotopy, could theoretically improve the binaural integration. The Greenwood map offers a relatively precise function describing the physiological place-frequency relation in the human cochlea [19]. With CI, the place-frequency function is different from the physiological cochlear tonotopy and a high inter-individual variability exists since the coverage of the cochlear duct by the electrode array is partial and variable. With time and training, a tolerance to the shift between the electric and acoustic stimuli in terms of temporal and spatial coding appears [18]. In many implantees, a perceptual fusion is observed for two sounds with very different pitches presented simultaneously to both ears [20,21].
In a standard CI fitting, the default frequency allocations to active electrodes are not modified and only the sensation of loudness is adjusted [22,23]. Modifying the frequency allocations may improve speech discrimination and music perception [18,24,25], but this type of fitting would involve too many parameters and is not practiced in routine [26]. Moreover, its effect on bimodal binaural patients has not been reported to our knowledge.
We hypothesized that reallocating the frequency bands in the CI would lead to a better fusion of the central binaural information, an improved hearing in noise, and a higher sound quality in bimodal binaural conditions. Binaural redundancy is one of the binaural advantages that could be addressed with bimodal rehabilitation. Binaural redundancy could increase loudness via binaural summation but could also improve the detection threshold and frequency differences, and as a result, the speech recognition in noise [1]. Adults who use bimodal hearing devices seem to benefit from the binaural redundancy [3,12,27] but not always [28]. An inter-aural mismatch may significantly limit this effect. In NH subjects listening to bilateral CI simulations with varying virtual electrode positions [29], the maximum binaural summation benefit for speech in quiet and in noise was observed when the inter-aural mismatch of the virtual electrode positions was ≤1 mm.
Moreover, inter-aural mismatch has also been shown to limit speech understanding in noise when signal and noise are spatially separated [10,30]. This is due to distortions of ITD and ILD [31,32].
A place-matched frequency mapping based on electrode location could be hampered by the difficulty to determine the amount of neural survival or local electric stimulation interactions in the cochlea for an individual [10,33]. ITD was used to evaluate inter-aural place mismatch [31,33] and the results were close in accordance with CT scan estimates, but the studies were limited to SSD and bilateral CI patients. No study has been carried out with bimodal patients with CI and contralateral HA.
In the bimodal context, patients wear a HA on the contralateral ear with various signal processing programs (number of channels, frequency bandwidths, etc.). Since the number of fitting combinations is very high, artificial intelligence can be employed to search this vast domain for the best solution.
Evolutionary algorithms (EA) are a family of algorithms inspired by Darwin’s theory of evolution [34,35]. Initial individuals, represented in our case by the set of frequency ranges for all electrodes (fMAPs), are submitted to the constraint of the environment (i.e., audiometric scores). Their adaptation, stochastic mixing of their genes (i.e., frequency band allocations), and the possibility of mutation (stochastic changes in the fMAP) yield offspring (new fMAPs) generation after generation (iterations). Each fMAP is submitted to an evaluation by a speech audiometric test in noise to obtain a score for each fMAP, represented by a word recognition score (WRS) out of ten. Based on this score, the best solutions are selected and combined to obtain more performant fMAPs. These algorithms provide a wide exploration of solutions in a predefined domain that could not otherwise be conducted in a timely fashion, even by an expert [36]. By comparison, the existing deterministic and probabilistic algorithms, such as the one used in the only computer-based fitting aid, the Fitting to Outcome Expert (FOX) system, tend to modify the settings to approach an ideal situation with predefined parameters (T- and M-levels, gains, [37,38]). Moreover, the frequency bands are not considered as a parameter [38].
The objective of the present study was to develop and evaluate a fitting protocol based on the CI frequency reallocation for bimodal binaural CI users with different CI brands using an interactive EA method.
2. Materials and Methods
2.1. Participants
Twenty-seven adults (10 men, 17 women) volunteered to participate in this monocentric and prospective study. All participants were unilateral CI users for a minimum of 6 months with functional contralateral hearing (normal hearing or HA). Their mean age was 58 ± 16.7 years (median: 64, range: 20–80 years). The average duration of the deafness before CI was 23.5 ± 15.8 years (median: 19, range: 1–55 years). In the implanted ear, patients wore various brands of CIs and coding strategies. In the contralateral ear, 23 participants wore a behind-the-ear HA which was fitted with a NAL-NL1 protocol and checked by their audiologist within the 3-month period before inclusion. Three participants had contralateral normal hearing (Table 1).
Table 1.
Patient characteristics.
| ID# | Etiology | Hearing Deprivation on Implanted Ear (Years) | Implant | Coding | Contralateral Hearing Aid |
|---|---|---|---|---|---|
| 1 | Otosclerosis | 55 | MED-EL | FS4 [39] | Chili SP7, Oticon |
| 2 | Congenital | 28 | MED-EL | FS4 | Legend 1786, Beltone |
| 3 | Congenital | 38 | COCHLEAR | ACE [40] | Naida Q70-SP, Phonak |
| 4 | Ménière | 17 | COCHLEAR | ACE | Cobalt 8+, Rexton |
| 5 | Congenital | 23 | MED-EL | HDCIS [41] | Normal |
| 6 | Congenital | 43 | OTICON | Crystalis XDP [42] | Nitro 7MI SP, Siemens |
| 7 | Congenital | 34 | COCHLEAR | ACE | Ambra SP, Phonak |
| 8 | Sudden SNHL | 1 | AB | HiRes Optima [43] | Insio 5bX, Siemens |
| 9 | Idiopathic | 16 | AB | HiRes Optima | UPSmart988 GN Resound |
| 10 | Lobstein’s disease | 42 | COCHLEAR | ACE | PHONAK Naida Q50 SP |
| 11 | Congenital | 39 | COCHLEAR | ACE | Siemens Signia Orion 2312 |
| 12 | Sudden SNHL | 18 | MED-EL | FS4 | Widex Moment |
| 13 | Congenital | 20 | COCHLEAR | ACE | Siemens Pure 500 |
| 14 | Sudden SNHL | 30 | COCHLEAR | ACE | Phonak Naida V90 UP |
| 15 | Sudden SNHL | 13 | MED-EL | FS4 | Starkey Livio 2400 |
| 16 | Otosclerosis | 2 | MED-EL | FS4 | Normal |
| 17 | Otosclerosis | 7 | COCHLEAR | ACE | Phonak Audeo B50 R |
| 18 | Neurofibromatosis type 2 | 19 | MED-EL | FS4 | Phonak Naida Q70 SP |
| 19 | Chronic otitis media | 42 | MED-EL | FS4 | No hearing aid |
| 20 | Perilymphatic fistula | 2 | COCHLEAR | ACE | Siemens Rexton Strata 2 |
| 21 | Perilymphatic fistula | 9 | COCHLEAR | ACE | Normal |
| 22 | Chronic otitis media | 50 | MED-EL | FS4 | Siemens Motion XS |
| 23 | Congenital | 32 | COCHLEAR | ACE | Gn Hearing Resound Alera 7 |
| 24 | Ménière | 2 | COCHLEAR | ACE | Belton Identity 86D |
| 25 | Meningitis | 30 | MED-EL | FS4 | Siemens Signia Pure 312 |
| 26 | Ménière | 17 | MED-EL | FS4 | Starkey Resound |
| 27 | Sudden SNHL | 5 | COCHLEAR | ACE | Belton Identity 66D |
2.2. Experimental Setup
At inclusion, the clinical and audiometric data were obtained, and each CI was fitted with an fMAP based on the EA. Other fMAPs already available on the processor were left unmodified. Participants were asked to use the EA-based fMAP as much as possible, but they were free to switch to their usual fMAPs ad lib. The second session was conducted 45 to 60 days later. Patients were again evaluated with a pure-tone, speech recognition test in quiet and noise in free-field-like conditions and questionnaires. The main criterion of the study was the improvement of the word recognition score (WRS) in noise with EA-based fMAP.
2.3. Audiometry
All evaluations were performed in the bimodal binaural condition in an audiometry booth with a calibrated audiometer (AC40®, Interacoustics, Middelfart, Denmark). The signal was delivered by a loudspeaker (Planet M, Elipson, Champigny, France) placed at the level of the head 1 m in front of the participant.
French Fournier lists were used for the speech audiometry in this study [44].
At the initial and the final evaluation, the audiometry tests included:
A pure-tone audiometry in free-field-like condition;
A speech recognition test in quiet with monosyllabic words providing the WRS in quiet;
A speech recognition test in noise: both signal and noise (white noise at 60 dB SPL) were delivered by the same loudspeaker;
In a preliminary trial with different lists of 20 words, the signal-to-noise ratio (SNR) was individually adapted (−7, 0, +5, or +10 dB) to obtain a percentage of WRS between 3/10 and 7/10. Every patient kept its individually adapted SNR at the same level through the follow-up. Two series of words were also administered for the initial and the final evaluations.
During the EA-based fitting, each generated fMAP was tested with a different series of 10 words to obtain a WRS in noise out of ten at the same level of SNR used for the evaluations.
No feedback was provided during speech recognition tests.
The improvement of the WRS in noise on 20 words at the initial and at the final evaluation was the main judgment criterion of the study.
2.4. Questionnaires
We also asked the participants to complete a quality-of-hearing questionnaire, APHAB (Abbreviated Profile of Hearing Aid Benefit) in its French version related to their handicap before and after the new CI fitting [45]. The questionnaire includes 24 questions on different everyday-life situations related to hearing function. They are divided into 4 categories: Ease of Communication (EC), Reverberation (RV), Background Noise (BN, communication in environments with high background noise), and Aversiveness (AV). It provides a global score and 4 subdomain scores. The Hearing Implant Sound Quality Index score (HISQUI19, [46]) comprises 19 questions on the sound quality perceived by the CI. Scores range from 19 (poor) to 133 points (excellent). In addition, a shortened Munich Music questionnaire (MMQ) [47] was administered including categorical ratings of “metallic”, “clear”, “pleasant”, and “natural” qualities of the musical sounds plus the following questions with forced categorical responses: How long do you listen to music since the last CI fitting? (<30 min/30–60 min/60–120 min/ >120 min); Can you distinguish between high and low notes? (Yes/no); Do you normally feed music directly into your speech processor? (Yes/no). Finally, patients rated the global sound quality by a Likert scale (natural sound and voices, auditory comfort in silence and in noise; scores ranging from 1, “not at all” to 5, “totally agree”).
2.5. Frequency Reallocation with the Evolutionary Algorithm
Evolutionary algorithms are calculation methods based on biological evolution. Among this family of algorithms, the most popular are genetic algorithms [48,49,50,51,52,53]. In this paper, we propose a hybrid algorithm at the intersection of a genetic algorithm and an evolutionary strategy. Indeed, we will manipulate real values and apply Gaussian mutations derived from evolutionary strategies, but we will also perform locus type crossovers, which are usually used in genetic algorithms. Finally, since the evaluation step is carried out without a mathematical evaluation function, but only based on the hearing test results, we consider this approach as an interactive evolutionary algorithm.
The general idea is to bring changes to a set of solutions in the optics of improving them by gradual changes and the assessment of the effects of these changes. During this process, the initial values can be changed randomly in a predefined range, especially to generate the initial set of solutions (parents). Later, limited random changes are also introduced in the process to create new solutions (crossover and mutations). This characteristic classes the evolutionary algorithms as stochastic [54]. This algorithm employs alternatively, the mathematical operators of initiation, evaluation, variation (by combination and mutation), selection, and replacement. Solutions (fMAPs in our study) are generated by combining the parent solutions based on their performance (speech recognition test in noise). Mathematically, the algorithm attempts to find the shortest way to the highest performance. It considers the previous combinations and their results to propose new solutions.
The general procedure was as follows (Figure 1 and Figure 2):
Input default settings to define the boundaries of the exploration space: exploration domain for each band was set at the lower limit of the same band (fLOW) to 1.2 times the upper limit (1.2 × fHIGH);
Random generation of an initial population in the range allowed for each electrode: 4 parents (i.e., 4 fMAPs P1, P2, P3, P4);
Evaluation of P1 to P4 by speech recognition in noise. Each fMAP obtains a score: SP1, SP2, SP3, SP4;
Input SP1 to 4;
- Evolutionary loop, until stop-criteria (number of generations = 3 in our study):
- Generation of children (3 individuals, first loop: C1, C2, C3);
- Selection of 2 individuals among the previous generation by tournament: Two individuals of the previous generation are randomly selected; the one with the highest probability is chosen. The previous process is repeated. In this way, two individuals are finally selected;
- Crossover: combining electrode settings from 2 parent fMAPs to obtain a child;
- Gaussian mutation: mutations can be applied to fLOW and fHIGH with a probability Pm in the predefined range;
- Evaluation of the 3 children by WRS yielding scores SCn (first loop: SC1, SC2, SC3);
- Input SCn;
- Selection of 4 individuals with the highest WRS among all generated individuals (for the first loop: 7 fMAPS, P1 to P4, and C1 to C3).
Output: The best fMAP (highest WRS) obtained during the evolutionary process.
Figure 1.
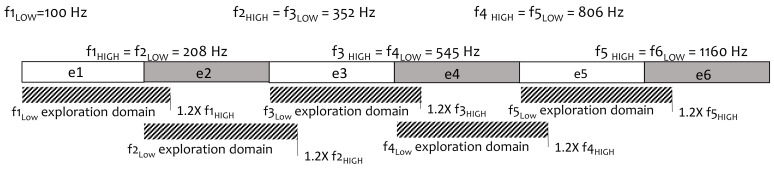
Frequency MAP fitting by an evolutionary algorithm (EA). An example with 5 electrodes (e1 to e5) for an MED-EL device is presented. The initial frequency intervals (fLOW and fHIGH in Hz) and the exploration domains for fHIGH (upper limit of each frequency band) by the algorithm are shown. The fLOW was set equal to fHIGH of the previous electrode to avoid discontinuity and overlap.
Figure 2.
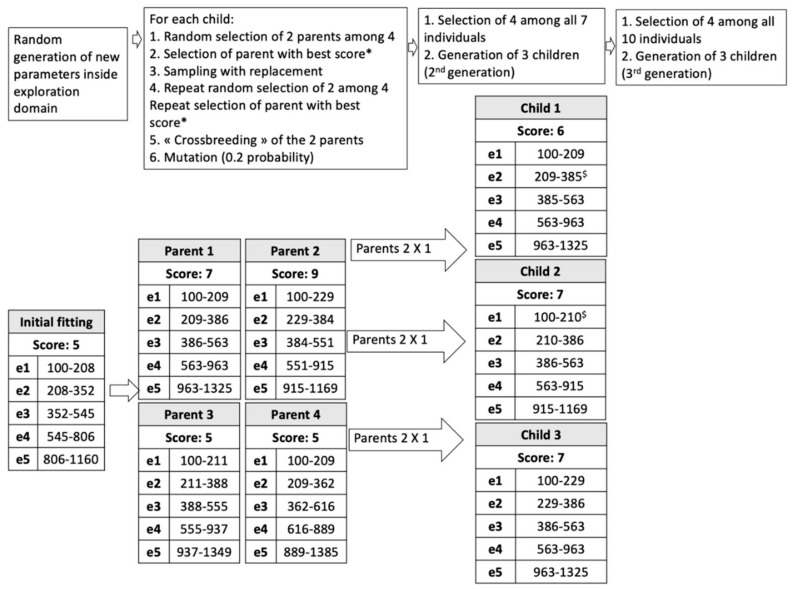
The process of parent and children generation is shown through an example of an MED-EL CI with 5 electrodes. Scores are obtained by speech recognition in noise in a binaural free-field-like condition. * In the case of a tie, the first selected individual is retained (fHIGH mutation $).
The algorithm was developed using MATLAB (2016a version, MathWorks, Natick, MA, USA) as described before [35]. In this algorithm, each fMAP represented an individual. The default fMAP (factory settings) was used to define the initial frequency bands. Initial fMAPs were generated by EA-based on these initial values. For a new fMAP, the upper limit of each frequency band (fHIGH) was determined by the EA in an exploration domain ranging from the lower limit of the same band (fLOW) to 1.2 × fHIGH (Figure 1).
Discontinuities and overlaps in the frequency domain were not permitted. Consequently, The fLOW of each frequency band was set equal to the fHIGH of the previous band. Larger values of overlapping and mutation probability would have created a total disruption of the original fMAP, requiring longer adaptation periods, a larger exploration domain with more generations, and longer tests. With these constraints, the default fMAP generated a random initial population of 4 parent fMAPs (Figure 2).
Each new fMAP was evaluated by WRS in noise (/10) and the result was fed to the algorithm. Two parent MAPs were randomly selected using a tournament selection. The best fMAP (based on WRS) became the first parent and the repetition of the same procedure produced the second parent. These two parents generated a child fMAP by combining their frequency bands (crossover) and applying mutations. During the combination, a variable proportion of the available frequency bands from one parent were combined with those from the second parent to form a new set of frequency bands for the offspring. The combination process did not modify the upper and lower frequency band limits. For the generation of child-fMAPs mutation was applied.
During a mutation, the upper-frequency band limit was modified in a stochastic manner. This modification was limited to the exploration domain [fLOW, 1.2 × fHIGH]. The mutation probability for a frequency band in each generated fMAP was set at 0.2, with a standard deviation of 0.1 × frequency band width (Gaussian mutation). The tournament selection was repeated 2 more times to generate 2 other couples of parent MAPs. For these selections, a sampling with replacement strategy was employed. Crossbreeding of each couple produced a child fMAP. Hence, the first generation of 3 child-fMAPs was created and tested by WRS in noise. To create the second generation, 4 parents were selected from 7 already generated fMAPs (4 parents and 3 children). The selection and crossbreeding generated 3 children for the second generation. Finally, for the third generation, 4 parents were selected from 10 generated fMAPs (4 parents and 6 children), and 3 children were obtained. The process was stopped after 3 generations. In total, 13 fMAPs including 4 parents and 9 children over 3 generations, were produced. In the end, the fMAP with the highest WRS was selected. In the case of 2 fMAPs with the same score, the one preferred by the patient based on sound quality was selected. This algorithm differed from the general scheme by the fact that the optimization cycle was halted after 3 generations, and not when an optimization criterion was reached. This specificity was imposed by the length of the procedure and the necessity of multiple speech audiometries, which could not be increased indefinitely.
2.6. Fitting Software Programs
BEPS+ research software (Advanced Bionics Research Center, Hannover, Germany) was used for frequency allocation in Advanced Bionics CIs. For all other CIs, routine clinical software was used. The minimal frequency fitting step was 1 Hz for MED-EL and Cochlear CIs, 62 Hz for Advanced Bionics CIs, and 131 Hz for the Oticon Medical CI. For Advanced Bionics and Oticon CIs, the closest frequency increments to the provided fMAP were selected. The minimum frequency band width was 62 Hz for Advanced Bionics and Cochlear CIs, 1 Hz for MED-EL CIs, and 131 Hz for the Oticon Medical CI. These values are obligatory and inherent to the fitting software, the coding strategy, or both.
2.7. Determination of Greenwood Frequency MAP in Individual Cochleae
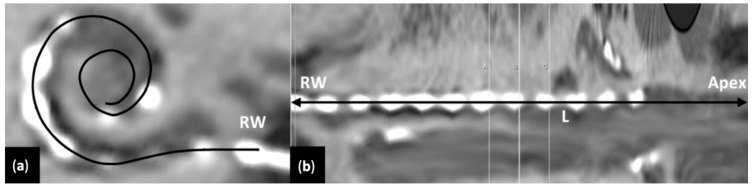
The postoperative CT scans were analyzed with Osirix (V4, Pixmeo, Geneva, Switzerland). The length of the cochlea from the round window (RW) to the apex and the position of each electrode from the apex were measured in millimeters (Figure 3). A tridimensional curved multiplanar reconstruction was created. The relative position of each electrode to the apex was expressed as the ratio of the distance between the electrode and the RW to the estimated length of the basilar membrane (in mm). The Greenwood equation was then applied to determine the corresponding tonotopic frequency [19]: F = 165.4 (102.1X − 0.88) where F is the frequency (Hz) and X is the relative distance of the electrode from the round window (distance from round window/entire length of the basilar membrane).
Figure 3.
Postoperative CT scans analysis and reconstruction: (a) Oblique view of the cochlea on a multiplanar reconstruction (MPR) with minimal intensity projection showed the full length of the electrode array; (b) A reconstruction of the image (a) in a curvilinear plane unfolded the cochlear spiral; The relative distance of each electrode to the round window (RW) was measured. Since the electrodes are not on the same plane, they cannot be all visualized on the MPR.
2.8. Statistics
Power calculations were carried out by G*Power (v. 1.3.6.9, Heinrich Heine Universität Düsseldorf, Düsseldorf, Germany, [55]). Based on reported studies on speech discrimination in noise in bimodal binaural patients, the inter-individual performance variability was estimated as 20% and a 15% variation of the WRS after EA-based fitting was anticipated. With β = 0.05, and α = 0.05, and for a two-tailed non-parametric paired comparison, 27 participants were required. All patients were included in an intention-to-treat analysis.
Statistical tests were conducted on Prism, version 8, GraphPad Software, San Diego, CA, USA, 2018. Paired comparisons of continuous variables with non-normal distributions were analyzed by Wilcoxon signed-rank test, and the results were expressed as mean ± standard error of mean, median, and range. ANOVA or mixed-model analysis were employed to compare center frequencies deduced from EA to those obtained from the Greenwood MAPs and the manufacturer’s default settings. Their normal distribution was verified by D’Agostino and Pearson’s test. The results were expressed as mean ± standard error of mean. A p-value < 0.05 was considered significant.
3. Results
All 27 participants performed the first and the second evaluation sessions and fully completed all evaluation steps. The average duration of the fitting session was 135 ± 30 min. Subjects 2 and 4 (Table 1) did not accept to finish the fitting due to the duration of the test: subject 2 completed the procedure up to the first fMAP of the second generation (8 fMAPs in total) and subject 4 completed the second fMAP of the first generation (6 fMAPs). In patients with MED-EL CIs, the most basal electrodes were deactivated because of a high impedance (patient 1 and 2: electrodes #12, patient 16: electrodes #11 and #12) or a vestibular response (patient 5: electrode #12). For subject 6 (Oticon Medical), the 3 most apical electrodes were deactivated during the first postoperative months because of unpleasant sounds. For the patient with Cochlear CIs, electrode #15 was deactivated because of high impedance (patient 10), and electrodes #1-5 were deactivated because of a vestibular response or no response (Patient 27). The fMAP proposed by the EA excluded the deactivated electrodes as a precondition.
3.1. Frequency Band Adjustments with Evolutionary Algorithm
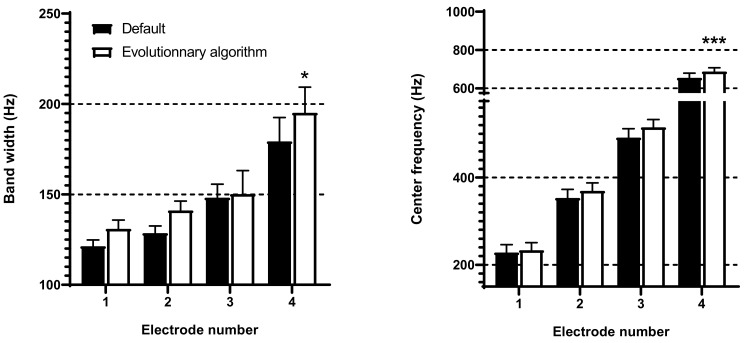
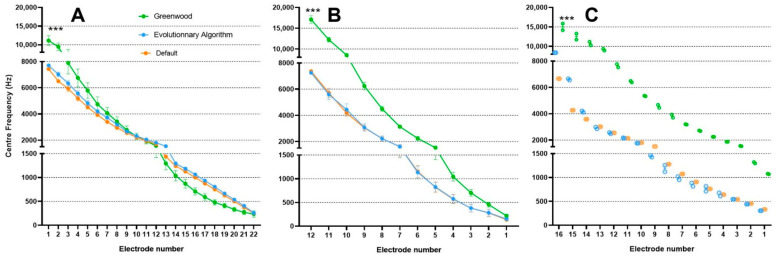
The algorithm suggested a different frequency allocation per electrode than the default setting. The EA yielded an enlargement of the bands in the low frequencies (4 most apical electrodes, Figure 3). Moreover, the EA shifted the center frequencies (Fc) of these apical electrodes toward higher values regardless of CI brand or the number of available electrodes (Figure 4).
Figure 4.
Effect of evolutionary algorithm (EA) after mutations and evolutions on frequency bandwidth, and center frequencies for the 4 most apical electrodes (electrode 1 representing the most apical as in MED-EL and Advanced Bionics CIs). Values are expressed as mean ± standard error of mean (n = 27). * p < 0.05 for the effect of EA on band width, and *** p < 0.001 for the effect of EA on center frequencies; in both analyses, p < 0.001 for the effect of electrode number and no significant interaction, two-way ANOVA.
In patients with Cochlear or Advanced Bionics CIs, several frequency bands were dramatically narrowed while the bands allocated to the neighboring electrodes were significantly widened, suggesting the detection and the resolution of channel interferences (e.g., electrode 16 in patient 3, Appendix A).
A postoperative CT scan was available for 15 patients (6 MED-EL, 2 Advanced Bionics, and 7 Cochlear). The center frequencies deduced from the Greenwood map were compared to those from the EA and the default fMAPs (Figure 5, Appendix B). Both EA-based and default fMAPs yielded lower center frequencies than the Greenwood map.
Figure 5.
Center frequencies of electrodes according to the Greenwood map calculated on the postoperative CT scan, the default setting, and the evolutionary algorithm (EA) in Cochlear ® (A, n = 7), MED-EL ® (B, n = 6) and Advanced bionics ® (C, n = 2) cochlear implants. Values are expressed as mean ± standard error of mean. For Advanced Bionic (panel C), individual values are depicted. Center frequencies according to the Greenwood map differed from default and EA settings. *** p < 0.001 for the effects of settings, electrode position, and interaction in all 3 brands, two-way ANOVA.
3.2. Audiometry
The best fMAP was not always obtained at the last generation (Table 2). In 6 patients, the first generation of fMAPs (parents), which were generated randomly in the predefined domain, yielded the best results. In the remaining cases, the best fMAP was among the first (n = 7), the second (n = 6), or the third (n = 8) generations. In 9 patients, several fMAPs yielded the same optimal WRS (patients 2–4, 8, 9, 11, 20, 21, 24). In these cases, the patient chose the fMAP among those with the highest WRS based on subjective quality of sound.
Table 2.
WRS for each fMAP generated by the evolutionary algorithm (P1–4 and C1–C9). Asterisk indicates the selected final fMAP. Initial and final WRS (45–60 days after fitting) were tested by 20 words and intermediate WRS by 10 words. All were expressed as a score out of ten. All tests were conducted at the same signal/noise ratio (SNR). Minus sign (-): the patient was not willing to test the fMAPs and abandoned the procedure.
| Patient Number | Initial WRS | SNR (dB HL) | Parents | 1st Generation | 2nd Generation | 3rd Generation | Final WRS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | ||||
| 1 | 4 | 10 | 6 | 8 | 6 | 6 | 8 | 6 | 9 * | 6 | 7 | 7 | 7 | 6 | 7 | 9 |
| 2 | 5 | 5 | 7 | 6 | 10 * | 9 | 4 | 6 | 10 | 9 | - | - | - | - | - | 6.5 |
| 3 | 4 | 10 | 6 | 4 | 5 | 5 | 5 | 5 | 6 | 8 * | 7 | 8 | 8 | 8 | 6 | 7.5 |
| 4 | 4 | 0 | 5 | 3 | 5 | 6 | 6 * | 4 | - | - | - | - | - | - | - | 6 |
| 5 | 4 | −7 | 4 | 4 | 5 | 8 | 6 | 7 | 6 | 5 | 5 | 9 * | 7 | 6 | 8 | 8 |
| 6 | 5 | 10 | 0 | 2 | 4 | 5 | 2 | 3 | 4 | 5 | 6 * | 5 | 4 | 0 | 5 | 5 |
| 7 | 3 | 5 | 4 | 7 | 6 | 7 | 5 | 7 | 9 * | 6 | 8 | 4 | 7 | 6 | 7 | 7.5 |
| 8 | 5.5 | 5 | 7 | 5 | 6 | 7 | 4 | 5 | 5 | 7 | 5 | 6 | 7 * | 6 | 6 | 7.5 |
| 9 | 5 | 10 | 4 | 6 | 8 * | 5 | 5 | 6 | 6 | 7 | 8 | 7 | 7 | 6 | 5 | 7.5 |
| 10 | 3 | 10 | 2 | 4 | 2 | 3 | 5 | 4 | 5 | 3 | 5 | 4 | 3 | 4 | 9 * | 2 |
| 11 | 6 | 10 | 6 | 9 | 7 | 5 | 5 | 6 | 8 | 6 | 8 | 8 | 8 | 9 * | 6 | 8 |
| 12 | 6 | −5 | 5 | 4 | 3 | 5 | 7 | 6 | 7 | 4 | 4 | 7 | 4 | 5 | 8 * | 7 |
| 13 | 3 | 0 | 6 | 2 | 5 | 5 | 4 | 7 | 6 | 2 | 6 | 7 | 4 | 6 | 8 * | 7 |
| 14 | 3 | 0 | 4 | 8 * | 6 | 2 | 4 | 3 | 6 | 2 | 4 | 3 | 6 | 5 | 5 | 8 |
| 15 | 4 | −5 | 5 | 2 | 4 | 6 | 8 * | 4 | 3 | 7 | 5 | 4 | 1 | 4 | 4 | 7 |
| 16 | 4 | −10 | 5 | 4 | 1 | 2 | 3 | 4 | 6 * | 4 | 5 | 4 | 2 | 3 | 5 | 7 |
| 17 | 5 | −5 | 3 | 5 | 8 * | 3 | 4 | 4 | 5 | 1 | 3 | 7 | 3 | 3 | 2 | 7 |
| 18 | 4 | −5 | 4 | 6 * | 2 | 5 | 2 | 2 | 3 | 5 | 1 | 5 | 5 | 4 | 5 | 7 |
| 19 | 3 | −5 | 3 | 5 | 4 | 4 | 8 * | 4 | 3 | 5 | 5 | 4 | 3 | 5 | 5 | 6 |
| 20 | 5 | −10 | 1 | 1 | 1 | 1 | 7 | 7 * | 4 | 5 | 5 | 7 | 5 | 6 | 5 | 5 |
| 21 | 4 | 0 | 2 | 5 | 2 | 2 | 3 | 4 | 4 | 2 | 3 | 2 | 5 | 5 * | 4 | 3 |
| 22 | 3 | 0 | 7 * | 3 | 5 | 5 | 6 | 5 | 4 | 4 | 4 | 2 | 2 | 6 | 6 | 7 |
| 23 | 3 | 10 | 4 | 2 | 4 | 2 | 1 | 3 | 7 | 8 * | 2 | 4 | 5 | 1 | 4 | 7 |
| 24 | 5 | 0 | 3 | 5 | 7 | 3 | 3 | 2 | 4 | 3 | 4 | 6 | 7 * | 3 | 2 | 6 |
| 25 | 5 | 0 | 4 | 1 | 3 | 4 | 5 | 4 | 3 | 3 | 6 * | 4 | 4 | 1 | 1 | 3 |
| 26 | 3 | 0 | 4 | 3 | 5 | 4 | 2 | 6 | 6 | 4 | 3 | 2 | 4 | 7 * | 4 | 6 |
| 27 | 4 | −5 | 2 | 3 | 3 | 4 | 5 | 3 | 3 | 6 * | 4 | 1 | 4 | 5 | 5 | 7 |
At the final evaluation, WRS in noise was significantly improved with EA (4.17 ± 0.97, median: 3.5, range: 2–5, with the default fMAP versus 6.46 ± 1.63, median: 7, range: 2–9 with the EA-based fMAP, n = 27, p = 0.0001, Wilcoxon sign-ranked test). The duration of hearing deprivation was not correlated to the initial or the final WRS (linear regression test, not significant, data not shown). At the final evaluation, the WRS in quiet remained unchanged (9.04 ± 2.01 initially, median: 10, range: 2–10 versus 9.22 ± 1.76, median 10, range 3–10 at the final test, n = 27, non-significant, Wilcoxon sign-ranked test). Similarly, the EA-based fitting did not alter the pure-tone average in the free-field-like condition (20.9 ± 9.4 dB HL, median: 20, range: 1.3–47.5, for the initial fitting versus 19 ± 7.2 dB, median 20, range: 1.3–32.5 at the final test, n = 27, non-significant, Wilcoxon sign-ranked test).
3.3. APHAB Questionnaire
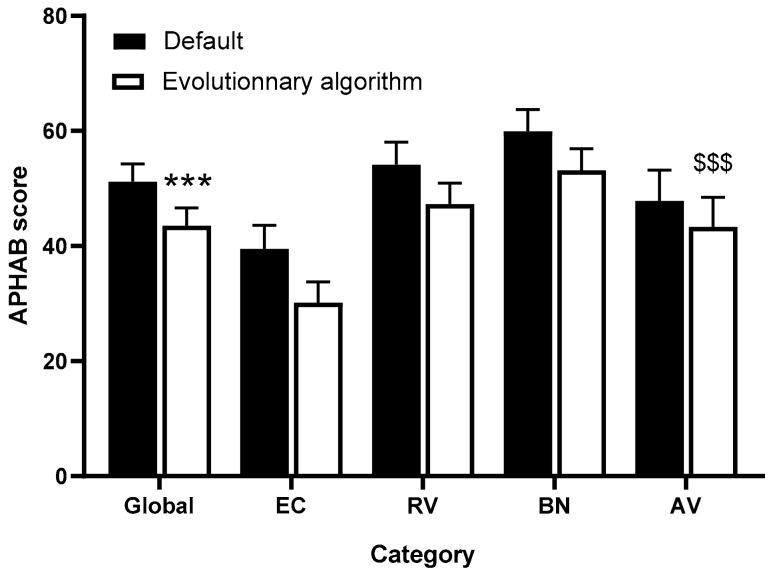
The quality of hearing, as assessed by APHAB, was significantly improved (50.4 ± 16.6, median: 55.5, range: 24.1–73.2, for the global score before versus 43.5 ± 16.8, median: 46.6, range: 10.2–66.6, after the EA fitting, n = 27, p = 0.002 Wilcoxon sign-ranked test). The quality of hearing was also significantly improved in EC, RV, and BN APHAB subdomains (Figure 6).
Figure 6.
APHAB questionnaire scores with default and evolutionary algorithm settings. Values are expressed as mean ± standard error of mean. *** p < 0.001 versus default for the global score, Wilcoxon signed-rank test. $$$ p < 0.001 for the effect of setting, and p < 0.01 for the effect of APHAB ranges, no significant interaction, 2-way ANOVA. EC = ease of communication, RV = reverberation, BN = background noise, AV = aversiveness.
3.4. HISQUI Questionnaire
The quality of hearing, as assessed by HISQUI, was significantly improved (72.2 ± 17.7, median: 70, range: 44–116 before versus 77.9 ± 21.5, median: 75, range: 45–121, after the EA fitting, n = 27, p = 0.034 Wilcoxon sign-ranked test).
3.5. Global Evaluation of the Sound Quality and Music Perception
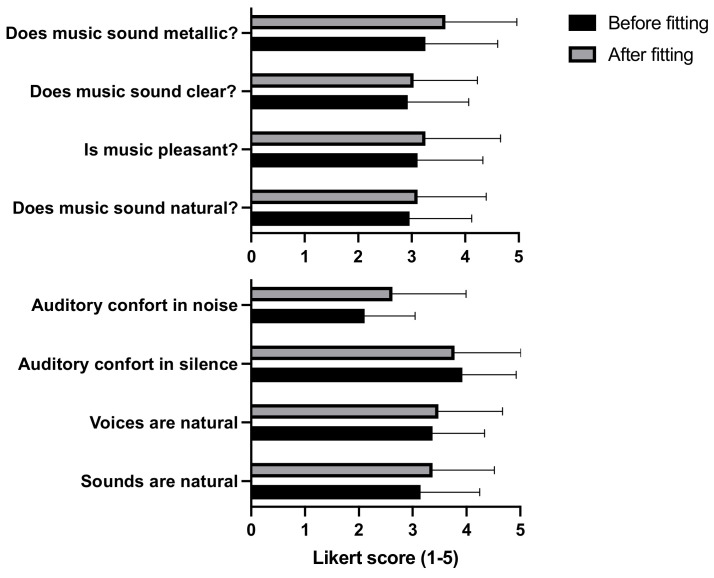
The music perception quality as evaluated by the MMQ rating, as well as the global sound quality, did not change, as judged by the Likert scale (Figure 7). Where 23 out of 27 participants (85%) preferred the new EA-based fitting to their previous fitting by the expert and chose to keep it after the study.
Figure 7.
Global evaluation of the sound quality and music perception with default and evolutionary algorithm settings. Values are expressed as mean ± standard error of mean. There is no significant effect of fitting on any item (Wilcoxon signed-rank test).
4. Discussion
Optimizing the fitting in CI users with bimodal binaural hearing can be challenging due to a high number of fitting parameters and combinations. We showed that the frequency band distribution by EA improves not only speech discrimination in noise but also the hearing-related quality of hearing, as judged by APHAB and HISQUI questionnaires. The EA-based fitting resulted in a widening of the frequency bands in the low frequencies and a global shift to higher frequencies than those proposed in the default setting, even farther from the original cochlear tonotopy. There were also individual alterations to the default fMAP, presumably depending on specific electrode nerve interactions. Neural survival and current spread could be reflected by these individual alterations because the measures used for the EA were evaluated directly on the patient and were dependent on its extrinsic characteristics [10]. The method was applicable to all different CI brands.
Speech recognition in noise is probably the most challenging and also one of the most relevant tasks for patients with hearing loss [2]. In case of bimodal or SSD patients, the intersubject variability for binaural results are very large [8]. Wess et al. proposed several possible explanations possible for this variability: (1) intrisinc characteristics of the individual listeners (neural survival, current spread, duration of deafness, lack of cortical plasticity) that cannot be addressed through signal processing; (2) extrinsic distorsions rectifiable via signal processing and/or mapping procedures including different processing times between CI and contralateral HA or NH ear and frequency mismatch between the CI and the contralateral HA or NH ear [10]. For example Zirn et al. [56] and Angermeier et al. [17] showed that sound Localization in Bimodal Listeners could be improved instantaneously when the device delay mismatch (between CI and contralateral HA) was reduced.
Binaural interaction in noisy conditions has been studied by simulating a CI in single-sided deafness [57]. This was obtained by delivering a vocoded speech with variable degrees of mismatch in one ear of eight normal-hearing individuals and evaluating the speech audiometry in noise. The authors show that binaural performances are similar or significantly better than the normal-hearing ear in all cases. Furthermore, in challenging conditions (speech-shaped noise) where the normal ear performance is constrained to the level of the CI performance, a frequency mismatch further degrades the performances, probably by disrupting the binaural interactions. These observations suggest that, in patients with bimodal hearing, reducing the pitch perception mismatch between the CI and the acoustic inputs might enhance hearing in noisy conditions [57].
Even in patients with single-sided deafness (SSD), the contribution of the CI to auditory performances is significant [58,59]. CI decreases the head-shadow effect [58]; increases speech understanding in noise, even in the S0N0 condition (frontal signal and noise) [58,60]; enhances the sound-source localization [58,61]; and improves the patient-reported outcome [62]. In our patients with SSD (patient number 5, 16, and 21), optimization also showed a significant improvement in speech discrimination, except for patient 21 who still felt a subjective improvement with EA fMAP and thus, finally chose it. In line with previous reports, this observation supports the idea that optimized binaural interactions increase performance even with one normal ear.
The idea behind the change in the frequency bands was to optimize the correspondence between the ears and the binaural hearing. Testing these patients in a monaural condition would have probably provided additional interesting data. By reducing channel interactions or by a better correspondence between frequency allocations and functional channels, the EA-based fMAP could also improve monaural hearing.
In a theoretical approach, many have attempted to address the issue of binaural optimization by restoring the pitch-place function of the implanted cochlea according to the original cochlear tonotopy [23,63]. But by looking closer at this problem, the location of the spiral ganglion may be more relevant to the CI stimulation, and its distribution map follows a distinct function from the Greenwood map [63,64]. Nevertheless, attempts to optimize the binaural hearing through frequency allocation according to either the Greenwood or the spiral ganglion map have yielded poor results in general, despite a few individual positive effects on speech discrimination in noise [24,63,65].
Several explanations can be advanced for this failure. The electrode array covers, at best, partially the cochlear apex coding for the low frequencies. Consequently, adjusting the fMAP to the Greenwood function means neglecting a significant part of the spectrum in low and mid frequencies [24]. In the case of shallow insertion, a full and slightly compressed spectral distribution seems to provide better results than a truncated fMAP following Greenwood [24]. Another reason is the number of functional channels (i.e., electrodes eliciting a distinctive pitch) in the implanted cochlea. Theoretically, to each electrode and frequency band should correspond a distinctive auditory nerve ending, but this assumption is far from true in many clinical situations, and frequencies allocated to “dead zones” are lost [66]. Moreover, channel interactions in these cases increase signal distortions and binaural mismatches [66]. Interestingly, the EA-based fitting program indicated an enlargement of frequency bands allocated to several electrodes in our series. We hypothesize that by minimizing the frequency allocation to the electrodes which do not stimulate a distinct neural population, the dissonance decreased, and the hearing improved as it has been already reported [14]. Finally, fitting based on the Greenwood map does not improve the binaural fusion in comparison to the default CI settings since complex central processing adaptations seem to modify the binaural interactions. In patients with bimodal binaural hearing or bilateral CI, two notes separated as far as three or four octaves presented simultaneously to both ears can be perceived with a similar pitch [20,21]. The extent of these alterations depends on many interconnected factors, such as ipsi- and contralateral auditory performances (i.e., speech discrimination, pitch resolution) and the hearing deprivation period [20,21].
In contrast to the theoretical approaches based on the Greenwood map, frequency band adjustments have been also tackled through a purely empirical approach [67,68,69]. By studying the correlations between speech and electrode discrimination abilities, several authors could show that low-frequency resolution is a significant factor for speech discrimination in quiet and noise [60,62]. Allocating most of the electrodes to low frequencies (9 out of 10 to frequencies < 2.6 kHz) improved only some aspects of hearing (e.g., vowel discrimination, speech in noise) at the expense of other performances, such as consonant discrimination [69]. Modifying this strategy by affecting only three additional electrodes to low frequencies in comparison to the default setting had small and variable effects [68]. EA appeared to be more performant than empirical systematic protocols by exploiting patient interaction at each step.
In line with studies that suggest that low-frequency resolution is determinant in speech discrimination, pitch-matching studies showed that perceived pitches with CI were lower than what was estimated by the place-pitch function in unilateral CI users with a normal contralateral hearing [70,71,72]. Several clinical studies demonstrated that the adaptation of the peripheral and the central tonotopies to the radical changes of frequency mapping after CI are possible in the majority. This adaptation drives the new tonotopy toward the frequency organization imposed by the CI [72,73,74,75]. Tonotopy adjustments can involve the entire cochlea or only a region [73]. Some patients may not adapt or adapt poorly to these modifications [73] and understanding the reasons for this maladaptation remains a challenge. However, recent results on SSD and bilateral CI suggest poor plasticity of the binaural system to mismatch [74].
In quiet, the maximum speech discrimination was not influenced by the EA-based fitting. This can be explained by the ceiling effect (9.04 ± 2.01 initially versus 9.22 ± 1.76 at the final test). The best fMAP was not always obtained at the last generation (Table 2). In six patients, the first generation of fMAPs (parents), which were generated randomly in the predefined domain, yielded the best results. In these cases, the algorithm could not further improve the result probably due to the ceiling effect again. Indeed, these patients were initially selected with contralateral functional hearing and consequently, had high performances in quiet. The other possible reason is that, while in quiet, patients may rely on their better ear, and in noise, improvement of binaural hearing has a measurable impact on the performance [75]. But if we limit the analyses to the patient who had their best fMAP after the parent generation, we still have a significant improvement for WRS in noise (mean difference = 2.86), APHAB and HISQUI scores, and no difference for tests in silence, which means that there is no reason to exclude those patients. Patients included during the second phase of the study also had WRS in noise with both their default fMAP and EA fMAP at six weeks. If we compare those scores, we still get a significant improvement with the EA fMAP, which confirms that there is an advantage of the EA fitting. Since different word lists were used at each test, a higher repetition of speech tests in noise versus only two tests in quiet does not affect its outcome and does not appear as a plausible cause of bias [76].
Although EA-based fitting procedure is long and can only be applied to motivated patients, conventional protocols of binaural pitch-matching are even more time-consuming, and more difficult [72,77]. Indeed, they require prolonged concentration and the ability to compare pitches of electrical and acoustic sound regardless of their timber, texture, and loudness [77,78]. Unlike these tedious tasks, we chose the discrimination of 10 monosyllabic words in noise which was short but relevant to our objective. On one hand, the performance of the algorithm depended on the reliability of the scores, on the other hand, short tests have the disadvantage of lower test-retest reliability [79]. This tradeoff appeared interesting since it produced a significant improvement in the hearing in noise.
Recently, inter-aural place mismatch was evaluated in bilateral CI and SSD patients with unilateral CI [78] using ITD discrimination (simultaneous bilateral stimulation), place-pitch ranking (sequential bilateral stimulation), and physical electrode location estimated by CT scans. The results showed that binaural processing may be optimized by using CT scan information to program the CI frequency allocation but not place-pitch ranking. However, the study was not carried out with bimodal users (CI + HA). Moreover, a place-matched frequency mapping based on electrode location could be limited due to the difficulty to determine neural survival at the site of each electrode or the electric interactions in the cochlea for individual patients [10]. EA is directly based on the speech discrimination in noise, and thus, might exploit its extrinsic characteristics to optimize the fitting.
The relationship between loudness and pitch can also add complexity to the modifications of frequency band allocations: pitch and loudness are both affected by the rate of stimulation [80,81,82]. Modifying the frequency allocation alters the loudness perception in a non-linear and unpredictable manner [82]. We did not control or investigate the loudness alterations induced by the frequency band shifts because adding loudness adjustments to frequency band modifications would have exponentially increased the possible combinations in the algorithm and would have made the protocol inapplicable. This could be a subject of future research and development in EA-based fitting.
In the future, the algorithm could be integrated into the fitting software to accelerate the procedure and improve its acceptability by the patients. Evaluation procedures other than the WRS, such as musical sound categorization tasks, could be evaluated to improve the process.
5. Conclusions
By modifying the default fMAP, the evolutionary algorithm increased the word recognition score in noise and improved APHAB and HISQUI scores. Most of the patients (23 out of 27) preferred the modified fMAP and kept it at the end of the study. These improvements were observed despite the heterogeneity of the CI brands and the contralateral ear condition. These results open insights on integrating this type of approach in standard CI fitting.
Acknowledgments
The authors thank Julie Eluecque-Toletti, Claude Gagneux, Adrian Travo, Geoffrey Guenser, and Cyril Cornu for their technical assistance during the fittings.
Appendix A
Table A1.
Frequency bands adjustments with the evolutionary algorithm. Values are expressed in Hertz, e# (electrode number), BW (Band Width), fLOW (Lower Limit, Hz), fHIGH (Upper Limit, Hz), CF (Center Frequency, Hz). -: deactivated electrodes.
| e# | Initial fLOW | Initial fHIGH | Final fLOW | Final fHIGH | Initial BW | Final BW | Initial CF | Final CF |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | ||||||||
| 1 | 100 | 208 | 100 | 195 | 108 | 95 | 154 | 147.5 |
| 2 | 208 | 352 | 195 | 332 | 144 | 137 | 280 | 263.5 |
| 3 | 352 | 545 | 332 | 484 | 193 | 152 | 448.5 | 408 |
| 4 | 545 | 806 | 484 | 655 | 261 | 171 | 675.5 | 569.5 |
| 5 | 806 | 1160 | 655 | 984 | 354 | 329 | 983 | 819.5 |
| 6 | 1160 | 1643 | 984 | 1619 | 483 | 635 | 1401.5 | 1301.5 |
| 7 | 1643 | 2303 | 1619 | 1917 | 660 | 298 | 1973 | 1768 |
| 8 | 2303 | 3208 | 1917 | 3048 | 905 | 1131 | 2755.5 | 2482.5 |
| 9 | 3208 | 4450 | 3048 | 4069 | 1242 | 1021 | 3829 | 3558.5 |
| 10 | 4450 | 6155 | 4069 | 6076 | 1705 | 2007 | 5302.5 | 5072.5 |
| 11 | 6155 | 8500 | 6076 | 8500 | 2345 | 2424 | 7327.5 | 7288 |
| 12 | - | - | - | - | - | - | - | - |
| Patient 2 | ||||||||
| 1 | 100 | 208 | 100 | 193 | 108 | 93 | 154 | 146.5 |
| 2 | 208 | 352 | 193 | 284 | 144 | 91 | 280 | 238.5 |
| 3 | 352 | 545 | 284 | 528 | 193 | 244 | 448.5 | 406 |
| 4 | 545 | 806 | 528 | 795 | 261 | 267 | 675.5 | 661.5 |
| 5 | 806 | 1160 | 795 | 1085 | 354 | 290 | 983 | 940 |
| 6 | 1160 | 1643 | 1085 | 1563 | 483 | 478 | 1401.5 | 1324 |
| 7 | 1643 | 2303 | 1563 | 2184 | 660 | 621 | 1973 | 1873.5 |
| 8 | 2303 | 3208 | 2184 | 2811 | 905 | 627 | 2755.5 | 2497.5 |
| 9 | 3208 | 4450 | 2811 | 4143 | 1242 | 1332 | 3829 | 3477 |
| 10 | 4450 | 6155 | 4143 | 5134 | 1705 | 991 | 5302.5 | 4638.5 |
| 11 | 6155 | 8500 | 5134 | 8500 | 2345 | 3366 | 7327.5 | 6817 |
| 12 | - | - | - | - | - | - | - | - |
| Patient 3 | ||||||||
| 1 | 188 | 313 | 188 | 298 | 125 | 110 | 250.5 | 243 |
| 2 | 313 | 438 | 298 | 409 | 125 | 111 | 375.5 | 353.5 |
| 3 | 438 | 563 | 409 | 471 | 125 | 62 | 500.5 | 440 |
| 4 | 563 | 688 | 471 | 617 | 125 | 146 | 625.5 | 544 |
| 5 | 688 | 813 | 617 | 722 | 125 | 105 | 750.5 | 669.5 |
| 6 | 813 | 938 | 722 | 871 | 125 | 149 | 875.5 | 796.5 |
| 7 | 938 | 1063 | 871 | 1001 | 125 | 130 | 1000.5 | 936 |
| 8 | 1063 | 1188 | 1001 | 1067 | 125 | 66 | 1125.5 | 1034 |
| 9 | 1188 | 1313 | 1067 | 1129 | 125 | 62 | 1250.5 | 1098 |
| 10 | 1313 | 1563 | 1129 | 1462 | 250 | 333 | 1438 | 1295.5 |
| 11 | 1563 | 1813 | 1462 | 1669 | 250 | 207 | 1688 | 1565.5 |
| 12 | 1813 | 2063 | 1669 | 1787 | 250 | 118 | 1938 | 1728 |
| 13 | 2063 | 2313 | 1787 | 1905 | 250 | 118 | 2188 | 1846 |
| 14 | 2313 | 2688 | 1905 | 2418 | 375 | 513 | 2500.5 | 2161.5 |
| 15 | 2688 | 3063 | 2418 | 3030 | 375 | 612 | 2875.5 | 2724 |
| 16 | 3063 | 3563 | 3030 | 3092 | 500 | 62 | 3313 | 3061 |
| 17 | 3563 | 4063 | 3092 | 3855 | 500 | 763 | 3813 | 3473.5 |
| 18 | 4063 | 4688 | 3855 | 4608 | 625 | 753 | 4375.5 | 4231.5 |
| 19 | 4688 | 5313 | 4608 | 5092 | 625 | 484 | 5000.5 | 4850 |
| 20 | 5313 | 6063 | 5092 | 5959 | 750 | 867 | 5688 | 5525.5 |
| 21 | 6063 | 6938 | 5959 | 6460 | 875 | 501 | 6500.5 | 6209.5 |
| 22 | 6938 | 7938 | 6460 | 7938 | 1000 | 1478 | 7438 | 7199 |
| Patient 4 | ||||||||
| 1 | 188 | 313 | 220 | 368 | 125 | 148 | 250.5 | 294 |
| 2 | 313 | 438 | 368 | 462 | 125 | 94 | 375.5 | 415 |
| 3 | 438 | 563 | 462 | 605 | 125 | 143 | 500.5 | 533.5 |
| 4 | 563 | 688 | 605 | 770 | 125 | 165 | 625.5 | 687.5 |
| 5 | 688 | 813 | 770 | 832 | 125 | 62 | 750.5 | 801 |
| 6 | 813 | 938 | 832 | 999 | 125 | 167 | 875.5 | 915.5 |
| 7 | 938 | 1063 | 999 | 1175 | 125 | 176 | 1000.5 | 1087 |
| 8 | 1063 | 1188 | 1175 | 1268 | 125 | 93 | 1125.5 | 1221.5 |
| 9 | 1188 | 1313 | 1268 | 1413 | 125 | 145 | 1250.5 | 1340.5 |
| 10 | 1313 | 1563 | 1413 | 1702 | 250 | 289 | 1438 | 1557.5 |
| 11 | 1563 | 1813 | 1702 | 2001 | 250 | 299 | 1688 | 1851.5 |
| 12 | 1813 | 2063 | 2001 | 2063 | 250 | 62 | 1938 | 2032 |
| 13 | 2063 | 2313 | 2063 | 2390 | 250 | 327 | 2188 | 2226.5 |
| 14 | 2313 | 2688 | 2390 | 2772 | 375 | 382 | 2500.5 | 2581 |
| 15 | 2688 | 3063 | 2772 | 3107 | 375 | 335 | 2875.5 | 2939.5 |
| 16 | 3063 | 3563 | 3107 | 3834 | 500 | 727 | 3313 | 3470.5 |
| 17 | 3563 | 4063 | 3834 | 4274 | 500 | 440 | 3813 | 4054 |
| 18 | 4063 | 4688 | 4274 | 5033 | 625 | 759 | 4375.5 | 4653.5 |
| 19 | 4688 | 5313 | 5033 | 5580 | 625 | 547 | 5000.5 | 5306.5 |
| 20 | 5313 | 6063 | 5580 | 6532 | 750 | 952 | 5688 | 6056 |
| 21 | 6063 | 6938 | 6532 | 7734 | 875 | 1202 | 6500.5 | 7133 |
| 22 | 6938 | 7938 | 7734 | 7938 | 1000 | 204 | 7438 | 7836 |
| Patient 5 | ||||||||
| 1 | 500 | 647 | 500 | 626 | 147 | 126 | 573.5 | 563 |
| 2 | 647 | 837 | 626 | 777 | 190 | 151 | 742 | 701.5 |
| 3 | 837 | 1083 | 777 | 973 | 246 | 196 | 960 | 875 |
| 4 | 1083 | 1401 | 973 | 1166 | 318 | 193 | 1242 | 1069.5 |
| 5 | 1401 | 1812 | 1166 | 1493 | 411 | 327 | 1606.5 | 1329.5 |
| 6 | 1812 | 2345 | 1493 | 2109 | 533 | 616 | 2078.5 | 1801 |
| 7 | 2345 | 3034 | 2109 | 2614 | 689 | 505 | 2689.5 | 2361.5 |
| 8 | 3034 | 3925 | 2614 | 3337 | 891 | 723 | 3479.5 | 2975.5 |
| 9 | 3925 | 5078 | 3337 | 4687 | 1153 | 1350 | 4501.5 | 4012 |
| 10 | 5078 | 6570 | 4687 | 5877 | 1492 | 1190 | 5824 | 5282 |
| 11 | 6570 | 8500 | 5877 | 8500 | 1930 | 2623 | 7535 | 7188.5 |
| 12 | - | - | - | - | - | - | - | - |
| Patient 6 | ||||||||
| 1 | 195 | 326 | 195 | 326 | 131 | 131 | 260.5 | 260.5 |
| 2 | 326 | 456 | 326 | 456 | 130 | 130 | 391 | 391 |
| 3 | 456 | 586 | 456 | 586 | 130 | 130 | 521 | 521 |
| 4 | 586 | 716 | 586 | 716 | 130 | 130 | 651 | 651 |
| 5 | 716 | 846 | 716 | 846 | 130 | 130 | 781 | 781 |
| 6 | 846 | 977 | 846 | 977 | 131 | 131 | 911.5 | 911.5 |
| 7 | 977 | 1107 | 977 | 1107 | 130 | 130 | 1042 | 1042 |
| 8 | 1107 | 1367 | 1107 | 1237 | 260 | 130 | 1237 | 1172 |
| 9 | 1367 | 1758 | 1237 | 1628 | 391 | 391 | 1562.5 | 1432.5 |
| 10 | 1758 | 2148 | 1628 | 2018 | 390 | 390 | 1953 | 1823 |
| 11 | 2148 | 2539 | 2018 | 2409 | 391 | 391 | 2343.5 | 2213.5 |
| 12 | 2539 | 3060 | 2409 | 2930 | 521 | 521 | 2799.5 | 2669.5 |
| 13 | 3060 | 3711 | 2930 | 3581 | 651 | 651 | 3385.5 | 3255.5 |
| 14 | 3711 | 4492 | 3581 | 4492 | 781 | 911 | 4101.5 | 4036.5 |
| 15 | 4492 | 5404 | 4492 | 5534 | 912 | 1042 | 4948 | 5013 |
| 16 | 5404 | 6185 | 5534 | 6576 | 781 | 1042 | 5794.5 | 6055 |
| 17 | 6185 | 7357 | 6576 | 7617 | 1172 | 1041 | 6771 | 7096.5 |
| 18 | - | - | - | - | - | - | - | - |
| 19 | - | - | - | - | - | - | - | - |
| 20 | - | - | - | - | - | - | - | - |
| Patient 7 | ||||||||
| 1 | 188 | 313 | 188 | 261 | 125 | 73 | 250.5 | 224.5 |
| 2 | 313 | 438 | 261 | 424 | 125 | 163 | 375.5 | 342.5 |
| 3 | 438 | 563 | 424 | 555 | 125 | 131 | 500.5 | 489.5 |
| 4 | 563 | 688 | 555 | 643 | 125 | 88 | 625.5 | 599 |
| 5 | 688 | 813 | 643 | 754 | 125 | 111 | 750.5 | 698.5 |
| 6 | 813 | 938 | 754 | 871 | 125 | 117 | 875.5 | 812.5 |
| 7 | 938 | 1063 | 871 | 933 | 125 | 62 | 1000.5 | 902 |
| 8 | 1063 | 1188 | 933 | 1095 | 125 | 162 | 1125.5 | 1014 |
| 9 | 1188 | 1313 | 1095 | 1157 | 125 | 62 | 1250.5 | 1126 |
| 10 | 1313 | 1563 | 1157 | 1408 | 250 | 251 | 1438 | 1282.5 |
| 11 | 1563 | 1813 | 1408 | 1470 | 250 | 62 | 1688 | 1439 |
| 12 | 1813 | 2063 | 1470 | 1667 | 250 | 197 | 1938 | 1568.5 |
| 13 | 2063 | 2313 | 1667 | 2048 | 250 | 381 | 2188 | 1857.5 |
| 14 | 2313 | 2688 | 2048 | 2255 | 375 | 207 | 2500.5 | 2151.5 |
| 15 | 2688 | 3063 | 2255 | 2603 | 375 | 348 | 2875.5 | 2429 |
| 16 | 3063 | 3563 | 2603 | 3442 | 500 | 839 | 3313 | 3022.5 |
| 17 | 3563 | 4063 | 3442 | 3634 | 500 | 192 | 3813 | 3538 |
| 18 | 4063 | 4688 | 3634 | 4079 | 625 | 445 | 4375.5 | 3856.5 |
| 19 | 4688 | 5313 | 4079 | 5133 | 625 | 1054 | 5000.5 | 4606 |
| 20 | 5313 | 6063 | 5133 | 5614 | 750 | 481 | 5688 | 5373.5 |
| 21 | 6063 | 6938 | 5614 | 6266 | 875 | 652 | 6500.5 | 5940 |
| 22 | 6938 | 7938 | 6266 | 7938 | 1000 | 1672 | 7438 | 7102 |
| Patient 8 | ||||||||
| 1 | 250 | 416 | 238 | 374 | 166 | 136 | 333 | 306 |
| 2 | 416 | 494 | 374 | 510 | 78 | 136 | 455 | 442 |
| 3 | 494 | 587 | 510 | 578 | 93 | 68 | 540.5 | 544 |
| 4 | 587 | 697 | 578 | 782 | 110 | 204 | 642 | 680 |
| 5 | 697 | 828 | 782 | 850 | 131 | 68 | 762.5 | 816 |
| 6 | 828 | 983 | 850 | 918 | 155 | 68 | 905.5 | 884 |
| 7 | 983 | 1168 | 918 | 1121 | 185 | 203 | 1075.5 | 1019.5 |
| 8 | 1168 | 1387 | 1121 | 1393 | 219 | 272 | 1277.5 | 1257 |
| 9 | 1387 | 1648 | 1393 | 1529 | 261 | 136 | 1517.5 | 1461 |
| 10 | 1648 | 1958 | 1529 | 2005 | 310 | 476 | 1803 | 1767 |
| 11 | 1958 | 2326 | 2005 | 2345 | 368 | 340 | 2142 | 2175 |
| 12 | 2326 | 2762 | 2345 | 2821 | 436 | 476 | 2544 | 2583 |
| 13 | 2762 | 3281 | 2821 | 3161 | 519 | 340 | 3021.5 | 2991 |
| 14 | 3281 | 3858 | 3161 | 5064 | 577 | 1903 | 3569.5 | 4112.5 |
| 15 | 3858 | 4630 | 5064 | 8054 | 772 | 2990 | 4244 | 6559 |
| 16 | 4630 | 8700 | 8054 | 8700 | 4070 | 646 | 6665 | 8377 |
| Patient 9 | ||||||||
| 1 | 250 | 416 | 238 | 374 | 166 | 136 | 333 | 306 |
| 2 | 416 | 494 | 374 | 510 | 78 | 136 | 455 | 442 |
| 3 | 494 | 587 | 510 | 578 | 93 | 68 | 540.5 | 544 |
| 4 | 587 | 697 | 578 | 646 | 110 | 68 | 642 | 612 |
| 5 | 697 | 828 | 646 | 782 | 131 | 136 | 762.5 | 714 |
| 6 | 828 | 983 | 782 | 850 | 155 | 68 | 905.5 | 816 |
| 7 | 983 | 1168 | 850 | 1054 | 185 | 204 | 1075.5 | 952 |
| 8 | 1168 | 1387 | 1054 | 1189 | 219 | 135 | 1277.5 | 1121.5 |
| 9 | 1387 | 1648 | 1189 | 1665 | 261 | 476 | 1517.5 | 1427 |
| 10 | 1648 | 1958 | 1665 | 1869 | 310 | 204 | 1803 | 1767 |
| 11 | 1958 | 2326 | 1869 | 2413 | 368 | 544 | 2142 | 2141 |
| 12 | 2326 | 2762 | 2413 | 2549 | 436 | 136 | 2544 | 2481 |
| 13 | 2762 | 3281 | 2549 | 3161 | 519 | 612 | 3021.5 | 2855 |
| 14 | 3281 | 3858 | 3161 | 5268 | 577 | 2107 | 3569.5 | 4214.5 |
| 15 | 3858 | 4630 | 5268 | 8054 | 772 | 2786 | 4244 | 6661 |
| 16 | 4630 | 8700 | 8054 | 8700 | 4070 | 646 | 6665 | 8377 |
| Patient 10 | ||||||||
| 1 | 188 | 313 | 188 | 354 | 125 | 166 | 250.5 | 271 |
| 2 | 313 | 438 | 354 | 448 | 125 | 94 | 375.5 | 401 |
| 3 | 438 | 563 | 448 | 658 | 125 | 210 | 500.5 | 553 |
| 4 | 563 | 688 | 658 | 816 | 125 | 158 | 625.5 | 737 |
| 5 | 688 | 813 | 816 | 927 | 125 | 111 | 750.5 | 871.5 |
| 6 | 813 | 938 | 927 | 1080 | 125 | 153 | 875.5 | 1003.5 |
| 7 | 938 | 1063 | 1080 | 1158 | 125 | 78 | 1000.5 | 1119 |
| 8 | - | - | - | - | - | - | - | - |
| 9 | 1068 | 1188 | 1158 | 1220 | 120 | 62 | 1128 | 1189 |
| 10 | 1188 | 1438 | 1220 | 1480 | 250 | 260 | 1313 | 1350 |
| 11 | 1438 | 1688 | 1480 | 1775 | 250 | 295 | 1563 | 1627.5 |
| 12 | 1688 | 1938 | 1775 | 2160 | 250 | 385 | 1813 | 1967.5 |
| 13 | 1938 | 2188 | 2160 | 2299 | 250 | 139 | 2063 | 2229.5 |
| 14 | 2188 | 2563 | 2299 | 2997 | 375 | 698 | 2375.5 | 2648 |
| 15 | 2563 | 2938 | 2997 | 3081 | 375 | 84 | 2750.5 | 3039 |
| 16 | 2938 | 3438 | 3081 | 4077 | 500 | 996 | 3188 | 3579 |
| 17 | 3438 | 3938 | 4077 | 4197 | 500 | 120 | 3688 | 4137 |
| 18 | 3938 | 4563 | 4197 | 4964 | 625 | 767 | 4250.5 | 4580.5 |
| 19 | 4563 | 5313 | 4964 | 5762 | 750 | 798 | 4938 | 5363 |
| 20 | 5313 | 6063 | 5762 | 6809 | 750 | 1047 | 5688 | 6285.5 |
| 21 | 6063 | 6938 | 6809 | 7702 | 875 | 893 | 6500.5 | 7255.5 |
| 22 | 6938 | 7938 | 7702 | 7938 | 1000 | 236 | 7438 | 7820 |
| Patient 11 | ||||||||
| 1 | 188 | 313 | 188 | 368 | 125 | 180 | 250.5 | 278 |
| 2 | 313 | 438 | 368 | 509 | 125 | 141 | 375.5 | 438,5 |
| 3 | 438 | 563 | 509 | 584 | 125 | 75 | 500.5 | 546.5 |
| 4 | 563 | 688 | 584 | 747 | 125 | 163 | 625.5 | 665.5 |
| 5 | 688 | 813 | 747 | 816 | 125 | 69 | 750.5 | 781.5 |
| 6 | 813 | 938 | 816 | 995 | 125 | 179 | 875.5 | 905.5 |
| 7 | 938 | 1063 | 995 | 1127 | 125 | 132 | 1000.5 | 1061 |
| 8 | 1063 | 1188 | 1127 | 1226 | 125 | 99 | 1125.5 | 1176.5 |
| 9 | 1188 | 1313 | 1226 | 1313 | 125 | 87 | 1250.5 | 1269.5 |
| 10 | 1313 | 1563 | 1313 | 1674 | 250 | 361 | 1438 | 1493.5 |
| 11 | 1563 | 1813 | 1674 | 1847 | 250 | 173 | 1688 | 1760.5 |
| 12 | 1813 | 2063 | 1847 | 2310 | 250 | 463 | 1938 | 2078.5 |
| 13 | 2063 | 2313 | 2310 | 2531 | 250 | 221 | 2188 | 2420.5 |
| 14 | 2313 | 2688 | 2531 | 3079 | 375 | 548 | 2500.5 | 2805 |
| 15 | 2688 | 3063 | 3079 | 3494 | 375 | 415 | 2875.5 | 3286.5 |
| 16 | 3063 | 3563 | 3494 | 4018 | 500 | 524 | 3313 | 3756 |
| 17 | 3563 | 4063 | 4018 | 4090 | 500 | 72 | 3813 | 4054 |
| 18 | 4063 | 4688 | 4090 | 4752 | 625 | 662 | 4375.5 | 4421 |
| 19 | 4688 | 5313 | 4752 | 5611 | 625 | 859 | 5000.5 | 5181.5 |
| 20 | 5313 | 6063 | 5611 | 6706 | 750 | 1095 | 5688 | 6158.5 |
| 21 | 6063 | 6938 | 6706 | 7846 | 875 | 1140 | 6500.5 | 7276 |
| 22 | 6938 | 7938 | 7846 | 7938 | 1000 | 92 | 7438 | 7892 |
| Patient 12 | ||||||||
| 1 | 70 | 170 | 70 | 170 | 100 | 100 | 120 | 120 |
| 2 | 170 | 300 | 170 | 325 | 130 | 155 | 235 | 247.5 |
| 3 | 300 | 469 | 325 | 487 | 169 | 162 | 384.5 | 406 |
| 4 | 469 | 690 | 487 | 702 | 221 | 215 | 579.5 | 594.5 |
| 5 | 690 | 982 | 702 | 1058 | 292 | 356 | 836 | 880 |
| 6 | 982 | 1368 | 1058 | 1453 | 386 | 395 | 1175 | 1255.5 |
| 7 | 1368 | 1881 | 1453 | 1919 | 513 | 466 | 1624.5 | 1686 |
| 8 | 1881 | 2564 | 1919 | 3076 | 683 | 1157 | 2222.5 | 2497.5 |
| 9 | 2564 | 3475 | 3076 | 3705 | 911 | 629 | 3019.5 | 3390.5 |
| 10 | 3475 | 4693 | 3705 | 4971 | 1218 | 1266 | 4084 | 4338 |
| 11 | 4693 | 6321 | 4971 | 6560 | 1628 | 1589 | 5507 | 5765.5 |
| 12 | 6321 | 8500 | 6560 | 8500 | 2179 | 1940 | 7410.5 | 7530 |
| Patient 13 | ||||||||
| 1 | 188 | 313 | 188 | 358 | 125 | 170 | 250.5 | 273 |
| 2 | 313 | 438 | 358 | 493 | 125 | 135 | 375.5 | 425.5 |
| 3 | 438 | 563 | 493 | 584 | 125 | 91 | 500.5 | 538.5 |
| 4 | 563 | 688 | 584 | 748 | 125 | 164 | 625.5 | 666 |
| 5 | 688 | 813 | 748 | 894 | 125 | 146 | 750.5 | 821 |
| 6 | 813 | 938 | 894 | 956 | 125 | 62 | 875.5 | 925 |
| 7 | 938 | 1063 | 856 | 1138 | 125 | 282 | 1000.5 | 997 |
| 8 | 1063 | 1188 | 1138 | 1318 | 125 | 180 | 1125.5 | 1228 |
| 9 | 1188 | 1313 | 1318 | 1541 | 125 | 223 | 1250.5 | 1429.5 |
| 10 | 1313 | 1563 | 1541 | 1808 | 250 | 267 | 1438 | 1674.5 |
| 11 | 1563 | 1813 | 1808 | 1870 | 250 | 62 | 1688 | 1839 |
| 12 | 1813 | 2063 | 1870 | 2373 | 250 | 503 | 1938 | 2121.5 |
| 13 | 2063 | 2313 | 2373 | 2490 | 250 | 117 | 2188 | 2431.5 |
| 14 | 2313 | 2688 | 2490 | 2793 | 375 | 303 | 2500.5 | 2641.5 |
| 15 | 2688 | 3063 | 2793 | 3216 | 375 | 423 | 2875.5 | 3004.5 |
| 16 | 3063 | 3563 | 3216 | 4002 | 500 | 786 | 3313 | 3609 |
| 17 | 3563 | 4063 | 4002 | 4447 | 500 | 445 | 3813 | 4224.5 |
| 18 | 4063 | 4688 | 4447 | 5017 | 625 | 570 | 4375.5 | 4732 |
| 19 | 4688 | 5313 | 5017 | 6196 | 625 | 1179 | 5000.5 | 5606.5 |
| 20 | 5313 | 6063 | 6196 | 6827 | 750 | 631 | 5688 | 6511.5 |
| 21 | 6063 | 6938 | 6827 | 7654 | 875 | 827 | 6500.5 | 7240.5 |
| 22 | 6938 | 7938 | 7654 | 7938 | 1000 | 284 | 7438 | 7796 |
| Patient 14 | ||||||||
| 1 | 188 | 313 | 188 | 315 | 125 | 127 | 250.5 | 251.5 |
| 2 | 313 | 438 | 315 | 512 | 125 | 197 | 375.5 | 413.5 |
| 3 | 438 | 563 | 512 | 688 | 125 | 176 | 500.5 | 600 |
| 4 | 563 | 688 | 688 | 761 | 125 | 73 | 625.5 | 724.5 |
| 5 | 688 | 813 | 761 | 936 | 125 | 175 | 750.5 | 848.5 |
| 6 | 813 | 938 | 936 | 1077 | 125 | 141 | 875.5 | 1006.5 |
| 7 | 938 | 1063 | 1077 | 1146 | 125 | 69 | 1000.5 | 1111.5 |
| 8 | 1063 | 1188 | 1146 | 1296 | 125 | 150 | 1125.5 | 1221 |
| 9 | 1188 | 1313 | 1296 | 1358 | 125 | 62 | 1250.5 | 1327 |
| 10 | 1313 | 1563 | 1358 | 1762 | 250 | 404 | 1438 | 1560 |
| 11 | 1563 | 1813 | 1762 | 1824 | 250 | 62 | 1688 | 1793 |
| 12 | 1813 | 2063 | 1824 | 2177 | 250 | 353 | 1938 | 2000.5 |
| 13 | 2063 | 2313 | 2177 | 2334 | 250 | 157 | 2188 | 2255.5 |
| 14 | 2313 | 2688 | 2334 | 2740 | 375 | 406 | 2500.5 | 2537 |
| 15 | 2688 | 3063 | 2740 | 3567 | 375 | 827 | 2875.5 | 3153.5 |
| 16 | 3063 | 3563 | 3567 | 4058 | 500 | 491 | 3313 | 3812.5 |
| 17 | 3563 | 4063 | 4058 | 4320 | 500 | 262 | 3813 | 4189 |
| 18 | 4063 | 4688 | 4320 | 5349 | 625 | 1029 | 4375.5 | 4834.5 |
| 19 | 4688 | 5313 | 5349 | 5579 | 625 | 230 | 5000.5 | 5464 |
| 20 | 5313 | 6063 | 5579 | 6595 | 750 | 1016 | 5688 | 6087 |
| 21 | 6063 | 6938 | 6595 | 7467 | 875 | 872 | 6500.5 | 7031 |
| 22 | 6938 | 7938 | 7467 | 7938 | 1000 | 471 | 7438 | 7702.5 |
| Patient 15 | ||||||||
| 1 | 70 | 170 | 70 | 197 | 100 | 127 | 120 | 133.5 |
| 2 | 170 | 300 | 197 | 354 | 130 | 157 | 235 | 275,5 |
| 3 | 300 | 469 | 354 | 480 | 169 | 126 | 384.5 | 417 |
| 4 | 469 | 690 | 480 | 816 | 221 | 336 | 579.5 | 648 |
| 5 | 690 | 982 | 816 | 1105 | 292 | 289 | 836 | 960.5 |
| 6 | 982 | 1368 | 1105 | 1394 | 386 | 289 | 1175 | 1249.5 |
| 7 | 1368 | 1881 | 1394 | 1985 | 513 | 591 | 1624.5 | 1689.5 |
| 8 | 1881 | 2564 | 1985 | 2805 | 683 | 820 | 2222.5 | 2395 |
| 9 | 2564 | 3475 | 2805 | 3965 | 911 | 1160 | 3019.5 | 3385 |
| 10 | 3475 | 4693 | 3965 | 7722 | 1218 | 3757 | 4084 | 5843.5 |
| 11 | 4693 | 6321 | 4722 | 6671 | 1628 | 1949 | 5507 | 5696.5 |
| 12 | 6321 | 8500 | 6671 | 8500 | 2179 | 1829 | 7410.5 | 7585.5 |
| Patient 16 | ||||||||
| 1 | 100 | 221 | 100 | 244 | 121 | 144 | 160.5 | 172 |
| 2 | 221 | 386 | 244 | 409 | 165 | 165 | 303.5 | 326.5 |
| 3 | 386 | 615 | 409 | 728 | 229 | 319 | 500.5 | 568.5 |
| 4 | 615 | 935 | 728 | 1015 | 320 | 287 | 775 | 871.5 |
| 5 | 935 | 1383 | 1015 | 1450 | 448 | 435 | 1159 | 1232.5 |
| 6 | 1383 | 2014 | 1450 | 2284 | 631 | 834 | 1698.5 | 1867 |
| 7 | 2014 | 2906 | 2284 | 3475 | 892 | 1191 | 2460 | 2879.5 |
| 8 | 2906 | 4169 | 3475 | 4569 | 1263 | 1094 | 3537.5 | 4022 |
| 9 | 4169 | 5959 | 4569 | 6309 | 1790 | 1740 | 5064 | 5439 |
| 10 | 5959 | 8500 | 6312 | 8500 | 2541 | 2188 | 7229.5 | 7406 |
| 11 | - | - | - | - | - | - | - | - |
| 12 | - | - | - | - | - | - | - | - |
| Patient 17 | ||||||||
| 1 | 188 | 313 | 188 | 337 | 125 | 149 | 250.5 | 262.5 |
| 2 | 313 | 438 | 337 | 501 | 125 | 164 | 375.5 | 419 |
| 3 | 438 | 563 | 501 | 563 | 125 | 62 | 500.5 | 532 |
| 4 | 563 | 688 | 563 | 751 | 125 | 188 | 625.5 | 657 |
| 5 | 688 | 813 | 751 | 882 | 125 | 131 | 750.5 | 816.5 |
| 6 | 813 | 938 | 882 | 1024 | 125 | 142 | 875.5 | 953 |
| 7 | 938 | 1063 | 1024 | 1227 | 125 | 203 | 1000.5 | 1125.5 |
| 8 | - | - | - | - | - | - | - | - |
| 9 | 1063 | 1313 | 1227 | 1397 | 250 | 170 | 1188 | 1312 |
| 10 | 1313 | 1563 | 1397 | 1808 | 250 | 411 | 1438 | 1602.5 |
| 11 | 1563 | 1813 | 1808 | 1984 | 250 | 176 | 1688 | 1896 |
| 12 | 1813 | 2188 | 1984 | 2203 | 375 | 219 | 2000.5 | 2093.5 |
| 13 | 2188 | 2563 | 2203 | 2653 | 375 | 450 | 2375.5 | 2428 |
| 14 | 2563 | 3063 | 2653 | 3505 | 500 | 852 | 2813 | 3079 |
| 15 | 3063 | 3563 | 3505 | 3900 | 500 | 395 | 3313 | 3702.5 |
| 16 | 3563 | 4188 | 3900 | 4315 | 625 | 415 | 3875.5 | 4107.5 |
| 17 | 4188 | 4938 | 4315 | 5275 | 750 | 960 | 4563 | 4795 |
| 18 | 4938 | 5813 | 5275 | 6519 | 875 | 1244 | 5375.5 | 5897 |
| 19 | 5813 | 6813 | 6519 | 7074 | 1000 | 555 | 6313 | 6796.5 |
| 20 | 6813 | 7938 | 7074 | 7938 | 1125 | 864 | 7375.5 | 7506 |
| 21 | - | - | - | - | - | - | - | - |
| 22 | - | - | - | - | - | - | - | - |
| Patient 18 | ||||||||
| 1 | 70 | 170 | 70 | 200 | 100 | 130 | 120 | 135 |
| 2 | 170 | 300 | 200 | 352 | 130 | 152 | 235 | 276 |
| 3 | 300 | 469 | 352 | 545 | 169 | 193 | 384.5 | 448.5 |
| 4 | 469 | 690 | 545 | 725 | 221 | 180 | 579.5 | 635 |
| 5 | 690 | 982 | 725 | 1098 | 292 | 373 | 836 | 911.5 |
| 6 | 982 | 1368 | 1098 | 1374 | 386 | 276 | 1175 | 1236 |
| 7 | 1368 | 1881 | 1374 | 2040 | 513 | 666 | 1624.5 | 1707 |
| 8 | 1881 | 2564 | 2040 | 2724 | 683 | 684 | 2222.5 | 2382 |
| 9 | 2564 | 3475 | 2724 | 3587 | 911 | 863 | 3019.5 | 3155.5 |
| 10 | 3475 | 4693 | 3587 | 4860 | 1218 | 1273 | 4084 | 4223.5 |
| 11 | 4693 | 6321 | 4869 | 6855 | 1628 | 1986 | 5507 | 5862 |
| 12 | 6321 | 8500 | 6855 | 8500 | 2179 | 1645 | 7410.5 | 7677.5 |
| Patient 19 | ||||||||
| 1 | 100 | 198 | 100 | 236 | 98 | 136 | 149 | 168 |
| 2 | 198 | 325 | 236 | 387 | 127 | 151 | 261.5 | 311.5 |
| 3 | 325 | 491 | 387 | 538 | 166 | 151 | 408 | 462.5 |
| 4 | 491 | 710 | 538 | 823 | 219 | 285 | 600.5 | 680.5 |
| 5 | 710 | 999 | 823 | 1147 | 289 | 324 | 854.5 | 985 |
| 6 | 999 | 1383 | 1147 | 1470 | 384 | 323 | 1191 | 1308.5 |
| 7 | 1383 | 1893 | 1470 | 2141 | 510 | 671 | 1638 | 1805.5 |
| 8 | 1893 | 2754 | 2141 | 2662 | 861 | 521 | 2323.5 | 2401.5 |
| 9 | 2574 | 3483 | 2662 | 3975 | 909 | 1313 | 3028.5 | 3318.5 |
| 10 | 3483 | 4698 | 3975 | 4727 | 1215 | 752 | 4090.5 | 4351 |
| 11 | 4698 | 6323 | 4727 | 7328 | 1625 | 2601 | 5510.5 | 6027.5 |
| 12 | 6323 | 8500 | 7328 | 8500 | 2177 | 1172 | 7411.5 | 7914 |
| Patient 20 | ||||||||
| 1 | 188 | 313 | 188 | 313 | 125 | 125 | 250.5 | 250.5 |
| 2 | 313 | 438 | 313 | 454 | 125 | 141 | 375.5 | 383.5 |
| 3 | 438 | 563 | 454 | 665 | 125 | 211 | 500.5 | 559.5 |
| 4 | 563 | 688 | 665 | 814 | 125 | 149 | 625.5 | 739.5 |
| 5 | 688 | 813 | 814 | 876 | 125 | 62 | 750.5 | 845 |
| 6 | 813 | 938 | 876 | 958 | 125 | 82 | 875.5 | 917 |
| 7 | 938 | 1063 | 958 | 1117 | 125 | 159 | 1000.5 | 1037.5 |
| 8 | 1063 | 1188 | 1117 | 1285 | 125 | 168 | 1125.5 | 1201 |
| 9 | 1188 | 1438 | 1285 | 1609 | 250 | 324 | 1313 | 1447 |
| 10 | 1438 | 1688 | 1609 | 1776 | 250 | 167 | 1563 | 1692.5 |
| 11 | 1688 | 1938 | 1776 | 2167 | 250 | 391 | 1813 | 1971.5 |
| 12 | 1938 | 2313 | 2167 | 2276 | 375 | 109 | 2125.5 | 2221.5 |
| 13 | 2313 | 2688 | 2276 | 2742 | 375 | 466 | 2500.5 | 2509 |
| 14 | 2688 | 3188 | 2742 | 2842 | 500 | 100 | 2938 | 2792 |
| 15 | 3188 | 3688 | 2842 | 2904 | 500 | 62 | 3438 | 2873 |
| 16 | 3686 | 4313 | 2904 | 4588 | 627 | 1684 | 3999.5 | 3746 |
| 17 | 4313 | 5063 | 4588 | 5492 | 750 | 904 | 4688 | 5040 |
| 18 | 5063 | 5938 | 5492 | 6541 | 875 | 1049 | 5500.5 | 6016.5 |
| 19 | 5938 | 6938 | 6541 | 7117 | 1000 | 576 | 6438 | 6829 |
| 20 | 6938 | 7935 | 7117 | 7938 | 997 | 821 | 7436.5 | 7527.5 |
| 21 | - | - | - | - | - | - | - | - |
| 22 | - | - | - | - | - | - | - | - |
| Patient 21 | ||||||||
| 1 | 188 | 313 | 188 | 313 | 125 | 125 | 250.5 | 250.5 |
| 2 | 313 | 438 | 313 | 447 | 125 | 134 | 375.5 | 380 |
| 3 | 438 | 563 | 447 | 578 | 125 | 131 | 500.5 | 512.5 |
| 4 | 563 | 688 | 578 | 781 | 125 | 203 | 625.5 | 679.5 |
| 5 | 688 | 813 | 781 | 893 | 125 | 112 | 750.5 | 837 |
| 6 | 813 | 938 | 893 | 973 | 125 | 80 | 875.5 | 933 |
| 7 | 938 | 1063 | 973 | 1161 | 125 | 188 | 1000.5 | 1067 |
| 8 | 1063 | 1188 | 1161 | 1223 | 125 | 62 | 1125.5 | 1192 |
| 9 | 1188 | 1313 | 1223 | 1327 | 125 | 104 | 1250.5 | 1275 |
| 10 | 1313 | 1563 | 1327 | 1829 | 250 | 502 | 1438 | 1578 |
| 11 | 1563 | 1813 | 1829 | 2016 | 250 | 187 | 1688 | 1922.5 |
| 12 | 1813 | 2063 | 2016 | 2446 | 250 | 430 | 1938 | 2231 |
| 13 | 2063 | 2313 | 2446 | 2599 | 250 | 153 | 2188 | 2522.5 |
| 14 | 2313 | 2688 | 2599 | 3001 | 375 | 402 | 2500.5 | 2800 |
| 15 | 2688 | 3063 | 3001 | 3562 | 375 | 561 | 2875.5 | 3281.5 |
| 16 | 3063 | 3563 | 3562 | 4189 | 500 | 627 | 3313 | 3875.5 |
| 17 | 3563 | 4063 | 4189 | 4688 | 500 | 499 | 3813 | 4438.5 |
| 18 | 4063 | 4688 | 4688 | 4866 | 625 | 178 | 4375.5 | 4777 |
| 19 | 4688 | 5313 | 4866 | 6232 | 625 | 1366 | 5000.5 | 5549 |
| 20 | 5313 | 6063 | 6232 | 6806 | 750 | 574 | 5688 | 6519 |
| 21 | 6063 | 6938 | 6806 | 7876 | 875 | 1070 | 6500.5 | 7341 |
| 22 | 6938 | 7938 | 7876 | 7938 | 1000 | 62 | 7438 | 7907 |
| Patient 22 | ||||||||
| 1 | 70 | 170 | 70 | 184 | 100 | 114 | 120 | 127 |
| 2 | 170 | 300 | 184 | 310 | 130 | 126 | 235 | 247 |
| 3 | 300 | 469 | 310 | 493 | 169 | 183 | 384.5 | 401.5 |
| 4 | 469 | 690 | 493 | 692 | 221 | 199 | 579.5 | 592.5 |
| 5 | 690 | 982 | 692 | 1163 | 292 | 471 | 836 | 927.5 |
| 6 | 982 | 1368 | 1163 | 1547 | 386 | 384 | 1175 | 1355 |
| 7 | 1368 | 1881 | 1547 | 2231 | 513 | 684 | 1624.5 | 1889 |
| 8 | 1881 | 2564 | 2231 | 2647 | 683 | 416 | 2222.5 | 2439 |
| 9 | 2564 | 3475 | 2647 | 4115 | 911 | 1468 | 3019.5 | 3381 |
| 10 | 3475 | 4693 | 4115 | 5439 | 1218 | 1324 | 4084 | 4777 |
| 11 | 4693 | 6321 | 5439 | 7050 | 1628 | 1611 | 5507 | 6244.5 |
| 12 | 6321 | 8500 | 7050 | 8500 | 2179 | 1450 | 7410.5 | 7775 |
| Patient 23 | ||||||||
| 1 | 188 | 313 | 188 | 361 | 125 | 173 | 250.5 | 274.5 |
| 2 | 313 | 438 | 361 | 507 | 125 | 146 | 375.5 | 434 |
| 3 | 438 | 563 | 507 | 584 | 125 | 77 | 500.5 | 545.5 |
| 4 | 563 | 688 | 584 | 755 | 125 | 171 | 625.5 | 669.5 |
| 5 | 688 | 813 | 755 | 885 | 125 | 130 | 750.5 | 820 |
| 6 | 813 | 938 | 885 | 1059 | 125 | 174 | 875.5 | 972 |
| 7 | 938 | 1063 | 1059 | 1214 | 125 | 155 | 1000.5 | 1136.5 |
| 8 | 1063 | 1188 | 1214 | 1310 | 125 | 96 | 1125.5 | 1262 |
| 9 | 1188 | 1313 | 1310 | 1372 | 125 | 62 | 1250.5 | 1341 |
| 10 | 1313 | 1563 | 1372 | 1738 | 250 | 366 | 1438 | 1555 |
| 11 | 1563 | 1813 | 1738 | 2050 | 250 | 312 | 1688 | 1894 |
| 12 | 1813 | 2063 | 2050 | 2130 | 250 | 80 | 1938 | 2090 |
| 13 | 2063 | 2313 | 2130 | 2368 | 250 | 238 | 2188 | 2249 |
| 14 | 2313 | 2688 | 2368 | 2956 | 375 | 588 | 2500.5 | 2662 |
| 15 | 2688 | 3063 | 2956 | 3676 | 375 | 720 | 2875.5 | 3316 |
| 16 | 3063 | 3563 | 3676 | 3805 | 500 | 129 | 3313 | 3740.5 |
| 17 | 3563 | 4063 | 3805 | 4538 | 500 | 733 | 3813 | 4171.5 |
| 18 | 4063 | 4688 | 4538 | 4975 | 625 | 437 | 4375.5 | 4756.5 |
| 19 | 4688 | 5313 | 4975 | 6196 | 625 | 1221 | 5000.5 | 5585.5 |
| 20 | 5313 | 6063 | 6196 | 6827 | 750 | 631 | 5688 | 6511.5 |
| 21 | 6063 | 6938 | 6827 | 7654 | 875 | 827 | 6500.5 | 7240.5 |
| 22 | 6938 | 7938 | 7654 | 7938 | 1000 | 284 | 7438 | 7796 |
| Patient 24 | ||||||||
| 1 | 188 | 313 | 188 | 315 | 125 | 127 | 250.5 | 251.5 |
| 2 | 313 | 438 | 315 | 517 | 125 | 202 | 375.5 | 416 |
| 3 | 438 | 563 | 517 | 641 | 125 | 124 | 500.5 | 579 |
| 4 | 563 | 688 | 641 | 781 | 125 | 140 | 625.5 | 711 |
| 5 | 688 | 813 | 781 | 942 | 125 | 161 | 750.5 | 861.5 |
| 6 | 813 | 938 | 942 | 1077 | 125 | 135 | 875.5 | 1009.5 |
| 7 | 938 | 1063 | 1077 | 1193 | 125 | 116 | 1000.5 | 1135 |
| 8 | 1063 | 1188 | 1193 | 1296 | 125 | 103 | 1125.5 | 1244.5 |
| 9 | 1188 | 1313 | 1296 | 1358 | 125 | 62 | 1250.5 | 1327 |
| 10 | 1313 | 1563 | 1358 | 1764 | 250 | 406 | 1438 | 1561 |
| 11 | 1563 | 1813 | 1764 | 1826 | 250 | 62 | 1688 | 1795 |
| 12 | 1813 | 2063 | 1826 | 2190 | 250 | 364 | 1938 | 2008 |
| 13 | 2063 | 2313 | 2190 | 2355 | 250 | 165 | 2188 | 2272.5 |
| 14 | 2313 | 2688 | 2355 | 2740 | 375 | 385 | 2500.5 | 2547.5 |
| 15 | 2688 | 3063 | 2740 | 3567 | 375 | 827 | 2875.5 | 3153.5 |
| 16 | 3063 | 3563 | 3567 | 4022 | 500 | 455 | 3313 | 3794.5 |
| 17 | 3563 | 4063 | 4022 | 4288 | 500 | 266 | 3813 | 4155 |
| 18 | 4063 | 4688 | 4288 | 5349 | 625 | 1061 | 4375.5 | 4818.5 |
| 19 | 4688 | 5313 | 5349 | 5579 | 625 | 230 | 5000.5 | 5464 |
| 20 | 5313 | 6063 | 5579 | 6537 | 750 | 958 | 5688 | 6058 |
| 21 | 6063 | 6938 | 6537 | 7467 | 875 | 930 | 6500.5 | 7002 |
| 22 | 6938 | 7938 | 7467 | 7938 | 1000 | 471 | 7438 | 7702.5 |
| Patient 25 | ||||||||
| 1 | 100 | 198 | 100 | 219 | 98 | 119 | 149 | 159.5 |
| 2 | 198 | 325 | 219 | 341 | 127 | 122 | 261.5 | 280 |
| 3 | 325 | 491 | 341 | 519 | 166 | 178 | 408 | 430 |
| 4 | 491 | 710 | 519 | 797 | 219 | 278 | 600.5 | 658 |
| 5 | 710 | 999 | 797 | 1052 | 289 | 255 | 854.5 | 924.5 |
| 6 | 999 | 1383 | 1052 | 1611 | 384 | 559 | 1191 | 1331.5 |
| 7 | 1383 | 1893 | 1611 | 2265 | 510 | 654 | 1638 | 1938 |
| 8 | 1893 | 2754 | 2265 | 2888 | 861 | 623 | 2323.5 | 2576.5 |
| 9 | 2574 | 3483 | 2888 | 3452 | 909 | 564 | 3028.5 | 3170 |
| 10 | 3483 | 4698 | 3452 | 5197 | 1215 | 1745 | 4090.5 | 4324.5 |
| 11 | 4698 | 6323 | 5197 | 7158 | 1625 | 1961 | 5510.5 | 6177.5 |
| 12 | 6323 | 8500 | 7158 | 8500 | 2177 | 1342 | 7411.5 | 7829 |
| Patient 26 | ||||||||
| 1 | 100 | 208 | 100 | 237 | 108 | 137 | 154 | 168.5 |
| 2 | 208 | 352 | 237 | 354 | 144 | 117 | 280 | 295.5 |
| 3 | 352 | 545 | 354 | 637 | 193 | 283 | 448.5 | 495.5 |
| 4 | 545 | 806 | 637 | 956 | 261 | 319 | 675.5 | 796.5 |
| 5 | 806 | 1160 | 956 | 1317 | 354 | 361 | 983 | 1136.5 |
| 6 | 1160 | 1643 | 1317 | 1891 | 483 | 574 | 1401.5 | 1604 |
| 7 | 1643 | 2303 | 1891 | 2644 | 660 | 753 | 1973 | 2267.5 |
| 8 | 2303 | 3208 | 2644 | 3459 | 905 | 815 | 2755.5 | 3051.5 |
| 9 | 3208 | 4450 | 3459 | 5033 | 1242 | 1574 | 3829 | 4246 |
| 10 | 4450 | 6155 | 5033 | 6365 | 1705 | 1332 | 5302.5 | 5699 |
| 11 | 6155 | 8500 | 6365 | 8500 | 2345 | 2135 | 7327.5 | 7432.5 |
| 12 | - | - | - | - | - | - | - | - |
| Patient 27 | ||||||||
| 1 | 188 | 313 | 188 | 328 | 125 | 140 | 250.5 | 258 |
| 2 | 313 | 438 | 328 | 490 | 125 | 162 | 375.5 | 409 |
| 3 | 438 | 563 | 490 | 599 | 125 | 109 | 500.5 | 544.5 |
| 4 | 563 | 813 | 599 | 927 | 250 | 328 | 688 | 763 |
| 5 | 813 | 1063 | 927 | 1176 | 250 | 249 | 938 | 1051.5 |
| 6 | 1063 | 1313 | 1176 | 1321 | 250 | 145 | 1188 | 1248.5 |
| 7 | 1313 | 1563 | 1321 | 1584 | 250 | 263 | 1438 | 1452.5 |
| 8 | 1563 | 1813 | 1584 | 1964 | 250 | 380 | 1688 | 1774 |
| 9 | 1813 | 2188 | 1964 | 2410 | 375 | 446 | 2000.5 | 2187 |
| 10 | 2188 | 2563 | 2410 | 2898 | 375 | 488 | 2375.5 | 2654 |
| 11 | 2563 | 3063 | 2898 | 3312 | 500 | 414 | 2813 | 3105 |
| 12 | 3063 | 3563 | 3312 | 4147 | 500 | 835 | 3313 | 3729.5 |
| 13 | 3563 | 4188 | 4147 | 4789 | 625 | 642 | 3875.5 | 4468 |
| 14 | 4188 | 4938 | 4789 | 5895 | 750 | 1106 | 4563 | 5342 |
| 15 | 4938 | 5813 | 5895 | 6430 | 875 | 535 | 5375.5 | 6162.5 |
| 16 | 5813 | 6813 | 6430 | 7256 | 1000 | 826 | 6313 | 6843 |
| 17 | 6813 | 7938 | 7256 | 7938 | 1125 | 682 | 7375.5 | 7597 |
| 18 | - | - | - | - | - | - | - | - |
| 19 | - | - | - | - | - | - | - | - |
| 20 | - | - | - | - | - | - | - | - |
| 21 | - | - | - | - | - | - | - | - |
| 22 | - | - | - | - | - | - | - | - |
Appendix B
Table A2.
The electrode position on post-operative CT scanner, center frequency deduced from the Greenwood map, default central frequency, and central frequency after fitting. BML (basilar membrane length) in mm, e# (electrode number), ERD (electrode to round window distance) in mm, GF (center frequency according to Greenwood equation) in Hz, DF (default center frequency) in Hz, EAF (Evolutionary Algorithm center frequency). -: deactivated electrodes.
| ID | CI Brand/Array | BML | e# | ERD | GF | DF | EAF |
|---|---|---|---|---|---|---|---|
| 1 | MEDEL/Flex 28 | 30.7 | 1 | 1.56 | 16,140.9 | 7327.5 | 7288 |
| 2 | 3.53 | 11,796.2 | 5302.5 | 5072.5 | |||
| 3 | 5.67 | 8379.27 | 3829 | 3558.5 | |||
| 4 | 7.76 | 5988.13 | 2755.5 | 2482.5 | |||
| 5 | 9.73 | 4351.89 | 1973 | 1768 | |||
| 6 | 11.78 | 3110.85 | 1401.5 | 1301.5 | |||
| 7 | 13.97 | 2160.84 | 983 | 819.5 | |||
| 8 | 16.04 | 1519.15 | 675.5 | 569.5 | |||
| 9 | 18.27 | 1026.09 | 448.5 | 408 | |||
| 10 | 20.3 | 705.461 | 280 | 263.5 | |||
| 11 | 22.41 | 464.833 | 154 | 147.5 | |||
| 2 | MEDEL/Flex 28 | 30.7 | 1 | 2.37 | 14,276.1 | 7327.5 | 6817 |
| 2 | 4.42 | 10,350.7 | 5302.5 | 4638.5 | |||
| 3 | 6.55 | 7399.64 | 3829 | 3477 | |||
| 4 | 8.45 | 5475.08 | 2755.5 | 2497.5 | |||
| 5 | 10.5 | 3945.21 | 1973 | 1873.5 | |||
| 6 | 12.5 | 2854.92 | 1401.5 | 1324 | |||
| 7 | 14.71 | 1984.74 | 983 | 940 | |||
| 8 | 16.67 | 1426.68 | 675.5 | 661.5 | |||
| 9 | 18.51 | 1036.59 | 448.5 | 406 | |||
| 10 | 20.47 | 726.911 | 280 | 238.5 | |||
| 11 | 22.32 | 509.428 | 154 | 146.5 | |||
| 3 | COCHLEAR/422 | 26.6 | 1 | 3.26 | 11,366.9 | 7438 | 7199 |
| 2 | 4.29 | 9401.1 | 6500.5 | 6209.5 | |||
| 3 | 5.09 | 8109 | 5688 | 5525.5 | |||
| 4 | 5.97 | 6888.73 | 5000.5 | 4850 | |||
| 5 | 6.85 | 5848.85 | 4375.5 | 4231.5 | |||
| 6 | 7.6 | 5084.86 | 3813 | 3473.5 | |||
| 7 | 8.4 | 4376.94 | 3313 | 3061 | |||
| 8 | 9.21 | 3757.73 | 2875.5 | 2724 | |||
| 9 | 10.02 | 3223.31 | 2500.5 | 2161.5 | |||
| 10 | 10.94 | 2704.49 | 2188 | 1846 | |||
| 11 | 11.76 | 2309.8 | 1938 | 1728 | |||
| 12 | 12.59 | 1965.93 | 1688 | 1565.5 | |||
| 13 | 13.53 | 1634.27 | 1438 | 1295.5 | |||
| 14 | 14.39 | 1376.69 | 1250.5 | 1098 | |||
| 15 | 15.06 | 1202.13 | 1125.5 | 1034 | |||
| 16 | 15.8 | 1032.51 | 1000.5 | 936 | |||
| 17 | 16.4 | 910.776 | 875.5 | 796.5 | |||
| 18 | 17.16 | 744.471 | 750.5 | 669.5 | |||
| 19 | 18.08 | 632.784 | 625.5 | 544 | |||
| 20 | 18.75 | 543.532 | 500.5 | 440 | |||
| 21 | 18.92 | 522.563 | 375.5 | 353.5 | |||
| 22 | 18.11 | 628.511 | 250.5 | 243 | |||
| 5 | MEDEL/Flex 28 | 34.2 | 1 | 2.02 | 15,503.9 | 7535 | 7188.5 |
| 2 | 4.12 | 11,483.7 | 5824 | 5282 | |||
| 3 | 6.34 | 8350.89 | 4501.5 | 4012 | |||
| 4 | 8.63 | 6000.88 | 3479.5 | 2975.5 | |||
| 5 | 10.47 | 4592.95 | 2689.5 | 2361.5 | |||
| 6 | 12.46 | 3430.86 | 2078.5 | 1801 | |||
| 7 | 14.17 | 2662.78 | 1606.5 | 1329.5 | |||
| 8 | 16.75 | 1804.41 | 1242 | 1069.5 | |||
| 9 | 18.91 | 1291.24 | 960 | 875 | |||
| 10 | 20.97 | 928.2 | 742 | 701.5 | |||
| 11 | 23.15 | 643.389 | 573.5 | 563 | |||
| 8 | AB/HiFOCUS Mid-scala | 28.6 | 1 | 2.22 | 14,160.8 | 6665 | 8377 |
| 2 | 3.32 | 11,732.9 | 4264 | 6559 | |||
| 3 | 4.1 | 10,265.3 | 3589.5 | 4112.5 | |||
| 4 | 4.9 | 8948.25 | 3021.5 | 2991 | |||
| 5 | 5.91 | 7520.72 | 2544 | 2583 | |||
| 6 | 6.86 | 6383.19 | 2142 | 2175 | |||
| 7 | 7.9 | 5330.47 | 1803 | 1767 | |||
| 8 | 8.93 | 4455.28 | 1517.5 | 1461 | |||
| 9 | 9.99 | 3700.4 | 1277.5 | 1257 | |||
| 10 | 10.8 | 3208.18 | 1075.5 | 1019.5 | |||
| 11 | 11.8 | 2686.5 | 905.5 | 884 | |||
| 12 | 12.8 | 2245.98 | 762.5 | 816 | |||
| 13 | 13.8 | 1873.97 | 642 | 680 | |||
| 14 | 14.8 | 1559.83 | 540.5 | 544 | |||
| 15 | 15.8 | 1294.56 | 455 | 442 | |||
| 16 | 16.8 | 1070.55 | 333 | 306 | |||
| 9 | AB/HiFOCUS Mid-scala | 27.5 | 1 | 1.5 | 15,849.7 | 6665 | 8377 |
| 2 | 2.5 | 13,270.6 | 4264 | 6661 | |||
| 3 | 3.46 | 11,186.7 | 3589.5 | 4214.5 | |||
| 4 | 4.46 | 9359.48 | 3021.5 | 2855 | |||
| 5 | 5.5 | 7770.99 | 2544 | 2481 | |||
| 6 | 6.5 | 6494.51 | 2142 | 2141 | |||
| 7 | 7.56 | 5365.4 | 1803 | 1767 | |||
| 8 | 8.32 | 4676.04 | 1517.5 | 1427 | |||
| 9 | 9.26 | 3941.48 | 1277.5 | 1121.5 | |||
| 10 | 10.44 | 3175.68 | 1075.5 | 952 | |||
| 11 | 11.27 | 2724.68 | 905.5 | 816 | |||
| 12 | 12.3 | 2249.21 | 762.5 | 714 | |||
| 13 | 13.28 | 1870.15 | 642 | 612 | |||
| 14 | 14.32 | 1533.28 | 540.5 | 544 | |||
| 15 | 15.08 | 1323.28 | 455 | 442 | |||
| 16 | 16.12 | 1077.81 | 333 | 306 | |||
| 11 | COCHLEAR/CI522 | 26.8 | 1 | 4.22 | 9578.87 | 7438 | 7892 |
| 2 | 4.45 | 9183.58 | 6500.5 | 7276 | |||
| 3 | 5.83 | 7127.34 | 5688 | 6158.5 | |||
| 4 | 6.35 | 6476.01 | 5000.5 | 5181.5 | |||
| 5 | 6.78 | 5981.71 | 4375.5 | 4421 | |||
| 6 | 9.29 | 3750.16 | 3813 | 4054 | |||
| 7 | 9.88 | 3356.77 | 3313 | 3756 | |||
| 8 | 10.8 | 2821.1 | 2875.5 | 3286.5 | |||
| 9 | 12.4 | 2077.21 | 2500.5 | 2805 | |||
| 10 | 13.4 | 1710.26 | 2188 | 2420.5 | |||
| 11 | 14.4 | 1403.89 | 1938 | 2078.5 | |||
| 12 | 15.2 | 1195.63 | 1688 | 1760.5 | |||
| 13 | 16.2 | 974.22 | 1438 | 1493.5 | |||
| 14 | 17.51 | 738.51 | 1250.5 | 1269.5 | |||
| 15 | 18.12 | 646.37 | 1125.5 | 1176.5 | |||
| 16 | 19.12 | 515.63 | 1000.5 | 1061 | |||
| 17 | 19.9 | 428.83 | 875.5 | 905.5 | |||
| 18 | 20.6 | 360.68 | 750.5 | 781.5 | |||
| 19 | 20.12 | 406.48 | 625.5 | 665.5 | |||
| 20 | 21.58 | 278.64 | 500.5 | 546.5 | |||
| 21 | 22.2 | 233.74 | 375.5 | 438.5 | |||
| 22 | 23.3 | 165.46 | 250.5 | 278 | |||
| 13 | COCHLEAR/CI522 | 23.2 | 1 | 1.71 | 14,434.22 | 7438 | 7796 |
| 2 | 2.42 | 12,428.74 | 6500.5 | 7240.5 | |||
| 3 | 3.39 | 10,127.08 | 5688 | 6511.5 | |||
| 4 | 4.17 | 8585.74 | 5000.5 | 5606.5 | |||
| 5 | 4.96 | 7260.21 | 4375.5 | 4732 | |||
| 6 | 5.6 | 6335.4 | 3813 | 4224.5 | |||
| 7 | 6.44 | 5294.51 | 3313 | 3609 | |||
| 8 | 7.19 | 4507.27 | 2875.5 | 3004.5 | |||
| 9 | 8.12 | 3687.41 | 2500.5 | 2641.5 | |||
| 10 | 9.15 | 2946.89 | 2188 | 2431.5 | |||
| 11 | 10.1 | 2391.39 | 1938 | 2121.5 | |||
| 12 | 11.2 | 1871.61 | 1688 | 1839 | |||
| 13 | 12 | 1561.82 | 1438 | 1674.5 | |||
| 14 | 13.1 | 1212,00 | 1250.5 | 1429.5 | |||
| 15 | 14 | 979.81 | 1125.5 | 1228 | |||
| 16 | 14.9 | 787.33 | 1000.5 | 997 | |||
| 17 | 15.7 | 644.06 | 875.5 | 925 | |||
| 18 | 16.7 | 495.5 | 750.5 | 821 | |||
| 19 | 17.3 | 420.14 | 625.5 | 666 | |||
| 20 | 18.2 | 323.39 | 500.5 | 538.5 | |||
| 21 | 19.2 | 235.16 | 375.5 | 425.5 | |||
| 22 | 20 | 176.69 | 250.5 | 273 | |||
| 14 | COCHLEAR/CI522 | 29.2 | 1 | 6.84 | 6562.82 | 7438 | 7702.5 |
| 2 | 7.92 | 5464.23 | 6500.5 | 7031 | |||
| 3 | 8.86 | 4655.58 | 5688 | 6087 | |||
| 4 | 9.64 | 4073.82 | 5000.5 | 5464 | |||
| 5 | 10.7 | 3394.54 | 4375.5 | 4834.5 | |||
| 6 | 11.7 | 2854.28 | 3813 | 4189 | |||
| 7 | 12.2 | 2615.9 | 3313 | 3812.5 | |||
| 8 | 13.1 | 2233.54 | 2875.5 | 3153.5 | |||
| 9 | 14 | 1904.12 | 2500.5 | 2537 | |||
| 10 | 14.7 | 1679.78 | 2188 | 2255.5 | |||
| 11 | 15.6 | 1427.04 | 1938 | 2000.5 | |||
| 12 | 16.7 | 1165.16 | 1688 | 1793 | |||
| 13 | 17.8 | 946.89 | 1438 | 1560 | |||
| 14 | 19 | 750.01 | 1250.5 | 1327 | |||
| 15 | 20 | 613.33 | 1125.5 | 1221 | |||
| 16 | 20.7 | 530.27 | 1000.5 | 1111.5 | |||
| 17 | 21.7 | 427.13 | 875.5 | 1006.5 | |||
| 18 | 22.6 | 347.84 | 750.5 | 848.5 | |||
| 19 | 23.4 | 286.62 | 625.5 | 724.5 | |||
| 20 | 24 | 245.74 | 500.5 | 600 | |||
| 21 | 25.1 | 180.58 | 375.5 | 413.5 | |||
| 22 | 25.9 | 140.11 | 250.5 | 251.5 | |||
| 15 | MEDEL/Flex 28 | 28.45 | 1 | 0.75 | 18,184.98 | 7410.5 | 7585.5 |
| 2 | 2.89 | 12,595.75 | 5507 | 5696.5 | |||
| 3 | 4.85 | 8985.88 | 4084 | 5843.5 | |||
| 4 | 7.07 | 6115.86 | 3019.5 | 3385 | |||
| 5 | 9.24 | 4184.53 | 2222.5 | 2395 | |||
| 6 | 11.2 | 2957.73 | 1624.5 | 1689.5 | |||
| 7 | 13.6 | 1918.25 | 1175 | 1249.5 | |||
| 8 | 16.4 | 1136.75 | 836 | 960.5 | |||
| 9 | 18.2 | 798.78 | 579.5 | 648 | |||
| 10 | 20.4 | 504.18 | 384.5 | 417 | |||
| 11 | 22.5 | 309.15 | 235 | 275.5 | |||
| 12 | 24.4 | 183.66 | 120 | 133.5 | |||
| 16 | MEDEL/Flex 28 | 28.2 | 1 | 0.87 | 17,791.38 | 7229.5 | 7406 |
| 2 | 2.39 | 13,675.99 | 5064 | 5439 | |||
| 3 | 4.68 | 9187.52 | 3537.5 | 4022 | |||
| 4 | 6.82 | 6320.85 | 2460 | 2879.5 | |||
| 5 | 8.7 | 4538.94 | 1698.5 | 1867 | |||
| 6 | 11 | 3012.26 | 1159 | 1232.5 | |||
| 7 | 13.2 | 2019.94 | 775 | 871.5 | |||
| 8 | 15.4 | 1339.44 | 500.5 | 568.5 | |||
| 9 | 18 | 805.29 | 303.5 | 326.5 | |||
| 10 | 20.2 | 506.49 | 160.5 | 172 | |||
| 11 | 22.3 | 309.32 | - | - | |||
| 12 | 24.2 | 182.85 | - | - | |||
| 17 | COCHLEAR/CI522 | 24.8 | 1 | 2.64 | 12,299.22 | - | - |
| 2 | 3.52 | 10,337.09 | - | - | |||
| 3 | 4.33 | 8805.66 | 7375.5 | 7506 | |||
| 4 | 5.19 | 7423.81 | 6313 | 6796.5 | |||
| 5 | 6.11 | 6180.84 | 5375.5 | 5897 | |||
| 6 | 6.91 | 5267.15 | 4563 | 4795 | |||
| 7 | 7.78 | 4422.64 | 3875.5 | 4107.5 | |||
| 8 | 8.67 | 3694.89 | 3313 | 3702.5 | |||
| 9 | 9.77 | 2953.55 | 2813 | 3079 | |||
| 10 | 10.8 | 2389.68 | 2375.5 | 2428 | |||
| 11 | 11.6 | 2023.53 | 2000.5 | 2093.5 | |||
| 12 | 12.6 | 1639.29 | 1688 | 1896 | |||
| 13 | 13.5 | 1352.02 | 1438 | 1602.5 | |||
| 14 | 14.5 | 1086.73 | 1188 | 1312 | |||
| 15 | 15.4 | 888.4 | - | - | |||
| 16 | 16.5 | 688.81 | 1000.5 | 1125.5 | |||
| 17 | 17.3 | 568.31 | 875.5 | 953 | |||
| 18 | 18.1 | 465.21 | 750.5 | 816.5 | |||
| 19 | 18.8 | 387.29 | 625.5 | 657 | |||
| 20 | 19.6 | 310.33 | 500.5 | 532 | |||
| 21 | 20.4 | 244.49 | 375.5 | 419 | |||
| 22 | 21.2 | 188.16 | 250.5 | 262.5 | |||
| 21 | COCHLEAR/CI522 | 28.1 | 1 | 5.48 | 7964.02 | 7438 | 7907 |
| 2 | 6.24 | 6969.87 | 6500.5 | 7341 | |||
| 3 | 7.33 | 5752.95 | 5688 | 6519 | |||
| 4 | 8.27 | 4872.01 | 5000.5 | 5549 | |||
| 5 | 9.34 | 4028.21 | 4375.5 | 4777 | |||
| 6 | 10.5 | 3272.96 | 3813 | 4438.5 | |||
| 7 | 11.3 | 2833.31 | 3313 | 3875.5 | |||
| 8 | 12.5 | 2277.55 | 2875.5 | 3281.5 | |||
| 9 | 13.6 | 1859.68 | 2500.5 | 2800 | |||
| 10 | 14.5 | 1571.98 | 2188 | 2522.5 | |||
| 11 | 15.4 | 1325.56 | 1938 | 2231 | |||
| 12 | 16.3 | 1114.49 | 1688 | 1922.5 | |||
| 13 | 17.4 | 897.19 | 1438 | 1578 | |||
| 14 | 18.2 | 763.09 | 1250.5 | 1275 | |||
| 15 | 19 | 646.23 | 1125.5 | 1192 | |||
| 16 | 19.8 | 544.4 | 1000.5 | 1067 | |||
| 17 | 20.7 | 445.41 | 875.5 | 933 | |||
| 18 | 21.6 | 360.62 | 750.5 | 837 | |||
| 19 | 22.7 | 273.33 | 625.5 | 679.5 | |||
| 20 | 23.2 | 238.79 | 500.5 | 512.5 | |||
| 21 | 24.1 | 183.65 | 375.5 | 380 | |||
| 22 | 24.9 | 141.31 | 250.5 | 250.5 | |||
| 24 | COCHLEAR/CI522 | 24.3 | 1 | 1.33 | 15,835.2 | 7438 | 7702.5 |
| 2 | 2.47 | 12,591.78 | 6500.5 | 7002 | |||
| 3 | 3.28 | 10,695.68 | 5688 | 6058 | |||
| 4 | 4.15 | 8972.32 | 5000.5 | 5464 | |||
| 5 | 4.84 | 7802.55 | 4375.5 | 4818.5 | |||
| 6 | 5.65 | 6619.38 | 3813 | 4155 | |||
| 7 | 6.53 | 5532.69 | 3313 | 3794.5 | |||
| 8 | 7.43 | 4601.63 | 2875.5 | 3153.5 | |||
| 9 | 8.44 | 3737.31 | 2500.5 | 2547.5 | |||
| 10 | 9.52 | 2986.43 | 2188 | 2272.5 | |||
| 11 | 10.3 | 2536.15 | 1938 | 2008 | |||
| 12 | 11.4 | 2008.96 | 1688 | 1795 | |||
| 13 | 12.2 | 1691.89 | 1438 | 1561 | |||
| 14 | 13.3 | 1330.67 | 1250.5 | 1327 | |||
| 15 | 14.1 | 1113.42 | 1125.5 | 1244.5 | |||
| 16 | 15.2 | 865.92 | 1000.5 | 1135 | |||
| 17 | 16 | 717.06 | 875.5 | 1009.5 | |||
| 18 | 16.8 | 590.11 | 750.5 | 861.5 | |||
| 19 | 17.7 | 469.49 | 625.5 | 711 | |||
| 20 | 18.5 | 378.97 | 500.5 | 579 | |||
| 21 | 19.4 | 292.97 | 375.5 | 416 | |||
| 22 | 20.5 | 206.76 | 250.5 | 251.5 | |||
| 26 | MEDEL/Flex 28 | 25.4 | 1 | 0 | 20,677.07 | - | - |
| 2 | 2.19 | 13,578.18 | 7327.5 | 7432.5 | |||
| 3 | 4.35 | 8951.27 | 5302.5 | 5699 | |||
| 4 | 5.24 | 7533.49 | 3829 | 4246 | |||
| 5 | 7.01 | 5336.82 | 2755.5 | 3051.5 | |||
| 6 | 9.25 | 3433.53 | 1973 | 2267.5 | |||
| 7 | 10.5 | 2675.59 | 1401.5 | 1604 | |||
| 8 | 12.1 | 1934.82 | 983 | 1136.5 | |||
| 9 | 14 | 1303.4 | 675.5 | 796.5 | |||
| 10 | 16 | 844.59 | 448.5 | 495.5 | |||
| 11 | 18.3 | 493.51 | 280 | 295.5 | |||
| 12 | 20.3 | 289.49 | 154 | 168.5 |
Author Contributions
Conceptualization, V.P., P.L. and A.B.G.; methodology, V.P.; software, P.L.; validation, A.B.G.; formal analysis, P.L. and L.S.A.G.; investigation, A.B.G.; resources, P.L.; data curation, V.P.; writing—original draft preparation, A.S. (Alexis Saadoun), A.S. (Antoine Schein) and A.B.G.; writing—review and editing, A.B.G.; visualization, A.B.G.; supervision, A.B.G.; project administration, A.B.G.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol was reviewed and approved by the Institutional Ethics Committee Board (CPP EST III, Nancy University Hospital, France, number: 2017-Jan-14444ND and CPP OUEST V, Rennes University Hospital, France, number: 2020-A00586-33).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
Vincent Péan is a full-time Medel employee. The authors declare no other commercial or financial relationships that could be construed as a potential conflict of interest in this research project.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Avan P., Giraudet F., Büki B. Importance of Binaural Hearing. Audiol. Neurotol. 2015;20:3–6. doi: 10.1159/000380741. [DOI] [PubMed] [Google Scholar]
- 2.Kong Y.-Y., Stickney G.S., Zeng F.-G. Speech and Melody Recognition in Binaurally Combined Acoustic and Electric Hearing. J. Acoust. Soc. Am. 2005;117:1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- 3.Morera C., Manrique M., Ramos A., Garcia-Ibanez L., Cavalle L., Huarte A., Castillo C., Estrada E. Advantages of Binaural Hearing Provided through Bimodal Stimulation via a Cochlear Implant and a Conventional Hearing Aid: A 6-Month Comparative Study. Acta Oto-Laryngol. 2005;125:596–606. doi: 10.1080/00016480510027493. [DOI] [PubMed] [Google Scholar]
- 4.Neuman A.C., Waltzman S.B., Shapiro W.H., Neukam J.D., Zeman A.M., Svirsky M.A. Self-Reported Usage, Functional Benefit, and Audiologic Characteristics of Cochlear Implant Patients Who Use a Contralateral Hearing Aid. Trends Hear. 2017;21:233121651769953. doi: 10.1177/2331216517699530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer E.C., Amlani A.M., Paiva D., Nozari L., Verret S. A Meta-Analysis to Compare Speech Recognition in Noise with Bilateral Cochlear Implants and Bimodal Stimulation. Int. J. Audiol. 2011;50:871–880. doi: 10.3109/14992027.2011.622300. [DOI] [PubMed] [Google Scholar]
- 6.Van Hoesel R.J.M. Contrasting Benefits from Contralateral Implants and Hearing Aids in Cochlear Implant Users. Hear. Res. 2012;288:100–113. doi: 10.1016/j.heares.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Zirn S., Arndt S., Aschendorff A., Wesarg T. Interaural Stimulation Timing in Single Sided Deaf Cochlear Implant Users. Hear. Res. 2015;328:148–156. doi: 10.1016/j.heares.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein J.G.W., Goupell M.J., Schuchman G.I., Rivera A.L., Brungart D.S. Having Two Ears Facilitates the Perceptual Separation of Concurrent Talkers for Bilateral and Single-Sided Deaf Cochlear Implantees. Ear Hear. 2016;37:289–302. doi: 10.1097/AUD.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spirrov D., Kludt E., Verschueren E., Büchner A., Francart T. Effect of (Mis)Matched Compression Speed on Speech Recognition in Bimodal Listeners. Trends Hear. 2020;24:2331216520948974. doi: 10.1177/2331216520948974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wess J.M., Brungart D.S., Bernstein J.G.W. The Effect of Interaural Mismatches on Contralateral Unmasking With Single-Sided Vocoders. Ear Hear. 2017;38:374–386. doi: 10.1097/AUD.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 11.Gifford R.H., Dorman M.F., McKarns S.A., Spahr A.J. Combined Electric and Contralateral Acoustic Hearing: Word and Sentence Recognition With Bimodal Hearing. J. Speech Lang. Hear. Res. 2007;50:835–843. doi: 10.1044/1092-4388(2007/058). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ching T.Y.C., van Wanrooy E., Dillon H. Binaural-Bimodal Fitting or Bilateral Implantation for Managing Severe to Profound Deafness: A Review. Trends Amplif. 2007;11:161–192. doi: 10.1177/1084713807304357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litovsky R.Y., Johnstone P.M., Godar S.P. Benefits of Bilateral Cochlear Implants and/or Hearing Aids in Children: Beneficios de Los Implantes Cocleares Bilaterales y/o Auxiliares Auditivos En Niños. Int. J. Audiol. 2006;45:78–91. doi: 10.1080/14992020600782956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok M., Grayden D., Dowell R.C., Lawrence D. Speech Perception for Adults Who Use Hearing Aids in Conjunction With Cochlear Implants in Opposite Ears. J. Speech Lang. Hear. Res. 2006;49:338–351. doi: 10.1044/1092-4388(2006/027). [DOI] [PubMed] [Google Scholar]
- 15.Kiefer J., Pok M., Adunka O., Stürzebecher E., Baumgartner W., Schmidt M., Tillein J., Ye Q., Gstoettner W. Combined Electric and Acoustic Stimulation of the Auditory System: Results of a Clinical Study. Audiol. Neurotol. 2005;10:134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- 16.Richard C., Ferrary E., Borel S., Sterkers O., Grayeli A.B. Interaction Between Electric and Acoustic Cues in Diotic Condition for Speech Perception in Quiet and Noise by Cochlear Implantees. Otol. Neurotol. 2012;33:30–37. doi: 10.1097/MAO.0b013e31823c9461. [DOI] [PubMed] [Google Scholar]
- 17.Angermeier J., Hemmert W., Zirn S. Sound Localization Bias and Error in Bimodal Listeners Improve Instantaneously When the Device Delay Mismatch Is Reduced. Trends Hear. 2021;25:233121652110161. doi: 10.1177/23312165211016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francart T., McDermott H.J. Psychophysics, Fitting, and Signal Processing for Combined Hearing Aid and Cochlear Implant Stimulation. Ear Hear. 2013;34:685–700. doi: 10.1097/AUD.0b013e31829d14cb. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood D.D. A Cochlear Frequency-position Function for Several Species—29 Years Later. J. Acoust. Soc. Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 20.Oh Y., Reiss L.A.J. Binaural Pitch Fusion: Binaural Pitch Averaging in Cochlear Implant Users With Broad Binaural Fusion. Ear Hear. 2020;41:1450–1460. doi: 10.1097/AUD.0000000000000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartling C.L., Fowler J.R., Stark G.N., Glickman B., Eddolls M., Oh Y., Ramsey K., Reiss L.A.J. Binaural Pitch Fusion in Children With Normal Hearing, Hearing Aids, and Cochlear Implants. Ear Hear. 2020;41:1545–1559. doi: 10.1097/AUD.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaerenberg B., Smits C., De Ceulaer G., Zir E., Harman S., Jaspers N., Tam Y., Dillon M., Wesarg T., Martin-Bonniot D., et al. Cochlear Implant Programming: A Global Survey on the State of the Art. Sci. World J. 2014;2014:501738. doi: 10.1155/2014/501738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veugen L.C.E., Chalupper J., Snik A.F.M., van Opstal A.J., Mens L.H.M. Frequency-Dependent Loudness Balancing in Bimodal Cochlear Implant Users. Acta Oto-Laryngol. 2016;136:775–781. doi: 10.3109/00016489.2016.1155233. [DOI] [PubMed] [Google Scholar]
- 24.Baskent D., Shannon R.V. Speech Recognition under Conditions of Frequency-Place Compression and Expansion. J. Acoust. Soc. Am. 2003;113:2064–2076. doi: 10.1121/1.1558357. [DOI] [PubMed] [Google Scholar]
- 25.Falcón-González J.C., Borkoski-Barreiro S., Limiñana-Cañal J.M., Ramos-Macías Á. Reconocimiento auditivo musical y melódico en pacientes con implante coclear, mediante nuevo método de programación de asignación frecuencial. Acta Otorrinolaringol. Esp. 2014;65:289–296. doi: 10.1016/j.otorri.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Maarefvand M., Blamey P.J., Marozeau J. Pitch Matching in Bimodal Cochlear Implant Patients: Effects of Frequency, Spectral Envelope, and Level. J. Acoust. Soc. Am. 2017;142:2854–2865. doi: 10.1121/1.5009443. [DOI] [PubMed] [Google Scholar]
- 27.Arndt S., Laszig R., Aschendorff A., Hassepass F., Beck R., Wesarg T. Cochlear Implant Treatment of Patients with Single-Sided Deafness or Asymmetric Hearing Loss. HNO. 2017;65:98–108. doi: 10.1007/s00106-016-0297-5. [DOI] [PubMed] [Google Scholar]
- 28.Williges B., Wesarg T., Jung L., Geven L.I., Radeloff A., Jürgens T. Spatial Speech-in-Noise Performance in Bimodal and Single-Sided Deaf Cochlear Implant Users. Trends Hear. 2019;23:233121651985831. doi: 10.1177/2331216519858311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon Y., Liu A., Fu Q.-J. Binaural Benefit for Speech Recognition with Spectral Mismatch across Ears in Simulated Electric Hearing. J. Acoust. Soc. Am. 2011;130:EL94–EL100. doi: 10.1121/1.3606460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goupell M.J., Stoelb C.A., Kan A., Litovsky R.Y. The Effect of Simulated Interaural Frequency Mismatch on Speech Understanding and Spatial Release From Masking. Ear Hear. 2018;39:895–905. doi: 10.1097/AUD.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein J.G.W., Stakhovskaya O.A., Schuchman G.I., Jensen K.K., Goupell M.J. Interaural Time-Difference Discrimination as a Measure of Place of Stimulation for Cochlear-Implant Users with Single-Sided Deafness. Trends Hear. 2018;22:233121651876551. doi: 10.1177/2331216518765514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francart T., Wiebe K., Wesarg T. Interaural Time Difference Perception with a Cochlear Implant and a Normal Ear. J. Assoc. Res. Otolaryngol. 2018;19:703–715. doi: 10.1007/s10162-018-00697-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dirks C.E., Nelson P.B., Winn M.B., Oxenham A.J. Sensitivity to Binaural Temporal-Envelope Beats with Single-Sided Deafness and a Cochlear Implant as a Measure of Tonotopic Match (L) J. Acoust. Soc. Am. 2020;147:3626. doi: 10.1121/10.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bäck T. Evolutionary Algorithms in Theory and Practice: Evolution Strategies, Evolutionary Programming, Genetic Algorithms. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- 35.Legrand P., Bourgeois-Republique C., Péan V., Harboun-Cohen E., Levy-Vehel J., Frachet B., Lutton E., Collet P. Interactive Evolution for Cochlear Implants Fitting. Genet. Program. Evolvable Mach. 2007;8:319–354. doi: 10.1007/s10710-007-9048-4. [DOI] [Google Scholar]
- 36.Ramesh A., Kambhampati C., Monson J., Drew P. Artificial Intelligence in Medicine. Ann. R. Coll. Surg. Engl. 2004;86:334–338. doi: 10.1308/147870804290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Govaerts P.J., Vaerenberg B., De Ceulaer G., Daemers K., De Beukelaer C., Schauwers K. Development of a Software Tool Using Deterministic Logic for the Optimization of Cochlear Implant Processor Programming. Otol. Neurotol. 2010;31:908–918. doi: 10.1097/MAO.0b013e3181dd160b. [DOI] [PubMed] [Google Scholar]
- 38.Meeuws M., Pascoal D., Bermejo I., Artaso M., De Ceulaer G., Govaerts P.J. Computer-Assisted CI Fitting: Is the Learning Capacity of the Intelligent Agent FOX Beneficial for Speech Understanding? Cochlear Implant. Int. 2017;18:198–206. doi: 10.1080/14670100.2017.1325093. [DOI] [PubMed] [Google Scholar]
- 39.Riss D., Hamzavi J.-S., Blineder M., Honeder C., Ehrenreich I., Kaider A., Baumgartner W.-D., Gstoettner W., Arnoldner C. FS4, FS4-p, and FSP: A 4-Month Crossover Study of 3 Fine Structure Sound-Coding Strategies. Ear Hear. 2014;35:e272–e281. doi: 10.1097/AUD.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 40.Vandali A.E., Whitford L.A., Plant K.L., Clark G.M. Speech Perception as a Function of Electrical Stimulation Rate: Using the Nucleus 24 Cochlear Implant System. Ear Hear. 2000;21:608–624. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Wilson B.S., Finley C.C., Lawson D.T., Wolford R.D., Eddington D.K., Rabinowitz W.M. Better Speech Recognition with Cochlear Implants. Nature. 1991;352:236–238. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- 42.Bozorg-Grayeli A., Guevara N., Bebear J.-P., Ardoint M., Saaï S., Hoen M., Gnansia D., Romanet P., Lavieille J.-P. Clinical Evaluation of the XDP Output Compression Strategy for Cochlear Implants. Eur. Arch. Otorhinolaryngol. 2016;273:2363–2371. doi: 10.1007/s00405-015-3796-1. [DOI] [PubMed] [Google Scholar]
- 43.Firszt J.B., Holden L.K., Reeder R.M., Skinner M.W. Speech Recognition in Cochlear Implant Recipients: Comparison of Standard HiRes and HiRes 120 Sound Processing. Otol. Neurotol. 2009;30:146–152. doi: 10.1097/MAO.0b013e3181924ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fournier J.E. Vocal audiometry, its technique and results. Rev. Otoneuroophtalmol. 1950;22:649–656. [PubMed] [Google Scholar]
- 45.Vincent C., Gagné J.-P., Leroux T., Clothier A., Larivière M., Dumont F.S., Gendron M. French-Canadian Translation and Validation of Four Questionnaires Assessing Hearing Impairment and Handicap. Int. J. Audiol. 2017;56:248–259. doi: 10.1080/14992027.2016.1263398. [DOI] [PubMed] [Google Scholar]
- 46.Amann E., Anderson I. Development and Validation of a Questionnaire for Hearing Implant Users to Self-Assess Their Auditory Abilities in Everyday Communication Situations: The Hearing Implant Sound Quality Index (HISQUI19) Acta Otolaryngol. 2014;134:915–923. doi: 10.3109/00016489.2014.909604. [DOI] [PubMed] [Google Scholar]
- 47.Brockmeier S.J., Nopp P., Vischer M., Baumgartner W., Stark T., Schoen F., Mueller J., Braunschweig T., Busch R., Getto M., et al. Correlation of speech and music perception in postlingually deaf Combi 40/40+ users. In: Kubo T., Takahashi Y., Iwaki T., editors. Cochlear Implants: An Update. Kugler Publications; The Hague, The Netherlands: 2002. pp. 459–464. [DOI] [Google Scholar]
- 48.Holland J.H. Adaptation in Natural and Artificial Systems an Introductory Analysis with Applications to Biology, Control, and Artificial Intelligence. MIT Press; Cambridge, MA, USA: 1992. [Google Scholar]
- 49.Goldberg D.E. Genetic Algorithms in Search, Optimization, and Machine Learning. Addison-Wesley Pub. Co.; Boston, MA, USA: 1989. [Google Scholar]
- 50.Schwefel H.-P., Rudolph G. Contemporary Evolution Strategies. In: Morán F., Moreno A., Merelo J.J., Chacón P., editors. Advances in Artificial Life. Volume 929. Springer; Berlin/Heidelberg, Germany: 1995. pp. 891–907. Lecture Notes in Computer Science. [Google Scholar]
- 51.Schwefel H.-P. Evolution and Optimum Seeking. Wiley; New York, NY, USA: 1995. (Sixth-Generation Computer Technology Series). [Google Scholar]
- 52.Emmerich M., Shir O.M., Wang H. Evolution Strategies. In: Martí R., Pardalos P.M., Resende M.G.C., editors. Handbook of Heuristics. Springer International Publishing; Cham, Switzerland: 2018. pp. 89–119. [Google Scholar]
- 53.Poli R., Langdon W.B., McPhee N.F., Koza J.R. A Field Guide to Genetic Programming. Lulu Press; Morrisville, NC, USA: 2008. [Google Scholar]
- 54.Li S., Kang L., Zhao X.-M. A Survey on Evolutionary Algorithm Based Hybrid Intelligence in Bioinformatics. BioMed Res. Int. 2014;2014:362738. doi: 10.1155/2014/362738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 56.Zirn S., Angermeier J., Arndt S., Aschendorff A., Wesarg T. Reducing the Device Delay Mismatch Can Improve Sound Localization in Bimodal Cochlear Implant/Hearing-Aid Users. Trends Hear. 2019;23:233121651984387. doi: 10.1177/2331216519843876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma N., Morris S., Kitterick P.T. Benefits to Speech Perception in Noise From the Binaural Integration of Electric and Acoustic Signals in Simulated Unilateral Deafness. Ear Hear. 2016;37:248–259. doi: 10.1097/AUD.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Firszt J.B., Holden L.K., Reeder R.M., Waltzman S.B., Arndt S. Auditory Abilities after Cochlear Implantation in Adults with Unilateral Deafness: A Pilot Study. Otol. Neurotol. 2012;33:1339–1346. doi: 10.1097/MAO.0b013e318268d52d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sladen D.P., Frisch C.D., Carlson M.L., Driscoll C.L.W., Torres J.H., Zeitler D.M. Cochlear Implantation for Single-Sided Deafness: A Multicenter Study: Cochlear Implantation for SSD. Laryngoscope. 2017;127:223–228. doi: 10.1002/lary.26102. [DOI] [PubMed] [Google Scholar]
- 60.Buss E., Dillon M.T., Rooth M.A., King E.R., Deres E.J., Buchman C.A., Pillsbury H.C., Brown K.D. Effects of Cochlear Implantation on Binaural Hearing in Adults With Unilateral Hearing Loss. Trends Hear. 2018;22:233121651877117. doi: 10.1177/2331216518771173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litovsky R.Y., Moua K., Godar S., Kan A., Misurelli S.M., Lee D.J. Restoration of Spatial Hearing in Adult Cochlear Implant Users with Single-Sided Deafness. Hear. Res. 2019;372:69–79. doi: 10.1016/j.heares.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson N.J., Dillon M.T., Buss E., Rooth M.A., King E.R., Bucker A.L., McCarthy S.A., Deres E.J., O’Connell B.P., Pillsbury H.C., et al. Subjective Benefits of Bimodal Listening in Cochlear Implant Recipients with Asymmetric Hearing Loss. Otolaryngol. Head Neck Surg. 2020;162:933–941. doi: 10.1177/0194599820911716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grasmeder M.L., Verschuur C.A., Batty V.B. Optimizing Frequency-to-Electrode Allocation for Individual Cochlear Implant Users. J. Acoust. Soc. Am. 2014;136:3313–3324. doi: 10.1121/1.4900831. [DOI] [PubMed] [Google Scholar]
- 64.Sridhar D., Stakhovskaya O., Leake P.A. A Frequency-Position Function for the Human Cochlear Spiral Ganglion. Audiol. Neurotol. 2006;11:16–20. doi: 10.1159/000095609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simpson A., McDermott H.J., Dowell R.C., Sucher C., Briggs R.J.S. Comparison of Two Frequency-to-Electrode Maps for Acoustic-Electric Stimulation. Int. J. Audiol. 2009;48:63–73. doi: 10.1080/14992020802452184. [DOI] [PubMed] [Google Scholar]
- 66.Moore B.C.J. Coding of Sounds in the Auditory System and Its Relevance to Signal Processing and Coding in Cochlear Implants. Otol. Neurotol. 2003;24:243–254. doi: 10.1097/00129492-200303000-00019. [DOI] [PubMed] [Google Scholar]
- 67.Henry B.A., McKay C.M., McDermott H.J., Clark G.M. The Relationship between Speech Perception and Electrode Discrimination in Cochlear Implantees. J. Acoust. Soc. Am. 2000;108:1269. doi: 10.1121/1.1287711. [DOI] [PubMed] [Google Scholar]
- 68.Leigh J.R., Henshall K.R., McKay C.M. Optimizing Frequency-to-Electrode Allocation in Cochlear Implants. J. Am. Acad. Audiol. 2004;15:574–584. doi: 10.3766/jaaa.15.8.5. [DOI] [PubMed] [Google Scholar]
- 69.McKay C.M., Henshall K.R. Frequency-to-Electrode Allocation and Speech Perception with Cochlear Implants. J. Acoust. Soc. Am. 2002;111:1036–1044. doi: 10.1121/1.1436073. [DOI] [PubMed] [Google Scholar]
- 70.Stakhovskaya O., Sridhar D., Bonham B.H., Leake P.A. Frequency Map for the Human Cochlear Spiral Ganglion: Implications for Cochlear Implants. JARO. 2007;8:220–233. doi: 10.1007/s10162-007-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vermeire K., Landsberger D.M., Van de Heyning P.H., Voormolen M., Kleine Punte A., Schatzer R., Zierhofer C. Frequency-Place Map for Electrical Stimulation in Cochlear Implants: Change over Time. Hear. Res. 2015;326:8–14. doi: 10.1016/j.heares.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorman M.F., Spahr T., Gifford R., Loiselle L., McKarns S., Holden T., Skinner M., Finley C. An Electric Frequency-to-Place Map for a Cochlear Implant Patient with Hearing in the Nonimplanted Ear. JARO. 2007;8:234–240. doi: 10.1007/s10162-007-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan C.-T., Martin B., Svirsky M.A. Pitch Matching between Electrical Stimulation of a Cochlear Implant and Acoustic Stimuli Presented to a Contralateral Ear with Residual Hearing. J. Am. Acad. Audiol. 2017;28:187–199. doi: 10.3766/jaaa.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernstein J.G.W., Jensen K.K., Stakhovskaya O.A., Noble J.H., Hoa M., Kim H.J., Shih R., Kolberg E., Cleary M., Goupell M.J. Interaural Place-of-Stimulation Mismatch Estimates Using CT Scans and Binaural Perception, But Not Pitch, Are Consistent in Cochlear-Implant Users. J. Neurosci. 2021;41:10161–10178. doi: 10.1523/JNEUROSCI.0359-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neher T. Characterizing the Binaural Contribution to Speech-in-Noise Reception in Elderly Hearing-Impaired Listeners. J. Acoust. Soc. Am. 2017;141:EL159–EL163. doi: 10.1121/1.4976327. [DOI] [PubMed] [Google Scholar]
- 76.Carbonell K.M. Reliability of Individual Differences in Degraded Speech Perception. J. Acoust. Soc. Am. 2017;142:EL461–EL466. doi: 10.1121/1.5010148. [DOI] [PubMed] [Google Scholar]
- 77.Carlyon R.P., Macherey O., Frijns J.H.M., Axon P.R., Kalkman R.K., Boyle P., Baguley D.M., Briggs J., Deeks J.M., Briaire J.J., et al. Pitch Comparisons between Electrical Stimulation of a Cochlear Implant and Acoustic Stimuli Presented to a Normal-Hearing Contralateral Ear. JARO. 2010;11:625–640. doi: 10.1007/s10162-010-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Green T., Faulkner A., Rosen S. Frequency Selectivity of Contralateral Residual Acoustic Hearing in Bimodal Cochlear Implant Users, and Limitations on the Ability to Match the Pitch of Electric and Acoustic Stimuli. Int. J. Audiol. 2012;51:389–398. doi: 10.3109/14992027.2011.642010. [DOI] [PubMed] [Google Scholar]
- 79.Cronbach L.J., Warrington W.G. Time-Limit Tests: Estimating Their Reliability and Degree of Speeding. Psychometrika. 1951;16:167–188. doi: 10.1007/BF02289113. [DOI] [PubMed] [Google Scholar]
- 80.Arnoldner C., Kaider A., Hamzavi J. The Role of Intensity Upon Pitch Perception in Cochlear Implant Recipients. Laryngoscope. 2006;116:1760–1765. doi: 10.1097/01.mlg.0000228214.02606.42. [DOI] [PubMed] [Google Scholar]
- 81.Arnoldner C., Riss D., Kaider A., Mair A., Wagenblast J., Baumgartner W.-D., Gstöttner W., Hamzavi J.-S. The Intensity–Pitch Relation Revisited: Monopolar Versus Bipolar Cochlear Stimulation. Laryngoscope. 2008;118:1630–1636. doi: 10.1097/MLG.0b013e3181799715. [DOI] [PubMed] [Google Scholar]
- 82.Neuhoff J.G., Wayand J., Kramer G. Pitch and Loudness Interact in Auditory Displays: Can the Data Get Lost in the Map? J. Exp. Psychol. Appl. 2002;8:17–25. doi: 10.1037/1076-898X.8.1.17. [DOI] [PubMed] [Google Scholar]