Abstract
Osteoporosis is characterized by an alteration of bone microstructure with a decreased bone mineral density, leading to the incidence of fragility fractures. Around 200 million people are affected by osteoporosis, representing a major health burden worldwide. Several factors are involved in the pathogenesis of osteoporosis. Today, altered intestinal homeostasis is being investigated as a potential additional risk factor for reduced bone health and, therefore, as a novel potential therapeutic target. The intestinal microflora influences osteoclasts’ activity by regulating the serum levels of IGF-1, while also acting on the intestinal absorption of calcium. It is therefore not surprising that gut dysbiosis impacts bone health. Microbiota alterations affect the OPG/RANKL pathway in osteoclasts, and are correlated with reduced bone strength and quality. In this context, it has been hypothesized that dietary supplements, prebiotics, and probiotics contribute to the intestinal microecological balance that is important for bone health. The aim of the present comprehensive review is to describe the state of the art on the role of dietary supplements and probiotics as therapeutic agents for bone health regulation and osteoporosis, through gut microbiota modulation.
Keywords: bone health, osteoporosis, microbiota, gut microbiota, dietary supplements, probiotics
1. Introduction
Poor bone health is a relatively silent condition that can develop in both adults and the elderly, inducing bone microstructural alterations and osteoporosis, with a decreased bone mineral density (BMD), leading to fragility fractures [1]. Around 200 million people worldwide are affected by osteoporosis [2]. Fragility fractures, due to low-energy trauma, might necessitate hospitalization and surgical interventions, with detrimental consequences, such as permanent disabilities and an increased risk of death [3]. Osteoporosis is associated with several risk factors—primarily endocrine disorders, medication, immobility, lifestyle, and hereditary genetic factors [4].
Today, altered intestinal homeostasis is being investigated as a potential additional risk factor for reduced bone health, and also as a novel therapeutic target [5]. Indeed, the intestinal microbial populations that live in symbiosis with their human host are collectively defined as the human gut microbiota [6,7], composed of more than 1000 different microorganisms, with the majority particular to each healthy subject, along with a common, stable, and abundant component of Bacteroidetes, Prevotellaceae, and Faecalibacterium species [8,9].
Alterations of the gut microbiota—called dysbiosis—which result in an imbalance between the types of microorganisms present in a person’s natural microflora, can dysregulate metabolic processes, leading to inflammatory bowel diseases [10], diabetes [11], malignancies [12], cardiovascular diseases [13], rheumatoid arthritis, and systemic lupus erythematosus [14].
Microbiota dysbiosis is a multifactorial condition that can be triggered by conditions such as stress, inflammation, and aging. In general, after healing of a diseased state, dysbiosis tends to be corrected naturally [15].
Environmental conditions associated with genetic factors of the host can modify their immunological profile and microbiota composition, which can unbalance the microbial community. For example, in animal models, quantitative trait loci encoding for interferons, interleukin-1 receptor-associated kinase 4 (IRAK-4),and transforming growth factor-beta 3 (TGF-β3) modulate the ratio of Bacteroidetes, Rikenellaceae, and Prevotellaceae, respectively [5,16].
Furthermore, the gut microbiota participates in the bone metabolic signaling pathways through the generation and translocation of metabolites. Bone is impacted by gut microbial dysbiosis that both impairs the intestinal absorption of calcium and dysregulates osteoclasts’ activity via the serum levels of insulin-like growth factor 1 (IGF-1) [17]. Moreover, microbiota alterations also reduce bone strength and quality [18,19], and affect the OPG/RANKL pathway in osteoclasts [20]. Furthermore, dysbiosis in mice also interferes with skeletal muscle mass and physical performance, altering the production of rapsyn and Lrp4—two crucial proteins in the functioning of neuromuscular junctions [21]. Similarly, the antibiotic and antiparasitic drug metronidazole causes gut dysbiosis, inducing skeletal muscle atrophy and impacting muscle chronometabolism [22].
Although gaps exist in the understanding of the pathophysiological mechanisms underpinning the interaction between gut microbiota and bone, it has been hypothesized that dietary supplements, prebiotics (non-digestible dietary fibers stimulating selective bacterial growth), and probiotics (microorganisms that might confer a health benefit to the host) contribute to the intestinal microecological balance, which is important for bone health [23,24,25]. Indeed, dietary supplements and probiotics might play a role in the management of osteoporosis, in conjunction with the already-known medical and lifestyle interventions.
For this purpose, we wrote the present comprehensive review after analyzing both animal and human-based model studies published in the past 20 years. Our survey focuses on the roles that dietary supplements and probiotics play as therapeutic agents for the regulation of bone health and osteoporosis, through gut microbiota modulation.
2. The Gut-Bone Axis
On the one hand, the gut microbiota shows variability depending on several conditions, such age, sex, diet, environmental conditions, and diseases [26,27]. On the other hand, bone is also a dynamic organ undergoing constant remodeling, maintaining bone mass and calcium serum level homeostasis [26].
Gut physiology and the gut microbial population are both involved in the regulation of bone metabolism at different levels. Gastrectomy causes a loss of BMD in both animal models and humans [28,29], and impaired gastric acid influences calcium metabolism and uptake [30]. In addition, several neuropeptides secreted by the gastrointestinal system (i.e., glucagon-like peptide-1, glucagon-like peptide-2, and ghrelin) enhance bone formation or inhibit bone resorption, whereas others (i.e., gastric inhibitory polypeptide, gastrin, and serotonin), in contrast, act as pro-osteoporotic factors [31,32]. Bacterial variance and the prevalence of individual microbial species in the gut of the host can alter micronutrients, calcium, and vitamin D absorption, and cause systemic inflammation [33].
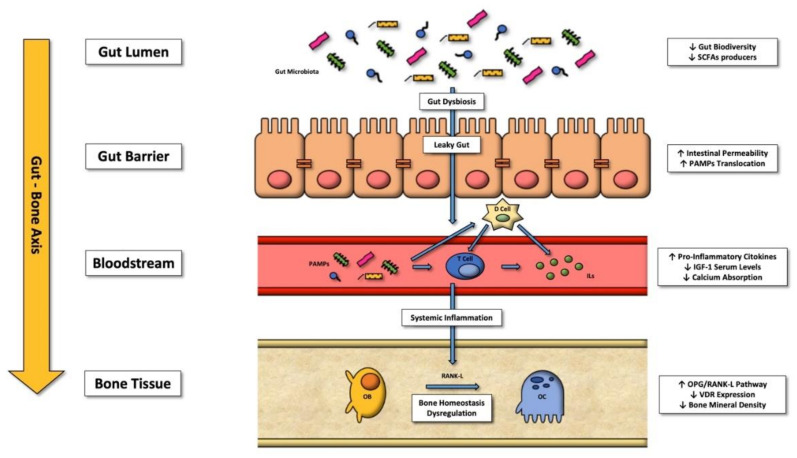
The relationship between the gut-bone axis and immunity is complex; under physiological conditions, the gut microbiota stimulates and develops the host’s innate and adaptive immune systems [34]. By contrast, gut microbiota dysbiosis can provoke inflammatory cytokine overproduction, as shown in animal models. Under dysbiosis, bacterial metabolites are delivered to the liver by the blood circulation and upregulate the hepatic immune response. In parallel, they also alter the bone marrow CD4+ T cells, with a resulting pro-osteoclastic effect [20] (see Figure 1 for further details).
Figure 1.
The gut-bone axis: pathways and factors starting from gut dysbiosis that determine bone metabolism alterations favoring osteoclasts. Abbreviations—SCFAs: short-chain fatty acids; PAMPs: pathogen-associated molecular patterns; D Cell: somatostatin-producing cells; T Cell: type of leukocyte that is an essential part of the immune system; OB: osteoblast; OC: osteoclast; RNK-L: receptor activator of nuclear factor-κB ligand; OPG: osteoprotegerin; VDR: vitamin D receptor; IGF-1: insulin-like growth factor one.
In a recent study involving 181 postmenopausal women, divided into 3 groups—normal BMD, osteopenia, and osteoporosis—fecal microbiota sample analysis revealed that the most prevalent microbial species in these conditions statistically correlated with altered bone metabolism [35]. In particular, Actinomyces and Clostridium species were more prevalent in subjects with osteoporosis, whereas Bacteroidetes and Firmicutes were more prevalent in healthy individuals. Interestingly, Actinomyces might be involved in the development of osteonecrosis of the jaw, whereas Firmicutes contribute to an estrogen analogue conversion, with anabolic effects on bone [36]. Interestingly, bacterial populations vary in osteopenia, primary osteoporosis, or secondary osteoporosis, but the relationship and prevalence between microbial species under these conditions are still debated [37]. To date, several substances—including trimethylamine N-oxide, N-acetyl-mannosamine, l-threonate, l-lysine, and short-chain fatty acids (SCFAs)—have been shown to modulate the gut microbiota, with a consequent impact on bone [37,38]. Therefore, the potential therapeutic role of dietary supplements and probiotics is intriguing and worthy of assessment in people affected by osteoporosis; such interventions might alter the gut microbiota composition, promoting bone health [24].
3. Effects of Dietary Supplements on the Gut Microbiota and Bone Health
Diet is the main determinant of the types and proportions of microorganisms in the gut microbiota, influencing bone health and wellness [39]. For example, a high-fat diet reduces microbiota biodiversity in mice, while a highly sweetened diet causes glucose intolerance [40]. From a nutritional point of view, the Mediterranean diet provides fibers, fermented dairy products, and polyphenols, which have beneficial effects on the gut microbiota in humans, reducing the Firmicutes and Bacteroidetes ratios and enhancing the levels of SCFAs [41], Together, these effects promote bone health and reduce fracture risk [42,43,44,45].
Compared to the known beneficial effects of the Mediterranean diet, it is still uncertain whether supplementation with prebiotics, proteins, peptides, amino acids, and micronutrients might modulate the gut microbiota composition to induce beneficial effects on bone health [46]. Current knowledge on this subject is summarized and discussed below.
3.1. Roles of Prebiotics
Prebiotics are non-digestible food ingredients that stimulate the growth and/or activity of bacteria colonizing the large bowel by acting as substrate for them [47]. In most cases, they are products of the enzymatic conversion of sugars, such as galactooligosaccharides (GOSs), fructooligosaccharides (FOSs), inulin, digestion-resistant starch, xylooligosaccharides (XOSs), and lactulose.
GOSs are composed of a chain of galactose (typically 2–8 units) with a terminal glucose, which fosters the growth of Bifidobacteria and Lactobacillus [47], which are known to increase the quantity and activity of osteoblasts [48,49]. Moreover, GOSs increase the intestinal absorption of calcium and magnesium, along with bone mineralization in animal models [50]. In postmenopausal women, their consumption leads to increased calcium absorption and stable urinary calcium excretion [51]. Lastly, in a double-blind trial, adolescent girls receiving 5 g of GOSs twice a day for 3 weeks increased both calcium absorption in the lower gut and fecal Bifidobacteria [52].
FOSs are composed of 3–10 fructose units, with the terminal fructose linked to a glucose residue. FOSs show similar effects to those of GOSs concerning the growth stimulation of Bifidobacteria [53], associated with an increase in colon concentrations of butyrate—an SCFA that, along with propionate, regulates the gut microbiota, in particular with respect to its action on bone metabolism. FOS ingestion has been correlated with increased bone strength, mineralization, and reduced bone resorption in animal models [54,55,56]. In another, more recent animal study, FOSs increased the maximum amount of bone mass—the so-called “peak bone mass”—and rised the serum level of butyrate in ovariectomized rats affected by bone loss caused by estrogen deficiency [57]. In humans, FOS supplementation (3.6 g/day for 12 months) coupled with calcium supplementation reduced the serum levels of bone turnover markers in postmenopausal women, with no effect on BMD [58].
Resistant starches are a subgroup of dietary fibers that reach the colon undigested, as the small intestine is not able to alter their composition. These fibers are utilized as substrates by the microbiota in the colon, and their fermentation produces SCFAs [59]; they also promote soya isoflavone production, and increase the ratio of Bifidobacteria, Lactobacillus species, and Bacteroides [60,61]. These starches reduce bone loss in ovariectomized mice [61], decrease inflammation, and interfere with the RANKL/OPG pathway [62].
XOSs are prebiotics composed of 2–7 xylose molecules fostering Bifidobacteria over Lactobacillus [63]; in mice, they increase calcium uptake by upregulating the expression of transient receptor potential vanillin receptor 6 (TRPV6) and Na+/Ca2+ exchanger in the duodenum, and increase BMD [64]; they also reduce bone loss in high-fat-diet insulin-resistant mice [65], and decrease inflammation markers.
Finally, oral supplementation of lactulose (20 gr/kg per 6 weeks) inhibits osteoclastogenesis, reduces bone resorption, and prevents ovariectomy-induced bone loss in mice [66], while increasing calcium and magnesium absorption in both men and postmenopausal women [67,68].
The prebiotics have in common the ability to be converted to SCFAs—including acetate, propionate, and butyrate—by the gut microbiota, increasing both their intestinal and serum levels, and lowering the intestinal pH. In an acidic environment, most minerals—such as magnesium and calcium—become more soluble; hence, their absorption is increased [69,70,71]. Butyrate also acts as a growth factor for enterocytes and colonocytes [72].
Moreover, SCFAs generated from prebiotics regulate the number and function of regulatory T cells in the colon, thereby controlling inflammation [73], and modulate the synthesis of IGF-1 involved in bone remodeling [74]. Therefore, supplementation with saccharides might have beneficial effects on bone metabolism, although further studies are warranted to generalize these findings (see Table 1 for further details).
Table 1.
Characteristics of studies investigating bone effects and gut microbiota modulation of supplementation with prebiotics.
| Authors | Journal | Year | Dietary Supplement |
Subjects | Pathways Investigated |
Gut Microbiota Modulation | Main Findings |
|---|---|---|---|---|---|---|---|
| Porwal et al. [55] |
Biomedicine & Pharmacotherapy | 2020 | Fructooligosaccharides | Animals | SCFA production | Not investigated | Fructooligosaccharides prevent ovariectomy-induced bone loss |
| Yan et al. [52] |
Carbohydrate Polymers | 2019 | Fructooligosaccharides | Animals | Osteoblast stimulation | Not investigated | Fructooligosaccharides increase bone mineral density and strength |
| Tanabe et al. [54] |
Journal of Agriculture and Food Chemistry | 2019 | Fructooligosaccharides | Animals | ↓ Serum CRP; SCFA production |
↑ Lactobacillus ↑ Bacteroides ↓ Clostridium |
Fructooligosaccharides reduce bone resorption and systemic inflammation |
| Slevin et al. [56] |
The Journal of Nutrition | 2014 | Fructooligosaccharides | Humans | Reduction in the number of bone turnover markers | Not investigated | Fructooligosaccharides reduce postmenopausal bone loss |
| Mathey et al. [53] |
Calcified Tissue International | 2004 | Fructooligosaccharides | Animals | SCFA production |
Not investigated | Fructooligosaccharides increase bone mineral density and strength |
| Whisner et al. [50] |
British Journal of Nutrition | 2013 | Galactooligosaccharides | Humans | SCFA production |
↑ Bifidobacteria | Galactooligosaccharides increase calcium absorption |
| Weaver et al. [48] |
Journal of Agricultural and Food Chemistry | 2011 | Galactooligosaccharides | Animals | SCFA production |
↑ Bifidobacteria | Galactooligosaccharides increase calcium absorption |
| Van den Heuvel et al. [49] |
The Journal of Nutrition | 2000 | Galactooligosaccharides | Humans | SCFA production | Not investigated | Galactooligosaccharides increase calcium absorption |
| Karakan et al. [65] |
Frontiers in Nutrition | 2021 | Lactulose | Humans | SCFA production | ↑ Bifidobacteria ↑ Lactobacillus ↓ Clostridium |
Lactulose increases calcium absorption |
| Chen et al. [64] |
Aging and Disease | 2020 | Lactulose | Animals | ↓ TNFα ↓ RANKL ↓ IL-17 ↑ IL-10 |
↓ Firmicutes ↑ Bacteroides |
Lactulose inhibits osteoclastogenesis and bone resorption |
| Seki et al. [66] |
Journal of
Nutritional Science and Vitaminology |
2007 | Lactulose | Humans | Not investigated | Not investigated | Lactulose increases calcium and magnesium absorption |
| Tousen et al. [60] |
Nutrients | 2019 | Resistant starch | Animals | ↓ IL-7R mRNA↑ IL-10 mRNA↓ RANKL | ↑ Bifidobacteria | Resistant starch prevents post-ovariectomy bone loss |
| Tousen et al. [59] |
British Journal of Nutrition | 2016 | Resistant starch | Animals | ↓ IL-7R mRNA | ↑ Bifidobacteria | Resistant starch prevents post ovariectomy bone loss |
| Tousen et al. [58] |
Metabolism | 2011 | Resistant starch | Animals | Increased isoflavone availability and estrogenic activities | ↑ Bifidobacteria | Resistant starch prevents post-ovariectomy bone loss |
| Gao et al. [62] |
Nutrients | 2020 | Xylooligosaccharides | Animals | ↑ Na+/Ca2+ exchanger 1 ↑ TRPV6 |
Not investigated | Xylooligosaccharides increase calcium absorption |
| Eaimworawuthikul et al. [63] |
European Journal of
Nutrition |
2020 | Xylooligosaccharides | Animals | Osteoclast inhibition |
Not investigated | Xylooligosaccharides reduce bone resorption in systemic inflammation |
Abbreviations—SCFA: short-chain fatty acid; CRP = C-reactive protein; IL-7R: interleukin-7 receptor; IL-10: interleukin-10; RANKL: receptor activator of nuclear factor kappa-Β ligand; TRPV6: transient receptor potential vanilloid subfamily member 6; TNFα: tumor necrosis factor-alpha; IL-17: interleukin-17; ↓ = lower; ↑ = higher.
3.2. Proteins, Peptides, and Amino Acids
Intake of dietary proteins—in particular those from dairy products—has been linked with bone size, bone mass, and strength [75]. Some peptides contained in these products solubilize calcium and promote its absorption by the intestine [75]. Furthermore, the production of hepatic IGF-1 is stimulated by aromatic amino acids that are abundant in fermented dairy products, and this growth factor regulates bone growth [76]. Indeed, a recent study suggested that consumption of fermented dairy products might slow the age-related cortical bone loss in non-weight-bearing bone sites, independent of total energy, calcium, or protein intake, in a cohort of healthy postmenopausal women [77]. Furthermore, ingestion of fermented dairy products was associated with increased bone mass and reduced risk of fracture in the elderly [78,79], partly due to its content in lactulose-derived products which, together with probiotics, reduce the serum levels of parathyroid hormone (PTH), promoting bone resorption and, consequently, bone resorption markers [80].
Among dairy products, kefir is a complex fermented product generated through the symbiotic fermentation of milk by lactic acid bacteria and yeasts encased in a protein-and-polysaccharide matrix. Kefir supplementation for 8 weeks in ovariectomized mice caused increased BMD, increased the number and thickness of bone trabeculae, increased bone volume, and promoted better mechanical properties and fracture toughness [81,82]. In the same study, effects on the variability of gut microbiota were also shown, with a normalization of fecal levels of Alloprevotella, Anaerostipes, Parasutterella, Romboutsia, Ruminococcus, and Streptococcus species that were all more abundant compared to the sham group [82]. Bacterial species might hydrolyze proteins in the intestine, although this process can be modified by diet [83]. A higher gut bacterial production of tyrosine and tryptophan, resulting in elevated levels of these amino acids, might increase osteoporosis [84], while reduced gut degradation of the branched-chain amino acids valine, leucine, and isoleucine—and, consequently, higher serum levels of them—was associated with the prevalence of specific gut microbiota species [85], and was inversely correlated with the occurrence of osteoporosis [86,87] (see Table 2 for further details).
Table 2.
Characteristics of studies investigating bone effects and gut microbiota modulation of supplementation with proteins, peptides, and amino acids.
| Authors | Journal | Year | Dietary Supplement |
Subjects | Pathways Investigated |
Gut Microbiota Modulation | Main Findings |
|---|---|---|---|---|---|---|---|
| Ling et al. [86] |
The Journal of Clinical
Endocrinology and Metabolism |
2021 | Amino acids | Humans | Not determined |
↓ Actinobacillus, ↓ Blautia, ↓ Oscillospira |
Valine, leucine, and isoleucine serum levels are inversely related to the occurrence of osteoporosis |
| Jennings et al. [85] |
Journal of Bone and Mineral
Research |
2016 | Amino acids | Humans | ↑ IGF1 | Not investigated | Alanine, arginine, glutamic acid, leucine, lysine, and proline increase BMD |
| Dawson-Hugler et al. [75] |
Osteoporosis
International |
2007 | Amino acids | Humans | ↑ IGF1 | Not investigated | Phenylalanine and histidine increase calcium absorption |
| Liu et al. [74] |
Food & Function | 2018 | Casein Phosphopeptides |
Animals | ↑ TRPV6 | Not investigated | Casein phosphopeptides increase calcium absorption and prevent bone resorption |
| Ong et al. [78] |
Advances in Nutrition | 2020 | Fermented dairy products | Humans | ↓ TNF-α ↓ IL-6 ↓ RANKL |
Not investigated | Fermented dairy products might reduce hip fracture risk |
| Biver et al. [76] |
Osteoporosis International | 2018 | Fermented dairyproducts | Humans | Action on calcium balance and decrease in secondary hyper parathyroidism. |
Not investigated | Fermented dairy products attenuate postmenopausal bone loss |
| Laird et al. [77] |
Osteoporosis International | 2017 | Fermented dairy products | Humans | Modulation of osteoclast numbers and activity | Not investigated | Fermented dairy products increase bone mineral density |
| Tu et al. [81] |
Nutrients | 2020 | Kefir peptides | Animals | ↓ TNF-α ↓ RANKL |
↑ Alloprevotella, ↑ Anaerostipes, ↑ Parasutterella, ↑ Romboutsia, ↑ Ruminococcus, ↑ Streptococcus |
Kefir peptides prevent ovariectomy-induced bone loss |
| Chen et al. [80] |
Osteoporosis International | 2014 | Kefir peptides | Animals | ↑TRPV6 | Not investigated | Kefir peptides prevent ovariectomy-induced bone loss |
Abbreviations—BMD: bone mineral density; RANKL: receptor activator of nuclear factor kappa-Β ligand; TRPV6: transient receptor potential vanilloid subfamily member 6; TNFα: tumor necrosis factor-alpha; IL-6: interleukin-6; IGF1: insulin growth factor-1; ↓ = lower; ↑ = higher.
3.3. Micronutrients
Several micronutrients other than calcium might play a role in bone mineralization and growth [88], and in some cases their levels are strictly linked to the gut microbiota composition [89].
Selenium deficiency can affect bone growth during development [90], and low serum values of this micronutrient have been correlated with low BMD in the elderly [91]. Supplementation with selenium modifies the gut microbiota composition, promoting a dose-dependent increase in bacterial variety [92]. Moreover, zinc is a micronutrient that participates in bone metabolism, as it affects collagen synthesis and alkaline phosphatase activity [93]; its deficiency is correlated with lower BMD in postmenopausal women [94]. In animal models, zinc supplementation increased the number of Lactobacillus, while its deficiency caused a reduced ratio of Firmicutes, with lower SCFA production [95,96]. Lastly, both iron deficit and excess were linked to osteoporosis [97]. Low iron levels increased the expression of the fibroblast growth factor 23 (FGF23) gene [98]. Transgenic mice overexpressing FGF23 in osteoblasts presented a rachitic bone phenotype with a bone mineralization deficit, and showed increased levels of osteoblast markers, but the numbers of osteoclasts were slightly reduced or unchanged [99]. Adamts1 was highly induced in rachitic bones of FGF23 transgenic mice, and participated in the degradation of non-mineralized bone matrix collagen [99]. Conversely, a blockade of FGF23 signaling improved the rachitic bone phenotypes [100]. Unsurprisingly, therefore, serum iron levels are linked to BMD, and an adequate intake of this micronutrient is recommended [101]. Iron intake modifies the gut microbiota, increasing Bacteroidetes and Bifidobacterium [102,103], when introduced with the diet. When supplemented at high doses, iron decreases Bifidobacterium, resulting in increased bacterial pathogenicity [104,105]. Therefore, among the several micronutrients with beneficial bone effects, selenium, zinc, and iron can impact the gut microbiota, strengthening the interaction between bone and gut microbial populations (see Table 3 for further details).
Table 3.
Characteristics of studies investigating bone effects and gut microbiota modulation of supplementation with micronutrients.
| Authors | Journal | Year | Dietary Supplement |
Subjects | Pathways Investigated |
Gut Microbiota Modulation | Main Findings |
|---|---|---|---|---|---|---|---|
| Qasem et al. [100] |
BMC
Pediatrics |
2017 | Iron | Humans | Lowering inflammation |
↑ Bifidobacteria
↑ Bacteroides |
Favorable effects of iron on bone might be mediated by the gut microbiome |
| Wang et al. [89] |
BMC
Musculoskeletal Disorders |
2019 | Selenium | Humans | Not determined |
↓ Parabacteroides
↓ Firmicutes |
Selenium deficiency is correlated with a higher prevalence of osteoporosis |
| Reed et al. [94] |
Nutrients | 2015 | Zinc | Animals | SCFA production Lowering of inflammation |
↑ Lactobacillus | Favorable effects of zinc on bone might be mediated by the gut microbiome |
Abbreviations—SCFA: short chain fatty acid; ↓ = lower; ↑ = higher.
4. Impact of Probiotics on the Gut Microbiota
Probiotics are defined as “live micro-organisms which, when given in adequate amounts, confer a health benefit to the host” [106], and are made available via yogurts, milk-based foods, powders, capsules, or oral solutions. Their main characteristics include acid and bile tolerance that confers survival in the gastrointestinal tract (GIT), phenotypic and genotypic stability, adhesion to the mucosal surface, antibiotic resistance, and production of antimicrobial factors able to inhibit known pathogens [107]. The more frequently administered probiotics include Lactobacillus, Bifidobacterium, Escherichia, Enterococcus, and Bacillus subtilis—but also yeasts such as Saccharomyces—at a concentration of approximately of 107 to 108 cells per gram of probiotic supplement, with a serving size of 100 to 200 mg [108]. Given the mechanism of action of probiotics, most studies have failed to report any impact on gut microbiota composition. In fact, the effects of probiotics do not lie in their ability to directly restore an altered gut microbiota composition, but rather in contributing genes and metabolites that directly influence epithelial and immune cells, promoting gut health through regulation of the intestinal pH and permeability, along with resistance to colonization by unwanted bacteria [109,110,111,112].
The beneficial effects of the administration of probiotics on bone tissue are widely recognized, such that they have been named by some authors “the new calcium and vitamin D” for bone health [108]. Several studies have demonstrated that probiotics (i.e., L. reuteri, L. paracasei, and L. helveticus) prevent bone loss in the ovariectomy-induced postmenopausal mouse model [113,114,115,116]. Lactobacillus reuteri administration prevents type-1-diabetes-induced osteoporosis by inhibiting TNFα-mediated suppression of Wnt10b; it also enhances bone density via increased osteoblast activity and decreased bone marrow adiposity [117]. Similarly, it was documented that Lactobacillus reuteri administration ameliorates trabecular bone loss, restoring Wnt10b suppression in the bones of mice with glucocorticoid-induced osteoporosis [118]. Wnt10b is thought to be an endogenous regulator of bone formation; one way whereby it increases osteoblastogenesis is by downregulating the expression of PPAR-γ [119].
A randomized double-blind controlled trial showed that the administration of a multispecies probiotic supplement for six months significantly decreased bone-specific alkaline phosphatase and collagen type 1 crosslinked C-telopeptide levels in postmenopausal women with osteopenia, slowing down the rate of bone turnover [120]. Similarly, another study reported beneficial effects of a novel red clover extract rich in isoflavone aglycones, and probiotics against postmenopausal osteopenia, characterized by an attenuation of estrogen-deficiency-related BMD loss and an improvement of bone turnover in women [121]. Furthermore, in a double-blind placebo-controlled study, 12-month daily administration of Lactobacillus reuteri decreased the loss of tibia total volumetric BMD in elderly women [122].
Therefore, probiotics might play a key role in bone tissue, through modulation of the gut microbiota, providing a health benefit in frail subjects, although their role still needs to be characterized further (see Table 4 for currently known roles).
Table 4.
Characteristics of the studies investigating bone effects and gut microbiota modulation of supplementation with probiotics.
| Authors | Journal | Year | Dietary Supplement |
Subjects | Pathways Investigated |
Gut Microbiota Modulation | Main Findings |
|---|---|---|---|---|---|---|---|
| Narva et al. [113] |
Annals of
Nutrition and Metabolism |
2007 |
Lactobacillus
helveticus |
Animals | Increasing bone formation | Not investigated |
Lactobacillus helveticus prevents ovariectomy-induced bone loss |
| Ohlsson et al. [114] |
PLoS
ONE |
2014 |
Lactobacillus
paracasei |
Animals | ↓ TNF-α ↓ IL-1β ↑ OPG |
Not investigated |
Lactobacillus paracasei prevents ovariectomy-induced bone loss |
| Schepper et al. [46] |
The Journal of Bone and Mineral
Research |
2020 |
Lactobacillus
reuteri |
Animals | ↑ Wnt10b | ↓ Clostridium |
Lactobacillus reuteri prevents glucocorticoid-induced bone loss |
| Nilsson et al. [120] |
Journal of
Internal Medicine |
2018 |
Lactobacillus
reuteri |
Humans | Not determined | Not investigated | Lactobacillus reuteri prevents bone loss |
| Zhang et al. [115] |
Endocrinology | 2015 |
Lactobacillus
reuteri |
Animals | ↓ TNF-α | Not investigated | Lactobacillus reuteri prevents type-1-diabetes-induced bone loss |
| Britton et al. [111] |
Journal of
Cellular Physiology |
2014 |
Lactobacillus
reuteri |
Animals | ↓ Trap5 ↓ RANKL ↑ CD4+ T-lymphocytes |
Promoting gut microbiota diversity |
Lactobacillus reuteri prevents Ovariectomy-induced bone loss |
| Jafarnejad et al. [118] |
Journal of the American
Nutrition Association |
2017 | Multispecies probiotic | Humans | ↓ PTH ↓ TNF-α |
Not investigated | Multispecies probiotic reduces bone turnover |
Abbreviations—RANKL: receptor activator of nuclear factor kappa-Β ligand; TNFα: tumor necrosis factor-alpha; Trap5: serum band 5 tartrate-resistant acid phosphatase; CD4+ T−: cluster differentiation 4 positive cT helper cells; IL-1β: interleukin-1 beta; OPG: osteoprotegerin; Wnt10b: Wnt family member 10B; PTH: parathyroid hormone; ↓ = lower; ↑ = higher.
5. Interventional Microbiota Modulation to Reduce Bone Loss
GIT and bone tissue interact with one another through a complex network modulated by the gut microbiota, in which osteoblasts, osteoclasts, and receptor activator of nuclear factor kappa-Β ligand (RANKL) participate [123]. Recently, several studies have focused on establishing the role of the gut microbiota in the onset of osteoporosis, considering its modulation as a potential therapeutic strategy to reduce bone loss [124,125,126]. For instance, restoring gut microbiota eubiosis has positive effects on the treatment of dysbiosis-related extraintestinal disorders—for instance, in bones and muscles—such as osteoporosis, osteoarthritis, and sarcopenia [123,124,127,128,129,130,131,132]. In particular, gut microbiota modulation can be achieved primarily through diet, but also with probiotics, prebiotics, synbiotics (mixtures of probiotics and prebiotics that beneficially affect the host), postbiotics (bioactive compounds produced by food-grade microorganisms during fermentation), and obviously also by antibiotics and fecal microbiota transplantation (FMT) [132,133,134,135].
Diet represents the main environmental factor contributing to the interindividual differences in gut microbiota composition in healthy people [134,135,136,137]. A balanced diet is fundamental for human health, to which a well-balanced complex microorganism ecosystem composed of more than 1014 bacteria—as well as fungi, viruses, phages, parasites, and archaea, which colonize the GIT—is a major contributor [41]. On the other hand, high-fat diets lead to a decrease in the numbers of “good bacteria”, such as Bacteroidetes, and an overgrowth of opportunistic pathogens, able to promote a disruption of the intestinal epithelial tight junctions. This leads to an increase in gut permeability, allowing the translocation of bacterial lipopolysaccharides into the systemic blood circulation (so called “leaky gut syndrome”), promoting systemic inflammation and insulin resistance [138,139]. In contrast, high-fiber diets and fructooligosaccharides are associated with an increased number of Bifidobacteria species producing SCFAs, such as acetate, propionate, and butyrate, which enhance glucose uptake and insulin sensitivity [140]. SCFAs also play a role in regulating bone homeostasis by downregulating both osteoclast activity that causes bone resorption and inflammation sustained by T-reg lymphocytes [141]. In this context, the adherence to a Mediterranean diet—rich in fibers, fermented dairy products, and polyphenols—is associated with lower hip fracture risk [142]. In association with diet, physical exercise has an impact on several cortical bone parameters and bone mechanical properties—possibly by modulating the intestinal microbial balance toward a decrease in the Firmicutes/Bacteroidetes ratio and an increase in the number of Actinobacteria phylum members, such as Bifidobacteriaceae. Modulating the gut microbiota with probiotics can promote the overgrowth of not only Lactobacillus and Bifidobacterium species, but also Faecalibacterium, Akkermansia, Ruminococcus, and Roseburia species, leading to a stimulation of SCFA production [143,144,145,146]. Furthermore, prebiotics exert a direct antiadhesive effect against pathogens by interacting with bacterial receptors, thereby inhibiting pathogen colonization [147,148]. Several experimental studies [149,150,151,152], both in animals and in humans, support the hypothesis that prebiotics play a key role in the intestinal absorption of calcium and magnesium by causing a decrease in the cecal pH, which results in an increase in BMD.
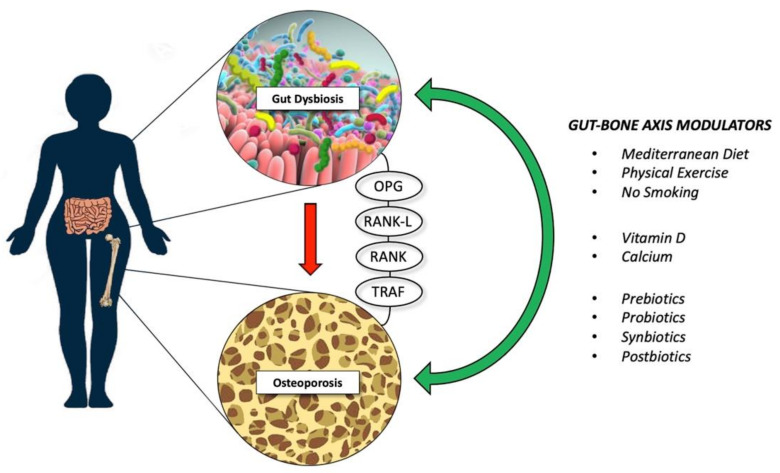
In the fast moving “-biotics” field, postbiotics have been defined recently as “bioactive compounds produced by food-grade micro-organisms during a fermentation process” [153]. Postbiotics is an umbrella term that includes metabolites, SCFAs, microbial cell fractions, functional proteins, extracellular polymeric substances (EPSs), cell lysates, teichoic acid, peptidoglycan-derived muropeptides, and pili-type structures, which may be used to promote health by modulating the gut microbiota [153]. In this context, only a few very recent studies [154,155,156] have shown how the administration of postbiotics ameliorates osteopenia and/or osteoporosis. A study of the effects of cell lysates and supernatants of five native probiotic strains (L. acidophilus, L. reuteri, L. casei, B. longum, and B. coagulans) in ovariectomized rats showed that the Bacillus-coagulans-derived-postbiotics had the best impact on bone homeostasis, ameliorating several parameters, such as reduced bone area, bone mineral content, and BMD caused by ovariectomy [153]. Furthermore, a corroborating study [155] using a murine model of postmenopausal-related osteoporosis reported an inhibitory effect of the postbiotic Lactobacillus curvatus 38-CS on RANKL-induced osteoclast differentiation and bone loss, by downregulating the TRAF6/NF-κB/MAPKs axis (see Figure 2 for further details).
Figure 2.
Lifestyle intervention and supplementation that might reduce bone loss; these modulators might exert their therapeutic effects through different known biological pathways, such as OPG, RANK, and TRAF. Abbreviations—OPG: osteoprotegerin; RANK-L: receptor activator of nuclear factor-κB ligand; RANK: receptor activator of nuclear factor κB; TRAF: tumor necrosis factor receptor-associated factor.
At this point, it is of interest to discuss the effects and consequences of antibiotics on the gut microbiota. Antibiotics are considered to be deep modulators of the gut microbiota, and the characteristics that can influence their negative or positive impact relate to antibiotic class, dosage, duration, and administration route [156]. On the one hand, their abuse causes several intestinal and extraintestinal disorders associated with gut microbiota impairment—especially Clostridium difficile infection. On the other hand, antibiotic therapy can provide a so-called “eubiotic” effect, increasing the abundance of beneficial bacteria, and therefore appears to be a reliable therapeutic strategy for several non-communicable disorders, such as hepatic encephalopathy or irritable bowel syndrome [156]. Antibiotic treatment, by modulating the gut microbiota, can influence bone mass. Animal studies have shown that short-term administration of low doses of antibiotics at weaning results in increased BMD and adiposity, with substantial taxonomic changes in terms of the gut microbiota [157]. Subsequent supporting results showed that a low dose of penicillin delivered from birth to weaning in mice was sufficient to transiently perturb the gut microbiota, inducing sustained effects on body composition with an increase in BMD [158]. In addition, treatment with tetracyclines was found to prevent ovariectomy-induced bone loss, reducing bone resorption modestly and stimulating bone formation substantially [159].
A more recent therapeutic strategy for the modulation of the gut microbiota is the transfer of donor stools to a recipient’s GIT—the so-called fecal microbiota transplants (FMTs), which have gained much recognition recently [160]. Considered to be an established treatment for recurrent Clostridium difficile infection, FMT is a potential therapy for inducing remission of mild–moderate ulcerative colitis [161]. Furthermore, its use has been debated in the literature for improving several extraintestinal conditions, including metabolic syndrome, modulation of responses to chemotherapy, eradication of multidrug-resistant organisms, and osteomuscular disorders [161]. In the context of bone disorders, FMT from young to aged rats improved senile osteoporosis in terms of bone volume, trabecular bone volume fraction, trabecular number, and trabecular thickness by restoring gut dysbiosis at the phylum and family levels [162]. Furthermore, FMT improved leaky gut syndrome in aged rats, upregulating the expression of the tight-junction proteins occludin, claudin, and ZO-1 [162]. Interestingly, transplantation of fecal material from mice treated with the corticosteroid prednisolone into naïve, untreated recipients caused bone loss, supporting a role of the gut microbiota in the development of glucocorticoid-induced osteoporosis [118]. However, further studies are required in order to clarify the emerging role of gut microbiota modulation in reducing bone loss to prevent osteoporosis.
6. Future Research Avenues and Perspectives for Osteoporosis Treatment
The current available osteoporosis treatments consist of antiresorptive agents that inhibit osteoclast activity, bone-forming compounds that stimulate osteoblasts, and dual-acting drugs that simultaneously inhibit bone resorption and enhance bone accrual [163,164,165]. In bone homeostasis, the Wnt/β-catenin and OPG/RANKL/RANK signaling pathways play crucial roles in bone anabolism and catabolism, respectively [166]. Antiresorptive treatments comprise bisphosphonates and denosumab—an antibody against RANKL [167,168].
Animal studies recently aimed to develop molecules capable of interfering even with the osteoclast–bone matrix proteins (αvβ3 integrin antagonists), acting as bone resorption inhibitors [169,170]. Furthermore, the anabolic bone accrual process can be stimulated by parathyroid hormone analogues (amino acids 1–34) [171]. Moreover, romosozumab is a novel antibody that inhibits sclerostin and, hence, interferes with the sclerostin/Wnt signaling pathway, and might have both anabolic and antiresorptive effects on bone [172]. In the same pathway, frizzled-related proteins (FRPs) are considered to be novel therapeutic targets, and miRNA-based therapies targeting FRPs are under development to prevent and treat osteoporosis [173].
The gut microbiota interferes to some extent with the Wnt signaling pathway, in particular promoting the renewal of hematopoietic stem cells [174]; moreover, it also participates in the OPG pathway and inhibits RANKL [175], possibly similarly to denosumab, but its mechanism is not fully understood. As discussed above, the gut microbiota enhances the intestinal absorption of calcium; interestingly, it might regulate bone metabolism through a calcium-dependent interaction with the Wnt/β-catenin signaling pathway, as demonstrated in animal models [176].
To date, knowledge on gut microbial interaction with the host—and even more so its potential regulatory role in bone health—remains partial [177]. Furthermore, we are aware that the interpretation of animal-model-based studies requires caution with respect to the extrapolation of results to humans, especially considering the clinical management of osteoporosis.
However, several dietary supplements and probiotics highlighted in this review might modulate bone health by acting on the same biological pathways used by some currently utilized drugs. Hence, it might be suggested that the exploration of combined supplement-drug approaches might help to maximize the anti-osteoporotic effects of these interventions.
Finally, it is worth noting that systemic inflammation and immune system effects are often neglected as therapeutic targets in osteoporosis, even though many natural and synthetic substances exert bone effects by acting on T cells or by decreasing the levels of proinflammatory cytokines.
In this review, we highlighted how gut microbiota modulation is appealing as a therapeutic intervention to counteract bone loss. In this context, dietary supplements, prebiotics, probiotics, postbiotics, and synbiotics should be explored further as useful adjuvant treatments for osteoporosis, especially with regards to targeting the most relevant biochemical and signaling pathways. Future human studies are warranted to confirm the hypotheses discussed, in order to build and provide the right support for the clinical management of the gut–bone axis and sustain bone health.
Author Contributions
Conceptualization, A.d.S. and W.W.; methodology, A.d.S. and W.W.; investigation, R.d.S. and C.C.; data curation, A.d.S., F.C. and W.W.; writing—original draft preparation, A.d.S., R.d.S. and C.C.; writing—review and editing, F.C. and W.W.; supervision, A.d.S. and W.W.; study submission: A.d.S. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marini F., Cianferotti L., Brandi M.L. Epigenetic Mechanisms in Bone Biology and Osteoporosis: Can They Drive Therapeutic Choices? Int. J. Mol. Sci. 2016;17:1329. doi: 10.3390/ijms17081329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisani P., Renna M.D., Conversano F., Casciaro E., Di Paola M., Quarta E., Muratore M., Casciaro S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 2016;7:171–181. doi: 10.5312/wjo.v7.i3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanis J.A., Norton N., Harvey N.C., Jacobson T., Johansson H., Lorentzon M., McCloskey E.V., Willers C., Borgström F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021;16:82. doi: 10.1007/s11657-020-00871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compston J.E., McClung M.R., Leslie W.D. Osteoporosis. Lancet. 2019;393:364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J., Lu Y., Wang Y., Ren X., Han J. The impact of the intestinal microbiome on bone health. Intractable Rare Dis. Res. 2018;7:148–155. doi: 10.5582/irdr.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson J., Garges S., Giovanni M., McInnes P., Wang L., Schloss J.A., Bonazzi V., McEwen J.E., Wetterstrand K.A., et al. NIH HMP Working Group The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagpal R., Yadav H., Marotta F. Gut microbiota: The next-gen frontier in preventive and therapeutic medicine? Front. Med. 2014;1:15. doi: 10.3389/fmed.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost F., Kacprowski T., Rühlemann M., Pietzner M., Bang C., Franke A., Nauck M., Völker U., Völzke H., Dörr M., et al. Long-term instability of the intestinal microbiome is associated with metabolic liver disease, low microbiota diversity, diabetes mellitus and impaired exocrine pancreatic function. Gut. 2021;70:522–530. doi: 10.1136/gutjnl-2020-322753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright E.K., Kamm M.A., Teo S.M., Inouye M., Wagner J., Kirkwood C.D. Recent advances in characterizing the gastrointestinal microbiome in Crohn’s disease: A systematic review. Inflamm. Bowel Dis. 2015;21:1219–1228. doi: 10.1097/MIB.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ussar S., Fujisaka S., Kahn C.R. Interactions between host genetics and gut microbiome in diabetes and metabolic syndrome. Mol. Metab. 2016;5:795–803. doi: 10.1016/j.molmet.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minemura M., Shimizu Y. Gut microbiota and liver diseases. World J. Gastroenterol. 2015;21:1691–1702. doi: 10.3748/wjg.v21.i6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pevsner-Fischer M., Blacher E., Tatirovsky E., Ben-Dov I.Z., Elinav E. The gut microbiome and hypertension. Curr. Opin. Nephrol. Hypertens. 2017;26:1–8. doi: 10.1097/MNH.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 14.Rosser E.C., Mauri C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J. Autoimmun. 2016;74:85–93. doi: 10.1016/j.jaut.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Ozaki D., Kubota R., Maeno T., Abdelhakim M., Hitosugi N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos. Int. 2021;32:145–156. doi: 10.1007/s00198-020-05728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKnite A.M., Perez-Munoz M.E., Lu L., Williams E.G., Brewer S., Andreux P.A., Bastiaansen J.W., Wang X., Kachman S.D., Auwerx J., et al. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS ONE. 2012;7:e39191. doi: 10.1371/journal.pone.0039191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan J., Herzog J.W., Tsang K., Brennan C.A., Bower M.A., Garrett W.S., Sartor B.R., Aliprantis A.O., Charles J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA. 2016;113:E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hathaway-Schrader J.D., Poulides N.A., Carson M.D., Kirkpatrick J.E., Warner A.J., Swanson B.A., Taylor E.V., Chew M.E., Reddy S.V., Liu B., et al. Specific Commensal Bacterium Critically Regulates Gut Microbiota Osteoimmunomodulatory Actions During Normal Postpubertal Skeletal Growth and Maturation. JBMR Plus. 2020;4:e10338. doi: 10.1002/jbm4.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castaneda M., Smith K.M., Nixon J.C., Hernandez C.J., Rowan S. Alterations to the gut microbiome impair bone tissue strength in aged mice. Bone Rep. 2021;14:101065. doi: 10.1016/j.bonr.2021.101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novince C.M., Whittow C.R., Aartun J.D., Hathaway J.D., Poulides N., Chavez M.B., Steinkamp H.M., Kirkwood K.A., Huang E., Westwater C., et al. Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health. Sci. Rep. 2017;7:5747. doi: 10.1038/s41598-017-06126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahiri S., Kim H., Garcia-Perez I., Reza M.M., Martin K.A., Kundu P., Cox L.M., Selkrig J., Posma J.M., Zhang H., et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019;11:eaan5662. doi: 10.1126/scitranslmed.aan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manickam R., Oh H.Y.P., Tan C.K., Paramalingam E., Wahli W. Metronidazole Causes Skeletal Muscle Atrophy and Modulates Muscle Chronometabolism. Int. J. Mol. Sci. 2018;19:2418. doi: 10.3390/ijms19082418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratajczak A.E., Rychter A.M., Zawada A., Dobrowolska A., Krela-Kaźmierczak I. Do Only Calcium and Vitamin D Matter? Micronutrients in the Diet of Inflammatory Bowel Diseases Patients and the Risk of Osteoporosis. Nutrients. 2021;13:525. doi: 10.3390/nu13020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behera J., Ison J., Tyagi S.C., Tyagi N. The role of gut microbiota in bone homeostasis. Bone. 2020;135:115317. doi: 10.1016/j.bone.2020.115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Floch M.H. Probiotics and Prebiotics. Gastroenterol. Hepatol. 2014;10:680–681. [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzoli R. Microbiota and Bone Health: The Gut-Musculoskeletal Axis. Calcif. Tissue Int. 2018;102:385–386. doi: 10.1007/s00223-018-0391-7. [DOI] [PubMed] [Google Scholar]
- 27.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., Nikkïla J., Monti D., Satokari R., Franceschi C., et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/annotation/df45912f-d15c-44ab-8312-e7ec0607604d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiskanen J.T., Kröger H., Pääkkönen M., Parviainen M.T., Lamberg- Allardt C., Alhava E. Bone mineral metabolism after total gastrectomy. Bone. 2001;28:123–127. doi: 10.1016/S8756-3282(00)00404-X. [DOI] [PubMed] [Google Scholar]
- 29.Tovey F.I., Hall M.L., Ell P.J., Hobsley M. A review of postgastrectomy bone disease. J. Gastroenterol. Hepatol. 1992;7:639–645. doi: 10.1111/j.1440-1746.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 30.Schinke T., Schilling A.F., Baranowsky A., Seitz S., Marshall R.P., Linn T., Blaeker M., Huebner A.K., Schulz A., Simon R., et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat. Med. 2009;15:674–681. doi: 10.1038/nm.1963. [DOI] [PubMed] [Google Scholar]
- 31.Hansen M.S., Frost M. Alliances of the gut and bone axis. Semin. Cell Dev. Biol. 2021;123:74–81. doi: 10.1016/j.semcdb.2021.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Karsenty G., Yadav V.K. Regulation of bone mass by serotonin: Molecular biology and therapeutic implications. Annu. Rev. Med. 2011;62:323–331. doi: 10.1146/annurev-med-090710-133426. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y.C., Greenbaum J., Shen H., Deng H.W. Association Between Gut Microbiota and Bone Health: Potential Mechanisms and Prospective. J. Clin. Endocrinol. Metab. 2017;102:3635–3646. doi: 10.1210/jc.2017-00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson C.T., Sharma V., Elmén L., Peterson S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015;179:363–377. doi: 10.1111/cei.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das M., Cronin O., Keohane D.M., Cormac E.M., Nugent H., Nugent M., Molloy C., O’Toole P.W., Shanahan F., Molloy M.G., et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology. 2019;58:2295–2304. doi: 10.1093/rheumatology/kez302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rafii F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites. 2015;5:56–73. doi: 10.3390/metabo5010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu Y., Yang R., Xu X., Zhou X. The microbiota-gut-bone axis and bone health. J. Leukoc. Biol. 2021;110:525–537. doi: 10.1002/JLB.3MR0321-755R. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y., Guo Y.L., Meng S., Gao H., Sui L.J., Jin S., Li Y., Fan S.G. Gut microbiota-dependent trimethylamine N-Oxide are related with hip fracture in postmenopausal women: A matched case-control study. Aging. 2020;12:10633–10641. doi: 10.18632/aging.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O’Connor E.M., Cusack S., Harris H.M., Coakley M., Lakshminarayanan B., O’Sullivan O., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 40.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 41.Gentile C.L., Weir T.L. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 42.Byberg L., Bellavia A., Larsson S.C., Orsini N., Wolk A., Michaëlsson K. Mediterranean Diet and Hip Fracture in Swedish Men and Women. J. Bone Miner. Res. 2016;31:2098–2105. doi: 10.1002/jbmr.2896. [DOI] [PubMed] [Google Scholar]
- 43.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 44.Palomeras-Vilches A., Viñals-Mayolas E., Bou-Mias C., Jordà-Castro M., Agüero-Martínez M., Busquets-Barceló M., Pujol-Busquets G., Carrion C., Bosque-Prous M., Serra-Majem L., et al. Adherence to the Mediterranean Diet and Bone Fracture Risk in Middle-Aged Women: A Case Control Study. Nutrients. 2019;11:2508. doi: 10.3390/nu11102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noel S.E., Mangano K.M., Mattei J., Griffith J.L., Dawson-Hughes B., Bigornia S., Tucker K.L. Dietary Approaches to Stop Hypertension, Mediterranean, and Alternative Healthy Eating indices are associated with bone health among Puerto Rican adults from the Boston Puerto Rican Osteoporosis Study. Am. J. Clin. Nutr. 2020;111:1267–1277. doi: 10.1093/ajcn/nqaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaiss M.M., Jones R.M., Schett G., Pacifici R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019;129:3018–3028. doi: 10.1172/JCI128521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whisner C.M., Castillo L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018;102:443–479. doi: 10.1007/s00223-017-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schepper J.D., Collins F.L., Rios-Arce N.D., Raehtz S., Schaefer L., Gardinier J.D., Britton R.A., Parameswaran N., McCabe L.R. Probiotic Lactobacillus reuteri Prevents Postantibiotic Bone Loss by Reducing Intestinal Dysbiosis and Preventing Barrier Disruption. J. Bone Min. Res. 2019;34:681–698. doi: 10.1002/jbmr.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J., Motyl K.J., Irwin R., MacDougald O.A., Britton R.A., McCabe L.R. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weaver C.M., Martin B.R., Nakatsu C.H., Armstrong A.P., Clavijo A., McCabe L.D., McCabe G.P., Duignan S., Schoterman M.H., van den Heuvel E.G. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011;59:6501–6510. doi: 10.1021/jf2009777. [DOI] [PubMed] [Google Scholar]
- 51.Van den Heuvel E.G., Schoterman M.H., Muijs T. Transgalactooligosaccharides stimulate calcium absorption in postmenopausal women. J. Nutr. 2000;130:2938–2942. doi: 10.1093/jn/130.12.2938. [DOI] [PubMed] [Google Scholar]
- 52.Whisner C.M., Martin B.R., Schoterman M.H., Nakatsu C.H., McCabe L.D., McCabe G.P., Wastney M.E., van den Heuvel E.G., Weaver C.M. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: A double-blind cross-over trial. Br. J. Nutr. 2013;110:1292–1303. doi: 10.1017/S000711451300055X. [DOI] [PubMed] [Google Scholar]
- 53.Bornet F.R., Brouns F., Tashiro Y., Duvillier V. Nutritional aspects of short-chain fructooligosaccharides: Natural occurrence, chemistry, physiology and health implications. Dig. Liver Dis. 2002;34:S111–S120. doi: 10.1016/S1590-8658(02)80177-3. [DOI] [PubMed] [Google Scholar]
- 54.Yan C., Zhang S., Wang C., Zhang Q. A fructooligosaccharide from Achyranthes bidentata inhibits osteoporosis by stimulating bone formation. Carbohydr. Polym. 2019;210:110–118. doi: 10.1016/j.carbpol.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 55.Mathey J., Puel C., Kati-Coulibaly S., Bennetau-Pelissero C., Davicco M.J., Lebecque P., Horcajada M.N., Coxam V. Fructooligosaccharides maximize bone-sparing effects of soy isoflavone-enriched diet in the ovariectomized rat. Calcif. Tissue Int. 2004;75:169–179. doi: 10.1007/s00223-004-0128-7. [DOI] [PubMed] [Google Scholar]
- 56.Tanabe K., Nakamura S., Moriyama-Hashiguchi M., Kitajima M., Ejima H., Imori C., Oku T. Dietary Fructooligosaccharide and Glucomannan Alter Gut Microbiota and Improve Bone Metabolism in Senescence-Accelerated Mouse. J. Agric. Food Chem. 2019;67:867–874. doi: 10.1021/acs.jafc.8b05164. [DOI] [PubMed] [Google Scholar]
- 57.Porwal K., Pal S., Kulkarni C., Singh P., Sharma S., Singh P., Prajapati G., Gayen J.R., Ampapathi R.S., Mullick A., et al. A prebiotic, short-chain fructo-oligosaccharides promotes peak bone mass and maintains bone mass in ovariectomized rats by an osteogenic mechanism. Biomed. Pharmacother. 2020;129:110448. doi: 10.1016/j.biopha.2020.110448. [DOI] [PubMed] [Google Scholar]
- 58.Slevin M.M., Allsopp P.J., Magee P.J., Bonham M.P., Naughton V.R., Strain J.J., Duffy M.E., Wallace J.M., Mc Sorley E.M. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J. Nutr. 2014;144:297–304. doi: 10.3945/jn.113.188144. [DOI] [PubMed] [Google Scholar]
- 59.Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 60.Tousen Y., Abe F., Ishida T., Uehara M., Ishimi Y. Resistant starch promotes equol production and inhibits tibial bone loss in ovariectomized mice treated with daidzein. Metabolism. 2011;60:1425–1432. doi: 10.1016/j.metabol.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Tousen Y., Matsumoto Y., Matsumoto C., Nishide Y., Nagahata Y., Kobayashi I., Ishimi Y. The combined effects of soya isoflavones and resistant starch on equol production and trabecular bone loss in ovariectomised mice. Br. J. Nutr. 2016;116:247–257. doi: 10.1017/S0007114516001537. [DOI] [PubMed] [Google Scholar]
- 62.Tousen Y., Matsumoto Y., Nagahata Y., Kobayashi I., Inoue M., Ishimi Y. Resistant Starch Attenuates Bone Loss in Ovariectomised Mice by Regulating the Intestinal Microbiota and Bone-Marrow Inflammation. Nutrients. 2019;11:297. doi: 10.3390/nu11020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z., Summanen P.H., Komoriya T., Finegold S.M. In vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp and Lactobacillus spp. Int. J. Food Sci. Nutr. 2015;66:919–922. doi: 10.3109/09637486.2015.1064869. [DOI] [PubMed] [Google Scholar]
- 64.Gao H., Zhou Z. Effect of Xylo-Oligosaccharides Supplementation by Drinking Water on the Bone Properties and Related Calcium Transporters in Growing Mice. Nutrients. 2020;12:3542. doi: 10.3390/nu12113542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eaimworawuthikul S., Tunapong W., Chunchai T., Suntornsaratoon P., Charoenphandhu N., Thiennimitr P., Chattipakorn N., Chattipakorn S.C. Altered gut microbiota ameliorates bone pathology in the mandible of obese-insulin-resistant rats. Eur. J. Nutr. 2020;59:1453–1462. doi: 10.1007/s00394-019-02002-8. [DOI] [PubMed] [Google Scholar]
- 66.Chen X., Zhang Z., Hu Y., Cui J., Zhi X., Li X., Jiang H., Wang Y., Gu Z., Qiu Z., et al. Lactulose Suppresses Osteoclastogenesis and Ameliorates Estrogen Deficiency-Induced Bone Loss in Mice. Aging Dis. 2020;11:629–641. doi: 10.14336/AD.2019.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karakan T., Tuohy K.M., Janssen-van Solingen G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021;8:672925. doi: 10.3389/fnut.2021.672925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seki N., Hamano H., Iiyama Y., Asano Y., Kokubo S., Yamauchi K., Tamura Y., Uenishi K., Kudou H. Effect of lactulose on calcium and magnesium absorption: A study using stable isotopes in adult men. J. Nutr. Sci. Vitam. 2007;53:5–12. doi: 10.3177/jnsv.53.5. [DOI] [PubMed] [Google Scholar]
- 69.Scholz-Ahrens K.E., Schaafsma G., van den Heuvel E.G., Schrezenmeir J. Effects of prebiotics on mineral metabolism. Am. J. Clin. Nutr. 2001;73((Suppl. S2)):459S–464S. doi: 10.1093/ajcn/73.2.459s. [DOI] [PubMed] [Google Scholar]
- 70.McCabe L., Britton R.A., Parameswaran N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr. Osteoporos. Rep. 2015;13:363–371. doi: 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolever T.M., Trinidad T.P., Thompson L.U. Short chain fatty acid absorption from the human distal colon: Interactions between acetate, propionate and calcium. J. Am. Coll. Nutr. 1995;14:393–398. doi: 10.1080/07315724.1995.10718527. [DOI] [PubMed] [Google Scholar]
- 72.Den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 74.Nath A., Molnár M.A., Csighy A., Kőszegi K., Galambos I., Huszár K.P., Koris A., Vatai G. Biological Activities of Lactose-Based Prebiotics and Symbiosis with Probiotics on Controlling Osteoporosis, Blood-Lipid and Glucose Levels. Medicina. 2018;54:98. doi: 10.3390/medicina54060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu G., Sun S., Guo B., Miao B., Luo Z., Xia Z., Ying D., Liu F., Guo B., Tang J., et al. Bioactive peptide isolated from casein phosphopeptides promotes calcium uptake in vitro and in vivo. Food Funct. 2018;9:2251–2260. doi: 10.1039/C7FO01709J. [DOI] [PubMed] [Google Scholar]
- 76.Dawson-Hughes B., Harris S.S., Rasmussen H.M., Dallal G.E. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos. Int. 2007;18:955–961. doi: 10.1007/s00198-006-0320-x. [DOI] [PubMed] [Google Scholar]
- 77.Biver E., Durosier-Izart C., Merminod F., Chevalley T., van Rietbergen B., Ferrari S.L., Rizzoli R. Fermented dairy products consumption is associated with attenuated cortical bone loss independently of total calcium, protein, and energy intakes in healthy postmenopausal women. Osteoporos. Int. 2018;29:1771–1782. doi: 10.1007/s00198-018-4535-4. [DOI] [PubMed] [Google Scholar]
- 78.Laird E., Molloy A.M., McNulty H., Ward M., McCarroll K., Hoey L., Hughes C.F., Cunningham C., Strain J.J., Casey M.C. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos. Int. 2017;28:2409–2419. doi: 10.1007/s00198-017-4049-5. [DOI] [PubMed] [Google Scholar]
- 79.Ong A.M., Kang K., Weiler H.A., Morin S.N. Fermented Milk Products and Bone Health in Postmenopausal Women: A Systematic Review of Randomized Controlled Trials, Prospective Cohorts, and Case-Control Studies. Adv. Nutr. 2020;11:251–265. doi: 10.1093/advances/nmz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rizzoli R. Dairy products and bone health. Aging Clin. Exp. Res. 2022;34:9–24. doi: 10.1007/s40520-021-01970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H.L., Tung Y.T., Chuang C.H., Tu M.Y., Tsai T.C., Chang S.Y., Chen C.M. Kefir improves bone mass and microarchitecture in an ovariectomized rat model of postmenopausal osteoporosis. Osteoporos. Int. 2015;26:589–599. doi: 10.1007/s00198-014-2908-x. [DOI] [PubMed] [Google Scholar]
- 82.Tu M.Y., Han K.Y., Chang G.R., Lai G.D., Chang K.Y., Chen C.F., Lai J.C., Lai C.Y., Chen H.L., Chen C.M. Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients. 2020;12:3432. doi: 10.3390/nu12113432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diether N.E., Willing B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet-Microbe-Host Interaction. Microorganisms. 2019;7:19. doi: 10.3390/microorganisms7010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo Y., Xie C., Li X., Yang J., Yu T., Zhang R., Zhang T., Saxena D., Snyder M., Wu Y., et al. Succinate and its G-protein-coupled receptor stimulates osteoclastogenesis. Nat. Commun. 2017;8:15621. doi: 10.1038/ncomms15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu W., Zhang L., Xia B., Tang S., Liu L., Xie J., Zhang H. Bioregional Alterations in Gut Microbiome Contribute to the Plasma Metabolomic Changes in Pigs Fed with Inulin. Microorganisms. 2020;8:111. doi: 10.3390/microorganisms8010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jennings A., MacGregor A., Spector T., Cassidy A. Amino Acid Intakes Are Associated with Bone Mineral Density and Prevalence of Low Bone Mass in Women: Evidence from Discordant Monozygotic Twins. J. Bone Min. Res. 2016;31:326–335. doi: 10.1002/jbmr.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ling C.W., Miao Z., Xiao M.L., Zhou H., Jiang Z., Fu Y., Xiong F., Zuo L.S., Liu Y.P., Wu Y.Y., et al. The Association of Gut Microbiota with Osteoporosis Is Mediated by Amino Acid Metabolism: Multiomics in a Large Cohort. J. Clin. Endocrinol. Metab. 2021;106:e3852–e3864. doi: 10.1210/clinem/dgab492. [DOI] [PubMed] [Google Scholar]
- 88.Karaaslan F., Mutlu M., Mermerkaya M.U., Karaoğlu S., Saçmaci Ş., Kartal Ş. Comparison of bone tissue trace-element concentrations and mineral density in osteoporotic femoral neck fractures and osteoarthritis. Clin. Interv. Aging. 2014;9:1375–1382. doi: 10.2147/CIA.S66354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rayman M.P. Selenium intake, status, and health: A complex relationship. Hormones. 2020;19:9–14. doi: 10.1007/s42000-019-00125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beukhof C.M., Medici M., van den Beld A.W., Hollenbach B., Hoeg A., Visser W.E., de Herder W.W., Visser T.J., Schomburg L., Peeters R.P. Selenium Status Is Positively Associated with Bone Mineral Density in Healthy Aging European Men. PLoS ONE. 2016;11:e0152748. doi: 10.1371/journal.pone.0152748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Xie D., Li J., Long H., Wu J., Wu Z., He H., Wang H., Yang T., Wang Y. Association between dietary selenium intake and the prevalence of osteoporosis: A cross-sectional study. BMC Musculoskelet. Disord. 2019;20:585. doi: 10.1186/s12891-019-2958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kasaikina M.V., Kravtsova M.A., Lee B.C., Seravalli J., Peterson D.A., Walter J., Legge R., Benson A.K., Hatfield D.L., Gladyshev V.N. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011;25:2492–2499. doi: 10.1096/fj.11-181990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Palacios C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006;46:621–628. doi: 10.1080/10408390500466174. [DOI] [PubMed] [Google Scholar]
- 94.Mutlu M., Argun M., Kilic E., Saraymen R., Yazar S. Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J. Int. Med. Res. 2007;35:692–695. doi: 10.1177/147323000703500514. [DOI] [PubMed] [Google Scholar]
- 95.Starke I.C., Pieper R., Neumann K., Zentek J., Vahjen W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014;87:416–427. doi: 10.1111/1574-6941.12233. [DOI] [PubMed] [Google Scholar]
- 96.Reed S., Neuman H., Moscovich S., Glahn R.P., Koren O., Tako E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients. 2015;7:9768–9784. doi: 10.3390/nu7125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Balogh E., Paragh G., Jeney V. Influence of Iron on Bone Homeostasis. Pharmaceuticals. 2018;11:107. doi: 10.3390/ph11040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Imel E.A., Liu Z., McQueen A.K., Acton D., Acton A., Padgett L.R., Peacock M., Econs M.J. Serum fibroblast growth factor 23, serum iron and bone mineral density in premenopausal women. Bone. 2016;86:98–105. doi: 10.1016/j.bone.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu L., Andersson G., Jonsson K.B., Melhus H., Lind T. Adamts1 is highly induced in rachitic bones of FGF23 transgenic mice and participates in degradation of non-mineralized bone matrix collagen. Biochem. Biophys. Res. Commun. 2013;430:901–906. doi: 10.1016/j.bbrc.2012.12.056. [DOI] [PubMed] [Google Scholar]
- 100.Kaneko I., Segawa H., Ikuta K., Hanazaki A., Fujii T., Tatsumi S., Kido S., Hasegawa T., Amizuka N., Saito H., et al. Eldecalcitol Causes FGF23 Resistance for Pi Reabsorption and Improves Rachitic Bone Phenotypes in the Male Hyp Mouse. Endocrinology. 2018;159:2741–2758. doi: 10.1210/en.2018-00109. [DOI] [PubMed] [Google Scholar]
- 101.Harris M.M., Houtkooper L.B., Stanford V.A., Parkhill C., Weber J.L., Flint-Wagner H., Weiss L., Going S.B., Lohman T.G. Dietary iron is associated with bone mineral density in healthy postmenopausal women. J. Nutr. 2003;133:3598–3602. doi: 10.1093/jn/133.11.3598. [DOI] [PubMed] [Google Scholar]
- 102.Qasem W., Azad M.B., Hossain Z., Azad E., Jorgensen S., Castillo San Juan S., Cai C., Khafipour E., Beta T., Roberts L.J., 2nd, et al. Assessment of complementary feeding of Canadian infants: Effects on microbiome & oxidative stress, a randomized controlled trial. BMC Pediatr. 2017;17:54. doi: 10.1186/s12887-017-0805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seura T., Yoshino Y., Fukuwatari T. The Relationship between Habitual Dietary Intake and Gut Microbiota in Young Japanese Women. J. Nutr. Sci. Vitam. 2017;63:396–404. doi: 10.3177/jnsv.63.396. [DOI] [PubMed] [Google Scholar]
- 104.Fang S., Zhuo Z., Yu X., Wang H., Feng J. Oral administration of liquid iron preparation containing excess iron induces intestine and liver injury, impairs intestinal barrier function and alters the gut microbiota in rats. J. Trace Elem. Med. Biol. 2018;47:12–20. doi: 10.1016/j.jtemb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 105.Kortman G.A., Dutilh B.E., Maathuis A.J., Engelke U.F., Boekhorst J., Keegan K.P., Nielsen F.G., Betley J., Weir J.C., Kingsbury Z., et al. Microbial Metabolism Shifts Towards an Adverse Profile with Supplementary Iron in the TIM-2 In vitro Model of the Human Colon. Front. Microbiol. 2016;6:1481. doi: 10.3389/fmicb.2015.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Araya M., Morelli L., Reid G., Sanders M., Stanton C. Guidelines for the Evaluation of Probiotics in Food. FAO; WHO Working Group; London, ON, Canada: 2002. [(accessed on 18 December 2021)]. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. [Google Scholar]
- 107.Collins F.L., Rios-Arce N.D., Schepper J.D., Parameswaran N., McCabe L.R. The Potential of Probiotics as a Therapy for Osteoporosis. Microbiol. Spectr. 2017;5:4. doi: 10.1128/microbiolspec.BAD-0015-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rizzoli R., Biver E. Are Probiotics the New Calcium and Vitamin D for Bone Health? Curr. Osteoporos. Rep. 2020;18:273–284. doi: 10.1007/s11914-020-00591-6. [DOI] [PubMed] [Google Scholar]
- 109.Wieërs G., Belkhir L., Enaud R., Leclercq S., Philippart de Foy J.M., Dequenne I., de Timary P., Cani P.D. How Probiotics Affect the Microbiota. Front. Cell Infect. Microbiol. 2020;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hill C., Guarner F., Reid G. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 111.Markowiak P., Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Satokari R. Modulation of Gut Microbiota for Health by Current and Next-Generation Probiotics. Nutrients. 2019;8:1921. doi: 10.3390/nu11081921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Britton R.A., Irwin R., Quach D., Schaefer L., Zhang J., Lee T., Parameswaran N., McCabe L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell Physiol. 2014;229:1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiang S.S., Pan T.M. Antiosteoporotic effects of Lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J. Agric. Food Chem. 2011;59:7734–7742. doi: 10.1021/jf2013716. [DOI] [PubMed] [Google Scholar]
- 115.Narva M., Rissanen J., Halleen J., Vapaatalo H., Vaananen K., Korpela R. Effects of bioactive peptide, valyl-prolyl-proline (VPP), and Lactobacillus helveticus fermented milk containing VPP on bone loss in ovariectomized rats. Ann. Nutr. Metab. 2007;51:65–74. doi: 10.1159/000100823. [DOI] [PubMed] [Google Scholar]
- 116.Ohlsson C., Engdahl C., Fak F., Andersson A., Windahl S.H., Farman H.H., Movérare-Skrtic S., Islander U., Sjögren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE. 2014;9:e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malmir H., Ejtahed H.S., Soroush A.R., Mortazavian A.M., Fahimfar N., Ostovar A., Esmaillzadeh A., Larijani B., Hasani-Ranjbar S. Probiotics as a New Regulator for Bone Health: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2021;2021:3582989. doi: 10.1155/2021/3582989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schepper J.D., Collins F., Rios-Arce N.D., Kang H.J., Schaefer L., Gardinier J.D., Raghuvanshi R., Quinn R.A., Britton R., Parameswaran N., et al. Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis. J. Bone Min. Res. 2020;35:801–820. doi: 10.1002/jbmr.3947. [DOI] [PubMed] [Google Scholar]
- 119.Bennett C.N., Longo K.A., Wright W.S., Suva L.J., Lane T.F., Hankenson K.D., MacDougald O.A. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jafarnejad S., Djafarian K., Fazeli M.R., Yekaninejad M.S., Rostamian A., Keshavarz S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J. Am. Coll. Nutr. 2017;36:497–506. doi: 10.1080/07315724.2017.1318724. [DOI] [PubMed] [Google Scholar]
- 121.Lambert M.N.T., Thybo C.B., Lykkeboe S., Rasmussen L.M., Frette X., Christensen L.P., Jeppesen P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017;106:909–920. doi: 10.3945/ajcn.117.153353. [DOI] [PubMed] [Google Scholar]
- 122.Nilsson A.G., Sundh D., Bäckhed F., Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018;284:307–317. doi: 10.1111/joim.12805. [DOI] [PubMed] [Google Scholar]
- 123.Seely K.D., Kotelko C.A., Douglas H., Bealer B., Brooks A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021;22:9452. doi: 10.3390/ijms22179452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Felizardo R.J.F., Watanabe I.K.M., Dardi P., Rossoni L.V., Câmara N.O.S. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacol. Res. 2019;141:366–377. doi: 10.1016/j.phrs.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 125.Gimigliano F., Moretti A., de Sire A., Calafiore D., Iolascon G. The combination of vitamin D deficiency and overweight affects muscle mass and function in older post-menopausal women. Aging Clin. Exp. Res. 2018;30:625–631. doi: 10.1007/s40520-018-0921-1. [DOI] [PubMed] [Google Scholar]
- 126.Quach D., Britton R.A. Gut Microbiota and Bone Health. Adv. Exp. Med. Biol. 2017;1033:47–58. doi: 10.1007/978-3-319-66653-2_4. [DOI] [PubMed] [Google Scholar]
- 127.de Sire R., Talocco C., Petito V., Lopetuso L.R., Graziani C., Gasbarrini A., Scaldaferri F. Microbiota and inflammatory bowel disease: An update. Recenti Progress. Med. 2018;109:570–573. doi: 10.1701/3082.30741. [DOI] [PubMed] [Google Scholar]
- 128.de Sire A., de Sire R., Petito V., Masi L., Cisari C., Gasbarrini A., Scaldaferri F., Invernizzi M. Gut-Joint Axis: The Role of Physical Exercise on Gut Microbiota Modulation in Older People with Osteoarthritis. Nutrients. 2020;12:574. doi: 10.3390/nu12020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Biver E., Berenbaum F., Valdes A.M., Araujo de Carvalho I., Bindels L.B., Brandi M.L., Calder P.C., Castronovo V., Cavalier E., Cherubini A., et al. Gut microbiota and osteoarthritis management: An expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO) Ageing Res. Rev. 2019;55:100946. doi: 10.1016/j.arr.2019.100946. [DOI] [PubMed] [Google Scholar]
- 130.de Sire R., Rizzatti G., Ingravalle F., Pizzoferrato M., Petito V., Lopetuso L., Graziani C., de Sire A., Mentella M.C., Mele M.C., et al. Skeletal muscle-gut axis: Emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva Gastroenterol. Dietol. 2018;64:351–362. doi: 10.23736/S1121-421X.18.02511-4. [DOI] [PubMed] [Google Scholar]
- 131.Pizzoferrato M., de Sire R., Ingravalle F., Mentella M.C., Petito V., Martone A.M., Landi F., Miggiano G., Mele M.C., Lopetuso L.R., et al. Characterization of Sarcopenia in an IBD Population Attending an Italian Gastroenterology Tertiary Center. Nutrients. 2019;11:2281. doi: 10.3390/nu11102281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nardone O.M., de Sire R., Petito V., Testa A., Villani G., Scaldaferri F., Castiglione F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021;12:694217. doi: 10.3389/fimmu.2021.694217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ianiro G., Bibbò S., Gasbarrini A., Cammarota G. Therapeutic modulation of gut microbiota: Current clinical applications and future perspectives. Curr. Drug Targe. 2014;15:762–770. doi: 10.2174/1389450115666140606111402. [DOI] [PubMed] [Google Scholar]
- 134.Cammarota G., Ianiro G., Bibbò S., Gasbarrini A. Gut microbiota modulation: Probiotics, antibiotics or fecal microbiota transplantation? Intern. Emerg. Med. 2014;9:365–373. doi: 10.1007/s11739-014-1069-4. [DOI] [PubMed] [Google Scholar]
- 135.Mullish B.H., Quraishi M.N., Segal J.P., Ianiro G., Iqbal T.H. The gut microbiome: What every gastroenterologist needs to know. Frontline Gastroenterol. 2020;12:118–127. doi: 10.1136/flgastro-2019-101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Milani C., Ferrario C., Turroni F., Duranti S., Mangifesta M., van Sinderen D., Ventura M. The human gut microbiota and its interactive connections to diet. J. Hum. Nutr. Diet. 2016;29:539–546. doi: 10.1111/jhn.12371. [DOI] [PubMed] [Google Scholar]
- 137.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;1:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murphy E.A., Velazquez K.T., Herbert K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:515–520. doi: 10.1097/MCO.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Graziani C., Talocco C., de Sire R., Petito V., Lopetuso L.R., Gervasoni J., Persichilli S., Franceschi F., Ojetti V., Gasbarrini A., et al. Intestinal permeability in physiological and pathological conditions: Major determinants and assessment modalities. Eur. Rev. Med. Pharm. Sci. 2019;23:795–810. doi: 10.26355/eurrev_201901_16894. [DOI] [PubMed] [Google Scholar]
- 140.Li L., Rao S., Cheng Y., Zhuo X., Deng C., Xu N., Zhang H., Yang L. Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. MicrobiologyOpen. 2019;8:e00810. doi: 10.1002/mbo3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ticinesi A., Lauretani F., Milani C., Nouvenne A., Tana C., Del Rio D., Maggio M., Ventura M., Meschi T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients. 2017;30:1303. doi: 10.3390/nu9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rizzoli R., Biver E., Brennan-Speranza T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9:606–621. doi: 10.1016/S2213-8587(21)00119-4. [DOI] [PubMed] [Google Scholar]
- 143.Fong W., Li Q., Yu J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39:4925–4943. doi: 10.1038/s41388-020-1341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dewulf E.M., Cani P.D., Claus S.P., Fuentes S., Puylaert P.G., Neyrinck A.M., Bindels L.B., de Vos W.M., Gibson G.R., Thissen J.P., et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112–1121. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Azcarate-Peril M.A., Ritter A.J., Savaiano D., Monteagudo-Mera A., Anderson C., Magness S.T., Klaenhammer T.R. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc. Natl. Acad. Sci. USA. 2017;114:E367–E375. doi: 10.1073/pnas.1606722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maier T.V., Lucio M., Lee L.H., VerBerkmoes N.C., Brislawn C.J., Bernhardt J., Lamendella R., McDermott J.E., Bergeron N., Heinzmann S.S., et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio. 2017;8:e01343-17. doi: 10.1128/mBio.01343-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shoaf K., Mulvey G.L., Armstrong G.D., Hutkins R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006;74:6920–6928. doi: 10.1128/IAI.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Monteagudo-Mera A., Rastall R.A., Gibson G.R., Charalampopoulos D., Chatzifragkou A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019;103:6463–6472. doi: 10.1007/s00253-019-09978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Orchard T.S., Larson J.C., Alghothani N., Bout-Tabaku S., Cauley J.A., Chen Z., LaCroix A.Z., Wactawski-Wende J., Jackson R.D. Magnesium intake, bone mineral density, and fractures: Results from the Women’s Health Initiative Observational Study. Am. J. Clin. Nutr. 2014;99:926–933. doi: 10.3945/ajcn.113.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alexy U., Remer T., Manz F., Neu C.M., Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am. J. Clin. Nutr. 2005;82:1107–1114. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- 151.Durosier-Izart C., Biver E., Merminod F., van Rietbergen B., Chevalley T., Herrmann F.R., Ferrari S.L., Rizzoli R. Peripheral skeleton bone strength is positively correlated with total and dairy protein intakes in healthy postmenopausal women. Am. J. Clin. Nutr. 2017;105:513–525. doi: 10.3945/ajcn.116.134676. [DOI] [PubMed] [Google Scholar]
- 152.Langsetmo L., Peters K.W., Burghardt A.J., Ensrud K.E., Fink H.A., Cawthon P.M., Cauley J.A., Schousboe J.T., Barrett-Connor E., Orwoll E.S., et al. Volumetric bone mineral density and failure load of distal limbs predict incident clinical fracture independent HR-pQCT BMD and failure load predicts incident clinical fracture of FRAX and clinical risk factors among older men. J. Bone Min. Res. 2018;33:1302–1311. doi: 10.1002/jbmr.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wegh C.A.M., Geerlings S.Y., Knol J., Roeselers G., Belzer C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019;20:4673. doi: 10.3390/ijms20194673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Montazeri-Najafabady N., Ghasemi Y., Dabbaghmanesh M.H., Ashoori Y., Talezadeh P., Koohpeyma F., Abootalebi S.N., Gholami A. Exploring the bone sparing effects of postbiotics in the post-menopausal rat model. BMC Complement. Med. Ther. 2021;21:155. doi: 10.1186/s12906-021-03327-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jang A.R., Park J.S., Kim D.K., Park J.Y., Ahn J.H., Kim D.Y., Lee T.S., Chang J.Y., Choi J.H., Park J.H. Cell-free culture supernatant of Lactobacillus curvatus Wikim38 inhibits RANKL-induced osteoclast differentiation and ameliorates bone loss in ovariectomized mice. Lett. Appl. Microbiol. 2021;73:383–391. doi: 10.1111/lam.13525. [DOI] [PubMed] [Google Scholar]
- 156.Ianiro G., Tilg H., Gasbarrini A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut. 2016;65:1906–1915. doi: 10.1136/gutjnl-2016-312297. [DOI] [PubMed] [Google Scholar]
- 157.Cho I., Yamanishi S., Cox L., Methé B.A., Zavadil J., Li K., Gao Z., Mahana D., Raju K., Teitler I., et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cox L.M., Yamanishi S., Sohn J., Alekseyenko A.V., Leung J.M., Cho I., Kim S.G., Li H., Gao Z., Mahana D., et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Williams S., Wakisaka A., Zeng Q.Q., Barnes J., Martin G., Wechter W.J., Liang C.T. Minocycline prevents the decrease in bone mineral density and trabecular bone in ovariectomized aged rats. Bone. 1996;19:637–644. doi: 10.1016/S8756-3282(96)00302-X. [DOI] [PubMed] [Google Scholar]
- 160.Bibbò S., Ianiro G., Gasbarrini A., Cammarota G. Fecal microbiota transplantation: Past, present and future perspectives. Minerva Gastroenterol. Dietol. 2017;63:420–430. doi: 10.23736/S1121-421X.17.02374-1. [DOI] [PubMed] [Google Scholar]
- 161.Waller K.M.J., Leong R.W., Paramsothy S. An update on FMT for the treatment of gastrointestinal diseases. J. Gastroenterol. Hepatol. 2021 doi: 10.1111/jgh.15731. online ahead of print . [DOI] [PubMed] [Google Scholar]
- 162.Ma S., Wang N., Zhang P., Wu W., Fu L. Fecal microbiota transplantation mitigates bone loss by improving gut microbiome composition and gut barrier function in aged rats. PeerJ. 2021;9:e12293. doi: 10.7717/peerj.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Biver E., Pepe J., de Sire A., Chevalley T., Ferrari S. Associations between radius low-frequency axial ultrasound velocity and bone fragility in elderly men and women. Osteoporos. Int. 2019;30:411–421. doi: 10.1007/s00198-018-4725-0. [DOI] [PubMed] [Google Scholar]
- 164.Bone H.G., Wagman R.B., Brandi M.L., Brown J.P., Chapurlat R., Cummings S.R., Czerwiński E., Fahrleitner-Pammer A., Kendler D.L., Lippuner K., et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 165.Iolascon G., de Sire A., Curci C., Paoletta M., Liguori S., Calafiore D., Gimigliano F., Moretti A. Osteoporosis guidelines from a rehabilitation perspective: Systematic analysis and quality appraisal using AGREE II. Eur. J. Phys. Rehabil. Med. 2021;57:273–279. doi: 10.23736/S1973-9087.21.06581-3. [DOI] [PubMed] [Google Scholar]
- 166.Yahiro Y., Maeda S., Morikawa M., Koinuma D., Jokoji G., Ijuin T., Komiya S., Kageyama R., Miyazono K., Taniguchi N. BMP-induced Atoh8 attenuates osteoclastogenesis by suppressing Runx2 transcriptional activity and reducing the Rankl/Opg expression ratio in osteoblasts. Bone Res. 2020;8:32. doi: 10.1038/s41413-020-00106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moretti A., de Sire A., Curci C., Toro G., Gimigliano F., Iolascon G. Effectiveness of denosumab on back pain-related disability and quality-of-life in patients with vertebral fragility fractures. Curr. Med. Res. Opin. 2019;35:151–155. doi: 10.1080/03007995.2018.1545636. [DOI] [PubMed] [Google Scholar]
- 168.Migliaccio S., Francomano D., Romagnoli E., Marocco C., Fornari R., Resmini G., Buffa A., Di Pietro G., Corvaglia S., Gimigliano F., et al. Persistence with denosumab therapy in women affected by osteoporosis with fragility fractures: A multicenter observational real practice study in Italy. J. Endocrinol. Investig. 2017;40:1321–1326. doi: 10.1007/s40618-017-0701-3. [DOI] [PubMed] [Google Scholar]
- 169.Lin T.H., Yang R.S., Tu H.J., Liou H.C., Lin Y.M., Chuang W.J., Fu W.M. Inhibition of osteoporosis by the αvβ3 integrin antagonist of rhodostomin variants. Eur. J. Pharmacol. 2017;804:94–101. doi: 10.1016/j.ejphar.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 170.Zur Y., Rosenfeld L., Keshelman C.A., Dalal N., Guterman-Ram G., Orenbuch A., Einav Y., Levaot N., Papo N. A dual-specific macrophage colony-stimulating factor antagonist of c-FMS and αvβ3 integrin for osteoporosis therapy. PLoS Biol. 2018;16:e2002979. doi: 10.1371/journal.pbio.2002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fan G., Zhao Q., Lu P., Chen H., Tan W., Guo W., Liu C., Liu J. Comparison between teriparatide and bisphosphonates for improving bone mineral density in postmenopausal osteoporosis patients: A meta-analysis. Medicine. 2020;99:e18964. doi: 10.1097/MD.0000000000018964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lau E., Dinavahi R., Woo Y.C., Wu C.H., Guan J., Maddox J., Tolman C., Yang W., Shin C.S. Romosozumab or alendronate for fracture prevention in East Asian patients: A subanalysis of the phase III, randomized ARCH study. Osteoporos. Int. 2020;31:677–685. doi: 10.1007/s00198-020-05324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gu H., Shi S., Xiao F., Huang Z., Xu J., Chen G., Zhou K., Lu L., Yin X. MiR-1-3p regulates the differentiation of mesenchymal stem cells to prevent osteoporosis by targeting secreted frizzled-related protein 1. Bone. 2020;137:115444. doi: 10.1016/j.bone.2020.115444. [DOI] [PubMed] [Google Scholar]
- 174.Yan H., Baldridge M.T., King K.Y. Hematopoiesis and the bacterial microbiome. Blood. 2018;132:559–564. doi: 10.1182/blood-2018-02-832519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhong X., Zhang F., Yin X., Cao H., Wang X., Liu D., Chen J., Chen X. Bone Homeostasis and Gut Microbial-Dependent Signaling Pathways. J. Microbiol. Biotechnol. 2021;31:765–774. doi: 10.4014/jmb.2104.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guo D., Liu W., Zhang X., Zhao M., Zhu B., Hou T., He H. Duck Egg White-Derived Peptide VSEE (Val-Ser-Glu-Glu) Regulates Bone and Lipid Metabolisms by Wnt/β-Catenin Signaling Pathway and Intestinal Microbiota. Mol. Nutr. Food Res. 2019;63:e1900525. doi: 10.1002/mnfr.201900525. [DOI] [PubMed] [Google Scholar]
- 177.Goodrich J.K., Di Rienzi S.C., Poole A.C., Koren O., Walters W.A., Caporaso J.G., Knight R., Ley R.E. Conducting a microbiome study. Cell. 2014;158:250–262. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.