Abstract
Simple Summary
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and deadliest cancer worldwide, with an overall survival (OS) rate, all stages combined, of still <10% at 5 years. For most patients with unresectable or recurrent PDAC, few therapeutic options are available with limited efficacy (median OS ≤ 12 months). Moreover, immune checkpoint inhibitors that have been tested so far in PDAC failed to improve patient survival. This resistance of PDAC to therapies is, in part, related to the dense tumor stroma, which creates a mechanical barrier around the tumor cells and generates high interstitial pressure, preventing appropriate vascularization and thus limiting exposure to treatments. In that PDAC tumoral microenvironment, the inflammatory cell infiltrates are unbalanced toward an immunosuppressive over pro-inflammatory phenotype. Therefore, there is an urgent need to better define the composition of PDAC stroma and key immune players in order to identify new therapeutic targets and strategies.
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and deadliest cancer worldwide with an overall survival rate, all stages combined, of still <10% at 5 years. The poor prognosis is attributed to challenges in early detection, a low opportunity for radical resection, limited response to chemotherapy, radiotherapy, and resistance to immune therapy. Moreover, pancreatic tumoral cells are surrounded by an abundant desmoplastic stroma, which is responsible for creating a mechanical barrier, preventing appropriate vascularization and leading to poor immune cell infiltration. Accumulated evidence suggests that PDAC is impaired with multiple “immune defects”, including a lack of high-quality effector cells (CD4, CD8 T cells, dendritic cells), barriers to effector cell infiltration due to that desmoplastic reaction, and a dominance of immune cells such as regulatory T cells, myeloid-derived suppressor cells, and M2 macrophages, resulting in an immunosuppressive tumor microenvironment (TME). Although recent studies have brought new insights into PDAC immune TME, its understanding remains not fully elucidated. Further studies are required for a better understanding of human PDAC immune TME, which might help to develop potent new therapeutic strategies by correcting these immune defects with the hope to unlock the resistance to (immune) therapy. In this review, we describe the main effector immune cells and immunosuppressive actors involved in human PDAC TME, as well as their implications as potential biomarkers and therapeutic targets.
Keywords: pancreatic ductal adenocarcinoma, tumoral microenvironment, immune cells, desmoplastic stroma
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and deadliest cancer worldwide, with a steadily increasing incidence, especially in industrialized countries [1,2]. Projections indicate that PDAC will be a leading cause of cancer mortality in Europe [1] and the United States [3] by 2030–2040. This increase in incidence is not fully explained by “classical” PDAC risk factors (i.e., age, tobacco smoking, diabetes mellitus, obesity/overweight, chronic pancreatitis, and genetics/family history). It also remains a major challenge in digestive oncology due to its high lethality despite many explored therapeutic strategies, with overall survival (OS) rate, all stages combined, of still <10% at 5 years [4]. Several well-established factors may account for this poor prognosis. First, curative surgical management of PDAC is not possible at the time of diagnosis for 85% of patients, owing to vascular invasion (locally advanced disease) or distant metastases [5]. Moreover, even when resectable, PDAC recurrence is often observed within the first two years after complete resection due to micrometastatic disease (present in 90% of patients), justifying systematic administration of adjuvant therapy in all resected patients [6]. For patients with unresectable or recurrent PDAC, therapeutic options are mainly based on combined chemotherapy regimens (FOLFIRINOX or Gemcitabine + nab-paclitaxel as first-line therapies) with limited efficacy (median OS ≤ 12 months) [6,7]. Finally, all immune therapies that have been tested so far in PDAC failed to improve patient survival. This resistance of PDAC to conventional therapies is, in part, related to the desmoplastic reaction, also known as stroma, that characterizes these tumors [8]. Indeed, this histopathological hallmark of PDAC is found in both primary PDAC and its metastases [9]. PDAC stroma is composed of extracellular matrix (ECM) and several cell types including cancer-associated fibroblasts (CAFs, which are responsible for ECM production) and inflammatory immune cells [10]. The dense ECM deposits create a mechanical barrier around the tumor cells and generate high interstitial pressure, preventing appropriate vascularization and thus limiting exposure to chemotherapy, leading to a hypoxic tumor microenvironment (TME) [11]. In this hypoxic TME, the inflammatory cell infiltrates are unbalanced toward an immunosuppressive over pro-inflammatory phenotype, with a high prevalence of myeloid-derived suppressor cells (MDSCs), M2 polarized macrophages, and regulatory T cells (Tregs), over M1 macrophages, dendritic cells, and effector CD4+ and CD8+ T lymphocytes [12].
Overall, PDACs are immune deserts or immune-excluded tumors, which can explain why immune checkpoint inhibitors (ICIs) and other immune approaches still did not show efficacy in PDAC [13].
In this review, we summarize current acquired knowledge about the immune landscape in human PDAC and its impact on survival and therapy in patients with PDAC.
2. The Key Role of Cancer-Associated Fibroblasts in PDAC Immune Macroenvironment
CAFs, through their ECM production and interactions with tumor and other stromal cells, play the central role of “orchestrators” of the PDAC microenvironment. Several cells of origin for CAFs have been proposed, including pancreatic stellate cells (PSCs). PSCs are a unique type of resident pancreatic cells that exist in normal pancreatic tissue in a quiescent form [14]. PSCs are engaged in several homeostatic roles under physiological conditions [15] (i.e., vitamin A storage, phagocytosis, immunity, and stimulation of amylase secretion from the exocrine pancreas) and also under pathological states as pancreatitis or PDAC in which they become activated [16,17]. Upon activation, they acquire a myofibroblastic, contractile phenotype (expressing alpha-smooth actin) and produce soluble factors (growth factors, cytokines) and ECM components [18].
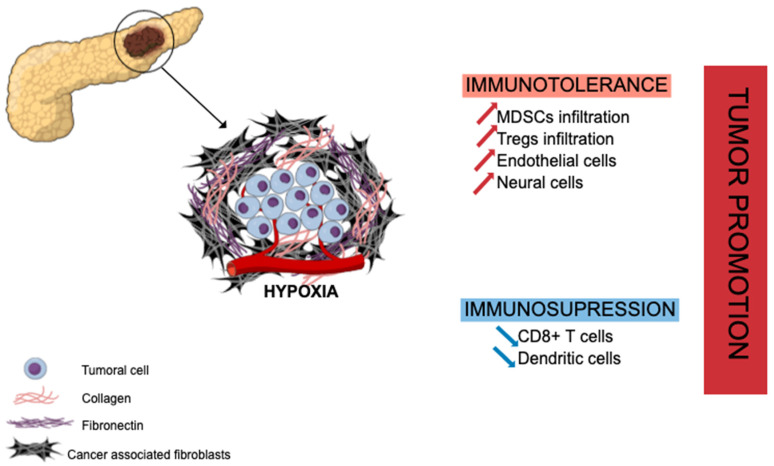
PDAC has a unique fibrotic TME, also known as desmoplastic stroma, illustrated in Figure 1 hereafter. This stroma is abundant (often representing the majority of tumor volume) and mainly composed of ECM proteins produced by CAFs (e.g., collagen, laminin, fibronectin) along with non-collagenous molecules (e.g., glycoprotein, proteoglycans, glycosaminoglycans, hyaluronic acid) [19]. This ECM enclosed various cell types, including different immune cells such as lymphocytes (rare), macrophages, mast cells, and myeloid-derived suppressor cells (MDSCs), along with endothelial and neuronal cells, to set up a growth permissive and immunotolerant microenvironment for pre-neoplastic pancreatic cells [20] (PanINs) and for pancreatic tumor cells [21]. The ECM is a dense meshwork of structural proteins, adaptor proteins, proteoglycans, and enzymes found in all tissues, where it provides biochemical and structural support for tissue homeostasis. In PDAC, there is a marked increase in the deposition of ECM [22]. Specifically, type I, III, and IV collagens are the main structural proteins constituting PDAC ECM. Pancreatic cancer cells induce desmoplastic responses within the tumor stroma by stimulating stromal fibroblasts to upregulate the expression of collagen family proteins and fibronectin in a paracrine manner [23]. Despite the hypoxia and hyaluronan-induced development of desmoplastic stroma, PDAC TME is composed of several immune cell populations that we will describe hereinafter. At early stages, effector cells such as natural killer (NK) cells, CD8+ T cells, and CD4+ T cells can be present and activated. Nevertheless, during the selection of resistant tumor cells and the development of the escape mechanism, PDAC TME induces the recruitment of monocytes and neutrophils, which then have acquired an anti-inflammatory phenotype [24]. The transformation of pro-inflammatory to anti-inflammatory in the TME increases the tumor growth and angiogenesis and correlates with poor survival, as well as systemic immunosuppression and malnutrition in patients.
Figure 1.
Extracellular matrix in pancreatic ductal adenocarcinoma and tumor growth promotion. Abundant stroma is mainly composed of cancer-associated fibroblasts (CAFs) and collagenous proteins leading to hypoxia, immune evasion, and immunotolerant state through recruitment of immunosuppressive cells (MDSCs, Tregs) and suppressive effect on effector immune cells (CD8+ T cells, dendritic cells). MDSCs: myeloid-derived suppressor cells; Tregs: regulatory T cells.
In PDAC, CAFs are strongly involved in the mechanism of immune evasion and the immunotolerant state toward pancreatic tumor cells through the cytokine-induced (IL-6, IL-11, TGF-β signaling) recruitment of immunosuppressive cells such as regulatory T cells (Treg cells) [21], myeloid-derived suppressor cells (MDSCs) [25,26] or programed death 1 ligand (PD-L1) mainly expressed on T cells [27]. CAFs and the hypoxia that they generate are also associated with a suppressive effect on effector immune cells as CD8+ T cells [28] or dendritic cells [29,30], hampering their infiltration into PDAC tumor areas. However, recent studies have highlighted that PDAC CAFs are heterogeneous, some of them being pro- and other anti-tumoral [31,32]. Several classifications have been proposed [33]. Therefore, a better understanding of CAF molecular and functional heterogeneity is required.
CAFs are involved in a growth-supportive TME in PDAC, especially through their complex interactions with immune cells, leading to an immunotolerant and unique immunosuppressive state. From a therapeutic perspective, despite the preclinical success of several agents targeting ECM (e.g., metalloproteinases inhibitors, hyaluronidase (PEGPH20)), this strategy failed to show any clinical activity in patients with advanced-stage PDAC [11]. A better understanding of those complex interactions might be useful to develop more effective immunotherapies against PDAC. Targeting the ECM alone is not effective in pancreatic cancer.
3. Effector Immune Cells in PDAC
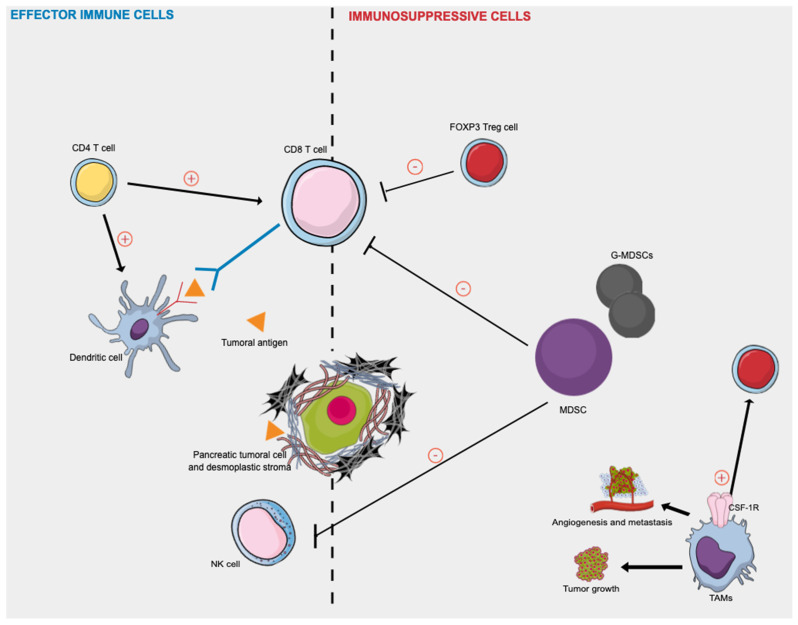
Figure 2 presented afterward summarizes the key effector immune cells and immunosuppressive actors in human PDAC.
Figure 2.
Effector immune cells and immunosuppressive cells in human pancreatic cancer. Activated CD8+ T cells attack tumor cells presenting tumor-associated antigens peptides on their surface, as dendritic cells. CD4+ T cells target tumor cells either directly by eliminating tumor cells through cytolytic mechanisms or indirectly by modulating the TME. Tregs and MDSCs cells play immunosuppressive roles in inhibiting the immune response against PDAC cells by directly inhibiting the anti-tumor functions of T and NK cells. The TAM phenotype is a consequence of the continuous presence of growth factors such as colony-stimulating factor-1 (CSF1) and its receptor (CSF-1R). TAMs release various growth factors and cytokines and promote tumor cell invasion, induce angiogenesis, suppress antitumor immunity, and facilitate tumor cell metastasis. CSF-1R: colony-stimulating factor-1 receptor; MDSC: myeloid-derived suppressor cells; NK: natural killer; TAMs = tumor-associated macrophages; Treg = regulatory T cell.
3.1. Tumor-Infiltrating Lymphocytes: CD8+ T and CD4+ T Cells
Tumor-infiltrating lymphocytes (TILs) are often observed in resected cancer tissue and are believed to participate in the host immune response against cancer [34,35]. They can be organized in tertiary lymphoid structures, which have been shown to have a positive prognostic impact [36].
Some TILs might be involved in restraining tumor development and progression. Intratumoral T cell infiltrates were shown to form an independent positive prognostic marker in colorectal cancer (CRC) [37] and also PDAC [38]. It could predict patients’ survival more efficiently than the TNM staging system (Immunoscore staging system) [37]. The most studied TILs are CD8+ T cells and CD4+ T cells, presented in Figure 2.
CD8+ T cells (cytotoxic T cells) are believed to identify cancer as a foreign body in a major histocompatibility complex class I-restricted manner [39]. Activated CD8+ T cells attack tumor cells presenting tumor-associated antigens peptides on their surface [39,40]. High levels of tumor infiltration with especially CD8+ lymphocytes are a characteristic of an immunogenically hot tumor, which better respond to immune checkpoint inhibitors [41].
CD4+ T cells play major roles in the host immune response against cancer and are required for efficacious antitumor immunity [42]. CD4+ T cells can target tumor cells in various ways, either directly by eliminating tumor cells through cytolytic mechanisms (perforin/granzyme B-dependent manner) or indirectly by modulating the TME [43].
In PDAC, total T lymphocytes (CD3) have been investigated in several studies [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. It is estimated that >80% of the CD3+ T cells are either CD4+ or CD8+ in tumoral pancreatic tissues [55]. Among those studies, CD3+ TIL rate was correlated to patient survival in 10 of them. High CD3+ TIL rate was significantly associated with better disease-free survival (DFS) and/or OS in 6 studies [45,46,51,53,58,59]. A summary of the described studies assessing CD3+ cells in PDAC is reported in Table 1.
Table 1.
Studies assessing CD3+ T cells expression in pancreatic ductal adenocarcinoma and the correlation with survival data.
| Reference | Number of Patients | Statistical Significance |
|---|---|---|
| Ryschich et al. 2005 [44] | 46 | NS |
| Tewari et al. 2013 [45] | 81 | Significantly associated with improved OS |
| Chen et al. 2014 [46] | 63 | Significantly associated with improved OS |
| Helm et al. 2014 [47] | 42 | NS |
| Zhou et al. 2015 [51] | 158 | Significantly associated with improved OS (RR = 0.611) |
| Hwang et al. 2016 [52] | 30 | NS |
| Lundgren et al. 2016 [53] | 175 | Significantly associated with improved OS (HR = 0.42) |
| Lohneis et al. 2017 [56] | 165 | NS |
| Ino et al. 2019 [58] | 241 | Significantly associated with improved OS and DFS |
| Miksch et al. 2019 [59] | 57 | Significantly associated with improved OS and DFS |
DFS: disease-free survival; HR: hazard ratio; NS: not significant; OS: overall survival; RR: relative risk.
CD4+ and/or CD8+ TILs assessment have also been the topic of several studies in PDAC [35,39,44,45,46,48,50,52,54,56,57,58,59,61,62,63,64,65,66,67,68,69,70,71]. Indeed, CD8+ T cells are the most studied T effectors in PDAC. Considerable lines of evidence suggest that a high CD8+ T cells infiltration in PDAC tumoral tissue is significantly associated with a better DFS and/or OS [39,50,54,56,58,59,62,63,65,67,68,69,70,71]. In the largest series of 165 patients operated on for PDAC [56], a high number of CD8+ lymphocytes was significantly and independently associated with longer DFS and OS in the overall study population. Median DFS/OS were 7.4/18.1 months for patients with a low number of CD8+ intratumoral lymphocytes (defined as ≤ 42 per 1 mm tissue core) and 12.7/25.2 months for patients with high CD8+ TILs numbers [56]. A summary of the described studies assessing CD4+ and/or CD8+ cells is reported in Table 2.
Table 2.
Studies assessing CD4 and CD8 T-cell expression in pancreatic ductal adenocarcinoma and the correlation with survival data.
| Reference | TILs | Number of Patients | Statistical Significance |
|---|---|---|---|
| Fukunaga et al. 2004 [39] | CD4 + CD8 | 80 | CD4/8 (+/+) significantly associated with better OS 5 years OS CD4/8 (+/+) = 48.4% versus 4.6% in CD4/8 (−/−) patients |
| Ryschich et al. 2005 [44] | CD4 + CD8 | 46 | NS |
| Tewari et al. 2013 [45] | CD8 | 81 | NS |
| Chen et al. 2014 [46] | CD8 | 63 | NS |
| Tang et al. 2014 [68] | CD8 | 160 | CD8+ cells significantly associated with better OS (HR = 0.56) |
| Castino et al. 2015 [48] | CD8 | 104 | NS |
| Karakhanova et al. 2015 [69] | CD4 + CD8 | 92 | CD4/8 (+/+) significantly associated with better DFS and OS |
| Liu et al. 2015 [71] | CD8 | 92 | CD8+ cells significantly associated with better OS (low versus high CD8: mean 14.2 months versus 31.0 months) |
| Wartenberg et al. 2015 [50] | CD8 | 110 | Reduce CD8+ cells are significantly associated with worse prognoses |
| Diana A et al. 2016 [62] | CD8 | 145 | CD8+ cells significantly associated with better PFS (low versus high CD8: mean OS = 23.7 versus 33.8 months) |
| Hwang et al. 2016 [52] | CD4 + CD8 | 30 | NS |
| Balachandran et al. 2017 [54] | CD8 | 166 | CD8+ cells significantly associated with better OS |
| Carstens et al. 2013 [55] | CD4 + CD8 | 132 | NS |
| Knudsen et al. 2017 [64] | CD8 | 109 | NS |
| Lohneis et al. 2017 [56] | CD8 | 165 | CD8+ cells significantly associated with better OS and DFS |
| Wang Z et al. 2017 [70] | CD4 + CD8 | 90 | CD4/8 (+/+) significantly associated with better OSmedian OS CD4/8 (+/+) = 28 months versus 15 months in CD4/8 (−/−) patients |
| Nizri et al. 2018 [65] | CD8 | 66 | CD8+ cells significantly associated with better OS(low versus high CD8: mean OS = 24.3 versus 36.8 months) |
| Pu et al. 2018 [66] | CD8 | 90 | NS |
| Sideras et al. 2018 [67] | CD8 | 148 | CD8+ cells significantly associated with better OS |
| Tahkola et al. 2018 [57] | CD8 | 108 | NS |
| Danilova et al. 2019 [61] | CD8 | 33 | NS |
| Hou et al. 2019 [63] | CD8 | 86 | CD8+ cells significantly associated with better OS(low versus high CD8: median OS = 10.9 versus 25.8 months) |
| Ino et al. 2019 [57] | CD4 + CD8 | 241 | CD4/8 (+/+) significantly associated with better OS |
| Miksch et al. 2019 [58] | CD8 | 57 | CD8+ cells significantly associated with better OS and DFS |
DFS: disease-free survival; NS: not significant; OS: overall survival; PFS: progression-free survival; TILs: tumor-infiltrating lymphocytes.
Those data suggest that TILs, especially CD4+ and CD8+ T cells effectors, are interesting and easily measurable indicators of PDAC immune TME. However, most PDACs are not infiltrated by cytotoxic CD8+ T cells. Several factors may be involved: low immunogenicity, T cell exclusion by other immune cells (immunosuppressive Treg, MDSCs). Moreover, most “hot” PDAC tumors in terms of CD8+ T cells are also infiltrated by macrophages, which are able to inhibit their cytotoxic activity [13].
3.2. Dendritic Cells
Dendritic cells (DCs, S100+ cells) are a diverse group of specialized antigen-presenting cells (APCs) [72]. DCs are able to induce antigen-specific immune responses and play major roles in tumor immunity, especially myeloid DCs (mDCs, CD11b+ cells). They are able to recognize tumor-associated antigens in tumor tissue and generate tumor-specific immunity, as described in Figure 2 [73]. DCs are rare in PDAC TME and are mainly located in the stroma peripheral to the tumor [74]. For the last decade, DCs have been a subject of interest in the study of PDAC immune TME [75] and have emerged as the immune cells of choice for the development of a vaccine against PDAC [76]. It was shown that circulating DCs or high-level DCs in PDAC tissues were significantly associated with better OS, whatever PDAC stage at diagnosis (resectable or metastatic disease) [77,78,79]. In a group of 42 patients with resected PDAC, Yamamoto et al. revealed that the OS rate in the high tissue-DCs group (DCs ≥ 3.5) was significantly longer than that in the low tissue-DCs group (p = 0.038, 1/2/3 year OS: 91%/70%/35% versus 67%/41%/27%) [79]. It was also demonstrated that the number of PDAC-infiltrating DCs was significantly higher in PDAC with CD4+ and CD8+ TILs infiltration [39]. These data suggest that if both CD4+ and CD8+ TILs are found in PDAC tissue, all key components of an antitumor immune response are present, from the presentation of tumor-associated antigens by DCs to the interaction of helper T cells and cytotoxic T cells to attack PDAC tumor cells [39].
PDAC is characterized by the reduced number and function of DCs, which impact antigen presentation and contribute to immune tolerance. As DCs are the most potent professional antigen-presenting cells, several ongoing studies use DC-based vaccines or agents promoting DC maturation (e.g., CD40 agonists) as potential immune therapies in PDAC.
3.3. Innate Lymphoid Cells
Innate lymphoid cells (ILCs) represent a heterogeneous group of cells that derive from common lymphoid progenitors in the bone marrow [80]. Five major groups of ILCs have been defined on the basis of their cytokine production patterns and developmental transcription factor requirements: natural killer (NK) cells, group 1 ILCs (ILC1s), group 2 ILCs (ILC2s), group 3 ILCs (ILC3s), and lymphoid tissue-inducer (LTi) cells [80].
Natural killer cells (NK cells, CD56+, CD3−) are large granular lymphocytes that are key components of the innate immune system [81]. The role of NK cells remains poorly understood, compared with other lymphocytes, especially T and B cells. Activation of NK cells mainly relies on the balance of signals received from inhibitory and activating cell surface receptors. In different cancer types, NK cells demonstrated potent antitumoral cytotoxicity, even if they appear to be highly heterogeneous between cancer types [82,83,84]. In human PDAC, few data are available but suggest antitumor activity. Indeed, to our knowledge, only one positive correlation was demonstrated between the percentage of NK cells in peripheral blood and recurrence-free survival in a few patients (n = 19) with resectable PDAC [85]. Patients who exhibited high NK levels before surgery significantly had later disease recurrence.
ILC1s, ILC2s, and ILC3s resemble the corresponding T helper cell subsets (T helper 1 (TH1), TH2, and TH17 cells, respectively) and produce cytokines that shape both innate and adaptive immune responses [80]. Recently, it was demonstrated that ILC2s infiltrate human PDAC to activate tissue-specific tumor immunity [86]. Using microarrays from formalin-fixed, paraffin-embedded from 45 PDAC short-term survivors (OS < 3 years) and from formalin-fixed, paraffin-embedded from 51 PDAC long-term survivors (OS ≥ 3 years), authors highlighted the fact that tumor ICL2 (TILC2s) were enriched in rare long-term PDAC survivors with “hot” tumors (activated CD8+ T-cell-enriched types) when compared with short-term survivors with “cold” tumors [86]. Moreover, higher TILC2 frequencies correlated with longer survival [86].
Although ILC (and more specifically ILC2s) could emerge as novel anticancer immune cells and potential targets for PDAC immunotherapy, their prognostic and predictive potential remains to be determined. Further investigations are needed to better understand ILC(2) role in PDAC and to confirm ILC2s as potential anticancer immune cells for PDAC immunotherapy.
From a therapeutic perspective, enhancing T-cell effector functions could be a promising therapeutic approach against human PDAC. Recent advances in the field of antigen identification, T-cell biology, and gene therapy have enabled the development of promising strategies such as chimeric antigen receptor T-cell (CAR-T cell) therapy and T-cell receptor T-cell (TCR-T) therapy to redirect T-cell antigen specificity and enhance T-cell function (adoptive T-cell therapy (ATCT)). However, ATCT remains unproven in human PDAC.
4. Immunosuppressive TME in PDAC
Figure 2 summarizes the key effector immune cells and immunosuppressive actors in human PDAC.
4.1. Regulatory T Cells
Regulatory T cells (Tregs) are major players in tumor immune suppression in PDAC. They can be identified based on forkhead box protein 3 (FOXP3) protein expression and high levels of interleukin-2 receptor alpha chain CD25 through immunohistochemistry in tumor tissues. There is abundant evidence to suggest that Tregs represent the main obstacle to successful tumor immunotherapy [87]. Indeed, Tregs accumulate in tumors and the peripheral blood of patients with cancer. Tregs are recruited to tumors sites where they suppress antitumor cytotoxic response by binding to DCs and preventing them from activating CD8+ T cells [88], as summarized in Figure 2. It has been demonstrated that Tregs contribute to inhibiting the immune response against PDAC cells, from the premalignant stage to invasive PDAC [89]. Moreover, in PDAC, a high prevalence of Tregs seems to be associated with poor prognosis and poor PDAC differentiation [68]. Treg infiltration in PDAC has been well studied since the 2000s. Several studies demonstrated that Tregs FOXP3+ immunostaining could be used as a prognostic biomarker and that it could be a crucial determinant of immunosuppressive TME in PDAC [90]. High Treg infiltrates in PDAC tissues were significantly associated with worse survival rates in PDAC [50,91] and conversely, low Tregs infiltrate rates were associated with better survival [68,70,71].
Increased peritumoral FOXP3+T-cell (Tregs cells) density is identified as an independent adverse prognostic factor in PDAC [50].
4.2. Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) are defined as a heterogeneous population of immature myeloid cells (CD15+, CD11b+) that accumulate during chronic inflammatory conditions such as cancer [92]. They have critical roles in the TME through immunosuppression [93], as they can directly inhibit the antitumor functions of T and NK cells (Figure 2). Their critical implication in different cancer types has been demonstrated [94,95,96]. MDSCs can be characterized into two major types—monocytic MDSCs (M-MDSCs, CD66b−) and granulocytic MDSCs (G-MDSCs, CD15+, CD66b+). Several CXC families of chemokines have been attributed major roles in MDSC recruitment. In human PDAC, it was demonstrated that high levels of CXCL5 correlate with both tumor-infiltrating CD15+ granulocytes and worse prognosis in patients with PDAC [97,98]. Indeed, in a cohort of 153 patients with resected PDAC, mean OS was significantly higher in the low CXCL5 expression group (immunostaining ≤ 50%) in comparison with the high CXCL5-expression group (immunostaining > 50%) (64.0 months versus 38.5 months, respectively) [97].
Moreover, interleukin-6 (IL-6) has been associated with the enrichment of immunosuppressive MDSCs in several tumor types [92]. The role of MDSCs in human pancreatic TME has also been recently explored [99,100]. In patients with PDAC, it was shown that the plasmatic level of circulating MDSCs was correlated with the stage of the patient’s disease [100,101]. Comparing cancer-free controls and patients with PDAC, Porembka et al. demonstrated that normal pancreatic tissue was free of MDSC infiltration, whereas patients with PDAC have significantly increased levels of MDSCs in the bone marrow and peripheral blood, which are avidly recruited to the primary PDAC sites [100]. MDSC mobilization was significantly most notable in patients with metastatic disease when compared with both normal controls and patients with resectable pancreatic cancer (respectively, 68.2% ± 3.6% versus 37.6% ± 3.6% versus 57.3% ± 3.5; p < 0.05 %) [100].
In addition to their main role in the antitumor response, MDSCs might be able to influence the production of Tregs, leading to cancer immunotolerance [102].
Although further studies are needed to confirm those data, MDSCs might be useful markers for PDAC detection and/or progression. They also represent potential therapeutic targets in PDAC oncogenesis.
4.3. B Cells
B cells (CD20+) are among important components of the immune infiltrate in solid tumors and are emerging as critical regulators of cancer progression [103]. B cells are known to critically regulate T-cell immune responses, but their role in cancer remains controversial.
One study investigated the role of B cells in the tumoral microenvironment of human PDAC [48]. In that study, the authors demonstrated first of all that human PDAC tissues showed considerable infiltration of CD20+ B lymphocytes, unlike normal pancreatic tissue. Moreover, B cells’ location in PDAC seems to display distinct spatial organization in two histologically distinct compartments: either in ectopic lymph node-like structures (or tertiary lymphoid tissue, B-TLT) [104] or interspersed at the tumor–stroma interface (B-TIL) [48]. Among the 104 PDAC tissue specimens they studied, they revealed that B cells were independently associated with prognosis. However, their prognostic value diverged according to their spatial distribution in the tissue [48]. Indeed, B-TLT were associated with better prognosis (OR = 0.24; 95% CI (0.08–0.71); p = 0.010), while B-TIL were associated to worse prognosis (OR = 2.56; 95% CI (0.91–7.23); p = 0.07) [48]. This result highlights B cells as prognostic variables in human PDAC and suggests that the influence of B cells on tumor progression changes whether they are confined within lymphoid tissue or are scattered at the tumor–stroma interface.
In PDAC, B cell behaviors seem to be tightly linked to their topological organization within the microenvironment. Further research is needed to better understand their role in PDAC oncogenesis and pancreatic cancer progression.
4.4. Tumor-Associated Macrophages
Macrophages are among the most prominent tumor-associated noncancer cell type in the TME, known as tumor-associated macrophages (TAMs). Current evidence suggests that TAMs engage in complex network interactions with cancer stem cells, cancer cells, endothelial cells, fibroblasts, T cells, B cells, and NK cells, as presented in Figure 2 [105]. TAMs receive signals from diverse cells within the tumor microenvironment and release various growth factors and cytokines and promote tumor cell invasion, induce angiogenesis, suppress antitumor immunity, and facilitate tumor cell metastasis [106]. TAMs are classically classified into two subtypes: M1-polarized macrophages and M2-polarized macrophages, even though this classification is debated. M1-polarized macrophages are characterized by a pro-inflammatory phenotype [106] (i.e., enhanced expression and release of IL-1β, TNF-α, IL-6, or IL-12), whereas M2-polarized macrophages (CD204/206; CD163) exhibit anti-inflammatory properties [106] (i.e., enhanced expression and secretion of immunosuppressive cytokines such as TGF-β1 and IL-10). TAM expression has been studied in three different studies in human PDAC. Kurahara et al. first described an elevated incidence of TAMs, in particular with an M2-polarized phenotype in human PDAC tissues, correlating with increased nodal lymphangiogenesis and poor prognosis [23,107]. Based on tissue specimens from 40 patients with resected PDAC, the authors showed that the nodal lymphatic vessel density had a strong association with the M2-polarized TAM density in the regional lymph nodes (p < 0.0001) [23]. They also demonstrated that the 5-year survival rate in the low CD163/204 group and the high CD163/204 group was 28.8% and 5.0%, respectively, with the prognosis significantly poorer in the high CD163/204 group, compared with the low CD163/204 group [107]. More recently, the same observation was made based on 112 cases of surgically resected PDACs [108]. Long-term survival patients (OS ≥ 60 months) display significantly lower densities CD68+ cells (total macrophages) and CD163+ (M2) macrophages than short-term survival cases (OS < 60 months; p = < 0.0001, and < 0.0001, respectively) [108].
From a therapeutic perspective, neutralizing immunosuppressive cells, which are major components of human PDAC TME, could be a potential therapeutic strategy by removing inhibitory factors that constrain T-cell responses. Unfortunately, a phase II clinical study evaluating the inhibition of colony-stimulating factor-1 receptor (CSF-1R, cabiralizumab, M2-like macrophage/TAMs inhibition) with nivolumab and chemotherapy in advanced PDAC patients did not improve progression-free survival, compared with chemotherapy alone (NCT03336216).
Those results highlight the crucial role of tumor-associated macrophages (TAMs) in human PDAC. Such observations could bring new therapeutic hopes, especially in tumors with high infiltration of immunosuppressive TAMs, owing to macrophage targeted drugs (e.g., anti-CSF1R, anti-CXCR2/CCR2), but their activity is hampered by “rescue” secretion of overlapping cytokines.
5. Discussion
PDAC TME is a complex network that is still being actively explored. At the immune level, it corresponds to the interactions between different effectors whose functions are still poorly understood, although several studies have been carried out to demystify it, in order to find new therapeutic approaches.
In addition to tumor cell-intrinsic mechanisms, different extrinsic factors may impact the human PDAC TME through immunomodulation. One of the most studied factors and rapidly evolving fields is the microbiome, which also drives the tolerogenic innate and adaptive immune programs in PDAC [109]. The species of bacteria found in human PDAC are distinct from those in the gut, with a greater relative abundance of Proteobacteria and reduced α-diversity and richness [109]. Lower intratumoral microbial diversity has been linked to reduced survival, whereas high bacterial diversity correlates with long-term survival in PDAC [110]. Moreover, an intratumoral microbiome signature highly predictive of long-term survival (>5 years) was developed and validated by Riquelme et al. (Pseudoxanthomonas–Streptomyces–Saccharopolyspora–Bacillus clausii) [110]. The microbiome in primary PDAC may exert potent suppressive influences on the inflammatory TME, establishing the tumor-promoting inflammatory program in PDAC by activating the expansion of MDSCs and anti-inflammatory M2-like tumor-associated macrophages (TAMs). These tolerogenic innate immune cells promote the differentiation of suppressive populations of CD4+ T cells and prevent the expansion of cytotoxic CD8+ T cells [109]. This suggests that microbiome manipulation might have an excellent potential to overcome the current lack of an effective treatment for chemo-resistant PDAC. Additionally, microbiome manipulation (e.g., fecal microbiota transplantation (FMT)) has been shown to favorably affect the response of PDAC tumors to immunotherapy in animal models [111]. In those animal models, FMT was able to differentially modulate the intratumor microbiome diversity and immune infiltration, with an increase in cytotoxic T cells and a decrease in MDSCs with feces from long-term survivors [110]. Some studies have also linked fungi to the development of PDAC and need further research [112].
Future research efforts are needed to better understand the function and phylogeny of microbes in PDAC using more comprehensive approaches and develop innovative therapeutic strategies. Further studies are also required to determine how microbiota impacts chemotherapy and immunotherapy, in order to generate novel and personalized therapeutic approaches for PDAC patients. Other extrinsic factors as PDAC virome are still under exploration, as well as the role of antibiotics/fungal ablation, chemotherapies, inflammatory pathologies (e.g., pancreatitis, obesity), and physical activity.
Another major difficulty is that human PDAC TME is dynamic and changes with the different stages of pancreatic oncogenesis [113]. This immune landscape evolution has been well described during the progression of intraductal papillary mucinous neoplasm (IPMN) to invasive PDAC in humans [113]. Pancreatic immune TME evolves from a diverse T-cell mixture, comprising CD8+ T cells, T helper 1 (Th1, involved in cellular immunity), and Th2 (involved in humoral immunity) as major players combined with Th17 (which produce pro-inflammatory cytokine as IL-17) and Treg cells in low-grade IPMN, to a Treg dominated immunosuppressive state in human invasive PDAC [113]. Moreover, the localization of T cells drastically changes during the natural history of IPMN progression to human invasive PDAC, but this last issue currently remains insufficiently documented [47,57,113]. PDAC TME heterogeneity is currently the subject of intense research. The recently developed method of combining immunity-related genes with clinical characteristics has been applied to predict prognosis, recurrence, and response to gemcitabine-based therapy ± ICI in PDAC. This might be a practical predictive tool to identify PDAC subtype benefitting from gemcitabine-based chemotherapy or potentially responding to PD1/PD-L1 blockade therapy [114,115].
Multiple clinical trials concerning immune-based therapies in PDAC have recently been conducted or are currently underway, so far with limited success. Mostly T-cell effector functions have been targeted in the development of cancer immunotherapies, but endogenous reactive T cells are limited, in quantity and quality, in pancreatic cancer. In PDAC, different immunology-based therapies are under exploration, especially therapeutic vaccines aimed to stimulate T- and B-cell production, and ICI targeting CTLA4, PD1, and programmed death ligand (PDL1). However, ICI targeting these three molecules are mostly ineffective in PDAC. A summary of the main studies assessing ICI in advanced PDAC is reported in Table 3. One explanation for ICI resistance could be the composition of immune cell infiltrate with a PDAC TME having potent immunosuppressive functions, which might interfere with ICI [24]. Therefore, human PDAC is one of the most immune-resistant tumor types, due to immunosuppression and T-cell exclusion from the earliest stages of PDAC development, leading to primary resistance to ICI. Moreover, PDAC is a complex and heterogeneous disease, with diverse clinical phenotypes. Another key of PDAC development and promotion remains in genomics and epigenomics changes, conferring distinct molecular, cellular, and clinical features that determine considerable inter- and intratumoral heterogeneity.
Table 3.
Studies assessing immune checkpoint inhibitors in advanced pancreatic ductal adenocarcinoma.
| References | Trial Phase | Therapy | Number of Patients | Clinical Outcomes |
|---|---|---|---|---|
| Laheru et al. 2008 [117] | II | GVAX + cyclophosphamide | 50 | Median survival: 4.3 months |
| Royal et al. 2010 [118] | II | Anti CTLA4 antibody (Ipilimumab) |
27 | No objective response |
| Brahmer et al. 2012 [119] | I (NCT00729664) |
Anti PD-L1 antibody (Nivolumab) | 14 | No objective response |
| Le et al. 2013 [120] | Ib (NCT00836407) |
Ipilimumab + GVAX | 15 | Median OS: 5.7 months |
| Le et al. 2015 [121] | II (NCT01417000) |
GVAX + cyclophosphamide | 61 | Median OS: 9.7 months |
| O’Reilly et al. 2019 [122] | II (NCT02558894) |
Anti PD-L1 antibody (Durvalumab) + Anti CTLA4 antibody (Tremelimumab) |
65 | No objective response |
| Renouf et al. 2020 [123] | II (NCT02879318) |
Gembitabine + Nabpaclitaxel ± Durvalumab + Tremelimumab | 180 | No objective response |
GVAX: granulocyte-macrophage colony-stimulating factor (GM-CSF) gene-transfected tumor cell vaccine; OS: overall survival.
In order to better consider PDAC immune TME as a potential therapeutic target, there is an urgent need to better understand its evolution during PDAC oncogenesis and to gain access to it. The main obstacle remains that desmoplastic reaction around pancreatic tumor islets, which acts similarly to a major physical barrier, limiting access to treatments. Recent treatment strategies focusing on that dense stroma to facilitate the distribution of systemic agents within the tumor microenvironment failed to improve both overall survival and progression-free survival [116]. Combined therapeutic approaches associating TME remodeling with better immunogenicity (e.g., T-cell antigen specificity, enhancing T-cell effector function, immunosuppressive actors’ inhibition), coupled with therapies adapted to the (epi)genetic profile of the tumors, could present a promising perspective in human PDAC.
6. Conclusions
The immune landscape of human PDAC is inextricably linked to the abundant desmoplastic stroma. That dense extracellular matrix contributes to the poor immunogenicity of PDAC, which impedes effector T-cell infiltration and thus promotes an immunosuppressive microenvironment.
Moreover, other extrinsic factors, especially the PDAC microbiome, may impact immune TME in human PDAC through immunomodulation.
There is an urgent need to better understand the interactions between tumor cells, immune cells, microbiome, and stromal components of pancreatic cancer. Further refining our knowledge of those interactions will be crucial to improve therapeutic strategies for human PDAC in the future.
Author Contributions
Manuscript writing and editing, M.M., V.H., M.S., G.G., B.C., L.P.-B., J.-P.B., C.N., and A.L. Figures design, M.M. Review and approval of the manuscript, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors have no conflict of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Partensky C., Bray F. More Deaths from Pancreatic Cancer than Breast Cancer in the EU by 2017. Acta Oncol. 2016;55:1158–1160. doi: 10.1080/0284186X.2016.1197419. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L., Wehner M.R., Matrisian L.M., Nead K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open. 2021;4:e214708. doi: 10.1001/jamanetworkopen.2021.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold M., Rutherford M.J., Bardot A., Ferlay J., Andersson T.M.-L., Myklebust T.Å., Tervonen H., Thursfield V., Ransom D., Shack L., et al. Progress in Cancer Survival, Mortality, and Incidence in Seven High-Income Countries 1995–2014 (ICBP SURVMARK-2): A Population-Based Study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strobel O., Neoptolemos J., Jäger D., Büchler M.W. Optimizing the Outcomes of Pancreatic Cancer Surgery. Nat. Rev. Clin. Oncol. 2019;16:11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 6.Neuzillet C., Gaujoux S., Williet N., Bachet J.-B., Bauguion L., Colson Durand L., Conroy T., Dahan L., Gilabert M., Huguet F., et al. Pancreatic Cancer: French Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC) Dig. Liver Dis. 2018;50:1257–1271. doi: 10.1016/j.dld.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.-L., Gourgou-Bourgade S., de la Fouchardière C., et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Ebelt N.D., Zamloot V., Manuel E.R. Targeting Desmoplasia in Pancreatic Cancer as an Essential First Step to Effective Therapy. Oncotarget. 2020;11:3486–3488. doi: 10.18632/oncotarget.27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whatcott C.J., Diep C.H., Jiang P., Watanabe A., LoBello J., Sima C., Hostetter G., Shepard H.M., Von Hoff D.D., Han H. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin. Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vonlaufen A., Joshi S., Qu C., Phillips P.A., Xu Z., Parker N.R., Toi C.S., Pirola R.C., Wilson J.S., Goldstein D., et al. Pancreatic Stellate Cells: Partners in Crime with Pancreatic Cancer Cells. Cancer Res. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 11.Ho W.J., Jaffee E.M., Zheng L. The Tumour Microenvironment in Pancreatic Cancer—Clinical Challenges and Opportunities. Nat. Rev. Clin. Oncol. 2020;17:527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padoan A., Plebani M., Basso D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019;20:676. doi: 10.3390/ijms20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilmi M., Bartholin L., Neuzillet C. Immune Therapies in Pancreatic Ductal Adenocarcinoma: Where Are We Now? World J. Gastroenterol. 2018;24:2137–2151. doi: 10.3748/wjg.v24.i20.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erkan M., Adler G., Apte M.V., Bachem M.G., Buchholz M., Detlefsen S., Esposito I., Friess H., Gress T.M., Habisch H.-J., et al. StellaTUM: Current Consensus and Discussion on Pancreatic Stellate Cell Research. Gut. 2012;61:172–178. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allam A., Thomsen A.R., Gothwal M., Saha D., Maurer J., Brunner T.B. Pancreatic Stellate Cells in Pancreatic Cancer: In Focus. Pancreatology. 2017;17:514–522. doi: 10.1016/j.pan.2017.05.390. [DOI] [PubMed] [Google Scholar]
- 16.Binkley C.E., Zhang L., Greenson J.K., Giordano T.J., Kuick R., Misek D., Hanash S., Logsdon C.D., Simeone D.M. The Molecular Basis of Pancreatic Fibrosis: Common Stromal Gene Expression in Chronic Pancreatitis and Pancreatic Adenocarcinoma. Pancreas. 2004;29:254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Apte M.V., Xu Z., Pothula S., Goldstein D., Pirola R.C., Wilson J.S. Pancreatic Cancer: The Microenvironment Needs Attention Too! Pancreatology. 2015;15:S32–S38. doi: 10.1016/j.pan.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Apte M.V., Park S., Phillips P.A., Santucci N., Goldstein D., Kumar R.K., Ramm G.A., Buchler M., Friess H., McCarroll J.A., et al. Desmoplastic Reaction in Pancreatic Cancer: Role of Pancreatic Stellate Cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Pothula S.P., Xu Z., Goldstein D., Pirola R.C., Wilson J.S., Apte M.V. Key Role of Pancreatic Stellate Cells in Pancreatic Cancer. Cancer Lett. 2016;381:194–200. doi: 10.1016/j.canlet.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Pandol S., Gukovskaya A., Edderkoui M., Dawson D., Eibl G., Lugea A. Epidemiology, Risk Factors, and the Promotion of Pancreatic Cancer: Role of the Stellate Cell: Pancreatic Cancer. J. Gastroenterol. Hepatol. 2012;27:127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pothula S.P., Pirola R.C., Wilson J.S., Apte M.V. Pancreatic Stellate Cells: Aiding and Abetting Pancreatic Cancer Progression. Pancreatology. 2020;20:409–418. doi: 10.1016/j.pan.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Bonnans C., Chou J., Werb Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurahara H., Takao S., Maemura K., Mataki Y., Kuwahata T., Maeda K., Sakoda M., Iino S., Ishigami S., Ueno S., et al. M2-Polarized Tumor-Associated Macrophage Infiltration of Regional Lymph Nodes Is Associated With Nodal Lymphangiogenesis and Occult Nodal Involvement in PN0 Pancreatic Cancer. Pancreas. 2013;42:155–159. doi: 10.1097/MPA.0b013e318254f2d1. [DOI] [PubMed] [Google Scholar]
- 24.Foucher E.D., Ghigo C., Chouaib S., Galon J., Iovanna J., Olive D. Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front. Immunol. 2018;9:1044. doi: 10.3389/fimmu.2018.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang D., Yuan Z., Xue X., Lu Z., Zhang Y., Wang H., Chen M., An Y., Wei J., Zhu Y., et al. High Expression of Galectin-1 in Pancreatic Stellate Cells Plays a Role in the Development and Maintenance of an Immunosuppressive Microenvironment in Pancreatic Cancer. Int. J. Cancer. 2012;130:2337–2348. doi: 10.1002/ijc.26290. [DOI] [PubMed] [Google Scholar]
- 26.Mace T.A., Bloomston M., Lesinski G.B. Pancreatic Cancer-Associated Stellate Cells: A Viable Target for Reducing Immunosuppression in the Tumor Microenvironment. OncoImmunology. 2013;2:e24891. doi: 10.4161/onci.24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey V., Storz P. Targeting the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Expert Rev. Anticancer Ther. 2019;19:473–482. doi: 10.1080/14737140.2019.1622417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ene–Obong A., Clear A.J., Watt J., Wang J., Fatah R., Riches J.C., Marshall J.F., Chin–Aleong J., Chelala C., Gribben J.G., et al. Activated Pancreatic Stellate Cells Sequester CD8+ T Cells to Reduce Their Infiltration of the Juxtatumoral Compartment of Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2013;145:1121–1132. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Q., Tian Y., Zhang J., Zhang H., Gu F., Lu Y., Zou S., Chen Y., Sun P., Xu M., et al. Functions of Pancreatic Stellate Cell-Derived Soluble Factors in the Microenvironment of Pancreatic Ductal Carcinoma. Oncotarget. 2017;8:102721–102738. doi: 10.18632/oncotarget.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y., Zhang C., Jiang K., Werner J., Bazhin A.V., D’Haese J.G. The Role of Stellate Cells in Pancreatic Ductal Adenocarcinoma: Targeting Perspectives. Front. Oncol. 2021;10:621937. doi: 10.3389/fonc.2020.621937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuzillet C., Tijeras-Raballand A., Ragulan C., Cros J., Patil Y., Martinet M., Erkan M., Kleeff J., Wilson J., Apte M., et al. Inter- and Intra-tumoural Heterogeneity in Cancer-associated Fibroblasts of Human Pancreatic Ductal Adenocarcinoma. J. Pathol. 2019;248:51–65. doi: 10.1002/path.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Öhlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A.S., Ponz-Sarvise M., Corbo V., Oni T.E., Hearn S.A., Lee E.J., et al. Distinct Populations of Inflammatory Fibroblasts and Myofibroblasts in Pancreatic Cancer. J. Exp. Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helms E., Onate M.K., Sherman M.H. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020;10:648–656. doi: 10.1158/2159-8290.CD-19-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balch C.M. Patterns of Human Tumor-Infiltrating Lymphocytes in 120 Human Cancers. Arch. Surg. 1990;125:200. doi: 10.1001/archsurg.1990.01410140078012. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L., Conejo-Garcia J.R., Katsaros D., Gimotty P.A., Massobrio M., Regnani G., Makrigiannakis A., Gray H., Schlienger K., Liebman M.N., et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 36.Hiraoka N., Ino Y., Yamazaki-Itoh R., Kanai Y., Kosuge T., Shimada K. Intratumoral Tertiary Lymphoid Organ Is a Favourable Prognosticator in Patients with Pancreatic Cancer. Br. J. Cancer. 2015;112:1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galon J. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 38.Peng J., Sun B.-F., Chen C.-Y., Zhou J.-Y., Chen Y.-S., Chen H., Liu L., Huang D., Jiang J., Cui G.-S., et al. Single-Cell RNA-Seq Highlights Intra-Tumoral Heterogeneity and Malignant Progression in Pancreatic Ductal Adenocarcinoma. Cell Res. 2019;29:725–738. doi: 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukunaga A., Miyamoto M., Cho Y., Murakami S., Kawarada Y., Oshikiri T., Kato K., Kurokawa T., Suzuoki M., Nakakubo Y., et al. CD8+ Tumor-Infiltrating Lymphocytes Together with CD4+ Tumor-Infiltrating Lymphocytes and Dendritic Cells Improve the Prognosis of Patients with Pancreatic Adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 40.van der Leun A.M., Thommen D.S., Schumacher T.N. CD8+ T Cell States in Human Cancer: Insights from Single-Cell Analysis. Nat. Rev. Cancer. 2020;20:218–232. doi: 10.1038/s41568-019-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galon J., Bruni D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 42.Borst J., Ahrends T., Bąbała N., Melief C.J.M., Kastenmüller W. CD4+ T Cell Help in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy R., Celis E. Multiple Roles for CD4+ T Cells in Anti-Tumor Immune Responses. Immunol. Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 44.Ryschich E., Nötzel T., Hinz U., Autschbach F., Ferguson J., Simon I., Weitz J., Fröhlich B., Klar E., Büchler M.W., et al. Control of T-Cell-Mediated Immune Response by HLA Class I in Human Pancreatic Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005;11:498–504. [PubMed] [Google Scholar]
- 45.Tewari N., Zaitoun A.M., Arora A., Madhusudan S., Ilyas M., Lobo D.N. The Presence of Tumour-Associated Lymphocytes Confers a Good Prognosis in Pancreatic Ductal Adenocarcinoma: An Immunohistochemical Study of Tissue Microarrays. BMC Cancer. 2013;13:436. doi: 10.1186/1471-2407-13-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y., Zhao H., Zhu D., Zhi Q., He S., Kuang Y., Li D., Zhang Z., Song S., Zhang L., et al. The Coexpression and Clinical Significance of Costimulatory Molecules B7-H1, B7-H3, and B7-H4 in Human Pancreatic Cancer. OncoTargets Ther. 2014:1465. doi: 10.2147/OTT.S66809. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Helm O., Mennrich R., Petrick D., Goebel L., Freitag-Wolf S., Röder C., Kalthoff H., Röcken C., Sipos B., Kabelitz D., et al. Comparative Characterization of Stroma Cells and Ductal Epithelium in Chronic Pancreatitis and Pancreatic Ductal Adenocarcinoma. PLoS ONE. 2014;9:e94357. doi: 10.1371/journal.pone.0094357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castino G.F., Cortese N., Capretti G., Serio S., Di Caro G., Mineri R., Magrini E., Grizzi F., Cappello P., Novelli F., et al. Spatial Distribution of B Cells Predicts Prognosis in Human Pancreatic Adenocarcinoma. OncoImmunology. 2016;5:e1085147. doi: 10.1080/2162402X.2015.1085147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang D.D., Gao J., Wang S., Yuan Z., Ye N., Chong Y., Xu C., Jiang X., Li B., Yin W., et al. Apoptosis and Anergy of T Cell Induced by Pancreatic Stellate Cells-Derived Galectin-1 in Pancreatic Cancer. Tumor Biol. 2015;36:5617–5626. doi: 10.1007/s13277-015-3233-5. [DOI] [PubMed] [Google Scholar]
- 50.Wartenberg M., Zlobec I., Perren A., Koelzer V.H., Gloor B., Lugli A., Karamitopoulou E. Accumulation of FOXP3+T-Cells in the Tumor Microenvironment Is Associated with an Epithelial-Mesenchymal-Transition-Type Tumor Budding Phenotype and Is an Independent Prognostic Factor in Surgically Resected Pancreatic Ductal Adenocarcinoma. Oncotarget. 2015;6:4190–4201. doi: 10.18632/oncotarget.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L., Niu Z.-Y., Liang Z.-Y., Zhou W.-X., You L., Wang M.-Y., Yao L.-T., Liao Q., Zhao Y.-P. HLA-G Impairs Host Immune Response and Predicts Poor Prognosis in Pancreatic Cancer. Am. J. Transl. Res. 2015;7:2036–2044. [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang H.K., Kim H.-I., Kim S.H., Choi J., Kang C.M., Kim K.S., Lee W.J. Prognostic Impact of the Tumor-Infiltrating Regulatory T-Cell (Foxp3+)/Activated Cytotoxic T Lymphocyte (Granzyme B+) Ratio on Resected Left-Sided Pancreatic Cancer. Oncol. Lett. 2016;12:4477–4484. doi: 10.3892/ol.2016.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundgren S., Warfvinge C.F., Elebro J., Heby M., Nodin B., Krzyzanowska A., Bjartell A., Leandersson K., Eberhard J., Jirström K. The Prognostic Impact of NK/NKT Cell Density in Periampullary Adenocarcinoma Differs by Morphological Type and Adjuvant Treatment. PLoS ONE. 2016;11:e0156497. doi: 10.1371/journal.pone.0156497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Australian Pancreatic Cancer Genome Initiative. Balachandran V.P., Łuksza M., Zhao J.N., Makarov V., Moral J.A., Remark R., Herbst B., Askan G., Bhanot U., et al. Identification of Unique Neoantigen Qualities in Long-Term Survivors of Pancreatic Cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carstens J.L., Correa de Sampaio P., Yang D., Barua S., Wang H., Rao A., Allison J.P., LeBleu V.S., Kalluri R. Spatial Computation of Intratumoral T Cells Correlates with Survival of Patients with Pancreatic Cancer. Nat. Commun. 2017;8:15095. doi: 10.1038/ncomms15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lohneis P., Sinn M., Bischoff S., Jühling A., Pelzer U., Wislocka L., Bahra M., Sinn B.V., Denkert C., Oettle H., et al. Cytotoxic Tumour-Infiltrating T Lymphocytes Influence Outcome in Resected Pancreatic Ductal Adenocarcinoma. Eur. J. Cancer. 2017;83:290–301. doi: 10.1016/j.ejca.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Tahkola K., Mecklin J.-P., Wirta E.-V., Ahtiainen M., Helminen O., Böhm J., Kellokumpu I. High Immune Cell Score Predicts Improved Survival in Pancreatic Cancer. Virchows Arch. 2018;472:653–665. doi: 10.1007/s00428-018-2297-1. [DOI] [PubMed] [Google Scholar]
- 58.Ino Y., Oguro S., Yamazaki-Itoh R., Hori S., Shimada K., Hiraoka N. Reliable Evaluation of Tumor-Infiltrating Lymphocytes in Pancreatic Cancer Tissue Biopsies. Oncotarget. 2019;10:1149–1159. doi: 10.18632/oncotarget.26646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miksch R.C., Schoenberg M.B., Weniger M., Bösch F., Ormanns S., Mayer B., Werner J., Bazhin A.V., D’Haese J.G. Prognostic Impact of Tumor-Infiltrating Lymphocytes and Neutrophils on Survival of Patients with Upfront Resection of Pancreatic Cancer. Cancers. 2019;11:39. doi: 10.3390/cancers11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orhan A., Vogelsang R.P., Andersen M.B., Madsen M.T., Hölmich E.R., Raskov H., Gögenur I. The Prognostic Value of Tumour-Infiltrating Lymphocytes in Pancreatic Cancer: A Systematic Review and Meta-Analysis. Eur. J. Cancer. 2020;132:71–84. doi: 10.1016/j.ejca.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Danilova L., Ho W.J., Zhu Q., Vithayathil T., De Jesus-Acosta A., Azad N.S., Laheru D.A., Fertig E.J., Anders R., Jaffee E.M., et al. Programmed Cell Death Ligand-1 (PD-L1) and CD8 Expression Profiling Identify an Immunologic Subtype of Pancreatic Ductal Adenocarcinomas with Favorable Survival. Cancer Immunol. Res. 2019;7:886–895. doi: 10.1158/2326-6066.CIR-18-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diana A., Wang L.M., D’Costa Z., Allen P., Azad A., Silva M.A., Soonawalla Z., Liu S., McKenna W.G., Muschel R.J., et al. Prognostic Value, Localization and Correlation of PD-1/PD-L1, CD8 and FOXP3 with the Desmoplastic Stroma in Pancreatic Ductal Adenocarcinoma. Oncotarget. 2016;7:40992–41004. doi: 10.18632/oncotarget.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou Y.-C., Chao Y.-J., Hsieh M.-H., Tung H.-L., Wang H.-C., Shan Y.-S. Low CD8+ T Cell Infiltration and High PD-L1 Expression Are Associated with Level of CD44+/CD133+ Cancer Stem Cells and Predict an Unfavorable Prognosis in Pancreatic Cancer. Cancers. 2019;11:541. doi: 10.3390/cancers11040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knudsen E.S., Vail P., Balaji U., Ngo H., Botros I.W., Makarov V., Riaz N., Balachandran V., Leach S., Thompson D.M., et al. Stratification of Pancreatic Ductal Adenocarcinoma: Combinatorial Genetic, Stromal, and Immunologic Markers. Clin. Cancer Res. 2017;23:4429–4440. doi: 10.1158/1078-0432.CCR-17-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nizri E., Sternbach N., Bar-David S., Ben-Yehuda A., Gerstenhaber F., Ofir T., Wolf I., Weiner G., Lahat G., Klausner J. T-Helper 1 Immune Response in Metastatic Lymph Nodes of Pancreatic Ductal Adenocarcinoma: A Marker For Prolonged Survival. Ann. Surg. Oncol. 2018;25:475–481. doi: 10.1245/s10434-017-6237-0. [DOI] [PubMed] [Google Scholar]
- 66.Pu N., Zhao G., Yin H., Li J., Nuerxiati A., Wang D., Xu X., Kuang T., Jin D., Lou W., et al. CD25 and TGF-β Blockade Based on Predictive Integrated Immune Ratio Inhibits Tumor Growth in Pancreatic Cancer. J. Transl. Med. 2018;16:294. doi: 10.1186/s12967-018-1673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sideras K., Biermann K., Yap K., Mancham S., Boor P.P.C., Hansen B.E., Stoop H.J.A., Peppelenbosch M.P., van Eijck C.H., Sleijfer S., et al. Tumor Cell Expression of Immune Inhibitory Molecules and Tumor-Infiltrating Lymphocyte Count Predict Cancer-Specific Survival in Pancreatic and Ampullary Cancer: Immune Inhibitory Molecules in Pancreatic and Ampullary Cancer. Int. J. Cancer. 2017;141:572–582. doi: 10.1002/ijc.30760. [DOI] [PubMed] [Google Scholar]
- 68.Tang Y., Xu X., Guo S., Zhang C., Tang Y., Tian Y., Ni B., Lu B., Wang H. An Increased Abundance of Tumor-Infiltrating Regulatory T Cells Is Correlated with the Progression and Prognosis of Pancreatic Ductal Adenocarcinoma. PLoS ONE. 2014;9:e91551. doi: 10.1371/journal.pone.0091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karakhanova S., Ryschich E., Mosl B., Harig S., Jäger D., Schmidt J., Hartwig W., Werner J., Bazhin A.V. Prognostic and Predictive Value of Immunological Parameters for Chemoradioimmunotherapy in Patients with Pancreatic Adenocarcinoma. Br. J. Cancer. 2015;112:1027–1036. doi: 10.1038/bjc.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Z., Zhao J., Zhao H., A S., Liu Z., Zhang Y., Liu X., Wang F. Infiltrating CD4/CD8 High T Cells Shows Good Prognostic Impact in Pancreatic Cancer. Int. J. Clin. Exp. Pathol. 2017;10:8820–8828. [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L., Zhao G., Wu W., Rong Y., Jin D., Wang D., Lou W., Qin X. Low Intratumoral Regulatory T Cells and High Peritumoral CD8+ T Cells Relate to Long-Term Survival in Patients with Pancreatic Ductal Adenocarcinoma after Pancreatectomy. Cancer Immunol. Immunother. 2016;65:73–82. doi: 10.1007/s00262-015-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 73.Steinman R.M. Decisions About Dendritic Cells: Past, Present, and Future. Annu. Rev. Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 74.Dallal R.M., Christakos P., Lee K., Egawa S., Son Y.-I., Lotze M.T. Paucity of Dendritic Cells in Pancreatic Cancer. Surgery. 2002;131:135–138. doi: 10.1067/msy.2002.119937. [DOI] [PubMed] [Google Scholar]
- 75.Deicher A., Andersson R., Tingstedt B., Lindell G., Bauden M., Ansari D. Targeting Dendritic Cells in Pancreatic Ductal Adenocarcinoma. Cancer Cell Int. 2018;18:85. doi: 10.1186/s12935-018-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J., Shangguan J., Eresen A., Li Y., Wang J., Zhang Z. Dendritic Cells in Pancreatic Cancer Immunotherapy: Vaccines and Combination Immunotherapies. Pathol.-Res. Pract. 2019;215:152691. doi: 10.1016/j.prp.2019.152691. [DOI] [PubMed] [Google Scholar]
- 77.Tjomsland V., Sandström P., Spångeus A., Messmer D., Emilsson J., Falkmer U., Falkmer S., Magnusson K.-E., Borch K., Larsson M. Pancreatic Adenocarcinoma Exerts Systemic Effects on the Peripheral Blood Myeloid and Plasmacytoid Dendritic Cells: An Indicator of Disease Severity? BMC Cancer. 2010;10:87. doi: 10.1186/1471-2407-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirooka S., Yanagimoto H., Satoi S., Yamamoto T., Toyokawa H., Yamaki S., Yui R., Inoue K., Michiura T., Kwon A.-H. The Role of Circulating Dendritic Cells in Patients with Unresectable Pancreatic Cancer. Anticancer Res. 2011;31:3827–3834. [PubMed] [Google Scholar]
- 79.Yamamoto T., Yanagimoto H., Satoi S., Toyokawa H., Yamao J., Kim S., Terakawa N., Takahashi K., Kwon A.-H. Circulating Myeloid Dendritic Cells as Prognostic Factors in Patients with Pancreatic Cancer Who Have Undergone Surgical Resection. J. Surg. Res. 2012;173:299–308. doi: 10.1016/j.jss.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 80.Chiossone L., Dumas P.-Y., Vienne M., Vivier E. Natural Killer Cells and Other Innate Lymphoid Cells in Cancer. Nat. Rev. Immunol. 2018;18:671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 81.Guillerey C. NK Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020;1273:69–90. doi: 10.1007/978-3-030-49270-0_4. [DOI] [PubMed] [Google Scholar]
- 82.Wu S.-Y., Fu T., Jiang Y.-Z., Shao Z.-M. Natural Killer Cells in Cancer Biology and Therapy. Mol. Cancer. 2020;19:120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Cheng Y., Xu Y., Wang Z., Du X., Li C., Peng J., Gao L., Liang X., Ma C. Increased Expression of Programmed Cell Death Protein 1 on NK Cells Inhibits NK-Cell-Mediated Anti-Tumor Function and Indicates Poor Prognosis in Digestive Cancers. Oncogene. 2017;36:6143–6153. doi: 10.1038/onc.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamei R., Yoshimura K., Yoshino S., Inoue M., Asao T., Fuse M., Wada S., Kuramasu A., Furuya-Kondo T., Oga A., et al. Expression Levels of UL16 Binding Protein 1 and Natural Killer Group 2 Member D Affect Overall Survival in Patients with Gastric Cancer Following Gastrectomy. Oncol. Lett. 2017;15:747–754. doi: 10.3892/ol.2017.7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoshikawa M., Aoki T., Matsushita H., Karasaki T., Hosoi A., Odaira K., Fujieda N., Kobayashi Y., Kambara K., Ohara O., et al. NK Cell and IFN Signatures Are Positive Prognostic Biomarkers for Resectable Pancreatic Cancer. Biochem. Biophys. Res. Commun. 2018;495:2058–2065. doi: 10.1016/j.bbrc.2017.12.083. [DOI] [PubMed] [Google Scholar]
- 86.Moral J.A., Leung J., Rojas L.A., Ruan J., Zhao J., Sethna Z., Ramnarain A., Gasmi B., Gururajan M., Redmond D., et al. ILC2s Amplify PD-1 Blockade by Activating Tissue-Specific Cancer Immunity. Nature. 2020;579:130–135. doi: 10.1038/s41586-020-2015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Curiel T.J. Tregs and Rethinking Cancer Immunotherapy. J. Clin. Investig. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krishnamoorthy M., Lenehan J.G., Burton J.P., Maleki Vareki S. Immunomodulation in Pancreatic Cancer. Cancers. 2020;12:3340. doi: 10.3390/cancers12113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hiraoka N., Onozato K., Kosuge T., Hirohashi S. Prevalence of FOXP3+ Regulatory T Cells Increases during the Progression of Pancreatic Ductal Adenocarcinoma and Its Premalignant Lesions. Clin. Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 90.Wang X., Lang M., Zhao T., Feng X., Zheng C., Huang C., Hao J., Dong J., Luo L., Li X., et al. Cancer-FOXP3 Directly Activated CCL5 to Recruit FOXP3+Treg Cells in Pancreatic Ductal Adenocarcinoma. Oncogene. 2017;36:3048–3058. doi: 10.1038/onc.2016.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ino Y., Yamazaki-Itoh R., Shimada K., Iwasaki M., Kosuge T., Kanai Y., Hiraoka N. Immune Cell Infiltration as an Indicator of the Immune Microenvironment of Pancreatic Cancer. Br. J. Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weber R., Groth C., Lasser S., Arkhypov I., Petrova V., Altevogt P., Utikal J., Umansky V. IL-6 as a Major Regulator of MDSC Activity and Possible Target for Cancer Immunotherapy. Cell. Immunol. 2021;359:104254. doi: 10.1016/j.cellimm.2020.104254. [DOI] [PubMed] [Google Scholar]
- 93.Pergamo M., Miller G. Myeloid-Derived Suppressor Cells and Their Role in Pancreatic Cancer. Cancer Gene Ther. 2017;24:100–105. doi: 10.1038/cgt.2016.65. [DOI] [PubMed] [Google Scholar]
- 94.Chun E., Lavoie S., Michaud M., Gallini C.A., Kim J., Soucy G., Odze R., Glickman J.N., Garrett W.S. CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function. Cell Rep. 2015;12:244–257. doi: 10.1016/j.celrep.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Danilin S., Merkel A.R., Johnson J.R., Johnson R.W., Edwards J.R., Sterling J.A. Myeloid-Derived Suppressor Cells Expand during Breast Cancer Progression and Promote Tumor-Induced Bone Destruction. OncoImmunology. 2012;1:1484–1494. doi: 10.4161/onci.21990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martens A., Wistuba-Hamprecht K., Foppen M.G., Yuan J., Postow M.A., Wong P., Romano E., Khammari A., Dreno B., Capone M., et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016;22:2908–2918. doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li A., King J., Moro A., Sugi M.D., Dawson D.W., Kaplan J., Li G., Lu X., Strieter R.M., Burdick M., et al. Overexpression of CXCL5 Is Associated With Poor Survival in Patients With Pancreatic Cancer. Am. J. Pathol. 2011;178:1340–1349. doi: 10.1016/j.ajpath.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nywening T.M., Belt B.A., Cullinan D.R., Panni R.Z., Han B.J., Sanford D.E., Jacobs R.C., Ye J., Patel A.A., Gillanders W.E., et al. Targeting Both Tumour-Associated CXCR2+ Neutrophils and CCR2+ Macrophages Disrupts Myeloid Recruitment and Improves Chemotherapeutic Responses in Pancreatic Ductal Adenocarcinoma. Gut. 2018;67:1112–1123. doi: 10.1136/gutjnl-2017-313738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goedegebuure P., Mitchem J.B., Porembka M.R., Tan M.C.B., Belt B.A., Wang-Gillam A., Gillanders W.E., Hawkins W.G., Linehan D.C. Myeloid-Derived Suppressor Cells: General Characteristics and Relevance to Clinical Management of Pancreatic Cancer. Curr. Cancer Drug Targets. 2011;11:734–751. doi: 10.2174/156800911796191024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Porembka M.R., Mitchem J.B., Belt B.A., Hsieh C.-S., Lee H.-M., Herndon J., Gillanders W.E., Linehan D.C., Goedegebuure P. Pancreatic Adenocarcinoma Induces Bone Marrow Mobilization of Myeloid-Derived Suppressor Cells Which Promote Primary Tumor Growth. Cancer Immunol. Immunother. 2012;61:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu X.-D., Hu J., Wang M., Peng F., Tian R., Guo X.-J., Xie Y., Qin R.-Y. Circulating Myeloid-Derived Suppressor Cells in Patients with Pancreatic Cancer. Hepatobiliary Pancreat. Dis. Int. 2016;15:099–105. doi: 10.1016/S1499-3872(15)60413-1. [DOI] [PubMed] [Google Scholar]
- 102.Marigo I., Dolcetti L., Serafini P., Zanovello P., Bronte V. Tumor-Induced Tolerance and Immune Suppression by Myeloid Derived Suppressor Cells. Immunol. Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 103.Bindea G., Mlecnik B., Tosolini M., Kirilovsky A., Waldner M., Obenauf A.C., Angell H., Fredriksen T., Lafontaine L., Berger A., et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 104.Di Caro G., Castino G.F., Bergomas F., Cortese N., Chiriva-Internati M., Grizzi F., Mantovani A., Marchesi F. Tertiary Lymphoid Tissue in the Tumor Microenvironment: From Its Occurrence to Immunotherapeutic Implications. Int. Rev. Immunol. 2015;34:123–133. doi: 10.3109/08830185.2015.1018416. [DOI] [PubMed] [Google Scholar]
- 105.Wang N., Wang S., Wang X., Zheng Y., Yang B., Zhang J., Pan B., Gao J., Wang Z. Research Trends in Pharmacological Modulation of Tumor-associated Macrophages. Clin. Transl. Med. 2021;11 doi: 10.1002/ctm2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Helm O., Held-Feindt J., Grage-Griebenow E., Reiling N., Ungefroren H., Vogel I., Krüger U., Becker T., Ebsen M., Röcken C., et al. Tumor-Associated Macrophages Exhibit pro- and Anti-Inflammatory Properties by which They Impact on Pancreatic Tumorigenesis: Role of Macrophages in Pancreatic Cancer. Int. J. Cancer. 2014;135:843–861. doi: 10.1002/ijc.28736. [DOI] [PubMed] [Google Scholar]
- 107.Kurahara H., Shinchi H., Mataki Y., Maemura K., Noma H., Kubo F., Sakoda M., Ueno S., Natsugoe S., Takao S. Significance of M2-Polarized Tumor-Associated Macrophage in Pancreatic Cancer. J. Surg. Res. 2011;167:e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 108.Sadozai H., Acharjee A., Eppenberger-Castori S., Gloor B., Gruber T., Schenk M., Karamitopoulou E. Distinct Stromal and Immune Features Collectively Contribute to Long-Term Survival in Pancreatic Cancer. Front. Immunol. 2021;12:643529. doi: 10.3389/fimmu.2021.643529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leinwand J., Miller G. Regulation and Modulation of Antitumor Immunity in Pancreatic Cancer. Nat. Immunol. 2020;21:1152–1159. doi: 10.1038/s41590-020-0761-y. [DOI] [PubMed] [Google Scholar]
- 110.Riquelme E., Zhang Y., Zhang L., Montiel M., Zoltan M., Dong W., Quesada P., Sahin I., Chandra V., San Lucas A., et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178:795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arsenijevic T., Nicolle R., Bouchart C., D’Haene N., Demetter P., Puleo F., Van Laethem J.-L. Pancreatic Cancer Meets Human Microbiota: Close Encounters of the Third Kind. Cancers. 2021;13:1231. doi: 10.3390/cancers13061231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tijeras-Raballand A., Hilmi M., Astorgues-Xerri L., Nicolle R., Bièche I., Neuzillet C. Microbiome and Pancreatic Ductal Adenocarcinoma. Clin. Res. Hepatol. Gastroenterol. 2021;45:101589. doi: 10.1016/j.clinre.2020.101589. [DOI] [PubMed] [Google Scholar]
- 113.Roth S., Zamzow K., Gaida M.M., Heikenwälder M., Tjaden C., Hinz U., Bose P., Michalski C.W., Hackert T. Evolution of the Immune Landscape during Progression of Pancreatic Intraductal Papillary Mucinous Neoplasms to Invasive Cancer. EBioMedicine. 2020;54:102714. doi: 10.1016/j.ebiom.2020.102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qian H., Li H., Xie J., Lu X., Li F., Wang W., Tang X., Shi M., Jiang L., Li H., et al. Immunity-Related Gene Signature Identifies Subtypes Benefitting From Adjuvant Chemotherapy or Potentially Responding to PD1/PD-L1 Blockage in Pancreatic Cancer. Front. Cell Dev. Biol. 2021;9:682261. doi: 10.3389/fcell.2021.682261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicolle R., Gayet O., Duconseil P., Vanbrugghe C., Roques J., Bigonnet M., Blum Y., Elarouci N., Armenoult L., Ayadi M., et al. A Transcriptomic Signature to Predict Adjuvant Gemcitabine Sensitivity in Pancreatic Adenocarcinoma. Ann. Oncol. 2021;32:250–260. doi: 10.1016/j.annonc.2020.10.601. [DOI] [PubMed] [Google Scholar]
- 116.Van Cutsem E., Tempero M.A., Sigal D., Oh D.-Y., Fazio N., Macarulla T., Hitre E., Hammel P., Hendifar A.E., Bates S.E., et al. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2020;38:3185–3194. doi: 10.1200/JCO.20.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Laheru D., Lutz E., Burke J., Biedrzycki B., Solt S., Onners B., Tartakovsky I., Nemunaitis J., Le D., Sugar E., et al. Allogeneic Granulocyte Macrophage Colony-Stimulating Factor–Secreting Tumor Immunotherapy Alone or in Sequence with Cyclophosphamide for Metastatic Pancreatic Cancer: A Pilot Study of Safety, Feasibility, and Immune Activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S., Sherry R.M., Topalian S.L., Yang J.C., Lowy I., et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brahmer J.R., Tykodi S.S., Chow L.Q.M., Hwu W.-J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Le D.T., Lutz E., Uram J.N., Sugar E.A., Onners B., Solt S., Zheng L., Diaz L.A., Donehower R.C., Jaffee E.M., et al. Evaluation of Ipilimumab in Combination With Allogeneic Pancreatic Tumor Cells Transfected With a GM-CSF Gene in Previously Treated Pancreatic Cancer. J. Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Le D.T., Wang-Gillam A., Picozzi V., Greten T.F., Crocenzi T., Springett G., Morse M., Zeh H., Cohen D., Fine R.L., et al. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes –Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. JCO. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Reilly E.M., Oh D.-Y., Dhani N., Renouf D.J., Lee M.A., Sun W., Fisher G., Hezel A., Chang S.-C., Vlahovic G., et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1431. doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Renouf D.J., Knox J.J., Kavan P., Jonker D., Welch S., Couture F., Lemay F., Tehfe M., Harb M., Aucoin N., et al. LBA65—The Canadian Cancer Trials Group PA.7 Trial: Results of a Randomized Phase II Study of Gemcitabine (GEM) and Nab-Paclitaxel (Nab-P) vs GEM, Nab-P, Durvalumab (D) and Tremelimumab (T) as First Line Therapy in Metastatic Pancreatic Ductal Adenocarcinoma (MPDAC) Ann. Oncol. 2020;31:S1195. [Google Scholar]