Introduction
Infections of the central nervous system (CNS) are among the most devastating infectious diseases worldwide and often result in medical emergencies that require prompt management. Pathogens may access the CNS by crossing the blood–brain barrier (BBB), which normally protects the CNS from microbial invasion, or via transneuronal routes that bypass the BBB. A broad array of infectious agents can cause CNS infections in the meningeal or parenchymal compartments (Fig 1, Table 1). Infection of the cerebrospinal fluid (CSF) and its surrounding meninges, termed meningitis, is accompanied with the acute onset of fever, headache, and neck stiffness. Infection of the CNS parenchyma leads to encephalitis, which clinically involves fever, neuropsychological impairment, and seizures. By contrast, CNS infection confined to small areas of focal lesions or abscesses are more likely to occur in immunocompromised individuals. Here, we summarize the etiologies of these potentially vaccine-preventable infections, their transmission routes, and the recent advances in understanding the mechanisms of CNS invasion by different neurotropic pathogens.
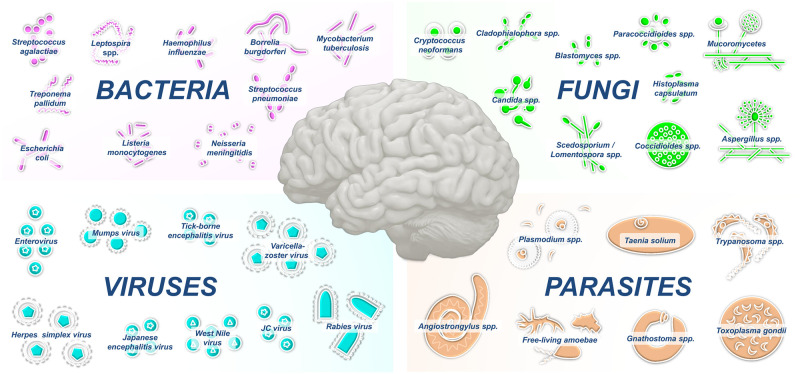
Fig 1. Compilation of prominent bacteria, fungi, viruses, and parasites that infect the CNS.
CNS, central nervous system.
Table 1. Etiology and epidemiology of CNS infections.
| Pathogen | Geographic distribution | Transmission route | Demographics | Clinical presentation |
|---|---|---|---|---|
| Bacteria | ||||
| Streptococcus agalactiae | Worldwide | Vertical transmission (mother to child) through the birth canal | Neonates | Meningitis |
| Escherichia coli | ||||
| Neisseria meningitidis | Inhalation (droplets produced by coughing or sneezing) | Children and adults | ||
| Streptococcus pneumoniae | ||||
| Haemophilus influenzae | Elderly and immunocompromised individuals | |||
| Listeria monocytogenes | Transplacental | Elderly, immunocompromised, (neonates) | Meningitis, rhombencephalitis | |
| Mycobacterium tuberculosis | Worldwide; vast majority in Africa/Asia | Inhalation (Flugge droplets) | Children and adults Immunocompromised (HIV infected patients) |
Meningitis, cerebral tuberculomas |
| Treponema pallidum (neurosyphilis) | Worldwide | Direct contact (sexual) | Adults | Meningoencephalitis; General paresis; Tabes dorsalis |
| Leptospira spp. | Worldwide | Contact with infected mammals (rodents) | Children and adults | Meningitis; Meningoencephalitis; Myelitis |
| Borrelia burgdorferi (neuroborreliosis) | North America and Eurasia | Arthropod borne (tick: Ixodes spp.) | Children and adults | Encephalitis; Meningitis; Encephalopathy |
| Viruses | ||||
| EV | Worldwide | Fecal/oral Inhalation (EV-D68) |
Children and adults | Meningitis Encephalitis (rare) |
| HSV | Worldwide | Skin/mucosa | Children (mainly HSV1) and adults (mainly HSV2) Immunocompromised |
Meningitis Encephalitis |
| VZV | Worldwide | Skin/mucosa Inhalation |
Adults (mostly immunocompromised) | Encephalitis; Meningitis (rare); Myelitis |
| Mumps virus | Worldwide | Inhalation | Children and adults (mostly unvaccinated) | Meningitis; Myelitis; |
| Rabies virus | Worldwide; vast majority in Africa/Asia | Contact with infected mammal (dogs) | Children and adults | Encephalitis |
| WNV | Worldwide | Arthropod borne (mosquito: Culex spp.) | Children and adults (mostly elderly population) | Meningitis; Encephalitis |
| JEV | Asia, Australia, and western Pacific | Arthropod borne (mosquito: Culex spp.) | Children and adults (mostly pediatric population) | Encephalitis |
| TBEV | Central and northern Europe | Arthropod borne (tick: Ixodes ricinus) | Children and adults | Encephalitis |
| JCV | Worldwide | Inhalation | Adults with severe immune deficiency | PML |
| Fungi | ||||
| Candida spp. (neurocandidiasis) | Worldwide (human commensal) | Nosocomial (neurosurgery, CNS devices) | Preterm neonates, children, and adults | Meningoencephalitis; brain abscesses |
| Cryptococcus neoformans (neurocryptococcosis) | Worldwide; frequent in Europe | Inhalation (bird droppings) | Immunocompromised (especially for CD4+ T-cell counts <100/mm3) | Meningoencephalitis |
| Aspergillus spp. | Worldwide | Inhalation | Immunocompromised | Brain abscesses (frequently secondary to lung infections) |
|
Scedosporium spp. Lomentospora spp. |
Worldwide | Inhalation | Children and adults (mostly immunocompromised) | Brain abscesses (secondary to lung infections or near-drowning) |
| Mucoromycetes (Rhizopus, Lichtheimia, Mucor …) | Worldwide | Inhalation | Immunocompromised | Brain abscesses (frequently secondary to sinus infections) |
| Histoplasma capsulatum (histoplasmosis) | Central and eastern United States (var. capsulatum); Africa (var. duboisii) | Inhalation (bird or bat droppings) | Children and adults (immunocompetent) | Meningitis, meningoencephalitis, and brain abscesses |
| Blastomyces spp. (blastomycosis) | North America | Inhalation (soil dust) | Children and adults (immunocompetent) | Meningitis, meningoencephalitis, and brain abscesses |
| Coccidioides spp. (coccidioidomycosis) | Southwest US, Mexico, and South America | Inhalation (soil dust) | Children and adults (immunocompetent) | Meningitis, meningoencephalitis, and brain abscesses |
| Paracoccidioides spp. (paracoccidioidomycosis) | Central and South America | Inhalation (soil dust) | Children and adults (immunocompetent) | Brain abscesses and meningitis |
| Dematiaceous molds (phaeohyphomycosis) | Worldwide | Inhalation | Children and adults (immunocompetent) | Brain abscesses |
| Parasites | ||||
| Toxoplasma gondii | Worldwide | Ingestion of tissue cysts or sporulated oocysts Organ transplant Blood transfusion |
Immunocompromised (especially for CD4+ T-cell counts <200/mm3) | Encephalitis and brain abscesses |
| Trypanosoma brucei (sleeping disease) | Africa | Arthropod borne (Tsetse flies, Glossinidae) | Children and adults | Mental and behavioral disorders, sleep and sleep–wake cycles disturbances |
| Plasmodium spp. (cerebral malaria) | Tropical and subtropical regions | Arthropod borne (Mosquito: Anopheles spp.) | Children and adults | Impaired consciousness and coma |
| Taenia solium (neurocysticercosis) | Africa, Asia, and Latin America | Fecal–oral | Children and adults (mostly from resource-limited countries) | Intracerebral cysts (epilepsy) |
| Angiostrongylus spp. (neuroangiostrongyliasis) | Southeast Asia, Oceania, and the Americas | Ingestion of terrestrial mollusks (snails/slugs) | Children and adults | Eosinophilic meningitis; Intracranial hemorrhage |
| Gnathostoma spp. (gnathostomiasis) | Southeast Asia, Japan Korea, and Latin America | Food borne (mostly raw freshwater fish, amphibians, and reptiles) | Children and adults | Eosinophilic meningitis; Intracranial hemorrhage |
| Free-living amoebae (Acanthamoeba, Balamuthia and Naegleria) | Worldwide | Inhalation (contaminated water) | Immunocompromised (Acanthamoeba, Balamuthia) Immunocompetent (Naegleria) |
Granulomatous amebic encephalitis (Acanthamoeba, Balamuthia) Primary amebic meningoencephalitis (Naegleria) |
CNS, central nervous system; EV, enterovirus; HSV, herpes simplex virus; JCV, JC virus; JEV, Japanese encephalitis virus; PML, progressive multifocal leukoencephalopathy; TBEV, tick-borne encephalitis virus; VZV, varicella-zoster virus; WNV, West Nile virus.
Bacterial infections
The CNS may be infected by a wide variety of bacteria (Fig 1, Table 1). The spectrum of these infections varies from focal infections, such as brain abscesses, to generalized entities such as meningoencephalitis. Contiguous spread from the upper airways, hematogenous spread from another primary site, and direct inoculation through trauma or surgery can contribute to the development of a CNS bacterial infection. The etiology of bacterial meningitis varies according to age group and immune status. The most frequent infective agents affecting newborns in the first week, Streptococcus agalactiae and Escherichia coli, are replaced by Streptococcus pneumoniae and Neisseria meningitidis by the sixth week [1]. Subsequently, S. pneumoniae remains the most common bacterial agent, followed by N. meningitidis and Listeria monocytogenes [1,2]. Haemophilus influenzae type B (Hib) used to be a leading cause of pediatric meningitis in the pre-Hib vaccination era [3]. Similarly, the introduction of pneumococcal and meningococcal conjugate vaccines has substantially reduced the burden of bacterial meningitis. Gram-negative bacilli and Staphylococcus spp. are the most common causes of nosocomial CNS infections.
Atypical bacteria can also reach the brain. Mycobacterium tuberculosis causes tuberculous meningitis, which originates from a pulmonary focus that spreads via the lymphatic system but also intracranial tuberculomas. The local inflammatory response leads to nerve palsy and alterations in the CSF and cerebral blood flow. The mortality rate in treated cases remains high, ranging from 20% to 67% [4]. Spirochetes are also responsible for CNS diseases that are mediated by local inflammatory responses. Treponema pallidum, a sexually transmitted pathogen, usually cause neurosyphilis in the late stage of infection [5]. Pathogenic Leptospira spp. cause leptospirosis, an acute febrile disease mainly transmitted by brown rats, eliciting neurological complications or meningoencephalitis [6]. Borrelia burgdorferi, an agent of a tick-borne Lyme borreliosis, causes encephalitis or meningitis associated with arthropathies [7].
Viral infections
Viral meningitis is by far the most frequent clinical presentation of CNS infections (Fig 1, Table 1). Enteroviruses (EVs), members of the Picornaviridae family, are involved in approximately 90% of cases [8]. EV-A71, EV-D68, and Coxsackievirus B are most frequently detected in patients with aseptic meningitis. Human parechoviruses (HPeVs), especially HPeV-3, are other Picornaviridae that are commonly responsible for meningitis. EVs can replicate in the upper respiratory and intestinal epithelial cells and then disseminate into the bloodstream and CNS through infected immune cells [9]. EV meningitis is usually a benign, self-limiting condition.
The Herpesviridae family is the second leading cause of viral meningitis. Herpes simplex virus 2 (HSV-2) is the predominant causal agent, but HSV-1, varicella-zoster virus (VZV), and Epstein–Barr virus (EBV) also cause meningitis. HSV-2 meningitis may occur as a result of primary infection or reactivation [10]. Mumps virus, causing parotitis and hearing loss, is one of the main causes of viral meningitis in unvaccinated populations [11].
In addition to meningitis, most of the aforementioned viruses are also capable of causing encephalitis. HSV-1 can cause necrotic acute encephalitis during reactivation in the adult population. Similarly, VZV encephalitis is common after reactivation of the virus (zoster). Other viruses are typically characterized as causal agents of encephalitis. Historically, rabies is the most popular viral brain infection and caused by various Lyssavirus species that are transmitted by dog bites in approximately 99% of human cases [12]. Animal control and vaccination programs aim to prevent infections that occur in approximately 150 countries and affect approximately 3 billion people.
Arthropod-borne viruses (arboviruses) are responsible for encephalitis in endemic areas. Over the years, however, climatic and ecological changes have altered the geographical distribution of arboviral infections. The West Nile virus (WNV) rapidly emerged in Northern America and Southern Europe during the 21st century. This Flavivirus is transmitted by mosquitoes and often cause encephalitis especially in elderly patients. The Japanese encephalitis virus (JEV) is another mosquito-borne Flavivirus and causes viral encephalitis in Asia. Tick-borne encephalitis virus (TBEV) is the third most common encephalitis-causing arbovirus. This Flavivirus is primarily transmitted to humans by the widespread hard tick species Ixodes ricinus [13]. Although the mortality rate is low, up to 30% of patients with TBEV encephalitis develop neurological sequelae. CNS diseases may be caused by other neurotropic viruses such as human immunodeficiency virus (HIV), cytomegalovirus, human herpesvirus 6, influenza virus, or measles virus [12].
Finally, JC virus (JCV), a polyomavirus that commonly establishes asymptomatic infection in the general population, is responsible for progressive multifocal leukoencephalopathy (PML), a fatal demyelinating disease of the CNS, in patients with severe immune deficiency. The development of new immunomodulatory and immunosuppressive drugs expanded the spectrum of conditions associated with PML [14].
Fungal infections
Unlike bacteria, fungi are eukaryotic (mostly saprophytic) organisms with membrane-bound nuclei that obtain nutrients from organic matter. Fungal infections of the CNS are commonly opportunistic, resulting from hematogenous dissemination in immunocompromised hosts. However, immunocompetent individuals are increasingly being reported as possible hosts for such infections. The fungal infections often originate from direct inoculation (e.g., trauma or surgery) of fungal spores.
Medically important fungi that invade the CNS include yeasts, molds (filamentous fungi), and dimorphic fungi (Fig 1, Table 1). CNS-infecting yeasts include a number of ubiquitous species, such as Candida spp. and Cryptococcus neoformans, the latter showing strong neurotropism. CNS-infecting molds include hyalohyphomycetes with septate hyphae (e.g., Aspergillus and Scedosporium/Lomentospora species) and the mucormycetes with nonseptate or sparsely septate hyphae (e.g., Mucor, Rhizopus, and Lichtheimia species). These fungi are distributed worldwide. Phaeohyphomycetes (dark molds) represent a third group of ubiquitous neurotropic molds (e.g., Cladophialophora bantiana, Exophiala dermatitidis, and Rhinocladiella mackenziei). Dimorphic fungi, such as Histoplasma, Blastomyces, Coccidioides, and Paracoccidioides, have a confined geographical distribution in the American continents and often infect the CNS [15].
Importantly, the morphology of the fungus influences the pathogenesis of CNS lesions. Fungi that develop as budding yeasts in vivo primarily cause meningitis (dimorphic fungi) or meningoencephalitis (Cryptococcus and Candida species). Those that exhibit yeast-to-hyphae transition (e.g., Candida albicans) can be more invasive, leading to necrosis and brain abscesses. Those that grow large hyphae (i.e., filamentous fungi) have a propensity for macrovascular invasion, causing hemorrhagic stroke, aneurysms, and cerebral abscesses. Cryptococcal meningoencephalitis is the most frequent fungal infection of the CNS, whereas candidiasis is the most common nosocomial infection. Aspergillosis and mucormycosis are relatively rare but devastating in immunosuppressed patients, while cerebral phaeohyphomycoses mainly occur in immunocompetent individuals [16]. Besides immunological disorders, some environmental, iatrogenic, and host-related factors may predispose an individual to the fungal CNS infection.
Parasitic infections
Parasitic diseases involving the CNS are major threats, especially in low- and middle-income countries. The causative agents include miscellaneous unicellular and multicellular organisms such as protozoa and worms, respectively (Fig 1, Table 1). Certain parasitic agents are highly dreaded in specific contexts, such as cerebral malaria in travelers returning from endemic regions with fever and any neurological symptoms or cerebral toxoplasmosis in patients infected with HIV. Parasitic infections of the CNS may be suspected in patients with nonspecific manifestations, such as meningitis, encephalitis, ventriculitis, myelitis, or brain abscess, with fever and headaches as chief complaints. The clinical presentation depends on the localization and size of the lesions, but distinct parasites may lead to the same symptomatology, making diagnosis challenging. Although a number of CNS parasitic infections are endemic in tropical countries, they are now spreading globally due to international migration and travel [17].
Nematode infections are the main cause of eosinophilic meningoencephalitis (especially Angiostrongylus and Gnathostoma species). In addition, neurocysticercosis, caused by larval cysts of the tapeworm Taenia solium, is the most common cause of epileptic seizures in low-income countries. In this respect, extraparenchymal forms (i.e., outside the brain tissue) result in high morbidity and mortality. In a pathophysiological perspective, the inflammatory response toward the larva is the hallmark of the disease and is supposed to contribute to BBB breakdown. Some protozoan species are also known for infecting the human CNS. This is notably the case of the flagellate Trypanosoma brucei, the etiological agent of African trypanosomiasis (sleeping sickness), which induces life-threatening meningoencephalitis. Although much rarer, the pathogenic free-living amoebae (Acanthamoeba, Balamuthia, and Naegleria species) are noteworthy causal agents due to their high case-fatality rates.
BBB crossing mechanisms of CNS-infecting pathogens
The human BBB is a neurovascular unit composed of brain microvascular endothelial cells (BMECs), pericytes, astrocytic end feet, microglia, and neurons. The presence of tight junctions (TJs) and adherens junctions (AJs) makes paracellular movements of even a small molecule extremely difficult [18]. Nevertheless, neurotropic pathogens can cross the BBB via (i) transcellular migration; (ii) paracellular migration; and/or (iii) a Trojan horse mechanism. In the transcellular mechanism, a pathogen binds to BMECs, then is taken up by BMECs through receptor-mediated endocytosis, is transported within a vacuole without fusion with lysosomes, and is finally released to the brain tissues. In the paracellular mechanism, a pathogen can traverse between BMECs by disrupting TJs and/or AJs, which can be facilitated by the induced expression of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, IL-6, and interferon gamma (IFNγ), in BMECs, pericytes, and astrocytes. In the Trojan horse mechanism, phagocytes infected with a pathogen cross the BBB paracellularly.
Several CNS-infecting bacteria, including E. coli, group B Streptococcus, S. pneumoniae, N. meningitidis, L. monocytogenes, and M. tuberculosis, cross the BBB transcellularly (see [19,20]). The latter 2 pathogens also cross the BBB via a Trojan horse mechanism. Bacterial surface adhesins are generally required for BBB crossing. These include CD48-interacting type I fimbrial adhesin FimH and gp96-interacting outer-membrane protein A in E. coli, a platelet-activating factor receptor-interacting cell wall phosphorylcholine in S. pneumoniae, laminin-binding protein, the fibrinogen-binding protein FbsA and invasion-associated gene A in S. agalactiae, and type IV pili PilC in N. meningitidis. In addition, several invasion proteins modulate host cytoskeleton regulation to promote transcellular traversal of bacterial pathogens.
Viruses can infect the CNS by either directly traversing the BBB through one of the mechanisms described above or by taking nonhematogenous routes, including retrograde axonal transport from peripheral nerves to the CNS and the nasal olfactory epithelium and neurons. In particular, CNS-infecting viruses can stimulate the production of pro-inflammatory cytokines and matrix metalloproteases in BMECs, astrocytes, and pericytes, which can destabilize TJs by activating the RhoA kinase pathway and promoting BBB crossing of neurotropic viruses [21,22].
Among the various neuroinfectious fungal pathogens, 2 pathogenic yeasts, C. albicans and C. neoformans, traverse the BBB transcellularly [23]. Inositol, which is abundantly present in the brain, is taken up by C. neoformans through inositol transporters Itr1a and Itr3c and induces the expression of hyaluronic acid (HA) synthase gene CPS1 in C. neoformans. The HA produced enhances the binding of the fungal pathogen to the CD44 glycoprotein in blood endothelial cells [24,25]. C. neoformans–derived extracellular microvesicles and the metalloprotease Mpr1 also contribute to the BBB crossing process of C. neoformans [26,27]. Recent systematic BBB crossing analyses of signature-tagged mutants and CRISPR/Cas-9–based gene deletion mutants in C. neoformans revealed that a variety of proteins involved in diverse biological functions are involved in BBB crossing and pathogen survival in the brain parenchyma [28]. In addition, C. neoformans can cross the BBB via Trojan horse mechanism [29]. C. albicans can cross the BBB by utilizing fungal invasins, Als3 and Ssa1, the former of which can interact with the gp96 receptor on BMECs [30].
The BBB crossing mechanisms of parasites are relatively well studied in Toxoplasma gondii and trypanosomes [31]. T. brucei can cross the BBB by expressing the cysteine protease cathepsin L (brucipain) that interacts with G-protein coupled receptors on host endothelial cells. T. gondii can cross the BBB in a Trojan horse mechanism. In this process, T. gondii secretes cyclophilin 18, which interacts with the chemokine receptor CCR5 present on phagocytic cells.
Toward new therapeutic approaches for treating CNS infections
Therapeutic options for CNS infections are highly limited, because the delivery of antimicrobial agents to the affected brain compartments is challenged by the structural complexity and tightness of the human BBB. To study the molecular mechanisms of microbial CNS invasion and expedite screening drugs for CNS infections and disorders, intensive efforts have been made to construct in vitro BBB models in the past years. The simplest but most widely used in vitro BBB model is a transwell system containing a monolayer of human BMECs with or without astrocytes [32]. Microfluidic devices have recently been developed to better reflect complex three-dimensional BBB structures [33]. Most recently, the human neurovascular unit (hNVU) chip, which contains all the necessary cellular and extracellular brain components, was used to examine the neurotropism and BBB penetration of C. neoformans [34]. Although screening and development of antimicrobial agents with a good BBB permeability are important, the application of BBB-penetrating conjugates could be a promising approach. In particular, a number of peptide-based BBB shuttles have been developed in the past decades [35–36]. These BBB shuttles could be applied to a variety of currently available antimicrobial agents in future.
In conclusion, recent advances in ex/in vivo and in vitro BBB and CNS models will not only facilitate understanding of the CNS infection pathophysiology, but also support the screening of novel antimicrobial agents for the treatment of microbial meningitis. Better treatment strategy targeting CNS infections is an essential prerequisite to improving the global management of these life-threatening microbial infections.
Funding Statement
This study was supported by National Research Foundation of Korea 2018R1A5A1025077, 2021M3A9I4021434 and 2021R1A2B5B03086596 (Y B). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22(Suppl 3):S37–62. doi: 10.1016/j.cmi.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 2.Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore). 1998;77:313–36. doi: 10.1097/00005792-199809000-00002 [DOI] [PubMed] [Google Scholar]
- 3.McIntyre PB, O’Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet. 2012;380:1703–11. doi: 10.1016/S0140-6736(12)61187-8 [DOI] [PubMed] [Google Scholar]
- 4.Slane VH, Unakal CG. Tuberculous meningitis. StatPearls. 2021. http://www.ncbi.nlm.nih.gov/books/NBK541015/ [PubMed]
- 5.Peeling RW, Mabey D, Kamb ML, Chen X-S, Radolf JD, Benzaken AS. Syphilis Nat Rev Dis Primers. 2017;3:17073. doi: 10.1038/nrdp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. doi: 10.1007/978-3-662-45059-8_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Summer G, Rupprecht TA. Neurologic manifestations of Lyme Borreliosis. Rev Neurol (Paris). 2019;175:417–9. doi: 10.1016/j.neurol.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 8.Rotbart HA. Viral meningitis. Semin Neurol. 2000;20:277–92. doi: 10.1055/s-2000-9427 [DOI] [PubMed] [Google Scholar]
- 9.Kohil A, Jemmieh S, Smatti MK, Yassine HM. Viral meningitis: an overview. Arch Virol. 2021;166:335–45. doi: 10.1007/s00705-020-04891-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright WF, Pinto CN, Palisoc K, Baghli S. Viral (aseptic) meningitis: A review. J Neurol Sci. 2019;398:176–83. doi: 10.1016/j.jns.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 11.Mallewa M, Vallely P, Faragher B, Banda D, Klapper P, Mukaka M, et al. Viral CNS infections in children from a malaria-endemic area of Malawi: a prospective cohort study. Lancet Glob Health. 2013;1:e153–60. doi: 10.1016/S2214-109X(13)70060-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozenberg F. Acute viral encephalitis. Handb Clin Neurol. 2013;112:1171–81. doi: 10.1016/B978-0-444-52910-7.00038-6 [DOI] [PubMed] [Google Scholar]
- 13.Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371:1861–71. doi: 10.1016/S0140-6736(08)60800-4 [DOI] [PubMed] [Google Scholar]
- 14.Saribas AS, Ozdemir A, Lam C, Safak M. JC virus-induced progressive multifocal leukoencephalopathy. Future Virol. 2010;5:313–23. doi: 10.2217/fvl.10.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockhart SR, Toda M, Benedict K, Caceres DH, Litvintseva AP. Endemic and other dimorphic mycoses in the Americas. J Fungi (Basel). 2021;7:151. doi: 10.3390/jof7020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velasco J, Revankar S. CNS Infections Caused by Brown-Black Fungi. J Fungi (Basel). 2019;5:E60. doi: 10.3390/jof5030060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyle CM. The returned traveler with neurologic manifestations: could my patient have a parasite? Curr Opin Infect Dis. 2021;34:245–54. doi: 10.1097/QCO.0000000000000732 [DOI] [PubMed] [Google Scholar]
- 18.Dickens D, Radisch S, Pirmohamed M. Chapter 5: Drug Transporters at the Blood–Brain Barrier. Drug Transporters. 2016;151–83. [Google Scholar]
- 19.Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol. 2008;6:625–34. doi: 10.1038/nrmicro1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Sorge NM, Doran KS. Defense at the border: the blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012;7:383–94. doi: 10.2217/fmb.12.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miner JJ, Diamond MS. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr Opin Immunol. 2016;38:18–23. doi: 10.1016/j.coi.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–93. doi: 10.1016/j.chom.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Góralska K, Blaszkowska J, Dzikowiec M. Neuroinfections caused by fungi. Infection. 2018;46:443–59. doi: 10.1007/s15010-018-1152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng H-K, Huang T-Y, Wu Y-J, Chen H-H, Liu C-P, Jong A. How Cryptococcus interacts with the blood-brain barrier. Future Microbiol. 2015;10:1669–82. doi: 10.2217/fmb.15.83 [DOI] [PubMed] [Google Scholar]
- 25.Vu K, Garcia JA, Gelli A. Cryptococcal meningitis and anti-virulence therapeutic strategies. Front Microbiol. 2019;10:353. doi: 10.3389/fmicb.2019.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S-H, Wu C-H, Chang YC, Kwon-Chung KJ, Brown RJ, Jong A. Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PLoS ONE. 2012;7:e48570. doi: 10.1371/journal.pone.0048570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vu K, Tham R, Uhrig JP, Thompson GR, Na Pombejra S, Jamklang M, et al. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio. 2014;5:e01101–14. doi: 10.1128/mBio.01101-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K-T, Hong J, Lee D-G, Lee M, Cha S, Lim Y-G, et al. Fungal kinases and transcription factors regulating brain infection in Cryptococcus neoformans. Nat Commun. 2020;11:1521. doi: 10.1038/s41467-020-15329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santiago-Tirado FH, Onken MD, Cooper JA, Klein RS, Doering TL. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. mBio. 2017;8:e02183–16. doi: 10.1128/mBio.02183-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Mittal R, Solis NV, Prasadarao NV, Filler SG. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog. 2011;7:e1002305. doi: 10.1371/journal.ppat.1002305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masocha W, Kristensson K. Passage of parasites across the blood-brain barrier. Virulence. 2012;3:202–12. doi: 10.4161/viru.19178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su Y, Sinko PJ. Drug delivery across the blood-brain barrier: why is it difficult? how to measure and improve it? Expert Opin Drug Deliv. 2006;3:419–35. doi: 10.1517/17425247.3.3.419 [DOI] [PubMed] [Google Scholar]
- 33.Maoz BM. Brain-on-a-Chip: Characterizing the next generation of advanced in vitro platforms for modeling the central nervous system. APL Bioeng. 2021;5:030902. doi: 10.1063/5.0055812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Lee K-T, Lee JS, Shin J, Cui B, Yang K, et al. Fungal brain infection modelled in a human-neurovascular-unit-on-a-chip with a functional blood-brain barrier. Nat Biomed Eng. 2021;5:830–46. doi: 10.1038/s41551-021-00743-8 [DOI] [PubMed] [Google Scholar]
- 35.Malakoutikhah M, Guixer B, Arranz-Gibert P, Teixidó M, Giralt E. ’À la carte’ peptide shuttles: tools to increase their passage across the blood-brain barrier. ChemMedChem. 2014;9(7):1594–601. doi: 10.1002/cmdc.201300575 [DOI] [PubMed] [Google Scholar]
- 36.Mendonça DA, Bakker M, Cruz-Oliveira C, Neves V, Jiménez MA, Defaus S, et al. Penetrating the Blood-Brain Barrier with New Peptide-Porphyrin Conjugates Having anti-HIV Activity. Bioconjug Chem. 2021. 16;32(6):1067–77. doi: 10.1021/acs.bioconjchem.1c00123 [DOI] [PMC free article] [PubMed] [Google Scholar]