Abstract
Introduction
Parasitic infections, especially intestinal protozoan parasites (IPPs) remain a significant public health issue in Africa, where many conditions favour the transmission and children are the primary victims. This systematic review and meta-analysis was carried out with the objective of assessing the prevalence of IPPs among school children in Africa.
Methods
Relevant studies published between January 2000 and December 2020 were identified by systematic online search on PubMed, Web of Science, Embase and Scopus databases without language restriction. Pooled prevalence was estimated using a random-effects model. Heterogeneity of studies were assessed using Cochrane Q test and I2 test, while publication bias was evaluated using Egger’s test.
Results
Of the 1,645 articles identified through our searches, 46 cross-sectional studies matched our inclusion criteria, reported data from 29,968 school children of Africa. The pooled prevalence of intestinal protozoan parasites amongst African school children was 25.8% (95% CI: 21.2%-30.3%) with E. histolytica/ dispar (13.3%; 95% CI: 10.9%-15.9%) and Giardia spp. (12%; 95% CI: 9.8%-14.3%) were the most predominant pathogenic parasites amongst the study participants. While E. coli was the most common non-pathogenic protozoa (17.1%; 95% CI: 10.9%-23.2%).
Conclusions
This study revealed a relatively high prevalence of IPPs in school children, especially in northern and western Africa. Thus, poverty reduction, improvement of sanitation and hygiene and attention to preventive control measures will be the key to reducing protozoan parasite transmission.
Author summary
Pathogenic intestinal protozoan parasites (IPPs) remain a major public health concern. Studies have documented that, the prevalence rates of protozoan infections are quite high in developing regions, particularly Africa and children are the primary victims. Despite numerous studies have been conducted on IPPs in school children in African countries, data on the burden of these infections in African school children have not yet been synthesised. This systematic review and meta-analysis was conducted to provide continent-wide prevalence of IPPs amongst African school children. Our study found that about 25.8% of the children had one or more species of intestinal protozoan parasites in their faecal specimens. E. histolytica/ dispar and Giardia spp. were the most predominant parasites amongst the study participants. The relatively high prevalence estimate of IPPs amongst African children and the considerable variation in the disease prevalence over the years, between and within countries and regions clearly indicates the needs to improve sanitation and hygiene, paying more attention to preventive control measures as well as poverty reduction which are the key to reducing protozoan parasite transmission.
Introduction
Despite the significant improvements in health facilities and quality of medical services in terms of diagnosis and mass treatment of parasitic diseases, most of them are still considered major public health problems [1,2]. Infections caused by intestinal protozoan parasites (IPPs) are among the most prevalent human diseases that affect a large section of poor communities particularly in developing countries [3,4]. They have been recognised as significant causes of gastrointestinal illnesses, malnutrition and substantial mortality. Several pathogenic protozoan parasites are responsible for the above health issues including Entamoeba histolytica/dispar, Giardia lamblia (also known as Giardia intestinalis and Giardia duodenalis), Cryptosporidium and Balantidium coli, which are the most common species associated with significant illnesses [3,5,6]. Infection by E. histolytica is considered the third most common cause of death after malaria and schistosomiasis [7]. In addtion, Cryptosporidium spp. and G. lamblia are important nonviral causes of diarrhoeal diseases in humans [8], while other species of intestinal protozoa are either not widely prevalent or non-pathogenic parasites.
Studies have documented that, the prevalence rates of protozoan infections are quite high in developing regions, particularly Africa, and people there are often infected with one or multiple protozoan parasites [9]. The high prevalence of the pathogenic and non-pathogenic protozoa in this continent is intimately related to poverty, poor environmental conditions, lack of access to clean water and adequate sanitation, inadequate hygiene practices and ignorance of health-promoting behaviours [10]. Despite people of all ages are at risk of being infected by intestinal protozoa, children are the most vulnerable and more likely to present with clinical symptom. Furthermore, school children aged 5–17 years are disproportionately affected and often heavily infected because of their habits of playing or handling infested soil, performing unhygienic toilet practices and eating or drinking with soiled hands [11].
Baseline data on the burden, distribution and trend of IPPs can provide essential evidence for implementation of effective prevention strategies in combating these neglected protozoan infections [12]. In this regard, the number of published articles on the epidemiology of IPPs have remarkably increased in recent years. Several studies have been conducted on IPPs in school children in African countries. Hence, there is a need for summarising and critically analysing the available studies to estimate the overall prevalence. To date, no systemic review or meta-analysis on the prevalence of IPPs among school children in Africa has been conducted. Thus, the present study aimed to synthesise existing data on the prevalence of IPPs among school children in Africa, in order to generate much needed, contemporary and reliable continent-wide estimates which might be helpful in the implementation of the relevant prevention and control measures.
Methods
This study systematically reviewed and analysed the published articles by using the meta-analysis approach to estimate the prevalence of intestinal protozoan parasites among school children in Africa. Literature search, selection of publications and reporting of results were conducted according to the PRISMA guidelines (S1 Checklist) [13]. The protocol of this systematic review and meta-analysis was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database. The registration number is CRD42021233119.
Search strategy
A comprehensive literature search was performed using all identifed keywords in four electronic databases (PubMed, Web of Science, Embase and Scopus) for the identification of relevant studies that report the prevalence of intestinal protozoan parasites among school children in Africa from January 2000 to December 2020. No language restriction was applied. Moreover, a manual search was conducted using references from retrieved articles for the identification of additional relevant studies that we might have missed. The detailed search strategy for all databases is presented in the S1 Table.
Data management and study selection
All identified articles were initially retrieved and managed using Endnote X8. After the removal of duplicates, relevant studies were selected independently by two authors (KH and AMS). The titles and abstracts of the retrieved studies were evaluated on the basis of the eligibility criteria. Subsequently, articles with any potential to be eligible for inclusion or any uncertainty about eligibility were further subjected to a full text review. Any disagreement or uncertainty was resolved by discussion, and when necessary, by a third reviewer (MAI). Furthermore, attempts have been made to gain missing data or to clarify any uncertainty with corresponding authors. Articles reporting the same research data/findings published in different formats/titles by the same author were counted only once.
Inclusion and exclusion criteria
The eligibility of full text articles to be included in this study was evaluated using the following inclusion criteria: (1) cross-sectional studies; (2) conducted in Africa and reporting prevalence of intestinal protozoan parasites; and (3) published between 1 January 2000 and 30 December 2020. The exclusion criteria were as follows: (1) case reports, reviews and studies without original data; (2) non cross-sectional studies; (3) overall prevalence was not reported and impossible to estimate on the basis of the results and confusing or unclear analysis results; (4) survey was conducted in a hospital or healthcare facilities; (5) and articles that had limited access and those of authors who did not respond to email two times.
Definition of intestinal protozoan infection and outcome measures
In the context of this study, an IPPs were defined as detection of one or more of the following intestinal parasites: E. histolytica/dispar, Giardia spp., Cryptosporidium spp., E. coli and other non-pathogenic protozoan parasites. The main outcome of this systematic review and meta-analysis was the estimated pooled prevalence of IPPs among school children in Africa. The prevalence of IPPs was defined as the proportion of positive samples to the total number of samples.
Data extraction
Relevant data from each eligible article was extracted and entered into a predefined Excel spreadsheet by the two authors (KH and AMS). Before the inclusion of data in the review, extracted information was checked twice by KH and MAI to ensure consistency and the absence of bias and minimise errors. The following data were extracted: first author’s name, year of publication, children enrolment time, country and region where the study was conducted, gender, diagnostic method, sample size, total number of cases, identified species and number of identified species. The United Nations Statistics Division (UNSD) African region (Northern, Eastern, Central, Western and Southern Africa) was assigned to each study according to the country of recruitment.
Quality assessment
The methodological quality of each included study was appraised by two independent authors (KH and MAI) using the Joana Brigg’s Institute (JBI) for prevalence studies [14], having nine checklist items with four options: ‘yes’, ‘no’, ‘unclear’ and ‘not applicable’ were used. The final score of each article was calculated according to the proportion of ‘yes’ answers. Studies were categorised as ‘high risk of bias’ (low quality), ‘moderate risk of bias’ (moderate quality) or ‘low risk of bias’ (high quality) when the overall score was ≤ 49%, 50%–69% or ≥70% respectively [15,16].
Data analysis
The prevalence estimate and corresponding 95% confidence interval (CI) were calculated for each included study. The prevalence data were then pooled through statistical meta-analysis with the restricted maximum likelihood (REML) method for random-effects model. A forest plot was generated to present the summarised results and heterogeneity among the included studies. Heterogeneity among studies was assessed using I2 statistics, in which I2 values of greater than 75% inidicated substantial heterogeneity [17]. The significance of heterogeneity was identified using Cochran’s Q-test. Publication bias was checked visually using a funnel plot and objectively using Egger’s regression test.
The potential sources of heterogeneity were further explored by subgroup analysis according to children enrolment time, detection method, region and sample size. Furthermore, the robustness of the pooled estimate was tested through sensitivity analysis according to the following strategies: (i) excluding small studies (n < 200); (ii) excluding moderate-quality studies (moderate risk of bias); (iii) excluding studies that used non-microscopic detection methods; and (iv) excluding outlier studies. Data analysis was performed and a plot was created using metaprop codes in the meta (version 4.15–1) and metafor (version 2.4–0) packages of R (version 3.6.3) in RStudio (version 1.3.1093).
Results
Study selection
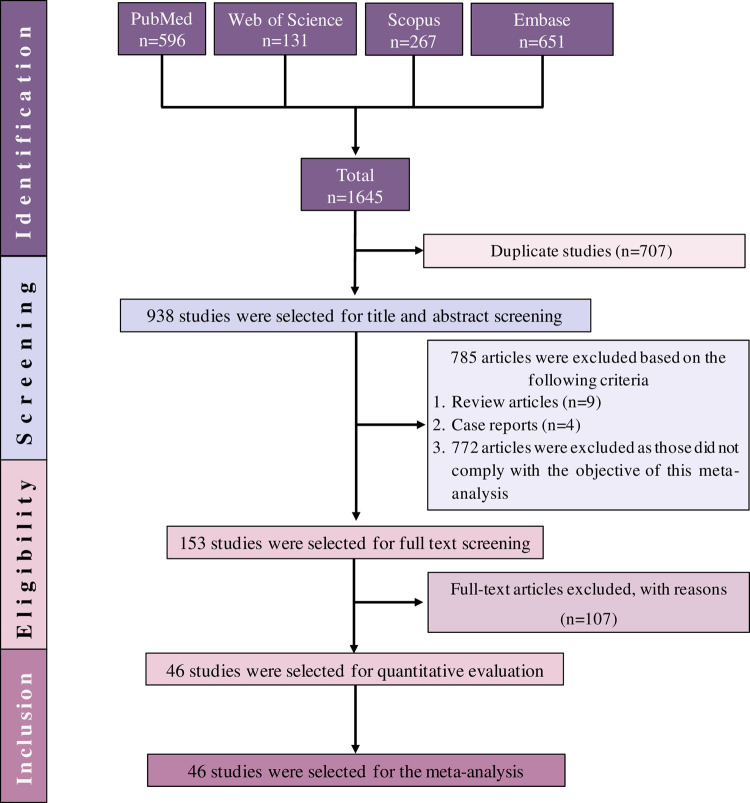
A total of 1,645 articles were initially identified form the four databases. After 707 duplicates were removed, another 785 studies were excluded from the remaining articles after title and/or abstract evaluation. Furthermore, 107 articles were further excluded during the full text assessment with reasons (S2 Table). Finally, only 46 (2.8%) of the articles met the eligibility criteria and included in the systematic review and meta-analysis (Fig 1).
Fig 1. PRISMA flow diagram of study selection.
Characteristics of included studies
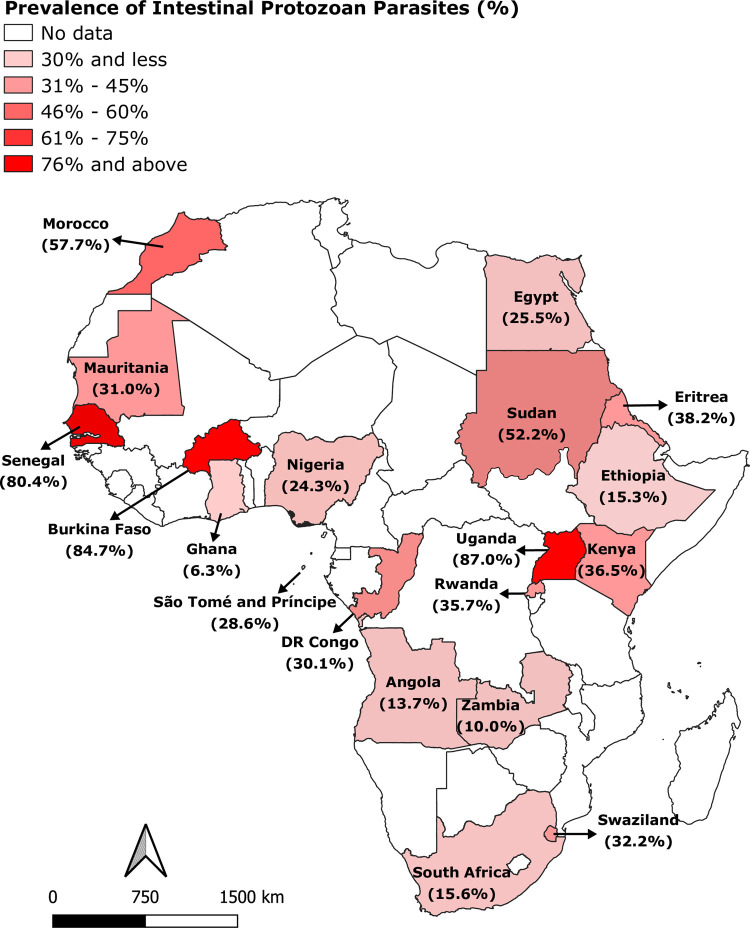
The detailed characteristics of the included studies are summarised in Table 1. The 46 eligible studies were conducted in 19 African countries, across the five UNSD regions of Africa. Ethiopia had the highest number of eligible studies (17 studies), followed by Nigeria (six studies) and South Africa (three studies). Two studies were conducted in each of the four countries, namely: Angola, Ghana, Kenya and Egypt, and one study was performed in each of the following countries: Burkina Faso, Democratic Republic of the Congo, DR of Sao Tome and Principe, Eritrea, Kingdom of Swaziland, Mauritania, Morocco, Rwanda, Senegal, Sudan, Uganda and Zambia. The included studies were school-based surveys and had cross-sectional study designs. A total of 29,968 school children aged 6–17 years were examined for the presence of IPPs. Microscopy was the predominant detection method for IPPs laboratory confirmation (40 studies). Molecular detection was used in four studies, similar to rapid diagnostic test. A range of protozoan parasites were detected across the studies, including: Entamoeba histolytica/ dispar, Giardia spp., Cryptosporidium spp., Entamoeba coli, Entoamoeba hartmanii, Cyclospora cayetanensis, Blastocystis hominis, Endolimax nana and Iodamoeba butschli. A map with the geographical distributions across the continent based on the studies included is presented in Fig 2.
Table 1. Major characteristics of the included studies.
| No | Study ID (references) | Publication Year | Country, place | Sample size (% female) | Cases | Methods | Reported parasites |
|---|---|---|---|---|---|---|---|
| 1 | Abdel-Aziz 2010 [18] | 2010 | Sudan, El dhayga, Central Sudan | 157 (47.8) | 83 | DWM and FECT | E. histolytica and G. lamblia |
| 2 | Abossie 2014 [19] | 2014 | Ethiopia, Chencha town, Southern Ethiopia | 400 (52.3) | 94 | DWM and FECT | E. histolytica/dispar and G. lamblia |
| 3 | Adams 2005 [20] | 2005 | South Africa, Cape Town | 3890 (49.8) | 673 | FECT | Giardia spp. |
| 4 | Adedoja 2015 [21] | 2015 | Nigeria, Pategi, Kwara State | 748 (50.8) | 197 | DWM and FECT | E. histolytica, E. coli, G. lamblia |
| 5 | Alemu 2019a [22] | 2019 | Ethiopia, Birbir, Southern Ethiopia | 351 (48.7) | 25 | DWM and FECT | E. histolytica/dispar and G. lamblia |
| 6 | Alemu 2019b [23] | 2019 | Ethiopia, Northwest | 273 (45.8) | 22 | DWM and FECT | E. histolytica/dispar and G. lamblia |
| 7 | Al-Shehri 2019 [24] | 2019 | Uganda, Gondar town | 254 (50.4) | 221 | qPCR | G. duodenalis |
| 8 | Amare 2013 [25] | 2013 | Ethiopia, Gondar town, Northwest | 405 (46.2) | 2 | DWM and FECT | G. lamblia and Entamoeba spp. |
| 9 | Awolaju 2009 [26] | 2009 | Nigeria, South-west | 312 (54.5) | 29 | DWM | E. histolytica |
| 10 | Ayogu 2015 [27] | 2015 | Nigeria, Enugu State | 450 (51.6) | 190 | DWM | E.histolytica |
| 11 | Baba 2012 [28] | 2012 | Mauritania, Gorgol, Guidimagha and Brakna | 1308 (57.8) | 405 | DWM | E. histolytica, E. coli, E. hartmani, G. intestinalis, E. nanus, Pseudolimax butchilii and C. mesnilii |
| 12 | Birhanu 2018 [29] | 2018 | Ethiopia, Pawe, Northwest Ethiopia | 422 (54.0) | 20 | DWM and FECT | G. lamblia |
| 13 | Bisangamo 2017 [30] | 2017 | Kiliba city, Eastern DR Congo | 602 (55.1) | 181 | DWM | E. histolytica, G. intestinalis and Trichomonas intestinalis |
| 14 | Chege 2020 [31] | 2020 | Kenya, Nakuru town | 96 (NR) | 40 | PCR | E. dispar, E. coli and G. intestinalis |
| 15 | de Alegria 2017 [32] | 2017 | Angola, Cubal, Southwestern | 230 (56.1) | 17 | FECT | G. lamblia, B. coli and E. histolytica/dispar |
| 16 | Dyab 2016 [33] | 2016 | Egypt, Aswan | 300 (43.3) | 58 | DWM, FECT and mZN stain |
E. histolytica, G. lamblia and Cryptosporidium spp. |
| 17 | Erismann 2016 [34] | 2016 | Burkina Faso, Plateau Central and Centre-Ouest regions | 385 (48.8) | 326 | DWM and FECT | E. histolytica/dispar, E. coli, Trichomonas intestinalis, B. coli and G. intestinalis |
| 18 | Eyamo 2019 [35] | 2019 | Ethiopia, Tula Sub-City, Southern | 384 (51.8) | 82 | FECT | G. duodenalis and E. histolytica/dispar |
| 19 | Fan 2012 [36] | 2012 | Kingdom of Swaziland, northwestern | 267 (56.9) | 86 | MIFC | G. lamblia, E. histolytica/dispar, B. hominis, E. coli, E. nana, C. mesnelli and Iodamoeba butschlii |
| 20 | Forson 2017 [37] | 2017 | Ghana, Accra | 300 (48.0) | 33 | DWM and FECT | G. lamblia and E. histolytica/dispar |
| 21 | Gebretsadik 2020 [38] | 2020 | Ethiopia, Harbu, North East | 400 (62.3) | 37 | DWM and FECT | E. histolytica and G. lamblia |
| 22 | Gelaw 2013 [39] | 2013 | Ethiopia, Gondar town | 304 (44.1) | 40 | DWM and FECT | E. histolytica/dispar and G. intestinalis |
| 23 | Gyang 2019 [40] | 2019 | Nigeria, Lagos city | 384 (51.0) | 202 | MIFC | E. histolytica/dispar, E. coli, G. duodenalis, E. nana and B. hominis |
| 24 | Hailegebriel 2017 [41] | 2017 | Ethiopia, Bahir Dar | 359 (50.7) | 134 | FECT | E. histolytica, G. lamblia and T. hominins |
| 25 | Hailegebriel 2018 [42] | 2018 | Ethiopia, Bahir Dar | 382 (49.5) | 66 | FECT | E. histolytica/dispar, G. lamblia and Isospora belli |
| 26 | Hall 2008 [43] | 2008 | Ethiopia, all 11 regions of Ethiopia | 7466 (50.2) | 239 | FECT | G. duodenalis |
| 27 | Heimer 2015 [44] | 2015 | Rwanda, Huye district | 622 (NR) | 222 | qPCR | G. duodenalis |
| 28 | Htun 2018 [45] | 2018 | South Africa, Port Elizabeth, South-eastern | 842 (49.4) | 114 | RDTs | C. parvum and G. intestinalis |
| 29 | Ibrahium 2011 [46] | 2011 | Egypt, Minia Governorate | 264 (64.8) | 84 | DWM and FECT | G. lamblia and E. coli |
| 30 | Ihejirika 2019 [47] | 2019 | Nigeria, Imo State, South Eastern | 300 (NR) | 32 | FECT | E. histolytica, E. coli and G. lambia |
| 31 | Jejaw 2015 [48] | 2015 | Ethiopia, Mizan-Aman, Southwest | 460 (50.4) | 36 | DWM and FECT | G. lamblia and E. histolytica/dispar/moshkovskii |
| 32 | Kesete 2020 [49] | 2020 | Eritrea, Ghindae town | 450 (52.2) | 172 | FECT | E. histolytica/dispar and G. duodenalis |
| 33 | Khaled 2020 [50] | 2020 | Senegal, northwestern | 731 (48.2) | 588 | qPCR | Blastocystis spp. |
| 34 | Legesse 2010 [51] | 2010 | Ethiopia, Adwa, Northern | 381 (56.2) | 7 | FECT | E. histolytica/dispar |
| 35 | Liao 2016 [52] | 2016 | DR of Sao Tome and Principe, Capital areas | 252 (52.0) | 72 | MIFC | E. histolytica/dispar, G. intestinalis and B. hominis |
| 36 | Mahmud 2013 [53] | 2013 | Ethiopia, Northern | 583 (53.5) | 286 | DWM and FECT | E. histolytica/dispar and G. lamblia |
| 37 | Müller 2016 [54] | 2016 | South Africa, Port Elizabeth | 934 (49.5) | 144 | RDTs | C. parvums and G. intestinalis |
| 38 | Nguyen 2012 [55] | 2012 | Ethiopia, Angolela | 664 (48.6) | 202 | FECT | G. lamblia and E. histolytica |
| 39 | Njambi 2020 [56] | 2020 | Kenya, Mwea, Central | 180 (50.0) | 59 | DWM | E. histolytica/dispar, E. coli and G. intestinalis |
| 40 | Oliveira 2015 [57] | 2015 | Angola, Lubango city, Huíla Province | 328 (56.4) | 66 | DWM | G. lamblia and E. histolytica/dispar |
| 41 | Opara 2012 [58] | 2012 | Nigeria, Akwa Ibom State | 405 (49.4) | 21 | DWM and FECT | G. lamblia and E. histolytica/dispar |
| 42 | Orish 2019 [59] | 2019 | Ghana, Volta Region | 550 (54.7) | 11 | DWM | Entamoeba spp. |
| 43 | Reji 2011 [60] | 2011 | Ethiopia, Adama town | 358 (57.3) | 57 | FECT | E. histolytica/dispar and G. lamblia |
| 44 | Sitotaw 2020 [61] | 2020 | Ethiopia, Sasiga District, Southwest | 383 (48.8) | 58 | DWM and FECT | E. histolytica and G. intestinalis |
| 45 | Tagajdid 2012 [62] | 2012 | Morocco, Salé city | 123 (NR) | 71 | MIFC | E. histolytica/dispar, G. intestinalis, E. nana, C. mesnilii and B. hominis |
| 46 | Tembo 2020 [63] | 2020 | Zambia, Lusaka | 329 (55.6) | 33 | DWM and FECT | G. duodenalis |
NR; not recorded, DWM; Direct wet mount, FECT: formalin-ether concentration technique, MIFC: Merthiolate-iodine-formaldehyde concentration, mZN stain; PCR: Polymerase chain reaction, qPCR: Real-time PCR, RDTs: Rapid Diagnostic Tests
Fig 2. Prevalence of intestinal protozoan parasites among school children in Africa.
Figure created by authors using QGIS software. Basemap source: https://www.diva-gis.org/Data.
Pooled prevalence of intestinal protozoan
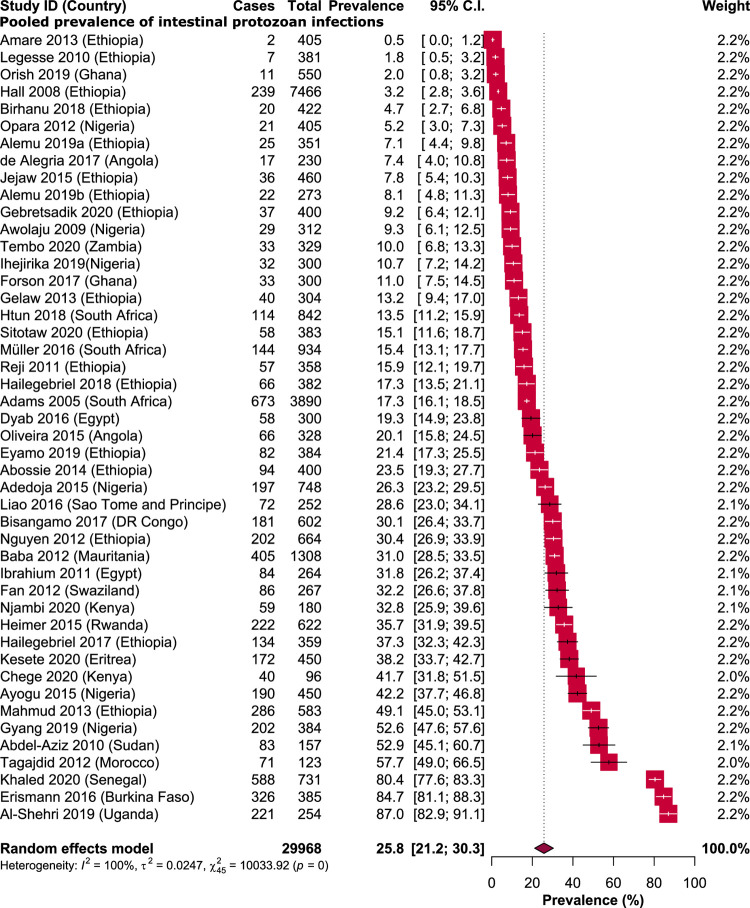
The prevalence of IPPs among school children in Africa ranged from 0.5% (95% CI: 0.0%-1.2%) in Ethiopia to 87% (95% CI: 82.9%-91.1%) in Uganda (24, 25). An overall prevalence of 25.8% (95% CI: 21.2%-30.3%) was obtained from 7731 school children infected with one or more species of IPPs. Substantial heterogeneity were seen across all the included studies (I2 = 100%, P < 0.001) (Fig 3).
Fig 3. Forest plot representing the pooled prevalence of intestinal protozoan infections among school children in Africa.
Quality assessment and publication bias
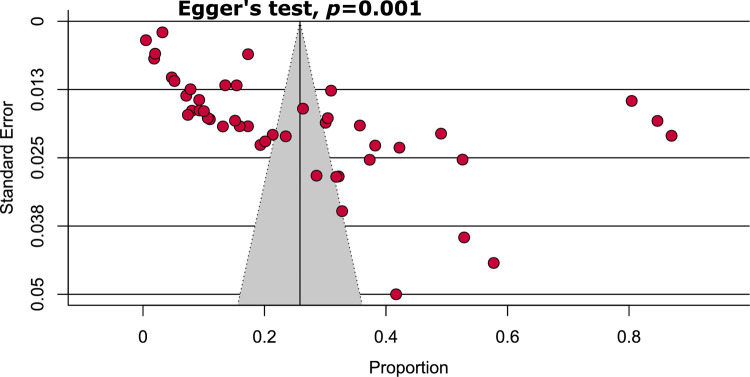
Information about the individual study quality assessment is presented in S3 Table. Briefly, 58.7% of the included studies were of high quality (low risk of bias), whereas the remaining 41.3% were of moderate quality. Funnel plot asymmetry indicated the existence of publication bias among the included studies (Fig 4). Similarly, regression-based Egger’s test revealed statistically significant publication bias (P = 0.001).
Fig 4. Funnel plot representing evidence of publication bias.
Subgroup analysis
With evidence of the substantial heterogeneity, subgroup analysis was performed. The results are shown in Table 2 and S1 Fig. According to children enrolment time, prevalence data were pooled into three-year periods for comparison. The prevalence of IPPs was gradually increased from 19.4% during the period between 2005 and 2010 to 23.5% in the next five years (2011–2015), and to 25.2% from 2016 to 2020. Among the UNSD African regions, Northern Africa had the highest prevalence (42.2%; CI: 22.7%-57.6%), followed by Western Africa (32.3%; 95% CI: 15.1%-49.5%), Eastern Africa (21.9%; 95% CI: 17.0% -26.8%) and Central Africa (21.5%; 95% CI: 10.1%-32.8%). Southern Africa had the lowest prevalence of 18.6% (95% CI: 14.5%-22.8%). Notably, remarkable differences in IPPs estimates obtained with laboratory detection methods were observed. A remarkably high overall estimate was observed when PCR or qPCR were used (61.4%; CI: 35.3–87.4%), and the pooled prevalence rates obtained through microscopy or RDTs were 22.7 (95% CI: 18.8–26.6%) or 14.5 (95% CI: 12.6–16.3%), respectively.
Table 2. Pooled prevalence of intestinal protozoan infections in different subgroups.
| Subgroups | Prevalence [95% CIs] (%) | Number of studies analysed | Total number of subjects | Heterogeneity | Publication Bias, Egger’s test (p-value) | ||
|---|---|---|---|---|---|---|---|
| I 2 | p-value | ||||||
| Children enrolment time | |||||||
| Year 2005–2010 | 19.4 [12.5–26.4] | 9 | 3,168 | 99% | <0.0001 | NA | |
| Year 2011–2015 | 23.5 [9.3–37.7] | 10 | 4,107 | 99% | <0.0001 | 0.45 | |
| Year 2016–2020 | 25.2 [6.9–43.4] | 9 | 3,314 | 100% | <0.0001 | NA | |
| Different regions of Africa | |||||||
| Northern Africa | 40.2 [22.7–57.6] | 4 | 844 | 97% | <0.0001 | NA | |
| Eastern Africa | 21.9 [17.0–26.8] | 23 | 15,906 | 99% | <0.0001 | 0.02 | |
| Central Africa | 21.5 [10.1–32.8] | 4 | 1,412 | 97% | <0.0001 | NA | |
| Western Africa | 32.3 [15.1–49.5] | 11 | 5,873 | 100% | <0.0001 | 0.20 | |
| Southern Africa | 18.6 [14.5–22.8] | 4 | 5,933 | 92% | <0.0001 | NA | |
| Countries | |||||||
| Ethiopia | 15.3 [11.7–19.0] | 17 | 13,975 | 99% | <0.0001 | <0.0001 | |
| Nigeria | 24.3 [10.7–37.8] | 6 | 2,599 | 99% | <0.0001 | NA | |
| South Africa | 15.6 [13.3–17.9] | 3 | 5,666 | 77% | 0.01 | NA | |
| Angola | 13.7 [1.2–26.2] | 2 | 558 | 95% | <0.0001 | NA | |
| Ghana | 6.3 [0.0–15.2] | 2 | 850 | 96% | <0.0001 | NA | |
| Kenya | 36. 5 [27.9–45.1] | 2 | 276 | 52% | 0.14 | NA | |
| Egypt | 25.5 [13.2–37.7] | 2 | 564 | 91% | <0.0001 | NA | |
| Burkina Faso | 84.7 [81.1–88.3] | 1 | 385 | NA | NA | NA | |
| DR Congo | 30.1 [26.4–33.7] | 1 | 602 | NA | NA | NA | |
| Eritrea | 38.2 [33.7–42.7] | 1 | 450 | NA | NA | NA | |
| Mauritania | 31.0 [28.5–33.5] | 1 | 1308 | NA | NA | NA | |
| Morocco | 57.7 [49.0–66.5] | 1 | 123 | NA | NA | NA | |
| Rwanda | 35.7 [31.9–39.5] | 1 | 622 | NA | NA | NA | |
| Sao Tome Principe | 28.6 [23.0–34.1] | 1 | 252 | NA | NA | NA | |
| Senegal | 80.4 [77.6–83.3] | 1 | 731 | NA | NA | NA | |
| Sudan | 52.9 [45.1–60.7] | 1 | 157 | NA | NA | NA | |
| Swaziland | 32.2 [26.6–37.8] | 1 | 267 | NA | NA | NA | |
| Uganda | 87.0 [82.9–91.1] | 1 | 254 | NA | NA | NA | |
| Zambia | 10.0 [6.8–13.3] | 1 | 329 | NA | NA | NA | |
| Different diagnostic methods | |||||||
| Microscopy | 22.7 [18.8–26.6] | 40 | 26,489 | 99% | <0.0001 | <0.0001 | |
| PCR or qPCR | 61.4 [35.3–87.4] | 4 | 1,703 | 99% | <0.0001 | NA | |
| Rapid diagnostic kit | 14.5 [12.6–16.3] | 4 | 1,776 | 21% | 0.16 | NA | |
| Different species | |||||||
| Giardia spp. | 12.0 [9.8–14.3] | 38 | 26,565 | 99% | <0.0001 | 0.0003 | |
| E. histolytica/ dispar | 13.3 [10.7–15.9] | 33 | 13,235 | 99% | <0.0001 | <0.0001 | |
| Entamoeba coli | 17.1 [10.9–23.2] | 9 | 3,788 | 97% | <0.0001 | NA | |
| Cryptosporidium spp. | 2.5 [1.8–3.2] | 3 | 2,076 | 3% | 0.35 | NA | |
CIs: Confidence intervals; NA: Not applicable.
Common intestinal protozoan infections among school children
Of the 46 included studies, Giardia spp. (38/46 [82.6%]) and E. histolytica/ dispar (33/46 [71.7%]) had the highest number of reports (Table 2). Similarly, E. histolytica/ dispar was the most common pathogenic protozoan parasite detected in children (13.3%; 95% CI: 10.9%-15.9%), followed by Giardia spp. (12%; 95% CI: 9.8%-14.3%) and Cryptosporidium spp. (2.5%; 95% CI: 1.8%-3.2%). Of the non-pathogenic protozoa, E. coli was the most common, with a prevalence of 17.1% (95% CI: 10.9%-23.2%).
Sensitivity analysis
Sensitivity analyses indicated that the exclusion of small studies, studies that used non-microscopic detection methods and outlier studies (Fig 5) did not significantly altered the summary of the pooled estimates. Prevalence rate remained within the 95% CI of the respective overall prevalence (Table 3 and S2 Fig). Despite that the removal of moderate-quality studies reduced the overall prevalence by 9.4%, it did not significantly reduce heterogeneity. Overall, the stability of IPPs prevalence validated the reliability and rationality of our analyses.
Fig 5. Galbraith plot depicting three outlier studies.
Table 3. Sensitivity analyses.
| Strategies of Sensitivity analyses | Prevalence [95% CIs] (%) | Difference of pooled prevalence compared to the main result | Number of studies analysed | Total number subjects | Heterogeneity | |
|---|---|---|---|---|---|---|
| I 2 | p-value | |||||
| Excluding small studies | 23.9 [19.2–28.6] | 1.9% lower (2.0% lower—1.7% lower) |
42 | 29,412 | 100% | <0.0001 |
| Excluding moderate-quality studies | 16.4 [12.9–19.8] | 9.4% lower (8.3% lower—10.5% lower) |
27 | 21,064 | 99% | <0.0001 |
| Excluding studies used non-microscopic detection methods | 22.7 [18.8–26.6] | 3.1% lower (2.4% lower—3.7% lower) |
40 | 26,489 | 99% | <0.0001 |
| Excluding outlier studies | 21.4 [18.2–24.6] | 4.4% lower (3.0% lower—5.7% lower) |
43 | 28,598 | 99% | <0.0001 |
CIs: confidence intervals
Discussion
Intestinal protozoan infections significantly contribute to the burden of gastrointestinal illnesses throughout Africa, where many conditions favour the transmission and children are the primary victims [64,65]. Here, we present the first systematic review and meta-analysis of the continent-wide prevalence of IPPs amongst school children. The current review compiled eligible data on the prevalence of IPPs from 29,968 school children reported in 46 studies conducted in 19 African countries. The prevalence rates of IPPs in African school children varied greatly amongst the included studies. According to Fig 2, the highest and lowest prevalence rates of IPPs were reported in studies conducted in Uganda (87%, 95% CI: 82.9%-91.1%) [24] and Ethiopia (0.5%, 95% CI: 0.0%-1.2%), respectively [25]. Such considerable variation is not surprising given that environmental conditions and socioeconomic status vary between and within the countries and different detection methods are used. In this review, the prevalence of IPPs amongst children was 25.8% (95% CI: 21.2%-30.3%), which could be due to poor hygiene given that the disease is transmitted via food, water and fingers that are contaminated with faeces. The relatively high number (7,731) of school children with IPPs in Africa in the present study is aligned with the 24.2% infection rate reported in Thailand [66]. However, our finding is higher than the data in Iran (16.9%) [67]. The difference might be attributed to the aforementioned reasons in addition to personal and cultural habits.
Significant decreasing trends of IPPs were observed amongst children in Nepal [68] and India [69], which could be due to improvement in sanitation and hygiene, socioeconomic development and establishing preventive control measures and control strategy. By contrast, the present findings revealed that the magnitude of IPPs gradually increased from 19.4% in 2005–2010 to 23.5% and 25.2% in 2011–2015 and 2016–2020, repectively. The increasing trend could be attributed to insufficient financial support, lack of political commitment and inadequate community involvement in implementation of effective strategies to reduce the infection in Africa [70].
The findings of this systematic review and meta-analysis indicated that northern and western Africa had the highest prevalence estimates (42.2% and 32.3%) than eastern (21.9%), central (21.5%) and southern Africa (18.6%). Whether exposure to IPPs through poor hygiene is higher in northern and western Africa than in other parts of the continent or/and whether it is related to environmental condition remains unknown. High prevalence of IPPs was also reported in eastern, central and southern Africa. Therefore, comparison of overall prevalence rates by regions may not provide sufficient detailed information, and additional studies are needed to further explore the sources of variation.
Africa consists of 54 countries, but IPPs was only reported in 19 countries. Ethiopia had the highest number of eligible studies (17 studies), with overall prevalence rate of 15.3%, which is lower than the 24.21% rate reported in 2020 by Tegen et al. [4] in the same geographical area. The second and third highest numbers of studies included were from Nigeria (six studies) and South Africa (three studies), respectively. Only two studies were reported in Angola, Ghana, Kenya and Egypt. Although the outcome of one study is inconclusive and cannot be generalised, only one eligible study was identified in the 12 remaining countries. Moreover, data from studies in 35 countries were unavailable because they did not met our eligibility criteria. Hence, further studies with different inclusion/exclusion criteria are needed, and scholars should focus on IPPs amongst school children in these countries.
Different parasitological techniques are used because of lack of gold standard test (with 100% accuracy) for detection of intestinal parasites. The prevalence estimate obtained by microscopy was lower (22.7%) than that achieved when using molecular methods (61.4%) but slightly higher than when using RDTs (14.5%). The differences in laboratory techniques used for IPPs diagnosis and the variations in the sensitivity and specificity even of same method could possibly be the reason for the observed disparity in the IPPs rates in the present study. The use of DNA-based methods for laboratory confirmation of intestinal parasites has been proven to be highly sensitive and specific [71,72]. This finding is evidenced by the significantly high prevalence (61.4%) of IPPs in the present study when PCR or qPCR was used. However, such methods require specialised equipment and technical expertise of personnel, which limit their use. As such, traditional stool examination (microscopy) is still widely used for diagnosis of protozoan parasites worldwide [9,73]. About 87% (40/46) of the included studies used microscopy as detection methods.
In this meta-analysis, nine types of protozoan parasites were identified; Giardia spp. (38/46 [82.6%]) and E. histolytica/ dispar (33/46 [71.7%]) were the most frequently reported parasites (Table 2). The predominance of both parasites is common in this region or in the other parts of the world. Studies from Saudi Arabia [74], Ethiopia [75], Sudan [76] and Yemen [77] reported supportive findings. The pooled prevalence rate of E. histolytica/ dispar in this meta-analysis was 13.3%, which is consistent with the 14.09% rate reported in Ethiopia [4] and 12.1% in the Philippines [78]. However, the prevalence rate in the present study was lower than that in studies conducted in Malaysia (20.4%) [79], Yemen (16.4) [80] and Tanzania 15% [81] but higher than that in studies in Bangladesh (3.83%) [82] and Thailand (3.7%) [83].
This study showed that 12% of school-aged African children were infected with Giardia spp. parasite. Similar infection rate (11.0%) was reported from Brazil [84], and a considerably higher prevalence rate was detected in Nepal (46.8%) [85]. Meanwhile, the infection rate in Bangladesh (6.01%) [82] and Thailand (4.9%) [83] was lower than the present finding. The variations in prevalence rates of E. histolytica/dispar and Giardia spp. might be attributed to low sanitation level, contamination of drinking water source, poor hand washing practices and consumption of raw vegetables.
Strengths and limitations
A key strength of this systematic review and meta-analysis is that it is the first to determine the pooled prevalence estimates of IPPs amongst school-aged children in the entire continent of Africa. Nevertheless, this review has its own limitation. The prevalence data were reported from only 19 of the 54 African countries, and the distribution of eligible studies was uneven across UNSD African regions, publication years and diagnostic methods. Given the limited sensitivity of microscopy to morphologically distinguish between samples infected with E. histolytica and those infected with other non-pathogenic Entamoeba species, the magnitude of the E. histolytica infection might be overestimated because the majority of the included studies used microscopy. Substantial heterogeneity was found across the primary studies, thus generalisations may have limited validity. Overall, the prevalence estimate may not fully represent the continent-wide prevalence of intestinal protozoan infection.
Conclusion
About 25.8% of school African children had one or more species of intestinal protozoan parasites in their faecal specimens. E. histolytica/ dispar and Giardia spp. were the most predominant parasites amongst the study participants. This review would be beneficial for understanding the IPPs status amongst African children and provide additional evidence that the burden of these parasites is still alarming. Thus, poverty reduction, improvement of sanitation and hygiene and attention to preventive control measures will be the key to reducing protozoan parasite transmission.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Subgroup analyses. Prevalence of intestinal protozoan infections among school children in Africa based on children enrolment time (A-C), different regions (D-G), countries (H-Z), diagnostic methods (AA-AC) and species (AD-AG).
(DOCX)
Sensitivity analysis by (A) excluding small studies, (B) excluding low- and moderate-quality studies, (C) excluding studies used non-microscopic diagnostic methods and (D) excluding outlier studies.
(DOCX)
Acknowledgments
We would like to thank Mr. Mohamad Zarudin Mat Said for his assistance in creating the Map using the QGIS software.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Abdullah I, Tak H, Ahmad F, Gul N, Nabi S, Sofi T. Predominance of gastrointestinal protozoan parasites in children: a brief review. Journal of Health Education Research and Development. 2016;4(4). [Google Scholar]
- 2.Hajissa K, Abd Elhafiz M, Eshag HA, Alfadel A, Nahied E, Dahab R, et al. Prevalence of schistosomiasis and associated risk factors among school children in Um-Asher Area, Khartoum, Sudan. BMC research notes. 2018;11(1):1–5. doi: 10.1186/s13104-017-3088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellanos-Gonzalez A, White A Jr, Melby P, Travi B. Molecular diagnosis of protozoan parasites by Recombinase Polymerase Amplification. Acta tropica. 2018;182:4–11. doi: 10.1016/j.actatropica.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tegen D, Damtie D, Hailegebriel T. Prevalence and Associated Risk Factors of Human Intestinal Protozoan Parasitic Infections in Ethiopia: A Systematic Review and Meta-Analysis. Journal of parasitology research. 2020;2020. doi: 10.1155/2020/8884064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajissa K, Abd Elhafiz M, Abd All T, Zakeia M, Eshag HA, Elnzer E, et al. Prevalence of Entamoeba histolytica and Giardia lamblia among schoolchildren in Um-Asher Area, Sudan. 2020. [Google Scholar]
- 6.Organization WH. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases: World Health Organization; 2010. [Google Scholar]
- 7.Ouattara M, N’Guéssan NA, Yapi A, N’Goran EK. Prevalence and spatial distribution of Entamoeba histolytica/dispar and Giardia lamblia among schoolchildren in Agboville area (Côte d’Ivoire). PLoS neglected tropical diseases. 2010;4(1):e574. doi: 10.1371/journal.pntd.0000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squire SA, Ryan U. Cryptosporidium and Giardia in Africa: current and future challenges. Parasites & vectors. 2017;10(1):1–32. doi: 10.1186/s13071-017-2111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haque R. Human intestinal parasites. J Health Popul Nutr. 2007;25(4):387–91. . eng. [PMC free article] [PubMed] [Google Scholar]
- 10.Ngowi HA. Prevalence and pattern of waterborne parasitic infections in eastern Africa: A systematic scoping review. Food and waterborne parasitology. 2020;20:e00089. doi: 10.1016/j.fawpar.2020.e00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alemu G, Abossie A, Yohannes Z. Current status of intestinal parasitic infections and associated factors among primary school children in Birbir town, Southern Ethiopia. BMC infectious diseases. 2019;19(1):1–8. doi: 10.1186/s12879-018-3567-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkari B, Hosseini G, Motazedian MH, Fararouei M, Moshfe A. Prevalence and risk factors of intestinal protozoan infections: a population-based study in rural areas of Boyer-Ahmad district, Southwestern Iran. BMC infectious diseases. 2016;16(1):1–5. doi: 10.1186/s12879-016-2047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. International journal of evidence-based healthcare. 2015;13(3):147–53. doi: 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 15.Hajissa K.; Marzan M.; Idriss M.I.; Islam M.A. Prevalence of Drug-Resistant Tuberculosis in Sudan: A Systematic Review and Meta-Analysis. Antibiotics. 2021, 10, 932. doi: 10.3390/antibiotics10080932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam MA, Alam SS, Kundu S, Hossan T, Kamal MA, Cavestro C. Prevalence of Headache in Patients With Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of 14,275 Patients. Frontiers in neurology. 2020;11. doi: 10.3389/fneur.2020.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C-T, Ang J-Y, Islam MA, Chan H-K, Cheah W-K, Gan SH. Prevalence of Drug-Related Problems and Complementary and Alternative Medicine Use in Malaysia: A Systematic Review and Meta-Analysis of 37,249 Older Adults. Pharmaceuticals. 2021;14(3):187. doi: 10.3390/ph14030187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Aziz MA, Afifi AA, Malik EM, Adam I. Intestinal protozoa and intestinal helminthic infections among schoolchildren in Central Sudan. Asian Pacific Journal of Tropical Medicine. 2010;3(4):292–3. [Google Scholar]
- 19.Abossie A, Seid M. Assessment of the prevalence of intestinal parasitosis and associated risk factors among primary school children in Chencha town, Southern Ethiopia. BMC Public Health. 2014;14(1). doi: 10.1186/1471-2458-14-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams VJ, Markus MB, Adams JFA, Jordaan E, Curtis B, Dhansay MA, et al. Paradoxical helminthiasis and giardiasis in Cape Town, South Africa: Epidemiology and control. African Health Sciences. 2005;5(2):131–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Adedoja A, Akanbi AA, Babatunde S. Asymptomatic intestinal protozoa in school age children in Pategi, Pategi LGA of Kwara State, Nigeria. African Journal of Infectious Diseases. 2015;9(2):39–42. English. [Google Scholar]
- 22.Alemu G, Abossie A, Yohannes Z. Current status of intestinal parasitic infections and associated factors among primary school children in Birbir town, Southern Ethiopia. BMC infectious diseases. 2019. Mar 19;19(1):270. doi: 10.1186/s12879-019-3879-5 . Pubmed Central PMCID: PMC6425597. Epub 2019/03/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alemu M, Anley A, Tedla K. Magnitude of Intestinal Parasitosis and Associated Factors in Rural School Children, Northwest Ethiopia. Ethiopian journal of health sciences. 2019. Jan;29(1):923–8. doi: 10.4314/ejhs.v29i1.14 . Pubmed Central PMCID: PMC6341440. Epub 2019/02/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Shehri H, James LaCourse E, Klimach O, Kabatereine NB, Stothard JR. Molecular characterisation and taxon assemblage typing of giardiasis in primary school children living close to the shoreline of Lake Albert, Uganda. Parasite epidemiology and control. 2019. Feb;4:e00074. doi: 10.1016/j.parepi.2018.e00074 . Pubmed Central PMCID: PMC6324016. Epub 2019/01/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amare B, Ali J, Moges B, Yismaw G, Belyhun Y, Gebretsadik S, et al. Nutritional status, intestinal parasite infection and allergy among school children in Northwest Ethiopia. BMC Pediatrics. 2013;13(1). doi: 10.1186/1471-2431-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awolaju BA, Morenikeji OA. Prevalence and intensity of intestinal parasites in five communities in south-west Nigeria. African Journal of Biotechnology. 2009;8(18):4542–6. [Google Scholar]
- 27.Ayogu RN, Okafor AM, Ene-Obong HN. Iron status of schoolchildren (6–15 years) and associated factors in rural Nigeria. Food & nutrition research. 2015;59:26223. doi: 10.3402/fnr.v59.26223 . Pubmed Central PMCID: PMC4424235. Epub 2015/05/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baba OASC Aminetou BM, Ba O, Mouhamedou K, Elhdj D, Samba H, et al. Prevalence of intestinal parasites among school children in the Gorgol, Guidimagha and Brakna area (Mauritania). Revue Francophone des Laboratoires. 2012;2012(440):75–8. [Google Scholar]
- 29.Birhanu M, Gedefaw L, Asres Y. Anemia among School-Age Children: Magnitude, Severity and Associated Factors in Pawe Town, Benishangul-Gumuz Region, Northwest Ethiopia. Ethiopian journal of health sciences. 2018. May;28(3):259–66. doi: 10.4314/ejhs.v28i3.3 . Pubmed Central PMCID: PMC6016356. Epub 2018/07/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisangamo CK, Mutwa PJ, Mbarambara PM. Profil des parasitoses intestinales chez les enfants d’âge scolaire de Kiliba (est de la RD Congo). Médecine et Santé Tropicales. 2017;27(2):209–13. doi: 10.1684/mst.2017.0686 [DOI] [PubMed] [Google Scholar]
- 31.Chege NM, Ondigo BN, Onyambu FG, Kattam AM, Lagat N, Irungu T, et al. The prevalence of intestinal parasites and associated risk factors in school-going children from informal settlements in Nakuru Town, Kenya. Malawi Medical Journal. 2020;32(2):80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Alegria M, Colmenares K, Espasa M, Amor A, Lopez I, Nindia A, et al. Prevalence of Strongyloides stercoralis and Other Intestinal Parasite Infections in School Children in a Rural Area of Angola: A Cross-Sectional Study. The American journal of tropical medicine and hygiene. 2017. Oct;97(4):1226–31. doi: 10.4269/ajtmh.17-0159 . Pubmed Central PMCID: PMC5637607. Epub 2017/08/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dyab AK, El-Salahy MM, Abdelmoneiem HM, Amin MM, Mohammed MF. PARASITOLOGICAL STUDIES ON SOME INTESTINAL PARASITES IN PRIMARY SCHOOL CHILDREN IN ASWAN GOVERNORATE, EGYPT. Journal of the Egyptian Society of Parasitology. 2016;46(3):581–6. [PubMed] [Google Scholar]
- 34.Erismann S, Diagbouga S, Odermatt P, Knoblauch AM, Gerold J, Shrestha A, et al. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasites and Vectors. 2016;9(1):1–14. doi: 10.1186/s13071-016-1835-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eyamo T, Girma M, Alemayehu T, Bedewi Z. Soil-Transmitted Helminths And Other Intestinal Parasites Among Schoolchildren In Southern Ethiopia. Research and reports in tropical medicine. 2019;10:137–43. doi: 10.2147/RRTM.S210200 . Pubmed Central PMCID: PMC6817342. Epub 2019/11/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan C-K, Liao C-W, Lyu S-Y, Sukati H, Ji D-D, Cho C-M, et al. Prevalence of intestinal parasitic infections among primary schoolchildren in areas devoid of sanitation in northwestern Kingdom of Swaziland, Southern Africa. pathogens and global health. 2012;106(1):60–2. doi: 10.1179/2047773211Y.0000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forson AO, Arthur I, Olu-Taiwo M, Glover KK, Pappoe-Ashong PJ, Ayeh-Kumi PF. Intestinal parasitic infections and risk factors: A cross-sectional survey of some school children in a suburb in Accra, Ghana. BMC Research Notes. 2017;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebretsadik D, Tesfaye M, Adamu A, Zewde G. Prevalence of Intestinal Parasitic Infection and Its Associated Factors Among School Children in Two Primary Schools in Harbu Town, North East Ethiopia: Cross-Sectional Study. Pediatric health, medicine and therapeutics. 2020;11:179–88. doi: 10.2147/PHMT.S252061 . Pubmed Central PMCID: PMC7297451. Epub 2020/07/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gelaw A, Anagaw B, Nigussie B, Silesh B, Yirga A, Alem M, et al. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC public health. 2013;13(1):304. doi: 10.1186/1471-2458-13-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gyang VP, Chuang TW, Liao CW, Lee YL, Akinwale OP, Orok A, et al. Intestinal parasitic infections: Current status and associated risk factors among school aged children in an archetypal African urban slum in Nigeria. Journal of Microbiology, Immunology and Infection. 2019;52(1):106–13. [DOI] [PubMed] [Google Scholar]
- 41.Hailegebriel T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. BMC Infectious Diseases. 2017;17(1). doi: 10.1186/s12879-017-2466-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hailegebriel T. Undernutrition, intestinal parasitic infection and associated risk factors among selected primary school children in Bahir Dar, Ethiopia. BMC Infectious Diseases. 2018;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall A, Kassa T, Demissie T, Degefie T, Lee S. National survey of the health and nutrition of schoolchildren in Ethiopia. Tropical Medicine and International Health. 2008;13(12):1518–26. English. doi: 10.1111/j.1365-3156.2008.02168.x [DOI] [PubMed] [Google Scholar]
- 44.Heimer J, Staudacher O, Steiner F, Kayonga Y, Havugimana JM, Musemakweri A, et al. Age-dependent decline and association with stunting of Giardia duodenalis infection among schoolchildren in rural Huye district, Rwanda. Acta tropica. 2015. May;145:17–22. doi: 10.1016/j.actatropica.2015.01.011 . Epub 2015/02/17. eng. [DOI] [PubMed] [Google Scholar]
- 45.Htun NSN, Odermatt P, Müller I, Yap P, Steinmann P, Schindler C, et al. Association between gastrointestinal tract infections and glycated hemoglobin in school children of poor neighborhoods in Port Elizabeth, South Africa. PLoS neglected tropical diseases. 2018;12(3):e0006332. doi: 10.1371/journal.pntd.0006332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibrahium FAA. Prevalence and predisposing factors regarding intestinal parasitic infections among rural primary school pupils at minia governorate, Egypt. Journal of Public Health in Africa. 2011;2(2):123–6. doi: 10.4081/jphia.2011.e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ihejirika OC, Nwaorgu OC, Ebirim CI, Nwokeji CM. Effects of intestinal parasitic infections on nutritional status of primary children in Imo State Nigeria. Pan African Medical Journal. 2019;33. doi: 10.11604/pamj.2019.33.34.17099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jejaw A, Zemene E, Alemu Y, Mengistie Z. High prevalence of Schistosoma mansoni and other intestinal parasites among elementary school children in Southwest Ethiopia: A cross-sectional study. BMC Public Health. 2015;15(1). doi: 10.1186/s12889-015-2459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kesete Y, Tesfahiwet H, Fessehaye G, Kidane Y, Tekle Y, Yacob A, et al. Assessment of Prevalence and Risk Factors for Intestinal Parasitosis, Malnutrition, and Anemia among School Children in Ghindae Area, Eritrea. Journal of tropical medicine. 2020;2020:4230260. doi: 10.1155/2020/4230260 . Pubmed Central PMCID: PMC7647778 publication of this paper. Epub 2020/11/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khaled S., Gantois N., Ly A. T., Senghor S., Even G., Dautel E., Dejager R., Sawant M., Baydoun M., Benamrouz-Vanneste S., Chabé M., Ndiaye S., Schacht A. M., Certad G., Riveau G. & Viscogliosi E. Prevalence and Subtype Distribution of Blastocystis sp. in Senegalese School Children. Microorganisms, 2020. 8(9), 1408. doi: 10.3390/microorganisms8091408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Legesse L, Erko B, Hailu A. Current status of intestinal Schistosomiasis and soiltransmitted helminthiasis among primary school children in Adwa Town, Northern Ethiopia. Ethiopian Journal of Health Development. 2010;24(3):191–7. [Google Scholar]
- 52.Liao CW, Fu CJ, Kao CY, Lee YL, Chen PC, Chuang TW, et al. Prevalence of intestinal parasitic infections among school children in capital areas of the Democratic Republic of Sao Tome and Principe, West Africa. African Health Sciences. 2016;16(3):690–7. WOS:000388112800008. doi: 10.4314/ahs.v16i3.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahmud MA, Spigt M, Mulugeta Bezabih A, López Pavon I, Dinant GJ, Blanco Velasco R. Risk factors for intestinal parasitosis, anaemia, and malnutrition among school children in Ethiopia. Pathogens and Global Health. 2013;107(2):58–65. English. doi: 10.1179/2047773213Y.0000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller I, Yap P, Steinmann P, Damons BP, Schindler C, Seelig H, et al. Intestinal parasites, growth and physical fitness of schoolchildren in poor neighbourhoods of Port Elizabeth, South Africa: A cross-sectional survey. Parasites and Vectors. 2016;9(1). doi: 10.1186/s13071-016-1761-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen NL, Gelaye B, Aboset N, Kumie A, Williams MA, Berhane Y. Intestinal parasitic infection and nutritional status among school children in Angolela, Ethiopia. Journal of Preventive Medicine and Hygiene. 2012;53(3):157–64. [PMC free article] [PubMed] [Google Scholar]
- 56.Njambi E, Magu D, Masaku J, Okoyo C, Njenga SM. Prevalence of Intestinal Parasitic Infections and Associated Water, Sanitation, and Hygiene Risk Factors among School Children in Mwea Irrigation Scheme, Kirinyaga County, Kenya. Journal of tropical medicine. 2020;2020:3974156. doi: 10.1155/2020/3974156 . Pubmed Central PMCID: PMC7238387 publication of this article. Epub 2020/05/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliveira D, Ferreira FS, Atouguia J, Fortes F, Guerra A, Centeno-Lima S. Infection by intestinal parasites, stunting and anemia in school-aged children from southern Angola. PLoS ONE. 2015;10(9). doi: 10.1371/journal.pone.0137327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Opara KN, Udoidung NI, Opara DC, Okon OE, Edosomwan EU, Udoh AJ. The Impact of Intestinal Parasitic Infections on the Nutritional Status of Rural and Urban School-Aged Children in Nigeria. International journal of MCH and AIDS. 2012;1(1):73–82. doi: 10.21106/ijma.8 . Pubmed Central PMCID: PMC4948163. Epub 2012/01/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orish VN, Ofori-Amoah J, Amegan-Aho KH, Osei-Yeboah J, Lokpo SY, Osisiogu EU, et al. Prevalence of polyparasitic infection among primary school children in the volta Region of Ghana. Open Forum Infectious Diseases. 2019;6(4). doi: 10.1093/ofid/ofz153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reji P, Belay G, Erko B, Legesse M, Belay M. Intestinal parasitic infections and malnutrition amongst first-cycle primary schoolchildren in Adama, Ethiopia. African Journal of Primary Health Care and Family Medicine. 2011;3(1). [Google Scholar]
- 61.Sitotaw B, Shiferaw W. Prevalence of Intestinal Parasitic Infections and Associated Risk Factors among the First-Cycle Primary Schoolchildren in Sasiga District, Southwest Ethiopia. Journal of parasitology research. 2020;2020. doi: 10.1155/2020/8681247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tagajdid R, Lemkhente Z, Errami M, El Mellouki W, Lmimouni B. [Prevalence of intestinal parasitic infections in Moroccan urban primary school students]. Bulletin de la Societe de pathologie exotique (1990). 2012. Feb;105(1):40–5. doi: 10.1007/s13149-011-0137-5 . Epub 2011/02/22. Portage parasitaire intestinal chez l’enfant scolarise a Sale, Maroc. fre. [DOI] [PubMed] [Google Scholar]
- 63.Tembo SJ, Mutengo MM, Sitali L, Changula K, Takada A, Mweene AS, et al. Prevalence and genotypic characterization of Giardia duodenalis isolates from asymptomatic school-going children in Lusaka, Zambia. Food and waterborne parasitology. 2020. Jun;19:e00072. doi: 10.1016/j.fawpar.2020.e00072 . Pubmed Central PMCID: PMC7125351. Epub 2020/04/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Omarova A, Tussupova K, Berndtsson R, Kalishev M, Sharapatova K. Protozoan parasites in drinking water: A system approach for improved water, sanitation and hygiene in developing countries. International journal of environmental research and public health. 2018;15(3):495. doi: 10.3390/ijerph15030495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma M, Sapkota J, Jha B, Mishra B, Bhatt CP. Prevalence of Intestinal Parasitic Infestation among Public School Children of a Community. JNMA: Journal of the Nepal Medical Association. 2020;58(225):293. doi: 10.31729/jnma.4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yanola J, Nachaiwieng W, Duangmano S, Prasannarong M, Somboon P, Pornprasert S. Current prevalence of intestinal parasitic infections and their impact on hematological and nutritional status among Karen hill tribe children in Omkoi District, Chiang Mai Province, Thailand. Acta tropica. 2018;180:1–6. doi: 10.1016/j.actatropica.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 67.Daryani A, Hosseini-Teshnizi S, Hosseini S-A, Ahmadpour E, Sarvi S, Amouei A, et al. Intestinal parasitic infections in Iranian preschool and school children: A systematic review and meta-analysis. Acta tropica. 2017;169:69–83. doi: 10.1016/j.actatropica.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 68.Kunwar R, Acharya L, Karki S. Decreasing prevalence of intestinal parasitic infections among school-aged children in Nepal: a systematic review and meta-analysis. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2016;110(6):324–32. doi: 10.1093/trstmh/trw033 [DOI] [PubMed] [Google Scholar]
- 69.Samanta S, Mehra S, Maiti T, Ghosh P, Ghosh SK. Socio-demographic correlates influencing the trend of intestinal parasitic infestation in a rural community of West Bengal, India. Journal of Public Health. 2012;20(4):405–12. [Google Scholar]
- 70.Chelkeba L, Mekonnen Z, Alemu Y, Emana D. Epidemiology of intestinal parasitic infections in preschool and school-aged Ethiopian children: a systematic review and meta-analysis. BMC public health. 2020;20(1):117. doi: 10.1186/s12889-020-8222-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ten Hove RJ, van Esbroeck M, Vervoort T, van den Ende J, van Lieshout L, Verweij JJ. Molecular diagnostics of intestinal parasites in returning travellers. European Journal of Clinical Microbiology & Infectious Diseases. 2009. 2009/09/01;28(9):1045–53. doi: 10.1007/s10096-009-0745-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verweij JJ, Stensvold CR. Molecular Testing for Clinical Diagnosis and Epidemiological Investigations of Intestinal Parasitic Infections. Clinical Microbiology Reviews. 2014;27(2):371–418. doi: 10.1128/CMR.00122-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki CTN, Gomes JF, Falcão AX, Shimizu SH, Papa JP, editors. Automated diagnosis of human intestinal parasites using optical microscopy images. 2013 IEEE 10th International Symposium on Biomedical Imaging; 2013 7–11 April 2013. [Google Scholar]
- 74.Zaglool DA, Khodari YA, Gazzaz ZJ, Dhafar KO, Shaker HA, Farooq MU. Prevalence of intestinal parasites among patients of Al-Noor specialist hospital, Makkah, Saudi Arabia. Oman medical journal. 2011;26(3):182. doi: 10.5001/omj.2011.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wondmieneh A, Gedefaw G, Alemnew B, Getie A, Bimerew M, Demis A. Intestinal parasitic infections and associated factors among people living with HIV/AIDS in Ethiopia: A systematic review and meta-analysis. Plos one. 2020;15(12):e0244887. doi: 10.1371/journal.pone.0244887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abd Elhafiz M, Hajissa K, Mohamed Z, Aal AAA. Prevalence of Intestinal Parasitic Infection among Children in Al-kalakla, Khartoum, Sudan. World Applied Sciences Journal. 2017;35(2):219–22. [Google Scholar]
- 77.Alyousefi NA, Mahdy MA, Mahmud R, Lim YA. Factors associated with high prevalence of intestinal protozoan infections among patients in Sana’a City, Yemen. PLoS One. 2011;6(7):e22044. doi: 10.1371/journal.pone.0022044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weerakoon KG, Gordon CA, Williams GM, Cai P, Gobert GN, Olveda RM, et al. Co-parasitism of intestinal protozoa and Schistosoma japonicum in a rural community in the Philippines. Infectious diseases of poverty. 2018;7(1):1–11. doi: 10.1186/s40249-017-0384-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ngui R, Hassan N-A, Nordin NMS, Mohd-Shaharuddin N, Chang LY, Teh CSJ, et al. Copro-molecular study of Entamoeba infection among the indigenous community in Malaysia: A first report on the species-specific prevalence of Entamoeba in dogs. Acta tropica. 2020;204:105334. doi: 10.1016/j.actatropica.2020.105334 [DOI] [PubMed] [Google Scholar]
- 80.Alharazi T, Bamaga OA, Al-Abd N, Alcantara JC. Intestinal parasitic infection: prevalence, knowledge, attitude, and practices among schoolchildren in an urban area of Taiz city, Yemen. AIMS Public Health. 2020;7(4):769. doi: 10.3934/publichealth.2020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palmeirim MS, Mrimi EC, Minja EG, Samson AJ, Keiser J. A cross-sectional survey on parasitic infections in schoolchildren in a rural Tanzanian community. Acta tropica. 2021;213:105737. doi: 10.1016/j.actatropica.2020.105737 [DOI] [PubMed] [Google Scholar]
- 82.Hossain MR, Musa S, Zaman RF, Khanum H. Occurrence of intestinal parasites among school going children of a slum area in Dhaka city. Bangladesh Journal of Zoology. 2019;47(1):67–75. [Google Scholar]
- 83.Pattanawong U, Putaporntip C, Kakino A, Yoshida N, Kobayashi S, Yanmanee S, et al. Analysis of DA locus of tRNA-linked short tandem repeats reveals transmission of Entamoeba histolytica and E. dispar among students in the Thai-Myanmar border region of northwest Thailand. PLOS Neglected Tropical Diseases. 2021;15(2):e0009188. doi: 10.1371/journal.pntd.0009188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seguí R, Muñoz-Antoli C, Klisiowicz DR, Oishi CY, Köster PC, de Lucio A, et al. Prevalence of intestinal parasites, with emphasis on the molecular epidemiology of Giardia duodenalis and Blastocystis sp., in the Paranaguá Bay, Brazil: a community survey. Parasites & vectors. 2018;11(1):1–19. doi: 10.1186/s13071-018-3054-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta R, Rayamajhee B, Sherchan SP, Rai G, Mukhiya RK, Khanal B, et al. Prevalence of intestinal parasitosis and associated risk factors among school children of Saptari district, Nepal: a cross-sectional study. Tropical medicine and health. 2020;48(1):1–9. doi: 10.1186/s41182-020-00261-4 [DOI] [PMC free article] [PubMed] [Google Scholar]