Abstract
The fields of regenerative medicine and tissue engineering offer new therapeutic options to restore, maintain or improve tissue function following disease or injury. To maximize the biological function of a tissue-engineered clinical product, specific conditions must be maintained within a bioreactor to allow the maturation of the product in preparation for implantation. Specifically, the bioreactor should be designed to mimic the mechanical, electrochemical and biochemical environment that the product will be exposed to in vivo. Real-time monitoring of the functional capacity of tissue-engineered products during manufacturing is a critical component of the quality management process. The present review provides a brief overview of bioreactor engineering considerations. In addition, strategies for bioreactor automation, in-line product monitoring and quality assurance are discussed.
Keywords: Bioreactor, Monitoring sensors and automation, Manufacturing, Tissue engineering, Regenerative medicine
Introduction
Tissue engineering for regenerative medicine is recognized as a promising field in the healthcare industry for offering therapies that restore, maintain or improve the function of tissues compromised by disease or injury [1, 2]. The field is rapidly transitioning from benchtop research and development toward clinical applications. Clinical products of regenerative medicine, particularly the tissue-engineered products, are generally comprised of cells (autologous or allogenic) combined with a porous biocompatible scaffold [3]. Following the seeding of these tissue scaffolds with cells, the cell-contained constructs are placed in a tissue or organ-specific bioreactor system for preconditioning and maturation prior to application in vivo. The bioreactor system is designed to provide the appropriate tissue-specific physiological conditions for the seeded cells to acclimate to the intended scaffold microenvironment. The bioreactor system facilitates the delivery of nutrients and oxygen and removes waste products, all within a contained sterile environment [2, 4]. During the phase of tissue construct maturation, cells proliferate to colonize the scaffold and reorganize to form a physiologically functional microarchitecture that resembles native tissue [5]. Environmental conditions in the bioreactor play a critical role in developing tissue constructs that mimic native tissues, as they determine the structural and functional characteristics of tissue constructs. Therefore, the design and manufacture a tissue-specific bioreactor presupposes the thorough knowledge of anatomical and functional characteristics of target tissues. For example, skeletal muscle tissue supports the body’s movements by unidirectional contraction and relaxation. As such, a bioreactor for skeletal muscle should provide a mechanism to promote unidirectional movement, which can be achieved by incorporating mechanical and/or electrical devices that stimulate the maturing skeletal muscle tissues to develop contractile functions.
Tissue- or organ-specific bioreactors are necessary to create tissue-engineered products for clinical applications, however, the availability of such bioreactors is limited. Currently, many research groups design and construct in-house custom-built bioreactor systems to meet specific product requirements [6]. The time and monetary cost associated with the development and production of custom-built bioreactors is extremely high, which limits their efficiency in providing clinical applications. Despite significant advances in cell culture bioreactor development over recent years, there is a dire need to develop standardized scalable bioreactors to support product development for regenerative medicine applications, as little progress has been made in this field. The next-generation bioreactors for clinical manufacturing in regenerative medicine will need to balance flexibility, cost, compatibility with automation and in-line quality assurance monitoring, as well as regulatory compliance.
This review discusses existing and emerging principles of the design, in-line monitoring and automation of bioreactors, subsequent quality assurance tools for engineered tissues and manufacturing/commercialization strategies for modular and configurable bioreactors intended for the development of clinical products in regenerative medicine.
Engineering parameters for bioreactor design
Conditioning tissue-engineered constructs in a bioreactor system requires more than supplying nutrients and oxygen to cells. Cellular constructs will only reach their highest functional potential if they mature under appropriate conditions that match their respective in vivo environment. Therefore, different constructs necessitate different types of stimulatory requirements, which may include the incorporation of chemical, electrical or mechanical stress-producing mechanisms in a hermetically sealed and contaminant-free culture environment. Therefore, specific engineering parameters such as material design, mass transfer, mechanical stimulation and electrical stimulation should be considered when building a tissue-specific bioreactor system (Fig. 1).
Fig. 1.
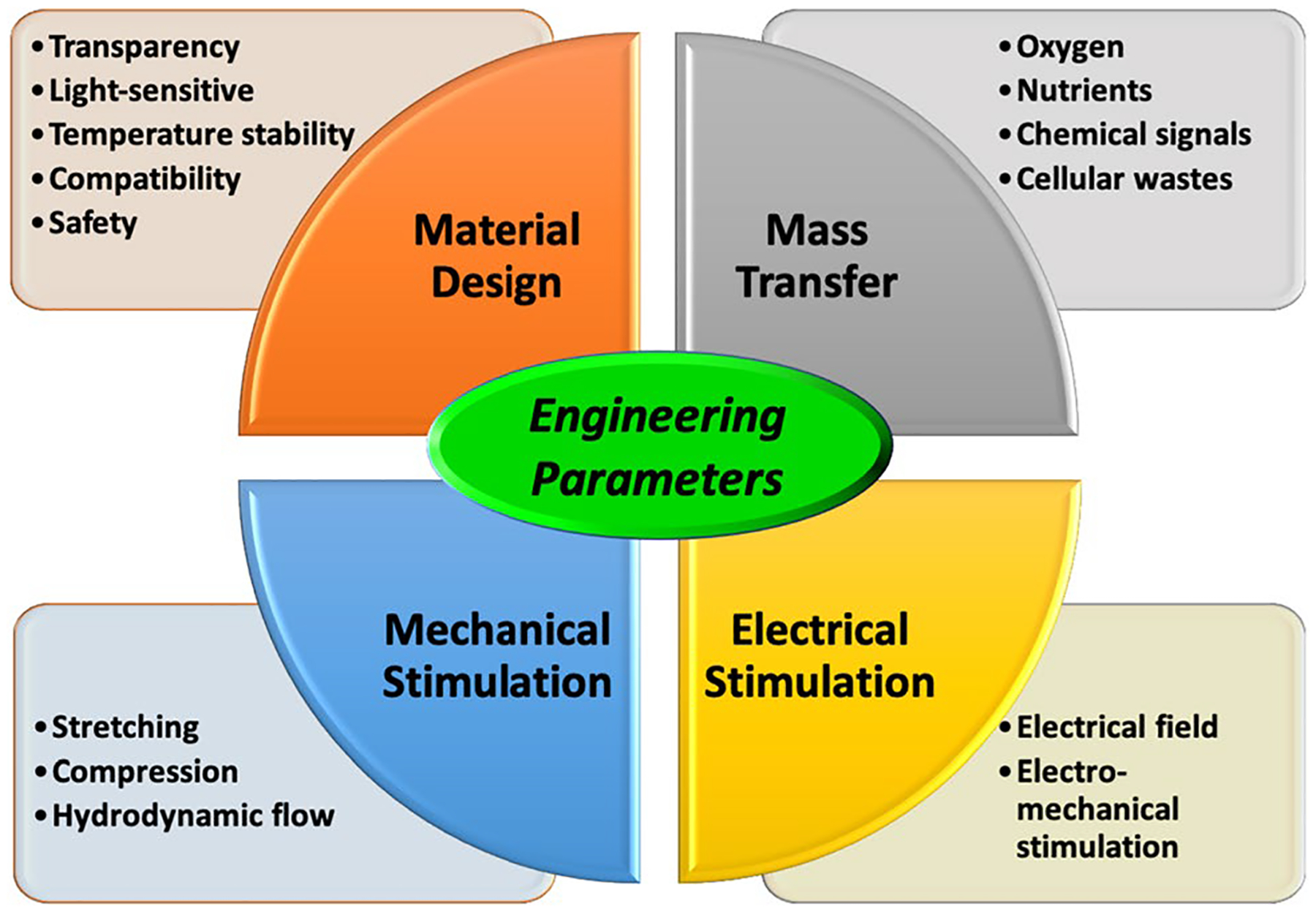
Schematic diagram of engineering parameters for bioreactor design
Bioreactor material design
An important parameter to regard in designing a bioreactor is the choice of appropriate materials for constructing the device. The initial factors that must be defined are the physical and chemical requirements for the materials to select as the various bioreactor components. When materials are being considered, it should be established whether they have to be transparent, light-sensitive, stable at appropriate temperatures and compatible with standard manufacturing techniques. In addition, the materials should normally be non-leachable, non-degradable, non-porous, non-protein binding and easily sterilized. Some of these criteria are summarized in Table 1.
Table 1.
Summary of criteria for the material selection process
| Criteria | Additional considerations |
|---|---|
| Transparency | Determine whether it is necessary for a component to be transparent |
| Light sensitivity | Identify the location and placement of the bioreactor |
| If using transparent material, the light sensitivity of the culture media and reagents used needs to be considered | |
| Non-leachable | Fluid path-specific, that should meet industry guidance/requirements, i.e., BPOG or USP for cell therapy products |
| Non-degradable | Can the device be degraded under specific conditions (e.g., hydrolysis in the culture medium)? |
| Non-porous | For fluid path materials, the adsorption characteristics of media components |
| Material longevity/durability | If the device will be used multiple times, how many sterilization cycles can the material withstand before degrading? |
| Is the device disposable? Single-use devices present fewer sterilization challenges | |
| Tissue culture compatibility | Does the material have known interactions with buffers, cell culture media and/or DMSO? |
| Temperature considerations | Will the device be inside an incubator? |
| Will the device have an external heat source? | |
| Will the device be exposed to humidity? | |
| Will the device be exposed to 4 °C, for example, during transportation? | |
| Will the device be put in storage? | |
| What is the shelf-life of the device? | |
| Sterilization technique | Can the material be autoclaved? |
| Can the material be gamma sterilized? | |
| Can the material be sterilized using EO gas? | |
| If so, is there a certain off-gas clearance time requirement? | |
| Will residual testing be required? | |
| Manufacturing considerations | Can the device be mass-produced? |
| What is the manufacturing design? | |
| Can the device be produced with a mold or a 3D Printer? | |
| This allows the device to be produced with limited seams | |
| It also helps with keeping the product hermetically sealed | |
| Material quality/consistency | Can the material be produced to a sufficient level of quality/consistency? |
| Is cGMP-grade material needed? | |
| Can materials be used that are not of medical grade? | |
| Is documentation to support material quality required? | |
| Supplier source | Materials are preferred that are not limited to a single supplier to avoid supply issues |
| Material safety | Hazards should be identified, including glass breakage, electric shock and the safety of any leachable chemicals |
| Transportability | If the device will be transported, the team should consider the device size, weight, durability, temperature sensitivity, integrity and leakage potential |
| The device may have to conform to ship testing guidelines like ISTA2A | |
| Mitigation of risks | Are any materials likely to be banned in the future? |
| Does it contain BPA? | |
| Are there any alternative materials that can be used in the same manufacturing process? |
BPOG, BioPhorum Operations Group; USP, United States Pharmacopeia; DMSO, Dimethyl Sulfoxide; EO, Ethylene Oxide; cGMP, Current Good Manufacturing Practice; ISTA, International Safe Transit Association
The most important concern in designing a bioreactor system is to find safe materials that meet these requirements for the manufacture of clinical products. Several materials previously approved by the Food and Drug Administration (FDA) have been used to construct medical devices, which materials should be considered first. Since the requirements for different bioreactors vary depending on the target tissue application, the materials selected for specific features may also vary. The benefits and limitations of materials previously used in medical devices and clinical manufacturing are summarized in Table 2.
Table 2.
List of materials previously used in various clinical devices and applications
| Material | Pros | Cons |
|---|---|---|
| Stainless steel | Compatible with human tissue | Requires chromium ion treatment to avoid leaching |
| Rigid | Decreased cell growth | |
| Glass | Common material for chambers | Decreased cell growth |
| Rigid | Breakable – safety issue | |
| Limited manufacturing techniques | ||
| C-Flex | Flexible | Decreased cell growth |
| Transparent | ||
| UV and autoclave sterilizable | ||
| Maximum temperature is 135 °C | ||
| Polycarbonate | Commonly used for bioreactor chambers [14] | Contains BPA and is capable of leaching |
| Transparent | Can become deformed | |
| Gas and autoclave sterilizable [10, 14] | Can become brittle when exposed to ionizing radiation | |
| Can be extruded or injected | ||
| Can be processed at room temperature | ||
| Ethylene propylene diene | Maximum temperature is 150 °C | Decreased cell growth |
| monomer (EPDM) | UV, gas and autoclave sterilizable | Opaque |
| Flexible | ||
| Polystyrene | Rigid | Non-biodegradable |
| Transparent | ||
| Commonly used for other disposable labware | ||
| Injection molding | ||
| Gas sterilizable | ||
| Acrylic (PMMA) | Transparent | Scratches easily |
| Rigid | ||
| Cell casting, injection molding, compression molding and extrusion | ||
| Less dense than glass | ||
| Higher impact strength than glass and polystyrene | ||
| Polyetherimide (PEI) | Common material for chambers | -Translucent |
| Translucent | ||
| 3D printable | ||
| UV, gas and autoclave sterilization | ||
| Polydimethylsiloxane (PDMS) | Common material for chambers | Malleable |
| Transparent | ||
| Gas and autoclave sterilizable | ||
| Can be molded | ||
| Polysulfone (PSF) | Common chamber material | Fabricated with BPA |
| Gas and autoclave sterilizable | Translucent | |
| Rigid | ||
| Injection molding, extrusion or hot forming | ||
| Polytetrafluoroethylene (PTFE) | Common chamber material | Opaque |
| UV, gas and autoclave sterilization | Expensive | |
| Compression molding | ||
| Polyether ether ketone (PEEK) | Common chamber material | Opaque |
| Gas and autoclave sterilizable | ||
| Commonly used in medical implants | ||
| Injection molding or extrusion | ||
| Polyglycolide (PGA) | Biodegradable | Manufactured as a fabric or thread |
| Used for absorbable sutures | ||
| Poly-4-hydroxybutyrate (P4HB) | Support for cell adhesion and type 1 collagen production | |
| Melting point temperature is 60 °C | ||
| Sterilized with ethylene oxide |
Mass transfer
Mass transfer, including that of oxygen and nutrients to the tissue construct, and metabolic waste removal play major roles in a bioreactor system. The absence of a vascular network in the engineered tissue construct is a principal limiting factor in maintaining construct cell survival during preconditioning and tissue maturation [10]. Nutrient and oxygen delivery to a tissue-engineered construct and the removal of metabolites occur primarily by diffusion [2]. Bioreactors that allow the effective transfer of critical factors such as oxygen, nutrients, chemical signals and cellular wastes have shown to enhance cellular microarchitecture and differentiation compared with constructs matured in a static culture [18]. Thus, a well-established bioreactor design that facilitates efficient mass transfer should be considered for enhanced cell survival and tissue maturation. Moreover, the design should incorporate mechanisms to control the physical and physiological environment in order to meet the specific needs of target tissues and organs. A comprehensive strategy for monitoring key elements (pH, O2, temp., etc.) and automated environmental control technologies will be discussed in the ‘Monitoring Sensors and Automation‘ section.
Mechanical stimulation
In functional tissue engineering, mechanical stimulation plays a key role in cellular proliferation and tissue maturation by accelerating the formation of appropriate tissue morphology and microarchitecture. Tissues are exposed to a variety of mechanical forces in the body, including stretching, compression and hydrodynamic flow [4]. Thus, it is essential to provide biomimetic physiological cues in the bioreactor to realize the desired tissue functions. The benefits of conditioning tissue constructs with mechanical stimulation have been widely studied in the tissue regeneration of muscle, bone, cartilage, skin, vessel and tendon/ligament [4].
The specific mechanical forces and means of application should be dependent on the tissue architecture and complexity [19]. For engineered skeletal muscle tissue, force can be applied by cyclic stretching along with a uni- or bidirectional vector [20]. For instance, the cyclic mechanical stimulation of engineered skeletal muscle constructs was shown to induce myosin heavy chain accumulation and contribute to myotube maintenance in a uniaxial bioreactor culture system [21]. Certain bioreactors for cartilage tissue constructs have been designed that apply dynamic compression loading and induce shear forces similar to those in the natural physiological loading of cartilage tissue [22]. Intermittent compression and the application of shear force significantly improved the tissue’s mechanical properties and enhanced the synthesis of extracellular matrix components, such as glycosaminoglycans and collagen [23, 24]. In bioreactors designed for creating engineered bone tissue, a combination of dynamic compression and perfusion has been demonstrated to improve cell viability and the synthesis of appropriate matrices within the engineered constructs [25]. The perfusion system also allows the enhanced mass transfer of nutrients and waste. Tissue-engineered bone constructs conditioned through both perfusion and cyclic compression showed higher alkaline phosphatase activity and significantly increased mineral deposition [26, 27]. Small-diameter vascular grafts have been engineered using pulsatile perfusion bioreactors. These vessel bioreactors apply pulsatile radial strain to promote collagen deposition, thus improve the mechanical properties of engineered blood vessels [28].
To accurately gauge the durability of tissues and prevent their damage, force sensors may be implemented [1, 29]. The mechanical limits for tissues can be measured with these sensors that provide a value representing the compressive or tensile force across the tissue sample. Different types of sensors can be installed for each tissue type to monitor health and maturation levels. These sensors provide data relating to the tissues’ functional capability and their expected performance in the intended biological roles.
Electrical stimulation
Electrical stimulation and combined electrical/mechanical stimulation are known to enhance the functional properties of engineered tissues. It can be achieved by creating an electrical field that passes through the tissue in the bioreactor’s culture chamber. Several studies have reported the enhanced functional maturation and differentiation of engineered tissue using this approach. In one specific study, electrical stimulation was applied to enhance the beating and elongation of cardiomyocytes. Increased cell proliferation and gap junction formation were evident in human adipose-derived stem cells induced toward the cardiomyocyte lineage [30]. Other studies have shown that capacitively coupled electrical fields stimulate bone cells to accelerate the biomineralization process in vitro [31]. In addition, bioreactors that incorporate synchronized electro-mechanical stimulation can induce the upregulation and enhanced alignment of contractile proteins in skeletal myoblasts [32].
Monitoring sensors and automation
In the development of an automated bioreactor, sensors are an essential component for the continuous monitoring of in vitro biological systems [33]. Sensors and automation allow the bioreactor to perform three main functions: environmental control, mechanical stimulation and mechanical characterization [23, 29]. A bioreactor with these capabilities will allow for developing a matured tissue construct that can perform its anticipated biological role following implantation.
Monitoring components
In cell cultures, it is critical to maintain the optimal media conditions that support cell survival and growth. Depending on the cell types involved, acidity (pH), dissolved oxygen, temperature and glucose/lactate are standard parameters monitored in a tissue culture [34]. These parameters require sensor technologies that provide accurate measurements to ensure a favorable culture environment [1]. Any value outside of the accepted normal value range should be alerted for corrective action. For instance, a pH sensor reading beyond the value of 6 to 8 could trigger the controller system to provide automated media exchange [34].
Acidity values (pH)
pH is a measure of free hydrogen ions in a solution relative to hydroxyl ion concentration [35] and is an indicator of how acidic or basic a solution is. The pH value can range from 0.0 to 14.0, with pure water being neutral at 7.0, and the lower the value, the more acidic the solution is [35]. Since pH is a logarithmic measurement, each point of difference indicates an order of magnitude difference in H+ concentration [35]. pH can be measured optically or electrochemically and is one of the most vital parameters to measure in a bioreactor. An electrochemical sensor compares the difference in electric potential between a pH electrode and a reference electrode, and the voltage difference is then converted to pH value. Optical sensors are typically comprised of a light sensitive mechanism (photoresistor, photodiode, camera, etc.) and a medium that is allowed to react with the environment in question, producing a color change which is detected by the light-sensitive mechanism. The change in color is referenced to its calibration value, and a pH reading is generated [36]. CO2 released by cells in the tissue will decrease the pH, which will make the media less hospitable for cells [37].
Dissolved oxygen (DO)
Oxygen is transported from the lungs throughout the body through reversible binding to hemoglobin [38]. Since a bioreactor will not have hemoglobin, dissolved oxygen (DO) in the media is gradually used up by cells [39]. DO content can vary greatly with temperature [40]. When the temperature rises, the increased energy of molecules in the media will cause the dissolved oxygen concentration to drop [40]. This can be measured both optically or electrochemically. Optical detection involves colorimetry, where a reaction with a reagent causes a color change. Electrochemical detection comprises probes that utilize redox reactions to generate a signal.
Temperature
Temperature is an important parameter affecting cellular metabolism and growth. The currently used measurement technologies vary. Thermal electromagnetic radiation is a more recent temperature measurement technology that provides reliable and accurate detection in a fluid medium. This technology relies on the black-body radiation emitted by the measured object [41]. Infrared thermometers allow for monitoring objects at a distance and give greater flexibility to thermometer placement in a bioreactor.
Glucose/lactate
Glucose is a monosaccharide used by all organisms as energy source and is vital to the survival and development of all types of culture. In blood glucose measurement, glucose oxidase is combined with reagents to measure hydrogen peroxide generation in a blood sample [42]. Several enzymatic methods are used to measure glucose concentration. The hexokinase/glucose-6-phosphate dehydrogenase (G6PD) method is used as a reference method, where glucose is phosphorylated by hexokinase with ATP G6P. This becomes 6-phosphogluconate while NAD+ is reduced to NADH, which can be detected via spectrophotometry at 340-nm wavelength [43].
Lactate is produced by the anaerobic metabolism of glucose in muscle tissue. This causes an increase in proton concentration inside the muscle cell and leads to decreased cellular pH and thus cellular acidosis [44]. Lactate can be measured both electrochemically and optically. Electrochemical sensors rely on the measurement of current passing between two electrodes, the magnitude of which is reliant on a redox product in the enzymatic reaction [44]. Optical lactate measurement is based on a similar reaction at the sensor surface that causes the depletion of a reactant or the formation of a product, leading to a change in fluorometry or chemiluminescence, which is detected by the optical sensor [44].
Sensing technologies
A biosensor can be defined as “a self-contained analytical device that combines a biological component with a physicochemical component for the detection of an analyte of biological importance” [45]. Biosensors are composed of three elements: (1) a detector that recognizes a stimulus, (2) a transducer that translates the stimulus to a digital signal and (3) a system that interprets the signal for the user [45]. Biosensors implemented in bioreactors are either used to monitor the media or the tissue. Sensors designed to monitor the media receive their signal through a reaction with the solute of interest. This can either trigger an electrochemical signal stimulating the sensor or create a detectable change in light.
Optical sensors
Optical biosensors have the advantage of the ability to monitor the target over distance while still providing an accurate result. This adds flexibility to the bioreactor design, as the space within the bioreactor does not need to accommodate the entire sensor. Optical biosensors are among the most common of sensors currently used for environmental monitoring, food safety and drug research [45]. They are better suited for small volumes, allowing for the rapid, sensitive and specific real-time detection of substances while not consuming any analyte [46]. They may be designed in a small platform affixed to the inside of a culture chamber that can be monitored externally [34].
Electrochemical sensors
Electrochemical biosensors require interaction with the media to detect the target molecule [45]. These sensors typically involve an electrode in which one pole of the probe is functionalized with a molecule that binds the compound of interest. The accumulation of this compound on the electrode surface increases resistance across the electrode in a predictable manner, which allows for the construction of a standard curve based on compound concentration in the media. By tracking the increase in resistance over time, calculating the derivative of this curve will indicate changes in the rate at which the compound of interest is being introduced into the media.
Sensor placement
Reading accuracy and thus culture quality can be affected by sensor placement [34]. The accuracy of sensors that rely on solute concentration is dependent on solute diffusion. As the tissue consumes nutrients and excretes wastes, solute concentration will form a gradient in a static culture environment [44]. Results for a sensor near the tissue will more closely resemble the intracellular environment, while a more distant sensor may better represent the system’s overall concentration [47]. In a non-static culture environment, the media is continuously redistributed, thereby reducing or eliminating this gradient. In a system where the media is circulating, readings can be taken by sensors distant from the biological construct. Sensors may also be located within the circulating media tubes connected to the culture chamber. In a recirculating system, the in-line sensors can provide up-to-date information on the culture chamber’s state while being distant enough from the culture chamber itself, which allows for a smaller and more streamlined chamber design. An appealing approach may be to place the sensors within the tissue itself, which would allow the sampling in the tissue interstitium that may be under conditions different from the media surrounding the construct.
Automated media exchange
Automation is an important aspect of a bioreactor—the exchange of media or tissue stimulation with minimal human interaction results in a lower likelihood of contamination. Automation can be applied to several aspects of bioreactor function, with a logical point of media exchange. In static bioreactors, this involves removing old media from the culture chamber, followed by the addition of fresh media. This is typically performed when a nutritional deficit occurs and waste accumulates. Replacing the media in the culture chamber removes waste products and replenishes oxygen, nutrients, and other factors needed for tissue survival and maturation [2, 48]. A bioreactor chamber lacking a media exchange mechanism may only support the cellular product for a short time [49]. Automated media exchange can be achieved by providing a port through which the spent fluid can exit and another where fresh fluid can be introduced. A pump enables the media to circulate continuously within the bioreactor system to keep tissues healthy. The media exchange can be maximized by strategically placing the ports; the optimal positioning of these ports can vary depending on the design of culture chamber. The most efficient method of media exchange is currently achieved by laminar flow within the bioreactor chamber [3], allowing for depleted media to be evacuated and replaced with fresh media while producing low levels of shear stress on the tissue [3]. Having inflow and outflow ports in the same region could create dead zones where media remains stagnant during the media exchange cycle. This makes media exchange inefficient and will necessitate more frequent fluid exchange cycles. Sharp corners in the bioreactor design could also produce similar results by disrupting fluid dynamics. Therefore, it is essential to consider flow dynamics when designing the bioreactor system for maximized tissue health [50].
While an integrated automated media exchange system in a bioreactor adds efficiency, the placement design of reservoirs for old and fresh media may affect the operational flow. The incorporation of a fresh media reservoir and waste media repository is straightforward but they require a large space. An alternative approach might be to have a single reservoir in which the rejuvenation of depleted media can be performed. This single recirculating reservoir could include dialysis or chelation to remove toxic products with in-line feed batch delivery to replenish nutrients. Alternatively, three reservoirs could be used for storing waste, fresh media and glucose-rich solution, respectively (Fig. 2).
Fig. 2.
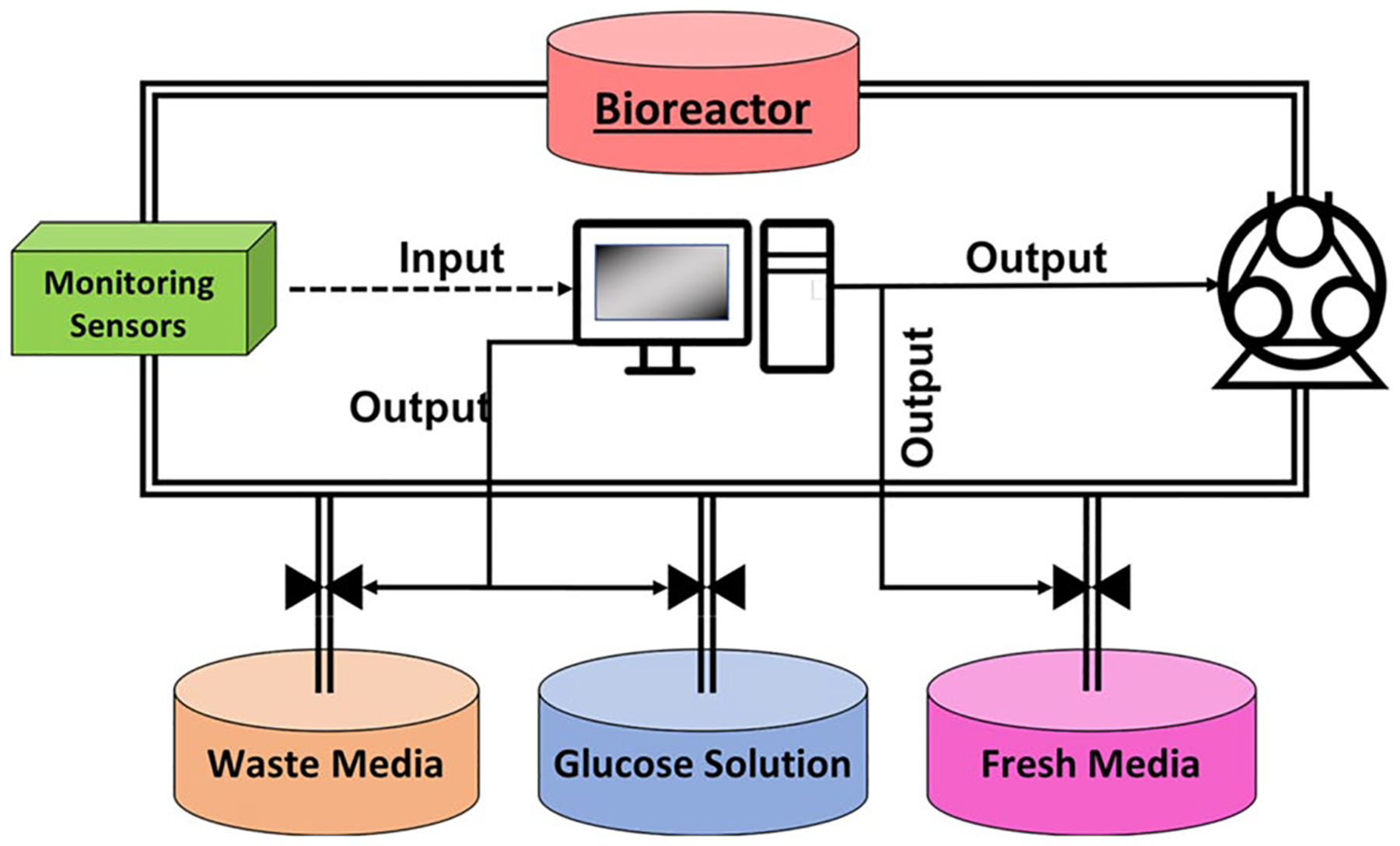
Example of tubing circuit used for the automated exchange of used media for fresh media
The depleted media reservoir collects the waste media expelled from the system as fresh media is introduced, maintaining a constant volume in the system. A separately stored glucose solution could be infused if glucose levels decreased below the threshold. During a regular media exchange utilizing the recirculating reservoir, the fresh and waste reservoirs can be serviced. This enables less frequent manipulation of the internal volume of the system. The point at which the bioreactor changes from recirculation to media exchange mode could be determined by the sensors controlling for the appropriate threshold values.
Evaluation of bioreactor-processed engineered tissue
The main performance measure of a bioreactor system for tissue engineering is the quality of tissue it can produce prior to implantation into a patient. Numerous tools are currently available to assess the maturation and viability of bioreactor-conditioned tissue, some of which are inevitably destructive to tissues, while others are able to assess them in real time.
Destructive analytical tools
Destructive analytical tools, which are common in most laboratory settings, include histological and molecular analyses, and quantitative and semi-quantitative microscopy. These methods are powerful in assessing tissue structure, function and viability. Modified histological techniques, such as immunohistochemistry (IHC) and immunocytochemistry (ICC), range in applicability from basic structural observation using general staining to detecting specific proteins using antibody stains that identify specific cellular components. Furthermore, high-contrast images can be produced using a variant of this technology called immunofluorescent staining. This method employs fluorescently tagged antibodies and imaging systems for qualitative and quantitative analyses of protein expression and organization at the cellular level. It is a highly versatile method of tissue analysis with adaptable resolution. Both IHC and ICC are frequently used to evaluate cell viability and proliferation. Assays applying bromodeoxyuridine (BrdU) and 5-ethynyl-2’-deoxyuridine (EdU) measure the incorporation of these compounds into newly synthesized DNA to detect cell proliferation. Another common measure of cell growth utilizes an antibody to the nuclear protein Ki67 that is strictly associated with proliferation. Carboxyfluorescein succinimidyl ester (CFSE) is a cell-permeable dye cleaved by living cells to emit green fluorescence. LIVE/DEAD assays simultaneously stain viable, dead, apoptotic or all cells using calcein-AM, propidium iodide or Hoechst 33342 accordingly. Lastly, trypan blue staining is used to identify dead cells that are permeable to the dye. The techniques of IHC and ICC can also assess tissue maturation by staining for more mature features of the tissue. For example, tissue-engineered muscle constructs matured in uniaxial bioreactors were examined using immunostaining with phalloidin and desmin that demonstrated the alignment of multinucleated myofibers [51, 52]. In these studies, the immunostaining of myosin heavy chains was also performed to evaluate muscle fiber formation and maturation [52]. In addition to the histological methods, several spectrophotometry-based assays are frequently used to assess cell viability. The three most commonly used assays are MTT, XTT and WST-1, which all use compounds that elicit a color change when applied to living cells. This color change is quantified by a spectrophotometer. It is important to note that in many bioreactor studies, IHC, ICC and spectrophotometry-based viability assays are often needed to complement non-invasive imaging analyses described below for validation.
Electron microscopy, a further commonly used method of evaluating tissue viability and maturation, utilizes electrons as a source of illuminating radiation in conjunction with an electron-optical lens system for image capture. This technique produces very high-resolution images of cellular and extracellular matrix components. In transmission electron microscopy (TEM), a high-energy electron beam is applied to a thin tissue section that is immune-labeled or otherwise stained with heavy metals. This creates an image akin to immunohistochemistry but with much higher contrast and resolution. In the evaluation of several cell culture bioreactors, TEM was used to assess the viability and maturation of cells in spheroids cultured in dynamic suspension [53, 54]. In one study, cells and their components were stained with heavy metals to visualize the presence of reversible DNA double-strand breaks, apoptotic cell death and proliferation [53]. In another study on the culture of human retinal organoids in a stirred-tank bioreactor, TEM was utilized to image the development of retina-like structures, such as the connecting cilium, outer segment and photoreceptor synapses indicative of tissue maturation [54]. TEM has also been employed to examine large-scale tissues cultured in bioreactors. In one such instance, TEM was used to show the maturation of adult human liver cells by identifying the reorganization of tissue features such as cell–cell junctions and bile canaliculi-like structures [55].
In scanning electron microscopy (SEM), a specimen’s surface is typically coated with a thin layer of gold and scanned by a focused electron beam. As the electron beams interact with different topographies on the specimen surface, differing amounts of energy are adsorbed, and the resulting pattern is captured as an image. The SEM technology has been used for tissue analysis to examine the formation of morphological features indicative of more mature tissue. For example, in the above-mentioned study on human retinal organoids, SEM was conducted to analyze the topographical features of whole retinal organoids, such as photoreceptor density and connecting cilium formation indicative of tissue development [54]. In another example, the formation of boney nodules in tissue-engineered bone cultured in a biaxial rotating bioreactor was imaged by SEM [56].
A non-imaging tool for tissue function analysis is the reverse transcriptase quantitative polymerase chain reaction (RT-qPCR/qPCR), which identifies and quantifies the amount of mRNA in a sample by amplifying the mRNA signal using a combination of PCR and fluorescent tagging. Usually, qPCR is coupled with IHC, Western blotting or protein quantification using enzyme-linked immunosorbent assay (ELISA) protocols that are designed to detect and quantify specific proteins in samples for validation. These tools have been employed to analyze tissue maturation based on that tissues produce different surface markers and proteins as they develop. For example, qPCR was utilized to detect the expression of early and late smooth muscle cell makers in an engineered tissue construct to track tissue maturation [57]. Furthermore, the formation of structural proteins indicative of tissue maturation can be assessed using RT-qPCR to detect collagen and aggrecan mRNA expression, as performed to evaluate cartilage tissue matured in a biaxial rotation bioreactor system [58]. Although histological imaging, electron microscopy, RT-qPCR and protein analyses are powerful tools for tissue evaluation, their major disadvantage is the risk of exposing the tissue or the bioreactor system to contamination. As a result, entire systems need to be dismantled in many cases, or tissues damaged to some degree must be sampled for biopsy. Due to these reasons, the evaluation of tissue in bioreactors is often carried out as an end-point analysis. Such analyses certainly provide essential and useful information regarding the quality of tissue produced in a bioreactor system. Nonetheless, if the end-goal of bioreactor application is to improve the implantation success or extend the study of tissues over time, methods of tissue analyses that do not expose the tissue to damage or contamination would be ideal, and perhaps even necessary.
Non-destructive analytical tools
Standard tools
The available non-destructive tools for structural and functional tissue evaluation feature varying degrees of applicability to bioreactor systems. Magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), single-photon emission computed tomography (SPECT) and ultrasound represent a range of options widely applied and readily available at biomedical facilities. These imaging modalities have been mostly used to monitor tissues and organs in vivo.
The MRI is an effective non-invasive technique for imaging tissue structural features, including fluid flow through tissue. It generates images by using powerful magnets to align protons within the tissue to a magnetic field, followed by pulsed radiofrequency currents that cause some protons to fall in and out of alignment, thus generating an emitted signal for analysis. In the clinic, functional MRI (fMRI) is commonly used to measure brain activity using algorithms to assess blood flow changes to different brain regions. For a similar purpose of flow detection, MRI was used to track the perfusion of media and nutrients through engineered tissues within bioreactor systems [59–62]. More recently, MRI has shown to be effective in the non-invasive evaluation of media flow through a smaller polymer chip-based bioreactor setup, showing the scalability of this tool for different bioreactor systems [63]. Although MRI was successfully used in these studies, the practicality of MRI in bioreactor systems is limited mainly by the requirement that bioreactor systems be built with MRI compatible parts. Additionally, MRI requires long acquisition times for high-resolution images on a small scale and has low contrast limiting the identification of complex structural components.
CT is a tool capable of generating higher resolution images while maintaining large imaging depths and fields of view. It uses multiple X-ray measurements from different angles non-invasively to produce cross-sectional images of scanned areas of a tissue. These image sections can be integrated by a computer to generate a three-dimensional image of the tissue to track gross structural changes that occur with tissue maturation. Several studies have used this technique to monitor 3D mineralization over time in a perfusion bioreactor [64–66]. While CT is a highly beneficial technique and has been previously used to evaluate some bioreactor systems, its extended use in tissue analysis is limited by concerns over possible damage to biological tissues due to the ionizing radiation from exposure to multiple X-rays [67].
Positron emission tomography (PET) is another instrument available in medical centers that provides an effective and non-invasively means of imaging the changes in tissue function. It is capable of detecting variations in metabolism, blood flow and regional chemical composition [68]. Though this method can provide robust information about tissue characteristics, PET alone has poor spatial resolution, thus it is often used in conjunction with CT in a technology known as single photon emission computed tomography (SPECT) to provide enhanced information on signal localization. By combining the advantages of real-time functional analysis of PET with the fine resolution images produced with CT, SPECT can provide a clearer picture of tissue functionality.
Of the tools readily available in the biomedical setting, ultrasound shows the highest potential for easy integration into bioreactor systems. Ultrasound transmits high-frequency sound waves into tissue that reflects them back based upon the interfacing densities within that tissue. The machine calculates the distance from the probe to the tissue density interface and the degree of density differential at the interface to construct a two-dimensional rendering of structure within a wedge of tissue. This technology can be integrated into bioreactor systems with relative ease for the real-time monitoring of tissue maturation [69]. In one example, matrix components were cultured within regenerating cartilage and their development was monitored successfully using a bioreactor system with integrated ultrasound [70]. Furthermore, vascular or Doppler ultrasound is a modified ultrasound technique that utilizes high-frequency sound waves to detect circulating red blood cells and their direction of flow to identify, for example, circulatory blockages and blood flow across heart valves. This technology can be used in bioreactor systems to analyze perfusion in constructs in real time and in a non-destructive and non-invasive manner [71]. Although this technique allows for the real-time tracking of tissue function, the associated low resolution and significant background noise in images, especially at greater image depths, currently limits the use of this technology.
Emerging optical analysis methods
In addition to the above non-destructive technologies, several emerging optical analysis methods have been identified as technologies particularly well suited for bioreactor systems. The below discussed optical analysis tools are not comprehensive, but include the most applicable ones to bioreactor systems, as they do not necessitate optical clearing of tissue and have adequate resolution and contrast. The first technology, optical coherence tomography (OCT), which is related to ultrasound in principle, uses light energy as a probe to identify tissue density interfaces to render an image with higher resolution than ultrasound. OCT can also be employed using fiber optics, enabling imaging probes with small diameters that can be easily integrated into bioreactor systems [72, 73]. For instance, this type of system has already been used to non-destructively assess the endothelialization of bioengineered electro-spun vessels in culture [74]. Based on the above studies, it is possible to imagine applications of OCT probes within a vessel or vessel-containing constructs in bioreactor systems. Moreover, in conjunction with polarization imaging, OCT can identify the fiber orientation of underlying tissues to monitor tissue maturation. Nevertheless, even though the resolution depth of OCT is improved, the problems similar to ultrasound of low contrast and decreasing resolution with tissue depth limit its use for the evaluation of surface features or within thin constructs.
Confocal microscopy is an additional optical tool commonly used to evaluate engineered tissue or cell-seeded scaffolds. It allows for a direct, non-invasive, serial optical sectioning of live specimens with little preparation akin to CT scans but without the X-ray radiation exposure. Using a small, focused beam of light (point illumination) and a pinhole to eliminate out-of-focus light, the microscope captures two-dimensional images at one narrow depth level at a time. Each two-dimensional image can have high resolution and contrast depending on the specific equipment used. Using immunofluorescent staining, these two-dimensional images can be used to identify specific immune-labeled markers of cell viability and maturation. In addition, the 2D images can be stacked using software to create a three-dimensional rendering, which is useful in determining gross tissue structure and fiber orientation. This technique has been used successfully in the clinical evaluation of various eye diseases and of tissue features of skin in vivo [75, 76]. Confocal microscopy and multiphoton microscopy, a variant technology that uses two-photon pulses instead of one to provide a more focused image, were used in a mechanical stretch bioreactor for non-invasive imaging and biomaterial screening [77]. The disadvantages of confocal microscopy arise from the machinery design that allows for high resolution and contrast. The pinhole that eliminates out-of-focus light causes a decrease in light intensity that requires longer exposure times; the wavelength of light thus produced limits the thickness of the focal plane. Due to these reasons, confocal microscopy is best suited for the surface profiling of relatively thin samples.
Raman spectroscopy is an optical analysis method with great potential application to bioreactor systems. This tool has recently gained much interest for use in vivo as it enables the analysis of the molecular composition and structure of tissue without destruction or alteration [78]. At a basic level, the machinery functions by measuring increases or decreases in scattered light wavelengths. It interacts with high-frequency vibrations within individual molecules to generate Raman spectroscopy graphs. From these data, a scattering spectrum unique to specific tissues or tissue constituents can be identified. Raman scattering spectra are often coupled with imaging systems like OCT or confocal imaging to gain more information on tissue differences. Several studies have demonstrated how Raman scattering spectra can differentiate between regions of heterogeneous tissue and track changes in close to real time. For example, early in vivo experiments using Raman spectroscopy demonstrated that this technique is very sensitive to lipid pools—being able to differentiate between subclasses of lipids such as triglycerides, cholesterol and different cholesterol esters [72]. When used in conjunction with OCT, atherosclerotic plaque morphology could be assessed rapidly in rabbit studies [79]. Furthermore, Raman spectroscopy has been exploited to detect molecular alterations in ulcerative colitis and Crohn’s disease with a classification accuracy of 95%. It has also been utilized to evaluate differences between biochemical characteristics of irritable bowel disease and normal colon [78]. This method accurately differentiated tumors from healthy tissue in lung, breast, gastrointestinal, urinary, brain, prostate and skin tissues [80–94]. These studies show the capacity of Raman spectroscopy to identify tissues at different stages of development and track tissue maturation in real time. Additionally, it can be coupled to the use of miniature probes, allowing for catheter-based implementation and making it more applicable to bioreactor systems. Raman spectroscopy has been used for the in-line monitoring of Escherichia coli and mammalian cells in culture bioreactors to determine viable cell numbers and total cell density in real time [95, 96]. However, while this technology has been well characterized for cell culture systems, it is still relatively untested in tissue culture bioreactor systems. The likely reason is that as with other non-destructive technologies discussed, this technology is currently limited to evaluating surface or thin structures due to loss of resolution at depth, as well as the high complexity of analysis that would need to be simplified for broader use in the field.
Spatial frequency domain imaging (SFDI), a label-free, non-destructive and non-invasive optically based technique, has come into focus over the recent years. It is a powerful tool that identifies spatial patterns of reflected light to identify and quantify unique structural properties of tissues, much like Raman spectroscopy. However, as an imaging technique, it can also recognize patterns of flow, structural changes, tissue component orientation and real-time three-dimensional morphology. It is well suited for future applications in bioreactor systems due to its ability to rapidly survey large areas of tissue with modest resolution while measuring endogenous contrast between different tissues at greater depths than other non-destructive optical techniques [97]. The utility of SFDI for future bioreactor systems is demonstrated through several published in vivo applications. Chronic wound studies highlighted the usefulness of SFDI to track tissue perfusion and oxygenation. These studies used SFDI to quantify and track hemoglobin oxygenation and distribution within the visible boundaries of wounds to identify unique circulation signatures associated with the wound areas [98]. Additionally, several studies showed that this technique can accurately identify ischemia and early markers of tissue necrosis. In several burn studies, SFDI was used in preclinical models to assess burn depth to develop better early predictors of burn severity [99–103]. In these researches, SFDI was proved to be one of the most sensitive and specific techniques for burn diagnosis [104]. Notably, one burn study used SFDI to monitor the infection status of burn injuries [99]. Furthermore, this method was evidenced by multiple groups to adequately measure ischemic onset in skin, kidney, bowel and liver, pointing to its possible application for assessing the viability of several different tissue types within bioreactor systems [105–108]. Finally, in cardiac studies, SFDI was employed to characterize soft tissue fiber orientation, highlighting its utility in assessing the structural maturation of tissue [109]. Taken together, the above findings demonstrate that SFDI may be a powerful tool to evaluate the structure, perfusion, contamination and overall health of constructs within bioreactors in real time. Though promising, much of this pre-clinical work has been exploratory. There is still room for further development in creating better algorithms for more accurate assessment, and in coupling this technology with other imaging techniques to allow for improved tissue evaluation [97].
Many methods have been devised for evaluating the maturation and viability of tissues cultured in bioreactor systems with varying degrees of utility (Table 3). Of these, emerging optically based analytical methods are well suited for non-destructive, label-free and adaptable analysis that is ideal for such systems. However, many areas need to be improved before a comprehensive optical monitoring strategy will be attained for bioreactor systems. The most important areas to improve are the depth-dependent lower resolution that limits the size of engineered tissues and the lack of algorithms specific for the types of constructs intended for maturation within next-generation bioreactor systems. Figure 3 provides a schematic of the overall differences between the destructive and non-destructive tissue analysis approaches that have been detailed in this section.
Table 3.
Summary of analytical tools for the evaluation of bioreactor processed tissue
| Analytical tool | Major applications | Resolution | Pros | Cons |
|---|---|---|---|---|
| Optical microscope [51, 52] | Analysis of scaffold architecture, cellular alignment, morphology, cellular junctions and intracellular components IHC/ICC analysis |
0.2 μm | Widely available in laboratory settings Relatively low-cost infrastructure for analysis Adaptable resolution |
Sample destruction during preparation Lacking 3D information Limited time points |
| TEM [53–55] SEM [54, 56] |
Analysis of scaffold architecture and cellular morphology | 0.2 nm (TEM) 1–5 nm (SEM) |
Very high-resolution images of inter- and intracellular components | Sample destruction during preparation Limited time points Maintenance |
| Spectroscopy | MTT assay XTT assay WST-1 assay |
N/A | Widely available Easy detection of viability |
Sample destruction during preparation Processing time can be lengthy |
| RT-qPCR/qPCR [57, 58] | mRNA content identification and quantification | N/A | Widely available Easy detection |
Sample destruction during preparation |
| MRI [59–63] | Analysis of ECM deposition, tissue oxygenation, mineralization and scaffold architecture | 60–125 μm | Non-invasive Capable of assessing real-time perfusion |
Sometimes requires the use of contrast agents Long acquisition time for high-resolution images Requires that bioreactor is made from MRI compatible materials |
| CT [64–67] PET [68] SPECT [68] |
Analysis of ECM deposition and mineralization, scaffold architecture and vascularization Analysis of cell metabolic activity, blood flow and regional chemical composition Cell tracking |
12–50 μm (CT) 1–3 mm (PET/SPECT) |
Non-invasive cross-sectional and 3D imaging High-resolution images of tissue structures* |
Requires drying of sample or use of contrast agents to obtain clear image Long data acquisition times *PET alone has poor spatial resolution Multiple X-rays used in CT may induce radiation damage |
| Ultrasound [69–71] | Analysis of tissue structure, ECM and mineral deposition Real time assessment of regeneration Doppler ultrasound is able to assess vascular flow |
250–500 μm | Non-invasive Readily available Real-time monitoring Can be easily integrated into bioreactor systems |
Noise in signal Limited tissue contrast Decreased resolution with image depth |
| OCT [72–74] | Optical biopsy Intra-arterial imaging |
Axial, 1–10 μm; lateral, 10 μm | Non-invasive Higher resolution than ultrasound Fiber optics allow for adaptable imaging probes Can be used with polarization imaging |
|
| Polarization imaging [73, 109] | Identification of fiber orientation, birefringent structures and specular reflection isolation | Depends on paired imaging technique | Can be paired with many different imaging modalities | Low performance as stand-alone technology |
| Confocal microscopy [73, 75, 76] | Histology and diagnostic pathology Cellular imaging Biochemical and molecular labeling |
< 1 μm | Non-invasive Can use intrinsic contrast, or with staining for increased contrast Capable of multi-channel fluorescence imaging |
Slow Limited optical depth (i.e., 200–300 μm in human skin) |
| Multi-photon microscopy [77] | Intrinisic contrast imaging of cells and tissues Structural and functional molecule labeling |
< 1 μm | Non-invasive Can use intrinsic contrast, or with staining for increased contrast Capable of higher resolution than confocal microscopy |
|
| Raman spectroscopy [78–96] | Monitor in vitro and in vivo tissue characteristics Spectral analysis of a variety of different tissue conditions |
Axial, integrated along depth; lateral, > 100 μm | Non-invasive Capable of real-time analysis Spectra can be created for many different and new tissue conditions Highly effective in differentiating between tissue regions/types Can be coupled to miniature probes |
Analytical complexity Low resolution depth limits use |
| SFDI [97–109] | Analysis of flow, structural changes, tissue component orientation and real-time three-dimensional morphology | Axial, integrated along depth; lateral, > 100 μm | Non-invasive High-speed image acquisition of large survey area Uses endogenous contrast Capable of greater resolution depth than other optical techniques Highly effective in differentiating between tissue regions/types |
Needs better algorithms for tissue analysis |
Bio-Design and Manufacturing (2022) 5:43–63
Fig. 3.
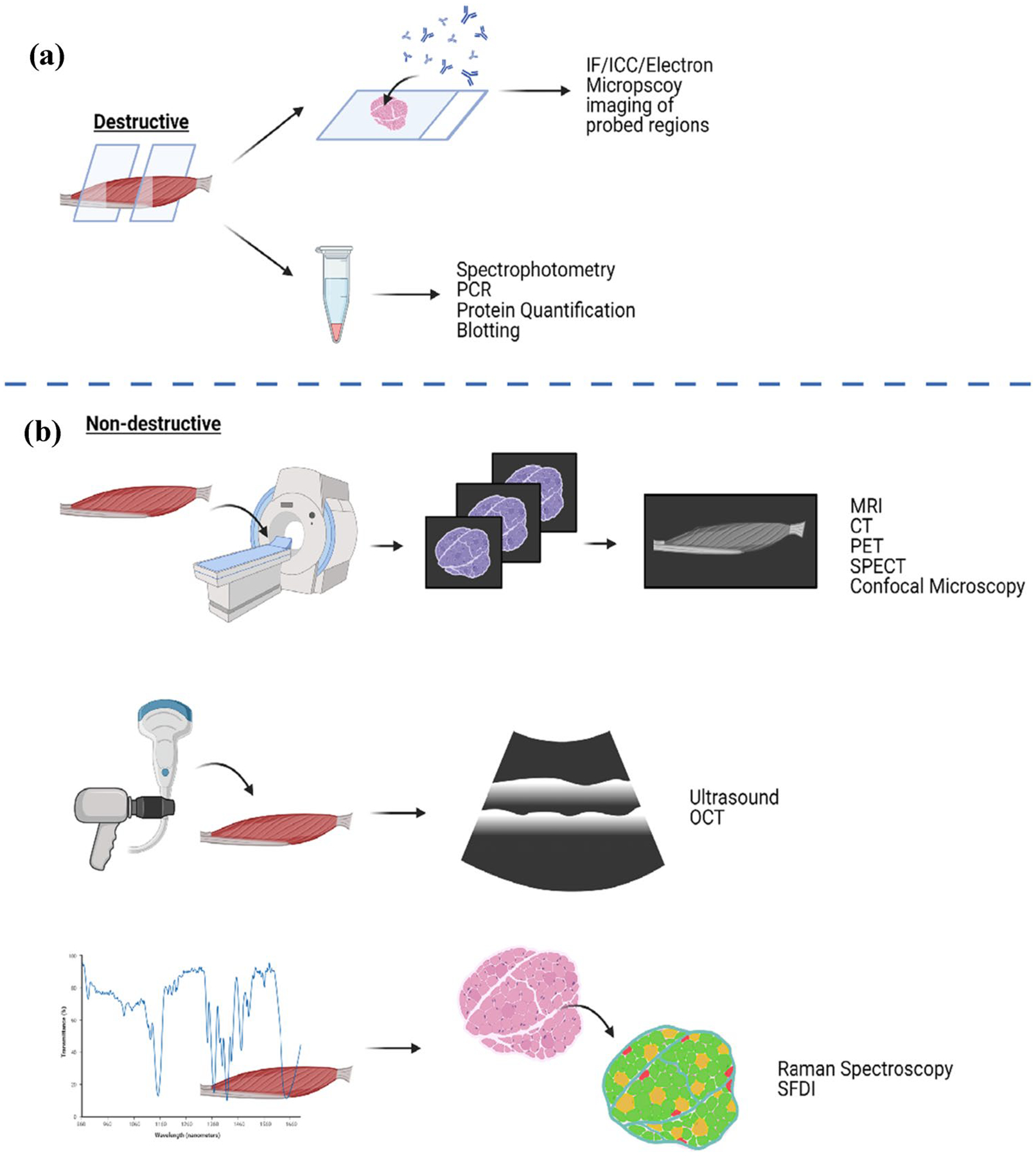
Schematic of destructive and non-destructive tissue evaluation methods. a The destructive analytical methods necessitate sectioning, lysis or a destructive staining of tissues. These methods focus on tagging surface markers on sections of tissues for imaging, or the collection of metabolites or cell components for quantitative analysis. b Non-destructive analytical methods can be built into bioreactors to evaluate tissues in real time without disturbing tissue growth. These methods utilize differences in molecular bond energy, tissue density, fiber orientation or light scattering patterns to identify differences between tissue regions. The illustration was created using BioRender.com
Manufacturing and commercialization
Construction aspects
Currently, the components of a bioreactor can be fabricated according to a variety of options. These options can be divided into traditional processes such as subtractive and net-shape processes, as well as relatively newer approaches like additive manufacturing. Although the latter has been traditionally used to fabricate intermediate prototypes of bioreactor components [110], this technology should still be considered since it is predicted to be used more widely as it becomes inexpensive and employs more advanced materials [111]. The subtractive manufacturing approach involves starting with a large amount of material and creating the final product by removing some of the material. CNC milling is a well-known example of subtractive manufacturing, which uses milling machines to slowly remove material from an initial block into the final part. The formative manufacturing approach involves using molds representing the negative volume of a component’s shape that can be filled with the desired material. Casting is an example where the liquid material is poured into a mold and allowed to solidify. Injection molding involves using a melted material to fill in a mold at high temperature and pressure. The main advantage of these approaches is that they allow similar parts to be mass-produced, however, they are not ideal for highly complex shapes. Traditional manufacturing techniques are well documented in the literature [112].
The additive manufacturing (AM) approach involves adding material in a layer-by-layer process, as shown in Fig. 4.
Fig. 4.
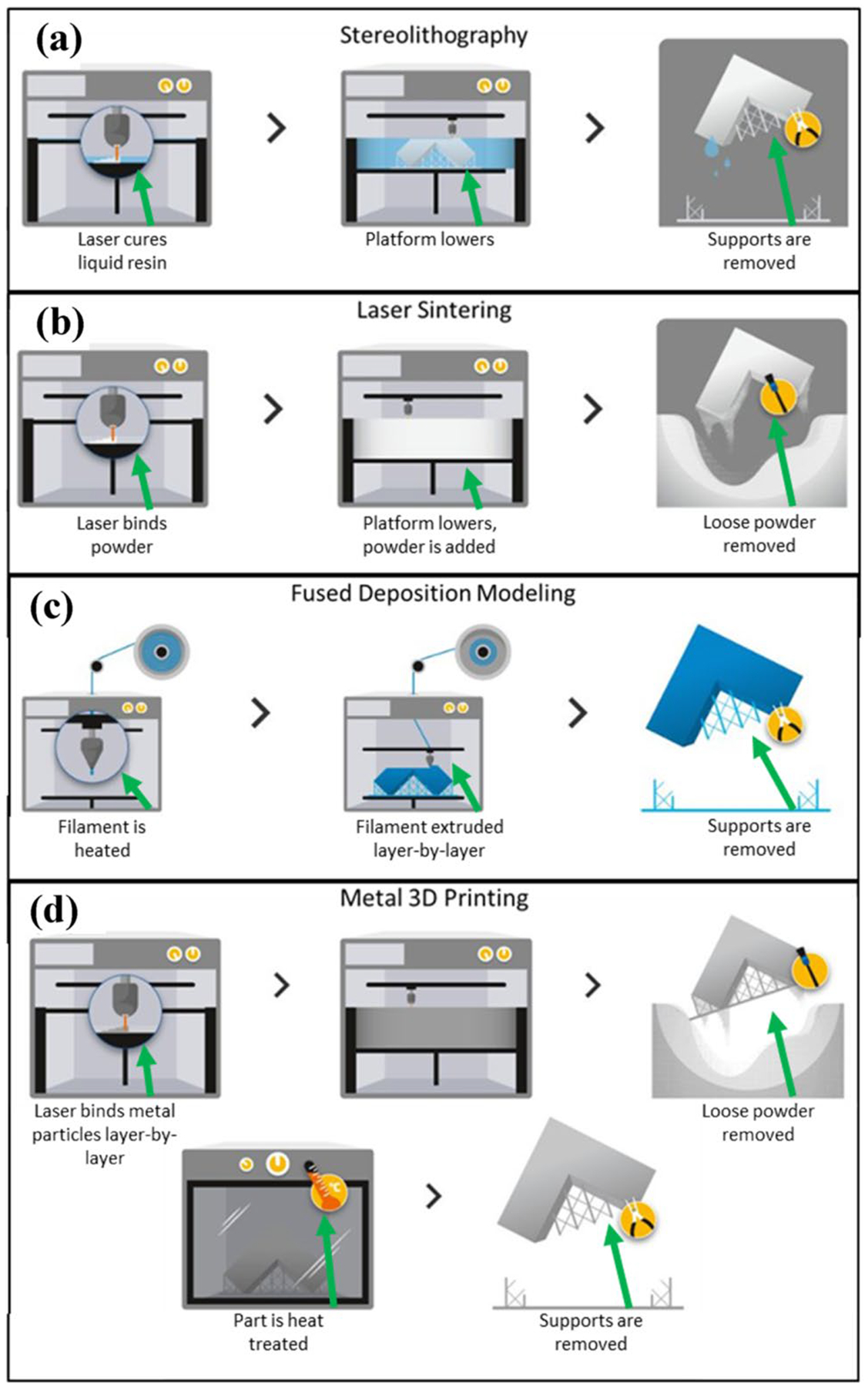
Various 3D printing technologies available for manufacturing. a The process where a stereolithographic printer cures resin with light. b The process where a laser sintering printer fuses powdered plastics in a bed to form objects. c The process where a fused deposition modeling printer creates objects by melting plastic filaments retrieved from a reel. d The process where a metal printer uses an energy source to fuse metal powder into objects. Reprinted with permission from Materialise [114], Copyright (2020) Materialise
Stereolithography is a type of AM that uses UV light to cure resins, which can produce parts at high resolution but fabricates slowly. Selective laser sintering is a kind of AM that uses lasers to melt powdered plastics in a bed into the final shape, which can also produce high-resolution parts at the cost of speed. Fused deposition modeling is an AM method that involves extruding heated plastics through a nozzle and depositing the material onto a platform, which prints parts relatively fast, but these parts have weaker properties. Metal printing is a relatively new form of AM that allows parts to be produced layer by layer directly using metals. Common types of metal printing include electron beam additive manufacturing (EBAM) and selective laser melting (SLM) [113], which can produce high-resolution parts with very strong mechanical properties. The benefits and drawbacks of each of these technologies are summarized in Table 4.
Table 4.
Summary of the benefits and limitations of various 3D printing technologies available for manufacturing
| Printing modality | Benefits | Limitations |
|---|---|---|
| Stereolithography | High resolution | Limited to UV curable materials |
| Robust material properties | Slow printing process | |
| High cost | ||
| Laser sintering | High-resolution prints | Slow printing process |
| Robust material properties | High cost | |
| Fuse deposition modeling | Low cost | Weak mechanical properties |
| Fast printing speeds | ||
| Metal 3D printing | High-resolution prints | Slow printing process |
| Strong mechanical properties | Extremely high cost | |
| Limited materials |
The layer-by-layer method of additive manufacturing has lower production rates than traditional manufacturing methods, but it enables the production of highly complex parts such as porous or more intricate designs [115].
Regulatory and standardization aspects
The main function of a bioreactor is to mature tissues, which will eventually be used as a clinical product. To bring this technology to the market, it is important to consider regulatory guidelines, since the bioreactor and its contents will interact with the final clinical product. The Food and Drug Administration (FDA) provides guidance for several classes of devices. Many similar predicate devices are listed as Class I devices, including spinner flasks, perfusion bioreactors, perfusion roller apparatuses, cell culture suspension systems and tissue culture flasks [111, 116, 117]. Under Class I devices, the FDA exempts most devices from premarket notification but does require that the device follows applicable current good manufacturing practices (cGMP) [117] and includes the 510(K) premarket submission to obtain premarket clearance. Class I devices that are labeled as sterile are not exempt from cGMP requirements [117]. The cGMP regulations for medical devices are found in CFR Title 21, Part 820. The requirements listed include creating a quality system, establishing a system for document control, implementing a system to identify and trace a product intended for surgical implantation, and much more [117]. In addition, for automated devices, design controls are required to ensure that the final product matches the intended function derived from user needs [118]. Figure 5 was taken from the FDA guidance documentation proposing a general methodology of identifying, verifying and validating the design requirements.
Fig. 5.
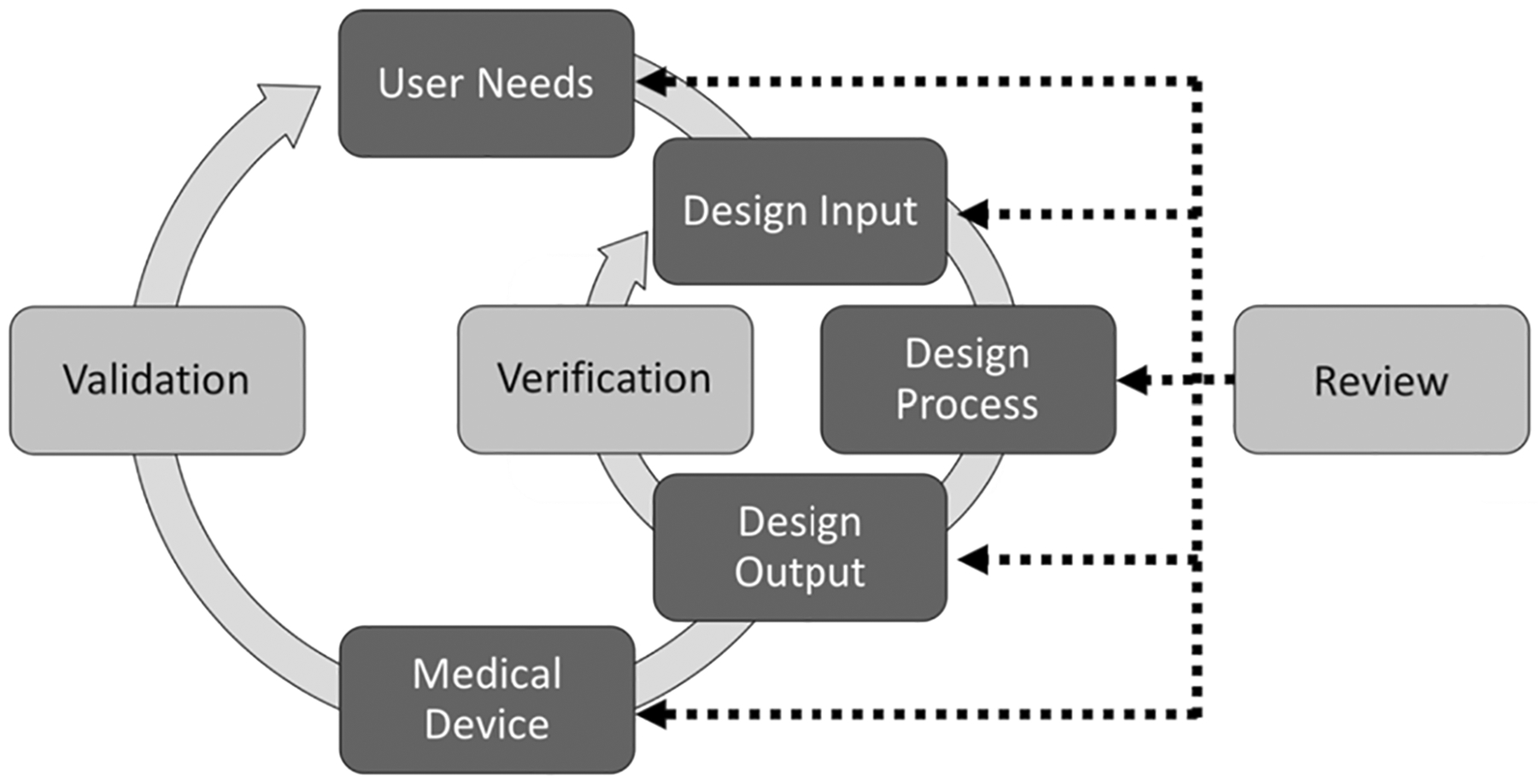
General process for design control as recommended by the FDA [117]
A bioreactor must also comply with ISO standards for similar devices. Fortunately, the FDA has worked to match cGMP guidelines with “ISO 13,485:1996…, which contains requirements for medical device manufacturers in addition to the general quality system requirements found in ISO 9001:1994” [117].
This is important as many end users of medical devices, such as hospitals, prefer medical devices certified with ISO 9001 standards. Due to this preference, many manufacturers follow “ISO 9001 and, where applicable, with ISO 13,485” [119] to ensure that their products meet these standards and are marketable to customers in the biopharmaceutical and regenerative medicine industries. Thus, following cGMP standards should make it easier for a medical device to obtain ISO certification.
Commercialization aspects
Although the scientific rationale for a bioreactor has been well documented, one area that lacks understanding is translating devices into commercialized products. A typical framework used to understand the market and readiness level is the 3C framework, which identifies information from companies, competitors and customers that can be used to justify the product [120]. As a company considers whether or not to invest funds into the research and development of a new device, it will gather data simultaneously for these categories. It will review its core competencies to ensure that its strengths can support the device being considered. In addition, data are gathered on competing products as a guide to understanding the cost and revenues that can be attained from selling the new product. By looking at data from companies with similar products, items like manufacturing costs and product pricing can be calculated or inferred. Lastly, a comprehensive knowledge of the needs of potential customers will help to define the potential market share that can be captured and the overall revenues that can be thus achieved. As reported in Global Market Insights in March 2019, the global bioreactor market was worth $700M in 2018 and will reach $2.2B by 2025, with a compound annual growth rate (CAGR) of 17% between this seven-year period (Fig. 6), due to “rising chronic disease prevalence and growing research in the field of biopharmaceuticals… and the increasing number of personalized medicines targeting a specific population will further boost industry expansion” [121]. To date, most bioreactors that are commercially available have been dedicated to cell culture or pharmaceutical purposes. The development of tissue bioreactors is considered to be in a pre-competitive stage.
Fig. 6.

Global bioreactors market trend, 2018–2025 (in billions of USD)
Thus, through the quick development and commercializing of a tissue bioreactor, a commercial entity can expect to capture an early market share from their competitors. This is a crucial task since studies have shown that profitability is correlated with market share [122]. Entering a market sooner also locks out a competitor’s solution because customers’ perceived risk of losing value is more significant than their perceived gain of value in a new product. This makes customers hesitate to change a vendor or solution [123].
Once these data points are mapped out, it is possible to start considering the STP framework, which Segments the customers, Targets the segment(s) that will return the most revenue and Positions the product message to these segments [124]. As referenced in Sartorius’ 2019 annual 10K report [119], traditional cell culture bioreactors are used by biopharmaceutical laboratories and upstream process development departments, which accounted for $1.37 billion in global revenues in 2019. The regenerative medicine industry was also identified as a future growth area. Thus, one can expect that customers in research laboratories will first adopt these technologies, followed by customers in commercial production. Therefore, a bioreactor should be developed to deliver the qualities preferred by these groups to help them capture their market share. Anecdotally, a customer in a research laboratory will find value in quality control systems that record values of pH, oxygen, glucose, etc., as well as in the ability to reproduce results from experiments. More reliable data are needed to ensure that customer’s needs are appropriately captured and quantified. These data can be obtained by conducting user requirement surveys and analyzing the responses to reinforce pre-existing concepts in addition to identifying any unnoticed trends in requirements. Presenting such data to commercial entities should give sufficient evidence to justify the value of investing in introducing a device into the market.
Conclusions
A well-established bioreactor design is crucial for the efficient growth and maturation of engineered tissue and for translating tissue engineering strategies into clinical applications. The bioreactor system provides a biomimetic physiological platform by delivering nutrients, eliminating cellular waste products, supplying oxygen and maintaining an appropriate pH range within a contained sterile environment. In addition, specific stimulation and conditioning processes within the bioreactor play a critical role in developing an optimal tissue microarchitecture, tissue functionality and tissue durability. By means of real-time monitoring the chemical components (e.g., pH, O2, glucose/lactate) of relevance to the culture of engineered tissue construct, bioreactors enable automatic and systematic control of the physical environment for the safe and reproducible culture of a specific tissue. During the maturation of engineered tissue in the bioreactor, it is essential to evaluate the functional tissue parameters using various technologies (destructive or non-destructive) to determine whether the tissue construct is suitable for patient implantation. The future utility of engineered tissue, required materials, as well as regulatory and commercialization aspects, should all be considered when evaluating how the novel bioreactor system may be implemented as a clinical manufacturing tool. Moreover, its success in engineered tissue manufacturing can be facilitated by intimate collaboration among academic scientists, industrial engineers and clinicians. For future clinical products, researchers need to further refine the bioreactor systems for scalability and ease of handling while minimizing contamination risks. These developmental efforts can strengthen the fundamental knowledge required to build efficient manufacturing models [1, 125]. Many critical considerations must be given to transition the bioreactor system from the laboratory level to the manufactured product for distribution, including safety, standardization, cost-effectiveness and regulatory compliant manufacturing processes. Moreover, a bioreactor-based tissue-engineered product must be validated to ensure its safety and efficacy before human use. It is evident that multidisciplinary team effort is necessary to build a novel and advanced bioreactor system capable of producing successful tissue-engineered products.
Acknowledgements
The authors would like to thank our Industry Collaborators as well as the Wake Forest Institute for Regenerative Medicine for the bioreactor research program. Funding was made possible by US Army Medical Research and Development Command through the Medical Technology Enterprise Consortium under Contract #W81XWH-15-9-0001.
Footnotes
Publisher's Disclaimer: Disclaimer: The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, by the U.S. Government.
Conflict of interest The authors declare that there is no conflict of interest.
Ethical approval This work does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Portner R, Nagel-Heyer S, Goepfert C et al. (2005) Bioreactor design for tissue engineering. J Biosci Bioeng 100(3):235–245. 10.1263/jbb.100.235 [DOI] [PubMed] [Google Scholar]
- 2.Salehi-Nik N, Amoabediny G, Pouran B et al. (2013) Engineering parameters in bioreactor’s design: a critical aspect in tissue engineering. Biomed Res Int 2013:762132. 10.1155/2013/762132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin I, Wendt D, Heberer M (2004) The role of bioreactors in tissue engineering. Trends Biotechnol 22(2):80–86. 10.1016/j.tibtech.2003.12.001 [DOI] [PubMed] [Google Scholar]
- 4.Ravichandran A, Liu Y, Teoh SH (2018) Review: bioreactor design towards generation of relevant engineered tissues: focus on clinical translation. J Tissue Eng Regen Med 12(1):e7–e22. 10.1002/term.2270 [DOI] [PubMed] [Google Scholar]
- 5.Mather ML, Morgan SP, Crowe JA (2007) Meeting the needs of monitoring in tissue engineering. Regen Med 2(2):145–160. 10.2217/17460751.2.2.145 [DOI] [PubMed] [Google Scholar]
- 6.Mathieu V, Chauvette G, Langelier E (2011) A roadmap for the design of bioreactors in mechanobiological research and engineering of load-bearing tissues. J Med Devices 5:041006–1 [Google Scholar]
- 7.Kannan RY, Salacinski HJ, Butler PE et al. (2005) Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater 74(1):570–581. 10.1002/jbm.b.30247 [DOI] [PubMed] [Google Scholar]
- 8.Pandey U, Bapat KN, Samuel G et al. (2005) Evaluation of 90Y phosphate particles as a possible radiation synoviorthesis agent. Nucl Med Commun 26(5):459–463. 10.1097/00006231-200505000-00011 [DOI] [PubMed] [Google Scholar]
- 9.Hassan MS, Kannan RY, Rehman N et al. (2005) Difficult adherent nail bed dressings: an escape route. Emerg Med J 22(4):312. 10.1136/emj.2003.012914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannan RY, Salacinski HJ, Sales K et al. (2005) The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials 26(14):1857–1875. 10.1016/j.biomaterials.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Wells C, Khangura S et al. (2017) CADTH Health Technology Assessments, in Proton Beam Therapy for the Treatment of Cancer in Children and Adults: A Health Technology Assessment. Canadian Agency for Drugs and Technologies in Health. https://www.ncbi.nlm.nih.gov/books/NBK531691/pdf/Bookshelf_NBK531691.pdf [PubMed] [Google Scholar]
- 12.Meidani Z, Farzandipour M, Davoodabadi A et al. (2017) Effect of reinforced audit and feedback intervention on physician behaviour: a multifaceted strategy for targeting medical record documentation. J R Coll Physicians Edinb 47(3):237–242. 10.4997/JRCPE.2017.305 [DOI] [PubMed] [Google Scholar]
- 13.Aizawa P, Karlsson G, Benemar C et al. (2007) Effect of different materials used in bioreactor equipments on cell growth of human embryonic kidney (HEK293) cells cultivated in a protein-free medium. In: Smith R (ed) Cell technology for cell products. Springer, Dordrecht [Google Scholar]
- 14.Lei Y, Ferdous Z (2016) Design considerations and challenges for mechanical stretch bioreactors in tissue engineering. Biotechnol Prog 32(3):543–553. 10.1002/btpr.2256 [DOI] [PubMed] [Google Scholar]
- 15.Lerman MJ, Lembong J, Muramoto S et al. (2018) The evolution of polystyrene as a cell culture material. Tissue Eng Part B Rev 24(5):359–372. 10.1089/ten.TEB.2018.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin DP, Williams SF (2003) Medical applications of poly-4-hydroxybutyrate: a strong flexible absorbable biomaterial. Biochem Eng J 16(2):97–105. 10.1016/S1369-703X(03)00040-8 [DOI] [Google Scholar]
- 17.Plunkett N, O’Brien FJ (2011) Bioreactors in tissue engineering. Technol Health Care 19:55–69. 10.3233/THC-2011-0605 [DOI] [PubMed] [Google Scholar]
- 18.Ng CP, Swartz MA (2006) Mechanisms of interstitial flow-induced remodeling of fibroblast-collagen cultures. Ann Biomed Eng 34(3):446–454. 10.1007/s10439-005-9067-3 [DOI] [PubMed] [Google Scholar]
- 19.Bilodeau K, Mantovani D (2006) Bioreactors for tissue engineering: focus on mechanical constraints. A comparative review. Tissue Eng 12(8):2367–2383. 10.1089/ten.2006.12.2367 [DOI] [PubMed] [Google Scholar]
- 20.Dennis RG, Smith B, Philp A et al. (2009) Bioreactors for guiding muscle tissue growth and development. Adv Biochem Eng Biotechnol 112:39–79. 10.1007/978-3-540-69357-4_3 [DOI] [PubMed] [Google Scholar]
- 21.Candiani G, Riboldi SA, Sadr N et al. (2010) Cyclic mechanical stimulation favors myosin heavy chain accumulation in engineered skeletal muscle constructs. J Appl Biomater Biomech 8(2):68–75 [PubMed] [Google Scholar]
- 22.Zhao J, Griffin M, Cai J et al. (2016) Bioreactors for tissue engineering: an update. Biochem Eng J 109:268–281. 10.1016/j.bej.2016.01.018 [DOI] [Google Scholar]
- 23.Ratcliffe A, Niklason LE (2002) Bioreactors and bioprocessing for tissue engineering. Ann N Y Acad Sci 961:210–215. 10.1111/j.1749-6632.2002.tb03087.x [DOI] [PubMed] [Google Scholar]
- 24.Hoenig E, Winkler T, Mielke G et al. (2011) High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng Part A 17(9–10):1401–1411. 10.1089/ten.TEA.2010.0395 [DOI] [PubMed] [Google Scholar]
- 25.Rauh J, Milan F, Gunther KP et al. (2011) Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev 17(4):263–280. 10.1089/ten.TEB.2010.0612 [DOI] [PubMed] [Google Scholar]
- 26.da Silva HM, Mateescu M, Damia C et al. (2010) Importance of dynamic culture for evaluating osteoblast activity on dense silicon-substituted hydroxyapatite. Colloids Surf B Biointerfaces 80(2):138–144. 10.1016/j.colsurfb.2010.05.040 [DOI] [PubMed] [Google Scholar]
- 27.Sikavitsas VI, Bancroft GN, Holtorf HL et al. (2003) Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci USA 100(25):14683–14688. 10.1073/pnas.2434367100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solan A, Mitchell S, Moses M et al. (2003) Effect of pulse rate on collagen deposition in the tissue-engineered blood vessel. Tissue Eng 9(4):579–586. 10.1089/107632703768247287 [DOI] [PubMed] [Google Scholar]
- 29.Viens M, Chauvette G, Langelier E (2011) Roadmap for the design of bioreactors in mechanobiological research and engineering of load-bearing tissues. J Med Devices 5(4):106 [Google Scholar]
- 30.Tandon N, Marsano A, Maidhof R et al. (2010) Surface-patterned electrode bioreactor for electrical stimulation. Lab Chip 10(6):692–700. 10.1039/b917743d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiesmann H, Hartig M, Stratmann U et al. (2001) Electrical stimulation influences mineral formation of osteoblast-like cells in vitro. Biochim Biophys Acta 1538(1):28–37. 10.1016/s0167-4889(00)00135-x [DOI] [PubMed] [Google Scholar]
- 32.Liao IC, Liu JB, Bursac N et al. (2008) Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Cell Mol Bioeng 1(2–3):133–145. 10.1007/s12195-008-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hambor JE (2012) Bioreactor design and bioprocess controls for industrialized cell processing: bioengineering strategies and platform technologies. BioProcess International 10(6):22–33 [Google Scholar]
- 34.Ge X, Hanson M, Shen H et al. (2006) Validation of an optical sensor-based high-throughput bioreactor system for mammalian cell culture. J Biotechnol 122(3):293–306. 10.1016/j.jbiotec.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 35.pH and Water. [cited 2020 Aug. 6]. https://www.usgs.gov/special-topic/water-science-school/science/ph-and-water?qt-science_center_objects=0#qtscience_center_objects
- 36.Tjandra A, Chang J, Ladame S et al. (2019) Optical sensors. In: Bioengineering innovative solutions for cancer. Elsevier Science & Technology [Google Scholar]
- 37.Zhou H, Purdie J, Wang T et al. (2009) PH measurement and a rational and practical PH control strategy for high throughput cell culture system. Biotechnol Prog 26(3):872–880 [DOI] [PubMed] [Google Scholar]
- 38.Pittman RN (2011) Oxygen gradients in the microcirculation. Acta Physiol (Oxf) 202(3):311–322. 10.1111/j.1748-1716.2010.02232.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US EPA OWOW. Indicators: dissolved oxygen. [cited 2020 Aug. 6]. https://www.epa.gov/national-aquatic-resource-surveys/indicatorsdissolved-oxygen.
- 40.A beginner’s guide to dissolved oxygen measurement [cited 2020 Jul. 25]. https://blog.hannainst.com/beginners-guide-to-dissolved-oxygen-measurement
- 41.Black body radiation. [cited 2020 Aug. 10]. http://galileo.phys.virginia.edu/classes/252/black_body_radiation.html
- 42.Mcmillin JM (1990) Blood Glucose. In: Walker HK, Hall WD, Hurst JW (eds) Clinical methods: the history, physical, and laboratory examinations. Butterworth-Heineman, Boston, pp 662–665 [PubMed] [Google Scholar]
- 43.Patolia S, Staros E. Glucose. [cited 2020 Aug 10]. https://emedicine.medscape.com/article/2087913-overview
- 44.Rassaei L, Olthuis W, Tsujimura S et al. (2014) Lactate biosensors: current status and outlook. Anal Bioanal Chem 406(1):123–137. 10.1007/s00216-013-7307-1 [DOI] [PubMed] [Google Scholar]
- 45.Ahmed S, Chauhan VM, Ghaemmaghami AM et al. (2019) New generation of bioreactors that advance extracellular matrix modelling and tissue engineering. Biotechnol Lett 41(1):1–25. 10.1007/s10529-018-2611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sud D, Mehta G, Mehta K et al. (2006) Optical imaging in microfluidic bioreactors enables oxygen monitoring for continuous cell culture. J Biomed Opt 11(5):050504. 10.1117/1.2355665 [DOI] [PubMed] [Google Scholar]
- 47.Rolfe P (2005) Chemical sensing and control in cell & tissue bioreactors. Conf Proc IEEE Eng Med Biol Soc 2005:7486–7489. 10.1109/IEMBS.2005.1616243 [DOI] [PubMed] [Google Scholar]
- 48.Gomes ME, Reis RL (2004) Tissue engineering: key elements and some trends. Macromol Biosci 4(8):737–742. 10.1002/mabi.200400094 [DOI] [PubMed] [Google Scholar]
- 49.Naing MW, Williams DJ (2011) Three-dimensional culture and bioreactors for cellular therapies. Cytotherapy 13(4):391–399. 10.3109/14653249.2011.556352 [DOI] [PubMed] [Google Scholar]
- 50.Selden C, Fuller B (2018) Role of bioreactor technology in tissue engineering for clinical use and therapeutic target design. Bioengineering (Basel) 5(2):32. 10.3390/bioengineering5020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machingal MA, Corona BT, Walters TJ et al. (2011) A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A 17(17–18):2291–2303. 10.1089/ten.TEA.2010.0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corona BT, Machingal MA, Criswell T et al. (2012) Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A 18(11–12):1213–1228. 10.1089/ten.TEA.2011.0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massai D, Isu G, Madeddu D et al. (2016) A versatile bioreactor for dynamic suspension cell culture. Application to the culture of cancer cell spheroids. PLoS ONE 11(5):e0154610. 10.1371/journal.pone.0154610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ovando-Roche P, West EL, Branch MJ et al. (2018) Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther 9(1):156. 10.1186/s13287-018-0907-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerlach JC, Zeilinger K, Grebe A et al. (2003) Recovery of preservation-injured primary human hepatocytes and nonparenchymal cells to tissuelike structures in large-scale bioreactors for liver support: an initial transmission electron microscopy study. J Invest Surg 16(2):83–92. 10.1080/08941930390194370 [DOI] [PubMed] [Google Scholar]
- 56.Zhang ZY, Teoh SH, Chong WS et al. (2009) A biaxial rotating bioreactor for the culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 30(14):2694–2704. 10.1016/j.biomaterials.2009.01.028 [DOI] [PubMed] [Google Scholar]
- 57.Harris LJ, Abdollahi H, Zhang P et al. (2011) Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res 168(2):306–314. 10.1016/j.jss.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van deWalle A, Wilhelm C, Luciani N (2017) 3D magnetic stem cell aggregation and bioreactor maturation for cartilage regeneration. J Vis Exp 122:55221. 10.3791/55221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thelwall PE, Neves AA, Brindle KM (2001) Measurement of bioreactor perfusion using dynamic contrast agent-enhanced magnetic resonance imaging. Biotechnol Bioeng 75(6):682–690. 10.1002/bit.10039 [DOI] [PubMed] [Google Scholar]
- 60.Hammer BE, Heath CA, Mirer SD et al. (1990) Quantitative flow measurements in bioreactors by nuclear magnetic resonance imaging. Nat Biotechnol 8(4):327–330. 10.1038/nbt0490-327 [DOI] [PubMed] [Google Scholar]
- 61.Donoghue C, Brideau M, Newcomer P et al. (1992) Use of magnetic resonance imaging to analyze the performance of hollowfiber bioreactors. Ann N Y Acad Sci 665:285–300. 10.1111/j.1749-6632.1992.tb42592.x [DOI] [PubMed] [Google Scholar]
- 62.Wolfe SP, Hsu E, Reid LM et al. (2002) A novel multi-coaxial hollow fiber bioreactor for adherent cell types. Part 1: hydrodynamic studies. Biotechnol Bioeng 77(1):83–90. 10.1002/bit.10081 [DOI] [PubMed] [Google Scholar]
- 63.Gottwald E, Kleintschek T, Giselbrecht S et al. (2013) Characterization of a chip-based bioreactor for three-dimensional cell cultivation via Magnetic Resonance Imaging. Z Med Phys 23(2):102–110. 10.1016/j.zemedi.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 64.Porter BD, Lin AS, Peister A et al. (2007) Noninvasive image analysis of 3D construct mineralization in a perfusion bioreactor. Biomaterials 28(15):2525–2533. 10.1016/j.biomaterials.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 65.Hagenmüller H, Hitz M, Merkle HP et al. (2010) Design and validation of a novel bioreactor principle to combine online micro-computed tomography monitoring and mechanical loading in bone tissue engineering. Rev Sci Instrum 81(1):014303. 10.1063/1.3284787 [DOI] [PubMed] [Google Scholar]
- 66.Appel AA, Larson JC, Garson AB et al. (2015) X-ray phase contrast imaging of calcified tissue and biomaterial structure in bioreactor engineered tissues. Biotechnol Bioeng 112(3):612–620. 10.1002/bit.25467 [DOI] [PubMed] [Google Scholar]
- 67.Smith-Bindman R, Lipson J, Marcus R et al. (2009) Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169(22):2078–2086. 10.1001/archinternmed.2009.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitehead TD, Nemanich ST, Dence C et al. (2013) A PET-compatible tissue bioreactor for research, discovery, and validation of imaging biomarkers and radiopharmaceuticals: system design and proof-of-concept studies. J Nucl Med 54(10):1812–1819. 10.2967/jnumed.113.119776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melchor J, Parnell WJ, Bochud N et al. (2019) Damage prediction via nonlinear ultrasound: a micro-mechanical approach. Ultrasonics 93:145–155. 10.1016/j.ultras.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 70.Rice MA, Waters KR, Anseth KS (2009) Ultrasound monitoring of cartilaginous matrix evolution in degradable PEG hydrogels. Acta Biomater 5(1):152–161. 10.1016/j.actbio.2008.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith LJ, Li P, Holland MR et al. (2018) FABRICA: a bioreactor platform for printing, perfusing, observing, & stimulating 3D tissues. Sci Rep 8(1):7561. 10.1038/s41598-018-25663-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tearney GJ, Boppart SA, Bouma BE et al. (1996) Scanning single-mode fiber optic catheter-endoscope for optical coherence tomography. Opt Lett 21(7):543–545. 10.1364/ol.21.000543 [DOI] [PubMed] [Google Scholar]
- 73.Goth W, Lesicko J, Sacks MS et al. (2016) Optical-based analysis of soft tissue structures. Annu Rev Biomed Eng 18:357–385. 10.1146/annurev-bioeng-071114-040625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gurjarpadhye AA, DeWitt MR, Xu Y et al. (2015) Dynamic assessment of the endothelialization of tissue-engineered blood vessels using an optical coherence tomography catheter-based fluorescence imaging system. Tissue Eng Part C Methods 21(7):758–766. 10.1089/ten.TEC.2014.0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel DV, McGhee CN (2007) Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques: a major review. Clin Exp Ophthalmol 35(1):71–88. 10.1111/j.1442-9071.2007.01423.x [DOI] [PubMed] [Google Scholar]
- 76.González S, Tannous Z (2002) Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol 47(6):869–874. 10.1067/mjd.2002.124690 [DOI] [PubMed] [Google Scholar]
- 77.Kluge JA, Leisk GG, Cardwell RD et al. (2011) Bioreactor system using noninvasive imaging and mechanical stretch for biomaterial screening. Ann Biomed Eng 39(5):1390–1402. 10.1007/s10439-010-0243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cordero E, Latka I, Matthäus C et al. (2018) In-vivo Raman spectroscopy: from basics to applications. J Biomed Opt 23(7):1–23. 10.1117/1.JBO.23.7.071210 [DOI] [PubMed] [Google Scholar]
- 79.Matthäus C, Dochow S, Egodage KD et al. (2018) Detection and characterization of early plaque formations by Raman probe spectroscopy and optical coherence tomography: an in vivo study on a rabbit model. J Biomed Opt 23(1):1–6. 10.1117/1.JBO.23.1.015004 [DOI] [PubMed] [Google Scholar]
- 80.Magee ND, Villaumie JS, Marple ET et al. (2009) Ex vivo diagnosis of lung cancer using a Raman miniprobe. J Phys Chem B 113(23):8137–8141. 10.1021/jp900379w [DOI] [PubMed] [Google Scholar]
- 81.McGregor HC, Short MA, McWilliams A et al. (2017) Real-time endoscopic Raman spectroscopy for in vivo early lung cancer detection. J Biophotonics 10(1):98–110. 10.1002/jbio.201500204 [DOI] [PubMed] [Google Scholar]
- 82.Yang Y, Li F, Gao L et al. (2011) Differential diagnosis of breast cancer using quantitative, label-free and molecular vibrational imaging. Biomed Opt Express 2(8):2160–2174. 10.1364/BOE.2.002160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abramczyk H, Imiela A, Brożek-Płuska B et al. (2019) Aberrant Protein Phosphorylation in Cancer by Using Raman Biomarkers. Cancers (Basel) 11(12):2017. 10.3390/cancers11122017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abramczyk H, Brozek-Pluska B, Jarota A et al. (2020) A look into the use of Raman spectroscopy for brain and breast cancer diagnostics: linear and non-linear optics in cancer research as a gateway to tumor cell identity. Expert Rev Mol Diagn. 10.1080/14737159.2020.1724092 [DOI] [PubMed] [Google Scholar]
- 85.Teh SK, Zheng W, Ho KY et al. (2010) Near-infrared Raman spectroscopy for early diagnosis and typing of adenocarcinoma in the stomach. Br J Surg 97(4):550–557. 10.1002/bjs.6913 [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Lin K, Zheng W et al. (2015) Simultaneous fingerprint and high-wavenumber fiber-optic Raman spectroscopy improves in vivo diagnosis of esophageal squamous cell carcinoma at endoscopy. Sci Rep 5:12957. 10.1038/srep12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin K, Wang J, Zheng W et al. (2016) Rapid fiber-optic Raman spectroscopy for real-time in vivo detection of gastric intestinal metaplasia during clinical gastroscopy. Cancer Prev Res (Phila) 9(6):476–483. 10.1158/1940-6207.CAPR-15-0213 [DOI] [PubMed] [Google Scholar]
- 88.Barman I, Dingari NC, Singh GP et al. (2012) Selective sampling using confocal Raman spectroscopy provides enhanced specificity for urinary bladder cancer diagnosis. Anal Bioanal Chem 404(10):3091–3099. 10.1007/s00216-012-6424-6 [DOI] [PubMed] [Google Scholar]
- 89.Kirsch M, Schackert G, Salzer R et al. (2010) Raman spectroscopic imaging for in vivo detection of cerebral brain metastases. Anal Bioanal Chem 398(4):1707–1713. 10.1007/s00216-010-4116-7 [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, Fan Y, He M et al. (2017) Accuracy of Raman spectroscopy in differentiating brain tumor from normal brain tissue. Oncotarget 8(22):36824–36831. 10.18632/oncotarget.15975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Devpura S, Barton KN, Brown SL et al. (2014) Vision 20/20: the role of Raman spectroscopy in early stage cancer detection and feasibility for application in radiation therapy response assessment. Med Phys 41(5):050901. 10.1118/1.4870981 [DOI] [PubMed] [Google Scholar]
- 92.Kourkoumelis N, Balatsoukas I, Moulia V et al. (2015) Advances in the in vivo Raman spectroscopy of malignant skin tumors using portable instrumentation. Int J Mol Sci 16(7):14554–14570. 10.3390/ijms160714554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao J, Zeng H, Kalia S et al. (2017) Using Raman spectroscopy to detect and diagnose skin cancer in vivo. Dermatol Clin 35(4):495–504. 10.1016/j.det.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 94.Teh SK, Zheng W, Ho KY et al. (2008) Diagnostic potential of near-infrared Raman spectroscopy in the stomach: differentiating dysplasia from normal tissue. Br J Cancer 98(2):457–465. 10.1038/sj.bjc.6604176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abu-Absi NR, Kenty BM, Cuellar ME et al. (2011) Real time monitoring of multiple parameters in mammalian cell culture bioreactors using an in-line Raman spectroscopy probe. Biotechnol Bioeng 108(5):1215–1221. 10.1002/bit.23023 [DOI] [PubMed] [Google Scholar]
- 96.Mehdizadeh H, Lauri D, Karry KM et al. (2015) Generic Raman-based calibration models enabling real-time monitoring of cell culture bioreactors. Biotechnol Prog 31(4):1004–1013. 10.1002/btpr.2079 [DOI] [PubMed] [Google Scholar]
- 97.Gioux S, Mazhar A, Cuccia DJ (2019) Spatial frequency domain imaging in 2019: principles, applications, and perspectives. J Biomed Opt 24(7):1–18. 10.1117/1.JBO.24.7.071613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saidian M, Lakey JRT, Ponticorvo A et al. (2019) Characterisation of impaired wound healing in a preclinical model of induced diabetes using wide-field imaging and conventional immunohistochemistry assays. Int Wound J 16(1):144–152. 10.1111/iwj.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nguyen TT, Ramella-Roman JC, Moffatt LT et al. (2013) Novel application of a spatial frequency domain imaging system to determine signature spectral differences between infected and noninfected burn wounds. J Burn Care Res 34(1):44–50. 10.1097/BCR.0b013e318269be30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nguyen JQ, Crouzet C, Mai T et al. (2013) Spatial frequency domain imaging of burn wounds in a preclinical model of graded burn severity. J Biomed Opt 18(6):66010. 10.1117/1.JBO.18.6.066010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mazhar A, Saggese S, Pollins AC et al. (2014) Noncontact imaging of burn depth and extent in a porcine model using spatial frequency domain imaging. J Biomed Opt 19(8):086019. 10.1117/1.JBO.19.8.086019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ponticorvo A, Burmeister DM, Yang B et al. (2014) Quantitative assessment of graded burn wounds in a porcine model using spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI). Biomed Opt Express 5(10):3467–3481. 10.1364/BOE.5.003467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burmeister DM, Ponticorvo A, Yang B et al. (2015) Utility of spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI) to non-invasively diagnose burn depth in a porcine model. Burns 41(6):1242–1252. 10.1016/j.burns.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ponticorvo A, Rowland R, Baldado M et al. (2019) Evaluating clinical observation versus Spatial Frequency Domain Imaging (SFDI), Laser Speckle Imaging (LSI) and thermal imaging for the assessment of burn depth. Burns 45(2):450–460. 10.1016/j.burns.2018.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gioux S, Mazhar A, Lee BT et al. (2011) First-in-human pilot study of a spatial frequency domain oxygenation imaging system. J Biomed Opt 16(8):086015. 10.1117/1.3614566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ponticorvo A, Taydas E, Mazhar A et al. (2015) Evaluating visual perception for assessing reconstructed flap health. J Surg Res 197(1):210–217. 10.1016/j.jss.2015.03.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nadeau KP, Ponticorvo A, Lee HJ et al. (2013) Quantitative assessment of renal arterial occlusion in a porcine model using spatial frequency domain imaging. Opt Lett 38(18):3566–3569. 10.1364/OL.38.003566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laughney AM, Krishnaswamy V, Rizzo EJ et al. (2013) Spectral discrimination of breast pathologies in situ using spatial frequency domain imaging. Breast Cancer Res 15(4):R61. 10.1186/bcr3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang B, Lesicko J, Sharma M et al. (2015) Polarized light spatial frequency domain imaging for non-destructive quantification of soft tissue fibrous structures. Biomed Opt Express 6(4):1520–1533. 10.1364/BOE.6.001520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Talo G, Turrisi C, Arrigoni C et al. (2018) Industrialization of a perfusion bioreactor: Prime example of a non-straightforward process. J Tissue Eng Regen Med 12(2):405–415. 10.1002/term.2480 [DOI] [PubMed] [Google Scholar]
- 111.Sladkova M, de Peppo G (2014) Bioreactor Systems for Human Bone Tissue Engineering. Processes 2(2):494–525. 10.3390/pr2020494 [DOI] [Google Scholar]
- 112.Kosky P, Balmer R, Keat W et al. (2013) Manufacturing engineering. In: Exploring engineering, pp 205–235 [Google Scholar]
- 113.Ngo TD, Kashani A, Imbalzano G et al. (2018) Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos Part B Eng 143:172–196. 10.1016/j.compositesb.2018.02.012 [DOI] [Google Scholar]
- 114.Ritzman TB, Banovich N, Buss KP et al. (2017) Facing the facts: The Runx2 gene is associated with variation in facial morphology in primates. J Hum Evol 111:139–151. 10.1016/j.jhevol.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 115.Pereira T, Kennedy JV, Potgieter J (2019) A comparison of traditional manufacturing vs additive manufacturing, the best method for the job. Procedia Manuf 30:11–18. 10.1016/j.promfg.2019.02.003 [DOI] [Google Scholar]
- 116.Eibl R, Kaiser S, Lombriser R et al. (2010) Disposable bioreactors: the current state-of-the-art and recommended applications in biotechnology. Appl Microbiol Biotechnol 86(1):41–49. 10.1007/s00253-009-2422-9 [DOI] [PubMed] [Google Scholar]
- 117.Kim DK, Sim BR, Kim JI et al. (2018) Functionalized silk fibroin film scaffold using beta-Carotene for cornea endothelial cell regeneration. Colloids Surf B Biointerfaces 164:340–346. 10.1016/j.colsurfb.2017.11.052 [DOI] [PubMed] [Google Scholar]
- 118.Design control guidance for medical device manufacturers (1997). https://www.fda.gov/regulatory-information/search-fdaguidance-documents/design-control-guidance-medical-device-manufacturers
- 119.Sartorius annual report (2019). https://www.sartorius.com/resource/blob/404288/a36a0ccff059aff8b76461baa5df69a4/sag-annual-report-2019-e-data.pdf
- 120.Ward D, Rivani E (2005) An overview of strategy development models and the Ward-Rivani model, Economics Working Papers. European school of Economics. Milan (Italy). https://econwpa.ub.uni-muenchen.de/econwp/get/papers/0506/0506002.pdf [Google Scholar]
- 121.Viola GM, Rolston KV, Butler C et al. (2019) Evaluation of current perioperative antimicrobial regimens for the prevention of surgical site infections in breast implant-based reconstructive surgeries. Plast Reconstr Surg Glob Open 7(7):e2342. 10.1097/GOX.0000000000002342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laverty Kevin J (2001) Market share, profits and business strategy. Manag Decis 39(8):607–618. 10.1108/EUM0000000005860 [DOI] [Google Scholar]
- 123.Tversky A, Kahneman D (1991) Loss aversion in riskless choice: a reference-dependent model. Q J Econ 106(4):1039–1061. 10.2307/2937956 [DOI] [Google Scholar]
- 124.Desarbo W, Blanchard S, Atalay S (2009) A new spatial classification methodology for simultaneous segmentation, targeting, and positioning (STP analysis) for marketing research. pp 75–103 [Google Scholar]
- 125.Wendt D, Riboldi SA, Cioffi M et al. (2009) Bioreactors in tissue engineering: scientific challenges and clinical perspectives. Adv Biochem Eng Biotechnol 112:1–27. 10.1007/978-3-540-69357-4_1 [DOI] [PubMed] [Google Scholar]


