Abstract
Simple Summary
While immune checkpoint inhibitors (ICIs) have become the standard of care for several types of cancer, they are closely associated with specific immune-related adverse events (irAEs). The decision to resume ICI treatment after its interruption due to irAEs is challenged by the need for tumor control versus the risk of occurrence of the same or different irAEs. Data regarding the safety of ICI resumption after irAE remain scarce, heterogeneous, and mostly based on small samples of patients or focused only on the recurrence rate of the same irAE. Herein, we provided a narrative review on the safety of ICI resumption after interruption due to irAE(s).
Abstract
Immune checkpoint inhibitors (ICIs) have become the standard of care for several types of cancer due to their superiority in terms of survival benefits in first- and second-line treatments compared to conventional therapies, and they present a better safety profile (lower absolute number of grade 1–5 adverse events), especially if used in monotherapy. However, the pattern of ICI-related adverse events is totally different, as they are characterized by the development of specific immune-related adverse events (irAEs) that are unique in terms of the organs involved, onset patterns, and severity. The decision to resume ICI treatment after its interruption due to irAEs is challenged by the need for tumor control versus the risk of occurrence of the same or different irAEs. Studies that specifically assess this point remain scarce, heterogenous and mostly based on small samples of patients or focused only on the recurrence rate of the same irAE after ICI resumption. Moreover, patients with grade ≥3 irAEs were excluded from many of these studies. Herein, we provide a narrative review on the field of safety of ICI resumption after interruption due to irAE(s).
Keywords: safety, immune-related adverse events, immune checkpoint inhibitors
1. Introduction
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies directed against cytotoxic T-lymphocyte antigen 4 (CTLA-4) (ipilimumab), programmed cell death protein 1 (PD-1) (pembrolizumab, nivolumab, cemiplimab) or the PD-1 ligand (PDL-1/2) (atezolizumab, durvalumab, and avelumab). ICIs are the standard of care for several types of cancers due to their superiority in terms of survival benefits as first- and second-line treatments, compared to conventional therapies [1,2,3,4,5,6].
The engagement of CTLA-4 in T cells during the induction phase of an anti-tumor immune response impedes T cell activation by inhibition of the co-stimulation signal, leading to anergy. Furthermore, engagement of PD-1 on effector T cells with PDL-1 in tumor-associated antigen-presenting cells promotes T cell exhaustion. ICIs, by releasing these inhibitory brakes of T cells, promote anti-tumor T cell activation and the maintenance of anti-tumor T cell effector function [7]. ICIs also work by activating other cells of the innate and adaptive immune system, leading to a coordinated and effective anti-tumoral response [1].
Compared to conventional therapy, ICIs are associated with a better safety profile (lower absolute number of grade 1–5 adverse events), especially if used in monotherapy [8]. However, the pattern of ICI-related adverse events is quite different, with the development of specific immune-related adverse events (irAEs) that are unique in terms of organs involved, onset patterns, and severity [7,9]. The mechanisms of these irAEs are related to the non-specific activation of the adaptive immune system [1]. Indeed, anti-CTLA-4 and anti-PD(L)-1 are associated with the reduced survival and inhibitory functions of CD4+ CD25+ regulatory T cells (Treg), an increased proportion of type 17 T helper cells (anti-CTLA-4) and cytokine production, in addition to the induction of cross-reactivity between anti-tumor T cells and antigens on healthy cells, leading to autoantibody production, tissue injury and auto-immune diseases [7].
IrAEs are highly heterogenous in terms of time taken until occurrence [10,11,12], type [8] and severity [13], depending on the ICI regimen [14] and/or cancer type [13]. Moreover, individual variations of the same cancer and ICI combination suggest a genetic background. However, to date, the risk factors for irAEs remain unknown [15].
Several recommendations have been published to help to manage irAEs [16,17]. Globally, grade 2/3 irAEs require corticosteroids and temporary ICI discontinuation, with the possibility of resuming when symptoms revert to grade ≤1, although permanent ICI discontinuation is recommended for grade 4 irAEs (except for endocrinopathies if controlled by hormone replacement). The decision to resume ICI treatment after grade ≥2 irAEs is challenged by the need for tumor control versus the risk of occurrence of the same or different irAEs. Studies having specifically assessed the safety of ICI retreatment after ICI discontinuation for irAEs remain scarce and heterogenous. Most are based on small samples of patients [18,19,20] or focused only on the recurrence rate of the same irAE after an ICI rechallenge [21]. Moreover, patients with grade ≥3 irAEs have been excluded from many of these studies [22,23,24,25]. To better understand the safety profile of ICI resumption after irAE occurrence, we conducted a narrative literature review to summarize the available data on the safety of ICI resumption after discontinuation for irAEs.
2. When Time and Words Matter to Define the Resumption of ICI(s) and Second irAEs
The first pitfalls when comparing data are the timeline of ICI rechallenge and second irAEs onset. The absence of definitions in terms of a timeline may lead to heterogeneous definitions of second irAEs. The experience from “natural” auto-immune diseases suggests that the break of tolerance may sometimes occur many years before onset of the auto-immune disease [26]. Due to the rapid onset of irAEs (a few weeks after the beginning of the treatment), it could be hypothesized that the break of tolerance at a cellular level occurred before ICI treatment, but without any clinical symptoms of auto-immune disease. As a result, the stereotypical kinetics of the first irAEs (skin irAEs between 3–7 weeks, pulmonary irAEs between 10–16 weeks, hepatitis between 6–15 weeks, colitis between 4–10 weeks, and endocrinopathies after 6 weeks for anti-PD-(L)1) [27] may be related to the activation and amplification of the preexisting autoimmune cells, which may differ depending on the type of antigen and the affinity of the selected T cell receptor (TCR), particularly to genetic polymorphisms and especially for PD-1 and PDL-1. However, how PD-1 or PDL-1 polymorphisms could influence irAE occurrence remains unknown. Furthermore, regarding the exposure to steroids or other immunosuppressant drugs for the first irAEs, up until now, no data have explored the deletion of latent auto-immune cells. This explains why treatment of the first irAEs does not mean that the immune system has been completely remodeled and that all latent auto-immune cells are destroyed. This raises the following question: Are second-irAE-related cells induced by the first exposure to ICI, the second or both? Of course, translational studies would help to answer these questions. From a clinical perspective, an indirect way to explore this condition would be to consider (i) the complete clearance of the first exposure to ICI, (ii) type of second irAE, (iii) and its time to onset after ICI resumption. If the first ICI is completely cleared and the onset of the second irAE occurs in the same timeframe as the first ICI exposure, then it is reasonable to think that the second irAE is clearly related to the rechallenge (i.e., a new auto-immune thyroid disorder occurring 8 weeks after second exposure to ICI). If the time lapse is shorter, it could be reasonable to believe that the latent auto-immune cells were stimulated by both exposures. If the time lapse is longer and the patient is exposed to steroids or immunosuppressants, it could be either that (1) latent auto-reactive cells expanded when exposed to the first ICI but were modulated by the treatment of the first irAE and restimulated by the second exposure to ICI, or that (2) latent auto-reactive cells expanded during the second exposition to ICI in an immunosuppressive environment. It could also help to distinguish a relapse of the first irAE after rechallenge from the onset of a second irAE with the same phenotype but driven by different autoreactive cells, if the relapse occurs a shorter time after rechallenge than the second irAE. Unfortunately, many irAEs were shown not to occur on the typical timeline after exposure to ICI or to ICI combinations. As a result, it is impossible to know if irAEs occurring after ICI resumption are clearly related to ICI second exposure or not. By default, irAEs occurring after ICI resumption were assumed to be related to the second exposure to ICIs (or combination of both exposures).
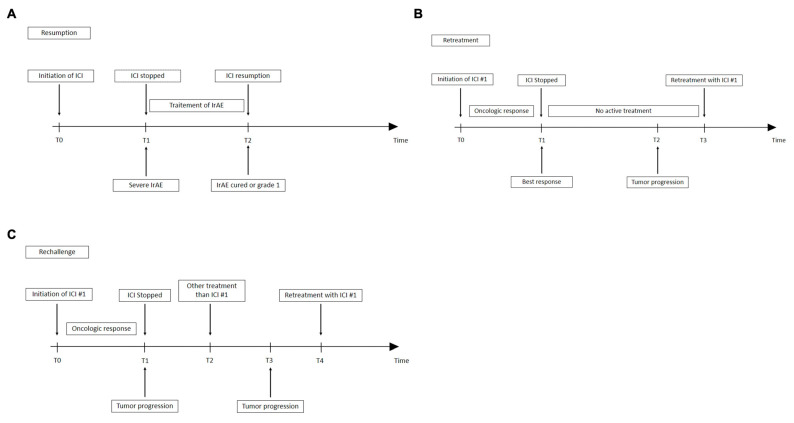
Moreover, most publications brought together what they termed resumption, rechallenge and retreatment in their evaluation of the ICI safety profile. Retreatment is the re-administration of the same therapeutic class following tumor relapse. Rechallenge is defined as reintroduction, after an intervening treatment of the same therapy to which the tumor has already proven to be resistant [28]. None of these definitions address the issue of restarting ICI after irAE, given that the treatment has not been completed, and that the tumor is not yet officially resistant to ICI. Resumption as the restarting after an interruption would be the best definition (Figure 1). To differentiate a postponed treatment from a resumption, the threshold of the biological activity of each ICI should be known. The postponed treatment would be defined as a reinjection of ICI while the level of ICI is above the threshold of the biological activity, whereas resumption would occur when the reinjection of ICI is below the threshold of the biological activity. As of now, no clear definition of resumption exists, a factor further complicating the interpretation of the onset of second irAEs. One solution would be to base the definition either on pharmacokinetic parameters (five half-lives), but that would be very restrictive (e.g., the half-life of nivolumab is about 21 days), or on a multiple of the recommended interval between two treatments.
Figure 1.
Differences between definitions of resumption (A), retreatment (B) or rechallenge (C) ICI: immune checkpoint inhibitor; irAE: immune-related adverse event; T: time; #1: number 1.
Table 1 summarizes the available data on the timing of ICI resumption after a first irAE, showing that it ranges from a median of 14 days [29] to 60 weeks [30]. It also bears mentioning that some studies have not reported this variable but provided a clear minimal time lapse between ICI discontinuation and ICI resumption. The heterogeneity of the data may lead to the consideration of a second irAE after either postponed or resumed treatment in the same publication. It is impossible to know whether or not the risk of a second irAE is the same in the postponed or in the resumption situation.
Table 1.
Summary of available definitions and timing of ICI resumption.
| Study | Resumption Definition | Median Time before Resumption |
|---|---|---|
| Abou Alaiwi et al. [31] | Dose interruption for at least 1 week due to irAEs | 0.9 (range: 0.2–31.6) months |
| Abu Sbeih et al. [32] | ICI resumption after suspension because of IMDC onset | 49 (IQR: 23–136) days |
| Allouchery et al. [33] | Discontinued ICI before rechallenge for a period at least equal to twice the duration of a cycle | 56 (IQR: 42–84) days |
| Bhatlapenumarthi et al. [30] | Rechallenge after irAE with the same drug and dose | 2–30 weeks depending on irAE type and severity |
| Brunot et al. [34] | Washout between ipilimumab and first dose of anti-PD-1 | 25 weeks (range: 2–194) |
| Cortazar et al. [35] | Readministration of the same or different ICI | 1.8 months (IQR: 1.2–11.0) after diagnosis of AKI- irAE |
| de Malet et al. [36] | Second-irAE treatment with ICIs after GI-irAE | 1.1 months (range: 0.1–32.6) after the end of GI irAE |
| Gobbini et al. [37] | At least 12 weeks after discontinuation because of toxicity, disease progression or clinical decision | NR |
| Gupta et al. [38] | Discontinuation then reinitiation with same, different class or ICI combination | 1.9 months (IQR: 1.1–4.0) after initial irAE |
| Li et al. [39] | ICI resumption after high-grade ICI hepatitis resolution | 51 days (IQR: 15–77) |
| Mouri et al. [40] | Treatment delay of longer than 4 weeks due to an irAE | NR |
| Patrinely et al. [29] | Initiation of ICI after hepatitis resolution | 14 days (median days) |
| Pollack et al. [41] | Anti-PD-1 resumption after combined anti-CTLA-4 and anti-PD-1 | 58 days (range: 14–395) |
| Santini et al. [42] | Treatment delay longer than one week between planned doses of ICI | 32 days (range: 7–177) |
| Simonaggio et al. [43] | Readministration of the same drug class in the same patient | NR |
| Ravi et al. [44] | At least 2 separate lines of ICI (alone or in combination with other therapies). | NR |
AKI: Acute kidney injury; GI: gastro-intestinal; ICI: immunological checkpoint inhibitor(s); IQR (interquartile range); irAE: immune-related adverse event; IMDC: immune-mediated diarrhea and colitis; NR: not reported.
In conclusion, the analysis of the available data shows that their heterogeneity may lead to a misinterpretation of the risk of a second irAE. That is why a data threshold of minimal biological activity for ICIs would help us to more precisely determine the factors related to postponed treatment, as opposed to resumption. Moreover, translational studies would help to calculate the percentage of irAEs related to immune stimulation from the first rather than the second ICI exposure.
3. Safety of Resumption of ICI after a First irAE
Only studies in English containing data on more than 20 patients with ICI resumption after ICI discontinuation for irAEs were retained. Table 2 summarizes these selected studies. We excluded one study because there were no data on the interrupted ICI and on the sub-group of patients with reinitiated ICI [45]. Finally, a total of 1563 patients from 18 studies received ICI resumption after ICI discontinuation due to irAEs. After the exclusion of the studies without details on the grade of initial irAEs or those including only patients with grade ≥3 irAEs [21,35,38,39], 47% (33.0–55.0) of the remaining patients with ICI resumption had grade ≥3 initial irAEs. As mentioned above, all irAEs occurring after ICI resumption were considered as second irAE.
Table 2.
Recurrence of irAEs after ICI resumption.
| Study | NOS Scale (*) | Malignancy | Number of Retreated Patients after 1st irAE | Rechallenge Type |
% of Any 2nd irAE (New + Same irAE) |
% of Same 2nd irAE | % of Permanent ICI Discontinuation |
|---|---|---|---|---|---|---|---|
| Abou Alaiwi et al. [31] | 8 | Metastatic RCC | 36 (G ≥3: 47%) |
Resumption: Anti-PD(L)1: 67% Combination (33%) |
50% (G ≥3: 38.9%) |
16.7% | 27.8% |
| Abu Sbeih et al. [32] † | 6 | Melanoma (54%) NSCLC (16%) Genitourinary (10%) Other solids (16%) Hematologic (4%) |
167 (G ≥3 colitis: 33%) †† |
Resumption: Anti-CTLA-4 (14%) Anti-PD(L)-1 (42.5%) Switch: Anti-CTLA-4 to anti-PD(L)-1 (38.5%) Anti-PD(L)-1 to anti-CTLA-4 (5%) |
NR | 34.1% (G ≥2 colitis: 46.6%) |
NR |
| Allouchery et al. [33] | 7 | Melanoma (44%) Lung (41%) RCC (6%) Lymphoma (3%) Others (6%) |
180 (G ≥3: 49%) |
Resumption: Same ICI or ICI combination (85%) Switch (15%) |
38.9% (G ≥3: 35%) |
27.2% | 26.1% |
| Bhatlapenumarthi et al. [30] | 6 | NSCLC (44%) SCLC (11%) Melanoma (18.5%) RCC (18.5%) Anaplastic thyroid (4%) Thymic (4%) |
27 (G ≥3: 26%) |
Resumption: Anti-PD-1 (93%) Anti-PDL-1 (7%) |
33.3% (G ≥3: 55.6%) |
7.4% | NR |
| Brunot et al. [34] | 7 | Metastatic melanoma | 56 (G ≥3: 100%) |
Switch: Anti-CTLA-4 to anti-PD-1 |
35.7% (G ≥3: 60%) |
14.3% | 8.9%) |
| Cortazar et al. [35] ††† | 8 | Melanoma (36%) ¶ Lung (26%) ¶ Genitourinary (17%) ¶ Other (21%) ¶ |
31 | Resumption with the same ICI (87%) | NR | 22.6% | NR |
| de Malet et al. [36] | 7 | Melanoma (75%) ¶ NSCLC (11%) † RCC (4%) ¶ Prostate (3%) ¶ Lymphoma (3%) ¶ Cervical (3%) ¶ Colorectal (1%) ¶ Ovarian (1%) ¶ |
26 (G ≥3: 61.5%) |
Resumption: Anti-CTLA-4 (23%) Anti-PD-1 (31%) Switch: Anti-CTLA-4 to anti-PD-1 (42%) Anti-PD-1 to anti-CTLA-4 (4%) |
46.2% (G ≥3: 16.7%) |
23.1% (G ≥3: 33.3%) |
NR |
| Dolladile et al. [21] | 6 | No detail about only rechallenged patients | 452 (Serious: 92%) |
PD(L)-1 (82%) CTLA-4 (5%) Combination (13%) |
33.2% | 28.8% | 4.6% treatment-related death |
| Gobbini et al. [37] | 8 | NSCLC | 58 (G ≥3: 47%) |
Resumption: Anti-PD(L)-1 (100%) |
14.8% * | 7.4% * | NR |
| Gupta et al. [38] ††† | 8 | Melanoma (32%) Genitourinary (23%) Lung (22%) Other (22%) |
121 (Stage ≥2: 78%) |
Resumption: (81%) Switch: (19%) |
NR | 16.5% (Stage ≥2: 80%) |
0% |
| Li et al. [39] | 7 | Melanoma | 31 (G ≥3: 100%) |
Resumption: Anti-PD-1 (84%) Anti-CTLA-4 (6.5%) Anti-PDL-1 (3%) Switch: Anti-CTLA-4 to anti-PD-1 (6.5%) |
48.4% (G ≥3: 20%) |
12.9% (G ≥3: 50%) |
19.3% |
| Menzies et al. [46] | 7 | Melanoma | 67 (G ≥3: 87%) |
Switch: Anti-CTLA-4 to anti-PD-1 (100%) |
34.3% (G ≥3: 60.9%) |
3% | 11.9% |
| Mouri et al. [40] | 8 | Stage III/IV NSCLC | 21 (G ≥3: 33%) |
Resumption: anti-PD-1 (100%) |
71.4% (G ≥3: 6.7%) |
NR | NR |
| Pollack et al. [41] | 7 | Metastatic melanoma | 80 (G ≥3: 69%) |
Resumption: Anti-PD-1 (100%) |
50% (G ≥3: 35%) |
17.5% (G ≥3: 50%) |
30% |
| Patrinely et al. [29] ‡ | 8 | Melanoma (84%) ¶ Lung (7%) ¶ RCC (3%) ¶ Squamous cell (1.2%) ¶ Other (4%)¶ |
66 (G ≥3: 39%) |
NR | 39.4% | 25.8% (G ≥3: 41.2%) |
NR |
| Ravi et al. [44] | 6 | RCC | 69 (G ≥3: 26%) |
Switch or resumption (no details on ICI) |
44.9% (G ≥3: 35.5%) |
NR | NR |
| Santini et al. [42] | 7 | NSCLC | 38 (G ≥3: 34%) |
Resumption: Anti-PD(L)-1 to anti-PDL-1 (63%) Anti-PD(L)-1 + anti-CTLA-4 to anti-PDL-1 (37%) |
52.6% (G ≥3: 40%) |
26.3% (G ≥3: 60%) |
5.2% treatment-related death |
| Simonaggio et al. [43] | 6 | Melanoma (28%) Lung (15%) Lymphoma (15%) Colorectal (15%) Other (30%) |
40 (G ≥3: 55%) |
Resumption: Anti-PD(L)-1 (100%) |
55% (G ≥3: 61.9%) |
42.5% | NR |
* Among the 27 patients with grade ≥3 irAEs (no data available on the 31 patients with grade 1–2 irAEs); † Focus only on ICI-related IMBC; †† Among the 138 patients with documented colitis; ††† Focus only on ICI-related AKI; ¶ Among patients with overall irAE (no data available on the irAE rechallenged sub-group); ‡ Focus only on ICI-related hepatitis; AKI: Acute kidney injury; CTLA-4: cytotoxic T-lymphocyte antigen 4; irAE: immune-related adverse event; G: grade; NSCLC: non-small-cell lung cancer; IMDC: immune-mediated diarrhea and colitis; NOS: Newcastle Ottawa Scale; PD(L)-1: programmed cell death protein(ligand)- 1; NR: not reported; RCC: renal cell cancer; SCLC: small-cell lung cancer.
Even though all of these studies are retrospective, they all present a Newcastle Ottawa Scale score ≥6, reflecting high quality. Most are multicentric, except for four studies [30,39,40,42]. Only four studies contained data of more than 100 patients with ICI resumption [21,32,33,38].
Aside from heterogeneity in the timing range of ICI resumption (Table 1), the studies were heterogeneous in terms of malignancy type and ICI regimen. Moreover, different studies assessed the safety of ICI resumption through the perspective of cancer type (i.e., renal cell carcinoma, melanoma, non-small-cell lung cancer) [31,34,37,39,40,41,42,44,46], irAE type (i.e., acute kidney injury, immune-mediated diarrhea and colitis (IMDC)) [32,35,38] or both [21,29,30,33,36,43].
The recurrence rate of any irAE after ICI resumption was 45.6% (36.5–50.0), of which 37.2% (30.7.56.7) were grade ≥3. The recurrence rate of the same irAE was 22.6% (15.4–26.8), of which 50.0% (41.2–50.0) were grade ≥3 (24/51). After the exclusion of fatal irAEs, irAE recurrence led to permanent ICI discontinuation in 19.4% (10.4–26.9) (100/571) of rechallenged patients.
Very few data are available on the risk factors associated with irAE recurrence. One study showed that the first irAEs occurred earlier in the patients who experienced new or recurrent irAEs after ICI resumption (9 vs. 15 weeks, p = 0.04) [43]. Contrary results were observed in another study with a longer duration between ICI discontinuation and ICI resumption (112 vs. 63 days, p = 0.01) [33]. Data on organ-specific irAEs are more consistent. For example, patients with initial gastrointestinal irAEs were more likely to have recurrent grade ≥2 irAEs after ICI resumption compared to those with no gastrointestinal irAEs [21,33]. By contrast, initial endocrine disorders were less likely to recur [21,33]. Only one study, which focused on IMDC, reported in a multivariate analysis that the risk factors associated with IMDC recurrence were initial grade ≥3 IMDC (OR 2.19, 95% CI (0.66–7.29), p = 0.20), initial need for immunosuppressive therapy (OR 3.22, 95% CI (1.08–9.62), p = 0.019) and longer duration of initial IMDC symptoms (OR 1.01, 95% CI (1.00–1.03), p = 0.031) [32]. The resumption of ICI combination after initial ICI combination was anecdotal insofar as the aim of resumption is to maintain ICI treatment. Most of the resumption scenarios were to (re)start with anti PD(L)-1 therapy after at least one combination of either anti-CTLA-4- or anti PD(L)-1-related irAE. We recently showed that the rechallenge of the same ICI drug or the same ICI combination was associated with a lower rate of irAE recurrence (77.1% vs. 90%, p = 0.02) [33]. In addition, Pollack et al. concluded that patients who experienced colitis or hypophysitis after an anti-PD-1/anti-CTLA-4 combination could safely resume anti-PD-1 [41]. The resumption of anti-CTLA-4 after anti-CTLA-4-related irAE or ICI combination was reported in fewer than 70 patients [21,32,36,39]. Abu-Sbeih et al. reported the recurrence of IMDC in 44% of patients who received anti-CTLA-4 resumption compared to 32% who received anti-PD(L)-1, and reduced risk of IMDC recurrence in the anti-PD(L)-1 group compared to the anti-CTLA-4 resumption group in multivariate analysis (odds ratio (OR) 0.30; 95% CI 0.11–0.81; p = 0.019) [32].
Overall, despite a sizable heterogeneity between the studies, these results show that the recurrence rates of irAE remain globally concordant with relatively tight ranges. They highlight (i) that more than half of patients with ICI discontinuation for irAE did not have irAE recurrence after ICI resumption; (ii) that the second irAE is often different from the first irAE [30,31,34,39,41,46]; (iii) that the second irAE seems no more severe than the first irAE; and (iv) the possibility of managing second irAEs while continuing ICI in most patients.
However, these results must be interpreted with caution given the retrospective design of the studies, heterogeneity of the timing of resumption and low sample sizes. All in all, they give an overview of ICI resumption after irAEs.
4. Unmet Medical Needs
Several general or organ-specific recommendations have been published to help to manage irAEs [17,47,48,49,50,51,52,53,54,55,56,57,58]. Generally, ICI should be continued with close monitoring for grade 1 irAEs, except for some neurologic, hematologic, and cardiac toxicities. Grade 2/3 irAEs require corticosteroids (0.5–1 or 1–2 mg/kg a day of prednisone equivalent, respectively) and temporary ICI discontinuation with the consideration of resuming when symptoms revert to grade ≤1. If symptoms do not improve within 48–72 h of high-dose steroids, various immunosuppressants (e.g., infliximab, rituximab) may be offered depending on the organ involved. ICI dose adjustments are not recommended. Finally, the permanent discontinuation of ICI is recommended for grade 4 irAEs (except for endocrinopathies if controlled by hormone replacement) [17]. However, given the relatively limited knowledge of irAEs and the lack of robust available data (mostly heterogeneous, retrospective, and small-scale studies), these recommendations are currently based only on expert opinion with a low level of evidence (C). The benefit/risk ratio of resumption for neurologic or cardiac involvements that can be potentially life-threatening is clearly in favor of the discontinuation of the ICIs. For gastro-intestinal toxicities, the balance is unclear due to the high risk of recurrence of irAEs; it is based mostly on the oncologic situation and the patient’s motivation. For the other irAEs, if the oncologic situation favors the resumption of the ICI, we encourage oncologists to discuss with their patients the resumption of ICI with the close management of potential second irAEs.
The heterogeneity of irAEs further complicates their management. In fact, irAEs can involve any organ [8], mostly within 2–16 weeks of ICI initiation [12], but some cases have been reported only a few days after ICI initiation or ≥1 year after the last ICI infusion [10,11]. The severity and type irAEs also vary depending on the ICI regimen. More precisely, grades ≥3 irAEs were more frequent, with anti-CTLA-4 compared to anti-PD-1 (31% vs. 10%) in a systematic review [13]. Combination therapies were associated with a greater risk and up to fivefold shorter median time until irAE compared to monotherapy (32 days vs. 146 days) [14]. Moreover, tumor-specific patterns of irAEs were also reported (i.e., melanoma associated with more dermatological/gastrointestinal irAEs and fewer cases of pneumonitis than other cancers treated by ICI) [37,59], suggesting that different organ-specific microenvironments could drive specific irAEs. Finally, inter-individual variations in the development of irAEs for the same cancer and the same ICI regimen suggest an additional genetic background [15] with a predisposition to autoimmunity (i.e., CTLA-4 and PDL-1 polymorphisms that are associated with autoimmune disorders [60,61]. Whether pre-existing autoimmune disease (AID) is a risk factor for irAEs remains controversial, as these patients were excluded from clinical trials. A meta-analysis of 14 retrospective studies showed a pooled incidence of AID flare and de novo irAEs, whatever the grade, of 35% and 33%, respectively, with no differences between patients with or without immunosuppressive therapy at the start of an ICI [62]. Two more recent retrospective studies of 27 and 47 patients with pre-existing AID treated by an ICI showed an overall AID exacerbation in 26 to 52% of patients, respectively. More than half of these patients required immunosuppressive drugs and 14 to 42% underwent ICI discontinuation [30,63]. Finally, a significant higher risk of irAEs or severe irAEs among patients with pre-existing AID compared to patients without pre-existing AID and treatment by ICI were reported [59,64]. Conversely, van der Kooij et al. reported an overall incidence of grade ≥3 irAEs as being similar in patients with or without preexisting AID, except for severe colitis among patients with preexisting inflammatory bowel disease [65]. These results suggest that irAEs are common in patients with preexisting AID but that they are often mild and manageable, without discontinuing therapy.
Identifying predictive risk factors not only for overall irAE occurrence, but also for irAE recurrence, is a challenging but necessary research perspective. Some clinical factors have been linked to the development of irAEs, such as preexisting AID, the use of CTLA-4 inhibitors and grade ≥3 renal insufficiency [64,66]. Obesity was reported as independently associated with irAEs whatever the grade, grade ≥3 irAEs [67] and ICI discontinuation [67] in patients treated with pembrolizumab [67,68], but this association was not confirmed with atezolizumab [69] or ipilimumab [70]. Additionally, a recent review summarized the available data on biomarkers linked to ICI-related toxicities [71]. Importantly, while these studies assessed the risk factors of irAE occurrence after the first ICI treatment, very few and sometimes conflicting data are available regarding the risk factors of irAE recurrence, as discussed above [32,33,41,43]. In the same way, while the type of irAEs after the first ICI treatment is generally well-described (e.g., colitis, hypophysitis and rash are more frequently described with anti-CTLA-4 and pneumonitis, hypothyroidism, arthralgia and vitiligo with anti-PD-1) [13], data remain unclear and heterogeneous on the type of second irAEs according to the type of ICI resuming. Finally, one of the main limitations to knowledge of ICI resumption is the lack of clear data on overall survival (OS), tumor by tumor, or compared within a group of tumors with homogenous prognosis. A growing body of evidence suggests that irAE occurrence is predictive of anti-PD(L)-1) response with a marked improvement in progression-free survival, OS and overall response rate in patients with irAE compared to those without [72]. Moreover, some specific irAEs are associated with enhanced survival, cutaneous irAEs being one of the best documented examples [73,74,75,76,77]. However, to date, whether or not specific irAE-related factors (severity, timing of onset, therapeutic intervention, irAE recurrence) are associated with increased OS remains unknown and questions the need for ICI resumption after ICI discontinuation due to irAE. The aim of resumption is to offer the patient the best available treatment for their cancer without knowing its impact on OS, whereas it is certain that the quality of life is impacted due to the number of infusions and risk of second irAE(s).
Combinatorial approaches, such as ICIs associated with chimeric antigen receptor-T (CAR-T) cells, are currently being investigated to improve antitumor effects, and to mitigate toxicities. However, only a few trials have been performed and most are proofs of concept with small numbers of included patients [78,79]. Adequate prospective and pharmacovigilance studies are needed to assess the safety of such combinations from a long-term perspective.
5. Conclusions
ICI resumption after irAE should always be discussed in a multidisciplinary team meeting in light of the usefulness of rechallenge, patient comorbidities and risk of recurrence of first irAE(s). However, although few data are available, ICI resumption is not recommended in clinical practice in some organ-specific irAEs (i.e., myocarditis and/or the central nervous system), especially if severe. ICI resumption should be regarded cautiously after gastrointestinal irAEs due to a high risk of irAE recurrence. Further prospective observational studies are therefore needed to assess risk factors of irAE recurrence to rapidly identify at-risk patients and modalities of ICI resumption and should be coupled with translational studies to improve knowledge of the physiopathology of irAEs.
Acknowledgments
The authors would like to thank Jeffrey Arsham for his help with editing.
Author Contributions
Conceptualization: M.A., M.P. and M.M.; Data collection: M.A., M.P. and M.M.; Formal analysis: M.A., C.B., M.-C.P.-P., P.R. and M.M.; Writing—original draft preparation, M.A., C.B., M.P. and M.M.; Writing—review and editing, M.A., C.B., M.-C.P.-P., P.R. and M.M.; Supervision, M.A. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 2.Ferris R.L., Blumenschein G., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmunt J., de Wit R., Vaughn D.J., Fradet Y., Lee J.-L., Fong L., Vogelzang N.J., Climent M.A., Petrylak D.P., Choueiri T.K., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst R.S., Baas P., Kim D.-W., Felip E., Pérez-Gracia J.L., Han J.-Y., Molina J., Kim J.-H., Arvis C.D., Ahn M.-J., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 6.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos-Casals M., Brahmer J.R., Callahan M.K., Flores-Chávez A., Keegan N., Khamashta M.A., Lambotte O., Mariette X., Prat A., Suárez-Almazor M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primer. 2020;6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C., Chen Y.-P., Du X.-J., Liu J.-Q., Huang C.-L., Chen L., Zhou G.-Q., Li W.-F., Mao Y.-P., Hsu C., et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangadhar T.C., Vonderheide R.H. Mitigating the toxic effects of anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2014;11:91–99. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 10.Yoest J.M. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: A short review. ImmunoTargets Ther. 2017;6:73–82. doi: 10.2147/ITT.S126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parakh S., Cebon J., Klein O. Delayed autoimmune toxicity occurring several months after cessation of anti-PD-1 therapy. Oncologist. 2018;23:849–851. doi: 10.1634/theoncologist.2017-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber J.S., D’Angelo S.P., Minor D., Hodi F.S., Gutzmer R., Neyns B., Hoeller C., Khushalani N.I., Miller W.H., Lao C.D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 13.Khoja L., Day D., Wei-Wu Chen T., Siu L.L., Hansen A.R. Tumour-and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017;28:2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 14.Kanjanapan Y., Day D., Butler M.O., Wang L., Joshua A.M., Hogg D., Leighl N.B., Razak A.R.A., Hansen A.R., Boujos S., et al. Delayed immune-related adverse events in assessment for dose-limiting toxicity in early phase immunotherapy trials. Eur. J. Cancer. 2019;107:1–7. doi: 10.1016/j.ejca.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Sandigursky S., Mor A. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr. Rheumatol. Rep. 2018;20:65. doi: 10.1007/s11926-018-0770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer J.R., Lacchetti C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., Chau I., Ernstoff M.S., Gardner J.M., Ginex P., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider B.J., Naidoo J., Santomasso B.D., Lacchetti C., Adkins S., Anadkat M., Atkins M.B., Brassil K.J., Caterino J.M., Chau I., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 2021;36:1714. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 18.Isik B., Alexander M.P., Manohar S., Vaughan L., Kottschade L., Markovic S., Lieske J., Kukla A., Leung N., Herrmann S.M. Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int. Rep. 2021;6:1022–1031. doi: 10.1016/j.ekir.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weill A., Delyon J., Descamps V., Deschamps L., Dinulescu M., Dupuy A., Célérier P., Nardin C., Aubin F., Le Corre Y., et al. Treatment strategies and safety of rechallenge in the setting of immune checkpoint inhibitors-related myositis: A national multicentre study. Rheumatology. 2021;60:5753–5764. doi: 10.1093/rheumatology/keab249. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Zhao Y., Zhang X., Si X., Song P., Xiao Y., Yang X., Song L., Shi J., Zhao H., et al. Clinical characteristics and management of immune checkpoint inhibitor-related pneumonitis: A single-institution retrospective study. Cancer Med. 2021;10:188–198. doi: 10.1002/cam4.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolladille C., Ederhy S., Sassier M., Cautela J., Thuny F., Cohen A.A., Fedrizzi S., Chrétien B., Da-Silva A., Plane A.-F., et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:865. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiarion-Sileni V., Pigozzo J., Ascierto P.A., Simeone E., Maio M., Calabrò L., Marchetti P., De Galitiis F., Testori A., Ferrucci P.F., et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: The expanded access programme in italy. Br. J. Cancer. 2014;110:1721–1726. doi: 10.1038/bjc.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert C., Schadendorf D., Messina M., Hodi F.S., O’Day S. MDX010-20 investigators efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:2232–2239. doi: 10.1158/1078-0432.CCR-12-3080. [DOI] [PubMed] [Google Scholar]
- 24.Lebbé C., Weber J.S., Maio M., Neyns B., Harmankaya K., Hamid O., O’Day S.J., Konto C., Cykowski L., McHenry M.B., et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase ii studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014;25:2277–2284. doi: 10.1093/annonc/mdu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larkin J., Minor D., D’Angelo S., Neyns B., Smylie M., Miller W.H., Gutzmer R., Linette G., Chmielowski B., Lao C.D., et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in checkmate 037: A randomized, controlled, open-label phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:383–390. doi: 10.1200/JCO.2016.71.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arbuckle M.R., McClain M.T., Rubertone M.V., Scofield R.H., Dennis G.J., James J.A., Harley J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 27.Martins F., Sofiya L., Sykiotis G.P., Lamine F., Maillard M., Fraga M., Shabafrouz K., Ribi C., Cairoli A., Guex-Crosier Y., et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 28.Tonini G., Imperatori M., Vincenzi B., Frezza A.M., Santini D. Rechallenge therapy and treatment holiday: Different strategies in management of metastatic colorectal cancer. J. Exp. Clin. Cancer Res. CR. 2013;32:92. doi: 10.1186/1756-9966-32-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrinely J.R., McGuigan B., Chandra S., Fenton S.E., Chowdhary A., Kennedy L.B., Mooradian M.J., Palmeri M., Portal D., Horst S.N., et al. A multicenter characterization of hepatitis associated with immune checkpoint inhibitors. OncoImmunology. 2021;10:1875639. doi: 10.1080/2162402X.2021.1875639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatlapenumarthi V., Patwari A., Harb A.J. Immune-related adverse events and immune checkpoint inhibitor tolerance on rechallenge in patients with IrAEs: A single-center experience. J. Cancer Res. Clin. Oncol. 2021;147:2789–2800. doi: 10.1007/s00432-021-03610-w. [DOI] [PubMed] [Google Scholar]
- 31.Abou Alaiwi S., Xie W., Nassar A.H., Dudani S., Martini D., Bakouny Z., Steinharter J.A., Nuzzo P.V., Flippot R., Martinez-Chanza N., et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J. Immunother. Cancer. 2020;8:e000144. doi: 10.1136/jitc-2019-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Sbeih H., Ali F.S., Naqash A.R., Owen D.H., Patel S., Otterson G.A., Kendra K., Ricciuti B., Chiari R., De Giglio A., et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J. Clin. Oncol. 2019;37:2738–2745. doi: 10.1200/JCO.19.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allouchery M., Lombard T., Martin M., Rouby F., Sassier M., Bertin C., Atzenhoffer M., Miremont-Salame G., Perault-Pochat M.-C., Puyade M., et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J. Immunother. Cancer. 2020;8:e001622. doi: 10.1136/jitc-2020-001622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunot A., Grob J.-J., Jeudy G., Grange F., Guillot B., Kramkimel N., Mortier L., Le Corre Y., Aubin F.F., Mansard S., et al. Association of anti–programmed cell death 1 antibody treatment with risk of recurrence of toxic effects after immune-related adverse events of ipilimumab in patients with metastatic melanoma. JAMA Dermatol. 2020;156:982. doi: 10.1001/jamadermatol.2020.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortazar F.B., Kibbelaar Z.A., Glezerman I.G., Abudayyeh A., Mamlouk O., Motwani S.S., Murakami N., Herrmann S.M., Manohar S., Shirali A.C., et al. Clinical Features and outcomes of immune checkpoint inhibitor—Associated AKI: A multicenter study. J. Am. Soc. Nephrol. 2020;31:435–446. doi: 10.1681/ASN.2019070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Malet A., Antoni G., Collins M., Soularue E., Marthey L., Vaysse T., Coutzac C., Chaput N., Mateus C., Robert C., et al. Evolution and recurrence of gastrointestinal immune-related adverse events induced by immune checkpoint inhibitors. Eur. J. Cancer. 2019;106:106–114. doi: 10.1016/j.ejca.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Gobbini E., Toffart A.C., Pérol M., Assié J.-B., Duruisseaux M., Coupez D., Dubos C., Westeel V., Delaunay M., Guisier F., et al. Immune checkpoint inhibitors rechallenge efficacy in non–small-cell lung cancer patients. Clin. Lung Cancer. 2020;21:e497–e510. doi: 10.1016/j.cllc.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S., Short S.A.P., Sise M.E., Prosek J.M., Madhavan S.M., Soler M.J., Ostermann M., Herrmann S.M., Abudayyeh A., Anand S., et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer. 2021;9:e003467. doi: 10.1136/jitc-2021-003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M., Sack J.S., Rahma O.E., Hodi F.S., Zucker S.D., Grover S. Outcomes after resumption of immune checkpoint inhibitor therapy after high-grade immune-mediated hepatitis. Cancer. 2020;126:5088–5097. doi: 10.1002/cncr.33165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouri A., Kaira K., Yamaguchi O., Shiono A., Miura Y., Hashimoto K., Nishihara F., Murayama Y., Kobayashi K., Kagamu H. Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother. Pharmacol. 2019;84:873–880. doi: 10.1007/s00280-019-03926-y. [DOI] [PubMed] [Google Scholar]
- 41.Pollack M.H., Betof A., Dearden H., Rapazzo K., Valentine I., Brohl A.S., Ancell K.K., Long G.V., Menzies A.M., Eroglu Z., et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (IrAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann. Oncol. 2018;29:250–255. doi: 10.1093/annonc/mdx642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santini F.C., Rizvi H., Plodkowski A.J., Ni A., Lacouture M.E., Gambarin-Gelwan M., Wilkins O., Panora E., Halpenny D.F., Long N.M., et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol. Res. 2018;6:1093–1099. doi: 10.1158/2326-6066.CIR-17-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonaggio A., Michot J.M., Voisin A.L., Le Pavec J., Collins M., Lallart A., Cengizalp G., Vozy A., Laparra A., Varga A., et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5:1310. doi: 10.1001/jamaoncol.2019.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravi P., Mantia C., Su C., Sorenson K., Elhag D., Rathi N., Bakouny Z., Agarwal N., Zakharia Y., Costello B.A., et al. Evaluation of the safety and efficacy of immunotherapy rechallenge in patients with renal cell carcinoma. JAMA Oncol. 2020;6:1606. doi: 10.1001/jamaoncol.2020.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albandar H.J., Fuqua J., Albandar J.M., Safi S., Merrill S.A., Ma P.C. Immune-related adverse events (IrAE) in cancer immune checkpoint inhibitors (ICI) and survival outcomes correlation: To rechallenge or not? Cancers. 2021;13:989. doi: 10.3390/cancers13050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menzies A.M., Johnson D.B., Ramanujam S., Atkinson V.G., Wong A.N.M., Park J.J., McQuade J.L., Shoushtari A.N., Tsai K.K., Eroglu Z., et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 2017;28:368–376. doi: 10.1093/annonc/mdw443. [DOI] [PubMed] [Google Scholar]
- 47.Apalla Z., Nikolaou V., Fattore D., Fabbrocini G., Freites-Martinez A., Sollena P., Lacouture M., Kraehenbuehl L., Stratigos A., Peris K., et al. European recommendations for management of immune checkpoint inhibitors-derived dermatologic adverse events. The EADV task force “dermatology for cancer patients” position statement. J. Eur. Acad. Dermatol. Venereol. 2021:jdv.17855. doi: 10.1111/jdv.17855. [DOI] [PubMed] [Google Scholar]
- 48.Spain L., Tippu Z., Larkin J.M., Carr A., Turajlic S. How we treat neurological toxicity from immune checkpoint inhibitors. ESMO Open. 2020;4:e000540. doi: 10.1136/esmoopen-2019-000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q., Tang L., Zhou Y., He W., Li W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: Current understanding in characteristics, diagnosis, and management. Front. Immunol. 2021;12:663986. doi: 10.3389/fimmu.2021.663986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong Z., Wang Y. Immune checkpoint inhibitor–mediated diarrhea and colitis: A clinical review. JCO Oncol. Pract. 2020;16:453–461. doi: 10.1200/OP.20.00002. [DOI] [PubMed] [Google Scholar]
- 51.Dougan M., Blidner A.G., Choi J., Cooksley T., Glezerman I., Ginex P., Girotra M., Gupta D., Johnson D., Shannon V.R., et al. Multinational association of supportive care in cancer (MASCC) 2020 clinical practice recommendations for the management of severe gastrointestinal and hepatic toxicities from checkpoint inhibitors. Support. Care Cancer. 2020;28:6129–6143. doi: 10.1007/s00520-020-05707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shannon V.R., Anderson R., Blidner A., Choi J., Cooksley T., Dougan M., Glezerman I., Ginex P., Girotra M., Gupta D., et al. Multinational association of supportive care in cancer (MASCC) 2020 clinical practice recommendations for the management of immune-related adverse events: Pulmonary toxicity. Support. Care Cancer. 2020;28:6145–6157. doi: 10.1007/s00520-020-05708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi J., Anderson R., Blidner A., Cooksley T., Dougan M., Glezerman I., Ginex P., Girotra M., Gupta D., Johnson D., et al. Multinational association of supportive care in cancer (MASCC) 2020 clinical practice recommendations for the management of severe dermatological toxicities from checkpoint inhibitors. Support. Care Cancer. 2020;28:6119–6128. doi: 10.1007/s00520-020-05706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kostine M., Finckh A., Bingham C.O., Visser K., Leipe J., Schulze-Koops H., Choy E.H., Benesova K., Radstake T.R.D.J., Cope A.P., et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann. Rheum. Dis. 2021;80:36–48. doi: 10.1136/annrheumdis-2020-217139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omar N.E., El-Fass K.A., Abushouk A.I., Elbaghdady N., Barakat A.E.M., Noreldin A.E., Johar D., Yassin M., Hamad A., Elazzazy S., et al. Diagnosis and management of hematological adverse events induced by immune checkpoint inhibitors: A systematic review. Front. Immunol. 2020;11:1354. doi: 10.3389/fimmu.2020.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poto R., Marone G., Pirozzi F., Galdiero M.R., Cuomo A., Formisano L., Bianco R., Della Corte C.M., Morgillo F., Napolitano S., et al. How can we manage the cardiac toxicity of immune checkpoint inhibitors? Expert Opin. Drug Saf. 2021;20:685–694. doi: 10.1080/14740338.2021.1906860. [DOI] [PubMed] [Google Scholar]
- 57.Perazella M.A., Sprangers B. Checkpoint inhibitor therapy-associated acute kidney injury: Time to move on to evidence-based recommendations. Clin. Kidney J. 2021;14:1301–1306. doi: 10.1093/ckj/sfab052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrmann S.M., Perazella M.A. Immune checkpoint inhibitors and immune-related adverse renal events. Kidney Int. Rep. 2020;5:1139–1148. doi: 10.1016/j.ekir.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rose L.M., DeBerg H.A., Vishnu P., Frankel J.K., Manjunath A.B., Flores J.P.E., Aboulafia D.M. Incidence of skin and respiratory immune-related adverse events correlates with specific tumor types in patients treated with checkpoint inhibitors. Front. Oncol. 2020;10:570752. doi: 10.3389/fonc.2020.570752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zamani M.R., Aslani S., Salmaninejad A., Javan M.R., Rezaei N. PD-1/PD-L and autoimmunity: A growing relationship. Cell. Immunol. 2016;310:27–41. doi: 10.1016/j.cellimm.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Czaja A.J. Immune Inhibitory proteins and their pathogenic and therapeutic implications in autoimmunity and autoimmune hepatitis. Autoimmunity. 2019;52:144–160. doi: 10.1080/08916934.2019.1641200. [DOI] [PubMed] [Google Scholar]
- 62.Xie W., Huang H., Xiao S., Fan Y., Deng X., Zhang Z. Immune checkpoint inhibitors therapies in patients with cancer and preexisting autoimmune diseases: A meta-analysis of observational studies. Autoimmun. Rev. 2020;19:102687. doi: 10.1016/j.autrev.2020.102687. [DOI] [PubMed] [Google Scholar]
- 63.Hoa S., Laaouad L., Roberts J., Ennis D., Ye C., Al Jumaily K., Pope J., Nevskaya T., Saltman A., Himmel M., et al. Preexisting autoimmune disease and immune-related adverse events associated with Anti-PD-1 cancer immunotherapy: A national case series from the canadian research group of rheumatology in immuno-oncology. Cancer Immunol. Immunother. 2021;70:2197–2207. doi: 10.1007/s00262-021-02851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tully K.H., Cone E.B., Cole A.P., Sun M., Chen X., Marchese M., Roghmann F., Kilbridge K.L., Trinh Q.-D. Risk of immune-related adverse events in melanoma patients with preexisting autoimmune disease treated with immune checkpoint inhibitors: A population-based study using SEER-Medicare data. Am. J. Clin. Oncol. 2021;44:413–418. doi: 10.1097/COC.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 65.Van der Kooij M.K., Suijkerbuijk K.P.M., Aarts M.J.B., van den Berkmortel F.W.P.J., Blank C.U., Boers-Sonderen M.J., van Breeschoten J., van den Eertwegh A.J.M., de Groot J.W.B., Haanen J.B.A.G., et al. Safety and efficacy of checkpoint inhibition in patients with melanoma and preexisting autoimmune disease: A cohort study. Ann. Intern. Med. 2021;174:641–648. doi: 10.7326/M20-3419. [DOI] [PubMed] [Google Scholar]
- 66.Kartolo A., Sattar J., Sahai V., Baetz T., Lakoff J.M. Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol. Tor. 2018;25:e403–e410. doi: 10.3747/co.25.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cortellini A., Bersanelli M., Santini D., Buti S., Tiseo M., Cannita K., Perrone F., Giusti R., De Tursi M., Zoratto F., et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events. Eur. J. Cancer. 2020;128:17–26. doi: 10.1016/j.ejca.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 68.Eun Y., Kim I.Y., Sun J.-M., Lee J., Cha H.-S., Koh E.-M., Kim H., Lee J. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci. Rep. 2019;9:14039. doi: 10.1038/s41598-019-50574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kichenadasse G., Miners J.O., Mangoni A.A., Rowland A., Hopkins A.M., Sorich M.J. Association between body mass index and overall survival with immune checkpoint inhibitortherapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6:512–518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richtig G., Hoeller C., Wolf M., Wolf I., Rainer B.M., Schulter G., Richtig M., Grübler M.R., Gappmayer A., Haidn T., et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLoS ONE. 2018;13:e0204729. doi: 10.1371/journal.pone.0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Y., Fu Y., Zhu B., Wang J., Zhang B. Predictive biomarkers of immune checkpoint inhibitors-related toxicities. Front. Immunol. 2020;11:2023. doi: 10.3389/fimmu.2020.02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Das S., Johnson D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freeman-Keller M., Kim Y., Cronin H., Richards A., Gibney G., Weber J.S. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune-related adverse events and association with outcomes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teulings H.-E., Limpens J., Jansen S.N., Zwinderman A.H., Reitsma J.B., Spuls P.I., Luiten R.M. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 75.Chan L., Hwang S.J.E., Byth K., Kyaw M., Carlino M.S., Chou S., Fernandez-Penas P. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J. Am. Acad. Dermatol. 2020;82:311–316. doi: 10.1016/j.jaad.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 76.Indini A., Di Guardo L., Cimminiello C., Prisciandaro M., Randon G., De Braud F., Del Vecchio M. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J. Cancer Res. Clin. Oncol. 2019;145:511–521. doi: 10.1007/s00432-018-2819-x. [DOI] [PubMed] [Google Scholar]
- 77.Tang K., Seo J., Tiu B.C., Le T.K., Pahalyants V., Raval N.S., Ugwu-Dike P.O., Zubiri L., Naranbhai V., Carrington M., et al. Association of cutaneous immune-related adverse events with increased survival in patients treated with anti-programmed cell death 1 and anti-programmed cell death ligand 1 therapy. JAMA Dermatol. 2022 doi: 10.1001/jamadermatol.2021.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kverneland A.H., Chamberlain C.A., Borch T.H., Nielsen M., Mørk S.K., Kjeldsen J.W., Lorentzen C.L., Jørgensen L.P., Riis L.B., Yde C.W., et al. Adoptive cell therapy with tumor-infiltrating lymphocytes supported by checkpoint inhibition across multiple solid cancer types. J. Immunother. Cancer. 2021;9:e003499. doi: 10.1136/jitc-2021-003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kverneland A.H., Pedersen M., Westergaard M.C.W., Nielsen M., Borch T.H., Olsen L.R., Aasbjerg G., Santegoets S.J., van der Burg S.H., Milne K., et al. Adoptive cell therapy in combination with checkpoint inhibitors in ovarian cancer. Oncotarget. 2020;11:2092–2105. doi: 10.18632/oncotarget.27604. [DOI] [PMC free article] [PubMed] [Google Scholar]