Abstract
Current recommendations suggest that negative rapid Streptococcus pyogenes antigen tests be backed up with a culture, reflecting evidence that culture may have a higher sensitivity and also that testing of a second swab may yield a different (i.e., a positive) result because of variation in sample size or distribution. If the latter is common, the sensitivities of current antigen detection tests might be improved by simply increasing the amount of sample tested. The present study assessed the effect of antigen testing of two swabs extracted together compared to independent testing of each swab extracted separately for children with clinical pharyngitis. S. pyogenes grew from one or both swabs for 198 (37%) of 537 children. The combined culture was significantly (P < 0.05) more sensitive than culture of either swab alone. Compared to combined culture, antigen testing of two swabs extracted and tested together was significantly more sensitive than two single swab extractions (94.1 versus 80%; P = 0.03); however, the specificity was decreased (81.5 versus 89.8 to 92.7%; P < 0.05). This study suggests that sample size and/or uneven sample distribution may have influenced the apparent sensitivities of prior studies that compared antigen tests to a single plate culture. A strategy, such as the one used in the present study, that increases the sample size available for antigen testing (i.e., extraction of samples from both swabs) may improve detection rates to a level that will better approximate true disease status and obviate the need for backup cultures if specificity can be improved.
Since Breese et al. (4) first described throat swab culturing on sheep blood agar (SBA), there has been an evolving debate concerning the best method of bacteriologic identification and treatment of Streptococcus pyogenes. Recent studies have suggested that selective media should be the “gold standard” for diagnosis, although there are now several formulations which may have different levels of performance (9). However, despite the high degree of sensitivity of selective media, there remain several drawbacks with culture techniques, mainly in the form of a 24- to 48-h lag time that exists before a determination about the presence of S. pyogenes can be made.
With the advent of rapid antigen detection tests, this delay has been eliminated and, along with it, some of the related problems have been eliminated. In fact, numerous studies have demonstrated some unique advantages of using rapid S. pyogenes antigen detection tests. (i) In certain noncompliant populations, they have been shown to result in dramatically increased treatment rates. (ii) Antibiotic treatment can be initiated sooner. (iii) Lastly, they contribute less to the increasing levels of resistance to antibiotics because selective treatment can be administered according to the results of an immediate report of infection. However, despite these advantages and their routine use in clinical settings, rapid tests still lack diagnostic sensitivity compared to a rigorous gold standard. Numerous studies have revealed that various antigen detection tests have sensitivities between 70 and 90%, depending on the comparative standard and the study protocol used (9, 12). Unfortunately, differences in study populations, sampling techniques, the culture medium used, incubation conditions, and the personnel performing the tests (i.e., office employees versus trained laboratory technologists) make it difficult to compare one method with another method evaluated under different conditions.
The claim made for some rapid tests, that they are as good as a culture performed in the office, may deny the fact that the sensitivity of neither is sufficient that they can independently be relied upon (7). Thus, the American Academy of Pediatrics has recommended that all negative rapid S. pyogenes antigen detection tests be backed up with a culture (1). This advice reflects a belief that culture may have a higher degree of sensitivity (depending on the type of medium used) and also that testing of a second sample may yield a different (i.e., a positive) result because of a variation in sample size or distribution. If the latter is common, the sensitivities of current antigen detection tests might be improved by simply increasing the sample size. This study was designed to assess the effect of sample variation on the apparent gold standard and the effect of the inoculum on extraction and antigen testing of two swabs together compared to independent testing of either swab alone.
(This study was presented in part at the Pediatric Academic Societies Meeting, San Francisco, Calif., 4 May 1999.)
MATERIALS AND METHODS
Children who were between the ages of 4 and 15 years and who presented to a private pediatric office in Aurora, Colo., with clinical signs of S. pyogenes pharyngitis were considered for the study. Criteria for inclusion included fever, sore throat, and/or cervical adenitis and the absence of cough, rhinorrhea, lower respiratory infection, and otitis media. Treatment with antibiotics in the previous 7 days excluded a child from participation in the study.
Two throat swabs were vigorously rubbed simultaneously over the posterior pharynx and tonsils. One swab was streaked onto a standard 5% SBA plate and a bacitracin disc was applied. The second swab was similarly streaked onto a plate containing selective medium and trimethoprim-sulfamethoxazole (Remel). Both plates were incubated aerobically at 37°C for 18 to 24 h in order to simulate culture conditions in an office setting. Culture plates were coded with patient-specific numbers to blind the reader to the patient, the medium, and the antigen detection test result. Culture plates were examined within 18 to 24 h, and negative plates were incubated for an additional 24 h. Beta-hemolytic streptococci were presumptively classified as group A (S. pyogenes) on the basis of bacitracin susceptibility. Any discrepancies between antigen detection test and culture results were resolved by using a direct colony antigen test for the group A carbohydrate (Streptex). Immediately after streaking of the sample onto culture medium, rapid streptococcal antigen detection tests were performed with each of the two swabs by using the TestPac Plus (Abbott Diagnostics) antigen detection test according to the manufacturer's instructions. In a randomized fashion, for one-half of the study group two rapid tests were run, one for each swab. For the other half, only one rapid test was performed by using both swabs combined in a single extraction according to the instructions provided with the kit, with one exception; instead of using 3 drops of each solution for antigen extraction, we used 4 drops of each solution. Test results were graded on the basis of the intensity of the reaction, as follows: 0 (none), faint, 1 (definite but light), 2 (moderate), and 3 (dark). For purposes of initial analysis, those tests that gave a faint reaction were considered positive.
The gold standard for test comparison was the growth of S. pyogenes from either of the two swabs. Paired data were compared by the McNemar test, while unpaired data were compared by the chi-square test.
RESULTS
Overall, 537 children were included in this study, and one or both swabs from 198 (37%) of the children grew S. pyogenes. Compared to this combined-culture gold standard, swabs from 190 (96%) of the children grew S. pyogenes on SBA plates, and swabs from 179 (90.4%) of the children grew S. pyogenes on the selective medium. Although the majority grew on both plates, SBA proved to be significantly more sensitive than selective medium, and each culture alone was not as sensitive as the combined culture (Table 1).
TABLE 1.
Comparison of sensitivity and specificity of S. pyogenes culture and antigen detection techniques
| Method | Sensitivity (%)a | Specificitya |
|---|---|---|
| Culture | ||
| Combined plates | 198/198 (100)bc | 339/339 (100) |
| Plate A (SBA) | 190/198 (96)bd | 339/339 (100) |
| Plate B (selective) | 179/198 (90.4)cd | 339/339 (100) |
| Antigen tests | ||
| Swab A | 64/80 (80.0)e | 164/177 (92.7)g |
| Swab B | 63/79 (79.7)f | 159/177 (89.8)h |
| Combined swab extraction | 111/118 (94.1)ef | 132/162 (81.5)gh |
Values are number of positive specimens/total number of specimens (percent). Differences between methods indicated by the same superscript are statistically significant (P < 0.05) by the McNemar test for paired data or the chi-square test for unpaired sensitivities.
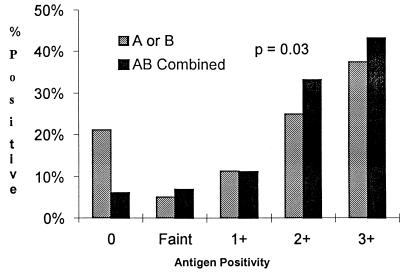
Each of the two swabs from 257 children was tested for antigen separately. Of these, 80 (31%) grew S. pyogenes on one or both of the corresponding culture plates. The first swab was always cultured on SBA and was positive for antigen for 64 (80%) of the 80 culture-positive swabs, and the second swab was always cultured on the selective medium and was positive for 63 (79%) of the culture-positive swabs. There was no difference in the sensitivity of the antigen detection test for either swab. For the other 280 children, both swabs were cultured separately and were then extracted together and tested by a single antigen detection test. Of these, 118 (42%) grew S. pyogenes and 111 (94.1%) were positive by the antigen detection test. The sensitivity of the combined extraction technique was significantly higher than that of the single-swab extraction technique (94.1 versus 80%; P = 0.03), although the specificity was lower (81.5 versus 89.8 to 92.7%; P < 0.05). The highest quantitative antigen detection test result for each child is shown in Fig. 1. The distribution for the combined extraction showed significantly fewer negative swabs and a shift toward stronger reactions (P = 0.03). If faintly reacting antigen detection test results were considered negative, the combined extraction technique was still significantly more sensitive than the single-swab extraction technique (87.3 versus 73.8%; P = 0.02), but the specificity was no longer significantly lower (96.3 versus 94.9%; P = 0.54).
FIG. 1.
Semiquantitative estimation of group A carbohydrate antigen detected from a single swab (A or B) compared to that of antigen detected from both swabs extracted together (AB) for children with pharyngitis.
DISCUSSION
This study addresses two important questions regarding S. pyogenes detection in throat swab specimens: what culture medium and/or technique should serve as the gold standard and whether an increase in sample size will increase the sensitivity of rapid antigen detection. We were surprised that, contrary to our previously reported results (13, 17), culture on selective medium was not superior to culture on SBA, although prior reports have suggested that results may vary depending both on the medium formulation and on the medium batch (9). In fact, reported sensitivities for the TestPack as well as other S. pyogenes antigen detection tests seem to vary inversely with the rigor of the culture techniques used, with anaerobic incubation (i.e., more rigorous culture environment) tending to yield lower sensitivities than aerobic incubation (8, 10, 11, 14, 15). This emphasizes the importance of the rigor of the definition of the comparative gold standard in assessing the clinical utility of a new diagnostic test. Even when performed in a clinical pathology laboratory, 10% of culture-positive S. pyogenes infections were missed by the antigen detection test, with the recommendation that backup culture be used for those specimens with antigen detection test-negative results (16).
Equally important as culture conditions, this study shows that culture of each of two swabs significantly increases the rate of detection of S. pyogenes over a culture of a single swab, documenting that sample size and an uneven organism distribution between swabs may each play a role in the discrepancies noted in studies that have compared one S. pyogenes detection test to another, each with a separate swab. This would explain why a number of studies have found that culture of two swabs separately and/or enhanced broth culture of the removed pledget may yield a higher rate of recovery than culture of a single swab, presumably because a single swab may have an insufficient inoculum size to adequately represent the entire sample (5, 7, 12). Those who propose that antigen detection tests need be only as good as a single-plate culture may be setting a standard that is too low; as such, tests may fail to detect organisms on those swabs with low inocula (2, 6).
This study also documents that a two-swab throat sampling and antigen extraction technique that increases the size of the inoculum extracted for antigen testing significantly improves antigen detection rates. By extracting both swabs together, we may have accounted for the uneven sample distributions between swabs and may have increased the antigen concentration in the extract. In either case, technology that permits testing of a larger or more complete throat swab specimen may have a threshold effect that could allow current antigen testing technology to more accurately approximate a rigorous culture gold standard. One consequence of an increased sensitivity is usually a decreased specificity, as was seen in this study if we included the faintly reacting test results in the analysis. The result was no longer significant when we considered faintly reacting specimens as negative and may have been due to the increased concentration of cross-reacting elements (other bacteria, chemicals) in the ad hoc extraction mixture that we used. If our sensitivity of 95% for two-swab extraction antigen detection compared to the result of a rigorous two-swab culture standard can be repeated while maintaining a high degree of specificity, backup culture of antigen-negative specimens might reasonably be eliminated. Other studies that increased the inoculum size either by preincubation of swabs (antigen amplification) (3) or by use of polycarbonate swabs (increased sample size) have shown antigen detection sensitivities which similarly approach this threshold.
The necessary culture gold standard for comparison of antigen test detection results should accurately distinguish those patients who truly have S. pyogenes colonization or infection in the throat and not set a lesser standard due to inferior culture conditions, sample variation, or inadequate sample size. In addition, a strategy, such as the one used in this study, that increases the sample size available for antigen testing (i.e., extraction of both swabs) may improve detection rates to a level that will better approximate true disease status and obviate the need for backup culture without requiring further advances in antigen detection technology.
ACKNOWLEDGMENT
The study was supported in part by a grant from Abbott Diagnostics.
REFERENCES
- 1.American Academy of Pediatrics. Group A streptococcal infections. In: Peter G, editor. 1997 red book: Report of the Committee on Infectious Diseases. 24th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 1997. pp. 483–494. [Google Scholar]
- 2.Anhalt J P, Heiter B J, Naumovitz D W, Bourbeau P P. Comparison of three methods for detection of group A streptococci in throat swabs. J Clin Microbiol. 1992;30:2135–2138. doi: 10.1128/jcm.30.8.2135-2138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourbeau P P, Heiter B J, Anhalt J P, Naumovitz D W. Comparison of direct specimen testing utilizing TestPack Strep A with testing of specimens following a two-hour broth enrichment. Diagn Microbiol Infect Dis. 1993;17:93–96. doi: 10.1016/0732-8893(93)90018-3. [DOI] [PubMed] [Google Scholar]
- 4.Breese B B, Disney F A, Talpey W. The nature of a small pediatric group practice. II. The incidence of beta hemolytic streptococcal illness in a private pediatric practice. Pediatrics. 1966;38:277–285. [PubMed] [Google Scholar]
- 5.Daly J A, Korgenski E K, Munson A C, Llausas-Magana E. Optical immunoassay for streptococcal pharyngitis: evaluation of accuracy with routine and mucoid strains associated with acute rheumatic fever outbreak in the intermountain area of the United States. J Clin Microbiol. 1994;32:531–532. doi: 10.1128/jcm.32.2.531-532.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerber M A, Randolph M F, Chanatry J, Wright L L, DeMeo K K, Anderson L R. Antigen detection test for streptococcal pharyngitis: evaluation of sensitivity with respect to true infections. J Pediatr. 1986;108:654–658. doi: 10.1016/s0022-3476(86)81036-8. [DOI] [PubMed] [Google Scholar]
- 7.Gerber M A, Tanz R R, Kabat W, Dennis E, Bell G L, Kaplan E L, Shulman S T. Optical immunoassay test for group A beta-hemolytic streptococcal pharyngitis. An office-based, multicenter investigation. JAMA. 1997;277:899–903. [PubMed] [Google Scholar]
- 8.Hoffmann S. Detection of group A streptococcal antigen from throat swabs with five diagnostic kits in general practice. Diagn Microbiol Infect Dis. 1990;13:209–215. doi: 10.1016/0732-8893(90)90061-y. [DOI] [PubMed] [Google Scholar]
- 9.Kellogg J A. Suitability of throat culture procedures for detection of group A streptococci and as reference standards for evaluation of streptococcal antigen detection kits. J Clin Microbiol. 1990;28:165–169. doi: 10.1128/jcm.28.2.165-169.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellogg J A, Bankert D A, Levisky J S. Comparison of the TestPack Strep A enzyme immunoassay system with anaerobically incubated cultures for detection of group A streptococci from oropharyngeal swabs. Am J Clin Pathol. 1987;88:631–634. doi: 10.1093/ajcp/88.5.631. [DOI] [PubMed] [Google Scholar]
- 11.Kellogg J A, Landis R C, Nussbaum A S, Bankert D A. Performance of an enzyme immunoassay test and anaerobic culture for detection of group A streptococci in a pediatric practice versus a hospital laboratory. J Pediatr. 1987;111:18–21. doi: 10.1016/s0022-3476(87)80335-9. [DOI] [PubMed] [Google Scholar]
- 12.Roe M, Kishiyama C, Davidson K, Schaefer L, Todd J. Comparison of BioStar Strep A OIA optical immune assay, Abbott TestPack Plus Strep A, and culture with selective media for diagnosis of group A streptococcal pharyngitis. J Clin Microbiol. 1995;33:1551–1553. doi: 10.1128/jcm.33.6.1551-1553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roe M H, Tolliver P R, Lewis P L, Todd J K. Primary plate identification of group A Streptococcus on a selective medium. Efficiency in an office practice. Am J Dis Child. 1984;138:589–591. doi: 10.1001/archpedi.1984.02140440073019. [DOI] [PubMed] [Google Scholar]
- 14.Schwabe L D, Gobbo A F, Gottschall R L, Randall E L. Comparison of TestPack Plus Strep A with selective and nonselective culture media for detection of group-A streptococci. Diagn Microbiol Infect Dis. 1991;14:367–372. doi: 10.1016/0732-8893(91)90062-k. [DOI] [PubMed] [Google Scholar]
- 15.Schwabe L D, Small M T, Randall E L. Comparison of TestPack Strep A test kit with culture technique for detection of group A streptococci. J Clin Microbiol. 1987;25:309–311. doi: 10.1128/jcm.25.2.309-311.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenjarla G, Kumar A, Dyke J W. TestPack Strep A kit for the rapid detection of group A streptococci on 11,088 throat swabs in a clinical pathology laboratory. Am J Clin Pathol. 1991;96:759–761. doi: 10.1093/ajcp/96.6.759. [DOI] [PubMed] [Google Scholar]
- 17.Tolliver P R, Roe M H, Todd J K. Detection of group A Streptococcus: comparison of solid and liquid culture media with and without selective antibiotics. Pediatr Infect Dis J. 1987;6:515–519. [PubMed] [Google Scholar]