Abstract
The conventional methods for identifying mycobacterial species are based on their phenotypic characterization. Since some problematic species are slow growers, their taxonomy takes several weeks or months to identify. The ribosomal DNA (rDNA) sequence-based identification strategy has been adopted to solve this problem. More recently, the gyrB sequences have been shown to be useful phylogenetic markers for the identification of species. We determined the gyrB sequences of 43 slowly growing strains belonging to 15 species in the genus Mycobacterium. The frequencies of base substitutions in the gyrB sequences were comparable to those in the 16S-23S rDNA internal transcribed spacer (ITS) sequences. The ITS sequences of four species belonging to the M. tuberculosis complex (M. tuberculosis, M. bovis, M. africanum, and M. microti) were 100% identical, while four synonymous substitutions were found in the gyrB sequences of these strains. Based on the differences found in the gyrB sequences, we developed PCR and PCR-restriction fragment length polymorphism methods to discriminate these species.
The increase in the incidence of infection by mycobacteria in humans, especially among immunocompromised patients, is a matter of serious concern to the public. In order to control this infection, rapid identification of the carriers of mycobacteria is a high priority. Intensive research efforts were made to develop rapid methods for identifying mycobacteria. Numerical taxonomic matrices and 16S ribosomal DNA (rDNA)-based phylogenetic analyses have provided a great deal of information on the systematics of mycobacteria (19, 24, 37). The 16S rDNA-based methods are currently widely accepted as rapid and accurate means for identifying mycobacteria (14, 15, 28). However, it was found that the distinctions between Mycobacterium kansasii and M. gastri, between M. malmoense and M. szulgai, between M. marinum and M. ulcerans, and between the members of the M. tuberculosis complex are difficult to make based on their 16S rDNA sequences since they are almost identical between these strains. To solve this problem, the sequences of the 16S-23S rDNA internal transcribed spacer (ITS) have been used to distinguish between M. kansasii and M. gastri (20). However, the ITS sequences are identical among the M. tuberculosis complex strains.
The M. tuberculosis complex consists of four closely related groups, M. tuberculosis, M. bovis, M. africanum, and M. microti, and they may be considered to be subspecies of M. tuberculosis (37). Their close relatedness has been demonstrated by DNA-DNA hybridization (1), isozyme analysis (5), and electrokaryotyping (5). However, their host range and pathogenicity are quite different. The host of M. tuberculosis and M. africanum is limited to humans (37), while M. bovis causes disease in a wide range of wild and domestic mammals, as well as in humans (18). M. microti has recently been reported to infect not only voles but also humans (32).
Several efforts have been made to differentiate slowly growing mycobacterial species by using protein-encoding genes such as the 32-kDa protein gene (22), dnaJ (26, 27, 34), hsp 65 (17, 25), the superoxide dismutase gene (21, 44), recA (31), and rpoV (3). However, DNA sequence analyses of these genes revealed them to be invariant among the members of the M. tuberculosis complex.
Yamamoto and Harayama (40) have proposed that gyrB could be a suitable phylogenetic marker for the identification and classification of bacteria (10, 41, 42). They have shown that the divergence of gyrB sequences reflected the taxonomical relationships in the genera Acinetobacter (42) and Pseudomonas (43). They have also shown that the average base substitution rate of 16S rDNA was 1% per 50 million years, while that of the gyrB genes at synonymous sites was 0.7 to 0.8% per one million years (41). The gyrB analyses of other bacterial genera have also resolved closely related strains (33, 35, 36, 39).
In this report, we analyzed the gyrB sequences of type strains and clinical isolates of slowly growing mycobacteria, including the M. tuberculosis complex, M. kansasii, M. gastri, M. avium, M. intracellulare, M. malmoense, and M. simiae. In parallel, we analyzed the ITS sequences of these strains and compared the results with those of the gyrB analysis. Furthermore, we developed PCR-based methods to differentiate four species of the M. tuberculosis complex.
MATERIALS AND METHODS
Strains and cultivation.
The strains used in this study are shown in Table 1. They were cultivated by using Ogawa's medium (Nissui Pharmaceutical Co.).
TABLE 1.
Source of DNA samples and accession numbers of gyrB sequences
| Strain | Genus and species | Remarks | Accession no.
|
|
|---|---|---|---|---|
| gyrB | ITS | |||
| KPM T801 | M. africanum | Type strain (ATCC 25420) | AB014192 | AB026699 |
| ATCC 25274 | M. asiaticum | AB014206 | AB026703 | |
| KPM 3012 | M. avium | Type strain (ATCC 25291) | AB014189 | AB026690 |
| KPM T704 | M. bovis | Type strain (ATCC 19210) | AB014184 | AB026693 |
| KPM T702 | M. bovis | BCG Japanese strain | AB014193 | |
| IKEDA | M. bovis | Cow isolate, Hokkaido | AB018554 | |
| KPM 3504 | M. gastri | Type strain (ATCC 15754) | AB014202 | AB026697 |
| KPM 3502 | M. gastri | Human isolate, 1982, Shimane | AB014294 | |
| KPM 3503 | M. gastri | Human isolate, 1982, Kyoto | AB014295 | |
| KPM 2201 | M. gordonae | Type strain (ATCC 14470) | AB014191 | AB026692 |
| KPM 3101 | M. intracellulare | Type strain (ATCC 13950) | AB014188 | AB026691 |
| KPM 1001 | M. kansasii | Type strain (ATCC 12478) | AB014204 | AB026695 |
| KPM 1004 | M. kansasii | Human clinical, 1990, Osaka | AB014301 | |
| KPM 1007 | M. kansasii | Human clinical, 1990, Osaka | AB014302 | |
| KPM KY256 | M. kansasii | Human clinical, 1991, Kyoto | AB014304 | |
| KPM KY761 | M. kansasii | Human clinical, 1991, Kyoto | AB014305 | |
| KPM KY798 | M. kansasii | Human clinical, 1991, Kyoto | AB014306 | |
| KPM 1988-1 | M. kansasii | Human clinical, 1988, Osaka | AB014307 | |
| KPM 3401 | M. malmoense | Type strain (ATCC 29571) | AB014187 | AB026696 |
| KPM 1201 | M. marinum | Type strain (ATCC 927) | AB014203 | AB026701 |
| KPM T901 | M. microti | Type strain (NCTC 8710) | AB014205 | AB026700 |
| KPM 2027 | M. scrofulaceum | Type strain (ATCC 19981) | AB014207 | AB026702 |
| KPM 1403 | M. simiae | Type strain (ATCC 25275) | AB014182 | AB026694 |
| KPM 2403 | M. szulgai | Type strain (NCTC 10831) | AB014185 | AB026704 |
| KPM T021 | M. tuberculosis | Type strain (ATCC 27294) | AB014194 | AB026698 |
| H37Rv | M. tuberculosis | Z80233 | ||
| H37Ra | M. tuberculosis | X78888 | ||
| KPM KY590 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014209 | |
| KPM KY631 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014210 | |
| KPM KY643 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014211 | |
| KPM KY673 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014212 | |
| KPM KY677 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014213 | |
| KPM KY678 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014214 | |
| KPM KY679 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014215 | |
| KPM KY682 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014216 | |
| KPM KY686 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014217 | |
| KPM KY697 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014218 | |
| KPM KY698 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014219 | |
| KPM KY699 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014220 | |
| KPM KY708 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014221 | |
| KPM KY709 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014230 | |
| KPM KY713 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014231 | |
| KPM KY715 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014240 | |
| KPM KY721 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014241 | |
| KPM KY741 | M. tuberculosis | Human clinical, 1987, Kyoto | AB014242 | |
Determination of gyrB gene sequences.
The protocol for determining the gyrB sequences was almost the same as that described by Yamamoto and Harayama except for the PCR primers (40). The primers used here are listed in Table 2, the location of each primer sequence being indicated by the numbering system for the M. tuberculosis gyrB sequence. A 1.2-kb gyrB segment was amplified by PCR by using the universal primers UP1TL and UP2rTL. To determine the nucleotide sequence of both strands of the gyrB segment, shorter fragments were amplified by using the PCR-amplified 1.2-kb gyrB segment as a template and two pairs of primers, either UP1EM13r plus QTK-21 or GGTH-r plus UP2r-21. The amplification reaction was subjected to 35 cycles with a denaturation step at 95°C for 1 min, an annealing step at 63°C for 1 min, and an extension step at 72°C for 2 min. Sequences were determined by using the universal primers for the M13 phage vector.
TABLE 2.
List of PCR primers used for gyrB analysis
| Primer | Nucleotide sequence (5′ to 3′) | Location of primersa |
|---|---|---|
| UP1TL | CAy GCn GGn GGn AAr TTy GA | 553–572 |
| UP2rTL | TCn ACr TCn GCr TCn GTC AT | 1831–1850 |
| UP1EM13r | CAG GAA ACA GCT ATG ACC AyG snG GnG GnA ArT Tyr A | 553–572 |
| QTK-21 | TGT AAA ACG ACG GCC AGT Ary TTn kyy TTn GTy TG | 1351–1367 |
| GGTH-r | CAG GAA ACA GCT ATG ACC GAn GGn GGn ACn CA | 1177–1190 |
| UP2r-21 | TGT AAA ACG ACG GCC AGT rTC nAC rTC nGC rTC nGT CAT | 1831–1850 |
| 675-T | AGA TCA AGC GCG ACG GGT AT | 656–675 |
| 756-G | GAA GAC GGG GTC AAC GGT G | 738–756 |
| 756-A | GAA GAC GGG GTC AAC GGT A | 738–756 |
| 1410-G | CCA GTG GGT CAG CTG TTC G | 1410–1428 |
| 1410-A | CCA GTG GGT CAG CTG TTC A | 1410–1428 |
| 1450-C | CCT TGT TCA CAA CGA CTT TCG C | 1450–1471 |
| 1450-A | CCT TGT TCA CAA CGA CTT TCG A | 1450–1471 |
| MTUBf | TCG GAC GCG TAT GCG ATA TC | 574–593 |
| MTUBr | ACA TAC AGT TCG GAC TTG CG | 1594–1613 |
| KG632f | GGT GTC TCG GTG GTC AAC GC | 613–632 |
| MK962r | GAC CTT GTG CGG GGC GGC GG | 962–981 |
| MG962r | CAC CTT GTG GGG GGC GGT GA | 962–981 |
Locations of primers are shown by the nucleotide number of gyrB of M. tuberculosis (GenBank accession number L27512).
Determination of ITS sequences.
The ITS sequences were determined by using the primers Ec16S.1390p (5′-TTGTACACACCGCCCGTC-3′) and Mb23S.44n (5′-TCTCGATGCCAAGGCATCCACC-3′) (20). The amplification reaction was subjected to 35 cycles with a denaturation step at 95°C for 1 min, an annealing step at 63°C for 1 min, and an extension step at 72°C for 1 min. The amplified DNA was purified, and its sequence was determined by using Ec16S.1390p and Mb23S.44n as sequence primers.
ETR typing.
Exact tandem repeat (ETR) typing was performed by using the combination of primers designated ETR-A and heat-treated crude lysates as DNA templates (7). The amplification reaction was subjected to 35 cycles with a denaturation step at 95°C for 1 min, an annealing step at 60°C for 1 min, and an extension step at 72°C for 2 min. Amplified fragment was analyzed by 1.5% agarose gel electrophoresis for typing.
Analysis of sequence data.
The gyrB sequences were aligned by using CLUSTAL W 1.7 (29), and the alignment was manually corrected. Phylogenetic analyses were performed by the PHYLIP version 3.5c package (6). Distance matrices based on Kimura's two-parameter model (12) were produced by using the DNADIST program, and a neighbor-joining tree was constructed by using the NEIGHBOR program. The resulting trees were depicted by using the TreeView version 1.5 package (16). The stability of the grouping was assessed by bootstrapping with the SEQBOOT, DNADIST, NEIGHBOR, and CONSENSE programs. A total of 1,000 bootstrapped trees were generated. Pairwise distances of the gyrB or ITS sequences were calculated by using the MEGA version 1.0 package (13).
gyrB-based species-specific PCR for the M. tuberculosis complex.
The gyrB sequences of four type strains belonging to the M. tuberculosis complex were aligned, and the four sites at which base substitutions occurred were found. Species-specific primers whose 3′ ends were at the base substitution sites were designed and named 675-T, 756-G, 756-A, 1410-G, 1410-A, 1450-C, and 1450-A (Table 2). The PCR reactions were performed in a final volume of 20 μl containing 1 μl of a boiled bacterial suspension, a reaction buffer (GeneAmp Kit; Perkin-Elmer), 1.25 U of AmpliTaq Gold DNA polymerase, a 0.1 mM concentration of each deoxynucleoside triphosphate, and 100 pmol of each primer. The reaction was subjected to 35 cycles of amplification, with a denaturation step at 95°C for 1 min and an annealing-extension step at 72°C for 1.5 min, by using a Progene thermal cycler (Techne). The amplified fragments were analyzed by 1.5% agarose gel electrophoresis.
gyrB-based species-specific PCR for M. kansasii and M. gastri.
The gyrB sequences of the type strains of M. kansasii and M. gastri were aligned with those of other mycobacterial and related species. The sequences specific to M. kansasii and M. gastri were then found. Species-specific primers whose 3′ ends corresponded to the species-specific sequences were designed and named KG632f, MK962r, and MG962r (Table 2). The PCR reactions were performed in a final volume of 20 μl containing 1 μl of a boiled bacterial suspension, a reaction buffer (GeneAmp Kit), 1.25 U of AmpliTaq Gold DNA polymerase, a 0.1 mM concentration of each deoxyribonucleoside triphosphate, and 100 pmol of each primer. The reaction was subjected to 30 cycles of amplification, with a denaturation step at 95°C for 1 min and an annealing-extension step at 68°C for 1.5 min, by using a Progene thermal cycler (Techne). The amplified fragments were analyzed by 1.5% agarose gel electrophoresis.
PCR-restriction fragment length polymorphism (RFLP) analysis.
The mycobacterial GyrB amino acid sequences were aligned, and two regions specific to the M. tuberculosis complex were found. Primers, which were designed from the specific amino acid sequences were named MTUBf and MTUBr (Table 2) and used for PCR. The PCR reaction was performed under the same conditions as those for species-specific PCR already described. The amplified fragments were digested with RsaI and TaqI, the digested fragments being analyzed by 2.0% agarose gel electrophoresis.
DNA-DNA hybridization.
The levels of DNA-DNA relatedness were determined fluorometrically by the method of Ezaki et al. (4) by using biotinylated DNA.
Nucleotide sequence accession numbers.
The gyrB and ITS sequences determined in this study have been deposited in DDBJ (DNA DataBank of Japan) under the accession numbers shown in Table 1.
RESULTS AND DISCUSSION
Phylogenetic analysis of slowly growing mycobacteria based on their gyrB sequences.
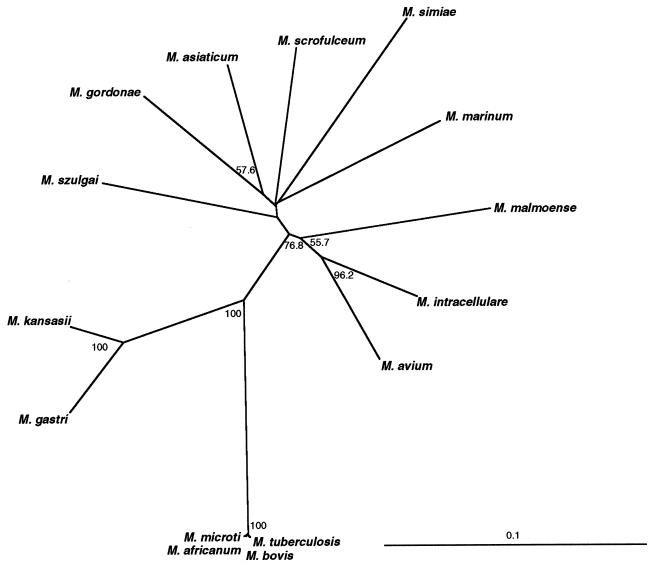
Primers UP1TL and UP2rTL were used to amplify ∼1.2-kb gyrB fragments by PCR from 15 mycobacterial species, and their nucleotide sequences were determined. The sizes of the amplified gyrB fragments were 1,257 bp for the M. tuberculosis complex, M. kansasii, and M. gastri, while those for the other species were longer by 6 bp. The alignment of the translated sequences showed that two residues were inserted in the N-terminal region: the amino acid sequences of the N terminus were Ser-Asp-(Ser or Ala)-Tyr-Ala-Ile-Ser for M. kansasii, M. gastri, and the M. tuberculosis complex, whereas they were Gly-Glu-Asn-Ser-Gly-Tyr-(Asn or Thr)-Val-Ser for the other species. The overall amino acid sequence identities ranged from 82 to 99%. The pairs showing more than 90% identity were M. kansasii and M. gastri (94%), M. avium and M. intracellulare (91%), and four species of the M. tuberculosis complex (99%). The other pairs showed <90% identity. The unrooted phylogenetic tree (Fig. 1) showed that the 15 mycobacterial species were well resolved by their gyrB sequences. Especially, branches of medically important species, such as M. kansasii, M. gastri, M. avium, M. intracellulare, and M. tuberculosis complex, were supported by high bootstrap values. The results indicated that the gyrB sequences would be useful for differentiation of those species.
FIG. 1.
Unrooted tree based on the gyrB sequences and showing the relationship between type strains of slowly growing mycobacteria. A neighbor-joining dendrogram was constructed. The numbers on the dendrogram are the percentages of occurrence in 1,000 bootstrapped trees; only values of >50% are shown.
As shown in Table 3, the resolution in the gyrB-based phylogeny was better than that in the ITS-based phylogeny, with some exceptions. The ITS sequence can evolve faster than 16S rDNA, as this region does not encode any biologically functional molecules, except for a role in pre-rRNA processing, and this sequence has been proposed to be suitable for differentiating closely related organisms (2, 9). In fact, Roth et al. (20) have shown that M. kansasii and M. gastri could be distinguished by using their ITS sequences. However, the ITS sequences of four species belonging to the M. tuberculosis complex were 100% identical. This means that the four species of the M. tuberculosis complex appear to have diverged quite recently, as has been indicated by Sreevatsan et al. (23).
TABLE 3.
Pairwise distance values for the gyrB sequences (lower left) and 16S-23S rDNA spacer (ITS) sequences (upper right)
| Strain | Pairwise distance in strain:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T704 | T021 | T801 | T901 | KPM 2201 | KPM 2403 | KPM 1001 | KPM 3504 | KPM 1201 | ATCC 25274 | KPM 3012 | KPM 3101 | KPM 2027 | KPM 3401 | KPM 1403 | |
| M. bovis T702 | 0.0000 | 0.0000 | 0.0000 | 0.1888 | 0.1205 | 0.1205 | 0.1285 | 0.1285 | 0.1526 | 0.2048 | 0.2016 | 0.1486 | 0.1526 | 0.1727 | |
| M. tuberculosis T021 | 0.0024 | 0.0000 | 0.0000 | 0.1888 | 0.1205 | 0.1205 | 0.1285 | 0.1395 | 0.1526 | 0.2048 | 0.2016 | 0.1486 | 0.1526 | 0.1727 | |
| M. africanum T801 | 0.0016 | 0.0008 | 0.0000 | 0.1888 | 0.1205 | 0.1205 | 0.1285 | 0.1395 | 0.1526 | 0.2048 | 0.2016 | 0.1486 | 0.1526 | 0.1727 | |
| M. microti T901 | 0.0024 | 0.0016 | 0.0008 | 0.1888 | 0.1205 | 0.1205 | 0.1285 | 0.1395 | 0.1526 | 0.2048 | 0.2016 | 0.1486 | 0.1526 | 0.1727 | |
| M. gordonae KPM 2201 | 0.1756 | 0.1739 | 0.1748 | 0.1756 | 0.1325 | 0.1205 | 0.1365 | 0.1004 | 0.1365 | 0.2008 | 0.1727 | 0.1727 | 0.1767 | 0.1446 | |
| M. szulgai KPM 2403 | 0.1715 | 0.1699 | 0.1707 | 0.1715 | 0.1230 | 0.0602 | 0.0683 | 0.0723 | 0.0964 | 0.1526 | 0.1406 | 0.1124 | 0.1165 | 0.1285 | |
| M. kansasii KPM 1001 | 0.1472 | 0.1448 | 0.1456 | 0.1448 | 0.1594 | 0.1610 | 0.0643 | 0.0683 | 0.0964 | 0.1687 | 0.1446 | 0.1205 | 0.1285 | 0.1245 | |
| M. gastri KPM 3504 | 0.1505 | 0.1497 | 0.1505 | 0.1497 | 0.1739 | 0.1675 | 0.0526 | 0.0723 | 0.1044 | 0.1365 | 0.1285 | 0.1004 | 0.1044 | 0.1205 | |
| M. marinum KPM 1201 | 0.1780 | 0.1764 | 0.1772 | 0.1780 | 0.1254 | 0.1262 | 0.1586 | 0.1683 | 0.1004 | 0.1687 | 0.1325 | 0.1245 | 0.1285 | 0.1084 | |
| M. asiaticum ATCC 25274 | 0.1739 | 0.1723 | 0.1731 | 0.1739 | 0.1011 | 0.1100 | 0.1537 | 0.1586 | 0.1173 | 0.1566 | 0.1285 | 0.1365 | 0.1526 | 0.1084 | |
| M. avium KPM 3012 | 0.1667 | 0.1650 | 0.1659 | 0.1667 | 0.1262 | 0.1295 | 0.1392 | 0.1553 | 0.1262 | 0.1214 | 0.0482 | 0.0884 | 0.1044 | 0.1205 | |
| M. intracellulare KPM 3101 | 0.1642 | 0.1626 | 0.1634 | 0.1642 | 0.1270 | 0.1141 | 0.1481 | 0.1602 | 0.1254 | 0.1116 | 0.0801 | 0.0803 | 0.0924 | 0.0924 | |
| M. scrofulaceum KPM 2027 | 0.1739 | 0.1723 | 0.1731 | 0.1739 | 0.1116 | 0.1214 | 0.1561 | 0.1675 | 0.1214 | 0.1133 | 0.1157 | 0.1084 | 0.0522 | 0.0884 | |
| M. malmoense KPM 3401 | 0.1650 | 0.1634 | 0.1642 | 0.1650 | 0.1481 | 0.1343 | 0.1578 | 0.1659 | 0.1497 | 0.1351 | 0.1222 | 0.1116 | 0.1303 | 0.1205 | |
| M. simiae KPM 1403 | 0.1820 | 0.1804 | 0.1812 | 0.1804 | 0.1343 | 0.1497 | 0.1634 | 0.1715 | 0.1392 | 0.1311 | 0.1432 | 0.1481 | 0.1319 | 0.1610 | |
Identification of M. kansasii and M. gastri by gyrB-targeted species-specific PCR.
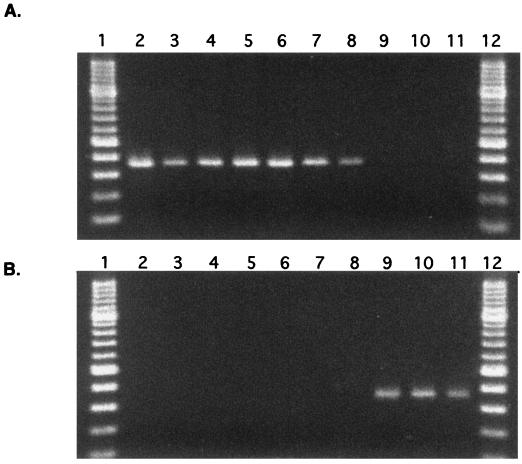
The gyrB sequences of the type strains of M. gastri and M. kansasii were 94.7% identical (1,191 of 1,257 bp). All except five substitutions were synonymous. The gyrB sequences of eight clinical isolates were determined to identify intraspecies variations in the gyrB sequences. The taxonomic identification of these isolates was done by DNA-DNA hybridization experiments: six of them were identified to be M. kansasii, and the remaining two were determined to be M. gastri. The gyrB sequences of these isolates were 100% identical to those of the type strains. Two stretches of DNA were found to be specific to M. kansasii and/or M. gastri when their gyrB sequences were aligned with those of 350 strains of high G+C gram-positive bacteria (H. Kasai and S. Harayama, unpublished data). Their locations corresponded to positions 613 to 632 and positions 962 to 981 of the M. tuberculosis gyrB sequence (GenBank accession number L27512). A set of primers adapted to these two specific sequences was designed. The forward primer was complementary to both the M. kansasii and M. gastri sequences, while two types of reverse primers, one specific to M. kansasii and the other specific to M. gastri, were prepared. As shown in Fig. 2, M. kansasii and M. gastri could be distinguished by PCR with these primer sets.
FIG. 2.
PCR for the amplification of gyrB to detect M. kansasii and M. gastri. Panels A and B show the specificity of M. kansasii-specific primers and of M. gastri-specific primers, respectively. Lanes: 1 and 12, 100-bp ladder molecular size markers; 2 and 9, PCR products from DNA of the type strains of M. kansasii and M. gastri; 3 to 8, PCR products from the cell lysate of clinical isolates identified as M. kansasii; 10 and 11, PCR products from the cell lysate of clinical isolates identified as M. gastri. The length of each amplified fragment was 368 bp.
gyrB sequences of strains in the M. tuberculosis complex.
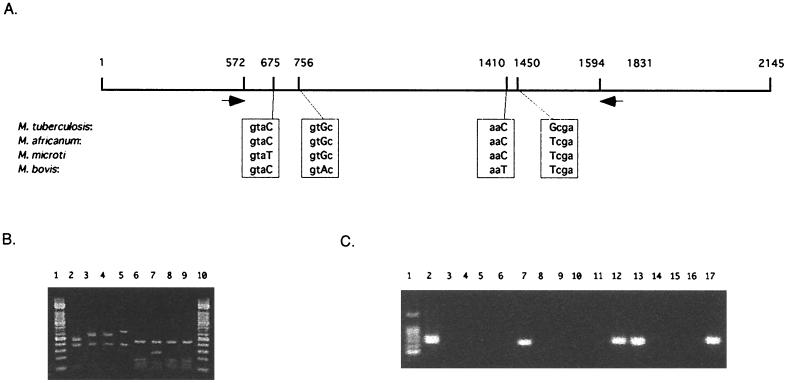
We determined the gyrB sequences of the type strains of M. tuberculosis, M. microti, M. africanum, and M. bovis. The obtained sequences were compared with each other, and we found substitutions at four different sites (Fig. 3A). At position 675, the gyrB sequence of M. microti was T, and the gyrB sequences of the other strains were C. At position 756, the gyrB sequence of M. bovis was A, and the gyrB sequences of the other strains were G. At position 1410, the gyrB sequence of M. bovis was T, and the gyrB sequences of the other strains were C. At position 1450, the gyrB sequence of M. tuberculosis was G, and the gyrB sequences of the other strains were T. All the substitutions were synonymous, three being transitions and one being a transversion. These substitutions are unlikely to have developed under the selective pressure of therapeutic drug treatment because drug resistance is acquired by nonsynonymous substitution. Therefore, these substitutions can be regarded as the result of naturally occurring divergent evolution of these members. Synonymous substitution may thus be useful to deduce the natural relationship among pathogenic bacteria since they are not subjected to selection by antibiotics.
FIG. 3.
Differentiation of members of the M. tuberculosis complex by using PCR and PCR-RFLP. (A) gyrB sequences of the members of the M. tuberculosis complex. The sequences of polymorphic loci are enclosed in boxes, and capital letters indicate substituted sequences. Black arrows show the positions of primers for the PCR-RFLP analysis. (B) PCR-RFLP patterns obtained with the type strains of the M. tuberculosis complex. Lanes: 1 and 10, 100-bp ladder molecular size markers; 2 to 9, RFLP patterns of PCR products from M. microti (lanes 2 and 6), M. tuberculosis (lanes 3 and 7), M. africanum (lanes 4 and 8), and M. bovis (lanes 5 and 9). Lanes 2, 3, 4, and 5 show RFLP patterns obtained by RsaI digestion; lanes 6, 7, 8, and 9 show RFLP patterns obtained by TaqI digestion. (C) Species-specific PCR amplification. Lanes: 1, 100-bp ladder molecular size markers; 2, 6, 10, and 14, PCR products from M. tuberculosis DNA; 3, 7, 11, and 15, PCR products from M. bovis DNA; 4, 8, 12, and 16, PCR products from M. microti DNA; 5, 9, 13, and 17, PCR products from M. africanum DNA; 2, 3, 4, and 5, PCR with 210-G and 442-C; 6, 7, 8, and 9, PCR with 756-A and 1410-A; 10, 11, 12, and 13, PCR with 756-G and 1410-A; 14, 15, 16, and 17, PCR with 675-T and 1410-G.
M. tuberculosis complex-specific PCR and differentiation of the members by PCR-RFLP.
A two-step method for differentiating the strains in the M. tuberculosis complex was developed. The first step involves detecting the M. tuberculosis complex, while the second step involves the differentiation of four species of the M. tuberculosis complex. The GyrB sequences of the M. tuberculosis complex were aligned with those of other mycobacterial species, and two unique sequences for the M. tuberculosis complex were found. A pair of primers, MTUB-f and MTUB-r, was then designed for specific amplification of the partial gyrB sequences from the M. tuberculosis complex. With these primers, the gyrB sequences were amplified from M. tuberculosis, but not from M. kansasii, M. gastri, M. abscessus, M. chelonae, or M. triviale (data not shown). The length of the amplified fragments was 1,020 bp. At the second step, the amplified DNA fragments were digested by RsaI or TaqI. As shown in Fig. 3B, M. bovis (lane 2) and M. microti (lane 5) could be differentiated from the other two species by the electrophorogram of the RsaI-digested fragments. Fragments of 500 and 700 bp can be found in the cases of M. bovis and M. microti, respectively, while 600-bp fragments were generated by RsaI digestion of the PCR products from M. tuberculosis and M. africanum. TaqI digestion of the M. tuberculosis gyrB fragment generated a 300-bp fragment, while a 150-bp fragment was generated from the other three species. Thus, RFLP analysis of the gyrB fragment could distinguish four species belonging to the M. tuberculosis complex.
Differentiation of members of the M. tuberculosis complex by species-specific PCR.
We were also able to differentiate the members of the M. tuberculosis complex by species-specific PCR. We designed PCR primers that allowed selective amplification of the gyrB fragments from each species of the M. tuberculosis complex. The sequences and their positions are listed in Table 2. According to the designs of these primers, the gyrB fragment of M. tuberculosis could be amplified only by using the primer set of 756-G and 1410-C, the gyrB fragment of M. bovis could be amplified only by using the primer set of 756-A and 1410-A, the gyrB fragment of M. africanum could be amplified only by using the primer set of 756-G and 1410-A, and the gyrB fragment of M. microti could be amplified by using both the 756-G and 1450-A set and the 675-T and 1410-G set. Combination of these primers enabled four species of the M. tuberculosis complex to be differentiated (Fig. 3C).
gyrB sequences of clinically isolated M. tuberculosis strains.
To evaluate the general applicability of the gyrB-based identification method, we analyzed the gyrB sequences of 18 M. tuberculosis strains isolated from patients, one M. bovis strain isolated from a cow, and the Japanese reference strain of BCG. Each M. tuberculosis strain was isolated from a different patient. They were classified into five different groups based on the variable numbers of tandem repeats in the ETR-A locus (7). Group 1 contained two strains (KPM KY590 and KPM 631), group 2 also contained two strains (KPM KY682 and KPM 721), and groups 3 and 4 each contained a single strain (KPM KY673 and KPM 679, respectively). The remaining 12 strains belonged to group 5. Thus, the strains studied were derived from at least five independent origins. The M. tuberculosis-specific substitutions found in the gyrB sequence of the type strain were conserved in all of the clinical isolates. Both the gyrB sequences of M. bovis isolated from a cow and the Japanese BCG strain were identical to that of the type strain of M. bovis, although the origins of these three strains were different. These results suggest that the synonymous substitutions found in M. tuberculosis and M. bovis are species specific. Besides these three common substitutions, we found three strain-specific base substitutions in the four M. tuberculosis strains. In the gyrB sequence of strain KPM KY673, the A at position 883 was found to be substituted by C, provoking the amino acid substitution from Ala to Pro. The A at position 1114 was found to be substituted by G in strain KPM KY679, while the G at position 1116 was found to be substituted by C in strains KPM KY678 and KPM H37Rv (GenBank accession number L27512). The latter two changes occurred in the same codon, ATG, provoking a change in the amino acid sequence from Met to Val or Ile. These two amino acid substitutions are found at positions that are highly conserved among the GyrB proteins of high G+C gram-positive bacteria. It is thus possible that the amino acid changes at these sites could confer some drug resistance to the strains.
Medical applications of gyrB sequences.
Many clinically significant strains are classified as slowly growing mycobacteria. As shown in this study, more substitutions were found in the gyrB sequences than in the 16S rDNA and ITS sequences of slowly growing mycobacterial strains. With the benefit of higher base substitution frequencies, PCR or PCR-RFLP of gyrB could be used for the species identification of clinically isolated mycobacteria. These methods are highly applicable to clinical medicine because DNA sequencing is not required for rapid identification of these species.
The gyrB-based methods have already shown to be useful for differentiation of closely related strains of other bacteria, such as Vibrio (33) and Bacillus (39) spp. For quantitation analysis, too, the gyrB-based methods would be more useful than 16S rDNA-based methods because the copy number of gyrB is single in almost all of the bacteria examined (35, 36), while that of 16S rDNA is variable. We have determined the gyrB sequences of various bacteria and deposited them in the gyrB database (11), which recently became accessible via the internet (http://www.mbio.co.jp/icb/icb.html/). The sequence data accumulated in the gyrB database will be useful for the development of bacterial diagnostic systems based on molecular methods such as PCR, PCR-RFLP, and high-density DNA probe arrays (8, 30).
ACKNOWLEDGMENTS
We are grateful to Isao Kawamoto for helpful suggestions. We thank Yukiko Itazawa and Yuhka Takahashi for their technical assistance.
This work was performed as part of The Industrial Science and Technology Frontier Program supported by New Energy and Industrial Technology Development Organization.
REFERENCES
- 1.Baess I. Deoxyribonucleic acid relatedness among species of slowly-growing mycobacteria. Acta Pathol Microbiol Scand. 1979;87:221–226. doi: 10.1111/j.1699-0463.1979.tb02430.x. [DOI] [PubMed] [Google Scholar]
- 2.Barry T, Colleran G, Glennon M, Dunican L K, Gannon F. The 16S/23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1991;1:51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Comicini S, Barbarini D, Telecco S, Bonno L, Marone P. Rapid identification of Mycobacterium tuberculosis and Mycobacterium avium by polymerase chain reaction and restriction enzyme analysis within sigma factor regions. New Microbiol. 1998;21:391–395. [PubMed] [Google Scholar]
- 4.Ezaki T, Hashimoto Y, Yabuuchi E. Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int J Syst Bacteriol. 1989;39:224–229. [Google Scholar]
- 5.Feizabadi M M, Robertson I D, Cousins D V, Hampson D J. Genomic analysis of Mycobacterium bovis and other members of the Mycobacterium tuberculosis complex by isozyme analysis and pulsed-field gel electrophoresis. J Clin Microbiol. 1996;34:1136–1142. doi: 10.1128/jcm.34.5.1136-1142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felsenstein J. PHYLIP (Phylogeny Inference Package), version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 7.Frothingham R, Meeker-O'Connell W A. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology. 1998;144:1189–1196. doi: 10.1099/00221287-144-5-1189. [DOI] [PubMed] [Google Scholar]
- 8.Gingeras T R, Ghandour G, Wang E, Berno A, Small P M, Drobniewski F, Alland D, Desmond E, Holodniy M, Drenkow J. Simultaneous genotyping and species identification using hybridization pattern recognition analysis of generic Mycobacterium DNA arrays. Genome Res. 1998;8:435–448. doi: 10.1101/gr.8.5.435. [DOI] [PubMed] [Google Scholar]
- 9.Gürtler V, Stanisich V A. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology. 1996;142:3–16. doi: 10.1099/13500872-142-1-3. [DOI] [PubMed] [Google Scholar]
- 10.Harayama S, Yamamoto S. Phylogenetic identification of Pseudomonas strains based on a comparison of gyrB and rpoD sequences. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C.: ASM Press; 1996. pp. 250–258. [Google Scholar]
- 11.Kasai H, Watanabe K, Elisabeth G, Bairoch A, Isono K, Harayama S. Genome informatics 1998. Tokyo, Japan: Universal Academy Press; 1998. Construction of the gyrB database for identification and classification of bacteria; pp. 13–21. [PubMed] [Google Scholar]
- 12.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetics analysis software for microcomputers. Comput Appl Biosci. 1994;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 14.Magdalena J, Supply P, Locht C. Specific differentiation between Mycobacterium bovis BCG and virulent strains of the Mycobacterium tuberculosis complex. J Clin Microbiol. 1998;36:2471–2476. doi: 10.1128/jcm.36.9.2471-2476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noordhoek G T, Kolk A H, Bjune G, Catty D, Dale J W, Fine P E. Sensitivity and specificity of PCR for detection of Mycobacterium tuberculosis isolates: a blind comparison study among seven laboratories. J Clin Microbiol. 1994;32:277–284. doi: 10.1128/jcm.32.2.277-284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 17.Plikaytis B B, Plikaytis B D, Yakurus M A, Bulter W R, Woodley C L, Silcox V A, Shinnick T M. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism. J Clin Microbiol. 1992;30:1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez J G, Mejia G A, Portillo P D, Patarroyo M E, Murillo L A. Species-specific identification of Mycobacterium bovis by PCR. Microbiology. 1995;141:2131–2138. doi: 10.1099/13500872-141-9-2131. [DOI] [PubMed] [Google Scholar]
- 19.Rogall T, Wolters J, Floher T, Böttger E C. Towards a phylogeny and definition of the species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990;40:323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- 20.Roth A, Fischer M, Hamid M E, Michalke S, Ludwig W, Mauch H. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J Clin Microbiol. 1998;36:139–147. doi: 10.1128/jcm.36.1.139-147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivannavar C T, Katoch V M, Sharma V D, Patil M A, Katoch K, Bharadwaj V P, Sharma R K, Bhatia A S, Agrawal B M. Determination of mycobacterial phylogeny on the basis of immunological relatedness of superoxide dismutases. Int J Syst Bacteriol. 1996;46:1164–1169. doi: 10.1099/00207713-46-4-1164. [DOI] [PubMed] [Google Scholar]
- 22.Soini H, Böttger E C, Viljanen M K. Identification of mycobacteria by PCR-based sequence determination of the 32-kilodalton protein gene. J Clin Microbiol. 1994;32:2944–2947. doi: 10.1128/jcm.32.12.2944-2947.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreevatsan S, Pan X, Stockbauer K E, Connel N D, Kreiswirth B N, Whittam T S, Musser J M. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;94:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stahl D A, Urbance J W. The division between fast- and slowly growing species corresponds to natural relationships among the mycobacteria. J Bacteriol. 1990;172:116–124. doi: 10.1128/jb.172.1.116-124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson D S, Kapur V, Stockbauer K, Pan X, Frothingham R, Musser J M. Subspecific differentiation of Mycobacterium avium complex strains by automated sequencing of a region of the gene (hsp65) encoding a 65-kilodalton heat shock protein. Int J Syst Bacteriol. 1997;47:414–419. doi: 10.1099/00207713-47-2-414. [DOI] [PubMed] [Google Scholar]
- 26.Takewaki S, Okuzumi K, Ishiko H, Nakahata K, Ohkubo A, Nagai R. Genus-specific polymerase chain reaction for the mycobacterial dnaJ gene and species-specific oligonucleotide probes. J Clin Microbiol. 1993;31:446–450. doi: 10.1128/jcm.31.2.446-450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takewaki S, Okuzumi K, Manabe I, Tanimura M, Miyamura K, Nakahata K, Yazaki Y, Ohkubo A, Nagai R. Nucleotide sequence comparison of the mycobacterial dnaJ gene and PCR-restriction fragment length polymorphism analysis for identification of mycobacterial species. Int J Syst Bacteriol. 1994;44:159–166. doi: 10.1099/00207713-44-1-159. [DOI] [PubMed] [Google Scholar]
- 28.Telenti A, Marchesi F, Bals M, Bally F, Böttger E C, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson J D, Higgins D G, Gibson T J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troesch A, Nguyen H, Miyada C G, Desvarenne S, Gingeras T R, Kaplan P M, Cros P, Mabilat C. Mycobacterium species identification and rifampin resistance testing with high-density DNA probe arrays. J Clin Microbiol. 1999;37:49–55. doi: 10.1128/jcm.37.1.49-55.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Soolingen D, Hoogenboezem T, de Haas P E W, Hermans P W M, Koedam M A, Teppema K S, Brennan P J, Besra G S, Portaels F, Top J, Shouls L M, van Embden J D A. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int J Syst Bacteriol. 1997;47:1236–1245. doi: 10.1099/00207713-47-4-1236. [DOI] [PubMed] [Google Scholar]
- 32.van Soolingen D, van der Zanden A G M, de Haas P E W, Noordhoek G T, Kiers A, Foudraine N A, Portaels F, Kolk A H J, Kremer K, van Embden J D A. Diagnosis of Mycobacterium microti infections among humans by using novel gene markers. J Clin Microbiol. 1998;36:1840–1845. doi: 10.1128/jcm.36.7.1840-1845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkateswaran K, Dohmoto N, Harayama S. Cloning and nucleotide sequence of the gyrB gene of Vibrio parahaemolyticus and its application in detection of this pathogen in shrimp. Appl Environ Microbiol. 1998;64:681–687. doi: 10.1128/aem.64.2.681-687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Victor T C, Jordaan A M, van Schalkwyk E J, Coetzee G J, van Helden P D. Strain-specific variation in the dnaJ gene of mycobacteria. J Med Microbiol. 1996;44:332–339. doi: 10.1099/00222615-44-5-332. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Yamamoto S, Hino S, Harayama S. Population dynamics of phenol-degrading bacteria in activated sludge determined by gyrB-targeted quantitative PCR. Appl Environ Microbiol. 1998;64:1203–1209. doi: 10.1128/aem.64.4.1203-1209.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe K, Teramoto M, Harayama S. An outbreak of nonflocculating catabolic populations caused by the breakdown of a phenol-digesting activated-sludge process. Appl Environ Microbiol. 1999;65:2813–2819. doi: 10.1128/aem.65.7.2813-2819.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wayne L G, Kubica G P. The mycobacteria. In: Sneath P H A, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams and Wilkins Co.; 1986. pp. 1435–1457. [Google Scholar]
- 38.Wayne L G, Good R C, Krichevsky M I, Blacklock Z, David H L, Dawson D, Gross W, Hawkins J, Jenkins P A, Juhlin I, Käppler W, Kleeberg H H, Levy-Frebault V, McDurmont C, Nel E E, Portaels F, Rüsch-Gerdes S, Schröder K H, Silcox V A, Szabo I, Tsukamura M, van den Breen L, Vergmann B, Yakrus M A. Third report of the cooperative, open-ended study of slowly growing mycobacteria by the International Working Group on Mycobacterial Taxonomy. Int J Syst Bacteriol. 1989;39:267–278. doi: 10.1099/00207713-41-4-463. [DOI] [PubMed] [Google Scholar]
- 39.Yamada S, Ohashi E, Agata N, Venkateswaran K. Cloning and nucleotide sequence analysis of gyrB of Bacillus cereus, B. thuringiensis, B. mycoides, and B. anthracis and their application to the detection of B. cereus in rice. Appl Environ Microbiol. 1999;65:1483–1490. doi: 10.1128/aem.65.4.1483-1490.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto S, Harayama S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol. 1995;61:1104–1109. doi: 10.1128/aem.61.3.1104-1109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto S, Harayama S. Phylogenetic analysis of Acinetobacter strains based on the nucleotide sequences of gyrB genes and on the amino acid sequences of their products. Int J Syst Bacteriol. 1996;46:506–511. doi: 10.1099/00207713-46-2-506. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto S, Bouvet P J M, Harayama S. Phylogenetic structures of the genus Acinetobacter based on the gyrB sequences: comparison with the grouping by DNA-DNA hybridization. Int J Syst Bacteriol. 1999;49:87–95. doi: 10.1099/00207713-49-1-87. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto S, Harayama S. Phylogenetic relationships of Pseudomonas putida strains deduced from the nucleotide sequences of gyrB, rpoD, and 16S rRNA genes. Int J Syst Bacteriol. 1998;48:813–819. doi: 10.1099/00207713-48-3-813. [DOI] [PubMed] [Google Scholar]
- 44.Zolg J W, Philippi-Schulz S. The superoxide dismutase gene, a target for detection and identification of mycobacteria by PCR. J Clin Microbiol. 1994;32:2801–2812. doi: 10.1128/jcm.32.11.2801-2812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]