Abstract
A recombinant Lyme borreliosis vaccine consisting of outer surface protein A (OspA) is commercially available for vaccination of humans against infection with Borrelia burgdorferi. Vaccination with OspA induces an antibody response that makes serologic interpretation of infection with B. burgdorferi difficult, especially by screening tests based on whole-cell preparations of B. burgdorferi. We show that an enzyme-linked immunosorbent assay with B. burgdorferi sensu stricto 50772, which lacks the plasmid encoding OspA and OspB, or a full-length recombinant OspC protein can identify patients infected with B. burgdorferi. We found that 69 and 65% of serum samples from patients with case-defined early Lyme borreliosis had anti-B. burgdorferi sensu stricto 50772 and anti-OspC reactivities, respectively. In addition, little or no reactivity was detected with sera obtained from individuals vaccinated with OspA. Unfortunately, 51 and 33% of sera from healthy patients and sera from patients with other illnesses were also reactive against B. burgdorferi sensu stricto 50772 and OspC, respectively. Although these assays can discriminate B. burgdorferi infection from vaccination with OspA, their lack of specificity highlights the necessity for confirming equivocal or positive reactivities with more specific serodiagnostic tests.
Lyme borreliosis, a multisystem illness caused by transmission of Borrelia burgdorferi sensu lato from Ixodes sp. ticks, is the most common vector-borne disease in the United States (5). Most cases of Lyme borreliosis occur in the northeastern and upper midwestern United States; however, cases have now been reported from 49 states. The widespread occurrence of cases of Lyme borreliosis has increased demand for serodiagnostic testing procedures with sufficient sensitivities and specificities to accurately detect infection with B. burgdorferi sensu lato. To date, a single sensitive and highly specific laboratory test is not widely available (2). In an effort to lower the rates of false-positive and false-negative serologic results, the Centers for Disease Control and Prevention (CDC) has advocated the use of a two-tiered approach for serodiagnosis of Lyme borreliosis (6, 7, 13). The first tier consists of a sensitive screening test such as an enzyme-linked immunosorbent assay (ELISA) or an indirect fluorescent-antibody test (IFA), followed by confirmation by Western blotting (WB).
Public concern about Lyme borreliosis has also stimulated efforts to develop an effective vaccine. Recent clinical trials of two Lyme borreliosis vaccines based on outer surface protein A (OspA) (23, 24) demonstrated that they could prevent Lyme borreliosis. These findings prompted the Food and Drug Administration (FDA) to approve a first-generation OspA vaccine for general use in 15- to 70-year-old individuals. Vaccination against Lyme borreliosis will likely become commonplace because of widespread public demand, despite recommendations to vaccinate only individuals at high risk of contracting the illness (25). The OspA vaccine provides protection in less than half of recipients before completion of the vaccine schedule of three injections over the course of 2 years. Thereafter, 78% of recipients are protected from infection, although the duration of protection is unknown. In addition, antigenically variant strains of B. burgdorferi sensu lato are found in the United States (18), and infection with these spirochetes could occur after vaccination. Thus, it is likely that individuals will still be evaluated for Lyme borreliosis, despite vaccination.
Serodiagnosis by the conventional two-tiered approach will be confounded in these patients because most screening tests use B. burgdorferi sensu lato which hyperexpress OspA, and vaccination induces seroreactivity against this protein. Therefore, false-positive reactivities will become more frequent. The necessity of monitoring the vaccination histories of individuals before performing a serologic evaluation will generate more confusion and further complicate the serodiagnosis of Lyme borreliosis.
In this investigation, we evaluated the performances of two ELISAs that may be useful as screening tests to more accurately detect early infection with B. burgdorferi. The sensitivity and specificity of the ELISA procedures with B. burgdorferi sensu stricto, which lacks the OspA and OspB genes, and a recombinant OspC were evaluated with serum samples from human subjects participating in a Lyme disease vaccine trial, patients with early Lyme borreliosis, and patients with other unrelated illnesses.
MATERIALS AND METHODS
Lyme disease sera.
Fifty-two serum samples from patients with Lyme borreliosis were obtained from Gundersen Lutheran Medical Center in La Crosse, Wis.; New York Medical College, Westchester County, N.Y.; or the New England Medical Center, Boston, Mass. All serum samples were from patients with clinically documented or culture-confirmed erythema migrans lesions.
Normal and potentially cross-reactive sera.
Normal sera were collected from 28 healthy adult volunteers 18 to 60 years of age residing in an area where Lyme borreliosis is endemic (3). Evidence of past exposure to B. burgdorferi was not detectable in 17 serum samples, while 11 serum samples had an IFA titer of 1:64 or more. Sera were also obtained from 26 individuals vaccinated and boosted with 30 μg of OspA during a phase III Lyme borreliosis vaccine study (23). Prior to enrollment, the sera of these participants were screened to ensure no serological evidence of previous exposure to B. burgdorferi sensu lato. After completion of the vaccination schedule, the sera of the vaccinees contained significant concentrations of anti-OspA antibodies (enzyme immunoassay range, 0.204 to 0.852 absorbance units). Other potentially cross-reactive sera were obtained from patients with cytomegalovirus antibodies (n = 30), Epstein-Barr virus (EBV) antibodies (n = 35), antinuclear antibodies (n = 10), or antibodies against Treponema pallidum (n = 15). Donors with Lyme borreliosis, healthy subjects, or donors of potentially cross-reactive sera had not received antimicrobial therapy during the previous 30 days.
Organisms.
B. burgdorferi sensu stricto B-31 or 50772, which lacks ospA and ospB and which expresses large amounts of OspC (1, 19), was grown to a concentration of approximately 5 × 107 organisms/ml in BSK medium at 35°C. After examination by dark-field microscopy, 500 μl aliquots were dispensed into 1.5-ml screw-cap tubes (Sarstedt, Newton, N.C.), sealed, and stored at −70°C until they were used. Escherichia coli JM109 (Promega, Madison, Wis.) and E. coli DH5α (Gibco BRL, Gaithersburg, Md.) were used in all cloning experiments.
Purification of recombinant OspC.
E. coli containing ospC was grown in 100 ml of 2× TY broth (19) containing ampicillin (100 μg/ml; Sigma Chemical Co., St. Louis, Mo.) for 12 h at 37°C, diluted 1:10 with 2× TY broth, and incubated for 1 h. Isopropyl-β-d-thiogalactopyranoside (final concentration, 0.1 μM; Sigma) was added to the culture, and the culture was incubated for an additional 4 h. The suspension was then centrifuged at 10,000 × g for 15 min at 4°C, resuspended in purification buffer containing 50 mM Tris (pH 8.0), 50 mM NaCl, 2 mM EDTA, and 0.1% Triton X-100, and lysed with a sonicator (model W350; Branson Sonic Power, Danbury, Conn.). The sonicated E. coli cells were centrifuged at 10,000 × g for 15 min, and the supernatant was passed over a column containing SoftLink resin (Promega) at a rate of 0.5 ml/min at 4°C. The column was then washed with 5 column volumes of purification buffer. Finally, OspC was eluted with 5 mM biotin (Sigma), and the recovered fractions were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE).
SDS-PAGE.
Eluted fractions containing OspC were boiled for 5 min, and 6 μg of total protein was loaded into individual wells of a 12% SDS-polyacrylamide gel. Protein concentrations were determined with a protein determination kit (Bio-Rad, Richmond, Calif.). The gels were run in an electrophoresis unit (SE600; Hoefer Scientific, San Francisco, Calif.) at 55 mA for 3 h with the buffer system of Laemmli (14). After electrophoresis, the gels were stained with 0.125% Coomassie blue.
ELISAs.
B. burgdorferi sensu stricto B-31 or 50772 was grown in BSK medium at 35°C to a density of approximately 5 × 107 organisms/ml. The spirochetes were centrifuged at 10,000 × g for 15 min, resuspended in phosphate-buffered saline (PBS), and disrupted with a sonicator. The protein concentrations were determined with a protein determination kit (Bio-Rad). Recombinant OspA was also prepared as described previously (17). Purified recombinant OspA, OspC, sonicated B. burgdorferi sensu stricto B-31, or sonicated B. burgdorferi sensu stricto 50772 was diluted to 1 μg/ml in coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3 [pH 9.6]); and 100-μl amounts were added to individual flat-bottom amine-binding microtiter wells (Costar, Cambridge, Mass.). The microtiter plates were incubated overnight at 4°C. After incubation, the plates were washed three times with PBS (pH 7.2) and blocked with PBS containing 0.05% Tween 20 (Sigma) and 1% bovine serum albumin (Sigma) for 1 h at room temperature with shaking. After blocking, the plates were washed again with PBS. Subsequently, 100-μl amounts of each patient serum sample diluted 1:100 in PBS-Tween was added to individual wells of OspC plates. The serum was diluted 1:400 in PBS-Tween before adding 100-μl amounts to individual wells of plates containing B. burgdorferi sensu stricto B-31 or 50772. The plates were incubated for 1 h at room temperature. Following incubation, the plates were washed three times with PBS, 100 μl of anti-human immunoglobulin M (IgM) or IgG horseradish peroxidase conjugate (Organon Teknika Cappel, Durham, N.C.) diluted 1:3,000 in PBS-Tween was added to each well, and the plates were reincubated at room temperature for 1 h. The plates were then washed three times with PBS, 100 μl of o-phenylenediamine phosphate (0.4 mg/ml; Sigma) was added to each well, and the plates were allowed to incubate at room temperature for 30 min. The reactions were stopped by adding 100 μl of 1 N H2SO4, and the absorbances at 490 nm (model EL 311; Bio-Tek Inc., Winooski, Vt.) were immediately determined.
Determination of sensitivity and interassay variation.
The success of the two-tiered system for the serodiagnosis of Lyme borreliosis is dependent on testing with a sensitive screening test followed by confirmation with a specific confirmatory test (13). Therefore, we set the cutoff value for a positive test as an absorbance at 490 nm of 0.05. The absorbance was set at 0.00 for the control sera from healthy subjects. To monitor interassay variation, a serum sample from a patient with early Lyme borreliosis containing anti-OspC antibodies (19) was included as a positive control. By using the ELISA for the detection of OspC antibodies, the positive control serum sample had mean optical densities of 0.898 (standard deviation, 0.014; coefficient of variation [CV], 1.5%) and 0.559 (standard deviation, 0.054; CV, 9.7%) for IgM and IgG, respectively. When B. burgdorferi sensu stricto 50772 was used, the mean optical densities were 0.719 (standard deviation, 0.114; CV, 15.8%) and 0.089 (standard deviation, 0.044; CV, 49.6%) for IgM and IgG, respectively.
Statistics.
Paired or unpaired Student's t tests were used to examine ELISA reactivities. P values less than or equal to 0.05 were considered significant.
RESULTS
Reactivities of sera from OspA vaccinees.
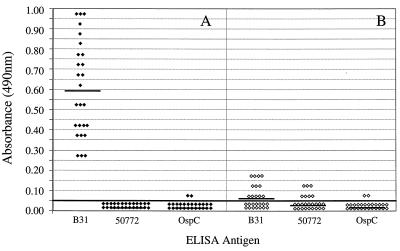
We determined the ELISA reactivities of B. burgdorferi sensu stricto B-31, an OspA-expressing organism frequently used as a serodiagnostic antigen (15), B. burgdorferi sensu stricto 50772, which expresses OspC (19) but not OspA, and OspC with serum from healthy individuals previously vaccinated and boosted with 30 μg of OspA. Low levels of IgM seroreactivity were detected in 12 (46%) of 26 serum samples from vaccinees when the ELISA for detection of B. burgdorferi sensu stricto B-31 was used (Fig. 1). IgM seroreactivity against B. burgdorferi sensu stricto 50772 was also detectable in seven (26%) serum samples; however, the mean reactivity was significantly (P < 0.05) decreased compared to the mean reactivity against B. burgdorferi sensu stricto B-31. When OspC was used, only two (8%) serum samples were reactive. The mean reactivity was also less (P = 0.06) than that detected with either B. burgdorferi sensu stricto B-31 or B. burgdorferi sensu stricto 50772. In contrast, 26 (100%) serum samples from vaccinees had significant levels of IgG antibodies against B. burgdorferi sensu stricto B-31. However, anti-OspA IgG antibodies were not detected when B. burgdorferi sensu stricto 50772 was used, and only two (8%) serum samples were slightly reactive against OspC.
FIG. 1.
IgG (A) or IgM (B) ELISA reactivities of sera from OspA vaccinees with B. burgdorferi sensu stricto B31, B. burgdorferi sensu stricto 50772, or OspC. Horizontal bars indicate the mean optical densities. The continuous horizontal bold line denotes the cutoff value for a positive test result.
Detection of early Lyme borreliosis.
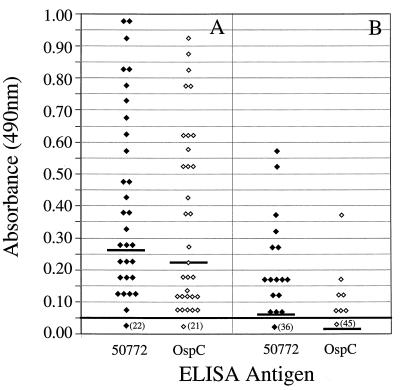
We next determined the abilities of B. burgdorferi sensu stricto 50772 and OspC to detect early Lyme borreliosis. Anti-B. burgdorferi sensu stricto 50772 or anti-OspC reactivities were detected in 36 (69%) and 34 (65%) of 52 serum samples from patients with early Lyme borreliosis, respectively (Fig. 2). Thirty (58%) of these serum samples had IgM antibodies and 16 (31%) serum samples had IgG antibodies against B. burgdorferi sensu stricto 50772. OspC detected IgM or IgG antibodies in 31 (60%) and 7 (13%) serum samples from patients with early Lyme borreliosis, respectively. In addition, the mean IgM and IgG reactivities by either ELISA did not differ significantly (P = 0.395). Thus, the abilities of B. burgdorferi sensu stricto 50772 or OspC to detect serologic evidence of infection with B. burgdorferi sensu lato during early Lyme borreliosis were similar.
FIG. 2.
IgM (A) or IgG (B) ELISA reactivities of sera from patients with case-defined early Lyme borreliosis when B. burgdorferi sensu stricto 50772 (⧫) or OspC (◊) was used. Horizontal bars indicate the mean optical densities. Numbers in parentheses indicate the numbers of serum samples that had reactivities of 0.05 or less. The continuous horizontal bold line denotes the cutoff value for a positive test result.
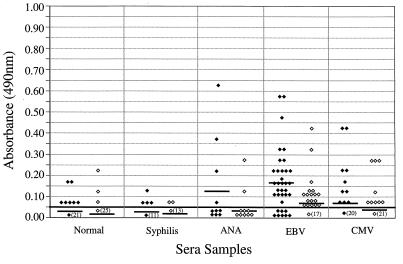
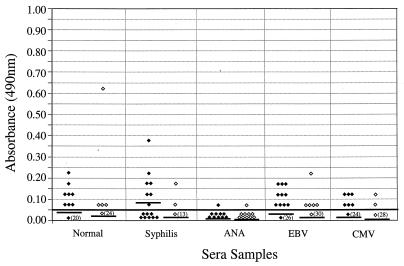
Reactivity of potentially cross-reactive sera.
We tested 28 serum samples from healthy individuals and 90 serum samples from patients with syphilis, antinuclear antibodies, cytomegalovirus antibodies, or EBV antibodies for IgM or IgG reactivity to B. burgdorferi sensu stricto 50772 and OspC (Fig. 3 and 4). In general, cross-reactivity was detected more frequently in serum samples evaluated for IgM reactivity (Fig. 3) than in serum samples evaluated for IgG reactivity (Fig. 4). Specifically, higher IgM reactivities were found in serum samples with antinuclear antibodies or antibodies to EBV and cytomegalovirus. When normal and syphilitic sera were compared for IgM and IgG reactivities, no significant differences were detected. In addition, no significant differences were detected when B. burgdorferi sensu stricto 50772 or OspC was used except with sera obtained from patients with EBV infection or syphilis. Higher levels of IgM reactivities were detected in patients with EBV antibodies when B. burgdorferi sensu stricto 50772 (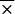 = 0.175) was used than when OspC was used (
= 0.175) was used than when OspC was used (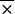 = 0.75). Likewise, IgG reactivity was elevated in sera from patients with syphilis when B. burgdorferi sensu stricto 50772 (
= 0.75). Likewise, IgG reactivity was elevated in sera from patients with syphilis when B. burgdorferi sensu stricto 50772 (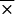 = 0.083) was used than when OspC was used (
= 0.083) was used than when OspC was used (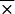 = 0.013).
= 0.013).
FIG. 3.
IgM ELISA reactivities of sera from healthy subjects (n = 28), syphilitic sera (n = 15), sera containing antinuclear antibodies (ANA; n = 10), and sera from patients infected with EBV (n = 35) or cytomegalovirus (CMV; n = 30) when the B. burgdorferi sensu stricto 50772 (⧫) or OspC (◊) ELISA was used. Horizontal bars indicate the mean optical densities. Numbers in parentheses indicate the numbers of serum samples that had reactivities of 0.05 or less. The continuous horizontal bold line denotes the cutoff value for a positive test result.
FIG. 4.
IgG ELISA reactivities of sera from healthy subjects (n = 28), syphilitic sera (n = 15), sera containing antinuclear antibodies (ANA; n = 10), and sera from patients infected with EBV (n = 35) or cytomegalovirus (CMV; n = 30) when B. burgdorferi sensu stricto 50772 (⧫) or OspC (◊) was used. Horizontal bars indicate the mean optical densities. Numbers in parentheses indicate the numbers of serum samples that had reactivities of 0.05 or less. The continuous horizontal bold line denotes the cutoff value for a positive test result.
DISCUSSION
The recent approval by FDA of an OspA vaccine is an important first step toward the prevention of Lyme borreliosis. The vaccine will likely be administered widely, especially in the upper midwestern and northeastern United States, where Lyme borreliosis is endemic. Vaccination should significantly reduce the morbidity associated with Lyme borreliosis. Unfortunately, the FDA-approved OspA vaccine was less than 50% effective in preventing infection with B. burgdorferi sensu lato after two injections and only 78% effective after the third injection (24). Sigal et al. (23) also reported efficacy of only 68% after two inoculations with another OspA Lyme borreliosis vaccine which is awaiting FDA approval. Other major concerns with OspA vaccination are the duration of protective immunity (17) and the number of booster vaccinations required to maintain sustained high levels of protective borreliacidal antibodies. There are reports of infections in humans (21), dogs (27), and rabbits (10) after vaccination. These results suggest that some OspA vaccinees will become infected with B. burgdorferi sensu lato. Accurate serodiagnosis will be severely compromised in these individuals, and the diagnostic uncertainty will be increased when patients with illness associated with a tick bite are evaluated.
Many clinical laboratories in the United States use the CDC-recommended two-tiered approach for the serodiagnosis of Lyme borreliosis recommended at the Second National Conference on the Serologic Diagnosis of Lyme Borreliosis (6). By this approach, serum is first screened by using a sensitive ELISA or IFA. To confirm the serodiagnosis of Lyme borreliosis, equivocal and positive serum specimens are then tested by a more specific IgM and IgG WB procedure (8, 9). Vaccination with OspA increases the number of false-positive results by the current ELISA and IFA screening procedures because most laboratories use B. burgdorferi sensu stricto isolates that express large amounts of OspA (2). If the number of OspA vaccinees becomes large, the increased cost of confirmatory testing will likely become prohibitive. A screening test that can discriminate between infection with B. burgdorferi sensu lato and vaccination is needed.
In this study, we evaluated the ability of B. burgdorferi sensu stricto 50772 and OspC ELISAs to detect early Lyme borreliosis. B. burgdorferi sensu stricto 50772 does not possess the ospA or ospB gene (1) but expresses relatively high concentrations of OspC (19). B. burgdorferi sensu stricto 50772 and OspC were chosen because of their ability to detect anti-OspC antibodies, which are among the first antibodies detectable during early Lyme borreliosis (8, 9, 11, 13, 19). Schwan et al. (22) and others (26) demonstrated that B. burgdorferi sensu lato upregulates OspC and concomitantly downregulates OspA shortly before spirochetes are transmitted to the host. Consequently, the detection of anti-OspC antibodies should be a reliable indicator of early infection with B. burgdorferi sensu lato, and detection should not be affected by vaccination with OspA.
We demonstrated that both B. burgdorferi sensu stricto 50772 and OspC could be used to detect early Lyme borreliosis. Sixty-nine percent and 65% of serum samples from patients with case-defined or culture-confirmed early Lyme borreliosis had anti-B. burgdorferi sensu stricto 50772 or anti-OspC reactivities, respectively. Magnarelli et al. (16) and Gerber et al. (12) also demonstrated that anti-OspC antibodies could be used to detect early Lyme borreliosis with sensitivities of 52 and 46%, respectively. Furthermore, we showed that little or no reactivity was detected in sera from individuals vaccinated with OspA when B. burgdorferi sensu stricto 50772 or OspC was used as the detection antigen. Recently, Zhang et al. (28) also demonstrated that an OspA-deficient B. burgdorferi sensu lato isolate could discriminate vaccination from infection.
Despite the elimination of OspA, B. burgdorferi sensu stricto 50772 and OspC were still highly nonspecific antigens. Similar numbers of false-positive reactions occurred with sera from healthy subjects, serum samples containing antinuclear antibodies, and sera from individuals infected with cytomegalovirus. In addition, B. burgdorferi sensu stricto 50772 was significantly more reactive with sera from patients with syphilis and EBV infection. The mean absorbance values for sera from patients with EBV infection did not differ significantly from the absorbance values obtained with sera from patients with early Lyme borreliosis. This is likely due to polyclonal stimulation of B cells by EBV (20). Some antibodies, for example, antibodies to the flagellar protein of B. burgdorferi sensu lato, may cause the cross-reactivity. The cross-reactivity observed with syphilitic serum is of less concern because clinicians can differentiate patients on the basis of clinical symptoms and treponemal antibody-specific tests. However, the high degree of cross-reactivity and the inability of B. burgdorferi sensu stricto 50772 to discriminate early Lyme disease from EBV infection may cause some confusion. Patients with EBV infection can present with clinical signs and symptoms similar to those of patients with Lyme borreliosis. Therefore, the OspC protein may be a more specific serodiagnostic antigen.
Collectively, our results and those of Zhang et al. (28) suggest that laboratories can eliminate cross-reactivity caused by vaccination against Lyme borreliosis by modifying screening tests used in the first tier of the two-tiered approach. OspC or B. burgdorferi sensu stricto 50772 ELISAs did not significantly react with serum from subjects vaccinated against Lyme disease. These tests also detected antibodies in over 60% of sera from patients with early Lyme borreliosis. However, both tests were highly nonspecific. The lack of specificity highlights the necessity of confirming positive findings by more specific serodiagnostic assays.
The most common confirmatory test for Lyme borreliosis is WB. It is important, however, that OspA vaccination may also increase the complexity of interpretation of WB. Most WB procedures also use OspA-expressing B. burgdorferi sensu lato as antigen. Detection of reactivities may be obscured by OspA that does not migrate as a single band but that reacts with anti-OspA antibody from vaccinees. Another option for confirming Lyme borreliosis serodiagnostically is by detection of borreliacidal antibodies. This procedure would not be affected by vaccination. We recently showed that viable B. burgdorferi sensu stricto 50772 could also be used to detect highly specific (>95%) borreliacidal antibodies without decreasing sensitivity (72%) by using a flow cytometric borreliacidal antibody test (4). In this method, viable B. burgdorferi sensu stricto 50772 is incubated with sera from patients with early Lyme borreliosis in the presence of complement. The enhanced specificity is due to the detection of only the antibodies that can specifically kill B. burgdorferi. In contrast, bound cross-reactive antibodies are readily detected on non-viable B. burgdorferi sensu stricto 50772 when exposed to conjugated anti-IgM or anti-IgG in the ELISA or IFA. Additional studies are being performed to determine whether this approach could eliminate the necessity of the two-tiered testing system.
REFERENCES
- 1.Anderson J F, Flavell R A, Magnarelli L A, Barthold S W, Kantor F S, Wallich R, Persing D H, Mathiesen D, Fikrig E. Novel Borrelia burgdorferi isolates from Ixodes scapularis and Ixodes dentatus ticks feeding on humans. J Clin Microbiol. 1996;34:524–529. doi: 10.1128/jcm.34.3.524-529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakken L L, Callister S M, Wand P J, Schell R F. Interlaboratory comparison of test results for detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists Proficiency Testing Program. J Clin Microbiol. 1997;35:537–543. doi: 10.1128/jcm.35.3.537-543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callister S M, Agger W A, Schell R F, Ellingson J L E. Borrelia burgdorferi infection surrounding La Crosse, Wis. J Clin Microbiol. 1988;26:2632–2636. doi: 10.1128/jcm.26.12.2632-2636.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callister S M, Jobe D A, Schell R F, Pavia C S, Lovrich S D. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a “gold standard”? Clin Diagn Lab Immunol. 1996;3:399–402. doi: 10.1128/cdli.3.4.399-402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Lyme disease surveillance—United States. Morbid Mortal Weekly Rep. 1995;45:481–484. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Recommendations for test performance and interpretations from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 7.Craven R B, Quan T J, Bailey R E, Dattwyler R, Ryan R W, Sigal L H, Steere A C, Sullivan B, Johnson B J B, Dennis D T, Gubler D J. Improved serodiagnostic testing for Lyme diseases: results of a multicenter serologic evaluation. Emerg Infect Dis. 1996;2:136–140. doi: 10.3201/eid0202.960211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 9.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley D M, Wang Y P, Wu X Y, Blanco D R, Lovett M A, Miller J N. Acquired resistance to Borrelia burgdorferi infection in the rabbit. Comparison between outer surface protein A vaccine- and infection-derived immunity. J Clin Invest. 1997;99:2030–2035. doi: 10.1172/JCI119371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: a role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber M A, Shapiro E D, Bell G L, Sampieri A, Padula S J. Recombinant outer surface protein C ELISA for the diagnosis of early Lyme disease. J Infect Dis. 1995;171:724–727. doi: 10.1093/infdis/171.3.724. [DOI] [PubMed] [Google Scholar]
- 13.Johnson B J B, Robbins K E, Bailey R E, Cao B L, Sviat S L, Craven R B, Mayer L W, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Magnarelli L A, Anderson J F, Johnson R C, Nadelman R B, Wormser G P. Comparison of different strains of Borrelia burgdorferi sensu lato used as antigens in enzyme-linked immunosorbent assays. J Clin Microbiol. 1994;32:1154–1158. doi: 10.1128/jcm.32.5.1154-1158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnarelli L A, Fikrig E, Padula S J, Anderson J F, Flavell R A. Use of recombinant antigens of Borrelia burgdorferi in serologic test for diagnosis of Lyme borrelosis. J Clin Microbiol. 1996;34:237–240. doi: 10.1128/jcm.34.2.237-240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padilla M L, Callister S M, Schell R F, Bryant G L, Jobe D A, Lovrich S D, DuChateau B K, Jensen J R. Characterization of the protective borreliacidal antibody response in humans and hamsters after vaccination with a Borrelia burgdorferi outer surface protein A vaccine. J Infect Dis. 1996;174:739–746. doi: 10.1093/infdis/174.4.739. [DOI] [PubMed] [Google Scholar]
- 18.Picken R N, Cheng Y, Han D, Nelson J A, Reddy A G, Hayden M K, Picken M A, Strle F, Bouseman J K, Trenholme G M. Genotypic and phenotypic characterization of Borrelia burgdorferi isolated from ticks and small animals in Illinois. J Clin Microbiol. 1995;33:2304–2315. doi: 10.1128/jcm.33.9.2304-2315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousselle J C, Callister S M, Schell R F, Lovrich S D, Jobe D A, Marks J A, Wieneke C A. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J Infect Dis. 1998;178:733–741. doi: 10.1086/515382. [DOI] [PubMed] [Google Scholar]
- 20.Schooley R T. Epstein-Barr virus (infectious mononucleosis) In: Mandell G L, Bennett J E, Dolin R, editors. Infectious diseases. New York, N.Y: Churchill Livingstone; 1990. [Google Scholar]
- 21.Schutzer S E, Luan J, Coyle P K. Detection of Lyme disease after OspA vaccine. N Engl J Med. 1997;337:794–795. doi: 10.1056/NEJM199709113371118. [DOI] [PubMed] [Google Scholar]
- 22.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein of Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Malawista S E the Recombinant Outer-Surface Protein A Lyme Disease Study Consortium. A vaccine consisting of recombinant Borrelia burgdorferi outer surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 24.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S the Lyme Disease Vaccine Study Group. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 25.Steigbigel R T, Benach J L. Immunization against Lyme disease—an important first step. N Engl J Med. 1998;339:263–264. doi: 10.1056/NEJM199807233390409. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straubinger R K, Chang Y, Johnson R H, Appel M J G. Sera from OspA-vaccinated dogs, but not those from tick-infected dogs, inhibit in vitro growth of Borrelia burgdorferi. J Clin Microbiol. 1995;33:2745–2751. doi: 10.1128/jcm.33.10.2745-2751.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Mathiesen D, Kolbert C P, Anderson J, Schoen R T, Fikrig E, Persing D H. Borrelia burgdorferi enzyme-linked immunosorbent assay for discrimination of OspA vaccination from spirochete infection. J Clin Microbiol. 1997;35:233–238. doi: 10.1128/jcm.35.1.233-238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]