Abstract
The optimization of antioxidant and anti-tyrosinase activity during jellyfish hydrolysate preparation was studied using a response surface methodology (RSM) with a face-centered composite design. The influence of the hydrolysis duration and the enzyme concentration on the IC50 of the DPPH and ABTS radical scavenging activity, ferric-reducing antioxidant power (FRAP), the degree of hydrolysis (DH), yield, and the IC50 value of tyrosinase inhibitory activity were determined. The optimum conditions for the production of jellyfish hydrolysate using alcalase (JFAH), flavourzyme (JFFH), or papain (JFPH) were achieved at hydrolysis times of 360, 345, or 360 min, respectively, and at an enzyme concentration of 5.0%. JFFH had the highest antioxidant and tyrosinase inhibitory activity. JFAH, JFFH, and JFPH concentrations of 2.5 mg/mL resulted in HaCaT cells (IC80) having a survival rate of 80%. The amino acid profile of JFFH contained about 43% hydrophobic and 57% hydrophilic amino acids, comprising Gly, Cys, Glx, Asx, which were dominant. The isolation of a peptide fraction from JFFH was carried out using ultrafiltration membranes (10, 3, and 1 kDa) and gel filtration chromatography. Fraction-III (1–3 kDa) showed the highest antioxidative and tyrosinase inhibitory activity.
Keywords: antioxidant activity, anti-tyrosinase activity, protein hydrolysate, jellyfish, alcalse, flavourzyme, papain, Lobonema smithii
1. Introduction
Tyrosinase, a copper-containing molecule, is an essential enzyme in melanin formation that accelerates the generation of melanin from tyrosine via oxidation. The enzyme is predominantly involved in two melanin production reactions, i.e., the monophenolase reaction involving the hydroxylation of tyrosine and the diphenolase reaction for the oxidation of 3,4-dihydroxyl-l-phenylalanine (l-DOPA) to dopaquinone [1]. Melanin is a pigment that is found in bacteria, fungals, plants, and mammals. Tyrosinase activity is necessary for the provision of melanosomes into keratinocytes, and melanogenesis modulators can directly affect this activity [2]. Therefore, limiting tyrosinase activity in cosmetics and medicine can prevent the browning of foods and melanin synthesis [3]. The development and screening of tyrosinase inhibitors to be used as cosmetics products and food additives has been of interest in the industrial sector [4,5,6].
Edible jellyfish have been consumed throughout Southeast Asia for more than a thousand years [7,8]. The total world capture production of edible jellyfish in 2015–2018 was estimated to be ~300,000 tonnes/year [9]. Most commercial jellyfish are processed using a traditional method involving alum salt dehydration before producing the product as being canned in brine, semi-dried, or dry-salted [8]. White jellyfish (Lobonema smithii) are one of the main edible species of jellyfish in Asia. In Thailand, it is used to produce a salted jellyfish product for both domestic consumption and for export to other consumer countries, i.e., Taiwan, China, Malaysia, South Korea, and Japan, with a value about USD 10 million annually [10,11]. Jellyfish are abundant in collagenous protein, have a high nutritional value, and is capable of pharmacological activities that might imply a wider range of applications in the food, cosmetic, and pharmaceutical industries [7,12]. In China, it has been utilized for the treatment of bronchitis, high blood pressure, tracheitis, asthma, and gastric ulcers [13]. Type I collagen and type A gelatin from jellyfish have been prepared and characterized for further applications [11,14,15]. Based on the supply and potential health benefits, jellyfish may be used to develop functional ingredients for health foods, including nutraceutical products.
Protein hydrolysates are chemically or enzymatically produced and yield free amino acids or short peptides. Hydrolysis using enzymes has been utilized to obtain nutritional and bioactive peptides [16,17]. The antioxidant and tyrosinase inhibition activity of protein hydrolysates and peptides from marine resources have shown strong antioxidant activity and have been applied as natural antioxidants [18,19]. They have also been shown to be tyrosinase inhibitors with low adverse side effects [4]. Jellyfish collagen hydrolysates and peptides demonstrate antioxidant activity [7,12,20,21], melanogenesis inhibition [12], tyrosinase inhibition [21], anti-fatigue activities [7], UV protection [14], and angiotensin converting enzyme (ACE) inhibitory activity [22]. However, these peptides from white jellyfish have not been characterized, nor have preparative methods been maximized for tyrosinase inhibitory and antioxidant activities.
The aim of this research was to develop value-added products from low-value salted white jellyfish from Thailand for potential applications in the food, cosmetics, and pharmaceutical industries. Therefore, the production of jellyfish hydrolysates with tyrosinase inhibitory and antioxidant activity was optimized using enzymatic hydrolysis and peptide isolation.
2. Materials and Methods
2.1. Raw Material and Preparation
Alum-salted jellyfish (Lobonema smithii) was obtained from Siam Jellyfish Co., Ltd., Samut Sakhon, Thailand. It was contained in a sealed high-density polyethylene (HDPE) bucket (size 18 kg). To desalt the jellyfish, the sample was initially cleaned under running tap water and was soaked in water (jellyfish:water = 1:2) overnight (12–14 h). The soaked jellyfish was cleaned under running tap water until the salt content in wash water was approximately zero. The salt content was measured using a salinometer (Master-S28 M, Atago®, Tokyo, Japan), and the water was drained for 30 min to obtain the desalted jellyfish. The jellyfish were ground using a blender (Daily Collection HR7627 Blender, Philips, Bangkok, Thailand) and were packed in Zip-lock polyethylene bags and stored at −18 to −20 °C until use (<2 months).
2.2. Chemical and Enzymes
Papain (≥3 activity units (AU)/mg), Alcalase 2.4 L[≥2.4 (AU)/g], flavourzyme (≥500 AU/g), tyrosinase from mushroom, 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferric chloride hexahydrate (FeCl3•6H2O), 2,2′azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,4,5-tripyridyl-triazine (TPTZ), 2,4,6-trinitrobenzenesulfonic acid (TNBS), kojic acid, and 3,4-dihydroxy-L-phenylalanine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS), penicillin, dulbecco’s modified eagle medium (DMEM), and l-glutamine were purchased from XL Biotec Co., Ltd. (Bangkok, Thailand). All of the chemicals and reagents used were of analytical grade.
2.3. Chemical Composition Analysis
The chemical composition of desalted jellyfish was determined according to the official AOAC method (2000): 934.01 moisture, 954.01 crude protein, 991.36 lipids, and 942.05 ash. Nitrogen was converted to crude protein using a conversion factor of 6.25.
2.4. Optimization of the Production of Jellyfish Hydrolysate (JFH)
The desalted jellyfish was hydrolyzed using three different proteases. The effect of the enzyme concentration (1–5% w/w protein content) and hydrolysis time (60–360 min) on the yield, DH, FRAP value, and antioxidant and tyrosinase inhibition activity were determined. For the hydrolysis process, a mixture of 50 g desalted jellyfish and 100 mL distilled water (w:v = 1:2) was terminated for endogenous enzymes by heating it at 95 °C for 15 min, cooling it to the optimum temperature of each enzyme (papain and flavozyme at 50 °C and alcalase at 60 °C), and hydrolyzing it following the experimental design. The reaction was carried out in a shaking incubator (Memmert WNB45, Schwabach, Germany) with constant agitation for 60–360 min (Table 1). After hydrolysis, the reaction of the hydrolysate solution was stopped by heating it at 95 °C for 15 min, and it was then cooled under running tab water. Thereafter, the hydrolysate solution was centrifuged at 5500× g for 15 min (1736R, LaboGene, Lynge, Denmark). The supernatant was lyophilized using a freeze-dryer (GRISRIANTHONG, GFD-3H, Samut Sakhon, Thailand). The jellyfish hydrolysate powder that was obtained was packed in Zip-lock polyethylene bags and kept at −20 °C until further investigation.
Table 1.
Experimental units and response variables for DH, yield, DPPH and ABTS radical scavenging activity, FRAP value, and tyrosinase inhibitory activity of jellyfish protein hydrolyzed using alcalase, flavourzyme, and papain.
| Enzyme | Treatment | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | %DH | %Yield | DPPH (IC50) (mg /mL) | ABTS (IC50) (mg /mL) |
FRAP
(mmol FeSO4 /g) |
Anti -Tyrosinase (IC50) (mg /mL) | ||
| Alcalase | 1 | 60 | 1 | 14.0 b ± 0.2 | 1.17 a ± 0.23 | 0.54 a ± 0.26 | 5.23 g ± 0.20 | 0.24 h ± 0.03 | 23.2 d ± 0.6 |
| 2 | 360 | 1 | 11.1 a ± 0.2 | 1.12 a ± 0.23 | 0.42 a ± 0.07 | 6.03 gh ± 1.69 | 0.19 e ± 0.03 | 22.9 c ± 0.5 | |
| 3 | 60 | 5 | 29.8 g ± 0.1 | 2.27 d ± 0.52 | 1.02 b ± 0.17 | 3.70 def ± 0.92 | 0.21 f ± 0.02 | 26.0 de ± 0.7 | |
| 4 | 360 | 5 | 25.5 e ± 0.1 | 2.93 f ± 0.80 | 2.15 cd ± 0.43 | 1.07 a ± 0.02 | 0.13 a ± 0.01 | 11.4 a ± 0.1 | |
| 5 | 60 | 3 | 28.4 f ± 0.1 | 2.03 d ± 0.32 | 2.35 d ± 0.18 | 3.17 de ± 0.57 | 0.22 g ± 0.07 | 13.7 a ± 1.0 | |
| 6 | 360 | 3 | 33.2 i ± 1.7 | 2.81 f ± 0.84 | 7.70 e ± 2.47 | 1.43 b ± 0.17 | 0.13 a ± 0.01 | 25.5 e ± 0.2 | |
| 7 | 210 | 1 | 19.7 c ± 0.1 | 1.43 b ± 0.12 | 1.50 bc ± 1.18 | 2.02 bc ± 1.63 | 0.19 e ± 0.03 | 30.8 g ± 0.7 | |
| 8 | 210 | 5 | 31.4 h ± 0.1 | 2.69 ef ± 0.46 | 0.92 ab ± 0.43 | 4.02 ef ± 1.04 | 0.17 c ± 0.02 | 28.9 fg ± 1.9 | |
| 9 | 210 | 3 | 28.2 f ± 0.1 | 2.97 f ± 0.11 | 2.40 d ± 0.36 | 4.63 f ± 0.23 | 0.14 b ± 0.01 | 23.9 d ± 0.1 | |
| 10 | 210 | 3 | 24.1 d ± 0.1 | 2.97 f ± 0.18 | 2.14 cd ± 0.27 | 5.21 g ± 0.40 | 0.13 a ± 0.01 | 25.7 de ± 1.8 | |
| 11 | 210 | 3 | 31.3 h ± 0.1 | 1.76 bc ± 0.32 | 1.14 b ± 0.08 | 2.97 c ± 0.30 | 0.18 d ± 0.02 | 20.5 b ± 0.1 | |
| Flavourzyme | 1 | 60 | 1 | 29.1 a ± 0.1 | 1.55 a ± 0.03 | 1.16 bc ± 0.28 | 11.1 ef ± 2.8 | 0.14 d ± 0.01 | 43.8 j ± 0.1 |
| 2 | 360 | 1 | 40.8 c ± 0.0 | 2.50 b ± 0.11 | 0.63 a ± 0.07 | 20.6 g ± 1.2 | 0.14 d ± 0.01 | 22.7 bc ± 0.5 | |
| 3 | 60 | 5 | 42.7 d ± 0.1 | 3.65 ef ± 0.24 | 2.22 e ± 0.88 | 9.76 e ± 0.91 | 0.13 c ± 0.01 | 29.4 f ± 0.5 | |
| 4 | 360 | 5 | 44.3 f ± 0.1 | 5.15 g ± 0.28 | 0.86 b ± 0.07 | 5.91 bc ± 0.14 | 0.14 d ± 0.01 | 13.5 cd ± 0.5 | |
| 5 | 60 | 3 | 43.0 e ± 0.0 | 2.88 b ± 0.14 | 1.75 d ± 0.24 | 7.74 d ± 0.88 | 0.14 d ± 0.01 | 30.1 gh ± 1.4 | |
| 6 | 360 | 3 | 45.7 g ± 0.3 | 3.97 f ± 0.15 | 2.57 ef ± 1.24 | 12.9 f ± 0.7 | 0.13 c ± 0.01 | 23.2 c ± 1.3 | |
| 7 | 210 | 1 | 43.1 e ± 0.1 | 3.30 c ± 0.12 | 2.41 ef ± 1.34 | 4.15 b ± 1.11 | 0.17 f ± 0.05 | 35.8 i ± 0.4 | |
| 8 | 210 | 5 | 38.1 b ± 0.1 | 5.63 g ± 1.18 | 4.66 h ± 0.17 | 2.49 a ± 0.31 | 0.15 e ± 0.02 | 33.5 h ± 0.2 | |
| 9 | 210 | 3 | 45.3 g ± 0.1 | 3.40 d ± 0.20 | 1.50 d ± 0.08 | 5.26 b ± 1.68 | 0.12 b ± 0.02 | 22.5 b ± 0.2 | |
| 10 | 210 | 3 | 44.9 f ± 0.1 | 3.57 e ± 0.12 | 2.71 f ± 0.41 | 6.03 c ± 0.14 | 0.11 a ± 0.02 | 20.7 a ± 0.7 | |
| 11 | 210 | 3 | 42.2 de ± 0.1 | 5.02 g ± 0.39 | 2.79 f ± 0.52 | 2.51 a ± 0.08 | 0.16 ef ± 0.06 | 24.4 e ± 0.8 | |
| Papain | 1 | 60 | 1 | 36.1 a ± 0.1 | 4.29 b ± 0.11 | 1.74 c ± 0.89 | 6.81 ef ± 0.47 | 0.14 a ± 0.00 | 46.2 h ± 1.5 |
| 2 | 360 | 1 | 37.1 b ± 0.1 | 5.41 e ± 0.09 | 1.60 bc ± 1.25 | 8.27 efg ± 1.10 | 0.15 b ± 0.01 | 29.4 f ± 0.4 | |
| 3 | 60 | 5 | 38.8 e ± 0.1 | 3.79 b ± 0.70 | 1.30 ab ± 0.57 | 3.42 b ± 0.16 | 0.16 c ± 0.07 | 31.8 g ± 0.1 | |
| 4 | 360 | 5 | 38.7 d ± 0.1 | 7.36 ij ± 0.40 | 0.89 a ± 0.26 | 5.12 c ± 0.77 | 0.16 c ± 0.01 | 24.2 d ± 0.7 | |
| 5 | 60 | 3 | 39.9 fg ± 0.0 | 2.70 a ± 0.92 | 0.87 a ± 0.24 | 2.65 a ± 0.01 | 0.13 a ± 0.01 | 28.4 e ± 0.1 | |
| 6 | 360 | 3 | 40.4 g ± 0.2 | 6.63 hi ± 0.44 | 1.79 cd ± 0.88 | 5.53 d ± 0.08 | 0.20 e ± 0.02 | 18.5 ab ± 0.5 | |
| 7 | 210 | 1 | 42.7 h ± 0.1 | 5.50 ef ± 0.75 | 1.40 b ± 0.20 | 5.28 cd ± 0.29 | 0.17 d ± 0.02 | 32.0 g ± 1.9 | |
| 8 | 210 | 5 | 37.8 c ± 0.1 | 7.91 j ± 0.16 | 1.95 d ± 1.30 | 5.03 c ± 0.25 | 0.20 e ± 0.02 | 22.5 c ± 1.0 | |
| 9 | 210 | 3 | 39.8 fg ± 0.1 | 6.12 fg ± 0.45 | 2.81 e ± 0.89 | 6.80 ef ± 0.46 | 0.17 d ± 0.01 | 17.5 a ± 0.9 | |
| 10 | 210 | 3 | 37.9 c ± 0.1 | 6.25 gh ± 0.23 | 2.94 f ± 0.41 | 6.70 ef ± 0.40 | 0.17 d ± 0.02 | 19.2 b ± 1.5 | |
| 11 | 210 | 3 | 39.6 f ± 0.2 | 7.05 i ± 0.35 | 1.99 d ± 0.32 | 6.67 e ± 0.57 | 0.16 c ± 0.01 | 19.5 b ± 0.2 | |
Note: Mean ± SD, X1: hydrolysis time (min), X2: enzyme concentration (%); different letters in the same column of each enzyme show significant differences (p ≤ 0.05).
2.5. The Degree of Hydrolysis (DH) Analysis
The DH of the jellyfish hydrolysate solution after hydrolysis was determined according to the method described by Mongkonkamthorn et al. [19]. The 125 µL sample was diluted with 2 mL Na3PO4 buffer (pH 8.2), and 1 mL TNBS solution (0.01% prepared with 0.2125 M phosphate buffer pH 8.2) was added. The mixture was shaken and kept in the dark at 50 °C for 30 min. After that, 2 mL of 0.1 M Na2SO3 was added into the mixture to terminate the activity, and the mixture was cooled at 25–27 °C for 15 min, and the absorbance of the mixture was measured at 420 nm using a microplate reader (VarioskanTM LUX, Thermo ScientificTM, Waltham, MA, USA). The α-amino group was estimated in terms of 2-Amino-4-methylpentanoic acid (l-leucine) (Sigma-Aldrich, St. Louis, MO, USA). The following equation was used to determine the DH (1).
| DH (%) = [(LH − L0)/(Lmax − L0)] × 100 | (1) |
where LH is the amount of Leu equivalence obtained from jellyfish hydrolysates. L0 is the amount of Leu equivalence in enzymatic hydrolysate at the initial time. Lmax is the total amount of Leu equivalence in the initial enzymatic hydrolysate taken after hydrolysis in 6 M hydrochloric acid at 100 °C for 12 h.
2.6. DPPH Radical Scavenging Activity
The scavenging activity of the DPPH radical was determined using the modified method of Ahn et al. [23]. The 100 µL sample was added 100 µL of DPPH solution (0.1 mM in 70% ethanol), and the mixture was kept in the dark at 25–27 °C for 30 min. Distilled water was used as the control and as a substitute sample. An absorbance was obtained at 517 nm using the microplate reader. The scavenging activity was expressed using Equation (2):
| Scavenging activity (%) = [(Acontrol − Asample)/Acontrol] × 100 | (2) |
Acontrol is the absorbance of the DPPH solution. Asample is the absorbance of the sample mixed with DPPH. The activity was presented as IC50 (sample concentration required to inhibit 50% of initial DPPH concentration). The IC50 value was estimated from the linear regression graph to optimize the process and µmol Trolox equivalents (TE)/g sample for the tested fractions.
2.7. ABTS Radical Scavenging Activity
The inhibition activity of the ABTS radical was determined in accordance with the method provided by Ahn et al. [23]. An ABTS solution (7 mM ABTS and 2.45 mM K2S2O8 were mixed together in a 1:1 (v/v) ratio) was prepared and allowed to activate in the dark for 16–18 h at 4 °C. Then, it was diluted with 70% ethanol to acquire an ABTS working solution (absorbance of 0.7 ± 0.05 at 734 nm). The 100 µL of the reaction mixture and 1.9 mL of the ABTS working solution was mixed and kept in the dark at 25–27 °C for 8 min. The resultant solution was obtained at 734 nm. The radical scavenging activity ABTS, and the IC50 value were calculated in a similar manner as in the DPPH analysis in order to optimize process and the µmol Trolox equivalents (TE)/g sample for the tested fractions.
2.8. Ferric Reducing Antioxidant Power (FRAP) Value
The FRAP value was measured following the method of Wangtueai et al. [24]. A fresh FRAP solution was produced by mixing of 20 mM FeCl3 and 6H2O in deionized water, 10 mM TPTZ solution in 40 mM HCl, and 300 mM acetate buffer (pH 3.6) at ratio of 1:1:10 (v:v:v). The FRAP solution was kept in a water bat at 37 °C for 30 min. An amount of 150 µL of the sample was mixed with 2.85 mL FRAP solution and was reacted for in the dark 30 min. The absorbance of the resultant solution was obtained at 593 nm. The FRAP values were expressed in mmol FeSO4/g sample. Additional dilutions were carried out if the FRAP value was at a higher range in the linear standard curve.
2.9. Tyrosianse Inhibitory Activity
The tyrosinase inhibition activity of jellyfish hydrolysate was measured according to a slightly modified version of the method discussed in Chan et al. [25]. A solution comprising 3.2 mM l-DOPA in 50 mM phosphate buffer (pH 6.8) was prepared. This reaction was generated in a multi-well plate. An amount of 10 µL of various concentrations of JFH was diluted with 40 µL of 50 mM K3PO4 buffer (pH 6.8) and 70 µL of tyrosinase (150 units/mL activity), and the mixture was allowed to react for 10 min. Then, 80 µL 3.2 mM L-DOPA was added to each well and incubated for another for 10 min. The absorbance of the resultant solution was measured at 475 nm using a multi-well plate reader. Kojic acid was used to as a positive control. The inhibitory activity of tyrosinase was expressed following Equation (3):
| Tyrosinase inhibitory activity (%) = {[ Acontrol − (Asample − Acolour)]/(Acontrol − Ablank)} × 100 | (3) |
where Ablank is the absorbance of deionized water. Acolour is an absorbance of a mixture comprising the sample with phosphate buffer. Acontrol is the absorbance of a mixture comprising buffer, tyrosinase, and L-DOPA without sample. Asample is the absorbance of solution resulting from the reaction.
The IC50 values were calculated in a similar manner as the DPPH analysis.
2.10. Amino Acid Analysis
The Amino acid composition of the jellyfish hydrolysate with flavourzyme hydrolysis (JFFH) powder was analyzed using HPLC (HP 1260, Fluorescence Detector, Agilent Technologies, Waldbronn, Germany) according to Herbert et al. [26]. The standard mixture of amino acids (Sigma-Aldrich, St. Louis, MO, USA) was applied for both identification and quantitation. The amino acid content was expressed in g amino acid/100 g of JFFH.
2.11. In Vitro Cytotoxicity Determination
Human keratinocytes cells (HaCaT cell line) were purchased from XL Biotec Co., Ltd. (Bangkok, Thailand). HaCaT cells were plated in DMEM containing 10% FBS, 2 mM glutamine, and 1% penicillin (100 U/mL) in a 5% CO2 atmosphere at 37 °C. The effects of jellyfish hydrolysate on HaCaT cell cytotoxicity and cell viability were measured using the MTT value determined by Pastorino et al. [27]. Briefly, HaCaT cells were seeded on a 96-well culture plate at 1 × 104 cell/well (100 µL) and were incubated 12 h to obtain cells that were attached to the substratum. The cells were treated with JFFH, JFAH, and JFPH at a various concentration (312.5, 625, 1250, 2500, and 5000 µg/mL), and some were left untreated (control) for 24 h at 37 °C. After incubation, 15 µL of the MTT solution (5 mg/mL in PBS) was added to each well. After subsequent incubation for 4 h, the purple-colored precipitates were obvious. The supernatant was removed, and the formazan precipitates were solubilized with the addition of 100 µL DMSO per well. Then, after 10 min of incubation, absorbances were obtained at 540 and 630 nm using a scanning multi-well microplate reader (SpectraMax i3x, Molecular Devices, CA, USA). The cell viability was expressed using Equation (4).
| % Cell viability = [(OD of treated)/(OD of control)] × 100 | (4) |
where OD treated is OD treated540 − OD treated630, OD control is OD control540 − OD control630.
2.12. Isolation of Antioxidant and Anti-Tyrosinase Peptides from Jellyfish Hydrolysate
2.12.1. Fractionation of Jellyfish Hydrolysate by Ultrafiltration
The JFFH were fractionated using an Amicon® stirred cell (Merck KGaA, Darmstadt, Germany) with an ultrafiltration membrane bioreactor system with a range of molecular weight cut-offs: 10, 3, and 1 kDa. Sample (10 g) was dissolved in deionized water (200 mL) (5% w:v) and fractionated using a nominal series of MWCO membranes: 1, 3 and 10 kDa. Four peptide fractions (fraction I (>10 kDa), fraction II (3–10 kDa), fraction III (1–3 kDa) and fraction IV (<1 kDa)) were collected and freeze dried. The JFFH fractions were analyzed to determine their antioxidant and tyrosinase inhibitory activity.
2.12.2. Gel Filtration Chromatography
The JFFH fraction with the highest anti-tyrosinase and highest antioxidant activity obtained from the ultrafiltration membrane was further separated using a gel filtration column (2.6 × 70 cm) with a Sephadex G-25 (GE Healthcare Bio-Science AB, Uppsala, Sweden). The low-pressure chromatography system (BioLogic LP system, Bio-Rad, Hercules, CA, USA) with a connected fraction collector (Biofrac Fraction Collector, Bio-Rad Laboratories) was used. In brief, 100 mg sample was dissolved in 2 mL distilled water, filtered through a 0.45 µm syringe filter PTFE (F13-PT045, Chrom Tech, MN, USA), injected onto a column, eluted with distilled water at a 0.5 mL/min flow rate, and collected with 3 mL eluted solution/fraction. The absorbances of the fractions were determined at 220 and 280 nm, as were the DPPH and ABTS radical scavenging activity, FRAP value, and tyrosinase inhibitory activity. The void volume of the column was measured using blue dextran (2000 kDa). Molecular-weight (MW) protein standards (tyrosine (181 Da), Gly-Try (238 Da), vitamin B12 (1356 Da), and insulin chain B (3496 Da)) were used for peptide MW estimation.
2.13. Experimental Design
The optimum conditions for jellyfish hydrolysis were determined using a response surface methodology with a face-centered composite design (two-factor three-levels). The independent variable effect (the hydrolysis time (X1, 60–360 min) and the enzyme concentration (X2, 1–5%)) on the DH, yield, IC50 value of the DPPH and ABTS radical scavenging and tyrosinase inhibition activity (mg/mL), and FRAP value (mmol FeSO4/g sample) were evaluated. The experiments with each enzyme consisted of 11 treatments (8 incomplete factorials and 3 replicated central points), as shown in Table 1. The design of the experimental, analysis and response surface plots were carried out using Design Expert software (version 11, Stat-Ease, Inc., Minneapolis, MN, USA). A full quadratic model for each response was obtained and expressed with real variables using following Equation (5):
| Yi = β0 + β1X1 + β2x2 + β11x12 + β22x22 + β12x1x2 + ε | (5) |
where Yi is the response variables, and x1 and x2 are the independent variables, whereas ε represents the random error, and β0, β1, β2, β11, β22, and β12 are the coefficients for the constant, linear, quadratic, and interaction terms.
One-way analysis of variance (ANOVA) was carried out using SPSS Statistical software version 17 (SPSS Inc., Chicago, IL, USA), and the means comparison (p ≤ 0.05) was conducted using Duncan’s new multiple range test (DMRT).
3. Results and Discussion
3.1. Chemical Composition of Jellyfish
The Chemical composition of the desalted jellyfish (rehydrated jellyfish) was 82.6% moisture, 13.1% crude protein, 4.1% ash, and 0.6% fat content. A previous report by Rodsuwan et al. [11] showed the proximate compositions of dried desalted jellyfish as being 70% protein, 10% moisture, 9% fat, and <1% ash content. The composition of the jellyfish tissue was largely determined based on the bodies of water in which these invertebrates were discovered. In dried Stomolophus meleagris, Cotylorhiza tuberculate, Rhizostoma pulmo, and Lobonema smithii, protein and ash were the predominant constituents, while fatty acids were more prevalent in zooxanthellate jellyfish [28,29,30].
3.2. Response Surface Models
The results of the hydrolysis time (X1) and alcalase, flavourzyme, and papain concentration (X2) on the jellyfish hydrolysate are shown in Table 1. A comprehensive response surface model was fitted using regression analysis. The regression coefficients for the full-2nd-order response surface models are shown in Table 2. Most of the models for each enzyme were significant (p ≤ 0.05) terms. The coefficients of determination (R2) were 0.78–0.99. Furthermore, the lack of fit for the models were not significant (p > 0.05), indicating relationships among the selected parameters as well as a high degree of confidence for explaining the effect of the variables in the observed data [31].
Table 2.
Full quadratic model of jellyfish hydrolysis using alcalase, flavourzyme, and papain.
| Enzyme | Responses | Quadratic Polynomial Model | R 2 | p-Value |
|---|---|---|---|---|
| Alcalase | DPPH (IC50) (mg/mL) | Y1 = −0.429 − 0.004X1 + 1.912X2 + 0.001X1X2 + 0.00001X12 − 0.335X22 | 0.8393 | 0.0469 |
| ABTS (IC50) (mg/mL) | Y2 = 3.74 + 0.019X1 − 0.172X2 − 0.0001X12 − 0.010X22 + 0.001X1X2 | 0.8377 | 0.0479 | |
| FRAP (mmol FeSO4/g) | Y3 = 0.316 − 0.001X1 − 0.065X2 − 0.00003X1X2 + 0.000001X12 + 0.010X22 | 0.9729 | 0.0006 | |
| %DH | Y4 = 0.93 + 0.028X1 + 13.9X2 − 0.0001X12 − 1.69X22 − 0.001X1X2 | 0.8282 | 0.0547 | |
| %Yield | Y5 = −0.071 + 0.004X1 + 1.09X2 − 0.00001X12 –0.145X22 + 0.001X1X2 | 0.886 | 0.021 | |
| Tyrosinase (IC50) (mg/mL) | Y6 = 19.6 + 0.218X1 − 9.14X2 − 0.001X12 + 1.53X22 − 0.01X1X2 | 0.8433 | 0.0442 | |
| Flavourzyme | DPPH (IC50) (mg/mL) | Y1 = 0.29 + 0.028X1 − 0.287X2 −0.0001X12 + 0.03X22 + 0.002X1X2 | 0.9367 | 0.0051 |
| ABTS (IC50) (mg) | Y2 = 12.9 –0.09X1 + 1.67X2 + 0.0003X12 − 0.37X22 − 0.002X1X2 | 0.8939 | 0.0177 | |
| FRAP (mmol FeSO4/g) | Y3 = 0.15 + 0.04X1 + 0.05X2 + 0.003X12 + 0.02X22 + 0.01X1X2 | 0.9913 | <0.0001 | |
| %DH | Y4 = 18.0 + 0.12X1 +4.60X2 − 0.002X12 − 0.16X22 − 0.01X1X2 | 0.9091 | 0.0122 | |
| %Yield | Y5 = 0.05 + 0.02X1 + 0.45X2 − 0.0001X12 + 0.01X22 + 0.01X1X2 | 0.9801 | 0.0003 | |
| Tyrosinase (IC50) (mg/mL) | Y6 = 59.6 − 0.16X1 − 7.39X2 + 0.002X12 + 0.26X22 + 0.014X1X2 | 0.9375 | 0.005 | |
| Papain | DPPH (IC50) (mg) | Y1 = 3.98 – 0.01X1 – 0.19X2 + 0.000002X12 – 0.05X22 + 0.001X1X2 | 0.9341 | 0.0056 |
| ABTS (IC50) (mg/mL) | Y2 = 7.80 + 0.01X1 – 0.17X2 – 0.00002X12 – 0.013X22 –0.001X1X2 | 0.9874 | <0.0001 | |
| FRAP (mmol FeSO4/g) | Y3 = 0.13 + 0.00002X1 – 0.01X2 + 0.0000003X12 + 0.01X22 – 0.00001X1X2 | 0.9253 | 0.0076 | |
| %DH | Y4 = 40.1 – 0.01X1 – 0.87X2 + 0.00002X12 + 0.07X22 + 0.002X1X2 | 0.7832 | 0.0924 | |
| % Yield | Y5 = 5.99 + 0.01X1 – 0.55X2 – 0.00001X12 + 0.05X22 + 0.002X1X2 | 0.9388 | 0.0047 | |
| Tyrosinase (IC50) (mg/mL) | Y6 = 39.2 – 0.04X1 – 4.01X2 + 0.0001X12 + 0.73X22 – 0.01X1X2 | 0.8818 | 0.0229 |
Note: Mean ± SD, X1: hydrolysis time (min), X2: enzyme concentration (%).
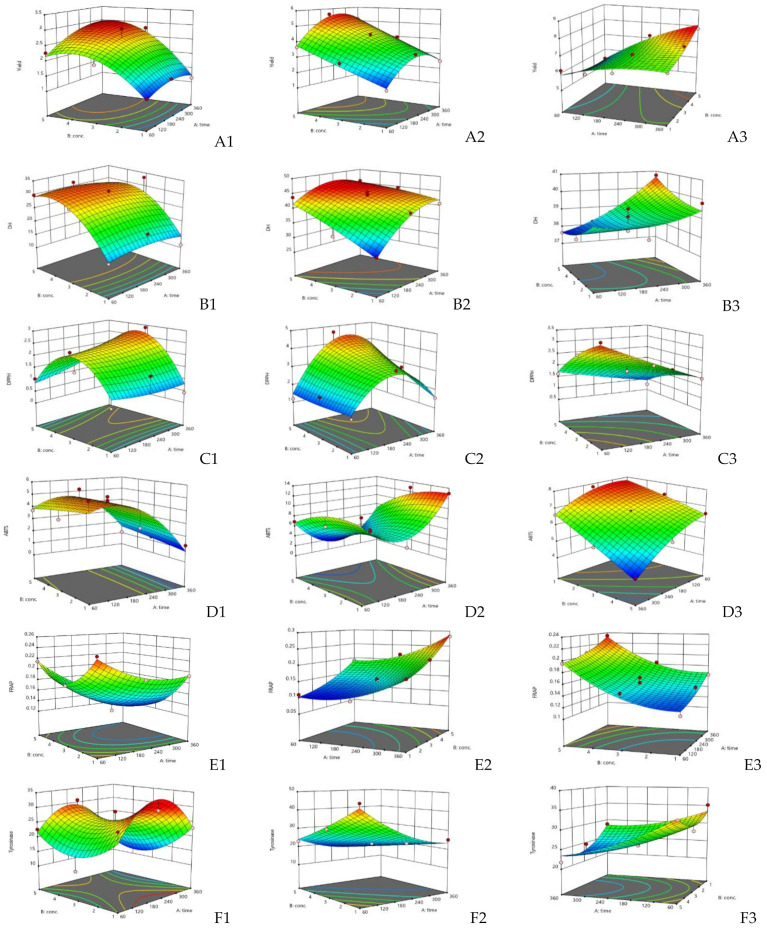
The plots of the response surface that were used to predict the effects of the hydrolysis time and concentration of alcalase, flavourzyme, or papain on the responses are shown in three-dimensional plots in Figure 1. For flavourzyme, both the hydrolysis time and enzyme concentration had the great effect on yield (Figure 1A1–A3), while the enzyme concentration and hydrolysis time and that most significant effect on alcalase and papain, respectively (Figure 2). Both independent factors were found to impact on yield in papain (Figure 3). Protein hydrolysate yield increased as the hydrolysis time and enzyme concentration increased. This might be due to the higher amount of peptide cleavage in the native protein during enzyme hydrolysis and the conversion to shorter or longer peptides, resulting in a higher hydrolysate yield. Similar results were also obtained in previous studies for eel protein hydrolysate [32] and herring muscle [33]. Various factors may have influenced hydrolysate yield, but type of enzyme used for hydrolysis had the greatest effect on the yield and properties [34]. This study ranks the hydrolysis yields of alcalase, flavourzyme, and papain, as shown in Table 1. Flavourzyme is a combination of endo- and exopeptidases that can produce both free amino acids and peptides, while alcalase and papain are endopeptidases that can hydrolyze protein with a high degree of specificity, particularly peptide bonds, and have a preference for uncharged residues [35,36].
Figure 1.
Response surface plots for DH, yield, IC50 of DPPH and ABTS radical scavenging, and tyrosinase inhibitory activity: and FRAP value using alcalase (A1–F1), flavourzyme (A2–F2), and papain (A3–F3) hydrolysis.
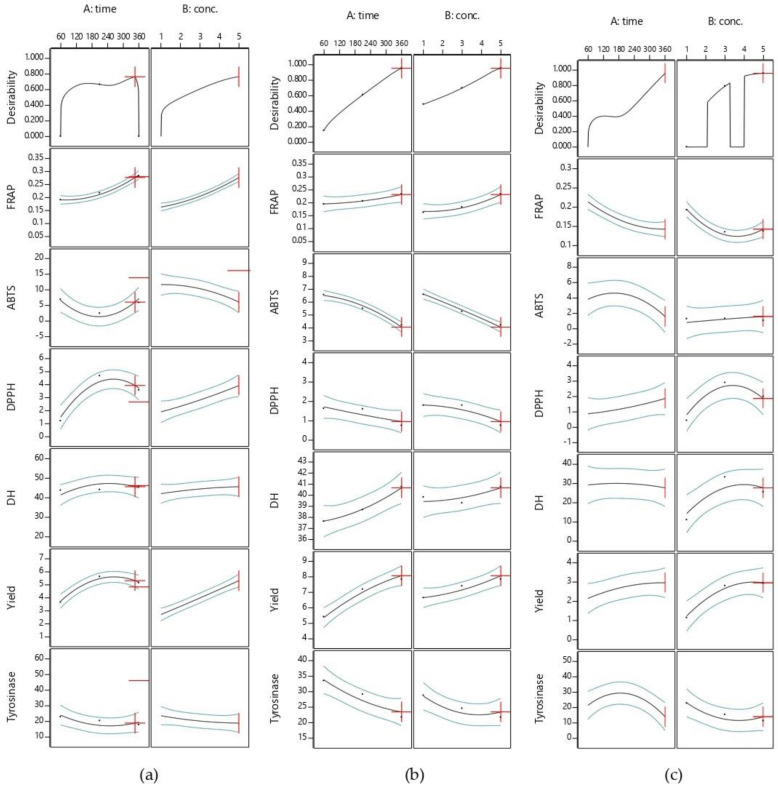
Figure 2.
Plots of the main effects of hydrolysis time (X1) and enzyme concentration (X2) on DH, yield, DPPH and ABTS radical scavenging, FRAP value, and tyrosinase inhibition activity using alcalase (a), flavourzyme (b) and papain (c) hydrolysis.
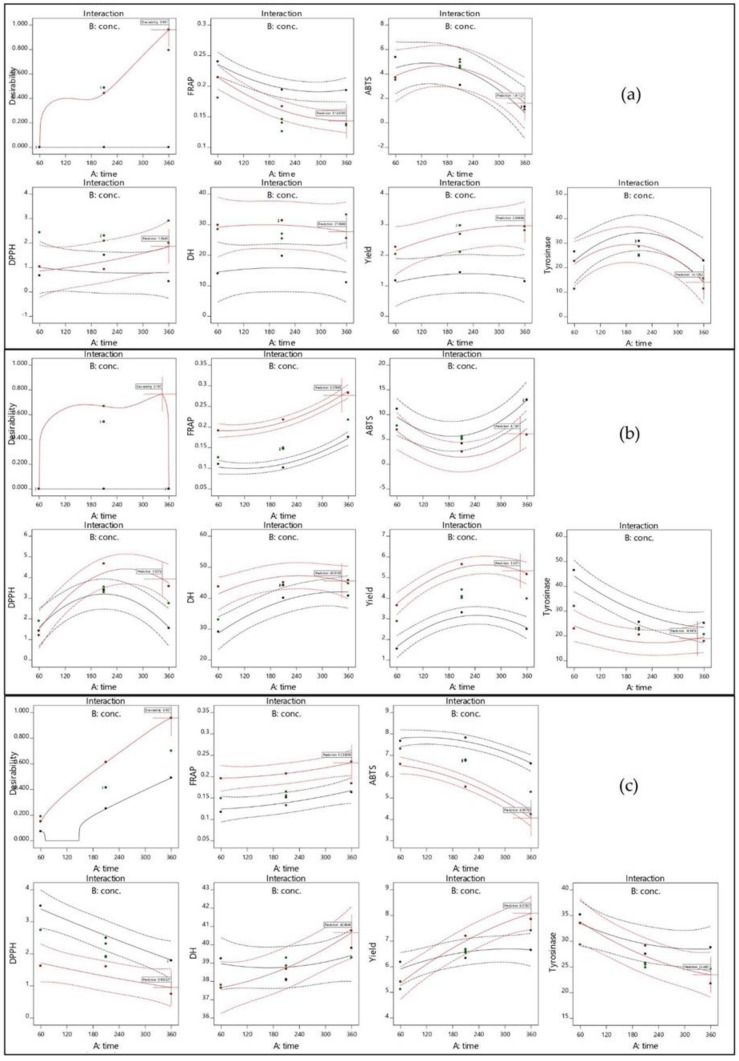
Figure 3.
Plot of the interaction effect for hydrolysis time (X1) and enzyme concentration (X2) on DH, yield, DPPH and ABTS radical scavenging, FRAP value, and tyrosinase inhibition activity using alcalase (a), flavourzyme (b), and papain (c) hydrolysis.
The effects of independent variables on the DH of JFH are shown in Figure 1B1–B3. The hydrolysis time had a significant effect on the DH for flavourzyme and papain, while the enzyme concentration had a significant effect on alcalase and flavourzyme (Figure 2). The DH increased as the enzyme concentration and hydrolysis time increased. The DH of jellyfish hydrolysate in this study was similar to those of previous studies, such as herring beluga viscera [37], tuna dark muscle by-products [17,38], and channel catfish skin [39]. Normally, the DH is higher when the reaction time is longer and increases rapidly at the beginning of a reaction before showing down and stabilizing [40,41]. The DH reflects the percentage of peptide bonds broken down by each protease. This allows the stage where number and size of peptides will have the best activity to be determined [40].
As shown in Figure 1C1–E3 exhibit the effects of the independent factors on the three responses to antioxidant activity: DPPH, ABTS, and FRAP, respectively. For flavourzyme and papain, the IC50 value of the DPPH radical scavenging activity was influenced by both independent variables. The duration of hydrolysis and the enzyme concentration demonstrated significant effects on the IC50 value of the ABTS radical scavenging activity of alcalase and flavourzyme hydrolysates, respectively, whereas both independent variables were caused significant effects in papain (Figure 2). However, the interaction between both independent variables affected the DPPH and ABTS for both flavourzyme and papain, respectively (Figure 3). Increasing the enzyme concentration and hydrolysis time increased the DH and led to good antioxidant activity in the jellyfish hydrolysates. The smaller peptides possessed more metal chelating and radical scavenging capabilities than the larger peptides, as per the results of Saidi et al. [41]. Different enzymes cleaved the peptide bonds at different positions in the protein structure, resulting in varying peptide sequences with various antioxidant activities [42]. Increasing the number of hydrophobic peptides showed a high reaction rate against free radicals [32]. After increasing the DH from 15 to 65%, a goby muscle hydrolysate short peptide with a stronger antioxidant was generated [43].
The effect of the independent variables on the IC50 value of the tyrosinase inhibition activity of jellyfish hydrolysate are shown in Figure 1F1–F3. Both the enzyme concentration and hydrolysis time had a significant effect on flavourzyme, while hydrolysis time significantly affected papain (Figure 2). The interaction effect for both independent variables on the tyrosinase inhibition activity was only in papain (Figure 3). Increased hydrolysis times and greater enzyme concentrations resulted in decreased IC50 values for tyrosinase inhibition activity (indicating strong tyrosinase inhibitory). Tyrosinase is a Cu2+-containing enzyme that plays an important role in melanogenesis and that could be catalyzed by quinones [44]. Therefore, antioxidative substances with the ability to prohibit the oxidative pathway and/or binding to the tyrosinase activity site (Cu2+) could inhibit the catalytic reaction of the enzymes. Polar or uncharged amino acid residues such as Ser and Cys are typically found in effective tyrosinase inhibitor peptides [4]. According to the report by Masuda et al. [45], some tyrosinase inhibitors also have strong antioxidant activity.
3.3. Multiple Response Optimization and Model Validation
The desirability function of the Design Expert Statistical program was used to optimize the hydrolysis conditions using multiple response optimization. Maximum targets were established for the DH, yield, and FRAP values, whereas minimum goals were set for the IC50 values for DPPH, ABTS radical scavenging, and tyrosinase inhibition activity. Table 3 shows the individual and composite desirability values of each response, assuming that all of the responses were evaluated equally. The results indicated that the optimal conditions for each enzyme were an enzyme concentration of 5% and hydrolysis times of 360, 345, or 360 min for alcalase (JFAH), flavourzyme (JFFH), and papain (JFPH), respectively. These optimal conditions were validated to obtain the experimental values. As indicated in Table 3, the predicted values were not substantially different from the experiment values (p > 0.05). The results indicated that they were good predictors. Based on the experimental values, some of which show better bioactivity than others, the most effective was JFFH, which was chosen for further research.
Table 3.
Optimized hydrolysis conditions and verification model for all responses of jellyfish hydrolysate using alcalase (JFAH), flavourzyme (JFFH), and papain (JFPH) hydrolysis.
| Enzyme | Value | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 (min) | X2 (%) | %DH | %Yield | DPPH (IC50) (mg/mL) |
ABTS (IC50) (mg/mL) |
FRAP (IC50) (mmol FeSO4/g) |
Tyrosinase Inhibitory (IC50) (mg/mL) | ||
| JFAH | Predicated value | 27.7 | 2.97 | 2.30 | 1.61 | 0.14 | 14.1 | ||
| Experimental value | 360 | 5 | 28.2 a ± 1.1 | 3.01 a ± 0.04 | 2.5 c ± 0.1 | 1.81 a ± 0.02 | 0.13 a ± 0.05 | 14.9 b ± 0.0 | |
| Composite desirability | 0.96 | ||||||||
| JFFH | Predicated value | 45.6 | 5.32 | 0.81 | 5.40 | 0.17 | 19.0 | ||
| Experimental value | 345 | 5 | 47.3 c ± 0.3 | 6.40 b ± 0.03 | 0.73 a ± 0.14 | 2.58 b ± 0.19 | 0.23 b ± 0.04 | 14.1 ab ± 0.1 | |
| Composite desirability | 0.96 | ||||||||
| JFPH | Predicated value | 40.7 | 7.45 | 0.95 | 4.30 | 0.23 | 23.5 | ||
| Experimental value | 360 | 5 | 41.8 b ± 0.5 | 7.21 bc ± 1.05 | 0.98 b ± 0.04 | 4.50 c ± 0.08 | 0.27 c ± 0.02 | 24.5 c ± 0.0 | |
| Composite desirability | 0.95 | ||||||||
Note: Mean ± SD, X1: hydrolysis time (min), X2: enzyme concentration (%); different superscripts in the same experimental values of response show significant differences (p ≤ 0.05).
3.4. Amino Acid Profile
JFFH’s amino acid profile presented in Table 4. Gly, Ala, Glx, Alx, and Pro were the most abundant amino acids in JFFH, accounting for around 18, 14, 13, 9, and 9% of the total amino acid composition, respectively. The hydrophobic amino acids Ala, Tyr, Val, Met, Cys, Ile, Phe, Trp, Leu, and Pro composed 43% of JFFH, whereas the other ~57% of JFFH comprised hydrophilic amino acids. The hydrophobic amino acid groups are important for bioactivity, especially in terms of antioxidant and antiproliferative activity of protein hydrolysates [46,47]. As a consequence, JFFH’s stronger antioxidant and tyrosinase inhibition activity might be related to its higher hydrophobic amino acid content. According to Schurink et al. [4], peptides possessing polar, uncharged amino acid residues, such as Ser and Cys, are similarly effective in inhibiting tyrosinase. The tyrosine-inhibitory activity might be related to a thiol group in a Cys-containing peptide chelating copper ions [48].
Table 4.
Amino acid content in jellyfish hydrolysate using flavourzyme hydrolysis (JFFH).
| Amino Acid | g Amino Acid/100 g of Sample |
|---|---|
| Asn + Asp | 5.00 |
| Gln + Glu | 6.96 |
| Ser | 1.85 |
| Thr | 1.46 |
| His | 0.23 |
| Gly | 9.74 |
| Ala | 4.50 |
| Arg | 3.53 |
| Tyr | 0.60 |
| Val | 1.62 |
| Met | 0.45 |
| Cys | 7.70 |
| Ile | 1.23 |
| Phe | 0.48 |
| Trp | 0.19 |
| Leu | 2.02 |
| Lys | 2.27 |
| Pro | 4.97 |
| Total amino acid | 54.76 |
3.5. Effect of Jellyfish Hydrolysate on Cell Viability
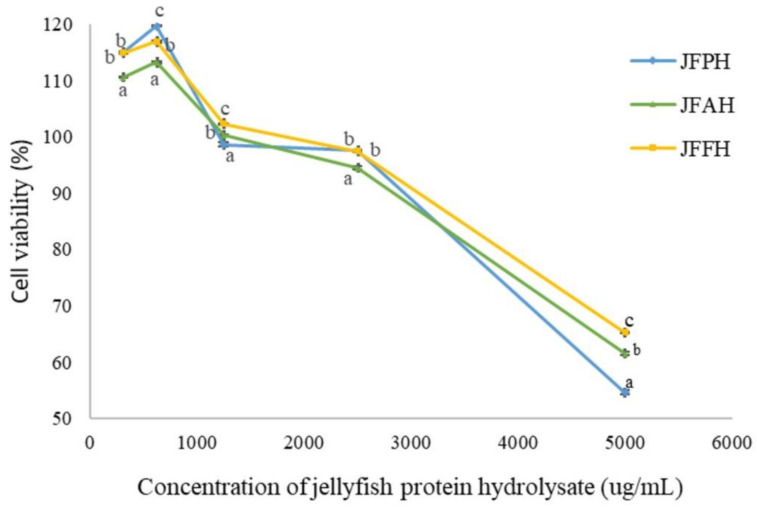
The effect on cell viability of jellyfish hydrolysate was evaluated based on the MTT value in the HaCaT cell lines, as shown in Figure 4. The cell viability was calculated compared to that of HaCaT that had been cell-cultured without jellyfish hydrolysate (control), reporting an 80% (IC80 value) survival rate. This result showed that the cell survival increased when the concentration increased from 0.313 to 0.625 mg/mL for all hydrolysates and decreased afterwards. The JFPH, JFAH, and JFFH concentrations of 0.313–1.25 mg/mL resulted in cell viability >100% as well as low cytotoxicity and potentially enhanced cell growth. Even at the highest dose (5 mg/mL), the jellyfish hydrolysate was non-cytotoxic. Domenico et al. [20] reported that the crude aqueous soluble protein (SP) of R. pulmo was able to diminish the cell viability of human epidermal keratinocyte to 60% when the SP was added >2.5 µg/mL. They also discovered that the fractioned SP with MW >30 kDa was cytotoxic at concentration doses as low as 1 mg/mL, while SP fractions with MW concentrations of 10–30 kDa and 3–10 kDa were non-cytotoxic, even at the maximum dosage (10 µg/mL). Human breast cancer cells (MCF-7), human hepatocellular carcinoma cells (HepG2), human normal fibroblasts (HFB4), breast cancer cells (MDA-MB231), and hepatocellular carcinoma (HCC) all demonstrated that the dose-dependent reduction in cell viability was dependent on the concentration of the extracts and the duration of the contact time [49,50]. The IC80 value of JFFH, JFAH, and JFPH was 2.5 µg/mL. Therefore, jellyfish hydrolysate demonstrated low toxicity to HaCaT at this concentration. However, further research is still required in order to assess the potential of jellyfish hydrolysate as functional ingredient in different applications.
Figure 4.
Cytotoxic effect of jellyfish hydrolysate prepared with alcalase (JFAH), flavourzyme (JFFH), and papain (JFPH) on human keratinocytes cells (HaCaT) at 24 h incubation.
3.6. Fractionation of JFFH
The JFFH was fractionated into four fractions: fraction-I (>10 kDa), fraction-II (3–10 kDa), fraction-III (1–3 kDa), and fraction-IV (<1 kDa), to identify which fractions exhibit functional properties. Table 5 showed the yield, antioxidant properties, and tyrosinase inhibition activity for each fraction. Fraction-III had the highest antioxidant activity and tyrosinase inhibition activity (p ≤ 0.05). These results are consistent with those determined by Mongkonkamthorn et al. [17] and Mongkonkamthorn et al. [19], who demonstrated that peptides with <1 kDa MW from tuna blood and dark meat had the strongest antioxidant activity. Zhuang et al. [12] reported jellyfish hydrolysate fraction HF-2 (1–3 kDa) has a tyrosinase inhibitory effect that is higher than 50% at 5 mg/mL as well as strong Cu2+ chelating activity. Prakot et al. [51] also reported a possible tyrosinase inhibitor in spotted babylon protein hydrolysate (MW < 5 kDa), reporting an IC50 value of 0.075 µg/mL.
Table 5.
Antioxidant and tyrosinase inhibition activity and yield from the fractionation of jellyfish hydrolysates using flavourzyme.
| MW (kDa) | Responses | |||||
|---|---|---|---|---|---|---|
| Fraction | ABTS (IC50) (mg/mL) | DPPH (IC50) (mg/mL) | FRAP (mmol FeSO4/g) | Tyrosinase Inhibitory (IC50) (mg/mL) | Yield (%) | |
| >10 kDa | I | 2.91 c ± 0.01 | 3.71 c ± 0.11 | 0.65 c ± 0.001 | 14.2 b ± 8.49 | 3.01 d ± 0.01 |
| 10–3 kDa | II | 1.15 b ± 0.01 | 0.85 a ± 0.03 | 0.27 b ± 0.001 | 9.35 a ± 0.27 | 2.18 c ± 0.02 |
| 3–1 kDa | III | 0.91 a ± 0.01 | 0.95 a ± 0.04 | 0.24 a ± 0.001 | 8.95 a ± 0.01 | 1.89 b ± 0.00 |
| < 1 kDa | IV | 0.89 a ± 0.01 | 1.11 b ± 0.01 | 0.28 b ± 0.01 | 12.6 b ± 0.14 | 0.73 a ± 0.00 |
Note: Mean ± SD, DPPH and ABTS radical scavenging activity, FRAP value and tyrosinase inhibitory activity; different letters in the same variables show significant differences (p ≤ 0.05).
3.7. Gel Filtration Chromatography
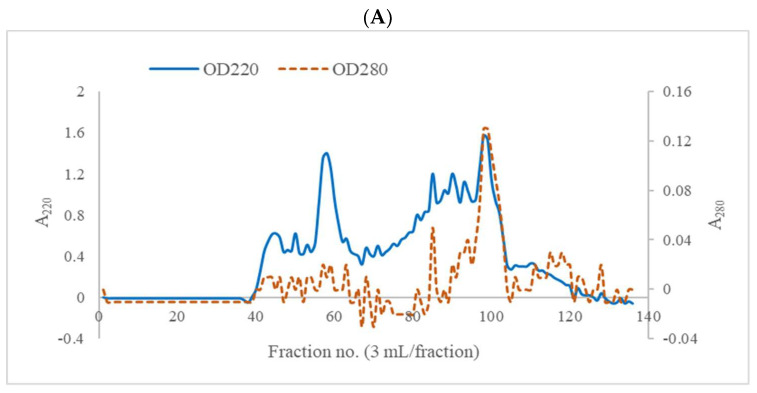
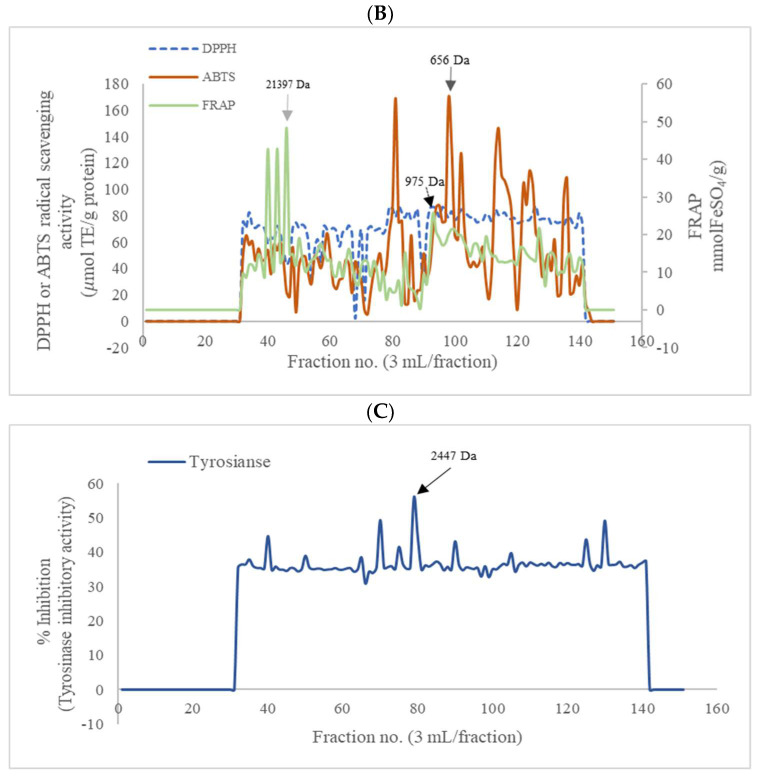
As represented in Figure 5A, the fraction-III of JFFH was further isolated using a Sephadex G-25 column. The peptide bonds and the proteins, peptides, or amino acids with aromatic rings were able to be monitored using A220 and A280, respectively [52]. Many individual peaks were observed at A280, indicating the presence of aromatic peptide or amino acid side chains. Fraction-III showed the same distinct peaks at A220 and A280 in fraction no. 97, which may indicate the presence peptide linkages and that aromatic amino acids rings are abundant (tyrosine and tryptophan). The DPPH and ABTS radical scavenging activity as well as the FRAP value (Figure 5B) and the tyrosinase inhibitory activity of the fractions (Figure 5C) were all investigated. Different fractions showed varying degrees of antioxidant activity and tyrosinase inhibition. Fraction no. 42, which exhibited the highest FRAP activity, contained peptides with an MW of 21,397 Da. The highest DPPH radical scavenging activity was shown in fraction no. 95 (peptide with MW of 807 Da). The strongest ABTS radical scavenging activity was in the compressed peptides with an MW of 656 Da. According to Wu et al. [53], antioxidant peptides from mackerel protein hydrolysate with a molecular weight of 1400 Da had higher activity than peptides with molecular weights of 900 Da or 200 Da. Mendis et al. [54] observed the strongest antioxidant activity in peptides from giant squid skin gelatin hydrolysate with an MW less than 3000 Da. The isolated peptides from short-sequence and low-MW (538–887 Da) tuna by-product hydrolysate showed strong antioxidant properties that affected the radical values and lipid oxidation inhibition [28]. Therefore, fraction-III, which was isolated from JFFH-containing peptides with an MW of 2448 Da, showed the strongest tyrosinase inhibitory activity. The hydrophobic Val, Ala, and/or Leu residues in potent tyrosinase inhibitory peptides are generally combined with Arg and/or Phe [4]. Sea cucumber (B. Vitiensis) demonstrated good tyrosinase inhibitory activity, with an IC50 value of 0.28 mg/mL [55]. Many studies have revealed that the amino acid composition and molecular weight distribution of certain peptides were associated with their antioxidant activity and strong metal chelation capacity [4,12,21].
Figure 5.
Elution profile of antioxidant and tyrosinase inhibitory peptide from fraction-III (MW 1–3 kDa) from JFFH using Sephadex G-25 gel filtration chromatography monitored at A220 and A280 (A). DPPH and ABTS radical scavenging and FRAP value (B) and tyrosinase inhibitory activity (C) of the different fractions.
4. Conclusions
The optimized conditions for the production of jellyfish hydrolysate using alcalase, flavourzyme, and papain were an enzyme concentration of 5%, with hydrolysis times of 360 min for alcalase, 345 min for flavourzyme, and 360 min for papain. The models that were generated fit the experimental values well. The jellyfish hydrolysate obtained flavourzyme hydrolysis (JFFH) contained hydrophobic and hydrophilic amino acids, which comprised about about 43 and 57% of the total amino acids, respectively. The IC80 value for HaCaT cell viability for JFAH, JFFH, and JFPH was 2.5 mg/mL. The fractionated peptides from JFFH fraction-III (MW of 1–3 kDa) had highest antioxidant activity and strongest tyrosinase inhibition activity. The peptides in JFFH with high ABTS radical scavenging, DPPH radical scavenging, and FRAP values were characterized as having MWs of 656, 807 and 21,397 Da, respectively, while the fractionated peptide with an MW of 2448 Da showed high tyrosinase inhibition activity. Therefore, it is possible to utilize salted jellyfish as a raw material for the preparation of natural antioxidants and as an anti-tyrosinase, meaning that it can potentially be used as functional ingredient in the food, cosmetic, and pharmaceutical industries.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Research Council of Thailand (NRCT), Chiang Mai University (Thailand), The Graduate School, and College of Maritime Studies and Management, Chiang Mai University, to the authors to allow them to conduct the practical work reported in this paper.
Author Contributions
Conceptualization, S.W.; methodology, S.W. and M.U.; validation, T.S., S.Y., S.M., J.M.R. and S.W.; formal analysis, M.U.; investigation, M.U. and S.W.; data curation, M.U.; writing-original draft preparation, M.U. and S.W.; writing-review and editing, T.S., S.W., and J.M.R.; supervision, S.Y., S.M., T.S., J.M.R., and S.W.; project administration, S.W.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was financial support from the National Research Council of Thailand (NRCT), Chiang Mai University (Thailand), The Graduate School, and College of Maritime Studies and Management, Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest related to this work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Song K.K., Huang H., Han P., Zhang C.H., Shi Y., Chen Q.X. Inhibitory effects of cis-and trans-isomers of 3,5-dihydroxystilbene on the activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 2006;342:1147–1151. doi: 10.1016/j.bbrc.2005.12.229. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.J., No J.K., Lee J.S., Kim M.S., Chung H.Y. Antimelanogenic activity of 3,4-dihydroxyacephenone: Inhibition of tyrosinase and MITF. Biosci. Biotechnol. Biochem. 2006;70:532–534. doi: 10.1271/bbb.70.532. [DOI] [PubMed] [Google Scholar]
- 3.Zolghadri S., Bahrami A., Khan M.T.H., Munoz-Munoz J., Garcia-Molina F., Garcia-Canovas F., Saboury A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019;34:279–309. doi: 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schurink M., van Berkel W.J.H., Wichers H.J., Boeriu C.G. Novel peptides with tyrosinase inhibitory activity. Peptides. 2007;28:485–495. doi: 10.1016/j.peptides.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Husni A., Jeon J.S., Um B.H., Han N.S., Chung D. Tyrosinase inhibition by water and ethanol extracts of a far eastern sea cucumber, Stichopus japonicus. J. Sci. Food Agric. 2011;91:1541–1547. doi: 10.1002/jsfa.4335. [DOI] [PubMed] [Google Scholar]
- 6.Fan Q., Jiang H., Yuan E.D., Zhang J.X., Ning Z.X., Qi S.J., Wei Q.J. Tyrosinase inhibitory effects and antioxidative activities of novel cinnamoyl amides with amino acid ester moiety. Food Chem. 2012;134:1081–1087. doi: 10.1016/j.foodchem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Ding J.F., Li Y.Y., Xu J.J., Su X.R., Gao X., Yue F.P. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocoll. 2011;25:1350–1353. doi: 10.1016/j.foodhyd.2010.12.013. [DOI] [Google Scholar]
- 8.Leone A., Lecci R.M., Milisenda G., Piraino S. Mediterranean jellyfish as novel food: Effects of thermal processing on antioxidant, phenolic, and protein contents. Eur. Food. Res. Technol. 2019;245:1611–1627. doi: 10.1007/s00217-019-03248-6. [DOI] [Google Scholar]
- 9.FAO . The State of World Fisheries and Aquaculture 2020. Sustainability in Action. FAO; Rome, Italy: 2020. [Google Scholar]
- 10.Kromfang I., Chikhunthod U., Karpilanondh P., Thumthanaruk B. Identification of volatile compounds in jellyfish protein hydrolysate. KMUTNB Int. J. App. Sci. Tech. 2015;8:153–161. doi: 10.14416/j.ijast.2014.10.003. [DOI] [Google Scholar]
- 11.Rodsuwan U., Thumthanaruk B., Kerdchoechuen O., Laohakunjit N. Functional properties of type A gelatin from jellyfish (Lobonema smithii) Food Sci. Technol. Int. 2016;23:507–514. [Google Scholar]
- 12.Zhuang Y., Sun L., Zhao X., Wang J., Hou H., Li B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum) J. Agric. Food Chem. 2009;89:1722–1727. doi: 10.1002/jsfa.3645. [DOI] [Google Scholar]
- 13.Yu H.H., Liu X.G., Xing R.E., Liu S., Guo Z.Y., Wang P.B. In vitro determination of antioxidant activity of proteins from jellyfish Rhopliema esculentum. Food Chem. 2006;95:123–130. doi: 10.1016/j.foodchem.2004.12.025. [DOI] [Google Scholar]
- 14.Zhuang Y., Hou H., Zhao X., Zhang Z., Li B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009;74:183–188. doi: 10.1111/j.1750-3841.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Duan R., Huang L., Song Y., Regenstein J.M. Characterisation of acid-soluble and pepsin-solubilised collagen from jellyfish (Cyanea nozakii Kishinouye) Food Chem. 2014;150:22–26. doi: 10.1016/j.foodchem.2013.10.116. [DOI] [PubMed] [Google Scholar]
- 16.Ahn C., Lee K., Je J. Enzymatic production of bioactive protein hydrolysates from tuna liver: Effects of enzymes and molecular weight on bioactivity. J. Food Sci. Technol. 2010;45:562–568. doi: 10.1111/j.1365-2621.2009.02166.x. [DOI] [Google Scholar]
- 17.Mongkonkamthorn N., Malila Y., Regenstein J.M., Wangtueai S. Enzymatic hydrolysis optimization for preparation of tuna dark meat hydrolysate with antioxidant and angiotensin I-converting enzyme (ACE) inhibitory activities. J. Aquat. Food. Prod. Technol. 2021;30:1090–1108. doi: 10.1080/10498850.2021.1974138. [DOI] [Google Scholar]
- 18.Karami Z., Akbari-Adergani B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019;56:535–547. doi: 10.1007/s13197-018-3549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mongkonkamthorn N., Malila Y., Yarnpakdee S., Makkhum S., Regenstein J.M., Wangtueai S. Production of protein hydrolysate containing antioxidant and angiotensin-I-converting enzyme (ACE) inhibitory activities from tuna (Katsuwonus pelamis) blood. Processes. 2020;8:1518. doi: 10.3390/pr8111518. [DOI] [Google Scholar]
- 20.Domenico S.D., Rinaldis G.D., Paulmery M., Piraino S., Leone A. Barrel jellyfish (Rhizostoma pulmo) as source of antioxidant peptides. Marine Drugs. 2019;17:134. doi: 10.3390/md17020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aziz N.A.A., Salim N., Zarei M., Saari N., Yusoff F.M. Extraction, anti-tyrosinase, and antioxidant activities of the collagen hydrolysate derived from Rhopilema hispidum. Prep. Biochem. Biotechnol. 2021;51:44–53. doi: 10.1080/10826068.2020.1789991. [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Zhang M., Jia A., Zhang Y., Zhu H., Zhang C., Sun Z., Liu C. Purification and characterization of angiotensin I converting enzyme inhibitory peptides from jellyfish Rhopilema esculentum. Food Res. Int. 2013;50:339–343. doi: 10.1016/j.foodres.2012.11.002. [DOI] [Google Scholar]
- 23.Ahn C.B., Kim J.G., Je J.Y. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014;147:78–83. doi: 10.1016/j.foodchem.2013.09.136. [DOI] [PubMed] [Google Scholar]
- 24.Wangtueai S., Siebenhandl-Ehn S., Haltrich D. Optimization of the preparation of gelatin hydrolysates with antioxidative activity from lizardfish (Saurida spp.) scales gelatin. Chiang Mai J. Sci. 2016;43:68–79. [Google Scholar]
- 25.Chan E.W.C., Lim Y.Y., Wong L.F., Lianto F.S., Wong S.K., Lim K.K., Loe C.E., Lim T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008;109:477–483. doi: 10.1016/j.foodchem.2008.02.016. [DOI] [Google Scholar]
- 26.Herbert P., Barros P., Ratola N., Alves A. HPLC determination of amino acids in must and port wine using OPA/FMOC derivatives. J. Food Sci. 2000;65:1130–1133. doi: 10.1111/j.1365-2621.2000.tb10251.x. [DOI] [Google Scholar]
- 27.Pastorino G., Marchetti C., Borghesi B., Cornara L., Ribulla S., Burlando B. Biological activities of the legume crops Melilotus officinalis and Lespedeza capitata for skin care and pharmaceutical applications. Ind. Crop. Prod. 2017;96:158–164. doi: 10.1016/j.indcrop.2016.11.047. [DOI] [Google Scholar]
- 28.Hsieh Y.H.P., Leong F.M., Rudloe J. Jellyfish as food. Hydrobiologia. 2001;451:11–17. doi: 10.1023/A:1011875720415. [DOI] [Google Scholar]
- 29.Leone A., Lecci R.M., Durante M., Meli F., Piraino S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa) Marine Drugs. 2015;13:4654–4681. doi: 10.3390/md13084654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muangrod P., Charoenchokpanich W., Rungsardthong V., Vatanyoopaisan S., Wonganu B., Roytrkul S., Thumthanaruk B. Effect of pepsin hydrolysis on antioxidant activity of jellyfish protein hydrolysate. EDP Sci. 2021;302:02010. doi: 10.1051/e3sconf/202130202010. [DOI] [Google Scholar]
- 31.Wangtueai S., Phimolsiripol Y., Vichasilp C., Regenstein J.M., Schoenlechner R. Optimization of gluten-free functional noodles formulation enriched with fish gelatin hydrolysates. LWT-Food Sci. Tech. 2020;133:10997. doi: 10.1016/j.lwt.2020.109977. [DOI] [Google Scholar]
- 32.Halim N.R.A., Sarbon N.M. A response surface approach on hydrolysis condition of eel (Monopterus sp.) protein hydrolysate with antioxidant activity. Int. Food Res. J. 2017;24:1081–1093. [Google Scholar]
- 33.Prabha J., Narikimelli A., Sajini M.I., Vincent S. Optimization for autolysis assisted production of fish protein hydrolysate from underutilized fish Pellona ditchela. Int. J. Energy Res. 2013;4:1863–1869. [Google Scholar]
- 34.Slizyte R., Rommi K., Mozuratyte R., Eck P., Five K., Rusta T. Bioactivities of fish protein hydrolysates from defatted salmon backbone. Biotechnol. J. 2016;11:99–109. doi: 10.1016/j.btre.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Je J.Y., Lee K.H., Ahn C.B. Antioxidant and antihypertensive protein hydrolysate produced from tuna liver by enzymatic hydrolysis. Food Res. Int. 2009;42:1266–1272. doi: 10.1016/j.foodres.2009.06.013. [DOI] [Google Scholar]
- 36.Yarnpakdee S., Benjakul S., Kristinsson H.G., Kishimura H. Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one-and two-step hydrolysis. J. Food Sci. Technol. 2015;53:3336–3349. doi: 10.1007/s13197-014-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molla A.E., Hovannisyan H.G. Optimization of enzymatic hydrolysis of visceral waste proteins of beluga Huso huso using Protamex. Int. Aquat. Res. 2011;3:93–99. [Google Scholar]
- 38.Saidi S., Deratani A., Amar R.B., Belleville M.P. Fractionation of tuna dark muscle hydrolysate by a two-step membrane process. Sep. Purif. Technol. 2013;108:28–36. doi: 10.1016/j.seppur.2013.01.048. [DOI] [Google Scholar]
- 39.Shun L., Lin L., Wei X., Jian-Feng L., Ying-Wang Y., Shao-Tong J. Optimization of hydrolysis conditions of gelatin from channel catfish skin. Food Sci. 2013;34:156–160. [Google Scholar]
- 40.Li B., Chen F., Wang X., Ji B.P., Wu Y. Isolation and identification of oxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization mass spectrometry. Food Chem. 2007;102:1135–1143. doi: 10.1016/j.foodchem.2006.07.002. [DOI] [Google Scholar]
- 41.Saidi V.M., Deratani A., Belleville M.P., Amar R.B. Antioxidant properties of peptide fractions from tuna dark muscle protein by-product hydrolysate produced by membrane fractionation process. Food Res. Int. 2014;65:329–336. doi: 10.1016/j.foodres.2014.09.023. [DOI] [Google Scholar]
- 42.Tavano O.L. Protein hydrolysis using proteases: An important tool for food biotechnology. J. Mol. Catal. B Enzym. 2013;90:1–11. doi: 10.1016/j.molcatb.2013.01.011. [DOI] [Google Scholar]
- 43.Nasri R., Jridi M., Lassoued I., Jemil I., Ben Slama-Ben Salam R., Nasri M., Karra-Haabouni M. The influence of the extent of enzymatic hydrolysis in antioxidative properties and ACE-inhibitory activity of protein hydrolysate by goby (Zosterisessoe ophiocephalus) muscle. Appl. Biochem. Biotechnol. 2014;173:1121–1134. doi: 10.1007/s12010-014-0905-3. [DOI] [PubMed] [Google Scholar]
- 44.Karioti A., Protopappa A., Megoulas N., Skaltsa H. Identification of tyrosinase inhibitors from Marrubrium velutinum and Marrubium cylleneum. Bioorg. Med. Chem. 2007;15:2708–2714. doi: 10.1016/j.bmc.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 45.Masuda T., Yamshita D., Takeda Y., Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliatica. Biosci. Biotechnol. Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- 46.Samarayanaka A.G.P., Li-Chan E.C.Y. Autolysis-assisted production of (FPH) with antioxidant properties from Pacific hake (Merluccius productus) Food Chem. 2008;107:768–776. doi: 10.1016/j.foodchem.2007.08.076. [DOI] [Google Scholar]
- 47.Picot L., Ravallec R., Fouchereau-Peron M., Vandanjon L., Jaouen P., Chaplain-Derouiniot M., Guerard F., Chabeaud A., LeGal Y., Alvarez O.M., et al. Impact of ultrafiltration and nanofiltration of an industrial fish protein hydrolysate on its bioactive properties. J. Sci. Food Agric. 2010;90:1819–1826. doi: 10.1002/jsfa.4020. [DOI] [PubMed] [Google Scholar]
- 48.Wu Z., Fernandez-Lima F.A., Russell D.H. Amino acid Influence on copper binding to peptides: Cysteine versus arginine. J. Am. Soc. Mass Spectrom. 2010;21:522–533. doi: 10.1016/j.jasms.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 49.Leone A., Lecci R.M., Durante M., Piraino S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculate modulates gap junction intercellular communication in human cell cultures. Marine Drugs. 2013;11:1728–1762. doi: 10.3390/md11051728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villanueva A. Hepatocellular carcinoma. N. Engl. J. Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 51.Prakot P., Chaitanawisuti N., Karnchanatat A. In vitro anti-tyrosinase activity of protein hydrolysate from spotted babylon (Babylonia areolata) Food Appl. Biosci. J. 2015;3:109–120. [Google Scholar]
- 52.Klompong V., Bemjakul S., Yachai M., Visessangun W., Shahidi F., Hayes K. Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe trevally (Selaroides leptolepis) J. Food Sci. 2009;74:C126–C133. doi: 10.1111/j.1750-3841.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 53.Wu H.C., Chen H.M., Shiau C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res. Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- 54.Mendis E., Rajapakse N., Byun H.G., Kim S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005;77:2166–2178. doi: 10.1016/j.lfs.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Nursid M., Marraskuranto E., Kuswardini A., Winanto T. Screening of tyrosinase inhibitor, antioxidant and cytotoxicity of dried sea cucumber from Tomoni Bay, Indonesia. Pharmacogn. J. 2019;11:555–558. doi: 10.5530/pj.2019.11.88. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.