Abstract
Twenty-four matched pairs of isolates of Pasteurella haemolytica and three matched pairs of isolates of Pasteurella multocida were isolated by using a nasal swab and a transtracheal swab from individual calves with clinical signs of bovine respiratory disease. The identity of each matched pair was confirmed biochemically and serologically. The similarity of the isolates obtained from a nasal swab and from a transtracheal swab was compared by using ribotyping and antibiotic susceptibility analyses. Although the calves were sampled only once with a nasal and a transtracheal swab, when both samples were bacteriologically positive the nasal swab identified the same bacterial species as the transtracheal swab 96% of the time. The nasal swab isolate was genetically identical to the transtracheal isolate in 70% of the matched pairs. Six different ribotypes were observed for the P. haemolytica isolates, while only one ribotype was observed for the limited number of P. multocida isolates. Of the six P. haemolytica ribotypes, two ribotypes predominated. All the paired isolates displayed similar susceptibility to ceftiofur, erythromycin, tilmicosin, trimethoprim-sulfamethoxazole, and florfenicol, with some minor variations for ampicillin and spectinomycin. These results suggest that a nasal swab culture can be predictive of the bacterial pathogen within the lung when the isolates are from an acutely ill animal and can be used to determine antibiotic susceptibility.
The occurrence of bovine respiratory disease (BRD) in recently weaned beef calves develops in a sudden and predictable fashion shortly after arrival at a feedlot (6, 15). Respiratory disease in commingled beef calves is a complex disease syndrome caused by many bacterial and viral agents and is influenced by management and environment (16). The most frequent etiologic bacterial agents from fatal cases of BRD are pasteurellae and, particularly, Pasteurella haemolytica (19).
Several investigators have examined the natural flora of feedlot calves to derive an association between recovered bacteria and disease. Some investigators sampled the upper airways of live animals, while others sampled lungs from animals that succumbed to BRD (7, 16, 18, 19). These studies demonstrated no correlation between the bacteria isolated from the upper respiratory tract and the disease-causing organism in the lungs, since only one site per animal was evaluated. A study that examined respiratory isolates from feedlot cattle with or without BRD by using nasopharyngeal and bronchoalveolar lavage cultures only determined bacteriological frequency and did not characterize the isolated bacteria to determine the degree of identity between the two isolates (1).
The purpose of this study was to determine whether pasteurellae isolated from nasal swabs are similar to those isolated from deeper within the respiratory system. The resulting information would allow the practitioner and laboratory diagnostician a reasonable degree of confidence that a nasal isolate would be representative of the disease-causing pathogen in the lung.
MATERIALS AND METHODS
Experimental animals.
The experimental animals were feeder calves purchased from livestock auctions in Mississippi during the fall of 1996. Purchasing these calves from livestock auctions increased the risk for contracting BRD. All calves were administered a vaccine for infectious bovine rhinotracheitis, bovine virus diarrhea, parainfluenza type III virus, and bovine respiratory syncytial virus (Horizon I Vac III; Bayer, Shawnee Mission, Kans.) and a seven-way clostridial bacterin (Ultrabac 7; Pfizer, Exton, Pa.) and were treated for internal (Synanthic; Fort Dodge Laboratory, Overland Park, Kans.) and external (Tiguvon; Bayer) parasites. After arrival at the feedlot, the animals were observed for clinical signs of BRD (i.e., depression, reduced feed intake, and a rectal temperature of ≥104°F). Calves with clinical signs indicative of BRD were pulled from pens, taken to a hospital facility for further evaluation, and sampled by using a guarded nasal swab (Medical Wire and Equipment Co., Ltd., Corsham, England) and a guarded transtracheal swab (Tiegland modified culture sterile swab, J-274; Jorgensen Labs, Loveland, Colo.).
Bacterial culture and identification.
A total of 40 paired swabs were transported to the Texas Veterinary Medical Diagnostic Laboratory (Amarillo, Tex.), where they were isolated and bacteriologically identified. The pasteurella isolates (n = 54) were subcultured onto blood agar plates and transported to Kansas State Diagnostic Laboratory. The identity of the isolates was confirmed biochemically and serologically by the Kansas State University, Department of Diagnostic Medicine and Pathobiology (Manhattan, Kans.).
Paired clinical isolates of Pasteurella multocida (n = 6) and P. haemolytica (n = 48) were serotyped, ribotyped, and tested for antibiotic susceptibility.
Serotyping. (i) P. multocida.
Type A cultures were identified by the staphylococcal hyaluronidase decapsulation test (5). Type D cultures were identified by the acriflavine test (4). An agar gel immunodiffusion test was performed to determine the somatic antigens of P. multocida isolates (12).
(ii) P. haemolytica.
A rapid plate agglutination test was performed to determine the capsule antigen of all isolates (9). Biotypes were determined based on arabinose and trehalose fermentation reactions.
Chromosomal DNA isolation.
Pasteurella isolates were inoculated into 5 ml of brain heart infusion broth (Difco, Detroit, Mich.) and incubated overnight at 37°C and 5% CO2. Bacteria were recovered by centrifugation, and the resulting pellet was washed with 10 ml of TE buffer (10 mM Tris, 1 mM EDTA). After the pellet was resuspended in 567 μl of TE sucrose buffer (25% sucrose in 50 mM Tris-HCl [pH 8.0]–50 mM EDTA–10 mM NaCl), the bacteria were disrupted with the addition of 30 μl of 10% sodium dodecyl sulfate (Sigma, St. Louis, Mo.) and 3 μl of proteinase K (20 mg/ml; Sigma) for 1 h at 37°C with frequent mixing. After incubation, 100 μl of 5 M NaCl and 80 μl of 10% cetyltrimethylammonium bromide (CTAB; Sigma) were added to the solution and incubated for 10 min at 65°C. Contaminating RNA was degraded with the addition of 20 μl of RNase A (10 mg/ml; Sigma) for 30 min at 55°C. The DNA was extracted with equal volumes of phenol-chloroform (1:1) until no protein precipitated. DNA was precipitated from the aqueous phase with 360 μl of ice-cold isopropanol, followed by centrifugation at 15,000 × g for 15 min. The DNA pellet was washed twice with 1.0 ml of 70% ice-cold ethanol, dried, and resuspended in 90 μl of TE buffer. Due to interference caused by CTAB in the isolation procedure, the DNA concentration and purity could not be reliably determined by UV spectrophotometry. Therefore, DNA concentration and purity were estimated by visualization on agarose gel by using known DNA concentration standards. The DNA preparations were stored at 4°C until needed.
Restriction endonuclease digestion.
Chromosomal DNA was digested with restriction endonucleases, PstI, PvuII, HaeIII, and HhaI (Promega, Madison, Wis.) according to the manufacturer's recommendation for 4 to 5 h. Briefly, the 20-μl digestion mixture consisted of approximately 3 μg of DNA, 10× enzyme buffer, 10 U of restriction enzyme, and water. Digestion was stopped by heating the solution for 10 min at 65°C, followed by cooling on ice for 5 min. To check the efficiency of digestion, 1 μl of the digestion mixture was electrophoresed in an agarose gel (0.7% agarose and 2 μl of ethidium bromide [10 mg/ml] in 40 ml of TBE [89 mM Tris borate, 2 mM EDTA; pH 8.3]) and visualized under an UV transilluminator. The remaining digestion sample (19 μl) was electrophoresed in 1% agarose. Electrophoresis was performed at room temperature at 40 V for 18 h. Digoxigenin-labeled DNA molecular weight marker II (Boehringer Mannheim, Indianapolis, Ind.) was used as a molecular weight standard.
Southern blotting.
After electrophoresis, the gel was depurinated in 250 ml of 0.25 M HCl for 9 min and then rinsed three times in distilled water. The fragments were denatured by incubation with 250 ml of 0.5 M NaOH for 30 min and then neutralized with 250 ml of 0.5 M Tris-HCl for at least 30 min. Fragments were transferred to a positively charged nylon membrane (Boehringer Mannheim) by the method of Southern (20). After transfer, the membrane was rinsed for 5 min in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), UV cross-linked (GS Gene Linker; Bio-Rad Laboratories, Richmond, Calif.), and stored at 4°C.
Probe preparation.
A nonradioactive labeling system (Genius System; Boehringer Mannheim) was used to incorporate digoxigenin-11–dUTP into first-strand cDNA. The cDNA was transcribed from a mixture of 16S and 23S rRNAs from Escherichia coli MRE600 by random priming by using reverse transcriptase. This probe was used to screen restriction fragment length polymorphisms of the gene encoding rRNA. The purified probes were stored at −20°C until used.
Hybridization.
Prehybridization and hybridization of the membrane were performed by using the Genius System as specified by the manufacturer (Boehringer Mannheim). Positive signals were detected by a color reaction of nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate with alkaline phosphatase-labeled anti-digoxigenin antibodies. The reaction was terminated by several water washes.
Antibiograms.
Each isolate was tested for susceptibility against antibiotics commonly used to treat BRD by using the automated Sensititre microtiter plate system (AccuMed Sensititre, Westlake, Ohio) according to the guidelines outlined by the National Committee for Clinical Laboratory Standards (NCCLS) (18).
RESULTS
Isolation rate.
Forty calves with diagnosed clinical BRD were sampled once each with a nasal and a transtracheal swab. Significant bacterial isolates were recovered from 38 of 40 calves. The bacteria recovered by using both sampling techniques are summarized in Table 1. The bacterial pathogens recovered from nasal and transtracheal swabs were the same 68.4% of the time. Each sampling procedure demonstrated approximately the same frequency (10%) of obtaining a negative culture.
TABLE 1.
Frequency of bacterial species recovered by using a nasal or a transtracheal swab
| Nasal swab isolate | Transtracheal swab isolate | No. of paired isolates |
|---|---|---|
| P. haemolytica | P. haemolytica | 17 |
| P. haemolytica and P. multocida | P. haemolytica | 4 |
| P. haemolytica | P. haemolytica and P. multocida | 2 |
| P. multocida | P. multocida | 3 |
| Negative | P. haemolytica | 5 |
| P. haemolytica | Negative | 3 |
| P. haemolytica and P. multocida | Negative | 1 |
| Negative | P. haemolytica and P. multocida | 1 |
| Negative | P. haemolytica and H. somnus | 1 |
| H. somnus | P. haemolytica | 1 |
Serotypes.
All P. haemolytica isolates reacted with antiserum to capsule antigen serotype 1 except one, which autoagglutinated and thus could not be typed by the plate agglutination assay. All P. haemolytica isolates belonged to biotype A.
All P. multocida isolates were positive for somatic antigen type 3 by agar gel immunodiffusion. Three of the four isolates were serotype A, while one isolate was negative for types A and D. However, the inability to type this strain may have been due to the loss of capsule due to in vitro culture.
Ribotyping.
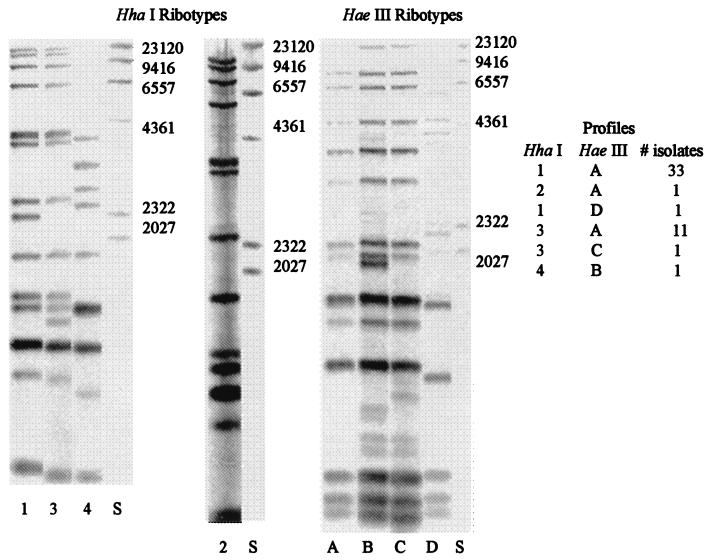
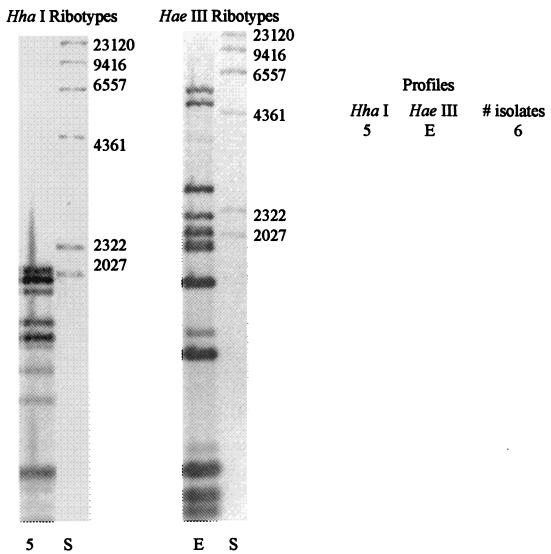
Four restriction enzymes were tested to determine which resulted in the highest level of differentiation among the pasteurella isolates. PvuII and PstI did not result in any differences among the isolates (data not shown). HaeIII and HhaI exhibited the greatest degree of differentiation and thus were selected for restriction digestion of the isolates prior to ribotyping. Digestion of P. haemolytica with HhaI or HaeIII resulted in four ribotype profiles with each enzyme. When combining profiles obtained with both enzymes, six distinct ribotype patterns were observed. All of the P. multocida pairs exhibited the same ribotype profile.
Among the P. haemolytica isolates, profile 1A represented 69%, while profile 3A represented 23% of all the isolates (Fig. 1). The remaining four profiles each represented only 2.1% of all the isolates. Among the P. haemolytica isolates, 16 of the 24 sets were identical, with 13 (54%) sets belonging to the 1A profile and 3 (12.5%) sets belonging to the 3A profile. Among the P. multocida isolates (Fig. 2), the three matched pairs exhibited the same ribotype. Combining all of the pasteurella isolates, 19 of the 27 (70%) matched pairs exhibited identical ribotype profiles.
FIG. 1.
Representative HaeI and HaeIII ribotypes of P. haemolytica isolated from clinical cases of BRD. HindIII-digested λ DNA (S) is used as a size marker. Letters and numbers are arbitrarily assigned. The figure was generated with Adobe Photoshop 5.5.
FIG. 2.
Representative HaeI and HaeIII ribotypes of P. multocida from clinical cases of BRD. HindIII-digested λ DNA (S) is used as a size marker. The figure was generated with Adobe Photoshop 5.5.
Antibiograms.
Table 2 presents the MIC for each isolate of a panel of antimicrobials used to treat BRD. For P. haemolytica isolates, MICs of ceftiofur, erythromycin, tilmicosin, trimethoprim-sulfamethoxazole, and florfenicol were low. For most isolates, ampicillin and spectinomycin MICs were high. For P. multocida isolates, the MICs of the panel of antibiotics were generally low, except those of spectinomycin for a few isolates.
TABLE 2.
Comparison of MICs of seven antibiotics for 27 matched isolate pairsa
| Isolate | Ribotype
|
MIC (μg/ml)
|
% Similarity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HhaI | HaeIII | Amp | Cef | Ery | Spec | Til | TM/SM | Flo | ||
| 56-N | 1 | A | 32 | 0.062 | 2 | 32 | 2 | 0.25/4.75 | 1 | 100 |
| 56-T | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | |
| 72-N | 1 | A | 32 | 0.062 | 2 | 32 | 2 | 0.25/4.75 | 1 | 86 |
| 72-T | 2 | A | 8 | 0.062 | 4 | 32 | 4 | 0.25/4.75 | 1 | |
| 54-N | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 54-T | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | |
| 202-N | 3 | A | 0.25 | 0.062 | 4 | 128 | 4 | 0.25/4.75 | 1 | 71 |
| 202-T | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | |
| 120-N | 3 | A | 8 | 0.062 | 1 | 16 | 2 | 0.25/4.75 | 1 | 86 |
| 120-T | 1 | A | 32 | 0.062 | 4 | 16 | 4 | 0.25/4.75 | 1 | |
| 68-N | 1 | A | 32 | 0.031 | 2 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 68-T | 1 | A | 32 | 0.062 | 2 | 32 | 2 | 0.25/4.75 | 2 | |
| 185-N | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 185-T | 1 | A | 32 | 0.062 | 4 | 32 | 2 | 0.25/4.75 | 0.5 | |
| 97-N | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 97-T | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | |
| 304-N | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | 71 |
| 304-T | 3 | A | 8 | 0.062 | 4 | 128 | 4 | 0.25/4.75 | 1 | |
| 65-N | 3 | A | 4 | 0.062 | 1 | 32 | 4 | 0.25/4.75 | 1 | 100 |
| 65-T | 3 | A | 8 | 0.062 | 1 | 16 | 4 | 0.25/4.75 | 1 | |
| 106-N | 1 | A | 32 | 0.062 | 2 | 32 | 2 | 0.25/4.75 | 1 | 100 |
| 106-T | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | |
| 128-N | 3 | A | 0.25 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | 86 |
| 128-T | 3 | A | 1 | 0.062 | 4 | 16 | 4 | 0.25/4.75 | 1 | |
| 187-N | 3 | A | 2 | 0.062 | 4 | 128 | 4 | 0.25/4.75 | 0.5 | 86 |
| 187-T | 4 | B | 8 | 0.062 | 2 | 64 | 4 | 0.25/4.75 | 0.5 | |
| 217-N | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | 86 |
| 217-T | 1 | A | 32 | 0.062 | 4 | 64 | 2 | 0.25/4.75 | 1 | |
| 244-N | 3 | A | 0.25 | 0.062 | 0.5 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 244-T | 3 | A | 0.25 | 0.062 | 1 | 16 | 2 | 0.25/4.75 | 1 | |
| 250-N | 3 | C | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 250-T | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | |
| 258-N | 1 | D | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 258-T | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | |
| 320-N | 1 | A | 32 | 0.062 | 4 | 128 | 4 | 0.25/4.75 | 1 | 86 |
| 320-T | 1 | A | 8 | 0.062 | 4 | 128 | 4 | 0.25/4.75 | 1 | |
| 117-N | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 117-T | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | |
| 219-N | 1 | A | 32 | 0.062 | 4 | 32 | 2 | 0.25/4.75 | 1 | 100 |
| 219-T | 1 | A | 32 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | |
| 220-N | 1 | A | 32 | 0.062 | 4 | 128 | 2 | 0.25/4.75 | 1 | 86 |
| 220-T | 3 | A | 8 | 0.062 | 4 | 128 | 4 | 0.25/4.75 | 1 | |
| 27-N | 1 | A | 0.5 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 0.5 | 100 |
| 27-T | 1 | A | 0.25 | 0.062 | 4 | 16 | 2 | 0.25/4.75 | 1 | |
| 86-N | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 86-T | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | |
| 99-N | 1 | A | 32 | 0.062 | 2 | 16 | 2 | 0.25/4.75 | 1 | 100 |
| 99-T | 1 | A | 32 | 0.062 | 4 | 16 | 4 | 0.25/4.75 | 1 | |
| 343-N | 5 | E | 8 | 0.062 | 4 | 64 | 2 | 0.25/4.75 | 1 | 71 |
| 343-T | 5 | E | 0.25 | 0.062 | 2 | 8 | 1 | 0.25/4.75 | 0.5 | |
| 258-N | 5 | E | 0.25 | 0.062 | 0.5 | 128 | 2 | 0.25/4.75 | 0.5 | 100 |
| 258-T | 5 | E | 0.25 | 0.062 | 0.5 | 128 | 2 | 0.25/4.75 | 0.5 | |
| 142-N | 5 | E | 0.25 | 0.062 | 0.5 | 8 | 2 | 0.25/4.75 | 0.5 | 100 |
| 142-T | 5 | E | 0.25 | 0.062 | 0.5 | 4 | 2 | 0.25/4.75 | 0.25 | |
The similarity of the MICs is based on whether the MICs for each isolate are within one twofold dilution of each other. Amp, ampicillin; Cef, ceftiofur; Ery, erythromycin; Spec, spectinomycin; TM/SM, trimethoprim-sulfamethoxazole; Flo, florfenicol.
Ceftiofur and tilmicosin have NCCLS-approved MIC breakpoints. All isolates were sensitive to ceftiofur (MIC of ≤2 μg/ml) and tilmicosin (MIC of ≤8 μg/ml). Ninety percent of the isolates (i.e., MIC90) were inhibited by 32 μg of ampicillin, 4 μg of erythromycin, 128 μg of spectinomycin, 0.25 and 4.75 μg of trimethoprim-sulfamethoxazole (respectively), and 1 μg of florfenicol per ml. Breakpoints for veterinary use have yet to be established for ampicillin, erythromycin, trimethoprim-sulfamethoxazole, spectinomycin, and florfenicol. Although a few paired isolates exhibited identical ribotypes, their antibiotic susceptibility profiles were different.
DISCUSSION
BRD is a complex syndrome with many etiologic agents. Bacterial pathogens are the most common agents but, currently, there is no efficient and simple test to determine the identity of the disease-causing pathogen ante mortem in the lungs. The goal of this study was to determine whether the identity of a nasal culture would be the same as or closely related to that of a culture obtained from a transtracheal swab of an animal with BRD. Validation of a procedure that correlates the identity of a pathogen in the nasal flora to the identity of the pathogen in the lungs during clinical BRD would enable the practitioner to determine the identity of the pathogens producing the respiratory condition in a particular group of animals.
In this study paired isolates, one from a nasal swab and the other from a transtracheal swab, were acquired from individual calves with clinical signs of BRD. Although the calves were sampled only once each with a nasal and a transtracheal swab, when both samples were bacteriologically positive, the nasal swab culture identified the same bacterial species as the transtracheal swab culture 96% of the time.
Previous research demonstrated that the P. haemolytica A1 serotype is most commonly isolated from stressed feeder calves (10). This serotype has been demonstrated to colonize the tonsils of healthy calves (11). During times of immunological stress, such as shipping and/or vaccination, P. haemolytica rapidly proliferates and is shed in nasal secretions (12). This increased proliferation has been shown to increase the number of aerosolized bacteria (14), thus allowing the bacteria to be aspirated deeper into the lung, and it also provides for potential airborne transmission to other calves. Therefore, such a mechanism would suggest that the isolate in the lung could be clonal in nature to the isolate recovered from the nasal culture.
The results of this study suggest that a nasal swab culture was genetically identical with the organism causing disease within the lung for 70% of the calves. From a therapeutic standpoint, antibiotic susceptibilities of the paired isolates are similar even though they may have different ribotypic profiles. An exception to this statement is the susceptibility to ampicillin and spectinomycin. Nine of eleven paired isolates that differed in their susceptibility to ampicillin differed by more than one dilution. A similar observation was noted for 5 of 12 paired isolates in their susceptibility to spectinomycin. The high MICs and variation observed with spectinomycin and ampicillin may be due to the presence of plasmids encoding antibiotic resistance. It has been previously reported that several plasmids are present in P. haemolytica serotype 1 (3). One plasmid encodes a β-lactamase conferring resistance against ampicillin, while another encodes streptomycin resistance which would provide cross-resistance to spectinomycin. Additionally, in vivo, pasteurellae may acquire or lose plasmids very readily while, in vitro, pasteurellae may have lost their plasmid(s) during passage prior to antibiotic susceptibility testing (7, 17).
Available data on therapeutic outcome, pharmacokinetics, and antimicrobial susceptibility testing provide the rational basis for MIC breakpoint and interpretative criteria (18). Strains designated susceptible can be inhibited by achievable serum or tissue levels of the dosage of antimicrobial agent. With intermediate strains MICs approach or exceed usually attainable blood or tissue levels, and the response rate tends to be lower than for susceptible strains. Resistant strains are not inhibited by the usually achievable systemic concentrations of the agent with normal dosage schedules and/or fall in the range where specific microbial resistance mechanisms are likely, and clinical efficacy has not been reliable in treatment studies. Using the results of this study, future breakpoints and interpretative criteria can be reliably determined with nasal isolates.
Although this study was done with a limited group of high-risk calves, several results are worth noting. A nasal culture of a clinically ill animal was predictive of the pathogen in the lungs and was genetically identical 70% of the time. The antibiotic susceptibility of the paired isolates against various antibiotics used to treat BRD was similar except for some minor variations for ampicillin and spectinomycin MICs. The predominance of two ribotypes among the clinically ill cattle is suggestive that either transmission of P. haemolytica occurred among the cattle during transport and commingling or else it simply reflected the farm origin of the calves. The results of this study suggest that a nasal swab culture can guide practitioners with some degree of confidence in the treatment of cattle clinically ill with bacterial pneumonia by providing an easily obtained culture that is representative of a lung isolate, especially with regard to antibiotic susceptibility.
ACKNOWLEDGMENTS
We thank the Texas Veterinary Medical Diagnostic Laboratory in Amarillo, Tex., for the original solution and identification of the BRD isolates. We also thank Chuck Scott for his graphic art expertise.
ADDENDUM IN PROOF
In June 1999 at the Veterinary Antimicrobial Susceptibility Testing meeting, MIC breakpoints for florfenicol for use in cattle were approved. The approved florfenicol MIC breakpoints for the BRD organisms M. haemolytica, P. multocida, and Haemophilus somnus are ≤2 μg/ml (sensitive), 4 μg/ml (intermediate), and ≥8 μg/ml (resistant).
The result of a recent publication (Int. J. Syst. Bacteriol. 49:67–86, 1999) has renamed P. haemolytica as Mannheimia haemolytica, reflecting its phylogenetic difference.
REFERENCES
- 1.Allen J W, Laurent V, Bateman K G, Rosendal S, Shewen P E, Physick-Sheard P. The microbial flora of the respiratory tract in feedlot calves: associations between nasopharyngeal and bronchoalveolar lavage cultures. Can J Vet Res. 1991;55:341–346. [PMC free article] [PubMed] [Google Scholar]
- 2.Briggs R E, Frank G H. Increased elastase activity in nasal mucus associated with nasal colonization by Pasteurella haemolytica in infectious bovine rhinotracheitis virus-infected calves. Am J Vet Res. 1992;53:631–635. [PubMed] [Google Scholar]
- 3.Briggs R E, Frank G H, Purdy C W, Zehr E S, Loan R W. Rapid spread of a unique strain of Pasteurella haemolytica serotype 1 among transported calves. Am J Vet Res. 1998;59:401–405. [PubMed] [Google Scholar]
- 4.Carter G R, Subronoto P. Identification of type D strains of Pasteurella haemolytica with acriflavin. Am J Vet Res. 1973;34:293–294. [PubMed] [Google Scholar]
- 5.Carter G R, Rundell S W. Identification of type A strains of P. multocida using staphylococcal hyaluronidase. Vet Rec. 1975;96:343. doi: 10.1136/vr.96.15.343. [DOI] [PubMed] [Google Scholar]
- 6.Church T, Radostits O M. A retrospective survey of diseases of feedlot cattle in Alberta. Can Vet J. 1981;22:27–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Corstvet R E, Panciera R J, Rinker H B, Starks B L, Howard C. Survey of tracheas of feedlot cattle for Haemophilus somnus and other selected bacteria. J Am Vet Med Assoc. 1973;163:870–873. [Google Scholar]
- 8.Eisenstein B I. New molecular techniques for microbial epidemiology and the diagnosis of infectious disease. Rev Infect Dis. 1990;161:595–602. doi: 10.1093/infdis/161.4.595. [DOI] [PubMed] [Google Scholar]
- 9.Frank G H, Wessman G E. Rapid plate agglutination procedure for serotyping Pasteurella haemolytica. J Clin Microbiol. 1982;7:142–145. doi: 10.1128/jcm.7.2.142-145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank G H, Smith P C. Prevalence of Pasteurella haemolytica in transported calves. Am J Vet Res. 1983;44:981–985. [PubMed] [Google Scholar]
- 11.Frank G H, Briggs R E. Colonization of the tonsils of calves with Pasteurella haemolytica. Am J Vet Res. 1992;53:481–484. [PubMed] [Google Scholar]
- 12.Heddleston K L, Wessman G E. Characteristics of Pasteurella multocida of human origin. J Clin Microbiol. 1975;1:377–383. doi: 10.1128/jcm.1.4.377-383.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen R, Pierson R E, Braddy P M, Saari D A, Lauerman L H, England J J, Keyvanfar H, Collier J R, Horton D P, McChesney A E, Bebitez A, Christie R M. Shipping fever pneumonia in yearling feedlot cattle. J Am Vet Med Assoc. 1978;160:500–506. [PubMed] [Google Scholar]
- 14.Jericho K W F, Lejeune A, Tiffin G B. Bovine herpesvirus-1 and Pasteurella haemolytica aerobiology in experimentally infected calves. Am J Vet Res. 1986;47:205–209. [PubMed] [Google Scholar]
- 15.Kelly A P, Janzen E D. A review of morbidity and mortality rates and disease occurrence in North American feedlot cattle. Can Vet J. 1986;27:497–499. [PMC free article] [PubMed] [Google Scholar]
- 16.Martin S W. Factors influencing morbidity and mortality in feedlot calves in Ontario. Vet Clin N Am (Large Anim Pract) 1983;5:74–86. [PubMed] [Google Scholar]
- 17.Murphy G L, Robinson L C, Burrows G E. Restriction endonuclease analysis and ribotyping differentiate Pasteurella haemolytica serotype A1 isolates from cattle within a feedlot. J Clin Microbiol. 1993;31:2303–2308. doi: 10.1128/jcm.31.9.2303-2308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M31-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.Schiefer B, Ward G E, Moffatt R E. Correlation of microbiological and histological findings in bovine fibrinous pneumonia. Vet Pathol. 1978;15:313–321. doi: 10.1177/030098587801500305. [DOI] [PubMed] [Google Scholar]
- 20.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 21.Thomson R G, Chandler S, Savan M, Fox M L. Investigation of factors of probable significance in the pathogenesis of pneumonic pasteurellosis in cattle. Can J Comp Med. 1975;39:194–207. [PMC free article] [PubMed] [Google Scholar]