Abstract
The effects of metal on pulmonary function are inconsistent, and abnormal distribution of metals can decrease lung function. However, the effects of metals exposure on chronic obstructive pulmonary disease (COPD) are still unclear. This study aims to explore the relationship between metal exposure and COPD risk. Cross-sectional data from the National Health and Nutrition Survey (NHANES) 2015–2016 was analyzed. Inductively coupled plasma dynamic reaction cell mass spectrometry (ICP-DRC-MS) was used to measure the metals concentration in the blood. The multiple linear regression and restricted cubic spline (RCS) were used to analyze the relationship between metals exposure and COPD risk. In this study, 1399 participants were included, of which 107 participants were diagnosed with COPD using self-reported chronic bronchitis, emphysema, and COPD. The second and third tertiles of copper increased the COPD risk by 1.98-fold (95% CI: 1.08–3.62) and 2.43-fold (95% CI: 1.32–4.48) compared with the first tertile, using p = 0.005 for the trend after adjusting for the covariates. RCS showed a positive linear correlation between copper and COPD risk (p = 0.006 for overall association) in all participants. When stratified by sex, the multi-factor analysis showed that the third tertile of copper increased male’s COPD risk by 3.42-fold (95% CI: 1.52–7.76), with p = 0.003 for the trend, and RCS also showed a positive linear correlation (p = 0.013 for overall association). Although RCS showed that selenium can reduce the COPD risk (p = 0.008 for overall association) in males, an association between selenium and COPD was not observed (p > 0.05). Our findings suggest that a high concentration of copper may increase COPD risk in males in the general US population, and more research is needed to explore its possible mechanism of action.
Keywords: metals, trace minerals, COPD, RCS, copper
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a disease characterized by persistent airflow limitation caused by large amounts of exposure to toxic particles and gases. It includes chronic bronchial and emphysema [1]. The most common symptoms of COPD are dyspnea, cough, and sputum production [2]. According to statistics from the World Health Organization (WHO), there are currently about 600 million people suffering from COPD in the world, and an average of about 2.7 million people die from COPD every year [3]. Due to the high prevalence, morbidity, and mortality of COPD, it has brought a great burden of disease to the world, it has always been one of our main public health issues [4]. COPD ranked 11th among the top 15 major diseases that caused the loss of disability-adjusted healthy life years in 1990, and by 2019, COPD rose to 6th. Additionally, 66% of the global burden of COPD and lung cancer comes from low- and middle-income countries [5]. Smoking, indoor air pollution (such as biofuels used for cooking and heating), outdoor air pollution, occupational dust, and chemicals are the main risk factors for COPD, among which smoking is the most important factor [6,7,8]. Therefore, the first measure to prevent and treat COPD is to quit smoking. In addition, vaccinations and drugs can also be used to prevent and treat COPD [2].
Metals may have different effects on pulmonary function. Copper, zinc, and selenium are essential trace elements for the human body. Copper in tap water was positively related to levels of both FVC and FEV1 among never smokers [9]. Xiao, Zhou et al. reported that copper can reduce the forced expiratory volume in 1 s [10]. Cadmium can enter the human body with tobacco drugs and can accumulate in the body, especially in the lungs [11,12], and the accumulation of cadmium can cause lung inflammation and a decline in lung function [13,14,15]. Zinc has an antagonistic effect on the toxicity of cadmium and copper [13,16]. A lack of zinc may lead to impaired immune function and tumor development [17,18,19]. An increased prevalence of obstructive lung disorder was observed among individuals with low zinc intake regardless of smoking status, and the adjusted odds of lung disorder are approximately 1.9 times greater for subjects in the lowest zinc-intake tertile than those in the highest tertile (odds ratio = 1.89, 95% confidence interval = 1.22–2.93) [20]. Selenium is related to the cellular antioxidant defense mechanism. Oxidative stress and chronic inflammation are important features in the pathogenesis of COPD [21]. However, the relationship between selenium and COPD is unclear. Studies have shown that the accumulation of manganese reduces the expression of cystic fibrosis transmembrane conductance regulator (CFTR), which is a chloride channel located in airway epithelial cells. CFTR plays an important role in maintaining the dynamic balance of airway surface fluid (ASL) volume and the physiological function of the lungs. If the expression decreases or the function is impaired, the lungs cause mucus accumulation, reduced bacterial clearance, and chronic infection and inflammation [22]. A study in NHANES 2007–2010 found a dose-dependent relationship between blood lead concentration and the risk of COPD [23].
Since the associations between metal exposure and trace minerals, and COPD remain unclear, and nothing is known about their dose–response relationship, we used the data from NHANES (2015–2016) to explore the relationship between metal and trace minerals, and COPD.
2. Materials and Methods
2.1. Study Population
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional survey based on the whole population of the United States. The study protocol was approved by the NCHS Research Ethics Review Board (Continuation of Protocol #2011-17), and all participants provided written informed consent. It combines questionnaire surveys and physical examinations to collect information about the health and nutrition of the American family population, including demographics, diet-related issues, physical examinations, laboratory examinations, and more [11].
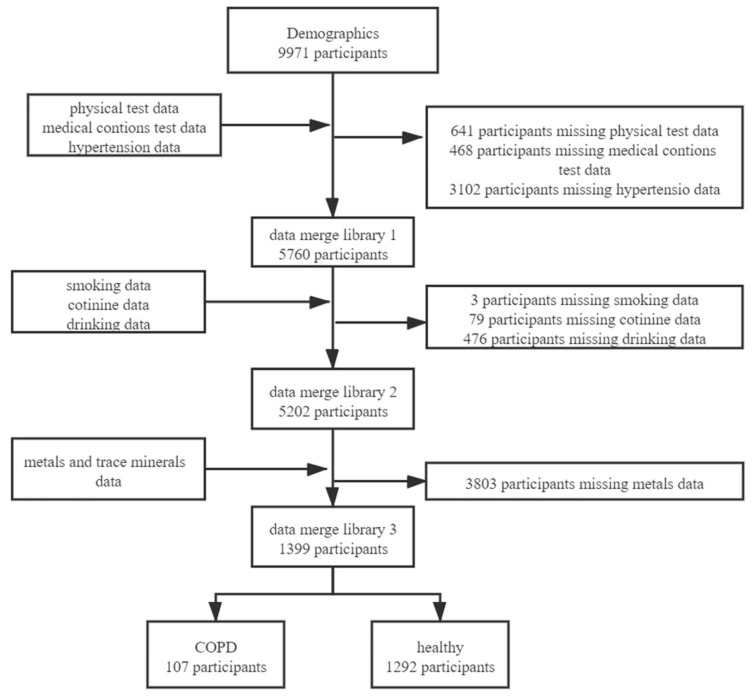
The data from NHANES 2015–2016 was analyzed, and the 9971 participants were screened. We merged the databases based on the unique identity of the survey subjects. After merging the databases, we excluded 8572 who had missing data in physical examination, medical conditions, drinking, smoking, second-hand smoke exposure, serum cotinine, hypertension, diabetes, and blood metal measurements. Finally, 1399 survey subjects were included in the study, including 694 males and 705 females (Figure 1).
Figure 1.
Flowchart for the inclusion of study participants.
2.2. Metal and Trace Mineral Measurements
The processed blood were stored under −20 °C conditions and sent to the laboratory for analysis. The levels of metals were measured using inductively coupled plasma dynamic reaction cell mass spectrometry (ICP-DRC-MS) by the Centers for Disease Control and Prevention in the US [24,25,26].
2.3. COPD
Current chronic bronchitis was defined by positive answers to the questions (1) “Has a doctor or other health professional ever told you that you have chronic bronchitis?” and (2) “Do you still have chronic bronchitis?” Emphysema was defined by positive answers to the questions: “Has a doctor or other health professional ever told you that you have emphysema?” COPD was defined by positive answers to the questions: “Has a doctor or other health professional ever told you that you have COPD?” [1].
2.4. Smoking and Secondhand Smoke Exposure
Smokers were divided into three groups: non-smokers, current smokers, and former smokers. Non-smokers were defined as smoking less than 100 cigarettes in their lifetime. Current smokers were defined as smoking more than 100 cigarettes in their lifetime and were still smoking. Former smokers were defined as smoking more than 100 cigarettes in their lifetime but not smoking now [20,27].
Participants who had been to jobs, bars, restaurants, other homes, and other indoor places and who had smoked cigarettes in these places were included in the secondhand smoke exposure group.
2.5. Drinking
Participants were divided into drinking groups and non-drinking groups according to whether they had drunk more than 12 alcoholic drinks in their lifetime [28].
2.6. Covariates
We included the covariates based on previous studies [3,29], which might be related to COPD or the concentration of metal. We collected basic information about participants through questionnaires, including gender; age; race (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, and other race); education (less than 9th grade, 9–11 grade, high school graduate, some college/AA degree, and college graduate); the ratio of family income to poverty and BMI (<18.5, 18.5–25, 25–30, ≥30); and hypertension and diabetes, which might be related to COPD or the concentration of metal [3,29].
2.7. Statistical Analysis
All analyses were performed with Stata version 15.0 (StataCorp LP, College Station, Texas, USA) and R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). RCS was implemented with the R package “rms” (version 6.1-1) (Frank Harrell, Nashville, Tennessee, USA). The continuous variables were represented by mean ± SD, non-normally distributed continuous variables were represented by the interquartile range (IQR), and categorical variables were represented by cases (n) and percentage (%). The Chi-square test was used to compare the demographic differences between the COPD cases and the control group, including age, gender, smoking, PIR, BMI, race, education, alcohol consumption, second-hand smoke exposure, high blood pressure, and diabetes. As the distributions of metals were right-skewed (Figure S1), the heavy metals were standardized via a natural ln (loge) transformation and the Wilcoxon rank-sum test was used to compare the metal concentration between the case group and the control group. A multiple logistic regression model was used to analyze the relationship between metals exposure and the risk of COPD. The metal exposure level was divided into tertiles (T1, T2, and T3 were the first, second, and third tertiles, respectively), T1 was used as a reference, and odds ratios (ORs) and 95% confidence intervals (95% CIs) were used to describe the relationship between metal exposure levels and diseases. RCS [30] was used to further analyze the relationship and trend between metal exposure and COPD because RCS not only can analyze the linear relationship between metals and the risk of COPD but also can reflect the nonlinear relationship between the two.
3. Results
3.1. Demographic Characteristics
In the study, 1399 participants were included; 107 participants with emphysema, chronic bronchitis, and COPD were classified as the COPD group; and 1292 were classified as the healthy group. There was a difference in the age distribution between the COPD group (58.53 ± 16.19) and the healthy group (48.81 ± 17.43) (p < 0.001). The results showed a significant difference in smoking between the two groups (p < 0.001). The proportion of former smoking in the COPD group (35.45%) was higher than that in the healthy group (23.08%). The COPD group had higher levels of serum cotinine (115.13 ± 166.73) than the control group (46.26 ± 108.12) (p < 0.001). Significant differences in race, BMI, education, the ratio of family income to poverty, secondhand smoke exposure, hypertension, and diabetes (p < 0.05) were also observed. The levels of lead, calcium, and copper in the COPD group were higher than those in the healthy group (p < 0.05). However, the levels of selenium in the COPD group were lower than in the healthy group (p < 0.05) (Table 1).
Table 1.
Demographic and socio-behavioral characteristics, metals level, and COPD disease status of the study population (N (%)).
| Variables | Healthy | COPD | p-Value |
|---|---|---|---|
| (N = 1292) | (N = 107) | ||
| Age | 48.81 ± 17.43 a | 58.53 ± 16.19 a | <0.001 *** |
| Sex | 0.828 | ||
| Male | 642 (49.69) | 52 (48.60) | |
| Female | 650 (50.31) | 55 (51.40) | |
| Race | <0.001 *** | ||
| Mexican American | 233 (18.03) | 8 (7.48) | |
| Other Hispanic | 167 (12.93) | 20 (78.69) | |
| Non-Hispanic White | 442 (34.21) | 60 (56.07) | |
| Non-Hispanic Black | 252 (19.50) | 16 (14.95) | |
| Other Race | 198 (15.33) | 3 (2.80) | |
| BMI (kg/m2) | 0.019 * | ||
| <18.5 | 18 (1.39) | 1 (0.93) | |
| 18.5–25 | 325 (25.15) | 16 (14.95) | |
| 25-30 | 437 (33.82) | 32 (29.91) | |
| ≥30 | 512 (39.63) | 58 (54.21) | |
| Education | 0.025 * | ||
| Less than 9th grade | 134 (10.37) | 17 (15.89) | |
| 9–11 grade | 142 (10.99) | 15 (14.02) | |
| High school graduate | 293 (22.68) | 26 (24.30) | |
| Some college/AA degree | 379 (29.33) | 35 (32.71) | |
| College graduate | 344 (26.63) | 14 (13.08) | |
| Ratio of Family Income to Poverty | 0.001 ** | ||
| Under standard level | 1071 (82.89) | 102 (95.33) | |
| Above standard level | 221 (17.11) | 5 (4.67) | |
| Smoking | <0.001 *** | ||
| Current smoking | 751 (58.13) | 31 (28.97) | |
| Non-smoking | 234 (18.11) | 38 (35.51) | |
| Former smoking | 307 (23.76) | 38 (35.51) | |
| Secondhand smoke exposure | 0.013 ** | ||
| Yes | 947 (75.39) | 69 (64.49) | |
| No | 318 (24.61) | 38 (35.51) | |
| Serum cotinine (ng/mL) | 0.03 (0.01, 4.08) b | 0.18 (0.01, 227) b | <0.001 *** |
| Drinking | 0.052 | ||
| Yes | 899 (69.58) | 84 (78.50) | |
| No | 393 (30.42) | 23 (21.50) | |
| Hypertension | <0.001 *** | ||
| Yes | 428 (33.13) | 67 (62.62) | |
| No | 864 (66.87) | 40 (37.38) | |
| Diabetes | <0.001 * | ||
| Yes | 198 (15.33) | 35 (32.71) | |
| No | 1094 (84.67) | 72 (67.29) | |
| Blood Pb (µg/dL) | 0.93 (0.59, 1.49) b | 1.20 (0.75, 2.02) b | <0.001 *** |
| Tertile 1 (0.05–0.71) | 445 (34.44) | 23 (21.50) | |
| Tertile 2 (0.72–1.32) | 431 (33.36) | 34 (31.78) | |
| Tertile 3 (1.33–23.51) | 416 (32.20) | 50 (46.73) | |
| Blood Cd (µg/L) | 0.29 (0.18, 0.50) b | 0.41 (0.23, 0.93) b | <0.001 *** |
| Tertile 1 (0.07–0.22) | 458 (35.45) | 26 (24.30) | |
| Tertile 2 (0.23–0.42) | 426 (32.97) | 28 (26.17) | |
| Tertile 3 (0.43–6.37) | 408 (31.58) | 53 (49.53) | |
| Blood Mn (µg/L) | 9.52 (7.72, 11.91) b | 9.72 (7.61, 11.83) b | 0.862 |
| Tertile 1 (2.31–8.30) | 427 (33.05) | 40 (37.38) | |
| Tertile 2 (8.31–10.96) | 439 (33.98) | 27 (25.23) | |
| Tertile 3 (10.97–56.56) | 426 (32.97) | 40 (37.38) | |
| Blood Se (µg/L) | 192.75 (178.99, 207.38) b | 189.26 (176.96, 201.26) b | 0.070 |
| Tertile 1 (119.87–183.22) | 427 (33.05) | 40 (37.38) | |
| Tertile 2 (183.23–201.75) | 423 (32.74) | 43 (40.19) | |
| Tertile 3 (201.76–318.33) | 442 (34.21) | 24 (22.43) | |
| Serum Cu (µg/dL) | 114.25 (99.15, 133.10) b | 124.30 (110.40, 145.00) b | <0.001 *** |
| Tertile 1 (52.90–105.00) | 454 (35.14) | 18 (16.82) | |
| Tertile 2 (105.01–126.60) | 425 (32.89) | 38 (35.51) | |
| Tertile 3 (126.61–306.60) | 413 (31.97) | 51 (47.66) | |
| Serum Zn (µg/dL) | 79.90 (69.80, 89.90) b | 80. 90 (72.50, 91.10) b | 0.408 |
| Tertile 1 (31.40–73.70) | 442 (34.21) | 27 (25.23) | |
| Tertile 2 (73.71–86.40) | 416 (32.20) | 48 (44.86) | |
| Tertile 3 (86.41–139.10) | 434 (33.59) | 32 (29.91) |
Note: *, p < 0.05; **, p < 0.01; ***, p < 0.001. a The continuous variables were represented by mean ± SD. b Non-normally distributed continuous variables were represented by IQR: P50(P25, P75).
3.2. The Association between Metals, Trace Mineral Exposure, and the Risk of COPD
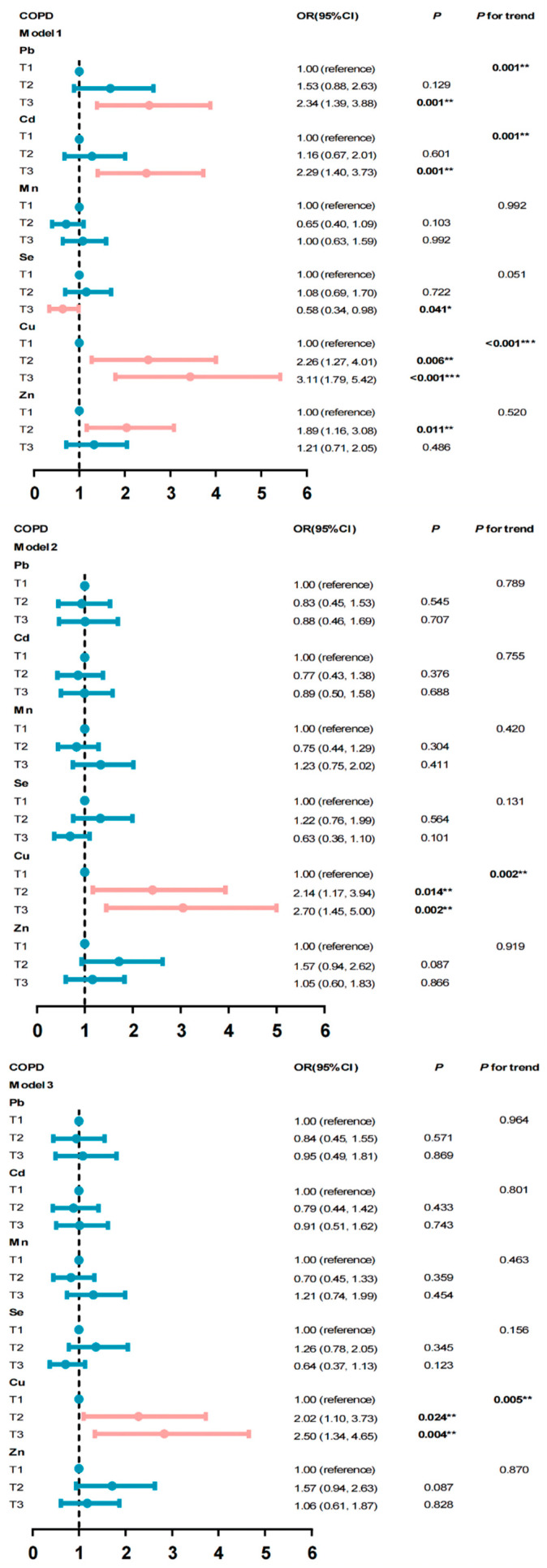
The second and third tertiles of copper increased the risk of COPD by 2.02 times and 2.50 times compared with the first tertile after adjusting for other covariates, respectively, the p-value for trend was 0.005 (T2 95% CI: 1.10–3.73; T3 95% CI: 1.34–4.65) (Figure 2). We further analyzed their relationships stratified by gender. The third tertile of copper only increased COPD risk in males by 3.31 times (p = 0.004, 95% CI: 1.47–7.44) compared with the lowest tertile, and the p-value for trend was 0.004 (Table 2).
Figure 2.
Association between COPD and metals. Model 1: unadjusted model; Model 2: adjusted for age, smoke, ratio of family income to poverty, BMI, sex, race, education, drink, second-hand smoke exposure, and serum cotinine; Model 3: adjusted for age, smoke, ratio of family income to poverty, BMI, sex, race education, drink, second-hand smoke exposure, serum cotinine, hypertension, and diabetes. * p < 0.05; ** p < 0.01; and *** p < 0.001.
Table 2.
Risk of COPD associated with level of metals in different genders.
| variables | Male | Female | ||||
|---|---|---|---|---|---|---|
| OR | p | 95% CI | OR | p | 95% CI | |
| Pb | ||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| T2 | 1.20 | 0.752 | 0.38–3.79 | 0.78 | 0.522 | 0.36–1.68 |
| T3 | 1.70 | 0.362 | 0.54–5.31 | 0.68 | 0.384 | 0.28–1.63 |
| p for trend | 0.279 | 0.388 | ||||
| Cd | ||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| T2 | 0.65 | 0.378 | 0.26–1.68 | 0.80 | 0.576 | 0.37–1.74 |
| T3 | 1.64 | 0.235 | 0.73–3.69 | 0.48 | 0.110 | 0.19–1.18 |
| p for trend | 0.160 | 0.109 | ||||
| Mn | ||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| T2 | 0.90 | 0.796 | 0.41–1.99 | 0.66 | 0.278 | 0.31–1.40 |
| T3 | 1.74 | 0.122 | 0.86–3.50 | 0.85 | 0.654 | 0.41–1.74 |
| p for trend | 0.147 | 0.696 | ||||
| Se | ||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| T2 | 0.86 | 0.683 | 0.43–1.75 | 1.79 | 0.092 | 0.91–3.53 |
| T3 | 0.48 | 0.078 | 0.22–1.08 | 0.83 | 0.633 | 0.38–1.81 |
| p for trend | 0.081 | 0.806 | ||||
| Cu | ||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| T2 | 1.65 | 0.200 | 0.77–3.52 | 2.53 | 0.113 | 0.80–7.96 |
| T3 | 3.31 | 0.004 ** | 1.47–7.44 | 2.38 | 0.128 | 0.78–7.28 |
| p for trend | 0.004 ** | 0.247 | ||||
| Zn | ||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| T2 | 1.43 | 0.332 | 0.69–2.96 | 1.81 | 0.124 | 0.85–3.86 |
| T3 | 0.72 | 0.423 | 0.32–1.62 | 1.70 | 0.195 | 0.76–3.80 |
| p for trend | 0.420 | 0.212 | ||||
Note: ** p < 0.01. The models were adjusted for age, smoke, ratio of family income to poverty, BMI, sex, race education, drink, second-hand smoke exposure, serum cotinine, hypertension, and diabetes.
3.3. Dose–Response Relationship between Metals and the Risk of COPD
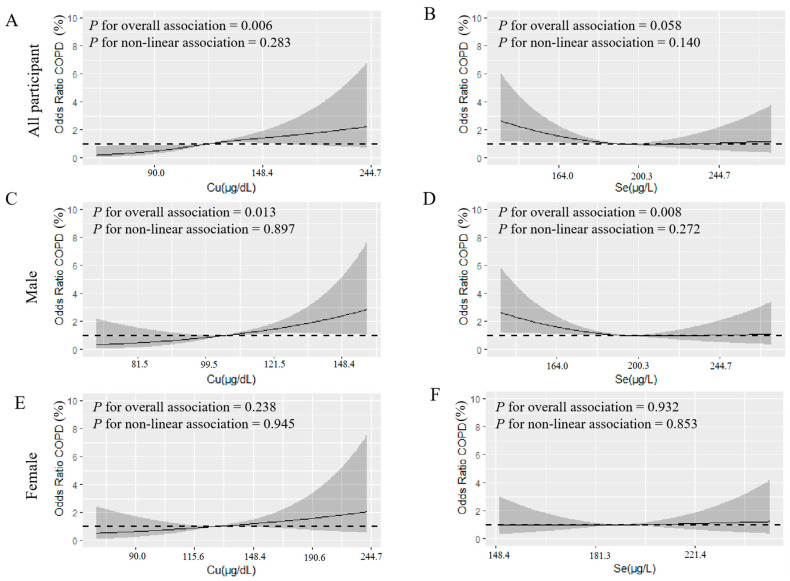
RCS showed that there was a linear relationship between ln(copper)- exposure and the risk of COPD (p = 0.006 for overall association) in all participants. As the ln(copper) level increased, the risk of COPD increased (Figure 3A). Both ln(copper) (p = 0.013 for overall association) and ln(selenium) (p = 0.008 for overall association) had linear relationships with the risk of COPD in males. As the ln(copper) level increased, the risk of COPD increased (Figure 3C); however, as the level of ln(selenium) increased, the risk of COPD decreased in males (Figure 3D). We did not observe this relationship in females (Figure 3E,F).
Figure 3.
(A–F) used restricted cubic spline modeling to analyze the relationship between metals, trace minerals, and COPD (ln-transformed). (A,B) is the relationship between copper, selenium, and COPD in all survey subjects, (C,D) is the relationship between copper, selenium, and COPD in males, and (E,F) is the relationship between copper, selenium, and COPD in females. The analysis adjusted for age, smoke, ratio of family income to poverty, BMI, sex, race education, drink, second-hand smoke exposure, serum cotinine, hypertension, and diabetes. The black solid line represents the combined restricted cubic spline curve model, and the shaded part represents the 95% CI of the combined curve.
4. Discussion
In our research, the high concentration of copper was positively correlated with COPD in males in the general US population using a multiple linear regression and restricted cubic spline modeling. While the risk of a male suffering from COPD decreased with the increase in selenium concentration (p = 0.008 for overall association). Studies have shown that copper is an important cofactor for some enzymatic reactions [9,31], and its mechanism may be related to Lysyl oxidase-like 2 (LOXL 2) [31,32,33]. LOXL 2 is a copper-dependent amine oxidase, which can activate lung fibroblasts through the TGF-β/Smad pathway, leading to pulmonary fibrosis, which in turn leads to impaired lung function. Selenium is related to the cellular antioxidant defense mechanism. Oxidative stress and chronic inflammation are important features in the pathogenesis of COPD [21]. Selenium is a cofactor of glutathione peroxidase, which can protect the human body from cell membrane damage mediated by reactive oxygen species and free radicals [34,35,36,37].
Selenium, cadmium, and lead are enriched in lung tissue [38], but copper, manganese, and zinc have not been reported. A study has shown that the median concentrations of lead, copper, manganese, selenium, copper, and zinc in the lung were 0.072, 0.026, 0.058, 0.11, 1.10, and 10.7 µg/g wet weight (ppm), respectively, among eight individuals in the age range 43 to 72 years [38]. The biological half-life of copper from the diet is 13–33 days, with bilary excretion being the major route of elimination [39]. Experiments of inhaled selenious acid and selenium metal aerosols in beagle dogs showed that the long-term component of the whole-body retention function for both inhaled aerosols had a half-life of about 34 days and accounted for about 20% of the initial selenium dose, and urine was the major route of excretion, accounting for 70 to 80% of the excreted selenium [40]. Therefore, one-time measures in serum of copper and blood of selenium are reflective of recent exposures.
The recommended intake of copper is 900 µg/day according to the Food and Nutrition Board (FNB) of the Institute of Medicine of the National Academy of Sciences [41]. The main dietary sources of copper are shellfish, seeds, nuts, offal, wheat bran cereals, whole grain products, and chocolate [41,42]. Although in our study, the concentration of the copper in the COPD group (124.30 µg/dL) was higher than the healthy group (114.25 µg/dL), they did not exceed the normal range (63.5–158.9 µg/dL) [43]. This is consistent with the results of Cen Jiang et al. [44]. Pearson’s research showed that copper had a strong negative correlation with lung function, and the higher serum copper levels reduce the forced expiratory volume in 1 s [45].
We found that copper only increased the risk of COPD in males, not females, but the serum copper in females (126.60 µg/dL) was higher than that in males (105.10 µg/dL) (p < 0.001) (Table S1). It might be the difference in sex hormones between males and females. Copper can activate lung fibroblasts through LOXL 2 activation of the TGF-β/Smad pathway, leading to pulmonary fibrosis, which in turn causes COPD [31,32,33]. Estrogen can inhibit the proliferation of fibroblasts through the Raf1-ERK-MAPK pathway [46,47]. The protective effect of estrogen might explain the sex-specific difference to copper. Therefore, based on our study, we suggest that the standard for serum copper concentration should be lowed and established according to gender.
The selenium intake of Americans exceeds 100 µg/d, which is much higher than the recommended intake (55 µg/d) [48] because of the high concentration of selenium in the soil. In addition, 18–19% of adults and children also use dietary supplements containing selenium [49]. The levels of selenium in the healthy group (192.75 µg/L) and COPD group (189.26 µg/L) were higher than normal concentrations (136.7 µg/L) [48]. Selenium is a cofactor of glutathione peroxidase, which can protect the human body from cell membrane damage mediated by reactive oxygen species and free radicals [34,35,36,37]. Wei Feng et al. [50] found that ln(selenium) has a positive linear relationship with the increase in lung function, but the COPD risk was not covered in the study. An RCT proved that selenium combined with vitamin C can alleviate the deterioration of COPD [51]. Our results also showed that selenium can reduce the risk of COPD.
There are some limitations to our study. First, because it was a cross-sectional study, the evidence for causality was not strong. Second, the data came from the NHANES, and it cannot represent the whole situation in the world and it needs to be verified in other populations. Third, there is no professional diagnosis of COPD by a doctor in NHANES. Although the definition of COPD depended on the participants’ answer to “Has a doctor told you have COPD, emphysema and chronic bronchitis?”, which is consistent with the previous studies about COPD using NHANES data, it might cause some bias in our studies.
In the 2015–2016 NHANES data, only copper and selenium exposure were related to the COPD in US male population. It is necessary to conduct in-depth research to verify this result and to investigate its potential mechanism. More research is expected to explore the relationship between metals and COPD, and the standard for serum copper concentration should establish by gender.
5. Conclusions
In our research, the high concentration of copper increased the COPD risk in males in the general US population using the multiple linear regression and restricted cubic spline modeling. While the risk of male suffering from COPD decreased with the increasing selenium level, we did not find associations between copper, selenium and the risk of COPD in female. We hope that there will be more research exploring the relationship between metals and COPD, and based on our study, we hope that the standard for serum copper concentration should be lowed and established according to gender.
Acknowledgments
Thanks to the data provided by the National Health and Nutrition Examination Survey (2015–2016) of the United States, which are used in epidemiological research and health science research to help formulate sound public health policies, to guide and design health plans and services, and to expand health knowledge.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19042085/s1. Figure S1: Distribution of exposure variables, Table S1: Level of metals in male and female.
Author Contributions
Q.F., X.W. and K.L.: Conceptualization, Writing—original draft, and Writing—review and editing. S.L.: Data curation and Resources. J.C.: Investigation and Resources. X.G.: Software and Validation. C.J.: Validation, Supervision, Writing—review and editing, and Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific funding from any funding agency.
Institutional Review Board Statement
The NHANES agreement has been reviewed and approved by the NCHS Research Ethics Committee. (approval code: Protocol #2011-17, approval date: 26 October 2017).
Informed Consent Statement
All participants provided written informed consent.
Data Availability Statement
All data in the article can be downloaded for free in the NHANES database from https://www.cdc.gov/nchs/nhanes/ (accessed on 1 May 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ibrahimou B., Azim S.I., Sun N. Interaction between blood lead level and chronic obstructive pulmonary disease (COPD) on risk of heart attack or stroke: USA NHANES, 2013–2014. Pulm. Pharmacol. Ther. 2019;58:101805. doi: 10.1016/j.pupt.2019.101805. [DOI] [PubMed] [Google Scholar]
- 2.Vogelmeier C.F., Criner G.J., Martinez F.J., Anzueto A., Barnes P.J., Bourbeau J., Celli B.R., Chen R., Decramer M., Fabbri L.M., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 3.Global Status of COPD. [(accessed on 2 October 2021)]. Available online: https://www.who.int/respiratory/copd/burden/zh/
- 4.López-Campos J.L., Tan W., Soriano J.B. Global burden of COPD. Respirology. 2016;21:14–23. doi: 10.1111/resp.12660. [DOI] [PubMed] [Google Scholar]
- 5.Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., Abbasi-Kangevari M., Abbastabar H., Abd-Allah F., Abdelalim A., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes L., Mesquita A. Biomass smoke exposure is the main risk factor for COPD in non smoking women in a developing country. Eur. Respir. J. 2013;42:2008. [Google Scholar]
- 7.Matheson M.C., Benke G., Raven J., Sim M.R., Kromhout H., Vermeulen R., Johns D.P., Walters E.H., Abramson M.J. Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax. 2005;60:645–651. doi: 10.1136/thx.2004.035170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Marco R., Accordini S., Marcon A., Cerveri I., Antó J.M., Gislason T., Heinrich J., Janson C., Jarvis D., Kuenzli N., et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am. J. Respir. Crit. Care Med. 2011;183:891–897. doi: 10.1164/rccm.201007-1125OC. [DOI] [PubMed] [Google Scholar]
- 9.Sparrow D., Silbert J.E., Weiss S.T. The relationship of pulmonary function to copper concentrations in drinking water. Am. Rev. Respir. Dis. 1982;126:312–315. doi: 10.1164/arrd.1982.126.2.312. [DOI] [PubMed] [Google Scholar]
- 10.Xiao L., Zhou Y., Cui X., Huang X., Yuan J., Chen W. Association of urinary metals and lung function in general Chinese population of Wuhan. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50:680–688. doi: 10.3760/cma.j.issn.0253-9624.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Pappas R.S., Fresquez M.R., Martone N., Watson C.H. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J. Anal. Toxicol. 2014;38:204–211. doi: 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piadé J.J., Jaccard G., Dolka C., Belushkin M., Wajrock S. Differences in cadmium transfer from tobacco to cigarette smoke, compared to arsenic or lead. Toxicol. Rep. 2015;2:12–26. doi: 10.1016/j.toxrep.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter P., Faroon O., Pappas R.S. Cadmium and cadmium/zinc ratios and tobacco-related morbidities. Int. J. Environ. Res. Public Health. 2017;14:1154. doi: 10.3390/ijerph14101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manca D., Ricard A.C., Trottier B., Chevalier G. Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology. 1991;67:303–323. doi: 10.1016/0300-483X(91)90030-5. [DOI] [PubMed] [Google Scholar]
- 15.Faroon O., Ashizawa A., Wright S., Tucker P., Jenkins K., Ingerman L., Rudisill C. Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry (US); Atlanta, GA, USA: 2012. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. [PubMed] [Google Scholar]
- 16.Anetor J., Ajose F., Anetor G., Iyanda A., Babalola B., Adeniyi F. High cadmium/zinc ratio in cigarette smokers: Potential implications as a biomarker of risk of prostate cancer. Niger. J. Physiol. Sci. 2008;23:41–49. doi: 10.4314/njps.v23i1-2.54921. [DOI] [PubMed] [Google Scholar]
- 17.Ibs K.H., Rink L. Zinc-altered immune function. J. Nutr. 2003;133:1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 18.Rink L., Kirchner H. Zinc-altered immune function and cytokine production. J. Nutr. 2000;130:1407S–1411S. doi: 10.1093/jn/130.5.1407S. [DOI] [PubMed] [Google Scholar]
- 19.Meydani S.N., Barnett J.B., Dallal G.E., Fine B.C., Jacques P.F., Leka L.S., Hamer D.H. Serum zinc and pneumonia in nursing home elderly. Am. J. Clin. Nutr. 2007;86:1167–1173. doi: 10.1093/ajcn/86.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y.S., Caffrey J.L., Chang M.H., Dowling N., Lin J.W. Cigarette smoking, cadmium exposure, and zinc intake on obstructive lung disorder. Respir. Res. 2010;11:53. doi: 10.1186/1465-9921-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman I., Kilty I. Antioxidant therapeutic targets in COPD. Curr. Drug Targets. 2006;7:707–720. doi: 10.2174/138945006777435254. [DOI] [PubMed] [Google Scholar]
- 22.Hassan F., Xu X., Nuovo G., Killilea D.W., Tyrrell J., Da Tan C., Tarran R., Diaz P., Jee J., Knoell D., et al. Accumulation of metals in GOLD4 COPD lungs is associated with decreased CFTR levels. Respir. Res. 2014;15:69. doi: 10.1186/1465-9921-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rokadia H.K., Agarwal S. Serum heavy metals and obstructive lung disease: Results from the National Health and Nutrition Examination Survey. Chest. 2013;143:388–397. doi: 10.1378/chest.12-0595. [DOI] [PubMed] [Google Scholar]
- 24.Thomas R. Practical Guide to ICP-MS: A Tutorial for Beginners. CRC Press; Boca Raton, FL, USA: 2008. [Google Scholar]
- 25.Tanner S.D., Baranov V.I. Theory, design, and operation of a dynamic reaction cell for ICP-MS. At. Spectrosc. Norwalk Conn. 1999;20:45–52. [Google Scholar]
- 26.Tanner S.D., Baranov V.I., Bandura D.R. Reaction cells and collision cells for ICP-MS: A tutorial review. Spectrochim. Acta Part B At. Spectrosc. 2002;57:1361–1452. doi: 10.1016/S0584-8547(02)00069-1. [DOI] [Google Scholar]
- 27.Navaneethan S.D., Mandayam S., Arrigain S., Rahman M., Winkelmayer W.C., Schold J.D. Obstructive and restrictive lung function measures and CKD: National Health and nutrition examination survey (NHANES) 2007–2012. Am. J. Kidney Dis. 2016;68:414–421. doi: 10.1053/j.ajkd.2016.03.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong X., Li S., Sun J., Zhang D. Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients. 2020;12:840. doi: 10.3390/nu12030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madrigal J.M., Persky V., Pappalardo A., Argos M. Association of heavy metals with measures of pulmonary function in children and youth: Results from the National Health and Nutrition Examination Survey (NHANES) Environ. Int. 2018;121:871–878. doi: 10.1016/j.envint.2018.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desquilbet L., Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 31.Wen X., Liu Y., Bai Y., Li M., Fu Q., Zheng Y. LOXL2, a copper-dependent monoamine oxidase, activates lung fibroblasts through the TGF-β/Smad pathway. Int. J. Mol. Med. 2018;42:3530–3541. doi: 10.3892/ijmm.2018.3927. [DOI] [PubMed] [Google Scholar]
- 32.Baldari S., Di Rocco G., Toietta G. Current biomedical use of copper chelation therapy. Int. J. Mol. Sci. 2020;21:1069. doi: 10.3390/ijms21031069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen R., de Brouwer B., Jan H., Wouters E.F. Copper as the most likely pathogenic divergence factor between lung fibrosis and emphysema. Med. Hypotheses. 2018;120:49–54. doi: 10.1016/j.mehy.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Bastola M.M., Locatis C., Maisiak R., Fontelo P. Selenium, copper, zinc and hypertension: An analysis of the National Health and Nutrition Examination Survey (2011–2016) BMC Cardiovasc. Disord. 2020;20:45. doi: 10.1186/s12872-020-01355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suadicani P., Hein H.O., Gyntelberg F. Serum selenium level and risk of lung cancer mortality: A 16-year follow-up of the Copenhagen Male Study. Eur. Respir. J. 2012;39:1443–1448. doi: 10.1183/09031936.00102711. [DOI] [PubMed] [Google Scholar]
- 36.Salmonowicz B., Krzystek-Korpacka M., Noczynska A. Trace elements, magnesium, and the efficacy of antioxidant systems in children with type 1 diabetes mellitus and in their siblings. Adv. Clin. Exp. Med. 2014;23:259–268. doi: 10.17219/acem/37074. [DOI] [PubMed] [Google Scholar]
- 37.Alkan F.A., Karis D., Cakmak G., Ercan A.M. Analysis of the relationship between hemorheologic parameters, aluminum, manganese, and selenium in smokers. Biol. Trace Elem. Res. 2019;187:22–31. doi: 10.1007/s12011-018-1352-8. [DOI] [PubMed] [Google Scholar]
- 38.Brune D., Nordberg G., Wester P.O. Distribution of 23 elements in the kidney, liver and lungs of workers from a smeltery and refinery in North Sweden exposed to a number of elements and of a control group. Sci. Total Environ. 1980;16:13–35. doi: 10.1016/0048-9697(80)90100-X. [DOI] [PubMed] [Google Scholar]
- 39.Barceloux D.G., Barceloux D. Copper. J. Toxicol. Clin. Toxicol. 1999;37:217–230. doi: 10.1081/CLT-100102421. [DOI] [PubMed] [Google Scholar]
- 40.Weissman S.H., Cuddihy R.G., Medinsky M.A. Absorption, distribution, and retention of inhaled selenious acid and selenium metal aerosols in beagle dogs. Toxicol. Appl. Pharmacol. 1983;67:331–337. doi: 10.1016/0041-008X(83)90316-2. [DOI] [PubMed] [Google Scholar]
- 41.Li S., Sun W., Zhang D. Association of zinc, iron, copper, and selenium intakes with low cognitive performance in older adults: A cross-sectional study from National Health and Nutrition Examination Survey (NHANES) J. Alzheimer’s Dis. 2019;72:1145–1157. doi: 10.3233/JAD-190263. [DOI] [PubMed] [Google Scholar]
- 42.Kies C. Food sources of dietary copper. Copper bioavailability and metabolism. Adv. Exp. Med. Biol. 1989;258:1–20. doi: 10.1007/978-1-4613-0537-8_1. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Copper Intake. [(accessed on 2 October 2021)]; Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/
- 44.Jiang C., Wu B., Xue M., Lin J., Hu Z., Nie X., Cai G. Inflammation accelerates copper-mediated cytotoxicity through induction of six-transmembrane epithelial antigens of prostate 4 expression. Immunol. Cell Biol. 2021;99:392–402. doi: 10.1111/imcb.12427. [DOI] [PubMed] [Google Scholar]
- 45.Pearson P., Britton J., McKeever T., Lewis S., Weiss S., Pavord I., Fogarty A. Lung function and blood levels of copper, selenium, vitamin C and vitamin E in the general population. Eur. J. Clin. Nutr. 2005;59:1043–1048. doi: 10.1038/sj.ejcn.1602209. [DOI] [PubMed] [Google Scholar]
- 46.Tofovic S.P., Zhang X., Jackson E.K., Zhu H., Petrusevska G. 2-methoxyestradiol attenuates bleomycin-induced pulmonary hypertension and fibrosis in estrogen-deficient rats. Vasc. Pharmacol. 2009;51:190–197. doi: 10.1016/j.vph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sathish V., Martin Y.N., Prakash Y. Sex steroid signaling: Implications for lung diseases. Pharmacol. Ther. 2015;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Selenium Intake. [(accessed on 2 October 2021)]; Available online: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- 49.Bailey R.L., Gahche J.J., Lentino C.V., Dwyer J.T., Engel J.S., Thomas P.R., Betz J.M., Sempos C.T., Picciano M.F. Dietary supplement use in the United States, 2003–2006. J. Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng W., Huang X., Zhang C., Liu C., Cui X., Zhou Y., Sun H., Qiu G., Guo H., He M., et al. The dose–response association of urinary metals with altered pulmonary function and risks of restrictive and obstructive lung diseases: A population-based study in China. BMJ Open. 2015;5:e007643. doi: 10.1136/bmjopen-2015-007643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isbaniah F., Wiyono W.H., Yunus F., Setiawati A., Totzke U., Verbruggen M. Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: Results from a randomized controlled trial. J. Clin. Pharm. Ther. 2011;36:568–576. doi: 10.1111/j.1365-2710.2010.01212.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in the article can be downloaded for free in the NHANES database from https://www.cdc.gov/nchs/nhanes/ (accessed on 1 May 2021).