Abstract
A novel microtiter assay for antifungal susceptibility testing was developed. This method has several potential advantages over the M27-A assay of the National Committee for Clinical Laboratory Standards. These include provision of MIC results within 6 to 19 h, graphical display of data, and the availability of objective quantitative endpoints. We refer to the method as the rapid susceptibility assay (RSA). RSA is based on substrate utilization by fungi in the presence of antifungal drugs. Substrate uptake is determined by a colorimetric method, which can be scored by analysis of data obtained from a microplate reader. Variables evaluated in the development of the RSA included inoculum size, incubation period, and efficacy with different classes of antifungal drugs and different yeast isolates. With the rapidly available and quantitative endpoints of the RSA, correlation of MICs and therapeutic drug doses can be evaluated more successfully than they can be evaluated by existing assays.
Numerous methods, in addition to the recently published M27-A assay of the National Committee for Clinical Laboratory Standards (9), have been described for antifungal susceptibility testing (7, 8, 12, 13). Only a few of these can be done within about 24 h to obtain a MIC endpoint. These few, however, rely on methods that involve either direct determination of cell mass or cell viability by fluorescence or indirect determination of cell mass by calorimetry, analysis of ATP production, or radiometry. Most of the rapid procedures require expensive equipment, and the slower tests, such as the NCCLS M27-A assay, involve subjective interpretation of results. We developed a rapid assay that uses inexpensive reagents and that requires only modest, common clinical laboratory equipment. In addition, the results are objective because the endpoint determinations are based on colorimetric differences, as assessed with a microplate reader.
The assay is based on the hypothesis that when susceptible fungi are exposed to an antifungal drug, their uptake of an exogenous substrate will be suppressed or inhibited. By measuring the amount of residual substrate in the medium compared to that for controls without drug, the susceptibility of a fungal isolate to an antifungal drug can be determined. MIC determinations obtained by evaluating substrate uptake are highly sensitive and should be more rapid than tests that rely on fungal growth. In addition to assessing the activities of antifungal agents against yeasts, as demonstrated by this report, this method should be applicable to the testing of antimicrobial agents against molds and nonfungal microorganisms, such as Mycobacterium spp. We refer to the method as the rapid susceptibility assay (RSA). In this paper we report on the development of the RSA for use with yeast isolates. We chose glucose as the prototypic substrate because of the universality of this carbon and energy source for most fungi of medical significance and the ease and sensitivity of glucose concentration measurement.
MATERIALS AND METHODS
The reagents, growth media, preparation of yeast inocula, selection of control yeast strains, and antifungal agents were in accordance with recommended guidelines established for performing antifungal susceptibility assays as described by the NCCLS M-27A protocol (9).
Reagents.
Unless otherwise noted the reagents were purchased from Sigma Chemical Company, St. Louis, Mo.
Medium.
RPMI 1640, prepared without bicarbonate, buffered with 0.165 M 3-(N-morpholino)propanesulfonic acid (MOPS), and adjusted to pH 7.0 with 10 M NaOH, was used in all susceptibility tests. This medium contains glucose at a concentration of 2 mg/ml. To vary the concentration of glucose, medium dilutions were made in MOPS-buffered RPMI 1640 without glucose, which is referred to as glucose-deficient medium.
Organisms, culture, and inoculum preparation.
Six yeast strains with established quality control (QC) performance (11) were used. They were Candida albicans ATCC 24433 and ATCC 90028, C. tropicalis ATCC 750, C. krusei ATCC 6258, and C. parapsilosis ATCC 22019 and ATCC 90018. The yeasts were inoculated onto Sabouraud dextrose agar plates from glycerol stocks stored at −20°C, incubated at 37°C, and passaged twice at 24- or 48-h intervals before use. Suspensions were prepared from 24-h cultures for C. albicans, C. tropicalis, and C. krusei and from 48-h cultures for C. parapsilosis. Cells from colonies were suspended in glucose-deficient medium, and the turbidity of each stock suspension was adjusted to match that of a 0.5 McFarland standard as read at 530 nm. At this turbidity, the yeast density was 3 × 106 to 5 × 106 CFU/ml. Dilutions from the stock suspension prepared in glucose-deficient medium are referred to as the inoculum dilutions and are twice the test density achieved by mixing the inoculum with the test drug. Unless otherwise stated, the inoculum test volume was 100 μl/well.
Antifungal agents.
Amphotericin B was obtained in powder form (catalog no. A-2411; Sigma), and fluconazole was a generous gift from Pfizer (lot no. Zb109-9200-15). A stock solution of each antifungal agent was prepared as recommended for the NCCLS M27-A protocol (9). To prepare susceptibility curves, the antifungal drugs were tested in the following ranges: amphotericin B, 0.03 to 16 μg/ml; and fluconazole, 0.008 to 64 μg/ml. Briefly, stock solutions consisting of amphotericin B at 1.6 mg of drug/ml of dimethyl sulfoxide and fluconazole at 1.28 mg of drug/ml of deionized water were stored at −80°C. From these stock solutions, initial dilutions of 1:50 and 1:10 of amphotericin B and fluconazole, respectively, were made, followed by preparation of twofold serial dilutions across the concentration range to be tested in medium containing 1 mg of glucose per ml. Dimethyl sulfoxide at 2% (vol/vol) was included in the dilutions of amphotericin B. A total of 100 μl of each antifungal agent at two times the test concentration was placed in duplicate wells of sterile 96-well plates (Corning Glass Works, Corning, N.Y.).
RSA.
Fungal inocula (100 μl) were added to each flat-bottom well of a microtiter plate containing two times the test concentration of each antifungal drug (100 μl/well). The plates were incubated at 35 to 37°C. During the initial assay development, results were determined at 3, 4, 6, and 19 h of incubation. Further experiments were limited to results that were obtained at 6 and 19 h. The amount of glucose remaining after incubation was detected by a modification of previously described methods (3, 10). Briefly, 50 μl of an enzyme substrate color mix containing 0.6 M sodium phosphate (pH 6.0), 360 μg of 4-amino antipyrine per ml, 490 μg of N-ethyl-N-sulfopropyl-m-toluidine per ml, 0.68 U of horseradish peroxidase per ml, and 0.4 U of glucose oxidase per ml was added to each well of the microtiter plate. In principle, hydrogen peroxide is released during the specific oxidation of glucose by glucose oxidase. The reaction of hydrogen peroxide with the chromogenic substrates is then catalyzed by the peroxidase. The intensity of the resulting purple color is proportional to the concentration of hydrogen peroxide, which is directly proportional to the amount of glucose in the culture medium. In our test, color was allowed to develop for 20 min to no more than 30 min. A dual-wavelength microplate reader (model 450; Bio-Rad Laboratories, Richmond, Calif.) was used to measure the optical density at 550 nm, with the baseline value for each well automatically established by a simultaneous reference reading at 655 nm. For development of the RSA, inoculum size and length of incubation were varied.
Susceptibility curves and RSA endpoint determination.
The six QC strains prepared as inoculum stock suspensions were tested at selected dilutions for their susceptibilities to amphotericin B and fluconazole. Each inoculum dilution was incubated with twofold serial dilutions of the test drug. For each test combination a plot of optical density versus antifungal concentration was constructed. Optical density was plotted on the ordinate, with the interval established by control wells containing yeast and no antifungal agent at the lower scale limit and by medium without yeast or antifungal agent at the upper scale limit. The drug concentration immediately preceding a drop in optical density from the highest point of the drug-sensitive area, equal to at least 10% of the detection interval, was selected as the quantitative endpoint (MICRSA). For each test organism MICRSAs obtained from selected inoculum dilutions were compared, and the values were compared to the published susceptibility values for the QC strains.
Optimal fungal inoculum range.
The inoculum stock suspension from each organism was serially diluted and was incubated in the presence of high-dose inhibitory concentrations of amphotericin B (16 μg/ml), fluconazole (64 μg/ml), or no drug. The cultures were incubated for 6 or 19 h, and color was developed and the optical density was determined as described above. The detection interval, defined as the difference in optical density between wells containing an inoculum and a high dose of antifungal agent and wells with an inoculum but no antifungal agent, was calculated and was plotted against the inoculum dilution.
Susceptibility curves determined by RSA were prepared by using an inoculum at dilutions that produced the maximum detection intervals with serial dilutions of each of the test antifungal agents. MICRSAs from these curves were compared to each other. In some cases inoculum dilutions that produced detection intervals less than the maximum were used, and their MICRSAs were compared with those described above. For each drug and organism combination the optimal fungal inoculum range was judged to be the greatest inoculum dilution range across which the MICRSA was stable within two adjacent drug concentrations.
RESULTS
Glucose measurement.
The glucose concentration that could be measured in a 200-μl volume of the assay medium with the described color mix formula ranged from 0.1 to 1.0 mg/ml (data not shown). Colorimetric determination of glucose concentration was unaffected by the presence of RPMI 1640 with MOPS, antifungal agents, or products of yeast metabolism (data not shown).
Time of incubation.
By 3 h of incubation with each of the test organisms at a 0.5 McFarland density, glucose uptake from the assay medium in the absence of antifungal agents was sufficient to reduce the optical density by 0.5 to 1.0 optical density units. The reduction was less for slower-growing organisms such as C. parapsilosis and was more for faster-growing organisms such as C. tropicalis. As expected, the amount of glucose utilization decreased with inoculum dilution and increased with longer incubation times. At ≥3 h of incubation, amphotericin B suppressed glucose uptake by C. albicans, and the suppression was drug dose dependent (data not shown). For fluconazole, a slower-acting drug (5), 6 h of incubation at 37°C was required for detection of an inhibition of glucose uptake by C. albicans. To establish a procedure with broad application to different classes of antifungal agents and to different organisms, variations in the protocol were explored. For further study, incubation times of 6 and 19 h were selected; these correspond to a single 8- to 10-h work shift and to an overnight test in a clinical laboratory, respectively.
Detection intervals.
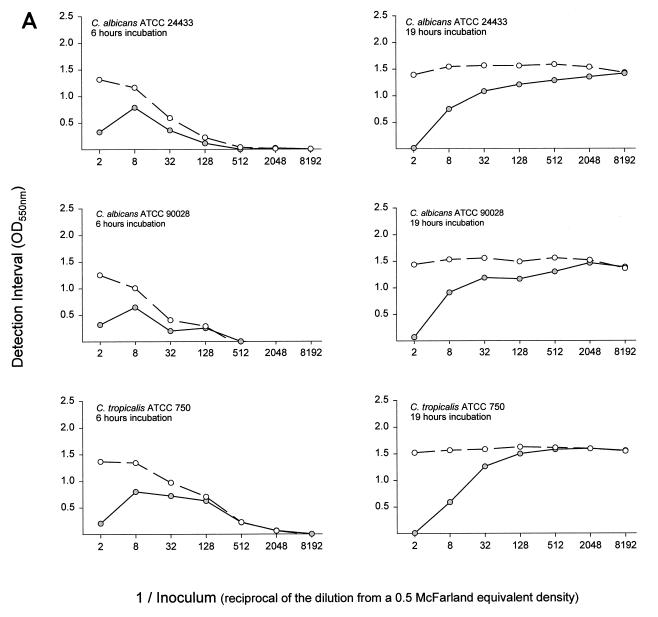
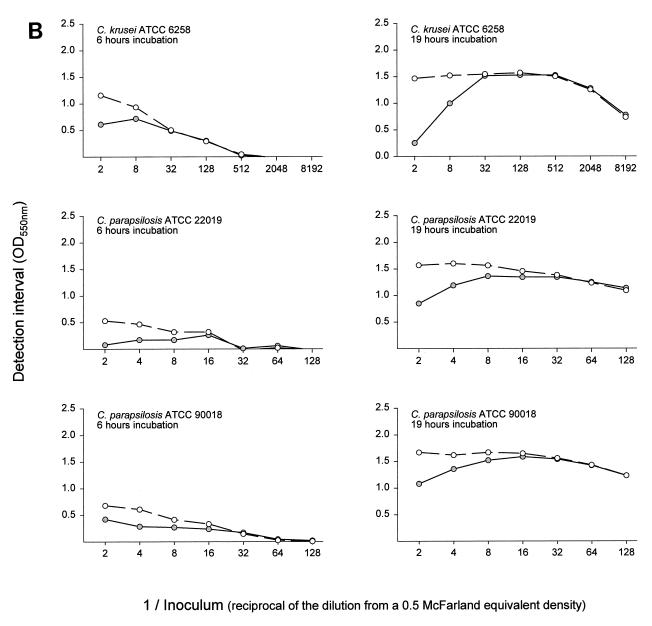
For a set of assay conditions the detection interval is the range of optical density over which a susceptibility curve can be generated. Detection interval curves for all drugs and organisms at both incubation times had the same basic shape (Fig. 1). The detection interval was small when the inoculum was in excess, as with C. albicans strains at a one-half inoculum dilution incubated for 19 h with fluconazole. Greater dilution of the inoculum resulted in an increased detection interval, to a maximum that for some assay conditions was sustained over a broad inoculum dilution range. Examples are C. albicans strains at dilutions of 1/8 through 1/2,048 incubated for 19 h with amphotericin B and C. tropicalis ATCC 750 at dilutions between 1/128 and 1/8,192 incubated for 19 h with fluconazole. At still greater inoculum dilutions, as with C. krusei ATCC 6258 at dilutions ≥1/2,048 incubated for 19 h or each organism incubated for 6 h, the inoculum size was insufficient for the organisms to utilize all of the glucose from the test medium, and the detection interval declined and approached zero.
FIG. 1.
Inocula from six yeast organisms were prepared in glucose-deficient medium as twofold or fourfold dilutions from a stock suspension with a density spectrophotometrically equivalent to that of a 0.5 McFarland standard. A total of 100 μl of each dilution was mixed with 100 μl of medium containing 1 mg of glucose per ml and no drug, 128 μg of fluconazole per ml, or 32 μg of amphotericin B per ml. After incubation at 37°C for 6 h (column of graphs on the left) or for 19 h (column of graphs on the right), 50 μl of enzyme substrate color mix containing glucose oxidase was added to each well. The color was allowed to develop for 20 to 30 min. The optical density was measured at 550 nm with a 655 nm reference filter. The detection interval (the difference in optical density between wells with an inoculum and a high dose of antifungal agent and wells with an inoculum but no antifungal agent) was calculated and was plotted against the inoculum dilution. Open circles, amphotericin B; filled circles, fluconazole; OD550, optical density at 550 nm.
RSA susceptibility curves and endpoints.
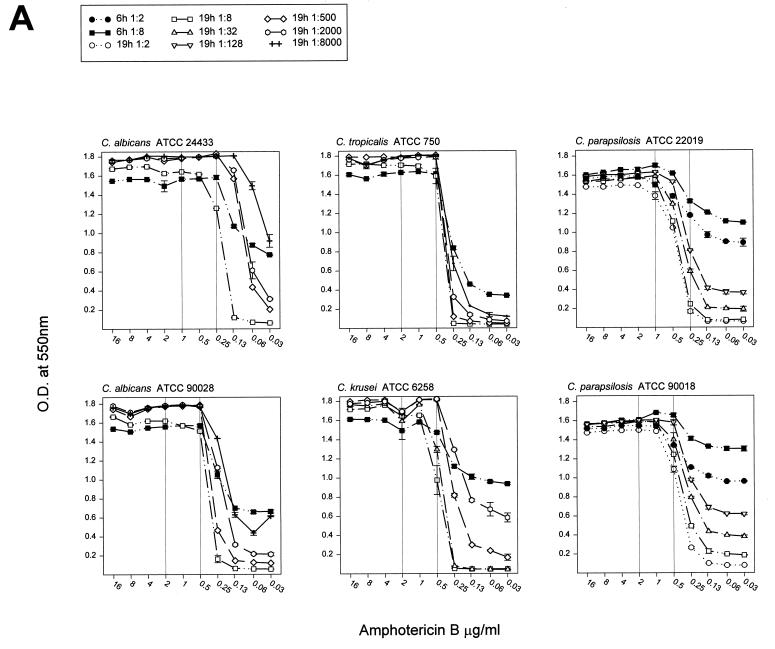
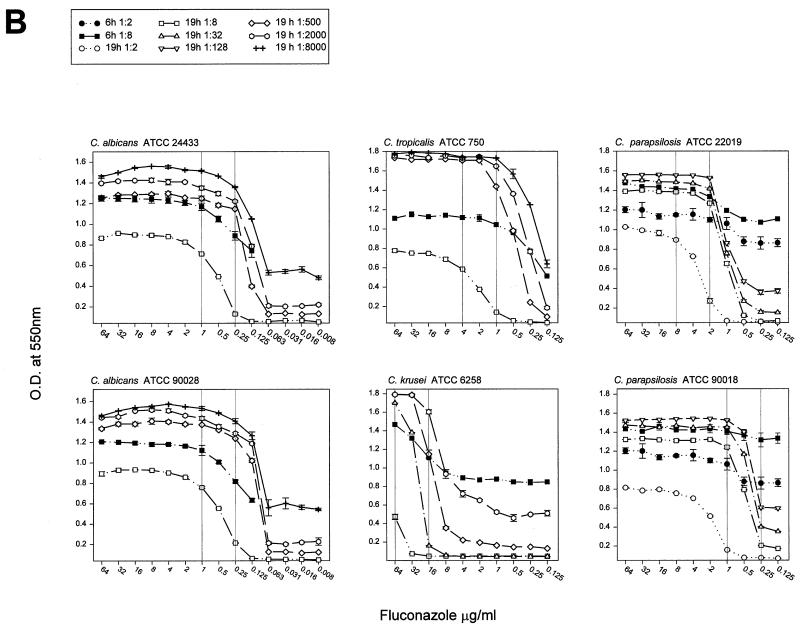
Susceptibility curves for the six QC organisms were determined by RSA with amphotericin B and fluconazole and were determined by using inoculum dilutions that yielded different detection intervals after 6 and 19 h of incubation (Fig. 2). For all drug and organism combinations, the curves were characterized from top to bottom by a plateau at the curve maximum, which represented the drug sensitivity area, a relatively steep decline through the dose-dependent sensitivity range, and a baseline representing the area of insufficient drug pressure. For all susceptibility curves, the MICRSAs are reported in Table 1 according to their respective inoculum dilutions. The endpoint for each curve was in the sharp transition between the drug-sensitive and the dose-dependent sensitivity regions. Exceptions were the curves for C. albicans and fluconazole (Fig. 2B), for which the MICRSA endpoints were within the more gradual arc of three to five drug concentrations which spanned the transition from drug sensitivity to dose-dependent sensitivity.
FIG. 2.
Inoculum dilutions in glucose-deficient medium were selected for the preparation of susceptibility curves. A total of 100 μl was added to wells containing 100 μl of twofold dilutions of amphotericin B (A) or fluconazole (B) prepared at twice the test concentration in medium containing 1 mg of glucose per ml. After 6 or 19 h of incubation, 50 μl of the color mixture was added to each well, the reaction was allowed to develop for 20 to 30 min, and the optical density was read at 550 nm (OD550). The optical density was plotted against the drug concentration, and susceptibility plots of all inoculum dilutions of an organism paired with an antifungal agent appear on one graph. Vertical reference lines were added to represent the published MIC range (11).
TABLE 1.
MICRSA endpoints determined from selected inoculum dilutionsa and selection of optimal fungal inoculum dilution rangeb
| Antifungal agent inoculum isolate | Time (h) of incubation at 37°C | MICRSA endpoints (μg/ml) for the following inoculum dilutions from the 0.5 McFarland equivalent stock suspension:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 8 | 32 | 128 | 512 | 2048 | 8192 | ||
| Amphotericin B | ||||||||
| C. albicans ATCC 24433 | 6 | 0.25b | ||||||
| 19 | 0.5 | 0.25 | 0.25 | 0.12 | ||||
| C. albicans ATCC 90028 | 6 | 0.5 | ||||||
| 19 | 0.5 | 0.5 | 0.5 | 0.5 | ||||
| C. tropicalis ATCC 750 | 6 | 0.5 | ||||||
| 19 | 0.5 | 0.5 | 0.5 | 0.5 | ||||
| C. krusei ATCC 6258 | 6 | 1.0 | ||||||
| 19 | 1.0 | 1.0 | 0.5 | 0.5 | ||||
| C. parapsilosis ATCC 22019 | 6 | 2.0 | 1.0 | |||||
| 19 | 1.0 | 1.0 | 1.0 | 0.5 | ||||
| C. parapsilosis ATCC 90018 | 6 | 1.0 | 0.5 | |||||
| 19 | 1.0 | 1.0 | 1.0 | 0.5 | ||||
| Fluconazole | ||||||||
| C. albicans ATCC 24433 | 6 | 2.0 | ||||||
| 19 | 2.0 | 0.5 | 0.5 | 0.5 | ||||
| C. albicans ATCC 90028 | 6 | 2.0 | ||||||
| 19 | 2.0 | 0.5 | 1.0 | 0.5 | ||||
| C. tropicalis ATCC 750 | 6 | 1.0 | ||||||
| 19 | 8 | 2.0 | 1.0 | 1.0 | ||||
| C. krusei ATCC 6258 | 6 | ≥64 | ||||||
| 19 | ≥64 | ≥64 | 32 | 32 | ||||
| C. parapsilosis ATCC 22019 | 6 | NCc | NC | |||||
| 19 | 8.0 | 4.0 | 2.0 | 2.0 | ||||
| C. parapsilosis ATCC 90018 | 6 | NC | NC | |||||
| 19 | 4.0 | 1.0 | 1.0 | 1.0 | ||||
MICRSAs were determined by using the indicated inoculum dilutions and incubation times.
For an organism and drug combination, boldface MICRSAs indicate the greatest inoculum dilution range for which the MICRSA was stable within two consecutive drug concentrations. These dilutions were judged to be optimal.
NC, MICRSA could not be determined.
A fluconazole MICRSA could not be called for C. parapsilosis strains at 6 h. Although these curves were shallow, as predicted by their small detection intervals, an area of dose-dependent sensitivity was observed coincident with the areas of dose-dependent sensitivity of curves from the 19-h incubations (Fig. 2B). However, changes in shape were not sufficiently dramatic for reliable identification of endpoints, and the replicate error rate was high. Incubation periods between 6 and 19 h for these organisms and fluconazole were not explored.
In some instances, the glucose remaining in the wells containing yeasts and no drug (controls) at 6 h exceeded that in the wells with yeast exposed to low concentrations of antifungal agents (data not shown). One possible explanation is that the initial reaction of the organisms to the presence of the drugs may be an immediate, increased uptake of the glucose. More than 6 h may be required for the action of the drugs to retard glucose utilization sufficiently to result in optical density values that exceed those for the controls. Despite this phenomenon, MICRSAs were in agreement with determinations from the 19-h curves if the inoculum was within the optimal dilution range.
Optimal inoculum dilution ranges.
For each isolate and drug pair, dilution of the inoculum resulted in a shift of the dose-dependent areas of the susceptibility curves (Fig. 2) and of the MICRSA endpoints to lower drug concentrations (Table 1).
For inoculum dilutions exceeding those which produced the maximum detection interval (Fig. 1), MICRSA endpoints were stable within two drug concentrations (Table 1). This range of inoculum dilutions was judged to be optimum, as defined in the Materials and Methods section. The endpoints achieved with an excess inoculum were frequently higher, and these dilutions were judged to be inappropriate for use in the RSA. For example, fluconazole MICRSA endpoints of 2, 1, 1, and 1 were determined for C. tropicalis ATCC 750 with inoculum dilutions of 1/512, 1/2,084, and 1/8,192 after 19 h and 1/8 at 6 h, respectively. However, the MICRSA was 8 μg/ml with a 1/8 inoculum dilution at 19 h (Table 1). The optimal inoculum dilution range for this organism is 1/8 for a 6-h test or 1/512 to 1/8,192 for a 19-h test. A 1/8 inoculum dilution should not be used in the 19-h test.
For the six test organisms and two antifungal agents, MICRSAs that match the criterion for selection of the inoculum dilution as optimal are in boldface (Table 1).
Endpoint agreement.
Evaluation of the test isolates in our laboratories under the NCCLS protocol produced susceptibility endpoints consistent with the published MICs (data not shown). For the two antifungal agents tested, the MICRSAs were within the published MIC range for each organism (Table 2). For fluconazole, MICRSAs tended to be higher than published values when the inoculum exceeded the optimal inoculum dilution range but were within the published range at the recommended optimal dilutions. That is, a susceptibility curve may be generated even when the inoculum concentration is too high, but it may yield a spurious high MICRSA.
TABLE 2.
Summary of optimal fungal inoculum dilutions for RSA and comparison of the MICRSAs to published MICs
| Antifungal agent | Isolate | RSA incubation time (h) | Optimal inoculum dilution range for the RSAa | MICRSA range (μg/ml) for optimal inoculum dilutions | Published (μg/ml)b MIC range |
|---|---|---|---|---|---|
| Amphotericin B | C. albicans ATCC 24433 | 6 | 1/8 | 0.25 | 0.25–1.0 |
| 19 | 1/512–1/8192 | 0.125–0.25 | |||
| C. albicans ATCC 90028 | 6 | 1/8 | 0.5 | 0.5–2.0 | |
| 19 | 1/8–1/8192 | 0.5 | |||
| C. tropicalis ATCC 750 | 6 | 1/8 | 0.5 | 0.5–2.0 | |
| 19 | 1/8–1/8192 | 0.5 | |||
| C. krusei ATCC 6258 | 6 | 1/8 | 1.0 | 0.5–2.0 | |
| 19 | 1/8–1/2048 | 0.5–1.0 | |||
| C. parapsilosis ATCC 22019 | 6 | 1/8 | 1.0 | 0.25–1.0 | |
| 19 | 1/2–1/128 | 0.5–1.0 | |||
| C. parapsilosis ATCC 90018 | 6 | 1/2–1/8 | 0.5–1.0 | 0.5–2.0 | |
| 19 | 1/2–1/128 | 0.5–1.0 | |||
| Fluconazole | C. albicans ATCC 24433 | 6 | NDc | 0.25–1.0 | |
| 19 | 1/512–1/8192 | 0.5 | |||
| C. albicans ATCC 90028 | 6 | ND | 0.25–1.0 | ||
| 19 | 1/512–1/8192 | 0.5–1.0 | |||
| C. tropicalis ATCC 750 | 6 | 1/8 | 1.0 | 1.0–4.0 | |
| 19 | 1/512–1/8192 | 1.0–2.0 | |||
| C. krusei ATCC 6258 | 6 | ND | 16.0–64.0 | ||
| 19 | 1/512–1/2048 | 32–64 | |||
| C. parapsilosis ATCC 22019 | 6 | ND | 2.0–8.0 | ||
| 19 | 1/8–1/128 | 2.0–4.0 | |||
| C. parapsilosis ATCC 90018 | 6 | ND | 0.25–1.0 | ||
| 19 | 1/8–1/128 | 1.0 |
The greatest inoculum dilution range across which the endpoint was stable within two consecutive drug concentrations.
Obtained from reference 11.
ND, no dilution evaluated filled the criteria; thus, a determination of an optimal inoculum for the RSA could not be made.
DISCUSSION
The inhibitory effect of antifungal drugs at the incubation endpoint of the NCCLS M27-A method is based on cell growth, which is measured in terms of culture turbidity. For cell growth to occur, the yeast must first and continually absorb sufficient exogenous nutrients. When yeast cells were exposed to antifungal drug pressure, their ability to absorb substrate (glucose) was inhibited, and this effect occurred in the absence of any cell turbidity change. The magnitude of substrate consumption was dependent on the degree of drug pressure (Fig. 2). These observations suggested that substrate consumption could form the basis of an RSA.
Several factors influence the sensitivity of RSA. These factors are not unique to the RSA but are common to those susceptibility assays that depend on metabolic activity to provide a detectable endpoint. Perhaps the most critical of these factors are inoculum density, organism growth rate, incubation time, and mechanism of drug action (1). Additional important factors specific to RSA include the concentration of the nutrient and the ability of the test organism to consume the nutrient.
Glucose was used as the nutrient for the development of the RSA. The maximum concentration of glucose in the assay was limited by the colorimetric saturation of the enzymatic detection system. Because the glucose concentration in the present assay did not exceed the recommended optional concentration used in the NCCLS M27-A methods (2 mg/ml) (9), we used the lowest concentration of glucose that produced near or complete colorimetric saturation. Such a concentration would allow the broadest range of glucose detection.
The goal of subsequent experiments was to determine whether assay conditions that would allow completion of the test in less than a standard work shift in a clinical laboratory (i.e., less than 9 h) and still retain “parity” with the NCCLS M27-A method could be defined. The yeast inoculum concentration and the treatment incubation time were found to influence the magnitude of the detection interval when cells were treated with antifungal drug. Not surprisingly, at lower fungal inoculum concentrations less glucose consumption occurred. Three regions of consumption activity were observed (Fig. 1). (i) When yeast cell concentrations were excessive, despite maximum glucose utilization, drug pressure was insufficient to suppress its consumption. The detection interval was small and a susceptibility curve could not be generated or the result was a spurious, high endpoint. (ii) Optimal yeast cell concentrations created a balance between the organism's capacity for glucose consumption and its susceptibility to drug pressure. These inoculum dilutions resulted in the maximum detection intervals and produced suitable susceptibility curves. (iii) When very low yeast cell concentrations were used, glucose consumption was insufficient to demonstrate drug pressure, and thus, the detection interval was inadequate for susceptibility curve preparation. The maximum detection interval was greater and occurred over a broader inoculum range when the incubation time was increased. These results indicate that the RSA can be modified to test new organisms and new antimicrobial agents such that a detection interval that will result in a reliable susceptibility test can be established.
When cells were exposed for 6 h to the rapidly acting drug amphotericin B, the detection interval was relatively large compared to the small detection interval achieved with the slower-acting drug fluconazole (Fig. 1). At drug concentrations exceeding the MIC (as determined by the NCCLS M27-A method), the difference in glucose consumption between treated and untreated cells approached the detection interval (Fig. 2). The steep slopes observed with cells that were exposed to amphotericin B for 6 h indicate a narrow interval over which the drug causes partial inhibition. They allow MICs that are similar to those produced from the 19-h test to be obtained. Cells treated with fluconazole for 6 h exhibited a lower slope than cells treated for 19 h. The concentration interval at which partial inhibition occurs was broader, and the same MIC was obtained after both incubation times only for C. tropicalis. These results indicate that for these isolates, susceptibility testing of amphotericin B can be accomplished by 6 h and that MICRSA results can be obtained for both drugs by 19 h. Additionally, for some drug and yeast species combinations, the MICRSA endpoint can be made distinct by increasing the treatment period. Further work is needed to determine if just an additional 1 or 2 h would be sufficient.
Treatment failures with the fungicidal drug amphotericin B are well known, despite the involvement of amphotericin B-susceptible etiologic agents as determined by the NCCLS methods. This may represent a limitation of the procedure because the NCCLS M27-A method is not well suited for detection of amphotericin B MICs (the MIC range is clustered between 0.25 and 1 μg/ml) and may fail to detect yeasts that are resistant to this drug (MIC, >1 μg/ml) (9). A graphical display of the inhibition curve for amphotericin B in the RSA demonstrates that there is, as noted above, a drug concentration interval at which partial inhibition or, conversely, partial glucose consumption occurs. These results suggest that the disparity between a MIC and the clinical outcome may be related to the partial inhibition zone. Because of the graphical display generated by RSA, it may be possible to refine the criterion used to define the MIC of amphotericin B. We are using mouse challenge experiments to evaluate how well MICRSA results correlate with the in vivo outcome of drug treatment.
In conclusion, the data demonstrate that measurement of glucose uptake can be used to predict the susceptibility of an organism to an antimicrobial agent. In many cases, this determination can be completed within one 8-h work shift, which is rapid by comparison to accepted methods that evaluate growth as an endpoint. Furthermore, the RSA method can be completed with commitments of materials, equipment, and technician time similar to those required for the NCCLS M27-A method. MICRSA results are in close agreement with published reference values for the six QC organisms. Experience with the RSA suggests that determinations can easily be made within a few hours for rapidly growing organisms and rapidly acting drugs. However, extension of the incubation protocols by a few additional hours or even overnight may yield more defined results with slower-growing yeast and filamentous organisms and/or slower-acting drugs.
The RSA is a technically simple and relatively rapid test whose applicability to a broad range of microorganisms and antimicrobial agents is under investigation.
ACKNOWLEDGMENTS
We thank Sheila Beery for able assistance in reviewing interpretation of the data.
We gratefully acknowledge Pfizer for the generous gift of the fluconazole (agreement UK-048,858) used in these studies.
REFERENCES
- 1.Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Lorian V, editor. Antimicrobics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 52–111. [Google Scholar]
- 2.Anaissie E, Paetznick V, Bodey G P. Fluconazole susceptibility testing of Candida albicans: microtiter method that is independent of inoculum size, temperature, and time of reading. Antimicrob Agents Chemother. 1991;35:1641–1646. doi: 10.1128/aac.35.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake D A, McLean N V. A colorimetric assay for the measurement of d-glucose consumption by cultured cells. Anal Biochem. 1989;177:156–160. doi: 10.1016/0003-2697(89)90031-6. [DOI] [PubMed] [Google Scholar]
- 4.Hazen K C, Brawner D L, Riesselman M H, Jutila M A, Cutler J E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991;59:907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazen K C, Wu G. Kill power of oral antifungals against dermatophytes. Pediatr Infect Dis J. 1999;18:200–204. doi: 10.1097/00006454-199902000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Kanbe T, Han Y, Redgrave B, Riesselman M H, Cutler J E. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph node tissue. Infect Immun. 1993;61:2578–2584. doi: 10.1128/iai.61.6.2578-2584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaRocco M. Recent developments in antifungal susceptibility testing. Clin Microbiol Newl. 1997;13:81–85. [Google Scholar]
- 8.McGinnis M R, Rinaldi M G. Antifungal drugs: mechanisms of action, drug resistance, susceptibility testing, and assays of activity in biological fluids. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 176–211. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Okajima T, Nakamura K, Zhang H, Ling N, Tanabe T, Yasuda T, Rosenfeld R G. Sensitive colorimetric bioassays for insulin-like growth factor (IGF) stimulation of cell proliferation and glucose consumption: use in studies of IGF analogs. Endocrinology. 1992;130:2201–2212. doi: 10.1210/endo.130.4.1372238. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller M A, Bale M, Buschelman B, Lancaster M, Espinel-Ingroff A, Rex J H, Rinaldi M G, Cooper C R, McGinnis M R. Quality control guidelines for National Committee for Clinical Laboratory Standards-recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J Clin Microbiol. 1995;33:1104–1107. doi: 10.1128/jcm.33.5.1104-1107.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller M A, Rinaldi M G. Antifungal susceptibility testing: current state of technology, limitations, and standardization. Infect Dis Clin N Am. 1993;7:435–444. [PubMed] [Google Scholar]
- 13.Warnock D W. Antifungal drug susceptibility testing. Curr Top Med Mycol. 1989;3:403–416. doi: 10.1007/978-1-4612-3624-5_12. [DOI] [PubMed] [Google Scholar]