Abstract
A standardized reference method for dermatophyte in vitro susceptibility testing is lacking. In a previous study, Norris et al. (H. A. Norris, B. E. Elewski, and M. A. Ghannoum, J. Am. Acad. Dermatol. 40(6, part 2):S9–S13) established the optimal medium and other growth variables. However, the earlier study did not address two issues: (i) selection of an optimal medium for conidial formation by dermatophytes and (ii) validation of the method with a large number of dermatophytes. The present study addresses these two points. To select which agar medium best supported conidial growth, representative isolates of dermatophytes were grown on different agars. Preliminary experiments showed that only oatmeal cereal agar supported the production of conidia by Trichophyton rubrum. We tested the abilities of 251 T. rubrum isolates to form conidia using three different cereal agars and potato dextrose agar. Overall, oatmeal cereal and rice agar media were comparable in their abilities to support T. rubrum conidial growth. Next, we used the oatmeal cereal agar for conidial formation along with the optimal conditions for dermatophyte susceptibility testing proposed by Norris et al. and determined the antifungal susceptibilities of 217 dermatophytes to fluconazole, griseofulvin, itraconazole, and terbinafine. Relative to the other agents tested, terbinafine possessed the highest antifungal activity against all of the dermatophytes. The mean ± standard error of the mean MICs of fluconazole, itraconazole, terbinafine, and griseofulvin were 2.07 ± 0.29, 0.13 ± 0.01, 0.002 ± 0.0003, and 0.71 ± 0.05 μg/ml, respectively. This study is the first step in the identification of optimal conditions that could be used for the standardization of the antifungal susceptibility testing method for dermatophytes. Inter- and intralaboratory agreement as well as clinical correlations need to be established.
In the last two decades the incidence of infections caused by dermatophytes and other fungi has increased considerably (1, 7, 11). With an increasing variety of drugs available for the treatment of dermatophytoses, the need for a reference method for the testing of the antifungal susceptibilities of dermatophytes has become apparent (3, 7, 9, 11). Establishment of a reference susceptibility testing method may allow the clinician to select the appropriate therapy for the treatment of infections caused by dermatophytic fungi. Recently, a standard method for antifungal susceptibility testing of yeasts has been established by the National Committee for Clinical Laboratory Standards (NCCLS;M27-A document) (6). This reference method for yeast is the first step in the establishment of a reliable, standardized, and clinically useful technique for the susceptibility testing of filamentous and dermatophytic fungi. Efforts to develop a reference method for broth dilution antifungal susceptibility testing of filamentous fungi are being pursued by NCCLS (1a, 8). This paper represents the first attempt at standardizing the antifungal susceptibility testing of dermatophytes.
In developing this method for antifungal susceptibility testing of dermatophytic fungi, many variables need to be considered. An earlier investigation by Norris et al. (7) evaluated inoculum size, temperature and duration of incubation, medium, and endpoint determination. Although the earlier study established many important variables concerning those conditions necessary for optimization of the susceptibility testing method for dermatophytes, it did not address which medium is appropriate for conidial formation. Identification of such a medium is critical since dermatophytes (particularly Trichophyton rubrum) are known to be poor producers of conidia (11). An isolate's inability to produce conidia will hamper our ability to determine the susceptibility or resistance of that particular isolate.
In this study, we established oatmeal cereal agar and rice agar as the optimal agar media for support of conidial growth. We also expanded the antifungal susceptibility findings of Norris et al. (7) by determining the antifungal susceptibilities of a larger number of dermatophytes isolates to four clinically used antifungal agents. The results from this study serve as a foundation for the development of a standardized susceptibility testing method for dermatophytes.
MATERIALS AND METHODS
Identification of an appropriate medium for production of conidia by various dermatophytes. (i) Agar.
Three types of agar media were initially used to evaluate the conidial growth of the selected dermatophyte isolates (see below): Mycosel agar with 1% yeast extract, potato dextrose agar (both from Becton Dickinson and Company, Cockeysville, Md.), and Heinz oatmeal cereal (H. J. Heinz Co., Pittsburgh, Pa.). On the basis of preliminary data, a continuation study evaluating a larger number of isolates for their conidial growth was performed by using two different cereal agars (mixed grains [H. J. Heinz Co.] and Beech-Nut Rice [Beech-Nut Nutrition Corp., St. Louis, Mo.]), along with oatmeal cereal agar and potato dextrose agar. To prepare the cereal agars, 100 g of the dried ingredients was mixed with 15 g of Bacto agar (Difco Laboratories, Detroit, Mich.). These components were then stirred into 1 liter of distilled water and were mixed thoroughly. The mixture was then autoclaved for 20 min and was then allowed to cool by placing it into a 10°C water bath. Once it was cooled, the mixture was autoclaved again for an additional 20 min. Preliminary experiments revealed that the hold time and the second autoclaving are critical to kill any Bacillus spores present in the mixture.
(ii) Organisms.
We initially tested 43 isolates of dermatophytes including 10 strains each of T. rubrum, Trichophyton mentagrophytes, and Trichophyton tonsurans, 7 strains of Epidermophyton floccosum, and 6 strains of Microsporum canis. The continuation study for conidial growth involved 251 strains of T. rubrum. All strains were clinical isolates obtained from nail or hair specimens received from clinicians by the Center for Medical Mycology, University Hospitals of Cleveland. Dermatophytes were identified to the species level by conventional methods (5). Isolates were stored at −80°C on potato dextrose agar slants until the time of use.
(iii) Determination of conidial growth.
Dermatophytes were grown on different agar media at 30°C for 4 to 7 days. At the end of the incubation period, cellophane tape preparations were used to quantitate the conidial formation microscopically. For each isolate the numbers of conidia were determined in five viewing fields. The average numbers of conidia were calculated for each strain. The ability of various media to support conidial formation was scored on a scale of from 0 to 3, where 0 implies no conidiation and 3 implies proliferative conidiation. The data were expressed as conidial formation as a percentage of that for T. rubrum isolates forming conidia on different agar media.
In vitro susceptibility testing. (i) Organisms.
We tested 217 isolates of dermatophytes including T. rubrum (n = 132), T. mentagrophytes (n = 32), T. tonsurans (n = 42), E. floccosum (n = 3), and M. canis (n = 8). Two American Type Culture Collection (ATCC; Rockville, Md.) quality control organisms were used: Candida parapsilosis ATCC 22019 and Paecilomyces variotti ATCC 22319. All strains were clinical isolates obtained from nail or hair specimens received from clinicians by the Center for Medical Mycology, University Hospitals of Cleveland. Dermatophytes were identified and were stored as described above.
(ii) Antifungal agents.
Four antifungal drugs, supplied by the manufacturers as powders, were used: fluconazole (Pfizer Pharmaceuticals Group, New York, N.Y.), terbinafine (Novartis, E. Hanover, N.J.), itraconazole (Janssen Research Foundation, Beerse, Belgium), and griseofulvin (Sigma Chemical Company, St. Louis, Mo.). Fluconazole was dissolved in sterile water, itraconazole and griseofulvin were dissolved in 100% dimethyl sulfoxide (Curtin Matheson Scientific Inc., Houston, Tex.), and terbinafine was dissolved in dimethyl sulfoxide with 5% Tween 80 (Curtin Matheson Scientific Inc., Houston, Tex.). All drugs were prepared as stock solutions of 1 mg/ml.
(iii) Medium.
RPMI 1640 (American Biorganics Inc., Niagara Falls, N.Y.) with l-glutamine but without sodium bicarbonate and buffered at pH 7.0 with 3-(N-morpholino)propanesulfonic acid, monosodium salt (MOPS), was the medium used for broth microdilution susceptibility testing.
(iv) Drug dilutions.
Serial twofold dilutions were prepared according to the NCCLS M27-A (6) proposed standard. Fluconazole and griseofulvin had MIC ranges of 0.13 to 64.0 μg/ml. Itraconazole and terbinafine had MIC ranges of 0.06 to 32.0 μg/ml.
(v) Inoculum preparation.
We prepared a standardized inoculum by counting the microconidia microscopically. Cultures were grown on oatmeal cereal agar slants for 7 days at 30°C. Sterile normal saline (85%) was added to the slant, and the culture was gently swabbed with a cotton tip applicator to dislodge the conidia from the hyphal mat. The suspension was transferred to a sterile centrifuge tube, and the volume was adjusted to 5 ml with sterile normal saline. The resulting suspension was counted on a hemocytometer and was diluted in RPMI 1640 to the desired concentration.
(vi) Broth microdilution testing.
Microdilution plates were set up in accordance with the NCCLS M27-A (6) reference method; the exception was the inoculum preparation, which was set up as described above. Column 1 was filled with 200 μl of medium to serve as a sterility control. Columns 2 through 11 were filled with 100 μl of the inoculum and 100 μl of the serially diluted antifungal agent. Column 12 was filled with 200 μl of the inoculum and served as a growth control.
(vii) Incubation time and temperature.
The microdilution plates were incubated at 35°C and were read visually after 4 days of incubation.
(viii) Endpoint criteria.
The MIC was defined as the point at which the organism was inhibited 80% compared with the growth in the control well. All isolates were run in duplicate and the results were read visually. For the two isolates tested with fluconazole as quality controls, MICs were within the expected range (for C. parapsilosis, 4.0 μg/ml; for P. variotti, 64.0 μg/ml) specified in document M-27A (6).
RESULTS
Comparison of conidial growth of common dermatophytes on different agar media.
Three types of agar media (potato dextrose, Mycosel with 1% yeast extract, and Heinz oatmeal cereal) were compared for their abilities to induce conidiation. The representative dermatophyte isolates tested were T. rubrum (n = 10), T. mentagrophytes (n = 10), T. tonsurans (n = 10), E. floccosum (n = 7), and M. canis (n = 6). All isolates of the last four species produced abundant conidia (≥26 conidia/field), irrespective of the medium used. The ability of T. rubrum to form conidia was medium dependent. Potato dextrose agar and Mycosel supported only limited growth of T. rubrum conidia at both 4 and 7 days of incubation; i.e., between 10 and 60% of the isolates were able to produce a small number of conidia (1 to 15 conidia/field). In contrast, by 4 days, oatmeal cereal agar promoted the production of a large number of conidia (≥26 conidia/field) from all T. rubrum isolates examined in this preliminary screen. Comparison of the abilities of various isolates to produce conidia at 4 and 7 days showed that 4 days was sufficient for growth of abundant conidia from all isolates of T. mentagrophytes, T. tonsurans, E. floccosum, and M. canis. T. rubrum, on the other hand, produced abundant conidia following growth for 4 and 7 days when it was cultured on oatmeal cereal but not potato dextrose or Mycosel (Table 1). On the last two media, conidial production by T. rubrum was enhanced when the organisms were incubated for 7 days. However, there was no conidial growth for 30 to 40% of the isolates even after 7 days of incubation.
TABLE 1.
Percentages of conidium-forming T. rubrum isolates on three types of agar media at 4 and 7 days of incubationa
| Medium | % of T. rubrum isolates with the following conidial growth at the indicated times:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No conidia
|
1 to 15 conidia/field
|
16 to 25 conidia/field
|
≥26 conidia/field
|
|||||
| 4 days | 7 days | 4 days | 7 days | 4 days | 7 days | 4 days | 7 days | |
| Heinz oatmeal cereal | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| Potato dextrose | 40 | 30 | 20 | 10 | 10 | 10 | 30 | 50 |
| Mycosel (1% yeast extract) | 40 | 40 | 60 | 30 | 0 | 10 | 0 | 20 |
A total of 10 isolates were tested.
Having identified oatmeal cereal agar as a potentially useful medium for conidial formation for all dermatophytic species tested, we wanted to test the ability of a large number of T. rubrum isolates (n = 251) to form conidia with three different cereal agars (oatmeal cereal, rice, and mixed grains) and potato dextrose agar. Overall, even though the oatmeal cereal agar was slightly more effective than rice in its ability to support production of conidia by approximately 84% of the T. rubrum isolates tested, these two media were comparable in their abilities to support T. rubrum conidial growth, as seen in Table 2. About 15% of T. rubrum isolates failed to produce conidia in any of the media tested.
TABLE 2.
Percentages of conidium-forming T. rubrum isolates on three types of cereal media agar and potato dextrose agar at 7 days of incubationa
| Medium | % of T. rubrum isolates with the following conidial growth:
|
|||
|---|---|---|---|---|
| No conidia | 1 to 15 conidia/field | 16 to 25 conidia/field | ≥26 conidia/field | |
| Heinz oatmeal cereal | 15.7 | 15.7 | 24.1 | 44.6 |
| Beech-Nut rice | 14.7 | 14.4 | 31.6 | 38.6 |
| Heinz mixed grains | 26.1 | 41.1 | 18.4 | 14.5 |
| Potato dextrose | 28.8 | 21.6 | 16.5 | 33.0 |
A total of 251 isolates were tested.
Determination of antifungal susceptibilities of 217 dermatophytes by using optimized conditions.
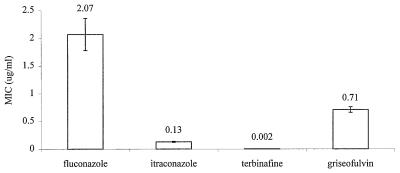
By using the optimized conditions the MICs of griseofulvin, itraconazole, terbinafine, and fluconazole for 217 dermatophyte isolates including T. rubrum (n = 132), T. mentagrophytes (n = 32), T. tonsurans (n = 42), E. floccosum (n = 3), and M. canis (n = 8) were determined. Our data show that terbinafine was the most active antifungal agent tested against dermatophytes. As shown in Fig. 1, the mean ± standard error of the mean MICs v were 2.07 ± 0.29, 0.13 ± 0.01, 0.002 ± 0.0003, and 0.71 ± 0.05 μg/ml for fluconazole, itraconazole, terbinafine, and griseofulvin, respectively. Additionally, the MIC at which 50% of isolates are inhibited (MIC50) and the MIC90 of terbinafine (MIC50, 0.001 μg/ml; MIC90, 0.004 μg/ml) were 130- and 250-fold lower, respectively, than those of the second most active antifungal agent (itraconazole), as seen in Table 3.
FIG. 1.
Mean MICs of fluconazole, itraconazole, terbinafine, and griseofulvin for 217 dermatophytes by the proposed method for in vitro susceptibility testing.
TABLE 3.
MIC50s and MIC90s of fluconazole, itraconazole, terbinafine, and griseofulvin for 217 dermatophytes
| Organism | Total no. of isolates | MIC (μg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluconazole
|
Itraconazole
|
Terbinafine
|
Griseofulvin
|
||||||
| 50% | 90% | 50% | 90% | 50% | 90% | 50% | 90% | ||
| T. rubrum | 132 | 1.0 | 2.0 | 0.13 | 0.5 | 0.001 | 0.002 | 0.5 | 2.0 |
| T. mentagrophytes | 32 | 2.0 | 16.0 | <0.06 | 0.5 | 0.001 | 0.001 | 0.13 | 0.25 |
| T. tonsurans | 42 | 1.0 | 8.0 | <0.06 | 0.06 | 0.002 | 0.008 | 0.5 | 4.0 |
| M. canis | 8 | 0.25 | 2.0 | <0.06 | 0.06 | 0.008 | 0.03 | 0.25 | 1.0 |
| E. floccosum | 3 | 4.0 | 4.0 | <0.06 | <0.06 | 0.015 | 0.015 | 1.0 | 2.0 |
| All dermatophytes | 217 | 1.0 | 4.0 | 0.06 | 0.25 | 0.001 | 0.004 | 0.5 | 2.0 |
DISCUSSION
A standardized dermatophyte susceptibility testing technique should encompass the following: an ideal growth medium, a specific protocol with reference to the initial inoculum size, a specific incubation time, a specific incubation temperature, and an MIC endpoint determination which is applicable to all dermatophytes. Another important factor for the determination of antifungal susceptibility which is particularly important for the testing of dermatophytes is the selection of the most appropriate medium that will support conidial growth. Norris et al. (7) were the first to identify the optimal parameters to be used in performing antifungal susceptibility testing of dermatophytes. In their study, variables such as growth medium, inoculum size, and length and temperature of incubation were addressed.
In this study, we showed that, of those media tested, oatmeal cereal agar and rice agar preparations are the most appropriate for the production of conidia from various dermatophyte species, specifically, T. rubrum. Identification of an appropriate medium for conidial production is of critical importance since an inability to produce spores will limit our ability to prepare the necessary inoculum for the initiation of testing. Although the majority of dermatophytes are capable of producing conidia in different media, the induction of conidiation by T. rubrum has proven to be difficult (7). Since T. rubrum is an important pathogen responsible for a significant number of dermatophyte infections (e.g., over 90% of nail infections are caused by this organism) (1), we decided to investigate the medium appropriate for induction of conidiation by this species. Our data suggest that both oatmeal cereal agar and rice agar could be adopted as media for the induction of conidiation for the standard in vitro dermatophyte susceptibility testing method. Unfortunately, approximately 15% of the isolates tested failed to produce conidia even in the most efficient media (oatmeal cereal and rice). Determination of the antifungal susceptibilities of these organisms will not be possible by currently accepted methods. Other conditions or media that may enhance the sporulation ability of these T. rubrum strains for which induction of sporulation is difficult should be investigated.
In this study we extended the findings of Norris et al. (7) and provided a more comprehensive investigation by testing a larger number of isolates. Using the proposed conditions, we were able to determine the susceptibility profiles of the antifungal agents used to treat dermatophyte infections. Importantly, this profile agrees with earlier reports (10) comparing the in vitro activities of various agents. For example, our data confirm earlier reports showing that terbinafine has the highest level of activity against dermatophytes (10). Furthermore, there was very little difference in the activity of terbinafine against different species, illustrating its uniformly high level of activity.
Our study shows that various parameters, RPMI 1640 medium, an incubation temperature of 35°C, an incubation time of 4 days, and an inoculum of 103 conidia/ml, are optimal for determination of the antifungal susceptibilities of dermatophytes. These conditions, along with our suggested agar medium (oatmeal cereal or rice), combine to provide optimal conditions by which one can obtain the MICs of antifungal agents for dermatophytes. Therefore, these proposed parameters form the foundation for a standardized antifungal susceptibility testing method for dermatophytes. A number of future studies that use these conditions are proposed. A larger sample of dermatophytes needs to be tested to determine the inter- and intralaboratory agreements of such a method. Additionally, MICs need to be correlated with clinical outcome to develop interpretive breakpoints for dermatophyte susceptibility testing.
ACKNOWLEDGMENT
We thank Michael Pfaller for useful comments and suggestions in reading our manuscript.
REFERENCES
- 1.Elewski B E, Charif M A. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172–1173. [PubMed] [Google Scholar]
- 1a.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing of filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georg L K, Camp L B. Routine nutritional tests for the identification of dermatophytes. J Bacteriol. 1956;74:113–121. doi: 10.1128/jb.74.2.113-121.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghannoum M A. Susceptibility testing of fungi and correlation with clinical outcome. J Chemother. 1997;9(Suppl. 1):19–24. [PubMed] [Google Scholar]
- 4.Jesenska Z. Comparative study of the growth inhibition of vegetative hyphae of some dermatophytes and keratinophilic fungi on glucose-peptone-agar with griseofulvin. Dermatol Monatsschr. 1979;165:292–299. [PubMed] [Google Scholar]
- 5.Larone D H. Medically important fungi: a guide to identification. 3rd ed. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 7.Norris H A, Elewski B E, Ghannoum M A. Optimal growth conditions for the determination of the antifungal susceptibility of three species of dermatophytes with the use of a microdilution method. J Am Acad Dermatol. 1999;40(6, part 2):S9–S13. doi: 10.1016/s0190-9622(99)70392-0. [DOI] [PubMed] [Google Scholar]
- 8.Odds F C, Van Gerven F, Espinel-Ingroff A, Bartlett M S, Ghannoum M A, Lancaster M V, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother. 1998;42:282–288. doi: 10.1128/aac.42.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 10.Ryder N S, Favre B. Antifungal activity and mechanism of action of terbinafine. Rev Contemp Pharmacother. 1997;8:275–287. [Google Scholar]
- 11.Weitzman I, Summerbell R C. The dermatophytes. Clin Microbiol Rev. 1995;8:240–259. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]