Abstract
Shift work may increase the risk for hypertension and arterial stiffness, potentially a consequence of disturbed sleep. The aim of this study was to investigate possible correlations between sleep length and spontaneous awakenings with selected cardiovascular risk factors in shift workers at an industrial plant. We examined 19 shift workers by means of blood pressure and arterial stiffness, measured as pulse wave velocity (PWV), prior to and after a 5-week shift period. Sleep patterns were monitored on a daily basis with the assistance of a smartphone-based sleep diary (the entire test period) and by actigraphy (limited to 2 weeks). The number of awakenings and total sleep time were calculated. Shorter sleep duration was associated with higher blood pressure and partly with higher PWV, indicating an increased risk of cardiovascular disease (CVD) with reduced sleep duration. Unexpectedly, a lower number of awakenings was associated with an increase in blood pressure, indicating a reduced risk of CVD. No other significant associations were determined. The results from the present study among shift workers in Norway could support the hypothesis that short sleep duration is associated with elevated blood pressure and arterial stiffness.
Keywords: shift work, night work, cardiovascular diseases, occupational health, sleep, sleep duration, hypertension
1. Introduction
Recent trends show a rapid increase in shift work and flexible schedules, but there is a shortage of studies evaluating the potential health effects in relation to this type of work schedule. In Europe 21% of the workforce is engaged in shift work, and 19% work at night [1].
Cardiovascular disease (CVD) is related to approximately one-third of deaths worldwide [2]. Hence, identifying modifiable risk factors for CVD is of great importance. A systematic review and meta-analysis reported an association between shift work and CVD [3]. It has been estimated that the risk of CVD, after an initial period of 5 years, increases by 7.1% for every subsequent 5 years of cumulative shift work exposure [4]. Shift workers are vulnerable to sleep disturbances/sleep disorders, predominantly among those working night shifts and early morning shifts [5]. Extended working hours can also be associated with excessive sleepiness [6] and lack of sleep [7].
Short sleep duration, or awakenings after sleep onset, has been associated with an increased risk of CVD [8]. Short sleep duration has also been associated with a higher risk of hypertension [9] and an increase in blood pressure (BP) among shift workers [10]. Keeping a sleep diary is a basic tool in insomnia research [11]. The present study used a consensus sleep diary [12] in combination with actigraphy. Activity-based sleep-wake monitoring or actigraphy is a key assessment tool in sleep medicine, but actigraphy should be complemented by a sleep diary [13].
Several mechanisms may account for an elevated CVD risk among shift workers These mechanisms include endocrinological disturbances [14], increased systemic inflammation [15,16], change to the immune system [17], or circadian disruption [18]. The detection of early manifestation of arterial stiffness is important to reduce the patients’ risk of CVD. Pulse wave velocity (PWV) is a measure of arterial stiffness and an independent predictor of cardiovascular disease and all-cause mortality in the general population [19]. Arterial stiffness is one of the earliest manifestations of vascular damage [20] and could coincide with frequent awakenings from sleep [21].
In a 3-year prospective study of shift workers in two industrial plants in Norway, we analyzed blood parameters, brachial blood pressure, resting heart rate and central blood pressure, augmentation pressure and index, and pulse wave velocity. We performed ultrasound measurement of the carotid arteries, and measured VO2max [22]. We found an association between shift work and risk factors for cardiovascular disease such as increased carotid intima media thickness and elevated C-reactive protein (CRP) [23]. The current study differed from many others due to the combination of day-to-day measurements of sleep, capturing acute sleep debt, and cardiac stiffness in a real-life situation.
The aim of the current study was to examine the possible inter-individual associations between sleep duration, number of awakenings and blood pressure and arterial stiffness in industrial shift workers.
2. Materials and Methods
The current study was part of a 3-year prospective follow-up study in relation to cardiovascular health effects of shift work, night shifts and long working hours among industrial workers (see published protocol [22]). Initially, 94 participants were recruited from two industrial plants in Norway. According to the protocol article preceding the current study [22], participants with severe cardiovascular and lung disease were excluded from the study. Two additional exclusion criteria were cancer and blood pressure exceeding 180/110 mmHg. In the current study, workers were invited to participate in a detailed follow-up study of sleep patterns over a 5-week shift period, including two examinations involving PWV and CVD risk factors. The participants worked rotating day, evening and night shifts lasting for 8 or 12 h. The participants worked on adjacent shifts and those with a high degree of compliance in a former study [24] were asked to participate. Nineteen of 30 eligible workers (two women) consented to participate. For a more detailed shift plan, see [23]. The examinations/tests took place in August 2019, after the participants had returned from their holiday. To minimize interference with the circadian rhythm, the participants were assessed for a minimum of 3 days and nights following the last preceding night shift. After the 5-week shift period, a second assessment was carried out following the same protocol. Data from the second assessment were used in the regression analysis.
Following 5 minutes of seated rest, blood pressure and resting heart rate in the left arm was measured 3 times at 1-min intervals by BpTRU® (Bp TRU medical devices, Coquitlam, BC, Canada). The average of three measurements of the seated systolic blood pressure (ssBP) and the seated diastolic blood pressure (sdBP) was calculated. Pulse wave velocity (PWV) was assessed by SphygmoCor XCEL® (AtCor Medical Pty Ltd., Sydney, Australia), according to the manufacturer’s recommendations, using the average result of the three measurements.
Sleep patterns were monitored using wrist-worn actigraphy (AX3, Axivity Ltd., Newcastle upon Tyne, UK) during a 2-week period and by a smartphone-based sleep diary derived from the Consensus Sleep Diary-Core for the duration of 5 weeks [12]. At 21.00 each evening, the participants received a text message, and by clicking on a URL, they accessed a web browser with questions concerning time going to sleep, sleep latency, time of final awakening, number of awakenings (NA), and wakefulness after sleep onset the night prior. Sleep duration was measured as total sleep time (TST) and calculated by subtracting sleep onset latency and wakefulness after sleep onset from the difference between the time going to sleep and the time of final awakening [25]. Similarly, an objectively based measurement was calculated by the use of actigraphy [26] for 2 weeks of the 5-week shift period.
Linear regression analyses were used to determine whether TST or NA were associated with the blood pressure and PWV measurements obtained following the 5-week shift period, using a between-subjects design. Systolic blood pressure, diastolic blood pressure and PWV were treated as dependent variables in separate analyses, one for each sleep variable (NA and TST assessed by diary or by actigraphy). The results are presented as crude models and as models adjusted for age and sex. Comparison of reliability in relation to NA and TST assessments was performed by intraclass correlation coefficient (ICC). All analyses were carried out using Stata v.16.1 (StataCorp LLC, College Station, TX, USA). The threshold for significance was α = 0.05.
3. Results
Table 1 illustrates the characteristics of the shift workers; age, body-mass index (BMI) and years working shifts, as well as blood pressure and PWV results from the examination prior to and following the 5-week shift period. The measured parameters were quite stable across the 5-week shift period.
Table 1.
Characteristics of shift workers before and after 5-week registration period.
| N | Mean | SD | Min, Max | |||
|---|---|---|---|---|---|---|
| Age (years) | 19 | 40.9 | 11.5 | 26, 58 | ||
| BMI (kg/m2) | 19 | 27.6 | 4.7 | |||
| Years in shift work | 19 | 16.3 | 10.3 | |||
| Prior to 5-Week Shift Period | Following 5-Week Shift Period | |||||
| N | Mean | SD | N | Mean | SD | |
| ssBP (mmHg) | 19 | 122.9 | 12.0 | 18 | 122.3 | 8.8 |
| sdBP (mmHg) | 19 | 84.4 | 7.1 | 18 | 82.7 | 6.9 |
| PWV (m/s) | 19 | 8.0 | 1.5 | 17 | 8.0 | 1.5 |
BMI: Body-mass index. ssBP: Sitting systolic blood pressure. sdBP: Sitting diastolic blood pressure. PWV: Pulse wave velocity.
3.1. Sleep Measurements from Diary and Actigraphy
The sleep diary was completed for 25.1 ± 7.9 (mean ± standard deviation) of 35 days (72%). Valid actigraphy data were available for 10.0 ± 2.5 of 14 days (71%). Subjects reported significantly fewer awakenings (NA) in the sleep diary, compared to NA measured by actigraphy (Table 2). Longer sleep duration (TST) was reported by diary, compared to TST measured by actigraphy (Table 2). The reliability comparison between diary and actigraphy measurements was ICC = 0.50 and ICC = 0.33 for TST and NA, respectively.
Table 2.
Sleep variables assessed by diary and actigraphy.
| Diary | Actigraphy | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | z | p | |
| NA (n) | 16 | 1.3 | 0.9 | 17 | 12.9 | 2.7 | 3.52 | 0.0004 |
| TST (hours) | 16 | 6.8 | 0.8 | 17 | 5.8 | 0.8 | 3.31 | 0.0009 |
z/p-value: Wilcoxon signed-rank test. NA: Number of awakenings, TST: total sleep time.
3.2. Association between Sleep Measurements and Cardiovascular Risk Factors
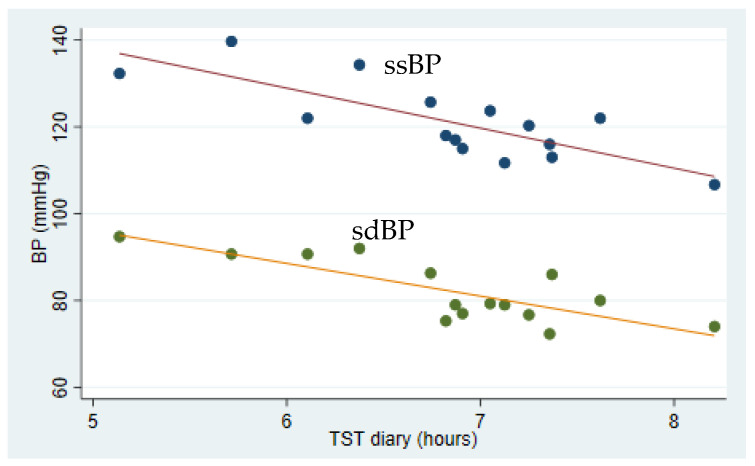
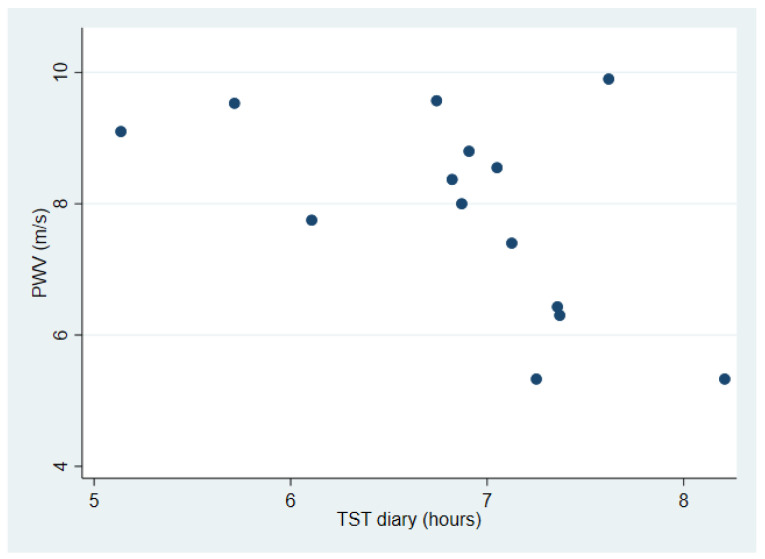
Table 3 shows the associations between sleep measurements, blood pressure and pulse wave velocity. A negative association between TST measured by diary and ssBP was identified, both in the crude (p < 0.001) and in the adjusted analysis (p = 0.005) (Figure 1). Similar associations were found between TST measured by/registered in diary and sdBP, both in the crude (p < 0.001) and adjusted analysis (p = 0.003). Furthermore, a negative association between TST registered in diary and PWV (p = 0.045) was identified, but limited to the crude analysis (Figure 2). A post hoc analysis showed a significant bivariate correlation between age and PWV (rho = 0.70, p = 0.0039). A negative association between NA measured by actigraphy and ssBP in the crude analysis (p = 0.031), and between NA and sdBP both in the crude (p = 0.001) and adjusted analysis (p = 0.008) was identified. NA measured by diary was not associated with blood pressure, nor was NA associated with PWV.
Table 3.
Associations between sleep and blood pressure.
| ssBP, mmHg | sdBP, mmHg | PWV | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | ||||||||||||||||
| Coeff. | p-Value | Coeff. | 95% CI | p-Value | R2 | Coeff. | p-Value | Coeff. | 95% CI | p-Value | R2 | Coeff. | p-Value | Coeff. | 95% CI | p-Value | R2 | ||||
| TST diary (hours) | −9.22 | <0.001 | −8.78 | −14.30 | −3.26 | 0.005 | 0.64 | −7.53 | <0.001 | −7.51 | −11.98 | −3.05 | 0.003 | 0.64 | −1.06 | 0.045 | −0.24 | −1.13 | 0.66 | 0.567 | 0.70 |
| TST actigraphy (hours) | −2.43 | 0.417 | −2.30 | −8.27 | 3.67 | 0.418 | 0.28 | −3.21 | 0.175 | −3.02 | −7.76 | 1.72 | 0.191 | 0.31 | −0.01 | 0.989 | 0.08 | −0.63 | 0.79 | 0.809 | 0.70 |
| NA diary (n) | −1.32 | 0.618 | −2.86 | −8.73 | 3.01 | 0.307 | 0.32 | −1.93 | 0.36 | −2.73 | −7.55 | 2.08 | 0.238 | 0.29 | 0.29 | 0.558 | 0.19 | −0.53 | 0.91 | 0.566 | 0.70 |
| NA actigraphy (n) | −1.73 | 0.031 | −1.46 | −3.43 | 0.51 | 0.132 | 0.38 | −1.93 | 0.001 | −1.95 | −3.28 | −0.62 | 0.008 | 0.57 | −0.22 | 0.143 | 0.06 | −0.18 | 0.30 | 0.593 | 0.71 |
Adjustment: Sex and age. R2: Goodness of fit.
Figure 1.
Association between average sleep duration and blood pressure among industrial shift workers. Sleep was measured as total sleep time by diary (TST diary) for 5 weeks preceding measurements of systolic and diastolic blood pressure (BP). A negative association between both systolic and diastolic blood pressure, and total sleep time in the adjusted analyses was identified (p = 0.005, R2 = 0.64 and p = 0.003, R2 = 0.64, respectively). Solid lines show linear prediction.
Figure 2.
Association between average sleep duration and pulse wave velocity (PWV) among industrial shift workers. Sleep was measured as total sleep time by diary (TST diary) for 5 weeks preceding measurements of systolic and diastolic blood pressure (BP).
4. Discussion
The results from the present study among rotating shift workers in industry suggest that shorter sleep duration, as measured by sleep diary, was associated with elevated blood pressure and arterial stiffness, reported by partly increased PWV, indicating an elevated CVD risk. Unexpectedly, more frequent number of awakenings after sleep onset was associated with lower blood pressure, indicating a reduced CVD risk.
The negative association between sleep duration and blood pressure is consistent with findings in several studies. A population-based prospective study including more than 9000 participants suggests that short sleep duration among shift workers could indicate a risk for hypertension [10]. That short sleep duration increases the risk for hypertension is also supported by a study among adults older than 40 years [27], and by a study among 578 Americans of similar BMI and age as the participants in the present study [28]. The regression coefficient was considerably smaller in the latter study (systolic BP −1.80 mmHg per hour sleep in the adjusted model), when compared to the present/current study (systolic BP −8.78 mmHg per hour sleep). This inconsistency may be explained by the limited number of participants in the current study, resulting in a greater degree of variation in blood pressure measurements. The findings of an additional American study, however, were limited to an association between sleep duration and blood pressure in African-American shift workers [29]. Other studies with contrasting findings are also available. A study among non-insomniac elderly subjects (72 ± 1 years) failed to identify an association between sleep duration, measured by the Pittsburg sleep quality index questionnaire, and hypertension [30]. The lack of association could be the result of the age range, relative to the present population.
We have previously shown a decrease in blood pressure following an 8-week physical activity initiative focusing on cardiorespiratory performance in the same group of workers [24], but these results may be temporary depending on the level of future physical activity. In the present study, self-reported short sleep duration was associated with elevated blood pressure inter-individually in a relatively small population of workers.
The present data partially support the perception that short sleep duration may be associated with arterial stiffness, measured as elevated PWV. However, the association was not to be found in the adjusted analysis (Table 3), suggesting age as a key confounder in the association between sleep duration and PWV. A post hoc analysis confirmed that age and PWV were positively correlated. That fact that PWV increases with age has been previously established [31]. However, other studies have shown that long-term exposure to night shift work [32] or shift work [33] seems to be associated with arterial stiffness, measured by pulse wave velocity (PWV). Comparing clockwise and counterclockwise shift work rotations failed to reveal any differences in PWV among male steel factory workers [34], a population quite comparable to the participants in the current study. As the present small number of participants exhibited a noticeable variance in sleep duration (Figure 1), the difference may be too insignificant to affect PWV, taking the age range into account. The above-mentioned studies, by Chen et al. [33] in particular, indicate that changes in PWV take years to develop. However, PWV values may, generally, be reduced as a result of weight loss, regular exercise, reduced salt intake and calorie restrictions [35]. Blood pressure treatment, statins and smoking cessation will also contribute to a reduction of arterial stiffness [20]. Interestingly, an association between shift work and CRP at baseline was found [23], which may be linked to sleep disturbances within the group. Sleep deprivation can cause an upregulation of genes controlling inflammation and thus CRP [17]. To further study the association between arterial stiffness, inflammation, PWV and shift work, a prospective design with long follow-up is necessary.
Frequent awakenings are one of the key elements in insomnia, and insomnia is a risk factor for hypertension [36]. The negative association between NA, assessed by actigraphy, and blood pressure suggests that a higher number of awakenings reduces blood pressure. This is a surprising finding. A possible explanation could be that the algorithm calculating the number of awakenings was too sensitive. This explanation is somewhat supported by the findings reported in Table 2, indicating that NA measured by actigraphy was tenfold compared to NA registered in the diary. Both sleep variables estimated by actigraphy differed significantly from those measured by the smartphone sleep diary. The actigraphy-based estimates of NA and TST were all within the range of those reported in a recent study comparing different algorithms [37]. In insomniacs, estimated TSTs are typically shorter if measured by diary than by polysomnography [38], contrary to the findings in the current study. Estimated TSTs were longer in diary registrations than those measured by actigraphy. Hence, the subjects may have a subjective experience of sleeping quite well. Offshore night shift workers have reported longer TSTs by diary than by actigraphy [39]. This may indicate a difference between insomniacs and regular shift workers. As actigraphy may register short sleep alterations not perceived as real awakenings by the subject, the actigraphy-based estimates of awakening may be more accurate. The significant difference between diary-based and actigraphy-based sleep measurements complicates the interpretation of the associations between sleep and risk of CVD.
The diary design of the current study represented a strength. It was likely to reduce recall bias, as participants were asked to recall sleep daily. Accurate sleep measures were then linked to the follow-up measurement of blood pressure and PWV. Another strength is that the same technicians performed all tests, providing the participants with identical guidelines in every session. The fact that the AX3 actigraphy algorithm calculating sleep duration [26] had not been validated by polysomnography for non-patient subjects, or for daytime sleepers, represents a limitation. A further limitation was the relatively low compliance between diary and actigraphy measurements (around 70%), as actigraphy measurements did not correspond exactly with diary measurements. Albeit a small group, the participants were all rotating shift workers selected as a result of high compliance in a previous sub-study [24]; consequently, the selection of healthy and highly motivated individuals into the study should be taken into consideration.
5. Conclusions
The results from the present study among a Norwegian population of industrial shift workers support the hypothesis that short sleep duration and number of awakenings are associated with an increase in blood pressure and possibly arterial stiffness. The current study had a relatively small sample size, and the findings may be somewhat limited to be considered representative of a population. To further study the relevant associations, a prospective design study with long follow-up is required.
Acknowledgments
We thank the workers at the industrial plant for participating in the study, Anne-Mari Gjestvang Moe for preprocessing actigraphy and sleep diary data, and Elisabeth Ødemark for proofreading the English manuscript.
Author Contributions
Conceptualization, M.S., E.G. and P.A.S.; methodology, E.G., M.S. and P.A.S.; software, Ø.S. and D.M.; validation, Ø.S., D.M., P.A.S. and M.S; formal analysis, D.M.; data curation, Ø.S.; writing—original draft preparation, M.S. and D.M.; writing—review and editing, all authors; visualization, D.M.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Regional Ethics Committee in Oslo, REC South-East B (Reference number: 2018/1258).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data protection issues, as data are still being collected from the subjects.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eurofound European Working Conditions Survey. [(accessed on 2 May 2020)]. Available online: https://www.eurofound.europa.eu/surveys/2020/european-working-conditions-survey-2020.
- 2.Deaton C., Froelicher E.S., Wu L.H., Ho C., Shishani K., Jaarsma T. The global burden of cardiovascular disease. Eur. J. Cardiovasc. Nurs. 2011;10:S5–S13. doi: 10.1016/S1474-5151(11)00111-3. [DOI] [PubMed] [Google Scholar]
- 3.Vyas M.V., Garg A.X., Iansavichus A.V., Costella J., Donner A., Laugsand L.E., Janszky I., Mrkobrada M., Parraga G., Hackam D.G. Shift work and vascular events: Systematic review and meta-analysis. BMJ. 2012;345:e4800. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torquati L., Mielke G.I., Brown W.J., Kolbe-Alexander T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand. J. Work Environ. Health. 2018;44:229–238. doi: 10.5271/sjweh.3700. [DOI] [PubMed] [Google Scholar]
- 5.Korsiak J., Tranmer J., Leung M., Borghese M.M., Aronson K.J. Actigraph measures of sleep among female hospital employees working day or alternating day and night shifts. J. Sleep Res. 2018;27:e12579. doi: 10.1111/jsr.12579. [DOI] [PubMed] [Google Scholar]
- 6.Son M., Kong J.O., Koh S.B., Kim J., Harma M. Effects of long working hours and the night shift on severe sleepiness among workers with 12-hour shift systems for 5 to 7 consecutive days in the automobile factories of Korea. J. Sleep Res. 2008;17:385–394. doi: 10.1111/j.1365-2869.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 7.Virtanen M., Ferrie J.E., Gimeno D., Vahtera J., Elovainio M., Singh-Manoux A., Marmot M.G., Kivimaki M. Long working hours and sleep disturbances: The Whitehall II prospective cohort study. Sleep. 2009;32:737–745. doi: 10.1093/sleep/32.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan B., Yang J., Zhao B., Fan Y., Wang W., Ma X. Objective Sleep Efficiency Predicts Cardiovascular Disease in a Community Population: The Sleep Heart Health Study. J. Am. Heart Assoc. 2021;10:e016201. doi: 10.1161/JAHA.120.016201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X., Zheng L., Wang J., Zhang X., Zhang X., Li J., Sun Y. Epidemiological evidence for the link between sleep duration and high blood pressure: A systematic review and meta-analysis. Sleep Med. 2013;14:324–332. doi: 10.1016/j.sleep.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Riegel B., Daus M., Lozano A.J., Malone S.K., Patterson F., Hanlon A.L. Shift Workers Have Higher Blood Pressure Medicine Use, But Only When They Are Short Sleepers: A Longitudinal UK Biobank Study. J. Am. Heart Assoc. 2019;8:e013269. doi: 10.1161/JAHA.119.013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buysse D.J., Ancoli-Israel S., Edinger J.D., Lichstein K.L., Morin C.M. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 12.Carney C.E., Buysse D.J., Ancoli-Israel S., Edinger J.D., Krystal A.D., Lichstein K.L., Morin C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Ulhoa M.A., Marqueze E.C., Burgos L.G., Moreno C.R. Shift work and endocrine disorders. Int. J. Endocrinol. 2015;2015:826249. doi: 10.1155/2015/826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amano H., Fukuda Y., Yokoo T., Yamaoka K. Interleukin-6 Level among Shift and Night Workers in Japan: Cross-Sectional Analysis of the J-HOPE Study. J. Atheroscler. Thromb. 2018;25:1206–1214. doi: 10.5551/jat.42036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavanello S., Stendardo M., Mastrangelo G., Bonci M., Bottazzi B., Campisi M., Nardini M., Leone R., Mantovani A., Boschetto P. Inflammatory Long Pentraxin 3 is Associated with Leukocyte Telomere Length in Night-Shift Workers. Front. Immunol. 2017;8:516. doi: 10.3389/fimmu.2017.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aho V., Ollila H.M., Rantanen V., Kronholm E., Surakka I., van Leeuwen W.M., Lehto M., Matikainen S., Ripatti S., Harma M., et al. Partial sleep restriction activates immune response-related gene expression pathways: Experimental and epidemiological studies in humans. PLoS ONE. 2013;8:e77184. doi: 10.1371/journal.pone.0077184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rüger M., Scheer F.A. Effects of circadian disruption on the cardiometabolic system. Rev. Endocr. Metab. Disord. 2009;10:245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlachopoulos C., Alexopoulos N., Stefanadis C. Aortic stiffness: Prime time for integration into clinical practice. Hell. J. Cardiol. 2010;51:385–390. [PubMed] [Google Scholar]
- 20.Kim H.-L., Kim S.-H. Pulse wave velocity in atherosclerosis. Front. Cardiovasc. Med. 2019;6:41. doi: 10.3389/fcvm.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallat R., Shah V.D., Redline S., Attia P., Walker M.P. Broken sleep predicts hardened blood vessels. PLoS Biol. 2020;18:e3000726. doi: 10.1371/journal.pbio.3000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunde L.-K., Skare Ø., Mamen A., Sirnes P.A., Aass H.C., Øvstebø R., Goffeng E., Matre D., Nielsen P., Heglum H.S.A. Cardiovascular Health Effects of Shift Work with Long Working Hours and Night Shifts: Study Protocol for a Three-Year Prospective Follow-Up Study on Industrial Workers. Int. J. Environ. Res. Public Health. 2020;17:589. doi: 10.3390/ijerph17020589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skogstad M., Mamen A., Lunde L.K., Ulvestad B., Matre D., Aass H.C.D., Ovstebo R., Nielsen P., Samuelsen K.N., Skare O., et al. Shift Work Including Night Work and Long Working Hours in Industrial Plants Increases the Risk of Atherosclerosis. Int. J. Environ. Res. Public Health. 2019;16:521. doi: 10.3390/ijerph16030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamen A., Øvstebø R., Sirnes P.A., Nielsen P., Skogstad M. High-intensity training reduces CVD risk factors among rotating shift workers: An eight-week intervention in industry. Int. J. Environ. Res. Public Health. 2020;17:3943. doi: 10.3390/ijerph17113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsifaraki M., Nilsen K.B., Christensen J.O., Wærsted M., Knardahl S., Bjorvatn B., Härmä M., Matre D. Sleep duration mediates abdominal and lower-extremity pain after night work in nurses. Int. Arch. Occup. Environ. Health. 2019;92:415–422. doi: 10.1007/s00420-018-1373-9. [DOI] [PubMed] [Google Scholar]
- 26.Borazio M., Berlin E., Kücükyildiz N., Scholl P., Van Laerhoven K. Towards benchmarked sleep detection with wrist-worn sensing units; Proceedings of the 2014 IEEE International Conference on Healthcare Informatics; Verona, Italy. 15–17 September 2014; pp. 125–134. [Google Scholar]
- 27.Faraut B., Touchette E., Gamble H., Royant-Parola S., Safar M.E., Varsat B., Leger D. Short sleep duration and increased risk of hypertension: A primary care medicine investigation. J. Hypertens. 2012;30:1354–1363. doi: 10.1097/HJH.0b013e32835465e5. [DOI] [PubMed] [Google Scholar]
- 28.Knutson K.L., Van Cauter E., Rathouz P.J., Yan L.L., Hulley S.B., Liu K., Lauderdale D.S. Association between sleep and blood pressure in midlife: The CARDIA sleep study. Arch. Intern. Med. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceïde M.E., Pandey A., Ravenell J., Donat M., Ogedegbe G., Jean-Louis G. Associations of short sleep and shift work status with hypertension among black and white Americans. Int. J. Hypertens. 2015;2015:697275. doi: 10.1155/2015/697275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sforza E., Saint Martin M., Barthelemy J.C., Roche F. Association of self-reported sleep and hypertension in non-insomniac elderly subjects. J. Clin. Sleep Med. 2014;10:965–971. doi: 10.5664/jcsm.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borlotti A., Khir A.W., Rietzschel E.R., De Buyzere M.L., Vermeersch S., Segers P. Noninvasive determination of local pulse wave velocity and wave intensity: Changes with age and gender in the carotid and femoral arteries of healthy human. J. Appl. Physiol. 2012;113:727–735. doi: 10.1152/japplphysiol.00164.2012. [DOI] [PubMed] [Google Scholar]
- 32.Jankowiak S., Backé E., Liebers F., Schulz A., Hegewald J., Garthus-Niegel S., Nübling M., Blankenberg S., Pfeiffer N., Lackner K. Current and cumulative night shift work and subclinical atherosclerosis: Results of the Gutenberg Health Study. Int. Arch. Occup. Environ. Health. 2016;89:1169–1182. doi: 10.1007/s00420-016-1150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C.-C., Shiu L.-J., Li Y.-L., Tung K.-Y., Chan K.-Y., Yeh C.-J., Chen S.-C., Wong R.-H. Shift work and arteriosclerosis risk in professional bus drivers. Ann. Epidemiol. 2010;20:60–66. doi: 10.1016/j.annepidem.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 34.Kantermann T., Duboutay F., Haubruge D., Kerkhofs M., Schmidt-Trucksäss A., Skene D.J. Atherosclerotic risk and social jetlag in rotating shift-workers: First evidence from a pilot study. Work. 2013;46:273–282. doi: 10.3233/WOR-121531. [DOI] [PubMed] [Google Scholar]
- 35.Donato A.J., Machin D.R., Lesniewski L.A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 2018;123:825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suka M., Yoshida K., Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J. Occup. Health. 2003;45:344–350. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 37.Lüdtke S., Hermann W., Kirste T., Beneš H., Teipel S. An algorithm for actigraphy-based sleep/wake scoring: Comparison with polysomnography. Clin. Neurophysiol. 2021;132:137–145. doi: 10.1016/j.clinph.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 38.McCall C., McCall W.V. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J. Sleep Res. 2012;21:122–127. doi: 10.1111/j.1365-2869.2011.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saksvik I.B., Bjorvatn B., Harvey A.G., Waage S., Harris A., Pallesen S. Adaptation and readaptation to different shift work schedules measured with sleep diary and actigraphy. J. Occup. Health Psychol. 2011;16:331–344. doi: 10.1037/a0022770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to data protection issues, as data are still being collected from the subjects.