Introduction
The age-adjusted prevalence of obesity (BMI >30) and severe obesity (BMI >40) among U.S. adults is 42.2% and 9.2%, respectively.1 The obesity epidemic has resulted in an increase in the proportion of patients with chronic liver disease due to nonalcoholic fatty liver disease (NAFLD, also referred to as metabolic associated fatty liver disease or MAFLD) and in the prevalence of obesity among patients with cirrhosis of all etiologies. The burden of obesity among patients with cirrhosis appears similar to the general population with a reported prevalence of 30%.2 A recent report revealed that 39% and 15% of transplant recipients in 2017 had Class I (BMI ≥ 30 kg/m2) or Class II (BMI ≥ 35 kg/m2) obesity, respectively, as compared to 21% and 12% in 2007.3 The clinical implications of the epidemic of obesity in liver disease are many, including its role as risk factor for de novo liver disease, progressive liver fibrosis and cirrhosis, and adverse outcomes among patients with established cirrhosis (Table 2).2,4,5
Table 2.
Best Practice Advice Statements for Bariatric Surgery in Obese Patients With Cirrhosis
| Target audience | Hepatologists, gastroenterologists, liver transplant surgeons, bariatric surgeons, anesthesiologists, critical care physicians, nutrition experts, and other subspecialists seeing patients with obesity and cirrhosis |
| Target population | Obese patients with cirrhosis undergoing bariatric surgery, liver transplantation, or both |
| Baseline risk factors for surgical morbidity and mortality | CTP score, MELD score, ASA physical status classification, BMI, visceral fat, sarcopenia, portal hypertension, centers performing low-volume surgeries, age, and non-hepatic comorbidities prevalent in this patient population (cardiovascular and cerebrovascular disease, type 2 diabetes mellitus, renal impairment, and restrictive lung disease) |
| Postoperative complications common in these patients | Hepatic decompensation, protein calorie malnutrition, portal vein thrombosis, malabsorption of nutrients, vitamins, and trace metals, impaired absorption of vital medications including immunosuppressive agents, potential inability to endoscopically evaluate the biliary tree or endoscopically access portal hypertensive gastropathy or varices in the excluded gastric remnant, and the onset of alcohol use disorders |
| Best Practice Advice statements | |
| 1 | Because obesity in patients with cirrhosis is a major risk factor for hepatic decompensation, portal vein thrombosis, hepatocellular carcinoma, and the development of acute on chronic liver disease, weight loss should be an important therapeutic goal for these patients. |
| 2 | The method and rapidity for obtaining a sustained loss of excess body fat in obese patients with cirrhosis need to be individualized and are dependent not only on the BMI but also the presence and degree of sarcopenia, edema/ascites, CSPH, whether the patient has compensated or decompensated cirrhosis, patient age, and potential candidacy for liver transplantation. |
| 3 | Weight management, ideally ≥10% total body weight loss, via lifestyle modification may decrease portal hypertension and histologic progression; however, success in implementing these interventions in clinical practice and uncertainties regarding durability of this approach limit the utility of this method for treatment of obese patients with cirrhosis. |
| 4 | Bariatric surgery should be considered in selected patients with compensated cirrhosis in an effort to reduce risk for hepatocellular carcinoma and improve survival. |
| 5 | Bariatric surgery in obese patients with cirrhosis should only be performed in those with compensated disease by an experienced surgeon at a high-volume bariatric center. Bariatric surgery in this patient population should only be performed after careful evaluation and management of extrahepatic comorbidities. |
| 6 | Assessment for CSPH should be included in the preoperative evaluation for bariatric surgery in patients with cirrhosis. Pending data to validate noninvasive testing for this purpose, cross-sectional imaging and upper endoscopy should be performed to evaluate features of CSPH. |
| 7 | Bariatric surgery may be associated with significant changes in alcohol drinking habits and deleterious changes in alcohol metabolism. The consequences of alcohol consumption after bariatric surgery among patients with cirrhosis warrant intensive assessment of these candidates preoperatively and long-term efforts to mitigate risk after surgery. |
| 8 | Currently approved endoscopic bariatric therapies include the intragastric balloon, a percutaneous gastric aspiration system, and endoscopic sleeve gastrectomy. Endoscopic bariatric therapies may have lower risk(s) compared with surgical approaches, although direct comparative studies to support this, as well as long-term efficacy data, are currently lacking. Endoscopic bariatric therapies should not be performed in patients with CSPH. |
| 9 | Programs offering bariatric surgical services for patients with cirrhosis must include a surgical and anesthesia team with experience in operating on patients with portal hypertension and cirrhosis as well as a medical team with experience in treating a postoperative patient with cirrhosis. Potential candidacy for liver transplantation should be determined as part of the preoperative assessment of obese patients with cirrhosis. |
| 10 | Because of preservation of endoscopic access to the biliary tree, gradual weight loss, and absence of malabsorption, the optimal bariatric surgical procedure for patients with cirrhosis is most likely a laparoscopic sleeve gastrectomy. The optimal timing is determined by the stage of liver disease. In decompensated liver disease, the only acceptable option at present is bariatric surgery concurrent with or after liver transplant. |
ASA, American Society of Anesthesiologists; BMI, body mass index; CSPH, clinically significant portal hypertension; CTP, Child-Turcotte-Pugh; MELD, Model for End-Stage Liver Disease.
Despite clear epidemiological risks of obesity and evidence that weight loss is beneficial in improving or reversing complications of liver disease,6,7 the management of obesity remains challenging. Bariatric surgery is the most effective and durable approach to obesity management and improves many associated comorbidities.8 However, surgical risk in these patients is increased relative to those without cirrhosis and tools for risk assessment and mitigation continue to evolve.9 Determining the risk to benefit ratio for bariatric surgery in individuals with cirrhosis is complex and hampered by a lack of randomized control trials. The goal of this review is to provide guidance to clinicians caring for patients with chronic liver disease and obesity, for bariatric surgical programs evaluating patients with advanced liver fibrosis, and for liver transplant programs aiming to optimize long-term outcomes in candidates with significant obesity.
This expert review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee (CPUC) and the AGA Governing Board to provide timely guidance on a topic of high clinical importance to the AGA membership, and underwent internal peer review by the CPUC and external peer review through standard procedures of Clinical Gastroenterology and Hepatology.
Methods
The authors have reviewed and summarized available data pertinent to performance of bariatric surgery in patients with cirrhosis in order to generate specific practice advice addressing key aspects of clinical management. Pubmed databases were queried to identify articles published from January 2000 to March 2020. We considered and selected peer-reviewed articles in the following order of relevance: RCTs, systematic reviews and meta-analyses, observational studies published in the English language, with additional priority given to comparative studies which were most recently published and eliminating citations with significant overlap in content.
How does obesity impact chronic liver disease?
Obesity is associated with significant morbidity and mortality with clinical decompensation rates of 14%, 31% and 43% in normal weight, overweight and obese cirrhotic patients, respectively.2 Obesity increases morbidity and mortality in patients with hepatitis C,10 alcoholic liver disease,11 and NAFLD (Table 2).12 Obesity is also associated with portal vein thrombosis,13 portal hypertension (HTN),7 hepatocellular carcinoma (HCC),14 and liver failure in acute on chronic liver disease.15 Although no longer an absolute contraindication for liver transplant (LT),16 obesity is associated with higher wait list mortality,17 increased post-operative complications,18 and may influence long-term survival post-LT.19 Since weight loss improves clinical outcomes,20 histology,21 portal HTN6 and the likelihood of receiving LT for patients on the waitlist,22 weight loss should be an important therapeutic goal for clinicians caring for these patients.
What factors should be considered in obesity management for patients with cirrhosis?
The assessment and strategies for weight loss in cirrhosis is mired by complex interactions among fat mass, visceral fat, edema/ascites and muscle mass that are not captured by BMI.16,18 Patients who have excess visceral fat suffer increased mortality, poor survival after LT, an increase in bacterial infections and sepsis related death.23,24 Sarcopenia (decreased muscle mass and strength) occurs in 40–70% of cirrhotic patients and adversely impacts survival, complications of cirrhosis, quality of life and post-LT outcomes.24,25 Sarcopenic obesity is associated with higher mortality rates, poor outcomes after LT, recurrent HCC, and perioperative complications.26 Although there are multiple methods to assess sarcopenia, CT measurement of the psoas muscle at the level of the third lumbar vertebrate is the most common. With appropriate software, muscle and adipose tissue can be quantified from routine surveillance exams for hepatocellular carcinoma.24 In addition to fat mass distribution and sarcopenia, patient variables critical to the determination of appropriate weight loss management in patients with cirrhosis include evidence of clinically significant portal HTN (BPA 6), history of decompensation (BPA 5), and potential liver transplant candidacy (BPA 9).
Considerations for non-invasive methods of weight management in cirrhosis
Achieving effective, durable weight loss is of paramount importance in all etiologies of liver disease though the bulk of clinical trials have been performed in NAFLD. In a meta-analysis of eight randomized controlled trials, patients achieving ≥ 3% weight loss had improvement in hepatic steatosis, ≥ 5% improved ballooning inflammation, ≥7 % had NASH resolution, and ≥10 % was associated with decreased fibrosis.27 Most of these trials employed intensive lifestyle interventions, frequently plagued by weight gain after completion. The SportDiet study, a 16 week intensive diet and exercise intervention in patients with cirrhosis, was safe, achieved durable (6 months) weight loss of 5% in 52% of patients and a 10% weight loss in 16%.6 There was also a reduction in portal hypertension with weight loss. Another prospective cohort of cirrhotic patients with a mean BMI of 40 kg/m2, employing a similar lifestyle intervention, was able to achieve and maintain a mean BMI of 33 kg/m2 prior to LT. Three years after LT 22% of these patients were able to maintain a BMI <35 kg/m2.28 These positive results notwithstanding, behavioral therapy is challenging to establish in routine clinical practice and optimally achieved by a multidisciplinary team, including physicians, nutritionists, psychologists and exercise physiologists. The most current obesity clinical practice guidelines emphasize caution in the consideration of weight-loss medications in patients with liver disease.29 Preliminary data for Liraglutide (a glucagon-like peptide-1 analog) in patients with NASH and advanced fibrosis appear promising.30 Additional studies are needed to establish the role of pharmacotherapy assisted weight loss in patients with cirrhosis.
While caloric restriction is a requisite for weight loss, vulnerability to muscle wasting must be anticipated when developing lifestyle management criteria for patients with cirrhosis. Per EASL guidelines, for cirrhotic patients with BMI>30 kg/m2 (corrected for water retention), nutritional and lifestyle changes should be implemented to achieve a progressive weight loss of >5–10%.31 To attain this goal, a modest but tailored hypocaloric diet (restriction of 500– 800 kcal/day) that includes a protein intake of ≥ 1.5 gram/kg per ideal weight/day is recommended. Periods of starvation should be avoided; frequent meals and a nighttime snack are recommended.
Best Practice Advice (see Table 1):
Table 1.
Risks Associated With Obesity in Chronic Liver Disease, Cirrhosis, and Liver Transplantation
| NAFLD | Non-NAFLD chronic liver disease | Cirrhosis | Liver transplant |
|---|---|---|---|
| Increased proportion of chronic liver disease due to NAFLD78 Increased number of LT registrants with cirrhosis due to NASH79 Increased death rates due to NAFLD12 Increased medical costs from NAFLD80 Risk factor for HCC in non-cirrhotic NASH81 |
Risk factor for fibrosis progression82,83 Increased morbidity/mortality in chronic hepatitis C infection4 Increased morbidity/mortality in alcohol-related liver disease11,84 |
Risk factor for decompensation2 Risk factor for portal vein thrombosis13 Risk factor for portal HTN7 Risk factor for cirrhosis-related mortality4 Risk factor for cirrhosis-related hospitalization4 Risk factor for HCC37,85 Risk factor for mortality from HCC86 Risk factor for liver failure in acute on chronic liver failure15 Increased mortality risk with septic shock87 |
Increasing prevalence of obesity in LT recipients18 Increased mortality among LT waitlist candidates17 Risk factor for perioperative complications18 Conflicting results with respect to long-term outcomes16,19 |
HCC, hepatocellular carcinoma; HTN, hypertension; LT, liver transplant; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
-
1
Since obesity in cirrhosis is a major risk factor for complications, weight loss should be an important goal for these patients.
-
2
The method and rapidity for obtaining a sustained loss of excess body fat in obese patients needs to be individualized.
-
3
Weight management, ideally 10%, via lifestyle modification may improve liver disease but success in implementing and sustaining this limits utility in treatment of obese patients with cirrhosis.
How might bariatric surgery improve outcomes for obese patients with cirrhosis?
Survival benefit with bariatric surgery has been demonstrated in general patient populations though data for improved transplant free survival in cirrhosis are lacking.32,33 Fibrosis regression may reduce risk for complications of chronic liver disease, including HCC and mortality.34 In a study of NASH patients undergoing serial liver biopsies at a mean interval of 4.6 years, a higher proportion of fibrosis regression occurred among patients who achieved weight loss ≥10% (63.2 % vs. 9.1 %; p = 0.001) and among those who underwent bariatric surgery (47.4 % vs. 4.5 %; p = 0.003).35 Weight loss can also decrease hepatic HVPG in patients with obesity and cirrhosis with greater declines reported among those with ≥10% weight loss (HVPG decrease −23.7 ± 19.9% vs. −8.2 ± 16.6%; P = 0.024).6 Long term data evaluating the impact of weight loss-induced improvement in fibrosis and portal HTN on liver-related morbidity and mortality are not currently available.
Obesity is a significant risk for the development of many types of cancer including HCC.36 Severity of obesity increases the risk of HCC in NAFLD with a 39% increase in risk for HCC per 5 unit increase in BMI in one meta-analysis.37 Data from a large propensity matched cohort found patients with severe obesity undergoing bariatric surgery (baseline BMI 47 kg/m2) demonstrated fewer incident cases of HCC (0.05% vs 0.34%, p = 0.03) compared to those without surgery (baseline BMI 46 kg/m2) over a median follow-up of 7.1 years.38 Data from the US Nationwide Inpatient Sample (NIS) database (2004–2014) were evaluated for discharges with a diagnosis of morbid obesity (n=2,881,414). Using a propensity-matched sample, patients with prior-bariatric surgery (n=267,082) demonstrated a lower prevalence of HCC (PR 0.11; 95% CI, 0.03–0.48; P < 0.001) and in-hospital mortality (0.1% versus 0.5%; OR 0.22; 95% CI, 0.20–0.26; P <0.001).39
Because of risks inherent to surgery for people with cirrhosis, including those specific to bariatric procedures (see BPA 10), the validation of pathophysiologically plausible benefits of this intervention on HCC risk and transplant free survival in this population through clinical research is currently an unmet need.
For obese compensated cirrhotic patients who cannot attain a desirable weight with lifestyle interventions, bariatric surgery may be considered. Bariatric surgery can achieve long term weight loss, improve metabolic co-morbidities, and diminish liver injury.40,41 Comorbidities associated with Metabolic Syndrome, particularly cardiovascular disease, should be evaluated in patients with cirrhosis being considered for bariatric surgery. The NIS study42 found the mortality risk of bariatric surgery in compensated cirrhosis to be slightly higher than patients without cirrhosis (0.9 % vs 0.3%) but significantly higher in decompensated cirrhosis (16.3%). The higher mortality rate with compensated cirrhosis may be justified given the benefits of weight loss in these patients as demonstrated in multiple other studies but needs to be acknowledged and addressed with potential candidates for surgery.8,40,41,43 Additional44–46 studies indicate that bariatric surgery is well tolerated and can be performed safely with minimal increased risk in well compensated, carefully selected obese patients with cirrhosis.
How should portal HTN be evaluated in potential bariatric surgical candidates with cirrhosis?
Evaluation for clinically significant portal hypertension (CSPH) is advised prior to any elective surgical procedure in patients with cirrhosis.9 Portal HTN may be assessed via transjugular pressure measurement (HVPG), imaging, esophagogastroduodenoscopy, endoscopic ultrasound, and MRI or ultrasound-based elastography. Baveno VI guidelines support avoidance of endoscopy for variceal screening based on elastography and platelet count to exclude CSPH,47 but this method has not been validated for use in patients being considered for bariatric surgery. An HVPG < 10 mmHg is an accepted threshold for safe segmental liver resection in cirrhosis.48 In a meta-analysis of liver resection for HCC, individuals with CSPH had significantly higher rates of postoperative mortality, complications, liver-related morbidity, liver failure, and poorer overall survival.49 While the risk for complications from CSPH following liver resection may differ from those occurring with bariatric surgery, absent validated data specific to bariatric surgery, the adoption of similar preoperative candidate criteria is prudent.
There are several small series reporting feasibility of bariatric surgery among patients with CSPH with low rates of post-operative mortality or portal HTN complications.46,50,51
Despite relatively low rates of complications reported in case series, performance of bariatric surgery on patients with evidence of CSPH should be restricted to selected medical centers with experience and resources for managing complications. The presence of CSPH should be evaluated prior to bariatric surgery in all patients with cirrhosis. Pending data to validate other modalities, cross sectional imaging and upper endoscopy should be performed to evaluate features of CSPH.
Best Practice Advice:
-
4
Bariatric surgery should be considered in selected patients with compensated cirrhosis to
How should alcohol use be evaluated in potential candidates for bariatric surgery?
Rates of alcohol use disorder (AUD) following bariatric surgery vary significantly due to heterogeneous methodology in ascertaining and defining AUD.52 The LABS-2 consortium reported prevalence of AUD (symptoms of alcohol dependence or the AUD Identification Test score ≥ 8) to increase over time following RYGB from approximately 7% pre-surgery to 16% at year 7, while remaining stable following LAGB between 6% and 8%.53 A meta-analysis found no increase in AUD 2 years following bariatric surgery but at 3 years pooled odds were 1.825 (95% CI, 1.53–2.178; P<0.001) with a significant increase in AUD, particularly with RYGB.54 Reported risk factors for post-operative AUD include regular or problematic alcohol use pre-surgery, male gender, younger age, tobacco or recreational drug use, lower interpersonal support and history of RYGB.55,56 In studies measuring blood alcohol concentration (BAC) following bariatric surgery (RYGB and SG) compared to pre-surgical control patients, time to peak BAC is significantly shorter, peak BAC is approximately doubled, and subjective feeling of intoxication is increased following both types of bariatric surgery.57
While there are limited data on risks and rates of AUD following bariatric surgery, there is sufficient evidence to recommend careful pre-operative assessment in all bariatric surgical candidates.58 Given the two-fold increase in BAC per drink equivalent following RYGB and SG, the threshold for clinically significant liver injury to occur in patients with pre-operative advanced fibrosis is likely quite low. Comprehension and retention of patient education regarding changes in alcohol metabolism and risks of post-bariatric alcohol are suboptimal59. As such, the evaluation of surgical candidates for history of AUD, reiterative education of candidates about risks related to post-bariatric alcohol use, and ongoing assessment for alcohol use following surgery is critical in these patients.
Best Practice Advice:
-
7.
Potential candidates for bariatric surgery with cirrhosis warrant intensive assessment for alcohol use disorder pre-operatively and long-term efforts to mitigate risk following surgery.
What considerations should be given to endoscopic bariatric therapies for obese patients with cirrhosis?
Endoscopic bariatric therapies are devices or procedures performed via endoscopy for the treatment of obesity. A broad spectrum of devices and procedures have shown efficacy in achieving weight loss via reduced calorie intake compared to lifestyle intervention or medical therapies.60 While there are no direct comparative studies, endoscopic bariatric procedures generally result in less weight loss than bariatric surgery but have a lower reported complication rate and shorter recovery time.60 The presence of CSPH (specifically, the presence of gastric and/or esophageal varices) is a contraindication for the use of all endoscopic devices.
By occupying space in the stomach, intra-gastric balloons inhibit intake and reduce gastric motility and are typically removed after 6 months.61 A recent meta-analysis demonstrated 25.4% excess body weight loss at 6 months post removal.62 Longer-term data are lacking but weight re-gain is expected. There are currently two FDA approved liquid filled balloons (Orbera and ReShape Integrated Dual Balloon System) and both list hepatic insufficiency or cirrhosis, as well as esophageal or gastric varices as contra-indications and deaths due to gastric perforation have been reported.63 The Obalon Balloon system is air filled, and does not list cirrhosis as a contraindication though the presence of gastric varices is listed as a contraindication. A published case series from India reported use of an intra-gastric balloon in 8 cirrhotic patients awaiting LT, 5 of whom were able to undergo LT following weight loss with the device.64 A recently published review highlights early data for endoscopic bariatric and metabolic therapies for patients with NAFLD, including intra-gastric balloon and the endoscopic sleeve gastrectomy.65 While the potential benefits of a less invasive approach are compelling, especially for those with sarcopenia and portal hypertension, it is important to note that the data for the use of endoscopic devices in patients with cirrhosis is very limited. A recently published paired biopsy series of 20 patients who had the intra-gastric balloon showed improved NASH activity scores in a majority of patients, though none of the patient had cirrhosis at the start of the study.66 Only 3 patients had improvement in fibrosis after the device removal, while 12 had no change and 5 had worsening fibrosis.
What are the essential programmatic criteria for performing bariatric surgery in people with cirrhosis?
The Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) has created a set of standards which define the best practices for bariatric centers of excellence.67 Central to these standards is the provision of care by a multi-disciplinary team with expertise in addressing the unique needs of patients with severe obesity. The standards require a bariatric surgeon meeting specific training and annual center volume requirements (50 cases annually for comprehensive bariatric centers), specialized anesthesia protocols, registered dieticians, licensed behavioral health providers, and physical therapists. Additionally, bariatric centers of excellence must have access to consultative services from cardiology, nephrology, and pulmonology.67
Beyond the aforementioned standards, bariatric care of patients with cirrhosis should include a surgical and anesthesia team with experience in operating on patients with portal hypertension and cirrhosis. A medical team with expertise in the perioperative care of patients with liver disease should optimize the management of liver specific complications which may arise in the post-operative period, as well as providing recommendations for pain management and other medication adjustments. Determination of potential for liver transplant candidacy should be made in advance of bariatric surgery, so that patients who are ineligible for transplant (and their families) have a clear understanding of this, avoiding the need for the medical team to address this issue urgently if the patient’s condition deteriorates postoperatively.9 If the bariatric procedure is being performed at a center which also performs liver transplantation, programs can determine what aspects of the evaluation would be mandated prior to committing to bariatric surgical candidacy. This becomes more complex when the bariatric procedure is performed at a center which does not perform transplantation, though at a minimum consultation with a hepatologist with transplant expertise is advised. For incidentally discovered cirrhosis at the time of bariatric surgery, transfer to a medical center with appropriate resources for cirrhosis care should be done in the event of any intra- or post-operative liver related complications.
What is the optimal timing and method of bariatric surgery for people with cirrhosis?
Adjustable gastric band involves (laparoscopic) placement of an adjustable prosthetic band around the upper stomach and is a purely restrictive operation which is now much less commonly performed due to reduced long-term efficacy, as well as concerns related to band erosion and displacement.68,69 Though there are limited data, the optimal procedure for patients with cirrhosis is likely laparoscopic sleeve gastrectomy. As compared to RYGB, sleeve gastrectomy (SG) preserves endoscopic access to the stomach and biliary tree and does not induce malabsorption. This results in a more gradual weight loss, which may be beneficial in the settings of cirrhosis and LT. Rapid weight loss and malabsorption after bariatric surgery in patients with NAFLD or steatohepatitis (NASH) may impair liver function. A randomized controlled trial of SG versus RYGB performed in patients with non-cirrhotic NAFLD demonstrated significantly improved liver chemistries at 12 months with SG compared to RYGB, while excess weight loss was similar in both groups.70 Another retrospective analysis of NAFLD patients undergoing SG (n =682) or gastric bypass (n = 355) found, at 12 months, those with SG had a significant lower ALT, while at 24 months the ALT was comparable between groups.71 Excess weight loss at 24 months was 75.9% for SG versus 80.1% for RYGB (P= .008). Finally, the efficacy of SG vs RYGB was compared in a matched case-control study of 34 noncirrhotic patients with both type II DM and a baseline elevated ALT72 Weight loss was similar, but ALT decreased more significantly SG compared to RYGB (ALT 17.8±8.8 vs. 31.1±11.2 U/L, p=0.003), with ALT becoming normal in 41% with SG compared to 0% with RYGB.
The optimal timing for surgery is related to the stage of disease. For patients with compensated cirrhosis, there are several case series reporting successful outcomes with laparoscopic SG. The largest is from University of California, San Francisco which reported on 32 patients with previous decompensated cirrhosis (compensated at the time of SG) with a median MELD score of 12. There were no deaths in the perioperative period and no liver-related morbidity. Seven patients improved, 14 underwent LT, 3 were transferred to other centers and 1 patient was ineligible for LT.73 An important note of caution, a recent analysis of 78 patients with cirrhosis with prior bariatric surgery evaluated for LT at a single center compared with a cohort of 156 patients without prior surgery matched by age, MELD, and underlying liver disease found a higher risk of de-listing and death on the list for those with prior bariatric surgery (33% versus 10% p= 0.03).74 Notably, those who underwent a RYGB were more likely to be delisted and had more sarcopenia than those with other types of bariatric surgery.
For patients with decompensated cirrhosis, the options are SG concurrent with or after transplant, with the goal of improved long-term post LT outcomes. The group at Mayo Clinic first reported a series of 7 patients undergoing a combined LT + SG compared to a group which was previously obese but were successful in reducing their BMI <35 kg/m2 and thus underwent LT alone.75 Those who underwent LT+SG attained and maintained significant weight loss with improved metabolic parameters compared to those who were successful with weight loss before LT who experienced more weight gain post LT. The hospital stay and complication rates were similar. A larger series with 3 year outcome data was recently published by the same group and demonstrated durable weight loss, favorable metabolic profiles and excellent graft and patient outcomes for the patients with the LT+SG procedure, compared to those with weight loss prior to LT.28 Two additional case reports also reported favorable outcomes.76,77 There are multiple small case series of 6–10 patients who underwent SG after transplant, and all reported acceptable outcomes though with longer OR times and increased complication rates.78–80 An advantage of combined LT+SG is that patients have only one procedure and recovery, though a disadvantage is the need to adapt to the challenges of LT and bariatric surgery simultaneously. The risk of complications with the combined approach compared to the risks with sequential operations is unknown. Patient preference should also be a consideration.
Best Practice Advice:
-
9.
Programs offering bariatric surgical services for patients with cirrhosis must include a surgical and anesthesia team with experience in operating on patients with portal hypertension and cirrhosis as well as a medical team with experience in treating a postoperative patient with cirrhosis. Potential candidacy for liver transplantation should be determined as part of the pre-operative assessment of obese patients with cirrhosis.
-
10.
The optimal bariatric surgical procedure for patients with cirrhosis is most likely a laparoscopic sleeve gastrectomy. The optimal timing is determined by the stage of liver disease. In decompensated liver disease, the only acceptable option at present is bariatric surgery concurrent with or following liver transplant.
Conclusion
The epidemic of obesity has led to a dramatic rise in the incidence of chronic liver disease due to NAFLD and deleterious effects on cirrhosis from non-NAFLD etiologies; weight loss has the potential to improve patient outcomes. Identification of safe and effective approaches to medically supervised weight loss in obese patients with cirrhosis is currently an unmet need. Bariatric surgery is an effective, durable therapy for obesity and associated comorbidities yet safe implementation in patients with cirrhosis poses unique challenges. A multidisciplinary approach at all phases of care is crucial for optimizing outcomes for obese patients with cirrhosis aiming to achieve gradual weight loss without compromise of lean muscle mass (see Figure 1). Pre-operative evaluation of bariatric surgical candidacy requires careful evaluation of portal hypertension and liver decompensation, and successful performance of bariatric surgery in this population requires appropriate resources to manage liver specific complications. The available evidence on the role of bariatric surgery for patients with cirrhosis is limited in scope, usually lacks risk-matched controls, and are based largely on single-center experiences; therefore, evidence-based recommendations are mostly lacking. Studies of larger cohorts with matched controls and longer follow up are needed to improve management of this challenging population.
Figure 1.
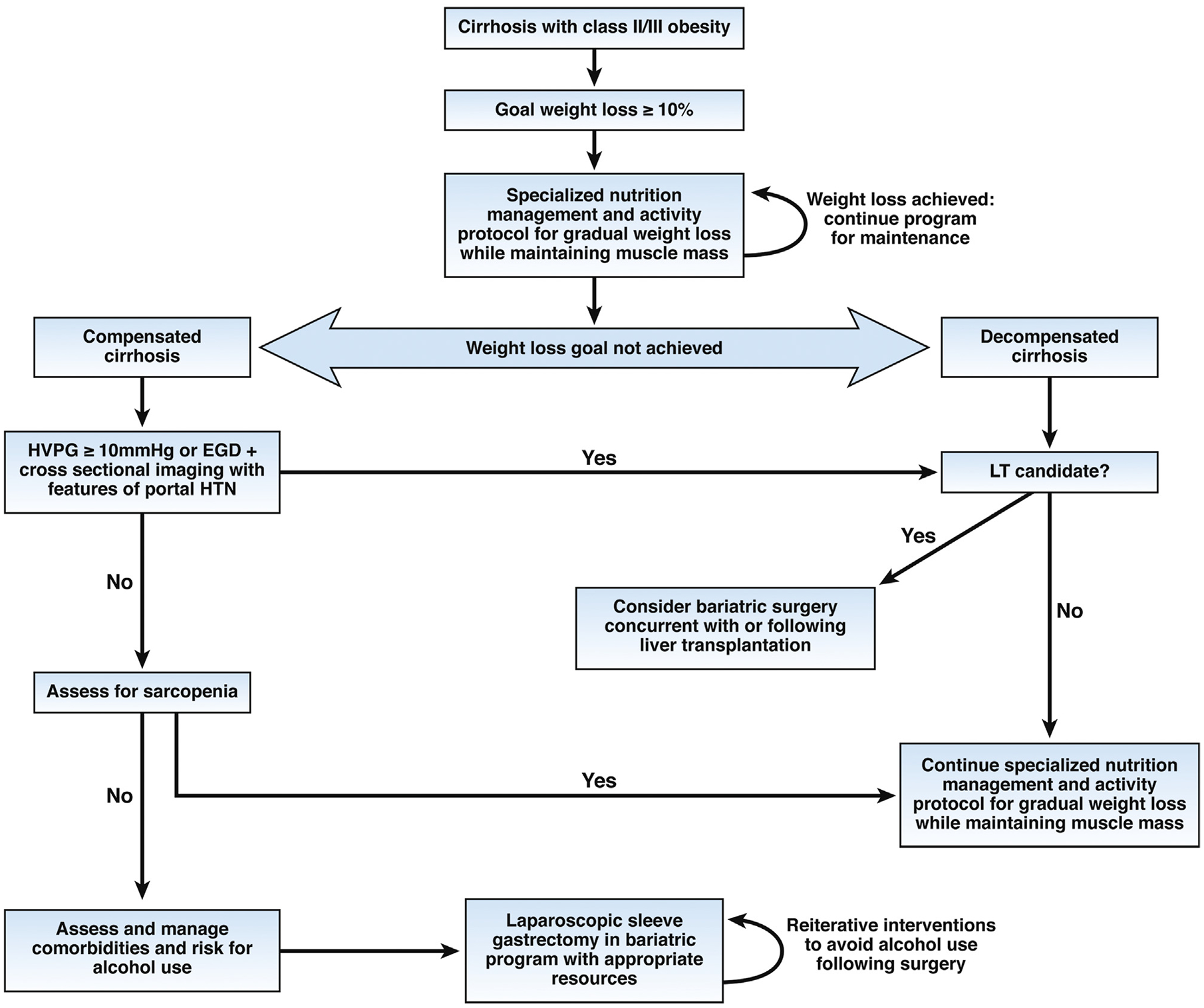
Algorithm for management of class II (BMI 35–39.9 kg/m2) and class III (BMI ≥40 kg/m2) obesity in individuals with cirrhosis. CSPH, clinically significant portal hypertension; EGD, esophagogastroduodenoscopy; HTN, hypertension; HVPG, hepatic venous pressure gradient; LT, liver transplant.
Grant Support:
JH: Regenerative Medicine for Prevention of Post-Transplant Biliary Complications. Funded by The Robert H. Yauk Charitable Trust Gift for Liver Transplant Research 2017 – 2020.
Abbreviations and Acronyms
- NAFLD
Nonalcoholic fatty liver disease
- MELD
Model for end stage liver disease
- ASA
American Society of Anesthesiologists Physical Status Classification
- CTP
Child-Turcotte-Pugh
- BMI
Body mass index
- HTN
Hypertension
- CSPH
Clinically significant portal HTN
- AUD
Alcohol use disorder
- AUDIT
Alcohol Use Disorders Identification Test
- RYGB
Roux-en-Y gastric bypass
- LAGB
Laparoscopic adjustable gastric banding
- TIPS
Transjugular intrahepatic portosystemic shunt
- LT
Liver Transplant
- VCTE
Vibration controlled transient elastography
- BAC
Blood alcohol concentration
Footnotes
Conflict of Interest Disclosures
Heather Patton: no disclosures
Julie Heimbach: no disclosures
Arthur McCullough: no disclosures
Writing Assistance: none
References
- 1.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018 Key Findings Data from the National Health and Nutrition Examination Survey., 2017. https://www.cdc.gov/nchs/products/index.htm. Accessed April 26, 2020.
- 2.Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology 2011;54:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant 2019;19:184–283. [DOI] [PubMed] [Google Scholar]
- 4.Harris R, Card TR, Delahooke T, et al. Obesity Is the Most Common Risk Factor for Chronic Liver Disease: Results From a Risk Stratification Pathway Using Transient Elastography. Am J Gastroenterol 2019;114:1744–1752. [DOI] [PubMed] [Google Scholar]
- 5.Ioannou GN, Weiss NS, Kowdley KV, et al. Is obesity a risk factor for cirrhosis-related death or hospitalization? A population-based cohort study. Gastroenterology 2003;125:1053–1059. [DOI] [PubMed] [Google Scholar]
- 6.Berzigotti A, Albillos A, Villanueva C, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology 2017;65:1293–1305. [DOI] [PubMed] [Google Scholar]
- 7.Berzigotti A, Abraldes JG. Impact of obesity and insulin-resistance on cirrhosis and portal hypertension. Gastroenterol Hepatol 2013;36:527–533. [DOI] [PubMed] [Google Scholar]
- 8.Chang SH, Stoll CRT, Song J, et al. The effectiveness and risks of bariatric surgery an updated systematic review and meta-analysis, 2003–2012. JAMA Surg 2014;149:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northup PG, Friedman LS, Kamath PS. AGA Clinical Practice Update on Surgical Risk Assessment and Perioperative Management in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol 2019;17:595–606. [DOI] [PubMed] [Google Scholar]
- 10.Lok ASF, Everhart JE, Chung RT, et al. Hepatic steatosis in hepatitis C: comparison of diabetic and nondiabetic patients in the hepatitis C antiviral long-term treatment against cirrhosis trial. Clin Gastroenterol Hepatol 2007;5:245–254. [DOI] [PubMed] [Google Scholar]
- 11.Mahli A, Hellerbrand C. Alcohol and Obesity: A Dangerous Association for Fatty Liver Disease. Dig Dis 2016;34:32–39. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Chang Y, Cho YK, et al. Obesity and Weight Gain Are Associated With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2019;17:543–550.e2. [DOI] [PubMed] [Google Scholar]
- 13.Ayala R, Grande S, Bustelos R, et al. Obesity is an independent risk factor for pre-transplant portal vein thrombosis in liver recipients. BMC Gastroenterol 2012;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Wang X, Wang J, et al. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer 2012;48:2137–2145. [DOI] [PubMed] [Google Scholar]
- 15.Sundaram V, Jalan R, Ahn JC, et al. Class III obesity is a risk factor for the development of acute-on-chronic liver failure in patients with decompensated cirrhosis. J Hepatol 2018;69:617–625. [DOI] [PubMed] [Google Scholar]
- 16.Leonard J, Heimbach JK, Malinchoc M, et al. The impact of obesity on long-term outcomes in liver transplant recipients - Results of the NIDDK Liver Transplant Database. Am J Transplant 2008;8:667–672. [DOI] [PubMed] [Google Scholar]
- 17.Schlansky B, Naugler WE, Orloff SL, et al. Higher Mortality and Survival Benefit in Obese Patients Awaiting Liver Transplantation. Transplantation 2016;100:2648–2655. [DOI] [PubMed] [Google Scholar]
- 18.Spengler EK, O’Leary JG, Te HS, et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation 2017;101:2288–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Son J, Stam SP, Gomes-Neto AW, et al. Post-transplant obesity impacts long-term survival after liver transplantation. Metabolism 2020;106:1–9. [DOI] [PubMed] [Google Scholar]
- 20.Everhart JE, Lok AS, Kim HY, et al. Weight-Related Effects on Disease Progression in the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial. Gastroenterology 2009;137:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367–378.e5. [DOI] [PubMed] [Google Scholar]
- 22.Segev DL, Thompson RE, Locke JE, et al. Prolonged waiting times for liver transplantation in obese patients. Ann Surg 2008;248:863–870. [DOI] [PubMed] [Google Scholar]
- 23.Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861–870. [DOI] [PubMed] [Google Scholar]
- 24.Carey EJ, Lai JC, Sonnenday C, et al. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 2019;70:1816–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huisman EJ, Trip EJ, Siersema PD, et al. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol 2011;23:982–989. [DOI] [PubMed] [Google Scholar]
- 26.Carey EJ. Sarcopenia in solid organ transplantation. Nutr Clin Pract 2014;29:159–170. http://www.ncbi.nlm.nih.gov/pubmed/24531627. Accessed April 11, 2020. [DOI] [PubMed] [Google Scholar]
- 27.Musso G, Cassader M, Rosina F, et al. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 2012;55:885–904. [DOI] [PubMed] [Google Scholar]
- 28.Zamora-Valdes D, Watt KD, Kellogg TA, et al. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology 2018;68:485–495. [DOI] [PubMed] [Google Scholar]
- 29.Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 2016;22:1–203. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–690. [DOI] [PubMed] [Google Scholar]
- 31.Merli M, Berzigotti A, Zelber-Sagi S, et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 2019;70:172–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White GE, Courcoulas AP, King WC, et al. Mortality after bariatric surgery: findings from a 7-year multicenter cohort study. Surg Obes Relat Dis 2019;15:1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reges O, Greenland P, Dicker D, et al. Association of bariatric surgery using laparoscopic banding, roux-en-y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA - J Am Med Assoc 2018;319:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glass LM, Dickson RC, Anderson JC, et al. Total Body Weight Loss of ≥10 % Is Associated with Improved Hepatic Fibrosis in Patients with Nonalcoholic Steatohepatitis. Dig Dis Sci 2015;60:1024–1030. [DOI] [PubMed] [Google Scholar]
- 36.Bertot LC, Adams LA. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol 2019;13:179–187. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Wang B, Shen F, et al. Body Mass Index and Risk of Primary Liver Cancer: A Meta‐Analysis of Prospective Studies. Oncologist 2012;17:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak M, Mehaffey JH, Hawkins RB, et al. Bariatric surgery is associated with reduction in non-alcoholic steatohepatitis and hepatocellular carcinoma: A propensity matched analysis. Am J Surg September 2019. [DOI] [PubMed] [Google Scholar]
- 39.Njei B, McCarty TR, Sharma P, et al. Bariatric Surgery and Hepatocellular Carcinoma: a Propensity Score-Matched Analysis. Obes Surg 2018;28:3880–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh T, Kochhar GS, Goh GB, et al. Safety and efficacy of bariatric surgery in patients with advanced fibrosis. Int J Obes 2017;41:443–449. [DOI] [PubMed] [Google Scholar]
- 41.Buchwald H, Estok R, Fahrbach K, et al. Trends in mortality in bariatric surgery: A systematic review and meta-analysis. Surgery 2007;142:621–635. [DOI] [PubMed] [Google Scholar]
- 42.Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:897–901. [DOI] [PubMed] [Google Scholar]
- 43.Dallal RM, Mattar SG, Lord JL, et al. Results of Laparoscopic Gastric Bypass in Patients with Cirrhosis. In: Obesity Surgery. Vol 14., 2004:47–53. http://www.ncbi.nlm.nih.gov/pubmed/14980033. Accessed April 11, 2020. [DOI] [PubMed] [Google Scholar]
- 44.Wolter S, Duprée A, Coelius C, et al. Influence of Liver Disease on Perioperative Outcome After Bariatric Surgery in a Northern German Cohort. Obes Surg 2017;27:90–95. [DOI] [PubMed] [Google Scholar]
- 45.Rebibo L, Gerin O, Verhaeghe P, et al. Laparoscopic sleeve gastrectomy in patients with NASH-related cirrhosis: a case-matched study. Surg Obes Relat Dis 2014;10:405–410; quiz 565. [DOI] [PubMed] [Google Scholar]
- 46.Pestana L, Swain J, Dierkhising R, et al. Bariatric surgery in patients with cirrhosis with and without portal hypertension: A single-center experience. Mayo Clin Proc 2015;90:209–215. [DOI] [PubMed] [Google Scholar]
- 47.De Franchis R, Abraldes JG, Bajaj J, et al. Expanding consensus in portal hypertension Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015:743–752. [DOI] [PubMed] [Google Scholar]
- 48.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: Prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018–1022. [DOI] [PubMed] [Google Scholar]
- 49.Choi SB, Kim HJ, Song TJ, et al. Influence of clinically significant portal hypertension on surgical outcomes and survival following hepatectomy for hepatocellular carcinoma: A systematic review and meta-analysis. J Hepatobiliary Pancreat Sci 2014;21:639–647. [DOI] [PubMed] [Google Scholar]
- 50.Hanipah ZN, Punchai S, McCullough A, et al. Bariatric Surgery in Patients with Cirrhosis and Portal Hypertension. Obes Surg 2018;28:3431–3438. [DOI] [PubMed] [Google Scholar]
- 51.Miñambres I, Rubio MA, de Hollanda A, et al. Outcomes of Bariatric Surgery in Patients with Cirrhosis. Obes Surg 2019;29:585–592. [DOI] [PubMed] [Google Scholar]
- 52.Ivezaj V, Benoit SC, Davis J, et al. Changes in Alcohol Use after Metabolic and Bariatric Surgery: Predictors and Mechanisms. Curr Psychiatry Rep 2019;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King WC, Chen JY, Courcoulas AP, et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis 2017;13:1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azam H, Shahrestani S, Phan K. Alcohol use disorders before and after bariatric surgery: a systematic review and meta-analysis. Ann Transl Med 2018;6:148–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spadola CE, Wagner EF, Dillon FR, et al. Alcohol and Drug Use Among Postoperative Bariatric Patients: A Systematic Review of the Emerging Research and Its Implications. Alcohol Clin Exp Res 2015;39:1582–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA - J Am Med Assoc 2012;307:2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Acevedo MB, Eagon JC, Bartholow BD, et al. Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4. Surg Obes Relat Dis 2018;14:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blackburn AN, Hajnal A, Leggio L. The gut in the brain: the effects of bariatric surgery on alcohol consumption. Addict Biol 2017;22:1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller-Matero LR, Coleman JP, LaLonde L, et al. Patient Recall of Education about the Risks of Alcohol Use Following Bariatric Surgery. Obes Surg 2019;29:2707–2710. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic Bariatric and Metabolic Therapies: New and Emerging Technologies. Gastroenterology 2017;152:1791–1801. [DOI] [PubMed] [Google Scholar]
- 61.Bazerbachi F, Vargas EJ, Abu Dayyeh BK. Endoscopic Bariatric Therapy: A Guide to the Intragastric Balloon. Am J Gastroenterol 2019;114:1421–1431. [DOI] [PubMed] [Google Scholar]
- 62.Saber AA, Shoar S, Almadani MW, et al. Efficacy of First-Time Intragastric Balloon in Weight Loss: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes Surg 2017;27:277–287. [DOI] [PubMed] [Google Scholar]
- 63.UPDATE: Potential Risks with Liquid-filled Intragastric Balloons - Letter to Health Care Providers | FDA. https://www.fda.gov/medical-devices/letters-health-care-providers/update-potential-risks-liquid-filled-intragastric-balloons-letter-health-care-providers-0. Accessed April 19, 2020.
- 64.Choudhary NS, Puri R, Saraf N, et al. Intragastric balloon as a novel modality for weight loss in patients with cirrhosis and morbid obesity awaiting liver transplantation. Indian J Gastroenterol 2016;35:113–116. [DOI] [PubMed] [Google Scholar]
- 65.Salomone F, Sharaiha RZ, Boškoski I. Endoscopic bariatric and metabolic therapies for non-alcoholic fatty liver disease: Evidence and perspectives. Liver Int 2020;40:1262–1268. https://pubmed.ncbi.nlm.nih.gov/32181573/. Accessed September 2, 2020. [DOI] [PubMed] [Google Scholar]
- 66.Bazerbachi F, Vargas EJ, Rizk M, et al. Intragastric Balloon Placement Induces Significant Metabolic and Histologic Improvement in Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.(No Title). https://www.facs.org/-/media/files/quality-programs/bariatric/2019_mbsaqip_standards_manual.ashx. Accessed April 19, 2020.
- 68.Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014;2014. https://pubmed.ncbi.nlm.nih.gov/25105982/. Accessed September 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koh CY, Inaba CS, Sujatha-Bhaskar S, et al. Laparoscopic Adjustable Gastric Band Explantation and Implantation at Academic Centers. J Am Coll Surg 2017;225:532–537. [DOI] [PubMed] [Google Scholar]
- 70.Kalinowski P, Paluszkiewicz R, Ziarkiewicz-Wróblewska B, et al. Liver Function in Patients with Nonalcoholic Fatty Liver Disease Randomized to Roux-en-Y Gastric Bypass Versus Sleeve Gastrectomy. Ann Surg 2017;266:738–745. [DOI] [PubMed] [Google Scholar]
- 71.Motamedi MAK, Khalaj A, Mahdavi M, et al. Longitudinal Comparison of the Effect of Gastric Bypass to Sleeve Gastrectomy on Liver Function in a Bariatric Cohort: Tehran Obesity Treatment Study (TOTS). Obes Surg 2019;29:511–518. [DOI] [PubMed] [Google Scholar]
- 72.Billeter AT, Senft J, Gotthardt D, et al. Combined Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Sleeve Gastrectomy or Gastric Bypass?—a Controlled Matched Pair Study of 34 Patients. Obes Surg 2016;26:1867–1874. [DOI] [PubMed] [Google Scholar]
- 73.Sharpton SR, Terrault NA, Posselt AM. Outcomes of Sleeve Gastrectomy in Obese Liver Transplant Candidates. Liver Transplant 2019;25:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Idriss R, Hasse J, Wu T, et al. Impact of Prior Bariatric Surgery on Perioperative Liver Transplant Outcomes. Liver Transplant 2019;25:217–227. [DOI] [PubMed] [Google Scholar]
- 75.Heimbach JK, Watt KDS, Poterucha JJ, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant 2013;13:363–368. [DOI] [PubMed] [Google Scholar]
- 76.Nesher E, Mor E, Shlomai A, et al. Simultaneous Liver Transplantation and Sleeve Gastrectomy: Prohibitive Combination or a Necessity? Obes Surg 2017;27:1387–1390. [DOI] [PubMed] [Google Scholar]
- 77.Tariciotti L, D’Ugo S, Manzia TM, et al. Combined liver transplantation and sleeve gastrectomy for end-stage liver disease in a bariatric patient: First European case-report. Int J Surg Case Rep 2016;28:38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Osseis M, Lazzati A, Salloum C, et al. Sleeve Gastrectomy After Liver Transplantation: Feasibility and Outcomes. Obes Surg 2018;28:242–248. [DOI] [PubMed] [Google Scholar]
- 79.Elli EF, Gonzalez-Heredia R, Sanchez-Johnsen L, et al. Sleeve gastrectomy surgery in obese patients post-organ transplantation. Surg Obes Relat Dis 2016;12:528–534. [DOI] [PubMed] [Google Scholar]
- 80.Morris MC, Jung AD, Kim Y, et al. Delayed Sleeve Gastrectomy Following Liver Transplantation: A 5-Year Experience. Liver Transplant 2019;25:1673–1681. [DOI] [PubMed] [Google Scholar]
- 81.Beste LA, Leipertz SL, Green PK, et al. Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001–2013. Gastroenterology 2015;149:1471–1482.e5. [DOI] [PubMed] [Google Scholar]
- 82.Goldberg D, Ditah IC, Saeian K, et al. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology 2017;152:1090–1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen AM, Van Houten HK, Sangaralingham LR, et al. Healthcare Cost and Utilization in Nonalcoholic Fatty Liver Disease: Real-World Data From a Large U.S. Claims Database. Hepatology 2018;68:2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ortiz V, Berenguer M, Rayon JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol 2002;97:2408–2414. [DOI] [PubMed] [Google Scholar]
- 86.Naveau S, Chaput JC, Fallik D, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology 2002;35:635–638. [DOI] [PubMed] [Google Scholar]
- 87.Parker R, Kim SJ, Im GY, et al. Obesity in acute alcoholic hepatitis increases morbidity and mortality. EBioMedicine 2019;45:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rui R, Lou J, Zou L, et al. Excess Body Mass Index and Risk of Liver Cancer: A Nonlinear Dose-Response Meta-Analysis of Prospective Studies. PLoS One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta A, Das A, Majumder K, et al. Obesity is Independently Associated with Increased Risk of Hepatocellular Cancer-related Mortality. Am J Clin Oncol Cancer Clin Trials 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kok B, Karvellas CJ, Abraldes JG, et al. The impact of obesity in cirrhotic patients with septic shock: A retrospective cohort study. Liver Int 2018;38:1230–1241. [DOI] [PubMed] [Google Scholar]


