Abstract
During cryopreservation, spermatozoa may suffer cold and cryo-induced injuries ―associated with alterations in cell defense systems― that are detrimental to their function and subsequent fertility. This study aimed to determine the efficacy of supplementing the semen freezing extender with the antioxidant reduced glutathione (GSH) in cattle. Semen was collected from four bulls and diluted in a freezing extender supplemented with or without GSH (0, 1, 5, and 10 mM) before the cooling step of the cryopreservation process. After thawing, the quality of the frozen-thawed semen was investigated for motility, viability, acrosomal and DNA integrity, and subsequent embryo development after in vitro fertilization of bovine oocytes. Additionally, semen from one of the bulls was used to analyze semen antioxidative potential, sperm penetration into oocytes, male pronucleus formation rate, and embryo DNA integrity. The sperm quality varied among bulls after GSH supplementation. One bull had decreased sperm total motility, and two bulls had decreased sperm DNA integrity. GSH supplementation had positive effects on embryo development for three bulls. Two of them showed both improved cleavage and blastocyst formation rates, while the other one only showed an improved cleavage rate. We observed positive effects on early male pronucleus formation and no negative effects on DNA integrity and cell number in blastocyst stage embryos. Although the effect varies depending on individual bulls and GSH concentration, GSH supplementation in semen may improve in vitro embryo production from frozen semen.
Keywords: Bull, Frozen-thawed semen, Reduced glutathione (GSH), Semen characteristics, Semen freezing extender
The utilization of frozen semen obtained from bulls with excellent genetic traits has been highly effective in increasing profitability and achieving genetic improvement owing to efficient calf production. In addition, the recent development of sperm sexing technology [1] has further heightened the practical benefit of frozen semen by allowing the breeders to obtain calves of the desired gender. However, conception rates after artificial insemination in cattle have been gradually declining over the recent decades in Japan and other countries [2, 3]. Generally, frozen-thawed semen has a lower quality manifested as decreased viability [4,5,6], motility [5,6,7], acrosome integrity [6,7,8], plasma membrane integrity [4, 6], and DNA integrity [4, 8] compared to the fresh semen. Cryopreservation-related injuries during the freezing and thawing processes decrease cellular metabolism, alter membrane permeability, and induce the loss of intracellular components [9]. These injuries are associated with excess production of reactive oxygen species (ROS) and a change in antioxidant defense systems in the cells [9].
In spermatozoa, ROS are generated metabolically and are necessary for sperm function [10]. However, excess levels of ROS cause oxidative damage, such as membrane lipid peroxidation and DNA fragmentation that adversely affect early embryo and subsequent fetal development after fertilization [11, 12]. Supplementing the freezing medium with antioxidants has been used in several species to reduce the detrimental effects of ROS on spermatozoa [13]. Reduced glutathione (l-γ-glutamyl-l-cysteinyl-glycine: GSH) is a biological antioxidant ubiquitously distributed in living cells that plays an important role in intracellular defense against oxidative stress [14]. GSH can act both as an antioxidant, using the reducing power of its sulfhydryl group, and as a cofactor for antioxidant enzymes. The cryopreservation process reduces the GSH content in spermatozoa from cattle [5, 15], pigs [16], and humans [17]. GSH supplementation in an egg yolk-based semen freezing extender (SFE) has been tested in several bovine breeds under different conditions [6, 8, 18,19,20]. However, the effect of GSH supplementation and effective concentration on different breeds and individuals of other mammalian species previously reported [21, 22] are not fully understood in bovine semen. Therefore, the present study focused on the individual differences in cattle semen quality to better understand the effects of GSH supplementation in SFE on frozen-thawed semen and the subsequent quality of embryos fertilized in vitro.
Materials and Methods
Reagents
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless indicated otherwise.
Preparation of frozen semen with or without GSH-supplemented SFE
Fresh semen was collected from four bulls: one Holstein bull (Bull 1) and three Japanese Black bulls (Bulls 2–4). Each semen sample was frozen to evaluate its quality and used in the in vitro fertilization (IVF) procedure (Supplementary Table 1). The animal experiments were approved by the Committee for the Care and Use of Experimental Animals at NILGS (No.1611BO11). Semen samples from these bulls were frozen, as previously reported [23]. Each ejaculate was divided into four equal portions and diluted with the first SFE to obtain a final (frozen step) concentration of 0, 1, 5, or 10 mM GSH. After equilibration at 4°C, an equal volume of the second SFE containing glycerol was added to obtain a final concentration of 6.5% glycerol. The final concentrations of diluted semen samples were 0.7–3.4 × 108 (Bull 1), 0.8–3.2 × 108 (Bull 2), 0.2–0.4 × 108 (Bull 3), and 0.5–3.2 × 108 (Bull 4) spermatozoa/ml. The osmolarity of SFE was measured using a vapor pressure osmometer (VAPRO 5520, Wescor, UT, USA) (Supplementary Table 2).
For sperm quality evaluation and IVF, frozen semen samples were thawed in a water bath at 38°C for 20 sec and were subjected to analysis and IVF protocols as described below.
Analysis of sperm motility
To evaluate sperm motility, frozen-thawed sperm aliquots from each group were diluted five-fold with Dulbecco’s phosphate-buffered saline without calcium chloride and magnesium chloride (PBS (–)). Samples from four bulls (three to eight semen lots) were then analyzed respectively using a computer-assisted sperm analysis (CASA) system (SMAS for Animal sperm, DITECT, Tokyo, Japan). For each measurement, a 10 µl aliquot was loaded onto a chamber (Makler counting chamber, Fujifilm Wako, Tokyo, Japan) at a depth of 10 µm. Approximately 900 spermatozoa from three videos taken at 60 frames per second were analyzed for each specimen. The following variables were analyzed: total percentage of motile sperm (MOT, %), average path velocity (VAP, μm/sec), straight-line velocity (VSL, μm/sec), curvilinear velocity (VCL, μm/sec), amplitude of lateral head displacement (ALH, μm), beat/cross frequency (BCF, Hz), straightness (STR = VSL/VAP × 100, %), linearity (LIN = VSL/VCL × 100, %), and wobble (WOB = VAP/VCL × 100, %). Data are presented as mean ± standard error for each group.
Evaluation of sperm acrosomal integrity and viability
The acrosome integrity of frozen-thawed spermatozoa was evaluated using fluorescein isothiocyanate-conjugated peanut agglutinin (FITC–PNA)/propidium iodide (PI) staining [24] (Fig. 1A). Samples from four bulls (three to four semen lots) were diluted respectively by 10-fold using FITC–PNA solution (final concentration: 1.0 µg/ml) to assess the acrosomal status and PI solution (final concentration: 6.0 µM) to assess the viability at 2 h after thawing, and then incubated for 20 min at 37°C. The unbound probes were removed by washing with PBS (–), and the precipitated spermatozoa were resuspended for mounting on a slide. The observations were performed under a fluorescence microscope (TE2000U, Nikon, Tokyo, Japan) at a magnification of 200 × using a B-2A filter (450–490 nm excitation filter, 505 nm dichroic mirror, 520 nm bandpass filter) for FITC–PNA and a G-2A filter (510–560 nm excitation filter, 575 nm dichroic mirror, 590 nm bandpass filter) for PI. Approximately 400 spermatozoa were observed on each prepared slide from five fields of vision. The viability and acrosome integrity were determined by counting PI (–) (Fig. 1A, b and c) or FITC–PNA (−) (Fig. 1A, b and d) spermatozoa per total spermatozoa. The acrosome integrity of living spermatozoa was determined by counting FITC–PNA (−) spermatozoa (Fig. 1A, b) per PI (−) spermatozoa. Data are presented as mean ± standard error for each group, and three to four replicates were used for each group.
Fig. 1.
Classification of spermatozoal integrity using staining methods. (A) Viability and acrosome integrity of bull spermatozoa evaluated with FITC-PNA/PI staining. a, Overall picture; b, FITC–PNA–/PI– (acrosome intact, live); c, FITC–PNA+ (damaged acrosome, live); d, PI+ (acrosome intact, dead); e, FITC–PNA+/PI+ (damaged acrosome, dead). (B) DNA fragmentation status of bull spermatozoa evaluated using TUNEL assay. a, Overall picture; b, TUNEL– (−, unfragmented DNA); c, TUNEL-partially positive (±; fragmented DNA); d, TUNEL-entirely positive (+; fragmented DNA). The image of phase–contrast and fluorescence picture were merged for observation.
Evaluation of sperm DNA integrity
Sperm DNA integrity was assessed using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, as described by Takeda et al. [25] (Fig. 1B). Fresh semen (four to six semen lots) was diluted using PBS (–), and frozen-thawed semen (from the same lots as fresh samples) was washed and diluted with PBS (–). Both semen samples were diluted to adjust the sperm concentration to 0.2 × 108 spermatozoa/ml. Diluted samples (15 µl) were fixed onto the slides using 2% paraformaldehyde and washed with PBS (–). Samples were permeabilized with 0.1% (v/v) Triton X-100 containing 0.1% (w/v) sodium citrate for 2 min on ice and then incubated in TUNEL reaction mixture from a commercial kit (In Situ Cell Death Detection Kit, fluorescein, Roche, Indianapolis, IN, USA) for 1 h at 37°C in a dark and humidified atmosphere. Positive TUNEL staining was observed under a fluorescence microscope (TE2000U, Nikon) using a B-2A filter. Approximately 800 spermatozoa were observed on each prepared slide from five fields of vision. Sperm DNA damage, expressed as DNA fragmentation index (DFI), was determined by the ratio of the entirely positive (+) (Fig. 1B, d) and partially positive (±) (Fig. 1B, c) stained spermatozoa. Data are presented as mean ± standard error for each group.
Measurement of semen antioxidative status
Frozen-thawed samples obtained from Bull 1 and 2 were used to evaluate antioxidative status. Fifty µl of each sample (0.8–1.9 × 108 spermatozoa/ml, one lot) was used for the biological antioxidant potential (BAP) test conducted by the analyzing company (Wismerll Co., Ltd., Tokyo, Japan). The BAP test is based on the ferric reducing ability of the plasma [26]. Samples were dissolved in a colored solution, initially prepared by mixing FeCl3 with a thiocyanate derivative. After incubation, the intensity of chromatic change that is directly proportional to the ability of the samples to reduce ferric ions (Fe3+) to ferrous ions (Fe2+), was photometrically read.
The levels of lipid peroxidation were assessed by determining malondialdehyde (MDA) production using a thiobarbituric acid reactive substances (TBARS) assay kit (OxiSelectTM TBARS Assay Kit, Cell Biolabs, CA, USA). Frozen-thawed samples from Bull 1 and 2 (four semen lots) were used for the TBARS assay. To avoid interference by the egg yolk, the extender was removed by centrifugation (700 × g, 5 min, 4°C). The pellets were resuspended in PBS (–) to adjust the sperm concentration to 1 × 108 spermatozoa/ml, followed by the addition of 1 × butylated hydroxytoluene. Further, the samples or MDA standards were mixed with a sodium dodecyl sulfate lysis solution. After 5 min of incubation, the thiobarbituric acid reagent was added, followed by incubation at 95°C for 60 min. Cooled and centrifuged supernatants were used for measurements. The level of fluorescence at 540 excitation/590 emission was determined using a microplate reader (GENios, Tecan, Maennedorf, Switzerland). The results are presented as µM MDA/ 108 spermatozoa.
IVF with frozen-thawed semen
IVF was performed in two laboratories—NILGS for Bull 1 and 2 and HLTRC for Bull 3 and 4. To examine the effect of different concentrations of GSH, 1 mM, 5 mM, and 10 mM (Bull 1: five lots, and Bull 2: four lots), and 5 mM and 10 mM (Bull 3 and 4, three lots) were used for IVF. Oocyte collection, in vitro maturation (IVM), IVF, and in vitro culture (IVC) were performed as described previously at NILGS (Bull 1 and 2) [27] and HLTRC (Bull 3 and 4) [28]. Briefly, bovine ovaries were obtained from a slaughterhouse and stored in PBS (–) with antibiotics until oocyte collection. Cumulus-oocyte complexes (COCs) were collected, and groups of 40–70 COCs were cultured for 22–24 h at 38.5°C under 5% CO2 in air. Matured oocytes were then co-cultured with washed sperm in 100 µL droplets of IVF medium (0.05–0.06 × 108 spermatozoa/ml) for 6 h at 38.5°C under 5% CO2 in air (10–20 oocytes per droplet). At the end of insemination, putative zygotes were completely denuded from cumulus cells and spermatozoa by gentle pipetting. IVC was performed in 200 μl droplets of IVD 101 medium (IFP, Yamagata, Japan) (Bull 1 and 2) or 50 μl droplets of modified m-SOF medium (Bull 3 and 4). Subsequently, 10–20 zygotes were placed in each culture drop and then cultured at 38.5°C under 5% O2 and 5% CO2 in air for 8–9 days (the day of IVF was considered to Day 0). Cleavage rates in each treatment group were recorded on Day 2 or 3. The rates of embryos developing to the blastocyst stage were recorded on Days 6–9.
Assessment of fertilization status
Fertilization status regarding sperm penetration and PN (pronucleus and pronuclei) formation was evaluated at 9 h and 18 h after IVF (the insemination timing, introducing sperm to oocytes considered to 0 h). Oocytes inseminated for 6 h with the semen samples from Bull 1 (presumptive zygotes: PZ) were used in the assay as previously described [29] with some modifications. Briefly, the PZ were randomly picked from each IVC drop, mounted on glass slides, and fixed in a mixture of acetic acid and ethanol (1:3) for at least 3 days. The fixed PZ were then stained with 1% (w/v) orcein in acetic acid, rinsed in a mixture of glycerol, acetic acid, and water (1:1:3), and then examined under a phase–contrast microscope (Axioskop, ZEISS, Oberkochen, Germany). The presence and number of male and female PN and/or sperm head(s) were then investigated in reference to a previous report [30]. Spermatozoa were considered to have the ability to penetrate and fertilize the oocytes when a male PN and/or sperm heads with attached sperm tails were observed inside the PZ, which were then classified as fertilized. The presence of both one sperm head and female chromosome or one male and one female PN indicated normal fertilization. The presence of more than two sperm heads or male PNs indicated polyspermy. Spermatozoa were considered to have the ability to decondense and form male PN when male PN was present in the cytoplasm of the PZ. Only male PN that had reached an enlarged size was added up to the number of male PN formations.
Evaluation of embryo quality
IVF embryos obtained from Bull 1 were assessed for embryo quality, represented as developmental competence and DNA integrity, at the expanded blastocyst stage on Day 7 or 8. The TUNEL assay was performed on the embryonic nuclei, combined with differential staining of inner cell mass (ICM) and trophectoderm (TE) cells, as previously described [31] with slight modifications. Embryos were partially permeabilized in 0.2% (v/v) Triton X-100 in PBS (–) and 0.02 mg/mL polyvinylpyrrolidone (PVP) solution (PVP-PBS). TE cells were stained with 30 µg/mL PI in PVP-PBS. Embryos were fixed and stained in PVP-PBS containing 4% (w/v) paraformaldehyde and 10 µg/ml Hoechst 33342. Embryos were then permeabilized in 0.1% (w/v) sodium citrate containing 0.1% (v/v) Triton X-100. After washing in PVP-PBS, the embryos were incubated in the TUNEL reaction mixture. Observations were performed under UV light with excitation at 365/10 nm and emission at 400 nm using an epifluorescence microscope (Eclipse E800; Nikon). A digital image of total cell number (DNA), TE cells, and TUNEL-labeled nuclei for each embryo was captured (NIS-Elements BR ver.4.30, Nikon), and the three images were composited using Adobe Photoshop Elements 2019 software. The number of cells and TUNEL-labeled nuclei were considered as an index of DNA damage and were determined using NIH ImageJ (v. 1.52a) software [32]. Data are presented as mean ± standard error, and 14–19 embryos were used for each group.
Statistical analysis
Differences among the GSH treatment groups for each bull (Fig. 2, Supplementary Tables 3 and 4) and differences among bulls in the 0 mM GSH control groups (Fig. 2, Supplementary Table 3) were analyzed by one-way analysis of variance (ANOVA) with the Tukey-Kramer test. Differences in DFI among GSH treatment groups in each bull and individual differences among bulls in fresh and 0 mM GSH control groups were analyzed using the Kruskal-Wallis test, followed by Dunn’s test (Fig. 3). Fertilization rates and corresponding fertilization status after IVF for each GSH concentration (Table 1), the developmental stages of blastocysts in each bull, and differences among bulls at 0 mM GSH (Table 2) were analyzed using the Chi-square test. Embryo quality at each GSH concentration was analyzed using the Kruskal-Wallis test, followed by the Mann-Whitney test (Table 3). Differences were considered statistically significant at P < 0.05. All statistical analyses were performed using R statistical software (version 3.6.1 and 4.0.2).
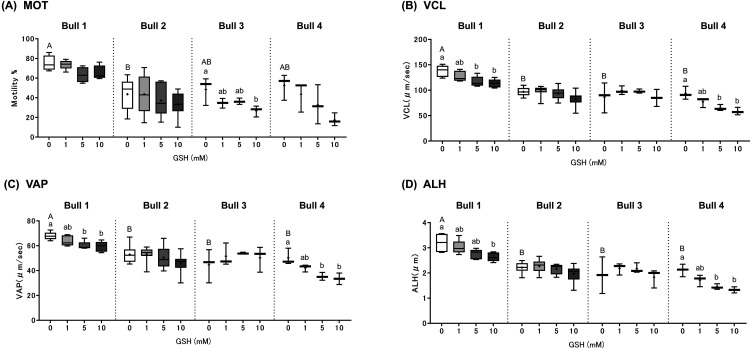
Fig. 2.
Effects of GSH supplementation on sperm motility in four bulls. Box plots represent (A) total motility (MOT, %), (B) curvilinear velocity (VCL, μm/sec), (C) average path velocity (VAP, μm/sec), and amplitude of lateral head displacement (ALH, μm) of frozen-thawed spermatozoa (n = 3–8 samples). Significant differences (P < 0.05) are represented by small letters (a, b) for differences among GSH concentrations for each bull and capital letters (A, B) for differences among bulls in the 0 mM GSH control group.
Fig. 3.
Effects of GSH supplementation on sperm DNA integrity (DNA fragmentation indices (DFI)) in fresh and frozen-thawed semen samples from four bulls. Fresh semen (Bull 1, n = 4; Bull 2, n = 6) and frozen-thawed semen (Bull 1, n = 4; Bull 2, n = 6; Bull 3, n = 3; Bull 4, n = 3) samples were evaluated for DFI using the TUNEL assay. DFI is represented as a percentage of TUNEL+ spermatozoa, with entirely (+, blue bar) or partially (±, red bar) positive fluorescent heads indicating fragmented DNA (Fig. 1b) and mean ± SE. Different letters indicate significant differences among the GSH concentrations in each bull (P < 0.05) as follows: a–b with blue: entirely positive sperm (%) and a-b with red: partially positive sperm (±). The differences in entirely positive DFI among bulls in fresh and 0 mM GSH control groups were analyzed, and significant differences were detected between Bull 1 and 2 (A–B: P < 0.05).
Table 1. Fertilization status of IVF embryos derived from frozen-thawed semen prepared with GSH-supplemented semen freezing extender.
| GSH concentration |
No. of oocyte (% total) |
No. of oocyte (% penetrated) |
|||||
|---|---|---|---|---|---|---|---|
| Sperm penetration | Normal fertilization | Polyspermic fertilization | Male PN formation |
||||
| 9 h after IVF | 18 h after IVF | ||||||
| 0 mM | 93 / 113 (82.3) | 65 / 93 (69.9) | 27 / 93 (29.0) | 17 / 41 (41.5) b | 50 / 52 (96.2) | ||
| 1 mM | 66 / 83 (79.5) | 42 / 66 (63.6) | 21 / 66 (31.8) | 18 / 25 (72.0) a | 40 / 41 (97.6) | ||
| 5 mM | 91 / 108 (84.3) | 56 / 91 (61.5) | 31 / 91 (34.1) | 26 / 32 (81.3) a | 58 / 59 (98.3) | ||
| 10 mM | 75 / 90 (83.3) | 49 / 75 (65.3) | 25 / 75 (33.3) | 34 / 40 (85.0) a | 34 / 35 (97.1) | ||
Frozen-thawed semen samples from Bull 1 were subjected to a fertilization status test. PN, pronuclei, and pronucleus. Samples were fixed at 9 h (three replicates) and 18 h (eight to ten replicates) after IVF. Spermatozoa were considered to have the ability to penetrate and fertilize when male PN and/or sperm heads with the contributing sperm tails were observed inside the oocyte. The presence of one sperm head and female chromosome or one male and one female PN were considered to indicate normal fertilization. Two or more sperm heads and/or male PNs were considered to indicate polyspermy. Spermatozoa were considered to have the ability to decondense and form male PN when enlarged male PN was present in the cytoplasm.
Table 2. In vitro development of IVF embryos derived from frozen-thawed semen prepared with GSH-supplemented semen freezing extender.
| Bull | GSH concentration |
Total number (No.) of oocytes cultured |
No. of developed embryos (%) |
|
|---|---|---|---|---|
| Cleaved | Blastocytes | |||
| 1 | 0 mM | 267 | 173 (64.8) b A | 63 (23.6) A |
| 1 mM | 245 | 177 (72.2) b | 69 (28.2) | |
| 5 mM | 288 | 188 (65.3) b | 84 (29.2) | |
| 10 mM | 264 | 212 (80.3) a | 66 (25.0) | |
| 2 | 0 mM | 237 | 45 (19.0) b C | 16 (6.8) b B |
| 1 mM | 265 | 86 (32.5) a | 28 (10.6) ab | |
| 5 mM | 182 | 61 (33.5) a | 24 (13.2) a | |
| 10 mM | 273 | 111 (40.7) a | 42 (15.4) a | |
| 3 | 0 mM | 154 | 114 (74.0) A | 49 (31.8) A |
| 1 mM | ND | ND | ND | |
| 5 mM | 152 | 117 (77.0) | 50 (32.9) | |
| 10 mM | 131 | 97 (71.7) | 40 (30.5) | |
| 4 | 0 mM | 240 | 136 (56.7) b B | 63 (26.3) b A |
| 1 mM | ND | ND | ND | |
| 5 mM | 225 | 176 (78.2) a | 93 (41.3) a | |
| 10 mM | 78 | 57 (71.7) a | 66 (32.2) ab | |
Data from 3–9 replicates. Values with different superscripts within the same column of each bull (a–b: P < 0.05) and among bulls in the 0 mM GSH control group (A–C: P < 0.05) were significantly different. ND = not determined.
Table 3. Quality of blastocysts derived from IVF with frozen-thawed semen prepared with GSH-supplemented semen freezing extender.
| GSH concentration |
No. of embryo | No. of cell |
ICM/Total (%) | No. of cells with damaged DNA (TU) |
||||
|---|---|---|---|---|---|---|---|---|
| ICM | TE | Total | TU-ICM | TU-TE | TU-Total | |||
| (TU-ICM/ICM%) | (TU-TE/TE%) | (TU-Total/Total%) | ||||||
| 0 mM | 19 | 33.7 ± 2.9 b | 115.7 ± 15.1 | 149.4 ± 16.1 | 24.8 ± 2.0 b | 1.5 ± 0.3 a | 5.4 ± 0.9 | 6.7 ± 0.9 |
| (4.5 ± 1.1) a | (5.5 ± 1) | (5.1 ± 0.8) | ||||||
| 1 mM | 14 | 43.4 ± 3.4 a | 112.4 ± 14.3 | 155.8 ± 16 | 29.6 ± 2.2 ab | 0.8 ± 0.4 ab | 5.1 ± 1.0 | 5.9 ± 1.1 |
| (1.8 ± 0.9) b | (5.5 ± 1.4) | (4.1 ± 0.8) | ||||||
| 5 mM | 18 | 44.0 ± 4.6 ab | 100.5 ± 10.9 | 144.5 ± 13.5 | 31.3 ± 2.1 a | 1.5 ± 0.4 ab | 5.3 ± 1.1 | 6.7 ± 1.3 |
| (3.1 ± 0.9) ab | (5.3 ± 1.1) | (4.5 ± 0.8) | ||||||
| 10 mM | 14 | 42.0 ± 4.6 ab | 96.9 ± 7.4 | 138.9 ± 8.4 | 30.5 ± 2.7 ab | 0.6 ± 0.3 b | 4.0 ± 1.0 | 4.6 ± 1.1 |
| (1.2 ± 0.5) b | (4.1 ± 0.8) | (3.2 ± 0.6) | ||||||
Frozen-thawed semen samples from Bull 1 were subjected to embryo quality evaluation. Six to ten replicates were used. Values are expressed as mean ± SE. ICM, inner cell mass; TE, trophectoderm; TU, TUNEL-positive. a–b: Different superscripts in the same parameter denote significant differences (P < 0.05) among the GSH concentration groups.
Results
Quality of frozen-thawed semen prepared with GSH-supplemented SFE
We measured the osmolality of fresh semen, control SFE, and GSH-SFE (GSH-supplemented SFE) to assess the physical impact on spermatozoa. Even though the sperm in GSH-SFE were exposed to double concentrations of GSH in the first dilution step, the osmolality was in the normal range (309.0–342.7 mmol/Kg), similar to that of the fresh semen (342.0–366.0 mmol/Kg) and control groups (300.0 mmol/Kg) (Supplementary Table 2).
The motility characteristics of frozen-thawed spermatozoa prepared with or without GSH-SFE were assessed using CASA. There were individual differences in five motility parameters (MOT, VCL, VAP, ALH, and WOB) in the initial scores of the 0 mM GSH control group (Fig. 2, P < 0.05, WOB: differences between Bull 1 (0.5%) and Bull 2 (0.6%)). In the case of GSH-SFE, there were no significant changes in motility parameters except MOT, VCL, VAP, and ALH (Fig. 2, P < 0.05). In all four bulls, increasing GSH concentration in SFE had an inversely affected MOT but, this relationship was only significant in Bull 3 with 10 mM GSH-SFE compared to the 0 mM GSH controls (Fig. 2A). A similar and significant inverse relationship between increasing GSH concentration and the other three motility parameters was also observed in Bull 1 with 10 mM GSH-SFE and Bull 4 in 5 and 10 mM GSH-SFE compared to 0 mM GSH controls (Fig. 2B, C, D). No significant differences were observed for Bull 2 semen. In total, there were no significant changes in motility or velocity of frozen-thawed semen prepared with 1 mM GSH-SFE but, a decrease in these characteristics was observed in the semen from three bulls (Bull 1, 3, and 4) with 10 mM GSH-SFE.
We analyzed the viability and acrosome integrity using FITC–PNA/PI staining (Supplementary Table 3). No individual differences among bulls were detected in the 0 mM control group. There were no significant differences in the percentage of viable spermatozoa (PI–), intact acrosome (FITC–PNA–), or viable spermatozoa with intact acrosome (FITC–PNA–/PI–) at any GSH concentrations in all bulls.
Sperm DNA integrity was also assessed using the TUNEL assay (Fig. 3). We assessed the initial differences in DFI among bulls and detected significant differences between Bull 1 and 2 in fresh (entirely (+) or partially (±) positive) and 0 mM GSH control (entirely positive, +) groups. Individual differences in DFI with increasing GSH concentration in SFE were observed in frozen-thawed samples among bulls. In Bull 1, the sperm DFI (entirely (+) or partially (±) positive) was higher in the 5 mM and 10 mM GSH-SFE groups than in their fresh semen counterparts (P < 0.05). In Bull 2, the DFI (entirely positive, +) at 10 mM GSH-SFE was higher than that of the fresh and 0 mM GSH-SFE (P < 0.05). There were no significant effects of GSH supplementation in SFE on sperm DNA integrity in Bull 3 and 4. The DFI (entirely positive, +) in Bull 2 and 4 showed a considerable increase (>20%) at 5 or 10 mM GSH supplementation. Overall, DFI increase due to high GSH supplementation concentration was observed, but the trend varied among the bulls.
We performed the BAP and TBARS assays to analyze the antioxidative status of frozen-thawed semen prepared with GSH-SFE. There was no difference in BAP and MDA levels among the different samples with GSH supplementation at different concentrations (Supplementary Table 4).
Taken together, GSH-SFE does not seem to have significant negative effects on frozen-thawed semen quality, except at high concentrations in some bulls.
IVF using frozen-thawed semen prepared with GSH-supplemented SFE
We evaluated the capacity for fertilization of semen prepared with GSH-SFE at 9 h and 18 h after IVF (Table 1). Data from Bull 1 showed three decreased motility parameters (Fig. 2) and increased DFI (Fig. 3) due to GSH supplementation. There were no significant differences in penetration rates, normal or polyspermic fertilization rates, and the male PN formation rates at 18 h after IVF, regardless of GSH concentration. The male PN formation rates evaluated at 9 h after IVF were significantly higher in the 1 mM (P < 0.05), 5 mM (P < 0.01), and 10 mM (P < 0.01) GSH-SFE groups than in the 0 mM GSH control.
The cleavage and blastocyst formation rates of IVF embryos derived from frozen-thawed semen prepared with or without GSH-SFE are shown in Table 2. At 0 mM GSH, there were individual differences among bulls in the cleavage and blastocyst formation rates (P < 0.05). GSH-SFE had positive effects on embryo cleavage and blastocyst formation in Bull 1, 2, and 4. Cleavage rates were significantly higher in embryos derived from 1 mM GSH-SFE in Bull 2 (P < 0.01), 5 mM GSH-SFE in Bull 2 and 4 (P < 0.01), and 10 mM GSH-SFE in Bull 1, 2, and 4 (P < 0.01) compared with the 0 mM GSH control. The cleavage rates of embryos derived from 10 mM GSH-SFE were higher than those from 1 mM and 5 mM GSH-SFE in Bull 1 (P < 0.05); however, cleavage rates were unaffected by GSH concentrations in Bull 2, 3, and 4. Increased blastocyst formation rates were observed in Bull 2 and 4 in embryos derived from GSH-SFE. In Bull 2, the highest blastocyst rate was observed in embryos derived from 10 mM GSH-SFE, whereas in Bull 4, 5 mM GSH-SFE resulted in the highest blastocyst rate. In Bull 1 and 3, there were no significant differences in blastocyst formation rates among the different GSH supplementation groups. Overall, GSH-SFE did not have any detrimental effects on embryo development, while its positive effect varied among bulls.
We then evaluated the quality of the IVF blastocysts derived from the frozen-thawed semen of Bull 1 based on the total cell number, relative ICM cell proportion, and the integrity of the DNA in the blastocyst cells (Table 3). The number of ICM cells in blastocysts derived from 1 mM GSH-SFE and the percentage of ICM/total cells in blastocysts derived from 5 mM GSH-SFE were significantly higher than those from the 0 mM GSH controls (P < 0.05). Blastocysts derived from 10 mM GSH-SFE had a lower DNA damage index in ICM cells compared with the 0 mM GSH controls. There was no significant difference among the GSH supplementation groups in the number or proportion of TUNEL-positive cells in TE and total cells. GSH-SFE increased the number of ICM cells and decreased the index of DNA damage in ICM cells of IVF blastocysts at different concentrations.
Discussion
In this study, by supplementing SFE with GSH, we aimed to improve the quality of frozen-thawed bull sperm, generally reduced during the freezing and thawing processes, and also to decrease the detrimental effects caused by ROS. Using semen from four bulls, we investigated whether GSH supplementation at varying concentrations of SFE affected the frozen-thawed sperm quality and subsequent embryo developmental competence after IVF. The results revealed that although sperm motility parameters or DNA integrity were decreased in all bulls at high concentrations of GSH-SFE, in vitro embryo development rates and the quality of those embryos were significantly improved in three of the bulls by GSH supplementation.
GSH supplementation has previously been reported to have positive effects on sperm motility in the bull [6, 20, 33] and other species [21, 22, 34], mainly due to a GSH-induced reduction in ROS. In our study, frozen-thawed semen prepared with GSH-SFE did not improve sperm motility in any of the bulls. All bulls showed a tendency for decreased sperm motility at 10 mM GSH-SFE, but this effect was significant for four out of five parameters in three bulls. Comparatively, most bull semen samples showed very little change in motility that agrees with similar reports of no effect of GSH on sperm motility in bulls [18, 19]. Other studies have shown that detrimental effects are occasionally observed under excess concentrations of GSH-SFE in equine [35] and ram [36] semen due to osmotic stress [36]. The deviation from the normal range of osmotic pressure is one of the reasons for motility loss in sperm [37]. We verified that the osmotic pressure applied in this study was within the normal range; therefore, it is unlikely that the motility loss with GSH supplementation in this study was a result of osmotic stress.
Differences in ROS sensitivity among ejaculated semen [38] and dose-dependent changes in antioxidative performance from 0 to 5 mM GSH supplementation have previously been reported [22]. Our results showed no effect of GSH treatment at different concentrations on BAP scores as an indicator of antioxidant power and MDA concentration as an indicator of lipid peroxidation. It was suggested that the GSH concentrations in this study did not affect the antioxidant capacity or cell stress caused by ROS.
Acrosome integrity is another indicator of sperm quality, and bull semen with low acrosome integrity often undergoes premature capacitation in vitro [39]. Our results showed no significant differences in sperm viability or acrosome integrity among all bulls at all tested GSH concentrations, agreeing with a previous report on ram semen [36]. Our results suggest that the improved IVF outcome is not necessarily related to semen quality as there was no significant increase in sperm viability or acrosome integrity with GSH-SFE.
In this study, differences in semen quality and IVF results among bulls were observed in fresh and frozen-thawed control semen samples. However, the effect of GSH varied among bulls regardless of the initial conditions. The individual variation in sperm DFI among the four bulls in this study is similar to that reported in a previous study on DFI fluctuation across ten bulls [25]. In Bull 1 and 2, DFI (entirely positive, +) in the 10 mM GSH-SFE group was higher than that of the fresh or 0 mM GSH control groups, and there was a trend of increasing DFI with increasing GSH concentrations. However, sperm viability evaluated using PI staining was not affected by GSH supplementation in SFE. Similarly, a decrease in DNA integrity, evaluated by the comet assay with 5 mM GSH supplementation in the thawing medium was previously reported in boar [40]. In contrast, positive effects of GSH treatment on DNA integrity and embryo production have been shown in Holstein bulls [41] and in vivo fertility in boar [42] and buffalo bulls [20]. Fresh semen from subfertile Holstein bulls has also been shown to have a higher DFI (> 20%) than that from the average or high fertility bulls (< 15%) [4]. In our study, none of the bulls had fresh and frozen-thawed control semen with high DFI (entirely positive, +), but two of the four bulls (Bull 2 and 4) showed high DFI (> 20% of entirely positive sperm, +) in frozen-thawed semen prepared with GSH-SFE. Sperm treatment with high concentrations of GSH may lead to increased DFI, especially in bulls that originally tend to have high DFI and might have adverse effects on conception rates. However, this will require further verification.
Although supplementation of GSH in SFE decreased DNA integrity in spermatozoa, it did not hinder fertilization and subsequent in vitro embryo development rates. Supplementation with GSH in SFE did not affect sperm penetration or normal fertilization rates. However, pronuclear formation rates at 9 h after IVF, cleavage rates, and embryo development rates increased in frozen-thawed semen prepared with GSH-SFE in three of the four bulls. Despite lower sperm DNA integrity in the two bulls, embryo production after IVF was still high. Interestingly, although the sperm DFI was increased by GSH supplementation, the DFI of embryos was reduced, and the ICM ratio was increased in blastocysts. Higher GSH concentration increased blastocyst formation rates, although these results varied among individual bulls.
Notably, the evaluated DFI was determined from the average value of the total spermatozoa in the sample, indicating that the DNA integrity of the semen sample that actually contributed to fertilization was not profiled. Moreover, spermatozoa categorized as “partially positive” represented fluorescence in the post-acrosomal region. This post-acrosomal region is regarded as the first component of the sperm head to solubilize within the ooplasm [43]. The mechanism of extracellular GSH increasing DFI in sperm, as well as the mechanism of improving embryo development after IVF, remain unknown.
Previous studies have shown that GSH contributes to redox reactions during fertilization and plays an important role in sperm decondensation by decreasing the disulfide bonds in sperm protamine [44, 45]. Sperm pretreatment with GSH before intracytoplasmic sperm injection improves the cleavage rate and quality of embryos and reduces disulfide bonds [33]. Hamilton et al. revealed the functional participation of GSTO2 (Glutathione-S-transferase omega 2) —a major enzyme that facilitates redox reactions through conjugation with GSH— in sperm decondensation and subsequent embryo development [46]. The importance of sperm decondensation in embryo development has also been reported [46]. The inhibition of GSTO2 causes a delay in early sperm nuclear decondensation at 2.5 h after insemination, resulting in decreased blastocyst formation rates [46]. In this study, male PN formation rates at 9 h after IVF were increased in the GSH-SFE groups. However, no differences were observed after 18 h. The earlier effects of GSH may be related to male PN formation that could be overlooked as a normal two-PN condition at 18 h. Moreover, GSH-SFE might play a role as a disulfide-reducing agent for sperm DNA that could be advantageous for early embryonic development stages, as previously reported [33]. Additional investigation is required regarding the increase in sperm DFI caused by GSH supplementation.
Our results revealed a variation in the effect of GSH-SFE on IVF embryo development between individual bulls. Two of the bulls showed both improved cleavage and blastocyst formation rate, while one other showed only an improved cleavage rate. Mammalian semen contains endogenous GSH, although the GSH concentration varies among individuals due to external and other factors [17, 47]. Intracellular GSH levels in bull semen vary in the range of 247–776 pmol/mg and decrease to 44–332 pmol/mg during cryopreservation with EYT extender [5, 15]. This large variation could be responsible for differences in the efficacy of supplemented GSH-SFE in individual bulls. It remains unclear whether it is possible to achieve both the general beneficial effects of GSH and those considered in this study, namely antioxidant activity, improved embryo development, and decreased DNA damage.
In conclusion, GSH supplementation in SFE has the potential to improve embryo development after IVF with frozen-thawed bull semen. However, the effective concentration of GSH supplementation in SFE may vary depending on the individual bull. Further studies are needed to clarify the mechanism of action of GSH treatment, as well as its advantages for calf production in bovine-assisted reproduction.
Conflict of interests
The authors have no conflicts of interest to disclose.
Supplementary
Acknowledgments
This work was supported by Ito Foundation Grants (H29-H31). The authors are grateful to the members of the Large Livestock Technical Team, Tsukuba Operation Unit 7, Technical Support Center of Central Region (NARO), technical staff at NILGS, Dr. Y Hirao, and Dr. S Akagi for their help.
References
- 1.Holden SA, Butler ST. Review: Applications and benefits of sexed semen in dairy and beef herds. Animal 2018; 12(s1): s97–s103. [DOI] [PubMed] [Google Scholar]
- 2.Barbat A, Le Mézec P, Ducrocq V, Mattalia S, Fritz S, Boichard D, Ponsart C, Humblot P. Female fertility in French dairy breeds: current situation and strategies for improvement. J Reprod Dev 2010; 56(Suppl): S15–S21. [DOI] [PubMed] [Google Scholar]
- 3.Dochi O, Kabeya S, Koyama H. Factors affecting reproductive performance in high milk-producing Holstein cows. J Reprod Dev 2010; 56(Suppl): S61–S65. [DOI] [PubMed] [Google Scholar]
- 4.Anzar M, He L, Buhr MM, Kroetsch TG, Pauls KP. Sperm apoptosis in fresh and cryopreserved bull semen detected by flow cytometry and its relationship with fertility. Biol Reprod 2002; 66: 354–360. [DOI] [PubMed] [Google Scholar]
- 5.Stradaioli G, Noro T, Sylla L, Monaci M. Decrease in glutathione (GSH) content in bovine sperm after cryopreservation: comparison between two extenders. Theriogenology 2007; 67: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 6.Gangwar C, Saxena A, Patel A, Singh SP, Yadav S, Kumar R, Singh V. Effect of reduced glutathione supplementation on cryopreservation induced sperm cryoinjuries in Murrah bull semen. Anim Reprod Sci 2018; 192: 171–178. [DOI] [PubMed] [Google Scholar]
- 7.Harayama H, Nishijima K, Murase T, Sakase M, Fukushima M. Relationship of protein tyrosine phosphorylation state with tolerance to frozen storage and the potential to undergo cyclic AMP-dependent hyperactivation in the spermatozoa of Japanese Black bulls. Mol Reprod Dev 2010; 77: 910–921. [DOI] [PubMed] [Google Scholar]
- 8.Shah N, Singh V, Yadav HP, Verma M, Chauhan DS, Saxena A, Yadav S, Swain DK. Effect of reduced glutathione supplementation in semen extender on tyrosine phosphorylation and apoptosis like changes in frozen thawed Hariana bull spermatozoa. Anim Reprod Sci 2017; 182: 111–122. [DOI] [PubMed] [Google Scholar]
- 9.Grötter LG, Cattaneo L, Marini PE, Kjelland ME, Ferré LB. Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post-thaw quality optimization. Reprod Domest Anim 2019; 54: 655–665. [DOI] [PubMed] [Google Scholar]
- 10.Rivlin J, Mendel J, Rubinstein S, Etkovitz N, Breitbart H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol Reprod 2004; 70: 518–522. [DOI] [PubMed] [Google Scholar]
- 11.Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev 2016; 28: 1–10. [DOI] [PubMed] [Google Scholar]
- 12.Lane M, McPherson NO, Fullston T, Spillane M, Sandeman L, Kang WX, Zander-Fox DL. Oxidative stress in mouse sperm impairs embryo development, fetal growth and alters adiposity and glucose regulation in female offspring. PLoS One 2014; 9: e100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amidi F, Pazhohan A, Shabani Nashtaei M, Khodarahmian M, Nekoonam S. The role of antioxidants in sperm freezing: a review. Cell Tissue Bank 2016; 17: 745–756. [DOI] [PubMed] [Google Scholar]
- 14.Adeoye O, Olawumi J, Opeyemi A, Christiania O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist Reprod 2018; 22: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilodeau JF, Chatterjee S, Sirard MA, Gagnon C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev 2000; 55: 282–288. [DOI] [PubMed] [Google Scholar]
- 16.Gadea J, Sellés E, Marco MA, Coy P, Matás C, Romar R, Ruiz S. Decrease in glutathione content in boar sperm after cryopreservation. Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004; 62: 690–701. [DOI] [PubMed] [Google Scholar]
- 17.Gadea J, Molla M, Selles E, Marco MA, Garcia-Vazquez FA, Gardon JC. Reduced glutathione content in human sperm is decreased after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology 2011; 62: 40–46. [DOI] [PubMed] [Google Scholar]
- 18.Tuncer PB, Bucak MN, Büyükleblebici S, Sarıözkan S, Yeni D, Eken A, Akalın PP, Kinet H, Avdatek F, Fidan AF, Gündoğan M. The effect of cysteine and glutathione on sperm and oxidative stress parameters of post-thawed bull semen. Cryobiology 2010; 61: 303–307. [DOI] [PubMed] [Google Scholar]
- 19.Perumal P, Selvaraju S, Selvakumar S, Barik AK, Mohanty DN, Das S, Das RK, Mishra PC. Effect of pre-freeze addition of cysteine hydrochloride and reduced glutathione in semen of crossbred Jersey bulls on sperm parameters and conception rates. Reprod Domest Anim 2011; 46: 636–641. [DOI] [PubMed] [Google Scholar]
- 20.Ansari MS, Rakha BA, Andrabi SM, Ullah N, Iqbal R, Holt WV, Akhter S. Glutathione-supplemented tris-citric acid extender improves the post-thaw quality and in vivo fertility of buffalo (Bubalus bubalis) bull spermatozoa. Reprod Biol 2012; 12: 271–276. [DOI] [PubMed] [Google Scholar]
- 21.Sinha MP, Sinha AK, Singh BK, Prasad RL. The effect of glutathione on the motility, enzyme leakage and fertility of frozen goat semen. Anim Reprod Sci 1996; 41: 237–243. [Google Scholar]
- 22.Yeste M, Estrada E, Pinart E, Bonet S, Miró J, Rodríguez-Gil JE. The improving effect of reduced glutathione on boar sperm cryotolerance is related with the intrinsic ejaculate freezability. Cryobiology 2014; 68: 251–261. [DOI] [PubMed] [Google Scholar]
- 23.Hamano S. Practice: Cryopreservation of Bull Semen. In: Takahashi Y (ed.), Artificial Insemination Manual for Cattle and Pig. Japan: AIAJ; 2016: 310–311. [Google Scholar]
- 24.Nagy S, Jansen J, Topper EK, Gadella BM. A triple-stain flow cytometric method to assess plasma- and acrosome-membrane integrity of cryopreserved bovine sperm immediately after thawing in presence of egg-yolk particles. Biol Reprod 2003; 68: 1828–1835. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Uchiyama K, Kinukawa M, Tagami T, Kaneda M, Watanabe S. Evaluation of sperm DNA damage in bulls by TUNEL assay as a parameter of semen quality. J Reprod Dev 2015; 61: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239: 70–76. [DOI] [PubMed] [Google Scholar]
- 27.Akagi S, Hosoe M, Matsukawa K, Ichikawa A, Tanikawa T, Takahashi S. Culture of bovine embryos on a polydimethylsiloxane (PDMS) microwell plate. J Reprod Dev 2010; 56: 475–479. [DOI] [PubMed] [Google Scholar]
- 28.Hidaka T, Fukumoto Y, Yamamoto S, Ogata Y, Horiuchi T. Variations in bovine embryo production between individual donors for OPU-IVF are closely related to glutathione concentrations in oocytes during in vitro maturation. Theriogenology 2018; 113: 176–182. [DOI] [PubMed] [Google Scholar]
- 29.Suttirojpattana T, Somfai T, Matoba S, Nagai T, Parnpai R, Geshi M. The effect of temperature during liquid storage of in vitro-matured bovine oocytes on subsequent embryo development. Theriogenology 2016; 85: 509–518.e1. [DOI] [PubMed] [Google Scholar]
- 30.Xu KP, Greve T. A detailed analysis of early events during in-vitro fertilization of bovine follicular oocytes. J Reprod Fertil 1988; 82: 127–134. [DOI] [PubMed] [Google Scholar]
- 31.Fouladi-Nashta AA, Alberio R, Kafi M, Nicholas B, Campbell KH, Webb R. Differential staining combined with TUNEL labelling to detect apoptosis in preimplantation bovine embryos. Reprod Biomed Online 2005; 10: 497–502. [DOI] [PubMed] [Google Scholar]
- 32.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oikawa T, Itahashi T, Yajima R, Numabe T. Glutathione treatment of Japanese Black bull sperm prior to intracytoplasmic sperm injection promotes embryo development. J Reprod Dev 2018; 64: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogata K, Sasaki A, Kato Y, Takeda A, Wakabayashi M, Sarentonglaga B, Yamaguchi M, Hara A, Fukumori R, Nagao Y. Glutathione supplementation to semen extender improves the quality of frozen-thawed canine spermatozoa for transcervical insemination. J Reprod Dev 2015; 61: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Oliveira RA, Wolf CA, de Oliveira Viu MA, Gambarini ML. Addition of glutathione to an extender for frozen equine semen. J Equine Vet Sci 2013; 33: 1148–1152. [Google Scholar]
- 36.Silva SV, Soares AT, Batista AM, Almeida FC, Nunes JF, Peixoto CA, Guerra MM. In vitro and in vivo evaluation of ram sperm frozen in tris egg-yolk and supplemented with superoxide dismutase and reduced glutathione. Reprod Domest Anim 2011; 46: 874–881. [DOI] [PubMed] [Google Scholar]
- 37.Guthrie HD, Liu J, Critser JK. Osmotic tolerance limits and effects of cryoprotectants on motility of bovine spermatozoa. Biol Reprod 2002; 67: 1811–1816. [DOI] [PubMed] [Google Scholar]
- 38.Simões R, Feitosa WB, Siqueira AFP, Nichi M, Paula-Lopes FF, Marques MG, Peres MA, Barnabe VH, Visintin JA, Assumpção MEO. Influence of bovine sperm DNA fragmentation and oxidative stress on early embryo in vitro development outcome. Reproduction 2013; 146: 433–441. [DOI] [PubMed] [Google Scholar]
- 39.Kuroda K, Fukushima M, Harayama H. Premature capacitation of frozen-thawed spermatozoa from subfertile Japanese black cattle. J Reprod Dev 2007; 53: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 40.Whitaker B, Carle B, Mukai T, Simpson A, Vu L, Knight J. Effect of exogenous glutathione supplementation on motility, viability, and DNA integrity of frozen-thawed boar semen. Anim Reprod 2008; 5: 127–131. [Google Scholar]
- 41.Hu T-X, Zhu H-B, Sun W-j, Hao H-s, Zhao X-m, Du W-h, Wang Z-L. Sperm pretreatment with glutathione improves IVF embryos development through increasing the viability and antioxidative capacity of sex-sorted and unsorted bull semen. J Integr Agric 2016; 15: 2326–2335. [Google Scholar]
- 42.Estrada E, Rodríguez-Gil JE, Rocha LG, Balasch S, Bonet S, Yeste M. Supplementing cryopreservation media with reduced glutathione increases fertility and prolificacy of sows inseminated with frozen-thawed boar semen. Andrology 2014; 2: 88–99. [DOI] [PubMed] [Google Scholar]
- 43.Zirkin BR, Soucek DA, Chang TSK, Perreault SD. In vitro and in vivo studies of mammalian sperm nuclear decondensation. Gamete Res 1985; 11: 349–365. [Google Scholar]
- 44.Cheng WM, An L, Wu ZH, Zhu YB, Liu JH, Gao HM, Li XH, Zheng SJ, Chen DB, Tian JH. Effects of disulfide bond reducing agents on sperm chromatin structural integrity and developmental competence of in vitro matured oocytes after intracytoplasmic sperm injection in pigs. Reproduction 2009; 137: 633–643. [DOI] [PubMed] [Google Scholar]
- 45.Delgado NM, Flores-Alonso JC, Rodríguez-Hernández HM, Merchant-Larios H, Reyes R. Heparin and glutathione II: correlation between decondensation of bull sperm cells and its nucleons. Arch Androl 2001; 47: 47–58. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton LE, Suzuki J, Aguila L, Meinsohn M-C, Smith OE, Protopapas N, Xu W, Sutovsky P, Oko R. Sperm-borne glutathione-S-transferase omega 2 accelerates the nuclear decondensation of spermatozoa during fertilization in mice. Biol Reprod 2019; 101: 368–376. [DOI] [PubMed] [Google Scholar]
- 47.Li TK. The glutathione and thiol content of mammalian spermatozoa and seminal plasma. Biol Reprod 1975; 12: 641–646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.