Significance
Kelp forests are declining worldwide due to varied combinations of environmental change and the trophic downgrading of urchin-controlling predators. These processes have increased the frequency and extent of rapid, nonlinear shifts to so-called urchin barrens whose ecological functioning and services are reduced relative to those of kelp forests. Understanding the factors that regulate kelp-forest tipping points and switches between states is key to their management. Here we demonstrate that substrate complexity (surface rugosity) determines both the existence of and dynamic transition between community states around San Nicolas Island, CA. Kelp-forest conservation and restoration efforts are growing internationally and may benefit from the consideration of substrate complexity in their strategies.
Keywords: alternative stable state, potential analysis, resilience, kelp forests, ecosystem stability
Abstract
The factors that determine why ecosystems exhibit abrupt shifts in state are of paramount importance for management, conservation, and restoration efforts. Kelp forests are emblematic of such abruptly shifting ecosystems, transitioning from kelp-dominated to urchin-dominated states around the world with increasing frequency, yet the underlying processes and mechanisms that control their dynamics remain unclear. Here, we analyze four decades of data from biannual monitoring around San Nicolas Island, CA, to show that substrate complexity controls both the number of possible (alternative) states and the velocity with which shifts between states occur. The superposition of community dynamics with reconstructions of system stability landscapes reveals that shifts between alternative states at low-complexity sites reflect abrupt, high-velocity events initiated by pulse perturbations that rapidly propel species across dynamically unstable state–space. In contrast, high-complexity sites exhibit a single state of resilient kelp–urchin coexistence. Our analyses suggest that substrate complexity influences both top-down and bottom-up regulatory processes in kelp forests, highlight its influence on kelp-forest stability at both large (island-wide) and small (<10 m) spatial scales, and could be valuable for holistic kelp-forest management.
Kelp-forest ecosystems exhibit rich and varied spatiotemporal dynamics. Prominent among these are dramatic shifts between kelp-dominated forests and so-called urchin barrens from which macroalgae are almost entirely absent due to intense urchin grazing (1, 2). Phase shifts between kelp and barren states have long been associated with structural changes to kelp-forest communities, such as the addition or removal of sea-urchin predators (3, 4) or changes in the environment such as shifting water temperatures (4–7). Kelp forests are also subject to stochastic perturbations such as large wave, marine disease, and anomalous warm water events that might perturb kelp forests between alternative stable states (8, 9). However, distinguishing phase shifts and alternative stable states is a major challenge (10). This is partially because both slow environmental change and relatively rapid stochastic perturbations often appear to act synergistically and with episodic urchin recruitment events that, due to their large regional extent, decouple rates of urchin grazing from the local density-dependent regulation of their populations (11, 12).
Although consensus is emerging that the maintenance of kelp-dominated forests is driven by a combination of top-down and bottom-up processes, the mechanisms underlying these processes—and hence the optimal means to control and avoid tipping points to the urchin-barren state—appear varied and often unclear (1, 13). For example, top-down processes contributing to kelp-forest stability include the effects of predators and disease on urchin grazing behavior and mortality rates (14–18), emphasizing the need for management strategies that preserve or restore top-down forms of urchin control (19, 20). On the other hand, bottom-up processes affecting kelp growth and senescence rates, and the retention of drift algae that urchins prefer to consume, are also known to contribute to kelp-forest stability, emphasizing management strategies that differ from those of direct urchin control (21–25). We hypothesize that substrate complexity modifies both top-down and bottom-up processes structuring urchin–kelp interactions, e.g., provisioning habitat for urchin predators and increasing the retention of drift algae for urchins.
Here we apply the perspective of stochastic dynamical systems to the study of kelp forests not to determine the specific mechanisms or feedbacks that underlie kelp-forest dynamics but rather to infer an environmental variable that influences their relative strength and net expression. The dynamical-systems perspective conceptualizes a system’s community states and dynamics using the ball-in-cup heuristic of stability and resilience (26, 27), formally described by a (quasi-)potential stability landscape (28, 29). A system with alternative stable states exhibits a multimodal landscape with two or more basins of attraction (cups) over which it travels in time due to endogenous drivers (e.g., species interactions) and external perturbations. Because most perturbations are directionally random and small, communities spend more time in states at the bottom of the attracting basins than they do on their slopes and cusps, with deeper and steeper-sloped basins corresponding to more stable and resilient community states whose dynamics are dominated by negative feedbacks (28). Previous work has utilized this characteristic of stochastic dynamical systems to make use of large-scale spatial variation in community structure to infer what biotic and environmental conditions may alter the stability of various ecological systems, including tropical and temperate forests and desert biomes (4, 30–32). For example, Scheffer et al. (33) used satellite-derived spatial variation in the frequency distributions of percentage of tree cover values to infer that boreal biomes exhibit between one and three different alternative stable states whose number and nature depend on mean July temperature, where empirical system–state frequency histograms represent negative potential (i.e., a mirror image of a ball-in-cup stability landscape reflected across the x axis). Similarly, Ling et al. (4) combined spatial survey data with translocation experiments to infer bistability in response to urchin densities in Tasmanian kelp forests. The approach underlying these inferences has been referred to as potential analysis (34).
Using spatially fixed and replicated long-term time series of kelp-forest community dynamics around San Nicolas Island, CA, we extended the application of potential analysis to include the temporal domain to more rigorously infer their condition-dependent stability landscapes and shifts in community structure. Our analyses reveal kelp-forest communities around San Nicolas Island to exhibit dramatic, perturbation-induced shifts between kelp-dominated forests and urchin-barren states only when the complexity of the underlying substrate is low and that similarly perturbed high-complexity substrates permit only a single persistent state of resilient kelp–urchin coexistence. We infer that substrate complexity at San Nicolas Island controls the relative strength of the many negative and positive feedbacks that have been described in kelp forests and that a greater understanding of its influences is likely to increase the effectiveness of management efforts seeking to conserve and restore their existence.
Methods and Results
Multimodality and Velocities of Community Movement.
San Nicolas Island is located in the Channel Islands off the southern California coast (N 33.25, W 119.50). We analyzed 38 y of biannual community data from 1980 to 2018 at six subtidal sites installed around the island at depths of 10 to 14 m. Each site comprises five fixed-location benthic transects (10 × 2 m) in which the abundance of seven key invertebrates and seven dominant macroalgae species (henceforth “kelp” for brevity) was monitored (35). We quantified the substrate complexity of each transect as its lengthwise linear relief measured using a 13-cm circumference electronic surveyor’s wheel (36). Because some sites exhibit informative variation in substrate complexity and dynamics among their transects, we present the results of analyses conducted at the transect scale rather than the site scale (37).
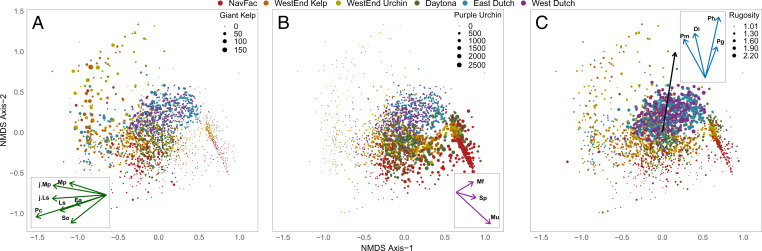
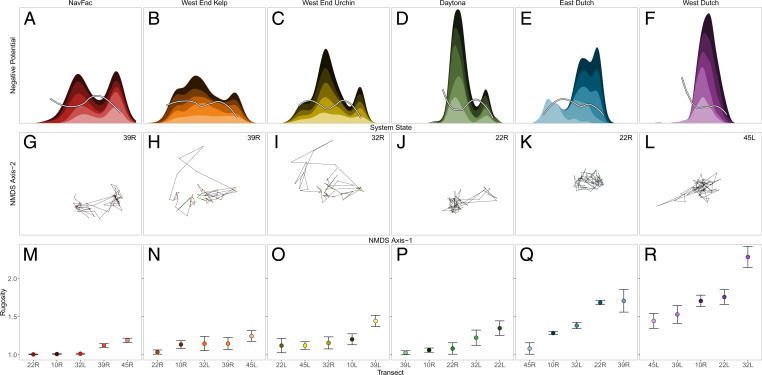
To reconstruct transect-level stability landscapes and evaluate their multimodality using potential analysis, we expanded upon ref. 37 and used nonmetric multidimensional scaling (NMDS) (38) to obtain a two-dimensional ordination of all transect-level species abundances through time. Axis 1 of this ordination encompassed 61.2% of the variation and effectively captured the predominant gradient of community structure ranging from kelp-dominated with almost no urchins (Fig. 1A), to a broad mixture of kelp and urchins centered around the axis origin (Fig. 1 A and B), to urchin barrens composed almost exclusively of urchins and no macroalgae (Fig. 1B). Axis 2 was primarily associated with nongrazing, predatory invertebrates, specifically sea stars (Fig. 1 C, Inset), and captured an additional 23.9% of variation. Due to their length and sampling frequency, each transect-level time series evidenced ample and consistent community variation relative to the broader range of community structures across all sites to enable the reconstruction of robust frequency distributions of community state. The multimodality of these frequency distributions along axis 1 was visualized using kernel densities and formally evaluated using Gaussian mixture models. These provided strong evidence for both unimodal and multimodal community state distributions among the 30 transects (Fig. 2 A–F and SI Appendix, Fig. S1 and Table S1).
Fig. 1.
Ordination of kelp-forest community dynamics in two-dimensional species space. Each point reflects community composition of a transect at a given timepoint. Point color identifies the transect’s site. Point size reflects a different variable in each panel: (A) giant kelp (adult Macrocystis pyrifera) abundance is negatively associated with axis 1 (Inset: the direction and strength of association of all algal taxa with ordination axes relative to the ordination center); (B) purple urchin (Strongylocentrotus purpuratus) abundance and presence are positively associated with axis 1 (Inset: the association of all grazing invertebrates with ordination axes); and (C) substrate rugosity is positively associated with axis 2 (main panel black arrow reflects the linear correlation of substrate rugosity with ordination axes (Inset: the association of nongrazing and predatory invertebrate taxa with ordination axes). The 14 benthic taxa were purple S. purpuratus (Sp) and red (Mesocentrotus franciscanus) (Mf) urchins, one gastropod grazer (Megastraea undosa) (Mu), four sea stars (the sunflower star [Pycnopodia heliathoides] [Ph], the giant spined star [Pisaster giganteus] [Pg], the leather star [Dermasterias imbricata] [Di], and the bat star [Patiria miniate] [Pm]), one macroalgal species in the Order Fucales (Stephanocystis osmundacea) (So), and six macroalgae in the Order Laminariales, including two juvenile stages (giant kelp [Macrocystis pyrifera] [Mp], juvenile [<1 m] giant kelp [j.Mp], [Pterygophora californica] [Pc], [Eisenia arborea] [Ea], Laminaria spp. [Ls], and young Laminariales [j.Ls]).
Fig. 2.
(A–F) Transect-specific stability landscapes stacked by site (transects differentiated by color hue) and state-dependent velocities of community shift (white lines loess smoothed with a span of 0.75). (G–L) Ordination of site-specific community dynamics in species space (as in Fig. 1) with the temporal dynamics of one focal transect visualized to highlight within- and between-basin movement. (M–R) Transect-specific estimates of substrate complexity (mean ±1 SE).
As inferred by potential analysis, some transects exhibited alternative stable states while others exhibited only a single stable state. Prior inferences based on potential analysis have relied on the assumption that low-frequency states reflect transient states en route to regions of stability. This assumption is not always warranted given the possibility of multigenerational transients and population cycles (39, 40), particularly in multidimensional systems (41). Therefore, and because our time series span many generations of the dominant kelp species, we next quantified velocities of community movement through two-dimensional ordination space to gain insight into the state-dependent nature of within- and between-basin perturbation effects and feedbacks. We expected rates of community change to be lowest and directionally random in regions of axis 1 reflecting centers of high-frequency community states and highest and directional (toward high-frequency centers) in regions reflecting low-frequency community states (42). These expectations were realized in all cases associated with urchin-barren and mixed kelp–urchin community states (Fig. 2 A–F and see SI Appendix, Fig. S1 for directionality), indicating that these high-frequency states indeed represent stable attractors resilient to most perturbations. Transitions between these states consistently entailed high-velocity, directional events (SI Appendix, Fig. S2 A and B), the mechanisms of which we return to below.
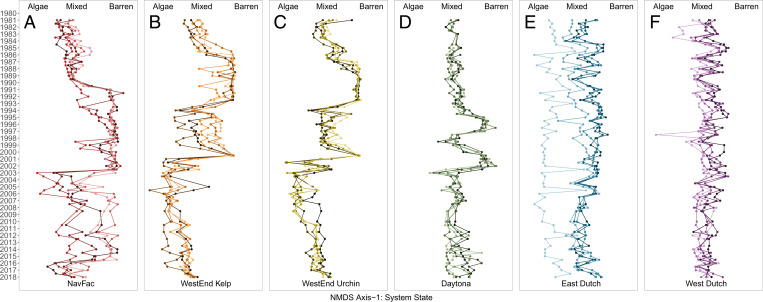
In contrast, our expectations were not realized for 10 transects at two sites inferred by potential analysis to exhibit the third high-frequency state, the algal-only state in which urchins were almost entirely absent (Fig. 2 H and I). It persisted for 2 to 8 y following large and rapid urchin declines, likely due to disease, and invariably transitioned back to the mixed kelp–urchin state in a smooth and continuous fashion (Fig. 3 B and C). We interpret the dynamics of these algal-only transects as reflecting multigenerational transient dynamics (39, 43), a finding potential analysis alone would not have resolved. One additional low-complexity transect (East Dutch 45R, Fig. 2E) exhibited the algal-only state for the entire duration of the time series, in marked contrast to the four other high-complexity transects of the same site that persistently exhibited the mixed kelp–urchin state (Figs. 2 E and Q and 3E).
Fig. 3.
(A–F) The temporal dynamics of system state (NMDS axis 1), with sites ordered from Left to Right by their average substrate complexity and where individual lines correspond to individual transects. Transitions between urchin-barren (“Barren”) and mixed kelp–urchin (“Mixed”) states (A–D), as well as from the urchin-barren state to the algal-only (“Algae”) state (A–C), represent high-velocity shifts. In contrast, transitions from the algal-only state to the mixed kelp–urchin state are smooth and continuous (B and C) after 2004. All types of shifts entail both synchronous and asynchronous events among transects and sites. The persistent algal-only state of the exceptional transect (light blue in E) is addressed in Discussion. The mixed kelp-urchin state exhibited 38 years of persistence (E and F) along transects with high substrate complexity.
Multimodality Determined by Substrate Complexity.
Transect-level estimates of substrate complexity (surface rugosity) varied markedly across the 30 transects, ranging from being highly structured and complex (linear relief m) to flat (linear relief = 10 m) (Fig. 2 M–R). Transects within a site tended to exhibit similar magnitudes of substrate complexity, but this was not always the case (e.g., Fig. 2 Q and R). Substrate complexity was clearly associated with axis 2 of the NMDS ordination (Pearson’s ), particularly for transects exhibiting the urchin-barren and mixed kelp–urchin community states (Fig. 1C).
Ordering sites and transects by their average substrate complexity evidenced that complexity is predictive of the kelp-forest stability landscape (SI Appendix, Fig. S2). While high-complexity transects (>15 m linear relief, rugosity >1.5) exhibited unimodal landscapes of persistent kelp–urchin coexistence, low-complexity transects (<15 m linear relief, rugosity <1.5) exhibited multimodal landscapes reflective of alternative stable states. Moreover, all transects exhibiting the algal-only transient state were low-complexity transects, including the single East Dutch 45R transect that exhibited a persistent algal-only state.
Discussion
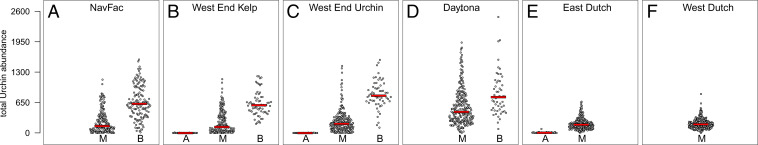
At San Nicolas Island (SNI), high-complexity sites and transects did not exhibit alternative stable states of community composition, instead exhibiting 38 y of stable kelp–urchin coexistence resilient to perturbation. Urchins were common in these transects (Fig. 4 E and F), but rather than forming fronts or grazing actively in the open, as urchins are known to do during urchin-barren formation (24), these urchins were consistently tucked away in crevices and self-created pits (44). In contrast, low-complexity transects exhibited both mixed kelp–urchin and urchin-barren states that persisted for up to 12 y, with transitions between them being higher-velocity events in both directions (Fig. 3 and SI Appendix, Figs. S1–S3). Urchins in these transects were observed to exhibit sedentary behavior when in the mixed kelp–urchin state, with urchin densities seen during mixed kelp–urchin periods overlapping considerably with those seen during urchin-barren periods (Fig. 4 A–D). Because high- and low-complexity sites are interspersed around the island, with adjacent sites of differing substrate complexity experiencing equivalent oceanographic conditions, these patterns are unlikely to be caused by unassessed covariates (see SI Appendix for discussions of chlorophyll a [SI Appendix, Fig. S4], sea temperature [SI Appendix, Fig. S5], wave height [SI Appendix, Fig. S6], and sea urchin predator abundance [SI Appendix, Fig. S7]). Instead, we hypothesize that high-complexity substrate permits stable kelp–urchin coexistence because it modifies the relative strength of both top-down and bottom-up regulatory feedbacks through an interplay of behavioral, interspecific, and oceanographic processes.
Fig. 4.
(A–F) Total red and purple urchin abundances partitioned by system state (transects combined by site) with sites arranged by increasing mean substrate complexity from Left to Right. Within each panel the algal-only state is represented by the letter A, the mixed kelp–urchin state by M, and the urchin-barren state by B. Red line segments delineate median urchin abundances. A high degree of overlap between the mixed kelp–urchin and urchin-barren states indicates that urchin density is not the exclusive driver of kelp-forest states.
Complexity Modifies the Strength of Urchin-Regulating Feedbacks.
We hypothesize that substrate-induced covariation between top-down and bottom-up effects on urchin behavior, recruitment, and mortality determines the propensity of kelp-forest communities to exhibit a single, resilient state versus multiple, alternative stables states between which switches occur. Urchin predators, such as sea stars and California sheephead (Semicossyphys pulcher), positively associate with high complexity at SNI (Fig. 1C and SI Appendix, Fig. S7). Their presence exerts direct mortality on urchins and modifies urchin behavior through a “landscape of fear” (14, 16, 45). High-complexity substrate also entraps drift algae in cracks, below ledges, and at the base of rocky outcrops. High-complexity substrate thereby retains and stabilizes the supply of drift, which urchins prefer to consume over live kelp, particularly during large wave events that otherwise result in net drift export (22, 46). Urchins persist during periods of relatively low drift availability following storms due to their high longevity even when starved (47). For low-complexity substrates, the net loss of drift during storms elicits the urchin behavioral shift to actively wander and graze upon live kelp (21, 23, 24). Because lower-complexity substrates also have lower abundances of slow-to-reproduce predators, active urchin grazing following drift loss proceeds largely unchecked, with increasingly strong feedback mechanisms—including lower local production of drift and a greater cover of encrusting algae that acts as a cue for urchin settlement (48)—stabilizing the urchin-barren state. Once in the urchin-barren state, large, density-dependent but stochastic disease outbreaks at high urchin densities (17) permit opportunities for kelp recovery. Low-complexity substrates are thereby predisposed to alternative stable states because the combination of low drift retention (a bottom-up effect) and low predator abundance (a top-down effect) promotes persistent changes in urchin behavior and demography.
Substrate complexity determines not only the number of kelp-forest alternative stable states but also how perturbations cause shifts between them. The high velocity required to move between alternative stable states (Fig. 2 and SI Appendix, Fig. S1) indicates that low-complexity transects exhibit a time-invariant, bimodal stability landscape with alternative stable attractors separated by dynamically unstable space (SI Appendix, Fig. S2). That is, transitions from one stable attractor to another require a pulse perturbation, such as rapid kelp and drift loss due to large wave events or urchin mass mortality due to disease (8, 49). Shifts between states occurred in both directions and occurred both synchronously and asynchronously at low-complexity sites around the island, even as high-complexity transects exhibited stable persistence (Fig. 3 and SI Appendix, Fig. S1). It is therefore unlikely that the existence of alternative stable states at low-complexity sites reflects forcing from changes in environmental drivers, including gradual press perturbation changes that alter the shape of the stability landscape itself. Instead, our results indicate that the localized effects of stochastic pulse perturbations are state dependent and are modified at small scales by the stabilizing feedbacks associated with substrate complexity.
The Algae-Only State as a Multigenerational Long Transient.
Potential analysis indicated the existence of a third alternative algal-only state (Fig. 2 B and C), but the velocity dynamics indicate this to be a multigenerational period of transient dynamics that inevitably and smoothly leads to the mixed kelp–urchin stable state upon the demographic recovery of urchins. For all but a single exceptional transect (discussed below), this algal-only state followed disease-related urchin mass mortality. Lacking nearly any observable urchins when in the algal-only state (Fig. 4 B and C), transects varied widely in kelp abundance, producing numerous instances of within-state high-velocity community movement that represent the vast majority of instances where transect position along axis 2 did not associate with substrate complexity (SI Appendix, Figs. S1 and S3). Fluctuations in kelp abundance decreased as urchins began recovering ∼6 to 8 y following their crash (Fig. 3 B and C). Whereas kelp reproduce and grow annually, urchins require several years to reach adult size (50); thus, we hypothesize that these transient dynamics are driven by the temporal lag between urchin mass mortality and the time required for local urchin recovery. Such dynamics are expected for slow–fast systems with strongly differing consumer and resource generation times (39). The multiyear nature of this transience highlights the limitation of potential analysis when additional temporal insight is lacking (51) and emphasizes the need for long-term monitoring to contextualize shifts in state and guide kelp-forest management and conservation (13, 52, 53).
Low-Complexity Dynamics Conditional upon Surrounding Heterogeneity.
Performing analyses at the transect level provided insight into variation that potential analysis would not have revealed at the site level (37), but also raises a question regarding the behavior of an exceptional transect. The transect, East Dutch 45R, is the only low-complexity transect to exhibit a persistent algal-only state (Fig. 2 E and Q). It experienced repeated perturbations from which it returned to the algal-only state with high velocity (Fig. 3E and SI Appendix, Fig. S2). These dynamics suggest that this transect’s algal-only state reflects a third stable attractor, rather than a long transient. This is an exception to our inference that substrate complexity is the sole predictor of kelp-forest stability at SNI, as we would expect this low-complexity transect to exhibit multimodality. We contend, however, that this exceptional transect reflects a deeper nuance to kelp-forest dynamics related to spatial scale, as it is the only low-complexity transect that is surrounded by otherwise high-complexity substrate. We hypothesize that the stabilizing effects of adjacent complex substrate spill over to confer this transect’s resilience. Larger expanses of low-complexity substrate—as surrounds all other low-complexity transects and sites of our study—lack this stabilizing spatial spillover. Manipulative experiments, such as urchin additions or the continual removal of drift from similar low-complexity areas that are surrounded by high-complexity substrate, are needed to test this hypothesis and determine the spatial scales to which the mechanism may apply.
Conclusions.
The processes and feedbacks that associate with substrate complexity undoubtedly extend well beyond those that we have discussed. For example, high-complexity transects are more species rich and exhibit a greater coverage of foliose red algae and sessile invertebrates than do low-complexity transects (35). As such, our results add to a rich ecological literature detailing the many means by which physical and biological complexity can modify species coexistence and the dynamics and functioning of ecological communities (54–57). It nonetheless remains an open question how globally widespread the importance of substrate complexity is, as changes in kelp-forest state certainly do occur irrespective of substrate complexity, especially at higher latitudes (4, 58–60). We speculate that many of these large-scale changes in kelp-forest state are driven by phase shifts rather than switches between alternative stable states. For example, in the Northeast Pacific, phase shifts between forested and urchin-barren states are driven by changing environmental conditions, specifically the presence or absence of sea otters (3, 61). Urchin predator diversity and environmental conditions that influence urchin recruitment and population structure also vary with latitude and across the globe (16, 50, 62). The importance of substrate complexity may thus be overridden by additional factors in region-specific ways.
That said, our findings bear two points of consideration for management and restoration efforts that seek to mitigate or reverse kelp-forest loss (63–65).
First, our work implies that both natural and artificial high-complexity reefs offer a means to increase the strength of stabilizing kelp-forest feedbacks. Reefs could be selected for conservation efforts or constructed to maximize the entrapment of locally produced and delivered drift algae, provide structure for urchins to shelter, and support a diversity of urchin-controlling predators. In the context of artificial reefs, we acknowledge there is no quick fix for ecological restoration (66) and that multiple interests are often at play (e.g., the desire to minimize man-made structures in marine protected areas). However, given our evidence that kelp-forest stability can vary at the scale of a 10 × 2-m transect and strong evidence that metapopulation dynamics driving kelp spore dispersal operate at much larger scales (67), we submit that strategically placed patchworks of natural and artificial reefs could serve as hotspots of emergent kelp-forest resilience.
Second, the large overlap between urchin densities in the mixed kelp–urchin and urchin-barren states (Fig. 4) emphasizes that urchin density alone is an insufficient predictor of urchin behavior and state stability. In particular, the rapid timescales of kelp and drift algae loss, and the rapid manner with which urchin behavior responds (24), indicate that bimodality in system state mirrors a bimodality in urchin grazing activity. Hence, the common practice of removing or culling urchins to reduce their abundance will decrease grazing rates only in the short term and will not alone restore feedback processes that confer kelp-forest stability. More specifically, the processes of kelp growth, reproduction, dispersal, senescence, and drift production, which are critical for achieving and stabilizing the mixed kelp–urchin state, as well as the counteracting processes of urchin immigration, settlement, and recruitment, which stabilize the barren state, are not affected by such direct, short-term means of urchin control. Instead, urchin removal is likely to be most effective for jump starting kelp recovery when efforts are focused upon high-complexity substrate and paired alongside local kelp-focused restoration (e.g., outplanting) and short-term drift enhancement to strategically protect out-planted kelp until local kelp growth and drift production are reestablished.
Materials and Methods
Time-Series and Community Analysis.
Five fixed-location transects at each of six sites around SNI were biannually surveyed from 1980 to 2018, yielding 30 location-specific time series of 14 taxa (1,973 transect surveys total due to some missed survey periods). See refs. 35 and 37 for additional details on the spatiotemporal structure of these data. Species abundances in the 1,973 × 14 community matrix were transformed to down-weight the influence of highly abundant purple urchins prior to calculation of a Bray–Curtis dissimilarity matrix. Nonmetric multidimensional scaling was performed on this dissimilarity matrix using the vegan package (v.2.5-4) (68) in R v.3.5.3 (69) and exhibited a stress of 0.18. Coefficients of determination (r2) were used to quantify the variance represented along each ordination axis and were obtained using PC-ORD v.7 (70).
Linear Relief Measurements.
We used a 13-cm circumference electronic surveyor’s wheel to measure the linear relief of each transect, averaging three replicate measurements per transect: down the lengthwise center and 1 m away on each side of the center. The association of mean complexity with axis 2 of the ordination was calculated using Pearson’s linear correlation coefficient in the vegan R package (68).
Potential Analysis and Multimodality.
Stability landscapes were represented as kernel density plots using the geom_density function of ggplot2 (71) with the default bandwidth (adjust ) for all but the two WestEnd sites (adjust , to better visualize the algal-only state); other bandwidths produced qualitatively similar results. Multimodality along axis 1 was formally evaluated univariately by Gaussian mixture models with the mclust package (v5.4.3) (72), allowing variable variances among the clusters (model option “V”). We also repeated our analyses after restricting the time series to either only fall or only spring surveys and obtained qualitatively similar results. State categorizations in Fig. 4 were delineated by maximum and minimum kernel density values.
Velocities of Community Movement.
Velocities were calculated in two-dimensional ordination space by dividing the Euclidean distance between two sequential sample points by the number of days elapsed between them. Their midpoint along axis 1 determined the community state against which velocities were plotted in Fig. 2.
Supplementary Material
Acknowledgments
We are indebted to the many individuals who monitored SNI over the years; particularly, James Bodkin, Linda Brown, Ken Collins, A. Keith Miles, Shannon Myers, Kristen Sanchez, and Amy Story; and to James Estes and Chris Harrold for their early dedication and leadership of the long-term monitoring program. Community data collection was supported by the US Geological Survey and the US Navy. Z.R. was supported by a NSF Graduate Research Fellowship (2016227734) and the Partnership for Interdisciplinary Studies of Coastal Oceans (PISCO). M.N. acknowledges the support of a David and Lucile Packard Foundation grant to PISCO. This is publication 522 from PISCO. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2103483119/-/DCSupplemental.
Data Availability
Time series and linear relief measurements of substrate complexity, code, and data have been deposited in GitHub (https://github.com/zhrandell/SubstrateComplexity). A project data release by the US Geological Survey is accessible on ScienceBase at https://doi.org/10.5066/P9Q6B625.
References
- 1.Ling S. D., et al., Global regime shift dynamics of catastrophic sea urchin overgrazing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 1–10 (2015). [Google Scholar]
- 2.Filbee-Dexter K., Scheibling R. E., Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 495, 1–25 (2014). [Google Scholar]
- 3.Estes J. A., Palmisano J. F., Sea otters: Their role in structuring nearshore communities. Science 185, 1058–1060 (1974). [DOI] [PubMed] [Google Scholar]
- 4.Ling S. D., Johnson C. R., Frusher S. D., Ridgway K. R., Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl. Acad. Sci. U.S.A. 106, 22341–22345 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson C. R., et al., Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J. Exp. Mar. Biol. Ecol. 400, 17–32 (2011). [Google Scholar]
- 6.Dayton P. K., Tegner M. J., Edwards P. B., Riser K. L., Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecol. Appl. 8, 309–322 (1998). [Google Scholar]
- 7.Tegner M., Dayton P., Sea urchins, El Ninos, and the long term stability of southern California kelp forest communities. Mar. Ecol. Prog. Ser. 77, 49–63 (1991). [Google Scholar]
- 8.Reed D. C., et al., Wave disturbance overwhelms top-down and bottom-up control of primary production in California kelp forests. Ecology 92, 2108–2116 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Rogers-Bennett L., Catton C. A., Marine heat wave and multiple stressors tip bull kelp forest to sea urchin barrens. Sci. Rep. 9, 15050 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudgeon S. R., Aronson R. B., Bruno J. F., Precht W. F., Phase shifts and stable states on coral reefs. Mar. Ecol. Prog. Ser. 413, 201–216 (2010). [Google Scholar]
- 11.Karatayev V. A., Baskett M. L., At what spatial scales are alternative stable states relevant in highly interconnected ecosystems? Ecology 101, e02930 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Uthicke S., Schaffelke B., Byrne M., A boom–bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol. Monogr. 79, 3–24 (2009). [Google Scholar]
- 13.Krumhansl K. A., et al., Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. U.S.A. 113, 13785–13790 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisaguirre J. H., et al., Trophic redundancy and predator size class structure drive differences in kelp forest ecosystem dynamics. Ecology 101, e02993 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton S. L., Caselle J. E., Exploitation and recovery of a sea urchin predator has implications for the resilience of southern California kelp forests. Proc. Biol. Sci. 282, 20141817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burt J. M., et al., Sudden collapse of a mesopredator reveals its complementary role in mediating rocky reef regime shifts. Proc. Biol. Sci. 285, 20180553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafferty K. D., Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol. Appl. 14, 1566–1573 (2004). [Google Scholar]
- 18.Smith J. G., et al., Behavioral responses across a mosaic of ecosystem states restructure a sea otter-urchin trophic cascade. Proc. Natl. Acad. Sci. U.S.A. 118, 1–7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estes J. A., et al., Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Ling S. D., Ibbott S., Sanderson J. C., Recovery of canopy-forming macroalgae following removal of the enigmatic grazing sea urchin Heliocidaris erythrogramma. J. Exp. Mar. Biol. Ecol. 395, 135–146 (2010). [Google Scholar]
- 21.Vanderklift M. A., Kendrick G. A., Contrasting influence of sea urchins on attached and drift macroalgae. Mar. Ecol. Prog. Ser. 299, 101–110 (2005). [Google Scholar]
- 22.Vanderklift M. A., Wernberg T., Detached kelps from distant sources are a food subsidy for sea urchins. Oecologia 157, 327–335 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Kriegisch N., Reeves S. E., Flukes E. B., Johnson C. R., Ling S. D., Drift-kelp suppresses foraging movement of overgrazing sea urchins. Oecologia 190, 665–677 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Harrold C., Reed D. C., Food availability, sea urchin grazing, and kelp forest community structure. Ecology 66, 1160–1169 (1985). [Google Scholar]
- 25.Foster M. S., Schiel D. R., Loss of predators and the collapse of southern California kelp forests (?): Alternatives, explanations and generalizations. J. Exp. Mar. Biol. Ecol. 393, 59–70 (2010). [Google Scholar]
- 26.Holling C. S., Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23 (1973). [Google Scholar]
- 27.Scheffer M., Carpenter S., Foley J. A., Folke C., Walker B., Catastrophic shifts in ecosystems. Nature 413, 591–596 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Nolting B. C., Abbott K. C., Balls, cups, and quasi-potentials: Quantifying stability in stochastic systems. Ecology 97, 850–864 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J. X., Aliyu M. D., Aurell E., Huang S., Quasi-potential landscape in complex multi-stable systems. J. R. Soc. Interface 9, 3539–3553 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota M., Holmgren M., Van Nes E. H., Scheffer M., Global resilience of tropical forest and savanna to critical transitions. Science 334, 232–235 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Aleman J. C., et al., Floristic evidence for alternative biome states in tropical Africa. Proc. Natl. Acad. Sci. U.S.A. 117, 28183–28190 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staver A. C., Archibald S., Levin S. A., The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Scheffer M., Hirota M., Holmgren M., Van Nes E. H., Chapin F. S. III, Thresholds for boreal biome transitions. Proc. Natl. Acad. Sci. U.S.A. 109, 21384–21389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livina V. N., Kwasniok F., Lenton T. M., Potential analysis reveals changing number of climate states during the last 60 kyr. Clim. Past 6, 77–82 (2010). [Google Scholar]
- 35.Kenner M. C., et al., A multi-decade time series of kelp forest community structure at San Nicolas Island, California (USA). Ecology 94, 2654 (2013). [Google Scholar]
- 36.Wilding T. A., Rose C. A., Downie M. J., A novel approach to measuring subtidal habitat complexity. J. Exp. Mar. Biol. Ecol. 353, 279–286 (2007). [Google Scholar]
- 37.Kenner M. C., Tinker M. T., Stability and change in kelp forest habitats at San Nicolas Island. West. N. Am. Nat. 78, 633–643 (2018). [Google Scholar]
- 38.Kruskal J. B., Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29, 1–27 (1964). [Google Scholar]
- 39.Hastings A., et al., Transient phenomena in ecology. Science 361, eaat6412 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Jäger C. G., Diehl S., Matauschek C., Klausmeier C. A., Stibor H., Transient dynamics of pelagic producer-grazer systems in a gradient of nutrients and mixing depths. Ecology 89, 1272–1286 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Sánchez P., van Nes E. H., Scheffer M., Climbing Escher’s stairs: A way to approximate stability landscapes in multidimensional systems. PLOS Comput. Biol. 16, e1007788 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamothe K. A., Somers K. M., Jackson D. A., Linking the ball-and-cup analogy and ordination trajectories to describe ecosystem stability, resistance, and resilience. Ecosphere 10, e02629 (2019). [Google Scholar]
- 43.Frank K. T., Petrie B., Fisher J. A., Leggett W. C., Transient dynamics of an altered large marine ecosystem. Nature 477, 86–89 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Russell M. P., Gibbs V. K., Duwan E., Bioerosion by pit-forming, temperate-reef sea urchins: History, rates and broader implications. PLoS One 13, e0191278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cowen R. K., The effects of sheephead (Semicossyphus pulcher) predation on red sea urchin (Strongylocentrotus franciscanus) populations: An experimental analysis. Oecologia 58, 249–255 (1983). [DOI] [PubMed] [Google Scholar]
- 46.Figurski J., “Patterns and sources of variation in drift algae and the ecological consequences for kelp forests,” PhD thesis, University of California, Santa Cruz, CA (2010).
- 47.Rogers-Bennett L., Chapter 19 the ecology of strongylocentrotus franciscanus and strongylocentrotus purpuratus. Dev. Aquac. Fish. Sci. 37, 393–425 (2007). [Google Scholar]
- 48.Taniguchi K., Kurata K., Maruzoi T., Suzuki M., Dibromomethane, a chemical inducer of larval settlement and metamorphosis of the sea urchin Strongylocentrotus nudus. Fish. Sci. 60, 795–796 (1994). [Google Scholar]
- 49.Miles A., Meslow E., Effects of experimental overgrowth on survival and change in the turf assemblage of a giant kelp forest. J. Exp. Mar. Biol. Ecol. 135, 229–242 (1990). [Google Scholar]
- 50.Ebert T. A., Demographic patterns of the purple sea urchin Strongylocentrotus purpuratus along a latitudinal gradient, 1985–1987. Mar. Ecol. Prog. Ser. 406, 105–120 (2010). [Google Scholar]
- 51.Bestelmeyer B. T., et al., Analysis of abrupt transitions in ecological systems. Ecosphere 2, art129 (2011). [Google Scholar]
- 52.Hughes B. B., et al., Long-term studies contribute disproportionately to ecology and policy. BioScience 67, 271–281 (2017). [Google Scholar]
- 53.Francis T. B., et al., Management implications of long transients in ecological systems. Nat. Ecol. Evol. 5, 285–294 (2021). [DOI] [PubMed] [Google Scholar]
- 54.MacArthur R. H., MacArthur J. W., On bird species diversity. Ecology 42, 594–598 (1961). [Google Scholar]
- 55.R. M. May, Stability and Complexity in Model Ecosystems (Princeton University Press, 1974), vol. 1. [Google Scholar]
- 56.Dayton P. K., Ecology of kelp communities. Annu. Rev. Ecol. Syst. 16, 215–245 (1985). [Google Scholar]
- 57.Bodkin J. L., Effects of kelp forest removal on associated fish assemblages in central California. J. Exp. Mar. Biol. Ecol. 117, 227–238 (1988). [Google Scholar]
- 58.Scheibling R., Increased macroalgal abundance following mass mortalities of sea urchins (Strongylocentrotus droebachiensis) along the Atlantic coast of Nova Scotia. Oecologia 68, 186–198 (1986). [DOI] [PubMed] [Google Scholar]
- 59.Hagen N. T., Destructive grazing of kelp beds by sea urchins in Vestfjorden, northern Norway. Sarsia 68, 177–190 (1983). [Google Scholar]
- 60.Steneck R. S., Leland A., McNaught D. C., Vavrinec J., Ecosystem flips, locks, and feedbacks: The lasting effects of fisheries on Maine’s kelp forest ecosystem. Bull. Mar. Sci. 89, 31–55 (2013). [Google Scholar]
- 61.Watson J., Estes J. A., Stability, resilience, and phase shifts in rocky subtidal communities along the west coast of Vancouver Island, Canada. Ecol. Monogr. 81, 215–239 (2011). [Google Scholar]
- 62.Steneck R. S., et al., Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459 (2002). [Google Scholar]
- 63.Morris R. L., et al., Key principles for managing recovery of kelp forests through restoration. Bioscience 70, 688–698 (2020). [Google Scholar]
- 64.Watanuki A., et al., Restoration of kelp beds on an urchin barren: Removal of sea urchins by citizen divers in southwestern Hokkaido. Bull. Fish. Res. Agen. 32, 83–87 (2010). [Google Scholar]
- 65.Gleason M., Zimmerman J., Ray J., Flores R., Contolini G., A Structured Approach for Kelp Restoration and Management Decisions in California (The Nature Conservancy, Arlington, VA, 2021). [Google Scholar]
- 66.Hilderbrand R. H., Watts A. C., Randle A. M., The myths of restoration ecology. Ecol. Soc. 10, 19 (2005). [Google Scholar]
- 67.Graham M. H., “Planktonic patterns and processes in the giant kelp Macrocystis pyrifera,” PhD thesis, University of California, San Diego/Scripps Institution of Oceanography, La Jolla, CA (2000).
- 68.Dixon P., Vegan, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930 (2003). [Google Scholar]
- 69.Core Team R, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2017). [Google Scholar]
- 70.McCune B., Mefford M., PC-ORD: Multivariate Analysis of Ecological Data. Version 7 (MJ Software Design, Gleneden Beach, OR, 2016). [Google Scholar]
- 71.Wickham H., ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, New York, NY, 2016). [Google Scholar]
- 72.Scrucca L., Fop M., Murphy T. B., Raftery A. E., mclust 5: Clustering, classification and density estimation using Gaussian finite mixture models. R J. 8, 289–317 (2016). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Time series and linear relief measurements of substrate complexity, code, and data have been deposited in GitHub (https://github.com/zhrandell/SubstrateComplexity). A project data release by the US Geological Survey is accessible on ScienceBase at https://doi.org/10.5066/P9Q6B625.