Significance
Since their discovery over 30 y ago, it has become clear that the superantigens (SAgs) are important virulence factors produced during severe Staphylococcus aureus–mediated disease including bacteremia. However, until the current study, it was unclear how these toxins manipulated the immune system to promote infection. Here, we have demonstrated that the SAgs can target a critical immune signaling molecule (interferon gamma), inducing overproduction that promotes bacterial survival by subverting the ability of macrophages to be able to kill the pathogen. This highlights SAg activity as a critical target for antistaphylococcal therapy to mitigate the impact of severe S. aureus disease.
Keywords: Staphylococcus aureus, superantigen, interferon gamma, macrophage
Abstract
Staphylococcus aureus is a foremost bacterial pathogen responsible for a vast array of human diseases. Staphylococcal superantigens (SAgs) constitute a family of exotoxins from S. aureus that bind directly to major histocompatibility complex (MHC) class II and T cell receptors to drive extensive T cell activation and cytokine release. Although these toxins have been implicated in serious disease, including toxic shock syndrome, the specific pathological mechanisms remain unclear. Herein, we aimed to elucidate how SAgs contribute to pathogenesis during bloodstream infections and utilized transgenic mice encoding human MHC class II to render mice susceptible to SAg activity. We demonstrate that SAgs contribute to S. aureus bacteremia by massively increasing bacterial burden in the liver, and this was mediated by CD4+ T cells that produced interferon gamma (IFN-γ) to high levels in a SAg-dependent manner. Bacterial burdens were reduced by blocking IFN-γ, phenocopying SAg-deletion mutant strains, and inhibiting a proinflammatory response. Infection kinetics and flow cytometry analyses suggested that this was a macrophage-driven mechanism, which was confirmed through macrophage-depletion experiments. Experiments in human cells demonstrated that excessive IFN-γ allowed S. aureus to replicate efficiently within macrophages. This indicates that SAgs promote bacterial survival by manipulating the immune response to inhibit effective clearing of S. aureus. Altogether, this work implicates SAg toxins as critical therapeutic targets for preventing persistent or severe S. aureus disease.
Staphylococcus aureus is an important bacterial pathogen that can exist as a harmless commensal. Yet once primary barriers have been breached, this pathobiont also has the propensity to cause an extraordinary range of superficial, invasive, and toxin-mediated diseases (1). This spectrum of disease can range from relatively simple soft-tissue infection to pneumonia or bacteremia that may lead to life-threatening sepsis (1, 2). S. aureus is one of the most common causes of sepsis and carries a high mortality rate of 20 to 40% that can double in the context of septic shock (2, 3). Widespread drug resistance, including both hospital- and community-associated methicillin-resistant S. aureus (MRSA), has further exacerbated treatment challenges with this important pathogen (4).
Key to the success of S. aureus as a pathogen is a vast array of virulence factors encoded both within the chromosome and on mobile genetic elements. These factors fall into several functional classes including adhesion factors (e.g., fibronectin-binding proteins A and B [FnbpA and FnbpB]) (5, 6), immunomodulatory proteins (e.g., staphylococcal protein A [Spa] or chemotaxis inhibitor protein of Staphylococcus [CHIPS]) (7), cytolytic toxins (e.g., alpha-hemolysin [Hla]) (8, 9), and superantigens (SAgs). The SAg family in S. aureus consists of at least 26 different paralogues that function by cross-linking major histocompatibility complex (MHC) class II molecules with the variable region of the T cell receptor (TCR) β-chain (Vβ); the unconventional interaction with these two key immune receptors occurs irrespective of the cognate antigen and results in the aberrant and widespread activation of T cells followed by proinflammatory cytokine release (10).
SAgs are the etiological agent of toxic shock syndrome, in which T cell activation caused by SAgs released from S. aureus triggers a systemic “cytokine storm” that can lead to hypotension and multiple organ failure and, in some cases, death. SAg activity has also been implicated in a number of other serious diseases including endocarditis, pneumonia, and bacteremia (11). Historically, it has been difficult to model the biological functions of SAg activity in vivo, as conventional murine strains are highly insensitive to these toxins. As a result, much of the pathogenesis work related to SAgs has been performed in rabbits, which are inherently sensitive to SAg activity (12–14). However, we have demonstrated that transgenic mice expressing the human leukocyte antigen (HLA)-DR4 are significantly more sensitive to SAg activity (15, 16). This allowed us to determine that staphylococcal SAgs are important during bloodstream infections leading to increased bacterial burdens within the liver (15).
In the current study, we deployed targeted antibody depletion protocols that demonstrated, during bloodstream infection, that SAgs target CD4+ T cells to produce pathogenic levels of the key cytokine interferon gamma (IFN-γ). IFN-γ promoted enhanced disease severity and bacterial burden in the liver, and excess IFN-γ levels during infection appeared to perturb liver macrophage activity to promote the survival of S. aureus.
Results
Transgenic HLA-DR4 C57BL/6 Mice Are Sensitive to SAgs Staphylococcal Enterotoxin B and Staphylococcal Enterotoxin C Activity and Can Be Used to Model S. aureus Bacteremia.
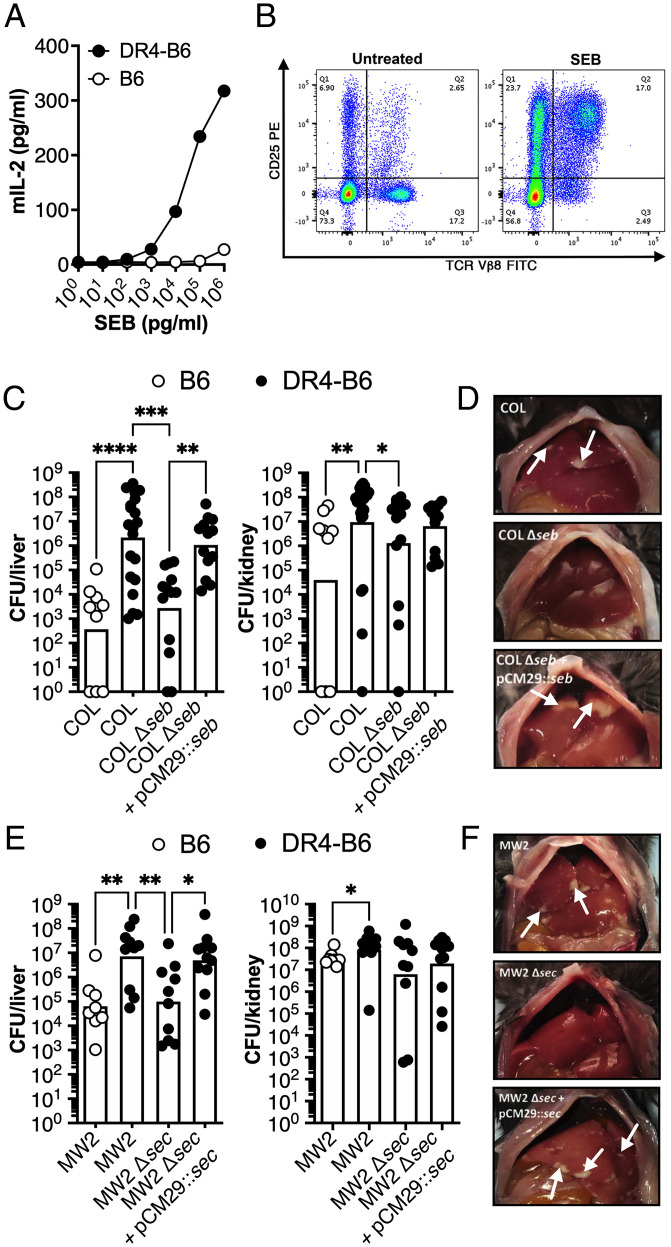
Previously, we demonstrated that S. aureus burden is promoted during murine bloodstream infections by the SAgs staphylococcal enterotoxin A (SEA) and staphylococcal enterotoxin-like W (SElW); however, the mechanism remained uncharacterized (15, 17). In the current study, we first utilized strain COL, a well-studied MRSA isolate from clonal complex (CC) 8 that produces the SAg staphylococcal enterotoxin B (SEB) (18). Splenocyte analysis from C57BL/6 (B6) or transgenic HLA-DR4 C57BL/6 (DR4-B6) animals identified that T cell activation (measured by the production of IL-2) to titrating doses of SEB was orders of magnitude more active on spleen cells from the DR4-B6 animals compared to conventional B6 mice (Fig. 1A). In addition, analysis of stimulated splenocytes using flow cytometry analysis demonstrated a massive expansion of Vβ8+ T cells, the major T cell targets of SEB in mice (19) (Fig. 1B). These cells represent ∼20% of the T cell repertoire in the DR4-B6 animals, and the majority of these were activated by SEB as measured by the up-regulation of CD25 (Fig. 1B). Together, these data demonstrate that, compared to conventional B6 mice, DR4-B6 mice are highly sensitive to SEB activity and can be used for the analysis of SEB activity in vivo.
Fig. 1.
SAgs SEB and SEC promote pathogenesis of S. aureus bacteremia in transgenic HLA-DR4 B6 animals. (A) IL-2 production of isolated splenocytes from conventional B6 (open dots) and transgenic DR4-B6 (solid dots) mice following stimulation with a titration of SEB protein. Data shown are from a representative experiment. (B) Activation of Vβ8+ T cells in DR4-B6 mice stimulated by SEB compared to an untreated control as determined by CD25 expression (quarter values represent total cell population). (C–F) B6 and DR4-B6 animals were inoculated i.v. with 5 × 106 CFUs and then killed at 96 hpi for S. aureus COL (C and D) and 72 hpi for S. aureus MW2 (E and F). Liver and kidney bacterial burden (C and E) was assessed in conventional B6 mice (open dots) or in transgenic DR4-B6 mice (solid dots). Each dot represents an individual mouse, and the bar indicates the geometric mean. Significant differences were determined using the Kruskal–Wallis test with the uncorrected Dunn’s test for multiple comparisons (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Representative livers from the infected mice from S. aureus COL and mutants (D) and S. aureus MW2 and mutants (F) with white arrows indicate the presence of liver lesions.
To determine if SEB contributed to pathogenicity in murine bacteremia, we infected B6 and DR4-B6 animals with S. aureus COL via tail-vein injection. We found that wild-type S. aureus COL was significantly more virulent in DR4-B6 mice with a much higher bacterial burden found in the liver and kidneys when compared to the B6 background (Fig. 1C). This was due to SEB activity, as the bacterial burden of the SEB-null mutant (COL Δseb) essentially phenocopied the data obtained from the B6 animals. Importantly, this phenotype could be complemented with COL Δseb containing pCM29::seb (Fig. 1 C and D). These data clearly demonstrate that SEB contributes to the pathogenicity of S. aureus COL during bloodstream infection.
To determine if additional SAgs other than SEB could also contribute to bacteremia, we expanded our analysis to include S. aureus MW2, a CC1 MRSA isolate that produces staphylococcal enterotoxin C (SEC) (20, 21). SEC is phylogenetically similar to SEB and has a similar Vβ activation profile in humans (10, 21). We successfully deleted the SEC gene in S. aureus MW2 and were able to complement the gene in trans (SI Appendix, Fig. S1). Like S. aureus COL, we found a significant increase in bacterial burden in the liver and kidneys in DR4-B6 animals compared to the B6 mice when infected with MW2 (Fig. 1E). Furthermore, deletion of sec from MW2 resulted in a significant reduction in bacterial burden and liver pathology (Fig. 1 E and F). These data indicate that both SEB and SEC produced from different S. aureus backgrounds can contribute to the pathogenesis of experimental bloodstream infection and that SAg-sensitive DR4-B6 mice are suitable for modeling SAg activity in the context of live S. aureus infection.
Depletion of CD4+ T Cells Results in Reduced Bacterial Burden in the Liver of S. aureus–Infected DR4-B6 Mice.
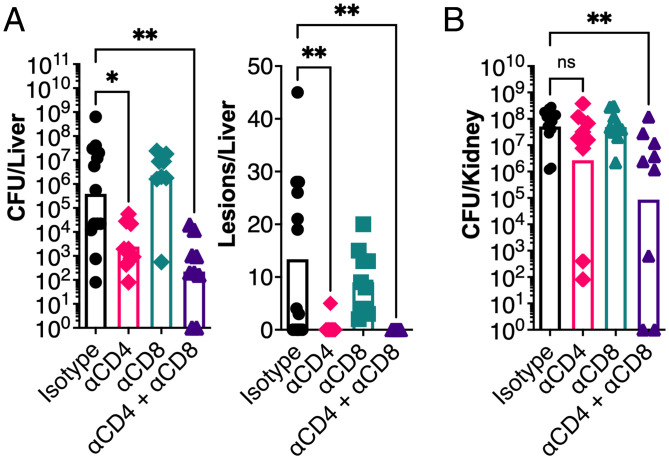
It has been well established that SAgs can target and activate different T cell subsets that express the appropriate TCR Vβ (10). For efficient nasopharyngeal infection, Streptococcus pyogenes required the expression of the SAg streptococcal pyrogenic exotoxin A (SpeA) (22), and depletion of T cells resulted in a markedly reduced bacterial burden, which phenocopied the deletion of the speA gene (23). In the current study, we used this T cell depletion strategy and applied it to our model of S. aureus bacteremia. We found that when CD4+ T cells were depleted, bacterial burden in the liver was significantly reduced, with a near-complete reduction of visible lesions, while depletion of CD8+ T cells had no impact (Fig. 2A). To reduce bacterial burden in the kidneys, depletion of CD4+ T cells was not sufficient and required the combined depletion of CD4+ and CD8+ T cells to observe a phenotype, suggesting a role for CD8+ cells in this organ (Fig. 2B). To determine if this effect was limited to conventional CD4+ T cells, we also depleted natural killer (NK) and invariant NKT cells using an anti-NK1.1 targeting antibody, according to a previously established protocol (24). In this case, there was no impact on bacterial burden or animal morbidity, indicating these cells likely do not play a role in this infection model (SI Appendix, Fig. S2). Altogether, these data indicate that bacterial burden in the liver during S. aureus bacteremia is promoted by the activity of conventional CD4+ T cells, likely due to activation by SEB.
Fig. 2.
CD4+ T cells promote bacterial burden during S. aureus bloodstream infection. In vivo T cell depletion in DR4-B6 mice was performed with monoclonal antibodies to deplete CD4+ (clone GK1.5) and/or CD8+ (clone YTS169.4) cells prior to i.v. infection with S. aureus COL. (A and B) In vivo liver bacterial burdens and pathology (A) and kidney bacterial burdens (B) were assessed 96 hpi. Each data point represents an individual mouse, and the bar indicates the geometric mean for CFUs/organ and the median for lesions/organ. Significant differences were determined using the Kruskal–Wallis test with the uncorrected Dunn’s test for multiple comparisons (*P < 0.05, **P < 0.01). ns, not significant.
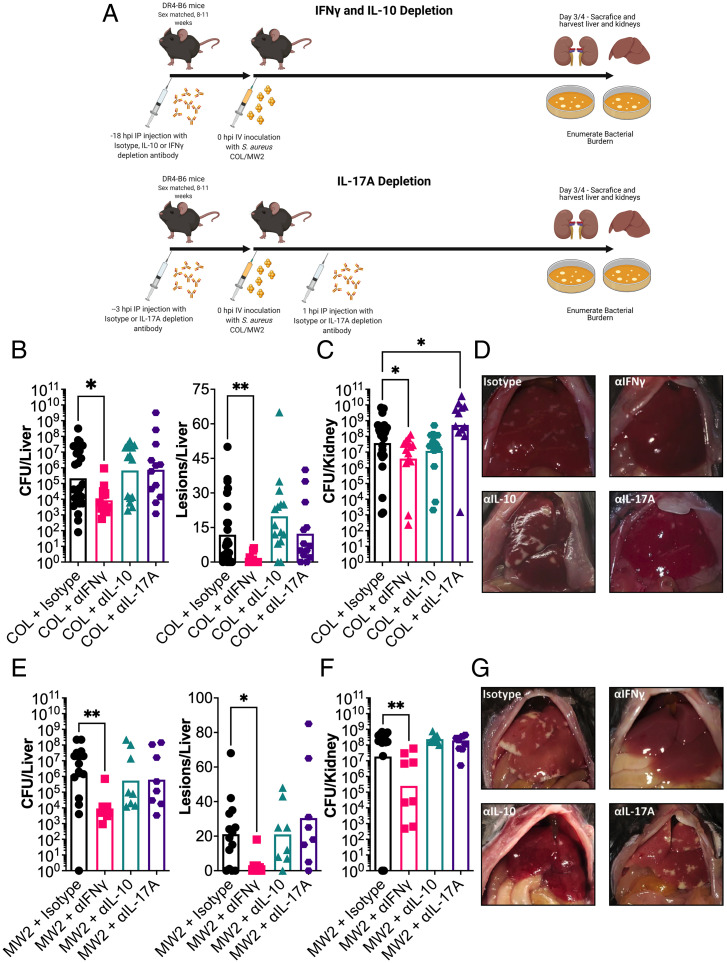
Blocking of IFN-γ Activity during Systemic S. aureus Infection Results in Reduced Disease Severity and Bacterial Burden.
Previous analyses have demonstrated that CD4+ T cells can be targeted by staphylococcal SAgs to result in the release of numerous cytokines including the key cytokines IFN-γ (also known as type II interferon), interleukin-10 (IL-10), and interleukin-17A (IL-17A) (10). These cytokines have antagonistic activity toward each other (25, 26) and, in the case of IL-17A and IFN-γ, have been shown to contribute to SEB-mediated morbidity during toxemia models in HLA-transgenic mice (27, 28). Together, this suggests that these key cytokines may contribute to SAg-mediated pathogenesis. To test this hypothesis, we used antibody depletion to block cytokine activity during bloodstream infection by both S. aureus COL and MW2 (Fig. 3A). We found that blocking IFN-γ resulted in a significant reduction in bacterial liver burden and liver pathology that phenocopied the deletion of seb or sec in S. aureus COL and MW2, respectively (Fig. 3 B, D, E, and G). However, depletion of either IL-10 or IL-17A had limited impact on the liver burden, suggesting that these cytokines do not promote bacterial burden in this model. Depletion of IFN-γ also resulted in lower bacterial burden in the kidneys, suggesting that the blocking of this cytokine reduced the overall severity of the infection (Fig. 3 C and F). It was also noted that bacterial burden in the kidneys increased once IL-17A was depleted but only for S. aureus COL. This suggests that IL-17A contributes to protection against kidney damage during bloodstream infection by S. aureus in the HLA-transgenic mouse model. Overall, these data indicate that, of the three cytokines tested, only IFN-γ promoted S. aureus burden during a bloodstream infection.
Fig. 3.
IFN-γ promotes liver abscess formation and bacterial burden during S. aureus bacteremia in DR4-B6 mice. (A) Schematic outlining in vivo cytokine depletion with monoclonal antibodies prior to i.v. infection of DR4-B6 mice. (B, C, E, and F) Bacterial burden and abscess formation in liver (B and E) and kidneys (C and F) at 96 hpi for S. aureus COL (B and C) or 72 hpi for S. aureus MW2 (E and F). Each dot represents an individual mouse, and the bar represents the geometric mean for CFUs/organ and the median for lesions/organ. Significant differences were determined using the Kruskal–Wallis test with the uncorrected Dunn’s test for multiple comparisons (*P < 0.05, **P < 0.01). (D and G) Representative livers are shown from the cytokine-treated mice from S. aureus COL (D) and S. aureus MW2 (G).
SAgs Drive Pathogenic Production of IFN-γ during S. aureus Bloodstream Infection.
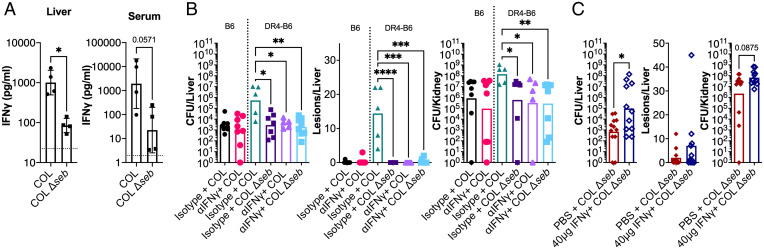
Several approaches were taken to determine if the promotion of bacterial burden by IFN-γ was mediated by the SAg toxins. First, we performed cytokine analysis on liver homogenates and serum from animals infected with the wild type or the SAg-deletion mutants at 24 h postinfection (hpi). Compared to wild-type S. aureus COL–infected mice, IFN-γ levels were ∼10-fold lower in livers from animals infected with S. aureus COL Δseb (Fig. 4A). There was also a clear trend for more IFN-γ in the serum for animals that were infected with the wild type, although this did not reach statistical significance (Fig. 4A).
Fig. 4.
SAgs promote pathogenic production of IFN-γ that supports bacterial burden. (A) DR4-B6 mice were inoculated i.v. with wild-type S. aureus COL or the COL Δseb deletion strain. At 24 hpi, animals were killed, livers and blood were harvested, and material was prepared for IFN-γ analysis. Each dot represents an individual mouse, the bar indicates the geometric mean, and error bars indicate the SD. The dotted line indicates the levels detected in an uninfected animal. (B) B6 and DR4-B6 mice were treated 18 h prior to infection with 250 µg isotype control or αIFN-γ antibody administered by i.p. injection and animals were infected i.v. with wild-type S. aureus COL or COL Δseb. (C) DR4-B6 animals were treated with 20 µg (40 µg total) recombinant murine IFN-γ or vehicle control (100 µL PBS) i.p. 2 h before and 1 h after i.v. infection with S. aureus COL Δseb. In both experiments (B and C), in vivo bacterial burden was assessed after 96 h in liver and kidneys and an assessment of gross pathological liver lesions was also performed. Each dot represents an individual mouse, and the bar indicates the geometric mean for CFUs/organ and the median for lesions/organ. Significant differences were determined using the Mann–Whitney U test or Kruskal–Wallis test with the uncorrected Dunn’s test for multiple comparisons (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Following from the cytokine analysis, we modified our infection model to characterize IFN-γ depletion under circumstances in which the SEB SAg from S. aureus COL was either absent or unable to function. In the SAg-insensitive B6 background, bacterial recovery from infected mice was comparatively low regardless of IFN-γ–depletion treatment, suggesting that this phenotype could only be observed in a SAg-sensitive environment (Fig. 4B). Indeed, when we repeated the IFN-γ depletion in the DR4-B6 background and included the COL Δseb construct, bacteria recovered from organs of the isotype or IFN-γ–depleted groups were similarly low, whereas wild-type infections treated with the isotype control antibody produced visible liver lesions and higher bacterial counts in both the liver and kidneys (Fig. 4B). These data demonstrate that a functioning seb gene is required to promote pathogenic IFN-γ activity.
Finally, to establish the link between SEB and IFN-γ, we aimed to determine if the addition of exogeneous IFN-γ could functionally complement the deletion of seb in S. aureus COL. In this experiment, animals were administered two 20-µg treatments of recombinant IFN-γ 2 h prior to infection and 1 h after. Treatment with exogenous IFN-γ resulted in a ∼2-log increase in bacterial burden in the liver when compared to the vehicle control (Fig. 4C). Curiously, very few lesions formed on the surface of the liver with this approach, suggesting that sustained SAg/IFN-γ activity is required for pathology to become evident (Fig. 4C). This demonstrates that the stimulation of pathogenic IFN-γ is a key function of SEB during bloodstream infection and, taken together with the previous data, suggests that a functional SAg must be present to elicit pathogenic production of IFN-γ.
IFN-γ Promotes Early Bacterial Survival during S. aureus Infection.
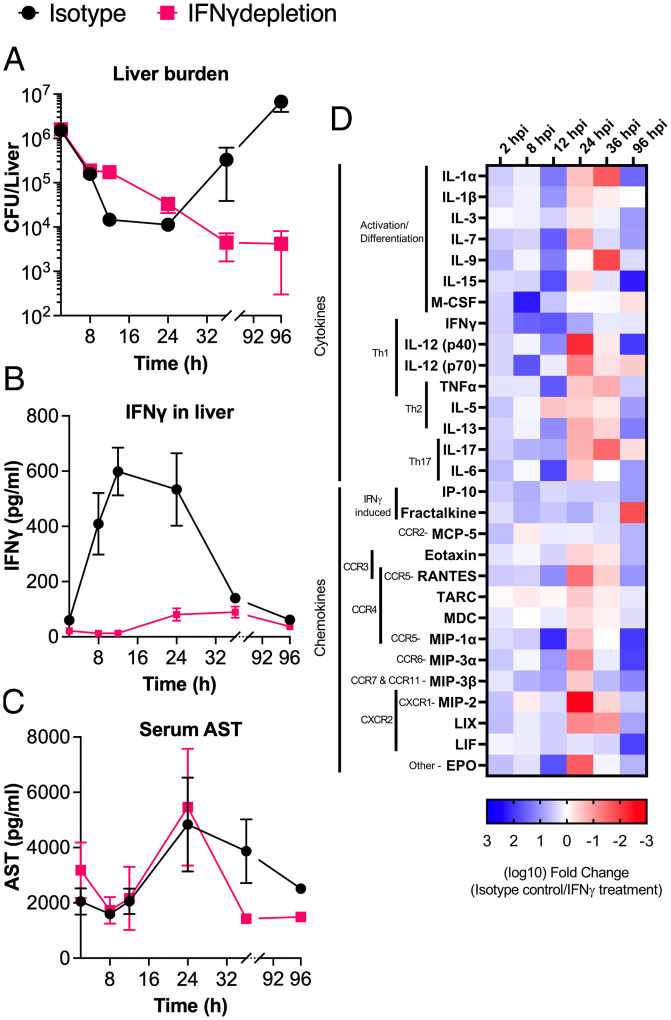
With it established that SAgs could drive the production of pathogenic levels of IFN-γ, we next wanted to determine when IFN-γ had the most impact during the disease course. Therefore, we determined bacterial burden at shorter time points (i.e., 2 to 36 hpi) in animals treated with αIFN-γ antibodies (monoclonal antibodies specific for IFN-γ) or an isotype control antibody (Fig. 5A). From these experiments, we determined that much of the infectious dose became trapped within the liver following tail-vein injection with ∼2 × 106 colony-forming units (CFU) at 2 hpi (∼40% of the dose), and this was followed by rapid clearance between 2 and 8 hpi in both groups. At 12 hpi, the rate of bacterial clearance was reduced in the αIFN-γ–treated animals but continued steadily with almost complete clearance of the bacteria by 96 hpi. Conversely, after 24 hpi in the livers of isotype antibody–treated mice, bacterial burdens rapidly expanded, reaching a level ∼3-logs higher by 96 hpi (Fig. 5A). We performed a repeat of this analysis with daily time points and were able to confirm the trajectories that were observed in the shorter time course (SI Appendix, Fig. S3). Together, these data indicate that IFN-γ produced during infection by wild-type S. aureus is contributing to the ability of the bacteria to avoid clearance by the immune system in the liver during the early stages of bloodstream infection.
Fig. 5.
SAg-induced IFN-γ promotes a proinflammatory environment that allows S. aureus to impede clearance during bloodstream infection. DR4-B6 mice were treated with isotype antibody or IFN-γ–depleting antibodies 18 h prior to infection with S. aureus COL. Following infection, three to four animals were killed from each group at the six time points shown, and liver and blood were harvested from each animal. (A) Bacterial burden was determined at each time point and is shown as mean CFU/liver ± SEM. (B) Liver homogenate was analyzed by multiplex cytokine array, and mean IFN-γ (pg/mL) ± SEM at each time point were determined for each time point. (C) Serum was analyzed by ELISA to determine the concentration of AST. Data shown are mean AST (pg/mL) ± SEM. (D) Multiplex cytokine/chemokine array analysis was conducted on liver homogenate recovered from each animal killed during the time course. Data shown represent the log10 fold change for each cytokine between isotype antibody– and IFN-γ–depleted groups that displayed significant differences by Student’s t test. Prior to comparison, data were normalized to an antibody-treated, uninfected animal killed at the same time point as their comparator. Blue color (i.e., positive values) indicates that more cytokine/chemokine is produced in the isotype antibody infection, and red color (i.e., negative values) indicates that more cytokine/chemokine is produced in the IFN-γ–depleted infection. Broad classification for each cytokine is indicated as well as potential binding partners for chemokines.
Proinflammatory Signaling Is Delayed and Less Intense When IFN-γ Is Blocked during Sepsis.
IFN-γ is a pleiotropic cytokine of the immune system, and its depletion during infection could impact numerous downstream signaling pathways during the response to S. aureus bloodstream infection. In isotype antibody–treated mice, the highest level of IFN-γ was observed in the liver between 12 and 24 hpi, in excess of 500 pg/mL (Fig. 5B). Additionally, we confirmed that αIFN-γ antibodies were able to reduce IFN-γ concentration during infection up to 36 hpi (Fig. 5B). We also analyzed serum samples for aspartate aminotransferase (AST) levels as a proxy for liver damage. These data indicate that there was limited change in liver damage during infection irrespective of IFN-γ levels; however, there did appear to be a faster drop in AST levels in the IFN-γ–depleted groups in the later time points (Fig. 5C), congruent with the reduced bacterial burden (Fig. 5A).
To gain a broader understanding of the cytokine and chemokine dynamics, liver homogenates were analyzed by multiplex cytokine array over the course of the experiment. This demonstrated that in earlier time points (2 to 12 hpi), many signaling molecules associated with inflammation were up-regulated during infections in which IFN-γ was produced at high levels (Fig. 5D). Strikingly, between 24 and 36 hpi, many cytokines and chemokines became reduced relative to the group treated with αIFN-γ antibodies, which directly correlated with the expansion of S. aureus (Fig. 5A) and was subsequently reversed again by 96 hpi (Fig. 5D). This suggests that an inflammatory environment favorable to bacterial proliferation is sustained for a longer period during infections in which IFN-γ production is relatively high. Altogether, we infer that pathogenic production of IFN-γ results in the rapid production of a proinflammatory environment in the liver that contributes to S. aureus survival during bloodstream infection.
Macrophage Activity in the Liver Is Subverted by SAg-Elicited IFN-γ Production.
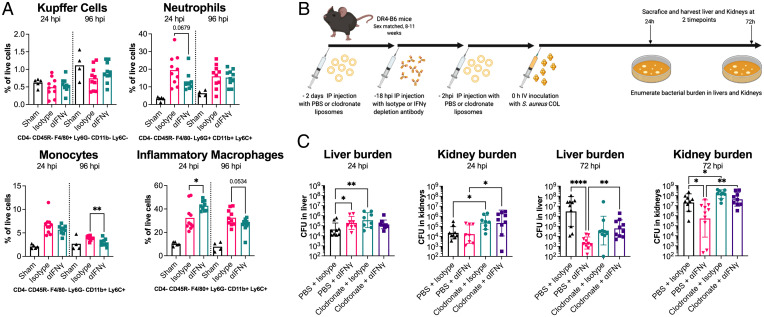
Immune cells, such as macrophages and neutrophils, are critical for clearance of S. aureus during infection (29, 30). The cytokine and chemokine analysis indicated that wild-type S. aureus infection in HLA-DR4 mice drives an IFN-γ–dependent proinflammatory signaling cascade in the livers of animals (Fig. 5D). To determine if this response had any impact on the phagocytic cell populations in the liver, we first phenotyped immune cells isolated from this organ using flow cytometry at 24 and 96 hpi infection with S. aureus COL (SI Appendix, Fig. S4 provides the gating strategy). We found few differences between the IFN-γ–depleted or control mice in terms of the resident macrophages (Kupffer cells) (Fig. 6A). For both monocytes and neutrophils, there were trends toward a higher percentage of these cells at 24 hpi in the isotype antibody–treated group; however, neither reached significance (Fig. 6A). For neutrophils, this had subsided to a similar level in both groups by 96 hpi, whereas monocytes had decreased in both groups by 96 hpi, but there were significantly fewer in the IFN-γ–depleted group at this time. We did detect significantly higher inflammatory macrophages in the IFN-γ–depleted mice at 24 hpi, although these were equivalent by 96 hpi (Fig. 6A).
Fig. 6.
Liver macrophages are the target of pathogenic IFN-γ production. (A) Flow cytometry–based phenotyping of immune cells isolated from livers of mice infected with S. aureus strain COL at 24 and 96 hpi. Control animals (sham) were treated with HBSS only. Cells were defined based on the staining profile listed below each graph and normalized to the percentage of live cells. Isotype antibody and αIFN-γ treatments were compared; each dot represents an individual mouse, and the bar indicates the mean. Significant differences were determined using an unpaired Welch’s t test (*P < 0.05, **P < 0.01). (B) Schematic outlining Clodronate liposome-based depletion of macrophages along with IFN-γ depletion used prior to i.v. infection of mice with S. aureus COL. (C) Bacterial burden in liver and kidneys at 24 and 72 hpi is shown. Each dot represents an individual mouse, and the bar represents the geometric mean for CFUs/organ. Significant differences were determined using the Kruskal–Wallis test with the uncorrected Dunn’s test for multiple comparisons (*P < 0.05, **P < 0.01, ****P < 0.0001).
The infection kinetics (Fig. 5A) and flow cytometry (Fig. 6A) analyses suggest that the presence of high levels of IFN-γ could impact the activity, recruitment, or differentiation of phagocytes, most likely inflammatory macrophages. To determine if these cells are the target of pathogenic IFN-γ production, we depleted macrophages in mice using clodronate containing liposomes (Clodrosome) and then performed S. aureus COL bloodstream infections with or without IFN-γ–blocking antibodies (Fig. 6B). Macrophage depletion had a negative impact on animal welfare, so end points were brought forward from 96 hpi to 72 hpi and bacterial burden was determined in both the kidneys and the liver. In the liver, we again observed, at 24 hpi in animals treated with the control liposomes, that IFN-γ depletion resulted in a higher bacterial burden (Fig. 6C). However, when we compared macrophage-depleted animals, the phenotype observed between the IFN-γ depletion and isotype antibody groups was abolished, indicating that macrophages are likely required for the IFN-γ–dependent phenotype. We also observed an increase in bacterial burden when macrophages were depleted, compared to animals treated with control liposomes and isotype antibody (Fig. 6C), indicating that these cells were important to restrict bacterial growth in the liver at this time point. In the kidneys, there was evidence that the depletion of macrophages likely resulted in greater bacterial “seeding” to this organ, although there was no difference due to IFN-γ depletion (Fig. 6C). The data from the 72-hpi time point confirmed the observation that macrophages were necessary for the IFN-γ phenotype, as again, we were able to observe a clear IFN-γ phenotype in both the kidneys and liver, yet this phenotype was mitigated by macrophage depletion (Fig. 6C). Together, these data demonstrate that the promotion of S. aureus burden by IFN-γ during bloodstream infection is mediated by macrophages.
High Levels of IFN-γ Allow for Increased Intracellular Replication of S. aureus inside Human Macrophages.
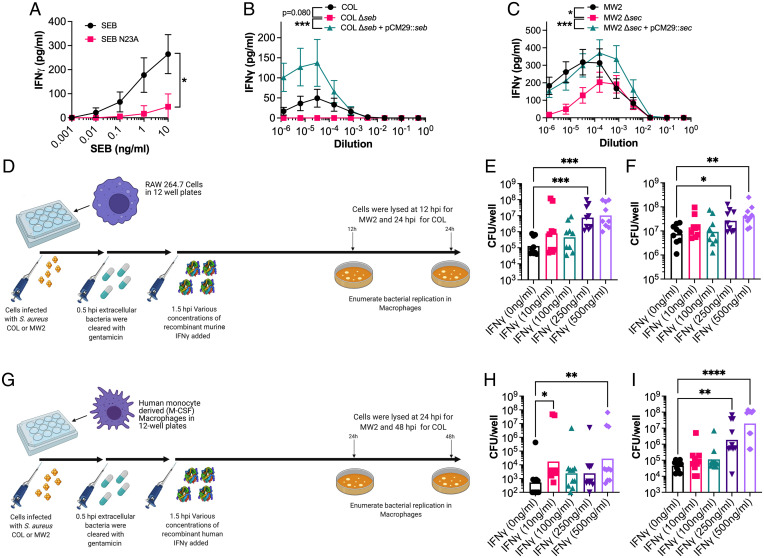
To determine if pathogenic IFN-γ had any impact in the human system, white blood cells from healthy human donors were analyzed for their responses to SAgs and IFN-γ. First, we wanted to confirm that SAgs can elicit IFN-γ from T cells through the engagement of the TCR. To do this, we compared IFN-γ production elicited by recombinant SEB protein compared to the site-directed mutant SEB-N23A. This mutant features a mutation within the TCR binding pocket resulting in a much lower ability to engage the TCR of its target cells (31). As expected, SEB-N23A elicited significantly lower IFN-γ from human peripheral blood mononuclear cells (PBMCs) compared with wild-type SEB (Fig. 7A). This confirmed that to elicit IFN-γ from human PBMCs, SEB must engage and activate the T cell through binding the TCR.
Fig. 7.
SEB and SEC elicit IFN-γ from human cells, and excessive concentration can promote increased extracellular replication of S. aureus in macrophages. (A) IFN-γ production by PBMCs from human blood following stimulation with a titration of SEB or the SEB-N23A proteins. (B and C) IFN-γ production by PBMCs from human blood following stimulation with a titration of supernatant from S. aureus COL (B) or MW2 (C) strains as indicated. Supernatants were taken from cultures grown for 8 h in BHI prior to use in these assays. Data shown (A–C) are mean ± SEM from eight donors. Significant differences were determined from the area under each curve using a paired Friedman test for multiple comparisons (*P < 0.05, ***P < 0.001). (D) Schematic outlining the procedure for intracellular S. aureus replication in RAW 264.7 murine macrophages after dosing with recombinant murine IFN-γ. (E and F) S. aureus recovered from RAW 264.7 cells after incubation at 24 h for strain COL (E) and 12 h for strain MW2 (F) with varying concentrations of recombinant IFN-γ. Each dot represents an individual experiment, and the bar represents the geometric mean for CFUs/well. (G) Schematic outlining the procedure for intracellular S. aureus replication in monocyte-derived human macrophages after dosing with recombinant human IFN-γ. (H and I) S. aureus recovered from human macrophages after incubation at 48 h for strain COL (H) and 24 h for strain MW2 (I) with varying concentrations of recombinant IFN-γ. Each dot represents macrophages from an individual human donor, and the bar represents the geometric mean for CFUs/well. Significant differences between 0 ng/mL of IFN-γ and other concentrations were determined using the Kruskal–Wallis test with the uncorrected Dunn’s test for multiple comparisons (*P < 0.05, **P < 0.01, ****P < 0.0001).
Next, we wanted to establish that our experimental strains (i.e., COL and MW2) could elicit IFN-γ production from human PBMCs. We stimulated human PBMCs with a titration of wild-type bacterial supernatants grown for 8 h in brain heart infusion (BHI) broth and included supernatants from the respective SAg-deletion and complemented strains. The data clearly indicated that both SEB and SEC, produced from COL and MW2, respectively, could drive IFN-γ production in human PBMCs (Fig. 7 B and C). The deletion of seb in COL eliminated the production of IFN-γ, while there was a significant decline in the potency of MW2 Δsec. The remaining IFN-γ production was still easily detectable at lower MW2 Δsec supernatant dilutions, suggesting that other SAgs encoded by MW2 (i.e., sea, selh, selk, sell, selq, selw, and selx) are also able to elicit the production of this cytokine.
As the murine model indicated that macrophages are likely the major target of SAg-induced IFN-γ, we infected both murine and human macrophages with S. aureus and dosed these cells with varying concentrations of recombinant IFN-γ (Fig. 7 D and G). With murine macrophages, we initially tested bone marrow–derived macrophages (BMDMs) from DR4-B6 animals but observed limited S. aureus replication in these cells (SI Appendix, Fig. S5). Therefore, we moved this analysis into RAW 264.7 cells (a murine macrophage cell line), which have been shown to be permissive to S. aureus replication (32). For both S. aureus COL and MW2, we saw an overall increase in intracellular bacterial replication when murine macrophages were dosed with high levels of IFN-γ, although this was most evident with S. aureus COL, which exhibited a ∼2-log increase as IFN-γ levels increased (Fig. 7 E and F). However, in human monocyte-derived macrophages, the IFN-γ phenotype was most evident with MW2, as this strain seemed to have an improved ability at replicating inside human macrophages and also exhibited a ∼2-log increase at the highest IFN-γ concentrations (Fig. 7 H and I). Notably, the high concentrations of IFN-γ did not impact macrophage viability, and the bacteria were not simply overgrowing dead macrophages (SI Appendix, Fig. S6). Together, these data indicate that SAg-induced, IFN-γ–mediated subversion of macrophages can occur in both the murine and human system and that this mechanism appears to impair the ability of macrophages to kill intracellular S. aureus.
Discussion
Bloodstream infections caused by S. aureus represent a significant challenge in the clinic, and SAgs have been demonstrated to be important in this disease (reviewed in refs. 2 and 11). However, it has remained unclear how SAgs promote S. aureus persistence during infection and, specifically, how these toxins manipulate the immune response (10). The weak activity of SAgs in murine models has been a serious challenge for our understanding as to how these toxins promote bacterial burden during infection. While rabbits are more sensitive to SAg activity and may be more physiologically appropriate, challenges include both cost and the availability of advanced immunological tools (33). Using the HLA-transgenic mouse model, we established that IFN-γ can be promoted to pathogenic levels by the SAgs SEB and SEC, showing how a key cytokine produced in response to SAg-mediated T cell activation dramatically promotes bacterial burden during a systemic infection. This research adds to a growing body of literature that explains how these toxins function to subvert different components of the immune system and challenges the waning dogma that these proteins are produced by the bacterium simply as an “immunological smoke screen” (reviewed in ref. 10).
The pathogenic potential of IFN-γ in S. aureus disease has been alluded to in other studies. Firstly, a model of wound infection indicated that S. aureus capsular polysaccharide was shown to elicit IFN-γ, which led to an increased recruitment of neutrophils (34). The authors postulated that higher neutrophil recruitment increased pathogenesis, as S. aureus was able to avoid neutrophil killing and survive intracellularly (34). Our cytokine/chemokine data are consistent with these findings (Fig. 5D), and while neutrophils were trending toward lower recruitment in the IFN-γ–depleted group at 24 hpi (Fig. 6A), it appeared to be monocytes and inflammatory macrophages, rather than neutrophils in the isotype antibody–treated group, that were recruited to the liver by 96 hpi (Fig. 6A). This variation in immune-cell recruitment could promote a niche for the bacteria to reside within in the liver. Furthermore, our data also indicated IFN-γ drove an increased production of the chemokine fractalkine (CX3CL1), which has been suggested to be able to skew liver macrophages toward a more suppressive state (35). Added to this, high levels of IFN-γ were able to induce apoptosis and affect the life cycle of hepatocytes, which can contribute to sustained inflammation in this organ (36). Together, these observations, both in this study and others, suggest that SAgs, through forcing the overproduction of IFN-γ, can modulate the liver environment to create a niche that is favorable for S. aureus survival.
The liver is an important organ during bacteremia, as circulating pathogens are frequently filtered and trapped by resident Kupffer cells (37). In addition, there have been several reports that demonstrate that S. aureus has evolved strategies to prevent this from occurring, including direct resistance to phagocytic killing by macrophages or through the release of Hla that can aggregate platelets to create ischemic areas in the liver and promote further bacterial growth (37, 38). It is important for the bacteria to establish in the liver, as bacteria surviving here can eventually seed other organs such as the kidneys (37, 39).
Given the pleotropic nature of IFN-γ, it is not surprising that several mechanisms may be at play in the liver to promote the growth of S. aureus. In addition to the other potential mechanisms of action defined in other studies, we have evidence that suggests a pathway in which macrophages are a clear target of pathogenic levels of IFN-γ. It has been suggested previously that macrophages may be influenced by the immune dysregulation caused by SAgs, as there were reports during the early stages of the menstrual toxic shock syndrome (mTSS) epidemic that found that macrophages from affected patients exhibited erythrophagocytosis (40). However, here we demonstrate that macrophage activity has been shown to be affected by high concentration of IFN-γ to support intracellular replication of S. aureus.
Our findings could also appear to be somewhat contradictory to several other studies that clearly demonstrate that IFN-γ contributes to the clearance of S. aureus during bloodstream infection (41, 42). Furthermore, the activity of memory CD4+ T cells supports the clearance of S. aureus by producing this cytokine along with other signals to coordinate this response (41). These studies were conducted in conventional mouse strains that are less vulnerable to the activity of staphylococcal SAgs and are more likely to represent what would occur in an immunocompetent individual with preexisting SAg-neutralizing antibodies. Indeed, this divergence is revealed by Brown et al., where mice previously exposed to S. aureus demonstrated an IFN-γ peak almost immediately after infection and subsequently dropped rapidly, whereas in mice that were not preexposed to S. aureus, IFN-γ was barely detected (41). This is contrary to what we have observed in SAg-mediated disease, as IFN-γ peaked later (24 hpi) and stayed high for much of the infection course. Altogether, this suggests that IFN-γ has a dual role during infection. Primarily, it is protective against S. aureus, but if manipulated to high and sustained levels, IFN-γ can act as a mediator to promote pathogenesis.
The implications of pathogenic IFN-γ production in human health are significant. As discussed, S. aureus is one of the most common causes of bloodstream infection, with disease often leading to life-threatening sepsis (2). Indeed, sepsis is a very serious concern in the clinic, contributing to nearly 20% of global annual deaths (43). One of the major challenges to treating sepsis is that without early intervention, this disease can rapidly move from a microbiologically mediated condition to an immunologically driven sequela, often resulting in antibiotic treatment being ineffective (3). The pathophysiology of sepsis has also proven to be highly complex, with many factors, including the invading pathogen, contributing to outcome. Death as an outcome of sepsis can occur both through acute inflammatory processes that lead to multiorgan failure as well as chronic immunosuppressive activity (44). In both cases, IFN-γ can contribute to these outcomes as a key promoter of the proinflammatory response or due to its absence leading to the dominance of immunosuppressive pathways (45, 46). Indeed, several studies have demonstrated that once a patient enters the immunosuppressive state of sepsis, therapy with IFN-γ may actually improve outcomes; however, the opposite may be true if administered too early (47, 48).
There is also evidence to suggest that this mechanism may be at play in the context of S. aureus vaccines and could be an important consideration for vaccine design. Karauzum et al. found that whole-cell vaccines in mice promoted disease and bacterial survival through the activity of a heavily skewed Th1 immune response (49). It appeared in this bloodstream infection model that disease was promoted by the vaccines and that this was mediated by excessive production of IFN-γ. It was also suggested that this study had significant parallels with the failure of a clinical trial using the IsdB subunit vaccine, which was intended to protect against bacteremia but instead had to be terminated early as it worsened patient outcome (49, 50). Altogether, this would suggest that S. aureus has evolved to take advantage of a human immune system whose responses have been skewed by the activity of IFN-γ and, further, evolved a family of toxins capable of driving this skewing itself.
In conclusion, we report the discovery that S. aureus SAgs can drive the production of IFN-γ during bloodstream infection to promote disease. Our analyses suggest that the pathogenic production of IFN-γ subverts macrophage activity, allowing the bacterium to persist within the liver and leading to increased morbidity. Furthermore, we were able to establish that this mechanism has implications for human health, as high levels of IFN-γ can promote bacterial intracellular replication in human macrophages. Altogether, this moves forward our understanding of the immunological factors at play during S. aureus–meditated sepsis in the context of pathogen-driven inflammation and may help inform the appropriate design of treatments and vaccines targeting S. aureus disease.
Materials and Methods
Human Ethics Statement.
Human venous blood was taken from healthy volunteer donors in accordance with human subject protocol 110859. The full study protocol was approved by the London Health Sciences Centre Research Ethics Board (University of Western Ontario, London, ON, Canada). Volunteers were recruited by a passive advertising campaign within the Department of Microbiology and Immunology at the University of Western Ontario, and following an outline of the risks, written informed consent was given by each volunteer before each sample was taken. Following sampling, blood was fully anonymized and no information regarding the identity of the donor, including sex and age, was retained.
Mice.
Male and female (8- to 11-wk-old) HLA-DR4-IE (DRB1*0401) humanized transgenic mice lacking endogenous mouse MHC-II on a C57BL/6 (B6) background (here referred to as DR4-B6 mice) (38), or B6 mice, were used for all in vivo infection experiments. DR4-B6 animals were bred on site at the University of Western Ontario, and B6 mice were purchased from the Jackson Laboratory (Stock No. 000664). Animals for experiments were housed in single-sex cages, which did not exceed four in number. During all breeding and experiments, mice were provided food and water ad libitum and appropriate enrichment was provided in all cages. All animal experiments were in accordance with the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals, and the animal protocol was approved by the Animal Use Subcommittee at the University of Western Ontario.
Bacterial Strains, Media, and Growth Conditions.
S. aureus strains listed in SI Appendix, Table S1 were grown aerobically at 37 °C in tryptic soy broth (TSB) (Difco) or BHI broth with shaking (250 rpm) supplemented with appropriate antibiotics. For solid-phase cultures, tryptic soy agar (TSA) was used (TSB containing 1.5% wt/vol agar) supplemented with the appropriate antibiotics. Escherichia coli strains were used as cloning hosts and were grown in Luria–Bertani (LB) broth (Difco) or LB agar supplemented with appropriate antibiotics at 37 °C with shaking (250 rpm). Growth curve analysis was performed using a Biotek Synergy H4 multimode plate reader.
Construction of MW2 Δsec Mutant.
Markerless deletion of sec in MW2 was performed using the pKOR1 allelic replacement system (51). The sec knockout was created as described previously (51), and candidate constructs were screened by PCR using primers SEC-screen-For and SEC-screen-Rev (SI Appendix, Table S2). See SI Appendix, SI Materials and Methods for more details.
Construction of pCM29::seb and pCM29::sec Complementation Plasmids.
SEB and SEC complementation plasmids for S. aureus SAg null mutants were created as previously described, with modifications (17). See SI Appendix, SI Materials and Methods for more details. After this step, ligations were transformed into E. coli SA30B (52) for appropriate methylation before transformation of sequence positive constructs into electrocompetent S. aureus using a protocol previously described (53).
Protein Expression and Purification.
Recombinant SEB was generated as described previously (54). Briefly, SEB was expressed with a His-tag in BL21 (DE3) E. coli and purified by nickel column chromatography. An attenuated mutant of SEB that has impaired binding to TCR was also purified. The mutant SEB carries an N→A point mutation at position 23 and is referred to as SEBN23A (31, 55). The experimental use of SEB was approved by the University of Western Ontario Biosafety Committee (application no. BIO-UWO-0155).
Murine Splenocyte Analysis.
The ability of murine cells to respond to SEB was determined using IL-2 production. Mouse spleens were removed and broken into a single-cell suspension, followed by red blood cell lysis in ammonium–chloride–potassium (ACK) buffer. The remaining cells were suspended in complete RPMI (cRPMI), containing RPMI (Invitrogen Life Technologies) supplemented with 10% fetal bovine serum (FBS) (Wisent Inc.), 100 μg/mL streptomycin, 100 U/mL penicillin (Gibco), 2 mM l-glutamine (Gibco), 1 mM sodium pyruvate (Gibco), 100 μM nonessential amino acids (Gibco), 25 mM Hepes (pH 7.2) (Gibco), and 2 μg/mL polymyxin B (Gibco). Cell suspensions were seeded into 96-well plates at a density of 1.1 × 106 cells/mL. Titrating concentrations of recombinant SEB were added to cells and incubated for 18 h at 37 °C with 5% CO2. Supernatants were assayed for IL-2 by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Thermo Fisher Scientific). For flow cytometry, cells were dual stained with phycoerythrin (PE)-conjugated anti-CD25 (clone PC61.5) (eBioscience) and fluorescein isothiocyanate (FITC)-conjugated anti-Vβ8 (clone KJ16) (eBioscience). Events were acquired using a FACSCanto II (BD Biosciences), and data were analyzed using FlowJo version 10.7.1 (TreeStar).
Staphylococcal Bacteremia Model.
Single bacterial colonies were picked from a TSA plate and grown in 3 mL TSB overnight (16 to 18 h). Cells were subsequently subcultured in TSB to an OD600 (optical density at 600 nm wavelength) of 0.1 and grown to postexponential phase (OD600 ∼3.0 to 3.5). The bacterial pellet was washed once and resuspended in Hank's balanced salt solution (HBSS) to an OD600 of 0.15 for strain COL and 0.1 for strain MW2, corresponding to ∼5 × 107 CFU/mL. Mice were injected via the tail vein with 5 × 106 CFU of S. aureus in a total volume of 100 μL. Mice were weighed and monitored daily. At various time points postinfection, mice were killed (maximum of 3 and 4 d for MW2 and COL, respectively), and the kidneys and liver were aseptically harvested. All organs were homogenized, plated on mannitol salt agar (Difco), and incubated at 37 °C overnight. S. aureus colonies were enumerated the following day with a limit of detection determined to be 3 CFU per 10 μL.
Antibody Depletion Protocols.
CD4+ and CD8+ T cells were depleted in animals according to a protocol described previously (23). Briefly, mice were injected with 300 µg of T cell–depleting antibodies (anti-CD4 [clone GK1.5, BioXCell], anti-CD8 [clone YTS169.4, BioXCell], or both at 150 µg each) or isotype antibody control (clone LTF-2, BioXCell) 7, 6, and 1 d before infection with S. aureus. For IL-17A depletion, mice were injected with a 200-µg dose of anti–IL-17A mAb (clone 17F3; BioXCell) or a mouse IgG1 isotype antibody control (clone MOPC-21, BioXCell) 3 h before S. aureus infection, then with a further 100-µg dose 1 h after infection, as described previously (28). For IL-10 and IFN-γ depletions, mice were treated with a 250-µg dose of anti–IL-10 mAb (clone JES5-2A5), anti–IFN-γ (clone XMG1.2, BioXCell), or Rat IgG1 isotype antibody control, anti-horseradish peroxidase (clone HRPN), 18 h prior to infection. NK cells were depleted in mice with 200 μg of anti‐NK1.1 mAb (clone PK136, BioXCell) or mouse IgG2a isotype antibody control (clone Cl.18, BioXCell) administered 18 h prior to infection, as described previously (55). All antibody doses were prepared in 100 to 200 µL PBS and administered by intraperitoneal (i.p.) injection.
Detection of Cytokines and Chemokines In Vivo.
At various time points postinfection, serum supernatants and livers were collected. Supernatants were obtained from whole livers by homogenization in HBSS supplemented with the complete protease inhibitor mixture (Roche). Samples were analyzed using Mouse Cytokine Array/Chemokine Array 44-Plex (MD44, Eve Technologies). AST levels were assessed from murine serum using a mouse AST ELISA Kit (Abcam).
IFN-γ Treatment of Mice during S. aureus Bacteremia.
DR4-B6 animals were infected with S. aureus COL Δseb intravenously (i.v.) at a dose of 5 × 106 CFU in 100 µL of HBSS prepared as described in the staphylococcal bactermia model section. At 2 h before and 1 h after infection, animals were treated with 20 µg recombinant murine IFN-γ (Sino Biological) in 100 μL PBS by i.p. injection (40 µg total). Control animals were treated with PBS only. Animals were monitored daily and killed at 96 hpi to harvest livers. Bacterial burden was determined as described in the staphylococcal bactermia model section.
Flow Cytometry Analysis of Murine Cells.
Livers were extracted from mice and pushed through a 0.7-µm cell strainer. Leukocytes were isolated from livers using a 33.75% Percoll gradient (GE Healthcare). Following isolation, red blood cells were lysed using ACK lysis buffer (Gibco) and washed with PBS containing 2% FBS. Following isolation, cells were stained and analyzed as outlined in SI Appendix, SI Materials and Methods.
Macrophage Depletion in Mice.
Macrophage depletion was based on a protocol previously described (56). Briefly, 200 µL Clodronate containing liposomes and control liposomes (Clodrosome + Encapsome [Encapsula Nano Sciences]) were administered to the mice i.p. 2 d and 4 h prior to infection with bacteria. At 18 h prior to infection, IFN-γ–depleting or control antibodies were also administered to the mice.
Detection of Human Cytokines from Stimulated Human Cells.
The ability of human cells to produce cytokines was determined from stimulated PBMCs. These cells were isolated from human blood by density-based centrifugation following layering of the blood onto Ficoll-Hypaque plus (GE healthcare). Cells were isolated and washed three times in RPMI (Gibco) and then resuspended in cRPMI. Cell suspension was seeded into 96-well plates to a final concentration of 1.0 × 106 cells/mL. Titrating concentrations of recombinant proteins or S. aureus supernatants were added to cells and incubated for 18 h at 37 °C with 5% CO2. Supernatants were assayed for IL-2 or IFN-γ by ELISA according to the manufacturer’s instructions (Thermo Fisher Scientific).
Macrophage Cultures and Infections.
RAW 264.7 (a murine macrophage cell line) cells were maintained in RPMI 1640 supplemented with 10% FBS and grown in 12-well plates with coverslips for experimental analysis. Primary human macrophages were derived from blood monocytes isolated from healthy human volunteers as previously described (32, 57). Briefly, mononuclear cells were isolated from blood with lympholyte-poly (Cedarlane Laboratories) according to the manufacturer’s instructions. Monocytes adhered to glass coverslips in 12-well plates (1.5 × 106 cells/well) and were subsequently cultured for 7 to 9 d in RPMI (Gibco) with 10% FBS (Wisent) and 0.5 ng/mL recombinant human macrophage colony-stimulating factor (M-CSF) (R&D Systems) to allow for differentiation of monocytes into macrophages. After 5 d of differentiation, adhered cells were washed with PBS and the medium was replaced with fresh RPMI + 10% FBS containing M-CSF. Macrophages were differentiated until day 7 and used experimentally until day 10.
For murine bone–derived macrophages, DR4-B6 mice were killed and leg bones were harvested for bone-marrow extraction. Bone marrow was flushed from the bones and washed with PBS prior to resuspension in RPMI supplemented with 5% FBS. Bone marrow–derived cells were adhered to glass coverslips in 12-well plates in a similar manner to human cells and stimulated with 10 ng/mL of murine M-CSF. Macrophages again were differentiated until day 7 and used experimentally until day 10.
Experimental infections for each type of macrophage followed the same protocol. S. aureus strains COL and MW2 were cultured overnight in TSB and then pelleted and resuspended in serum-free RPMI and diluted in serum-free RPMI to an OD600 of 0.5. Cells were infected with a multiplicity of infection (MOI) of 30 and, following inoculation, were centrifuged at 277 × g for 2 min and then incubated for 30 min at 37 °C in the presence of 5% CO2. Following phagocytosis, cells were treated with RPMI containing gentamicin (100 µg/mL) for 1 h at 37 °C to kill extracellular bacteria. Gentamicin was confirmed to be effective by plating supernatant of treatments onto TSA. Furthermore, minimal inhibitory concentration (MIC) for gentamicin with S. aureus MW2 was found to be 125 ng/mL, and for S. aureus COL, this value was below 62.5 ng/mL. After gentamicin treatment, macrophages were rinsed with PBS and incubated further in RPMI containing 10% FBS without antibiotic. At this point, recombinant human or murine IFN-γ (R&D Systems) was added at varying concentrations. Macrophages were incubated for 24 or 48 h following infection with S. aureus MW2 or COL, respectively, for derived macrophages or 12 or 24 h for infected RAW 264.7 cells. Enumeration of antibiotic-protected bacteria (i.e., intracellular bacteria) was performed by lysing infected macrophages with 0.1% (vol/vol) Triton X-100 in PBS. Macrophage lysates were serially diluted and plated on TSA for enumeration.
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism 9, and a P value <0.05 was considered statistically significant. For all bacterial burden CFU, analysis was performed with the nonparametric Mann–Whitney U test or Kruskal–Wallis test with an uncorrected Dunn’s test for multiple comparisons, depending on group numbers. Flow cytometry data were analyzed using Welch’s t test to determine significant differences between means of the isotype antibody and IFN-γ–depleted groups.
For the multiplex cytokine analysis heat map shown in Fig. 5D, each raw data point was normalized to a sample taken from an uninfected control animal that was killed at the same time point after treatment with the same antibody. Following normalization, the data from analysis at each time point were compared for statistical significance between the isotype antibody and IFN-γ depletion using a Student’s t test. All significant values were extracted, and the mean quantity of the cytokine/chemokine detected in the isotype antibody–treated animals was divided by the quantity detected in the IFN-γ–depleted group. These values were converted to log10 values to give fold change in positive and negative values that could be plotted on a heat map.
For the human cytokine analysis performed in Fig. 7 A–C, the area under each curve for each donor was determined. These values were then compared using a paired t test or paired Friedman test for multiple comparisons, depending on the number of groups. Paired tests were used due to the large variation observed between individual human donors.
Supplementary Material
Acknowledgments
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) (PJT-166050) to S.W.T. and J.K.M. D.E.H. acknowledges funding from CIHR Grant PJT-153308, and S.M.K. acknowledges funding from CIHR Grant PJT-175070. Figs. 3A, 6B, and 7 D and G were created using Biorender.com.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2115987119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Tong S. Y. C., Davis J. S., Eichenberger E., Holland T. L., Fowler V. G. Jr., Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwiecinski J. M., Horswill A. R., Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 53, 51–60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corl K. A., et al. , Delay in antibiotic administration is associated with mortality among septic shock patients with Staphylococcus aureus bacteremia. Crit. Care Med. 48, 525–532 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Turner N. A., et al. , Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 17, 203–218 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster T. J., Geoghegan J. A., Ganesh V. K., Höök M., Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 12, 49–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josse J., Laurent F., Diot A., Staphylococcal adhesion and host cell invasion: Fibronectin-binding and other mechanisms. Front. Microbiol. 8, 2433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koymans K. J., Vrieling M., Gorham R. D., van Strijp J. A. G., “Staphylococcal immune evasion proteins: Structure, function, and host adaptation” in Staphylococcus aureus: Microbiology, Pathology, Immunology, Therapy and Prophylaxis, Bagnoli F., Rappuoli R., Grandi G., Eds. (Springer International Publishing, Cham, 2017), pp. 441–489. [DOI] [PubMed] [Google Scholar]
- 8.Alonzo F. III, Torres V. J., The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 78, 199–230 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berube B. J., Bubeck Wardenburg J., Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins (Basel) 5, 1140–1166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuffs S. W., Haeryfar S. M. M., McCormick J. K., Manipulation of innate and adaptive immunity by staphylococcal superantigens. Pathogens 7, 53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaulding A. R., et al. , Staphylococcal and streptococcal superantigen exotoxins. Clin. Microbiol. Rev. 26, 422–447 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson G. J., et al. , A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog. 7, e1002271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuffs S. W., et al. , The Staphylococcus aureus superantigen SElX is a bifunctional toxin that inhibits neutrophil function. PLoS Pathog. 13, e1006461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salgado-Pabón W., et al. , Superantigens are critical for Staphylococcus aureus infective endocarditis, sepsis, and acute kidney injury. mBio 4, e00494-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu S. X., et al. , Superantigens subvert the neutrophil response to promote abscess formation and enhance Staphylococcus aureus survival in vivo. Infect. Immun. 82, 3588–3598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu S. X., Kasper K. J., Zeppa J. J., McCormick J. K., Superantigens modulate bacterial density during Staphylococcus aureus nasal colonization. Toxins (Basel) 7, 1821–1836 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vrieling M., et al. , Population analysis of Staphylococcus aureus reveals a cryptic, highly prevalent superantigen SElW that contributes to the pathogenesis of bacteremia. mBio 11, e02082-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill S. R., et al. , Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187, 2426–2438 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rellahan B. L., Jones L. A., Kruisbeek A. M., Fry A. M., Matis L. A., In vivo induction of anergy in peripheral V beta 8+ T cells by staphylococcal enterotoxin B. J. Exp. Med. 172, 1091–1100 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fey P. D., et al. , Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47, 196–203 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King J. M., Kulhankova K., Stach C. S., Vu B. G., Salgado-Pabón W., Phenotypes and virulence among Staphylococcus aureus USA100, USA200, USA300, USA400, and USA600 clonal lineages. mSphere 1, e00071-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasper K. J., et al. , Bacterial superantigens promote acute nasopharyngeal infection by Streptococcus pyogenes in a human MHC class II-dependent manner. PLoS Pathog. 10, e1004155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeppa J. J., et al. , Nasopharyngeal infection by Streptococcus pyogenes requires superantigen-responsive Vβ-specific T cells. Proc. Natl. Acad. Sci. U.S.A. 114, 10226–10231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabo P. A., et al. , Invariant natural killer T cells are pathogenic in the HLA-DR4-transgenic humanized mouse model of toxic shock syndrome and can be targeted to reduce morbidity. J. Infect. Dis. 215, 824–829 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Xu S., Cao X., Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 7, 164–174 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naundorf S., et al. , IL-10 interferes directly with TCR-induced IFN-gamma but not IL-17 production in memory T cells. Eur. J. Immunol. 39, 1066–1077 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Tilahun A. Y., Holz M., Wu T.-T., David C. S., Rajagopalan G., Interferon gamma-dependent intestinal pathology contributes to the lethality in bacterial superantigen-induced toxic shock syndrome. PLoS One 6, e16764 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabo P. A., et al. , Rapid and rigorous IL-17A production by a distinct subpopulation of effector memory T lymphocytes constitutes a novel mechanism of toxic shock syndrome immunopathology. J. Immunol. 198, 2805–2818 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaan A. N., Surewaard B. G. J., Nijland R., van Strijp J. A. G., Neutrophils versus Staphylococcus aureus: A biological tug of war. Annu. Rev. Microbiol. 67, 629–650 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Pidwill G. R., Gibson J. F., Cole J., Renshaw S. A., Foster S. J., The role of macrophages in Staphylococcus aureus infection. Front. Immunol. 11, 620339 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leder L., et al. , A mutational analysis of the binding of staphylococcal enterotoxins B and C3 to the T cell receptor beta chain and major histocompatibility complex class II. J. Exp. Med. 187, 823–833 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flannagan R. S., Heit B., Heinrichs D. E., Intracellular replication of Staphylococcus aureus in mature phagolysosomes in macrophages precedes host cell death, and bacterial escape and dissemination. Cell. Microbiol. 18, 514–535 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Salgado-Pabón W., Schlievert P. M., Models matter: The search for an effective Staphylococcus aureus vaccine. Nat. Rev. Microbiol. 12, 585–591 (2014). [DOI] [PubMed] [Google Scholar]
- 34.McLoughlin R. M., Lee J. C., Kasper D. L., Tzianabos A. O., IFN-γ regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 181, 1323–1332 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Aoyama T., Inokuchi S., Brenner D. A., Seki E., CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52, 1390–1400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horras C. J., Lamb C. L., Mitchell K. A., Regulation of hepatocyte fate by interferon-γ. Cytokine Growth Factor Rev. 22, 35–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surewaard B. G. J., et al. , Identification and treatment of the Staphylococcus aureus reservoir in vivo. J. Exp. Med. 213, 1141–1151 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surewaard B. G. J., et al. , α-Toxin induces platelet aggregation and liver injury during Staphylococcus aureus sepsis. Cell Host Microbe 24, 271–284.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorch S. K., et al. , Peritoneal GATA6+ macrophages function as a portal for Staphylococcus aureus dissemination. J. Clin. Invest. 129, 4643–4656 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larkin S. M., Williams D. N., Osterholm M. T., Tofte R. W., Posalaky Z., Toxic shock syndrome: Clinical, laboratory, and pathologic findings in nine fatal cases. Ann. Intern. Med. 96, 858–864 (1982). [DOI] [PubMed] [Google Scholar]
- 41.Brown A. F., et al. , Memory Th1 cells are protective in invasive Staphylococcus aureus infection. PLoS Pathog. 11, e1005226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y. X., Nilsson I. M., Tarkowski A., The dual role of interferon-γ in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology 93, 80–85 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudd K. E., et al. , Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 395, 200–211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Der Slikke E. C., An A. Y., Hancock R. E. W., Bouma H. R., Exploring the pathophysiology of post-sepsis syndrome to identify therapeutic opportunities. EBioMedicine 61, 103044 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotchkiss R. S., Monneret G., Payen D., Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 13, 260–268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero C. R., et al. , The role of interferon-γ in the pathogenesis of acute intra-abdominal sepsis. J. Leukoc. Biol. 88, 725–735 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nalos M., et al. , Immune effects of interferon gamma in persistent staphylococcal sepsis. Am. J. Respir. Crit. Care Med. 185, 110–112 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Payen D., et al. , Multicentric experience with interferon gamma therapy in sepsis induced immunosuppression. A case series. BMC Infect. Dis. 19, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karauzum H., et al. , Lethal CD4 T cell responses induced by vaccination against Staphylococcus aureus bacteremia. J. Infect. Dis. 215, 1231–1239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fowler V. G., et al. , Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: A randomized trial. JAMA 309, 1368–1378 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Bae T., Schneewind O., Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55, 58–63 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Monk I. R., Tree J. J., Howden B. P., Stinear T. P., Foster T. J., Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 6, e00308–e00315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monk I. R., Shah I. M., Xu M., Tan M.-W., Foster T. J., Transforming the untransformable: Application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3, e00277-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chau T. A., et al. , Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat. Med. 15, 641–648 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Hayworth J. L., et al. , CD1d-independent activation of mouse and human iNKT cells by bacterial superantigens. Immunol. Cell Biol. 90, 699–709 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Stritzker J., et al. , Enterobacterial tumor colonization in mice depends on bacterial metabolism and macrophages but is independent of chemotaxis and motility. Int. J. Med. Microbiol. 300, 449–456 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Flannagan R. S., et al. , Burkholderia cenocepacia disrupts host cell actin cytoskeleton by inactivating Rac and Cdc42. Cell. Microbiol. 14, 239–254 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.