Significance
Influenza infection elicits strong, long-lived protective antibodies, but most current influenza vaccines give weaker, short-lived protection. We noted that live virus is still replicating, making antigen and causing inflammation at 7 d postinfection (dpi), while an inactivated vaccine provides antigen for at most 4 dpi. We show that the generation of key T follicular helper cells (TFH) requires they recognize antigen locally at 6 dpi in the presence of ongoing viral infection. This creates a checkpoint that restricts TFH responses to dangerous infections that persist through the checkpoint. Using a live attenuated vaccine, akin to Flumist, we found that adding a second dose at 6 d generated a strong TFH response, suggesting an approach to improve vaccine strategies.
Keywords: T follicular helper cells, TFH, influenza, CD4, vaccination
Abstract
While influenza infection induces robust, long-lasting, antibody responses and protection, including the T follicular helper cells (TFH) required to drive B cell germinal center (GC) responses, most influenza vaccines do not. We investigated the mechanisms that drive strong TFH responses during infection. Infection induces viral replication and antigen (Ag) presentation lasting through the CD4 effector phase, but Ag and pathogen recognition receptor signals are short-lived after vaccination. We analyzed the need for both infection and Ag presentation at the effector phase, using an in vivo sequential transfer model to time their availability. Differentiation of CD4 effectors into TFH and GC-TFH required that they recognize Ag locally in the site of TFH development, at the effector phase, but did not depend on specific Ag-presenting cells (APCs). In addition, concurrent signals from infection were necessary even when sufficient Ag was presented. Providing these signals with a second dose of live attenuated influenza vaccine at the effector phase drove TFH and GC-TFH development equivalent to live infection. The results suggest that vaccine approaches can induce strong TFH development that supports GC responses akin to infection, if they supply these effector phase signals at the right time and site. We suggest that these requirements create a checkpoint that ensures TFH only develop fully when infection is still ongoing, thereby avoiding unnecessary, potentially autoimmune, responses.
T follicular helper cells (TFH) appear late in the CD4 effector response, and they drive the initiation of germinal centers (GCs) and resulting antibody (Ab) production (1). TFH are thus essential for strong immune responses generated by influenza infection and vaccines (2–4). Broad, highly protective Ab responses that last many years are generated by natural influenza infection but not by most influenza vaccines (5). Therefore, it is critical that we identify the signals that CD4 T effector cells receive during natural influenza infection that result in their robust differentiation into TFH and GC-TFH. Such insights could guide the design of superior influenza vaccine strategies and likely vaccines for other RNA viruses, such as HIV and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
After a sublethal dose of influenza infection in mice, viral titers remain at high levels in the lung, from 2 to 8 d postinfection (dpi), and the virus is cleared between 10 and 14 dpi (6). Antigen (Ag) is presented to CD4 T cells for over a week postinfection (7). However, Ag presentation after immunization with an inactivated vaccine is relatively short-lived with most presentation in the initial 3 d after immunization (8). The lack of a sustained Ag recognition and perhaps inflammation generated by ongoing infection may explain why TFH development and immunity after infection are far superior to those induced by vaccines (9).
Naive CD4 T cells primed by Ag-presenting cells (APCs) expand and differentiate to become various CD4 effector subsets, including TFH. During the effector phase, they clear virus via multiple synergizing mechanisms before contracting and becoming memory (10, 11). The initial priming requirements for TFH generation are well defined (12), but later requirements during the effector phase are not. Once they are generated and enter GCs, TFH repeatedly interact with B cells which drives both the GC B cell (GCB) response and further TFH differentiation, survival, and expansion (12). Several studies indicate that TFH development is enhanced by repeated immunization (13–15), but it is unclear if Ag recognition is required late during the effector phase or where it occurs. A new study suggests that T cell receptor (TCR) affinity for cognate Ag, during the GC response, determines TFH affinity, expansion, and contraction (16). Many studies have used in vivo immunization models, but few have analyzed more detailed mechanisms driving TFH development during the effector phase following infection.
Moreover, the role of infection, other than supplying Ag presentation for TFH development, is unclear. One study showed that effective TFH generation during lymphocytic choriomeningitis virus (LCMV) infection required ongoing infection (14), but whether infection supplied Ag or drove inflammation or both was not determined. This is important since vaccines, in a quest for low reactogenicity, often have only mild adjuvant activity. It is not clear what aspects of viral infection, during the effector phase of the immune response, act to drive the strong TFH development associated with infection. Here, we use an influenza infection model, where strong TFH responses develop, to identify factors driving TFH generation at the peak of the CD4 effector phase.
We showed that CD4 effectors generated during influenza A virus (IAV) infection need to recognize Ag during the effector phase at 6 to 8 dpi to escape default apoptosis and effectively form long-lived memory (7, 17). Here, we ask if late steps in the generation of TFH during infection also require cognate Ag recognition and/or other signals from infection, at this same “effector checkpoint.” We reason that if an infection is quickly cleared, or initial Ag is nonreplicating, both inflammation generated by pathogen recognition and Ag presentation will wane. If those signals are required for TFH development, it would create a checkpoint that would curtail TFH responses when infection is cleared and no longer a threat and act as a mechanism to prevent immunopathology and potential autoimmunity.
In this study, we isolate in vivo influenza infection-generated CD4 effectors and transfer them into second hosts where we modulate the availability of cognate Ag and signals from infection. This allows us to pinpoint which signals are required specifically at the CD4 effector phase, during in vivo infection, to induce optimal TFH generation. We find that CD4 effectors must recognize cognate Ag during the effector phase in spleen and draining lymph node (DLN) sites of TFH residency, to become full-fledged TFH including GC-TFH, and that they induce enhanced GCB formation. Multiple types of activated APCs can drive this transition, and it occurs even in the absence of B cells and GC. Moreover, there is an independent requirement for concurrent signals from infection to achieve optimal TFH generation. Thus, at the effector phase well after initial Ag priming, multiple signals derived from infection act in concert to drive the optimum generation of TFH. These requirements create a defined checkpoint that regulates TFH development. Our results from a polyclonal mouse model using live attenuated influenza vaccination to supply these signals suggest that these findings are relevant for designing vaccines that induce stronger TFH against influenza and likely other life-threatening respiratory viral infections, including the pandemic SARS-CoV-2.
Results
Compared to the Inactivated Vaccine, Influenza Infection Provides Extended Ag Presentation through the Initiation of the GC Response.
To understand the factors driving strong protection after infection compared to weaker protection from vaccines, we compared Ag presentation to CD4 T cells in each case. We transferred TCR Tg CD4 T cells that recognize influenza nucleoprotein NP311–325, crossed to the Nur77GFP reporter, into either influenza-infected mice or mice immunized with whole inactivated influenza (IIV). We analyzed green fluorescent protein (GFP) expression as an indicator of Ag presentation. As expected (7, 8), Ag presentation to CD4 T cells peaked at 5 to 7 dpi and declined slowly thereafter. In contrast, after IIV vaccination, Ag presentation peaked at 3 to 5 d followed by a sharper decline (SI Appendix, Fig. S1A).
We analyzed the kinetics of the TFH and the GC response after influenza infection by transferring naive OT-II CD4 T cells to mice primed with PR8-OVAII. At 5 to 6 dpi, few TFH were detected, they peaked at 7 to 8 dpi, and GCB formed after 6 to 7 dpi (SI Appendix, Fig. S1 B and C). Polyclonal CD4 and OT-II responses followed similar kinetics (SI Appendix, Fig. S1B). Thus, the peak of Ag presentation after infection (SI Appendix, Fig. S1A) correlated with the beginning of the GC response (SI Appendix, Fig. S1 B and C) and with the previously defined checkpoint for CD4 effector transition to memory (7, 17). We hypothesized that the infection and Ag presentation after 6 dpi, which is only weakly provided by IIV, is instrumental in driving superior TFH-driven immunity. We refer to this timeframe during the CD4 effector phase as the effector checkpoint or the “effector phase” throughout this study.
An In Vivo Model to Study the Role of Ag Presentation After 6 dpi during the Effector Phase.
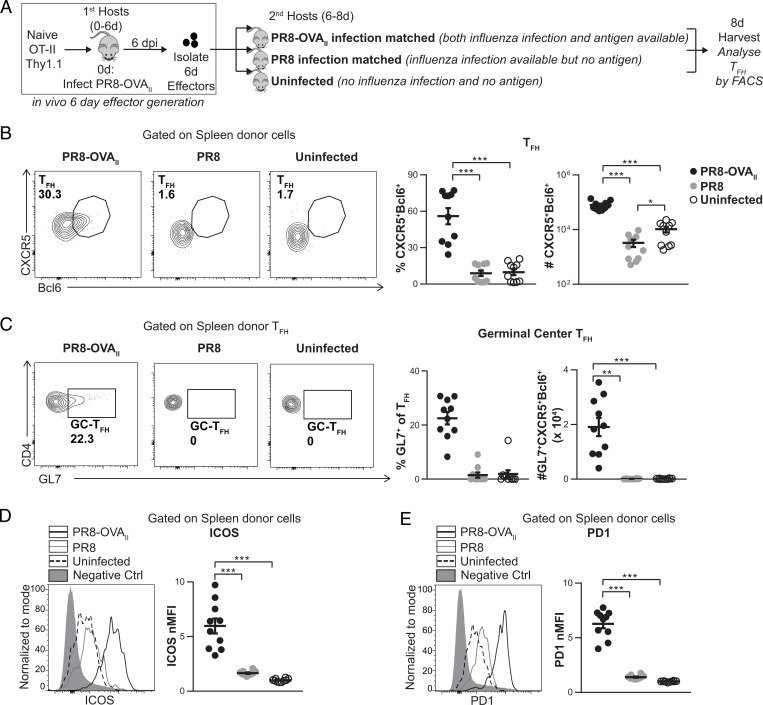
We used a sequential transfer approach (7) to manipulate signals available to CD4 effectors (Fig. 1A). We generated 6-dpi CD4 effectors in vivo by transferring naive HNT (specific for IAV hemagglutinin) or OT-II (specific for an OVA epitope) Thy1.1 CD4 T cells (6–8, 17) into primary hosts infected with PR8 or with PR8-OVAII (A/PR8/34 engineered to express the OVAII determinant in the PR8 hemagglutinin) influenza virus, respectively. After 6 d, we isolated the in vivo-generated donor CD4 effectors and transferred them into second hosts. The second hosts were either uninfected or infected 6 d previously (infection-matched), modeling intact physiology as closely as possible (Fig. 1A). With this model, we can directly control the availability of relevant signals in the second hosts specifically during the effector phase after 6 dpi. We modulated Ag availability in vivo by transferring Ag-pulsed APC (Ag/APC) into second hosts or infecting second hosts 6 d previously with influenza viruses (infection-matched). Thus, we could modulate the availability of signals from infection, while normalizing cognate Ag presentation, as done later (see Fig. 5A). In experiments using the sequential transfer model, we analyzed the transferred donor effector cells 2 to 4 d posttransfer (dpt; 8 to 10 dpi) and not later because TFH peak at 7 to 8 dpi (SI Appendix, Fig. S1B) and contract after 10 dpi.
Fig. 1.
Generation of TFH from CD4 effectors requires Ag recognition during the effector checkpoint. (A) Experimental design: Naive OT-II.Thy1.1+ cells were transferred into PR8-OVAII-infected mice (first hosts). At 6 dpi, OT-II.Thy1.1+ effectors were isolated from first hosts and transferred into the following groups of second hosts: 6-dpi PR8-OVAII-infected, 6-dpi PR8-infected, or uninfected mice. Donor cells were analyzed 8 dpi. FACS, fluorescence-activated cell sorting. (B) Percentage and numbers of spleen donor TFH (CXCR5+Bcl6+). (C) Percentage and number of spleen donor GC-TFH (GL7+CXCR5+Bcl6+). (D and E) Representative histogram of ICOS (D) and PD1 (E) expression by spleen donor cells (negative control: naive CD4 from uninfected mice). ICOS normalized median fluorescence intensity (nMFI) (D) and PD1 nMFI (E) expression by spleen donor cells. (A–E, n = 10 per group pooled, 2 independent experiments). Error bars represent SEM. Statistical significance was determined by two-tailed, unpaired Student’s t test (*P < 0.05, **P < 0.01, ***P < 0.001).
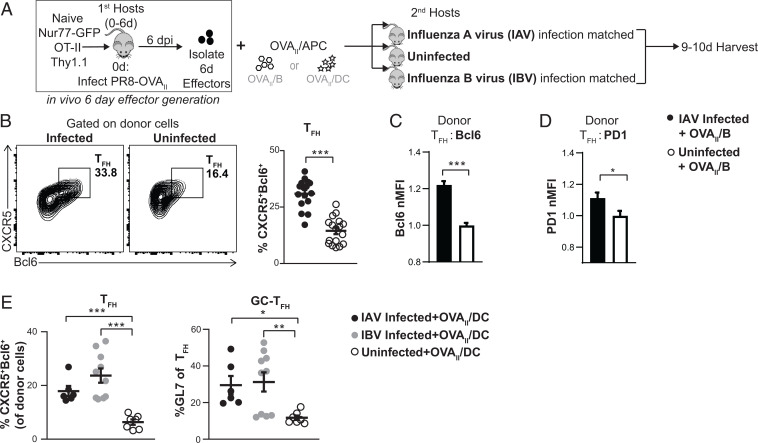
Fig. 5.
Ag independent signals from infection support TFH during the effector checkpoint. (A) Experimental design: In vivo-generated 6-d OT-II.Nur77GFP.Thy1.1+ effectors were transferred i.v. along with OVAII peptide-pulsed B cells (B–D) or OVAII peptide-pulsed DC (E) that were transferred both i.n. and i.v. into second hosts that were either infection-matched with IAV (PR8) or with IBV (B/Ann Arbor/1/66) or that were uninfected. (B–D) The 6-d effectors + OVAII/B were transferred into IAV infection-matched vs. uninfected hosts, 3 to 4 dpt. Spleens were analyzed by FACS for TFH generation from donor cells (B), Bcl6 normalized MFI of the TFH (C), and PD1 normalized MFI of the TFH (D). (B–D) n = 16 to 17 per group pooled from 5 independent experiments. (E) The 6-d effectors + OVAII/DC were transferred into IAV or IBV infection-matched or uninfected hosts, 4 dpt. Spleens were analyzed by FACS for TFH generation from donor cells. n = 6 to 10 per group pooled from 2 independent experiments. Error bars represent SEM. Statistical significance was determined by two-tailed, unpaired Student’s t test (*P < 0.05, **P < 0.01 and ***P < 0.001).
Cognate Ag Recognition, in the Presence of Infection at the Effector Checkpoint, Drives TFH Development.
We generated 6-dpi OT-II effectors in vivo as described above and transferred them into second hosts. The second hosts were either PR8-OVAII infection-matched (Ag and infection) or PR8 infection-matched (infection without Ag) or uninfected (neither infection nor Ag) (Fig. 1A). Donor TFH and GC-TFH generation were assessed at 2 dpt in the secondary lymphoid organs (SLOs), corresponding to 8 dpi. In the PR8-OVAII infection-matched positive controls, a strong donor TFH response developed in the spleen and DLN (Fig. 1B and SI Appendix, Fig. S2B). In contrast, in PR8 infection-matched hosts, where no cognate Ag presentation occurred, few if any donor TFH were seen and donor TFH numbers were reduced 25-fold in both the spleen (Fig. 1B) and the DLN (SI Appendix, Fig. S2B). Donor GC-TFH developed well in hosts with Ag and infection, while almost no GC-TFH were generated without Ag (Fig. 1C and SI Appendix, Fig. S2C). This suggests effectors have a strict requirement for Ag recognition during this phase, for development and/or maintenance of TFH and GC-TFH. PD1 and ICOS expression, markers associated with TFH function, were also strictly dependent on Ag recognition in the second host (Fig. 1 D and E and SI Appendix, Fig. S2 D and E).
At 6 dpi, there are already a minor fraction of CD4 effectors that express the TFH phenotype (CXCR5hiBcl6hi) (SI Appendix, Fig. S1B), suggesting that this cohort may undergo apoptosis or revert to a non-TFH state in the absence of cognate Ag presentation at 6 to 8 dpi. Thus, even when signals from infection were available in the PR8-infected hosts, development of TFH in the spleen and DLN required cognate Ag recognition during the effector checkpoint. A previous study showed that the transfer of 4-d effectors generated during LCMV infection into uninfected hosts did not support TFH development, but it did not distinguish between the need for infection vs. Ag recognition (14). Our data indicate that even when signals from infection are available, cognate Ag at the peak effector phase is needed to drive and sustain TFH and GC-TFH development.
TFH Function during Influenza Infection Is Dependent on Cognate Ag at the Effector Phase.
IL-21 produced by TFH promotes TFH differentiation and mediates the GC response (12). The proportion of donor effectors with the potential to secrete interleukin-21 (IL-21) was higher in second hosts with Ag than in those without Ag (Fig. 2A and SI Appendix, Fig. S2F). In contrast, secretion of Th1 cytokines, interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα), was not dependent on Ag and was in fact highest in uninfected hosts (SI Appendix, Fig. S2G). This indicates a selective dependence of TFH-associated programs, but not Th1 effector functions in the SLO, on Ag recognition during the effector checkpoint.
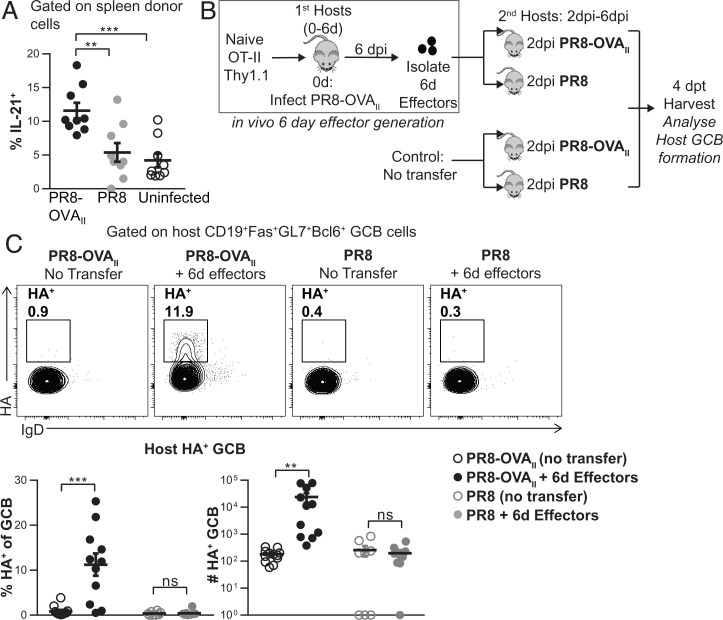
Fig. 2.
TFH function: IL-21 potential and enhanced GCB development is driven by Ag recognition during the effector phase. (A) Experiment design as in Fig. 1A. Percentage of spleen donor cells expressing intracellular IL-21 (n = 9 per group pooled, 2 independent experiments). (B) Experimental design: In vivo-generated 6-d OT-II.Thy1.1+ effectors were transferred into 2-dpi PR8-OVAII-infected or PR8-infected mice. Groups of 2-dpi PR8-OVAII-infected and PR8-infected mice, with no cells transferred served as negative controls. Spleens from these mice were analyzed 4 dpt. (C) Percentage and numbers of HA+ GCB. (n = 8 to 12 per group pooled, 2 to 3 independent experiments). Error bars represent SEM. Statistical significance was determined by two-tailed, unpaired Student’s t test (**P < 0.01, ***P < 0.001; ns, not significant).
To evaluate the impact of Ag recognition at the effector checkpoint on the key TFH function of enhancing GCB formation, we developed an in vivo GCB assay (Fig. 2B and SI Appendix, Methods). Endogenous host GCBs are undetectable from 2 to 6 dpi (SI Appendix, Fig. S1C), presumably because TFH have not yet fully formed (SI Appendix, Fig. S1B). We reasoned that if functional TFH were available earlier, they would accelerate GCB formation. Therefore, we transferred in vivo-generated 6-d OT-II effectors into hosts infected 2 d previously with either PR8 (infection only) or PR8-OVAII (infection and cognate Ag) and analyzed host GCB at 4 dpt (6 dpi) (Fig. 2B). The transfer of 6-d donor effectors into PR8-OVAII-infected mice caused a significant increase in total GCB formation (SI Appendix, Fig. S2H) and in influenza hemagglutinin-specific GCB formation (Fig. 2C) 4 d later. Transfer of the same cells to PR8-infected hosts, which lack OVAII Ag, did not boost GCB formation over the negative controls not receiving effectors. Thus, TFH induction of GCBs also requires that CD4 effectors recognize cognate Ag during the effector phase.
Multiple APC Subsets Effectively Present Ag at the Effector Checkpoint to Drive TFH.
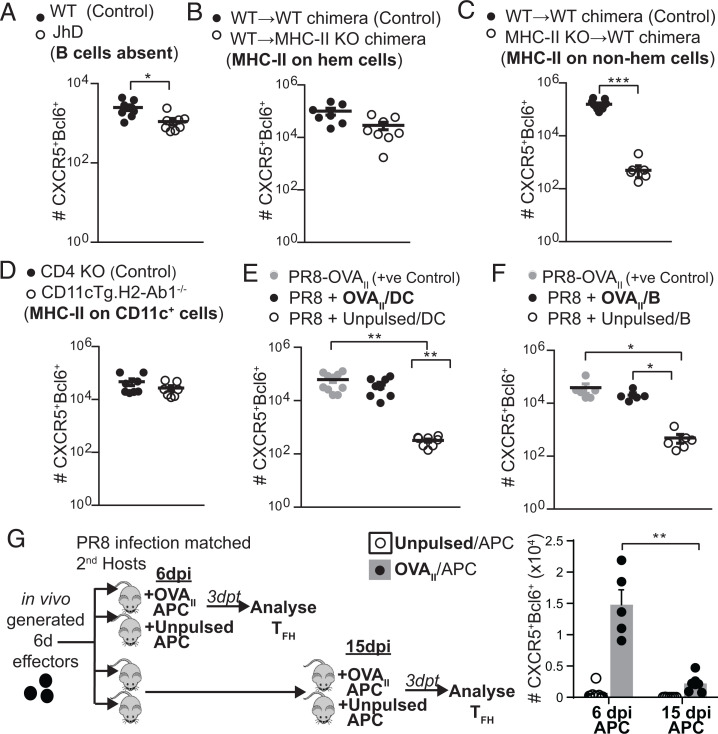
B cells become the major APC for TFH once they arrive in the follicular region of the SLO, which they repeatedly interact with for further differentiation (18). We considered the possibility that other APC subsets might be able to drive the effector to TFH differentiation at 6 dpi, if and when available. We evaluated the efficacy of different broad classes of APC at presenting Ag for TFH during the effector phase using B cell-deficient JhD mice, major histocompatibility complex II (MHC-II) KO bone marrow chimeras, and CD11cTg.H2-Ab1−/− mice which expressed MHC-II only on CD11c+ cells, which are all second hosts. In other experiments, we provided Ag by transferring in vitro-activated, Ag-pulsed, B cells or dendritic cells (DC) (SI Appendix, Fig. S3A).
To determine whether Ag-presenting B cells are essential to drive CD4 effectors to TFH, we transferred in vivo-generated 6-d HNT Thy1.1 (TCR Tg specific for the hemagglutinin (HA) epitope of the influenza strain) effectors into PR8 infection-matched B cell-deficient JhD mice (Fig. 3A and SI Appendix, Fig. S3B). Substantial numbers of TFH were generated in these hosts, although there was a twofold decrease in number compared to B cell replete hosts. This suggests that non-B cell APC can also drive TFH development from effectors.
Fig. 3.
Multiple APC subsets can present cognate Ag during the effector phase to drive TFH development from 6-d effectors. (A) Experimental design for A–F as depicted SI Appendix, Fig. S3A schematic: In vivo-generated 6-d OT-II.Thy1.1+ or 6-d HNT.Thy1.1+ effectors were transferred into PR8 infection-matched hosts (A), PR8-OVAII infection-matched hosts (B–D), or into PR8 infection-matched hosts together with OVAII/APC (E and F). Numbers of TFH (CXCR5+Bcl6+) generated were enumerated by flow cytometry, 2 to 4 dpt in each of these models. (A) JhD mice where B cells are absent or into WT control mice (n = 8 per group pooled, 2 independent experiments). (B) WT→ MHC-II KO (H2-Ab1−/−) bone marrow chimera mice that were made by transferring WT bone marrow into MHC-II KO irradiated hosts, where MHC-II is restricted to the hematopoietic compartment, or into WT→WT bone marrow chimera control mice (n = 7 to 8 per group pooled, 3 independent experiments). (C) MHC-II KO →B6 bone marrow chimera mice, where MHC-II is restricted to the nonhematopoietic compartment or into WT→WT bone marrow chimera control mice (n = 8 to 11 per group pooled, 3 independent experiments). (D) CD11cTg.H2-Ab1−/− mice where MHC-II is restricted to CD11c+ cells or into CD4 KO control mice (n = 7 to 11 per group pooled, 2 to 3 independent experiments). (E) WT mice with cognate Ag supplied via OVAII-pulsed BMDC vs. unpulsed BMDC controls (n = 8 to 10 per group pooled, 3 independent experiments). (F) WT mice with cognate Ag supplied via OVAII-pulsed B cells vs. unpulsed B cell controls (n = 5 to 6 per group pooled 2 independent experiments). (G) In vivo-generated 6-d OT-II.Thy1.1+ were transferred into PR8 infection-matched hosts. OVAII/APCs were transferred either on 6 d along with the effectors or on 15 d. Numbers of TFH (CXCR5+Bcl6+) generated were enumerated by flow cytometry, 3 dpt. For OVAII/APCs, we used in vitro-stimulated T-depleted splenocytes that contained a mixture of APC types, predominantly B cells, since we had determined the kind of APC was not important (n = 5 to 6 per group pooled 2 independent experiments). Error bars represent SEM. Statistical significance determined by two-tailed, unpaired Student’s t test (*P < 0.05, **P < 0.01, and ***P < 0.001).
To determine what alternate endogenous APCs were competent to present Ag, we transferred 6-d effectors into infection-matched BM chimeras in which MHC-II expression was restricted to either the hematopoietic compartment (wild type [WT] → MHC-II KO chimeras) (Fig. 3B and SI Appendix, Fig. S3C) or to the nonhematopoietic compartment (MHC-II knock out [KO]→ WT chimeras) (Fig. 3C and SI Appendix, Fig. S3D). Donor TFH recovery was equivalent in WT mice and those with MHC-II were restricted to the hematopoietic compartment (Fig. 3B and SI Appendix, Fig. S3C). However, very few TFH were found when Ag presentation was restricted to the nonhematopoietic compartment (Fig. 3C and SI Appendix, Fig. S3D), consistent with observations that few nonhematopoietic MHC-II+ cells present Ag in the SLO (19).
We next transferred 6-d effectors into PR8-OVAII infection-matched CD11cTg.H2-Ab1−/− mice (Fig. 3D and SI Appendix, Fig. S3E). There was no defect in donor TFH formation from these 6-d effectors, indicating that in infected mice, CD11c+ APCs are sufficient at the effector checkpoint to drive TFH development.
To compare the efficiency of DC (Fig. 3E and SI Appendix, Fig. S3F) and B cell (Fig. 3F and SI Appendix, Fig. S3G) Ag presentation, we pulsed each with Ag and transferred them with the 6-d effectors into PR8 infection-matched mice. The 6-d effectors gave rise to equivalent numbers of TFH when either B cells or DC presented Ag. These experiments indicate that multiple APC types, including APCs other than B cells, are effective at driving TFH development from 6-dpi effectors during influenza infection, as long as hematopoietic MHC-II+ presentation is available.
TFH begin to differentiate within the first few rounds of CD4 division (20), and thus, 6-d effectors contain partially differentiated pre-TFH effectors. It is possible that these pre-TFH can persist in the absence of signals from cognate Ag during the effector phase and are ready to develop into TFH later, if cognate-Ag becomes available. To evaluate this possibility, we transferred 6-d OT-II effectors into PR8 infection-matched hosts (Fig. 3G), so other signals from infection and GC responses were available, but cognate-Ag was not. As a positive control, we transferred OVAII/APC at 6 dpi. TFH developed from 6-d effectors when they were transferred together with OVAII/APC, as in Fig. 3 E and F. However, when we delayed the transfer of OVAII/APC to 15 dpi, sixfold to sevenfold fewer TFH were generated from the transferred 6-d effectors (Fig. 3G). This mirrored the contraction of total CD4 effectors, whose numbers were also reduced sevenfold to eightfold when the addition of OVAII/APC was delayed to 15 dpi (SI Appendix, Fig. S3H). This suggests that pre-TFH effectors undergo contraction in the absence of cognate Ag after 6 dpi. Thus, continuing Ag presentation through this effector checkpoint is essential for the 6-d effectors to avoid contraction and complete their differentiation into TFH, even if they receive the early signals and even if other signals from infection and from the GC are available.
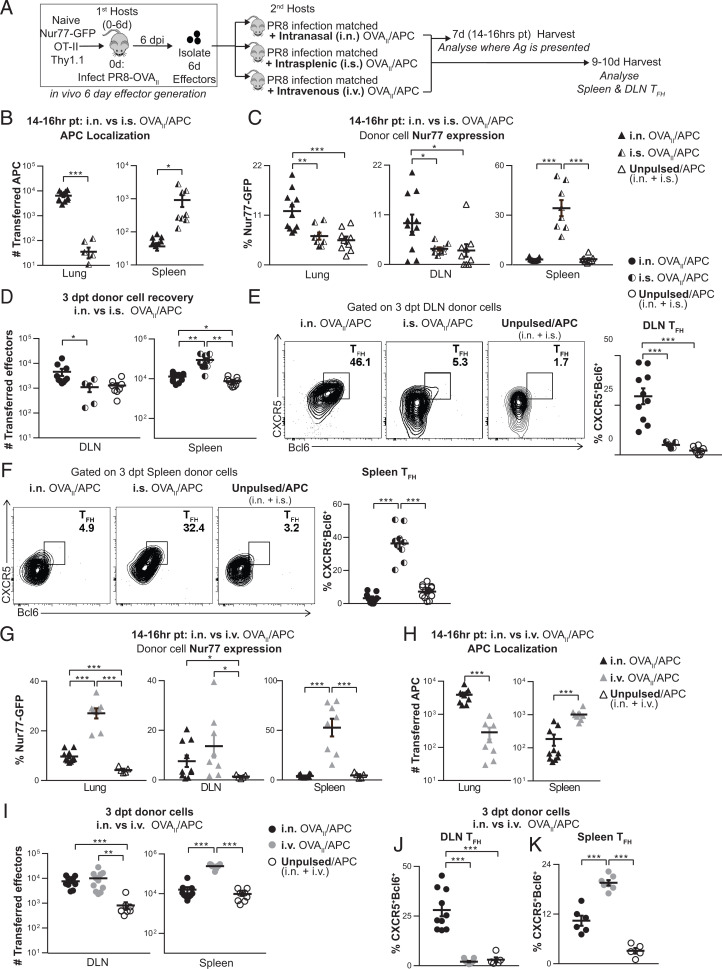
Ag Delivery by Different Routes Favors Ag Presentation in Distinct Sites and Selectively Drives TFH in DLN vs. Spleen.
TFH are restricted to the SLO (DLN and spleen) during primary infection and express signatures for residency in SLO (21–23). In the influenza model, we do not find TFH in the lung during the primary effector response, although they are found during the memory phase after 14 dpi and during secondary effector responses in the lung (24). To design vaccine delivery strategies that mimic the superior protection elicited after infection, we needed to understand if the site of Ag presentation during the effector phase plays a role in TFH generation.
We followed Ag/APC after different routes of transfer and evaluated where the CD4 effector interaction with Ag/APC needed to occur for TFH to develop in the spleen vs. the DLN (Fig. 4A). We transferred OT-II.Nur77GFP.Thy1.1+ 6-d effectors into PR8 infection-matched hosts with the OVAII/APC introduced by different routes and analyzed them 14 to 16 h posttransfer (Fig. 4 B and C). OVAII/APCs were found in the lung only after intranasal (i.n.) transfer and in the spleen only after intrasplenic (i.s.) transfer (Fig. 4B). Next, we examined Ag presentation as indicated by Nur77 expression in the transferred OT-II in the different sites. Transfer of OVAII/APC i.n. induced Nur77GFP expression in the DLN, and not in the spleen, while i.s. transfer induced Nur77GFP exclusively in donor cells recovered from the spleen and not in the DLN (Fig. 4C), indicating we had achieved localized Ag presentation.
Fig. 4.
Local Ag presentation during the effector phase is required for DLN and spleen TFH as shown by Ag delivery via i.n. vs. i.s vs. i.v. routes. (A) Experimental design: OVAII peptide-pulsed B cells (CD45.1+ or GFP+) were used as APCs and transferred into PR8 infection-matched hosts 6 dpi by either i.n., i.s., or i.v. routes. Unpulsed APCs were transferred both i.n. and i.s., or both i.n. and i.v., as negative controls. In vivo-generated 6-d OT-II.Nur77GFP.Thy1.1+ effectors were transferred by i.v. route. Mice were harvested 14 to 16 h posttransfer (pt), and donor cells were analyzed by flow cytometry. (B and H) Number of transferred APCs. (C and G) Donor cell Nur77GFP expression was analyzed by flow cytometry in the lung, DLN, and spleen. (n = 8 to 11 per group pooled, 3 independent experiments). (D–F, I, and J) Experiment was performed as in A, and mice were euthanized 3 to 4 dpt. (D and I) Total numbers of DLN and spleen donor effectors recovered (n = 5 to 12 per group pooled, 3 to 4 independent experiments). (E and J) DLN TFH formation from donor cells (n = 5 to 11 per group pooled, 2 to 3 independent experiments) (F and K) Spleen TFH formation from donor cells (n = 5 to 12 per group pooled, 2 to 4 independent experiments).
We found the total number of donor cells recovered in the DLN increased following i.n. but not i.s. OVAII/APC transfer and vice versa; the number of donor cells recovered in the spleen was increased following i.s. but not i.n. OVAII/APC transfer (Fig. 4D). In parallel, TFH developed from donor cells in the DLN only when OVAII/APCs were administered by the i.n. and not i.s. route (Fig. 4E and SI Appendix, Fig. S4A) and in the spleen only when OVAII/APCs were delivered by the i.s. and not i.n. route (Fig. 4F and SI Appendix, Fig. S4A). Thus, TFH development correlated with the location of Ag/APCs and with Ag presentation to the CD4 T cells. This implies that full TFH development requires that the CD4 effectors recognize Ag in the tissue in which they become resident, indicating local Ag presentation is needed.
We also tested if Ag/APCs delivered intravenously (i.v.) might result in presentation to CD4 effectors before they reached their SLO sites and would be sufficient to complete their differentiation into TFH. We i.v. transferred OVAII/APC with OT-II.Nur77GFP.Thy1.1+ 6-d effectors. After 14 to 16 h, donor effectors in both the spleen and DLN expressed Nur77GFP, indicating Ag recognition (Fig. 4G). However, after i.v. transfer, the APCs were found predominantly in the spleen with many fewer in the lung (Fig. 4H). OVAII/APCs transfer by the i.v. route, like transfer by i.n., increased the number of transferred effectors in the DLN, compared to the negative control (Fig. 4I). However, OVAII/APCs transferred i.v. supported donor spleen TFH but not DLN TFH generation (Fig. 4 J and K and SI Appendix, Fig. S4 B and C). This suggests that even if 6-d effectors have the opportunity to recognize Ag before migrating to their site of residence in the DLN (as here during i.v. transfer), this is not sufficient to induce the development of TFH in DLN because they require Ag recognition targeted to the specific SLO. These results emphasize that while many APCs may effectively present Ag, it is essential that during this effector checkpoint, the Ag is optimally delivered to the organ where the TFH will develop.
Signals from Infection during the Effector Phase Are Required to Support TFH.
Infection-generated signals activate APCs, but they also induce innate pathways that produce inflammatory signals independent of cognate Ag presentation (25). We reasoned that during influenza infection, abundant pathogen recognition signals continue through the effector checkpoint due to the continuing presence of live, replicating virus (6). The impact of Ag-independent signals from influenza infection during the effector checkpoint can be analyzed by normalizing cognate Ag presentation to CD4 effectors, using Ag/APC as the sole source of Ag (Fig. 5A). We transferred in vivo-generated 6-d OT-II.Nur77GFP.Thy1.1+ effectors along with OVAII/B cell APCs into IAV infection-matched or uninfected second hosts (Fig. 5A). After 14 to 16 h (SI Appendix, Fig. S5A), there was no significant difference in the number of transferred APCs or in Nur77GFP expression by the donor effectors in infected and uninfected mice, indicating that the transferred APCs present Ag equivalently in both hosts. Thus, the only variable was the presence of signals from influenza infection unrelated to Ag presentation. We found that the proportion of donor TFH at 3 to 4 dpt in the spleen of uninfected second hosts was lower than that in IAV-infected hosts (Fig. 5B). We analyzed the expression of signature proteins associated with TFH function and found that donor TFH in infected mice also expressed significantly higher levels of Bcl6, PD1, and ICOS (Fig. 5 C and D and SI Appendix, Fig. S5C) than in uninfected mice.
To additionally evaluate Ag-independent effects of infection, we used influenza B virus (IBV) infection (Fig. 5E and SI Appendix, Fig. S5 D–J). IBV has little homology with IAV (26). TFH and GC-TFH generation from transferred 6-d effectors in IBV-infected mice were higher than those in uninfected mice, with higher Bcl6 and ICOS expression, confirming a contribution of infection, independent of Ag presentation (SI Appendix, Fig. S5 D–G). We repeated the experiment using DC instead of B cells as the source of OVAII/APC to normalize Ag presentation (Fig. 5E and SI Appendix, Fig. S5 H–J) and found a similar dependence of TFH and GC-TFH generation on infection. The data indicate that optimum TFH development requires signals generated by infection at the effector checkpoint, which are independent from and in addition to signals from cognate Ag recognition.
In a Polyclonal Vaccine Model, Providing Both Ag and Infection Signals at the Effector Checkpoint Induces Strong TFH Responses Comparable to Influenza Infection.
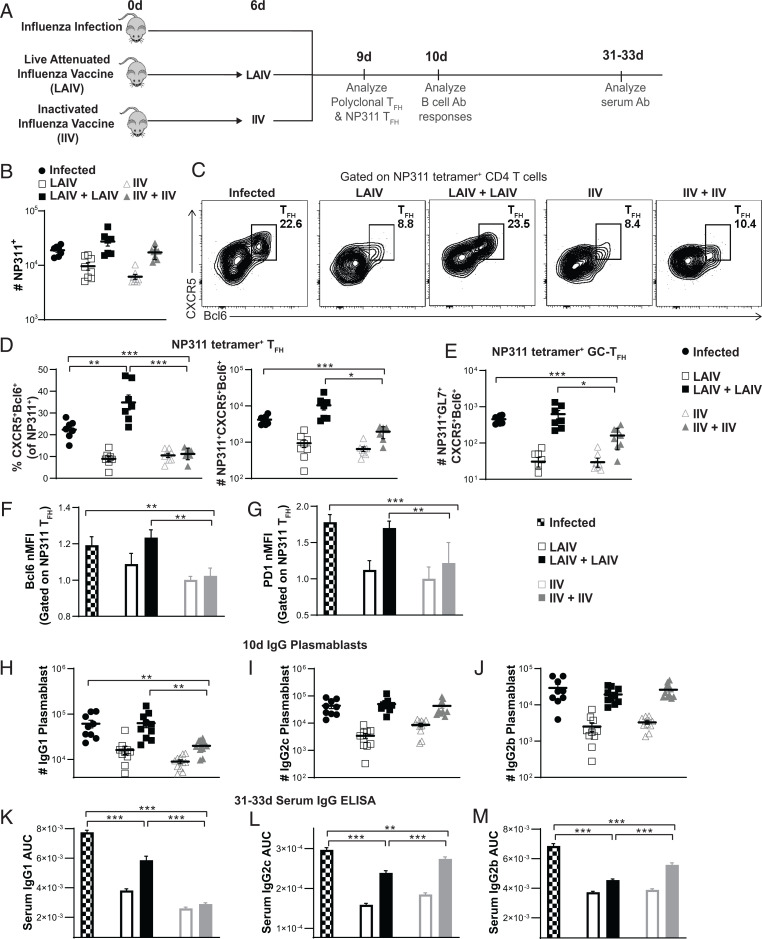
Our results indicate that optimal TFH development requires both Ag recognition and infection signals during the effector checkpoint. We postulate that when conventional vaccines do not provide these signals, they induce much less TFH than live infection. To explore this hypothesis in an in situ polyclonal response, we developed a model using the following two widely used vaccine approaches: nonreplicating inactivated influenza vaccine (IIV) and live attenuated influenza vaccine (LAIV) (27). We showed that IIV provides high-level Ag presentation only transiently (8) (SI Appendix, Fig. S1A). LAIV contains attenuated influenza viruses that are cold adapted, so replication is restricted to the upper respiratory tract and does not occur in the warmer lung environment (27). Since LAIV contains live replication-competent virus, it is likely to provide stronger infection signals than IIV. We treated mice with two doses of IIV (8) or LAIV (7) at d 0 and at d 6 to supply Ag presentation and signals from infection, for both priming and through the checkpoint (Fig. 6A). We compared the vaccine responses to the infection-generated TFH response. Virus-specific T cells were detected by tetramer staining for an immunodominant epitope in B6 mice, NP311.
Fig. 6.
Live attenuated influenza immunization that supplies both Ag and signals from infection at the effector checkpoint supports TFH generation, IgG plasmablast formation, and serum IgG levels near those of natural influenza infection. (A) Experimental design: One group of mice were infected with IAV. Two groups of mice were either given one dose of LAIV on d 0 or two doses on d 0 and d 6. Two groups of mice were either given one dose of IIV on d 0 or two doses on d 0 and d 6. Mice were euthanized at 9 d, 10 d or 31–33 d after infection/initial immunization. Virus-specific responses in the spleen were analyzed by staining with NP311 tetramer. (B) Total number of NP311+ cells in the spleen (C and D) TFH generation by NP311+ CD4 T cells. (E) GC-TFH generation by NP311+ CD4 T cells. (F and G) Normalized MFI of Bcl6 (F) and PD1 expression (G) by NP311+ TFH cells. (B–G) n = 7 to 8 per group pooled from 2 independent experiments. (H–J) IgG plasmablast generation in the spleen at 10 d was analyzed by FACS staining for IgG1+CD138+CD19+IgD−GL7− (H), IgG2c+CD138+CD19+IgD−GL7− (I), and IgG2b+CD138+CD19+IgD−GL7− (J). n = 9 to 11 per group pooled from 3 independent experiments. Statistical significance was determined by two-tailed, unpaired Student’s t test. (K–M) IgG serum Ab was analyzed by ELISA at 31 to 33 d. Area under the curve (AUC) analyses shown that were performed for IgG1 (K), IgG2c (L), and IgG2b (M) ELISA curves are in SI Appendix, Fig. S5 C–E. n = 8 per group pooled from 2 independent experiments. Statistical significance was determined by one-way ANOVA. Error bars represent SEM. (*P < 0.05, **P < 0.01 and ***P < 0.001).
Adding a second dose of either vaccine at the effector checkpoint increased the total number of NP311 tetramer+ cells by threefold over just one dose (Fig. 6B). Both the number of NP311 tetramer+ cells (Fig. 6B) and the number of GCB cells (SI Appendix, Fig. S6A), following a second dose of either vaccine at the checkpoint, were comparable to those generated during influenza infection. Two doses of LAIV resulted in a fourfold increase in the proportion of TFH compared to one dose, resulting in a higher proportion of TFH generation than during influenza infection (Fig. 6 C and D). Moreover, with two doses of LAIV, an equivalent number of TFH were generated as following influenza infection (Fig. 6D) which was over 10-fold higher than that with just one dose of LAIV at d 0. In contrast, the second dose of IIV did not increase the proportion of TFH generation over one dose (Fig. 6 C and D), even though the number of total NP311+ CD4 effectors generated were equivalent to those of a live infection (Fig. 6B). A similar effect on TFH generation was seen when analyzing the total polyclonal CD4 response (SI Appendix, Fig. S6B). Strikingly, the impact of adding a checkpoint dose of LAIV in inducing GC-TFH generation was even more pronounced. Two doses of LAIV induced 20-fold more GC-TFH than one dose and was equivalent to the levels induced by infection, while two doses of IIV did not induce levels equivalent to infection (Fig. 6E). Two doses of LAIV were also able to induce Bcl6 and PD1 expression in TFH to levels comparable to infection, but two doses of IIV did not (Fig. 6 F and G).
TFH are essential to induce IgG1 Ab in models of influenza immunization, while Th1 effectors are sufficient to induce IgG2 Ab (28). In line with those findings, two doses of LAIV induced IgG1 plasmablasts equivalent to those found after influenza infection, while two doses of IIV were unable to do so (Fig. 6H), thus mimicking TFH induction in Fig. 6D. Both IgG2c and IgG2b plasmablasts were induced by two doses of LAIV, as well as two doses of IIV (Fig. 6 I and J). This is expected since both strategies induced equivalent CD4 effector expansion (Fig. 6B). Similar patterns were also seen in levels of IgG1 and IgG2 serum Ab 1 mo after immunization (Fig. 6 K–M and SI Appendix, Fig. S6 C–E), with two doses of LAIV inducing enhanced IgG1 levels compared to one dose, while two doses of IIV did not induce more IgG1 than one dose (Fig. 6K). Two doses of LAIV and IIV were both able to induce enhanced IgG2c and IgG2b compared to one dose (Fig. 6 L and M). Several comprehensive B cell studies have also shown that TFH are critical for the increased potency of Ab generated (such as neutralizing ability, increased mutation diversity, increased affinity, increased avidity) and correlate with memory B cell responses that confer stronger protection against infection (20, 28). Our findings here indicate that the requirements established in a reductionist monoclonal TCR Tg transfer model translate readily to a vaccine model in which in situ polyclonal responses are analyzed. This supports the concept that it should be possible to achieve TFH-driven immunity nearly comparable to influenza infection by using vaccines that supply optimal Ag and infection signals during the checkpoint, in addition to during priming.
Discussion
Influenza infection generates long-lived Ab responses that provide much stronger protection against reinfection than current influenza vaccines (5). We interrogated aspects of live influenza infection that generate strong protection to provide insights into designing more effective vaccines. We identified the following three key mechanisms during the CD4 effector phase of an influenza infection that together drive full TFH development: sustained Ag presentation through the effector phase, Ag presentation in the site of TFH residence, and the presence of ongoing infection. We confirmed the relevance of these observations in a vaccine setting by showing that a double vaccination with LAIV at d 0 and d 6 induced the generation of high levels of TFH and GC-TFH comparable to those achieved by influenza infection.
We postulate that these requirements for effector differentiation provide a checkpoint mechanism that limits TFH generation and high affinity Ab to those situations where there is an ongoing threat from continuing infection. This should limit unnecessary, potentially harmful TFH responses, when they are not required for pathogen clearance. Indeed, in certain autoimmune diseases and chronic infections during which continuous Ag and inflammation persist, exaggerated TFH responses lead to exaggerated humoral responses (29, 30). When viral and likely other pathogens, persist at the effector phase of the response, it suggests that the early arms of the immune response have failed to clear virus, indicating a strong infection that warrants the development of immune memory (both CD4 memory [7, 17] and Ab production helped by TFH) for future protection. Thus, the checkpoint determines the generation of much of the long-term protection against future infections.
During polyclonal responses, new naive CD4 T cells are recruited throughout the response (6) and individual polyclonal cells have different propensities to become TFH because they express different TCRs (18). We circumvented these confounding factors by using TCR Tg CD4 T cells that give a more synchronized effector response. It also allowed us to use a sequential transfer model to study effector phase signals specifically after the 6-dpi timepoint.
In influenza-infected mice, 6 dpi marks the beginning of GC formation (31) (SI Appendix, Fig. S1 B and C). It also coincides with the checkpoint for CD4 memory, when CD4 effectors require Ag recognition to induce IL-2 production, which blocks apoptosis and initiates transition to memory (7, 17). The substantial diminution of TFH, when cognate-Ag presentation is absent from 6 to 15 d (Fig. 3G), suggests that Ag recognition also prevents the default contraction of the pre-TFH CD4 effectors, while inducing further TFH differentiation. Until recently, it was thought that TFH are largely quiescent during this time, but our results here and a recent study (16) indicate that TFH actively divide and are dynamically regulated during this phase.
Developing TFH requires repeated Ag recognition and costimulatory interactions once they reach the T-B border in the GC of the SLO (18), which are presumed to occur during their cognate interaction with GCBs. Thus, it was assumed that further differentiation of TFH including their development into GC-TFH depended on Ag recognition during the GC response. Indeed, it has recently been shown that already generated GC-TFH get selected for high affinity in the GC (16). It has been unclear if signals from viral infection are needed at this stage. In previous studies that provided evidence that infection during the effector phase contributes to TFH generation, it was not clear whether infection acted by supplying cognate Ag and/or by induction of infection-induced pathogen recognition signals (14). We find that at this pre-GC checkpoint, the requirement for infection after 6 dpi provides Ag-independent infection-derived signals, which must act together with Ag recognition to fully drive TFH and GC-TFH differentiation. The frequency of TFH, including GC-TFH, were enhanced when infection signals were present (Fig. 5). TFH generated in the absence of infection expressed lower levels of Bcl6, PD1, and ICOS. Bcl6low TFH rapidly lose proliferative potential and gradually gain migratory potential for egress from the LN (32). PD1 and ICOS control TFH tissue positioning, and if their expression is reduced in TFH, there is a breakdown of TFH and GC responses (33, 34). Our results complement recent research which shows that signals from bacterial infection can augment TFH (35).
The evidence that various activated MHC-II+ APCs, including DC and B cells, are able to drive donor effectors to develop into TFH (Fig. 3) is consistent with previous studies of APC subsets required during initial priming of TFH responses (13, 36, 37). Strikingly, TFH were generated from 6-d effectors even when Ag was presented only by DC (Fig. 3E and SI Appendix, Fig. S3F) and even in the absence of B cells and GC in JhD second hosts (Fig. 3A and SI Appendix, Fig. S3B). Thus, although B cells may be the major source of Ag for TFH once they enter GC in situ, other MHC-II+ APCs are sufficient to drive TFH development at the effector phase. Given the critical importance of TFH, this may allow the development of strong TFH responses even when GC responses are impaired or Ag-specific B cells are limited. This may be a useful strategy for the immune system to drive GC-independent B cell responses which benefit from TFH help, such as reactivation of previously generated memory B cells.
Aspects of the TFH developmental program are expressed early during CD4 effector activation when effectors begin to express Bcl6 within the first few rounds of cell division (12). Thus, the 6-d effectors we transfer include some pre-TFH at 6 dpi before the GC phase has begun (SI Appendix, Fig. S1 B and C). These 6-d pre-TFH likely have the potential to become TFH and GC-TFH, but our data here (25-fold reduction to negligible TFH levels in the absence of Ag) suggest they contract and fail to realize that potential unless they receive local signals from Ag recognition and infection again during the effector phase.
We were able to restrict Ag presentation to distinct sites by introducing the Ag-pulsed APCs by different routes (Fig. 4). Development of the SLO-resident TFH subsets require local Ag presentation in the tissue to which they will be restricted during the effector phase (Fig. 4 B–F), even if they also encountered Ag before entering the tissue (Fig. 4 G–J). This local Ag requirement is akin to that of T and B resident memory subsets that reside in nonlymphoid tissues (38–41). TFH in the SLO express universal tissue residency programs (22, 23), raising the possibility that the local Ag requirement may be necessary for inducing local residency. The transferred Ag-pulsed B cells introduced here by different routes localized dependably in tissues, but it is likely that soluble protein Ag in vaccines injected intramuscularly may become distributed more systemically. Further studies are needed to determine how routes of immunization impact the locations of Ag presentation. Given the critical functions of TFH that develop in the SLO, the need for local Ag presentation at the effector stage is likely important to consider during vaccine design.
TFH generation is a reliable indicator of protective Ab after influenza vaccination (2). Thus, vaccines that confer long-term effective Ab-mediated protection must, like natural influenza infection, provide the signals described here for pre-GC 6-d TFH to fully develop into TFH and GC-TFH. We note that mRNA lipid nanoparticle (LNP) vaccines, such as current mRNA COVID-19 vaccines, are highly effective against SARS-CoV-2 infection. These mRNA vaccines induce Ag production for an extended duration (42), and the LNPs are inflammatory (43) which may allow them to present Ag and provide inflammatory cues through the effector checkpoint and thus robustly induce TFH.
Unformulated soluble Ag/adjuvants in vaccines are rapidly cleared from the body (44), which may explain the low efficacy of several current, widely used influenza vaccines. Recent studies have supported the concept that vaccine design that allows for the sustained delivery of Ag, thus mimicking Ag presentation during natural infections, results in stronger immune responses (45). Several vaccine formulation strategies have been proposed to extend the kinetics of Ag presentation (44). An extended delivery approach using osmotic pumps or escalating dose delivery resulted in superior Ab responses to HIV vaccination (46). A microneedle patch that allowed for sustained Ag delivery over 2 wk also enhanced GC, TFH, and Ab responses (47). A hydrogel formulation that extended Ag delivery and supplied pathogen recognition receptor (PRR) signals resulted in similar increases in Ab titers, specifically IgG1 correlating to the induction of TFH (48). Our data suggest that extended delivery vaccines provide enhanced protection due to their induction of superior TFH responses by presenting Ag through the effector phase. We also find that an additional aspect, namely, signals from infection that are independent of cognate Ag, also play an important role in the robust TFH response seen during influenza infection. One way of supplying these signals is to use live attenuated vaccines. Here, we found that a vaccine regimen supplying Ag and infection signals, at priming and again at the effector checkpoint, could convert a weak response to conventional vaccination, which induced no TFH to a TFH response comparable to infection (Fig. 6). A second dose of IIV provided at the checkpoint was insufficient to induce TFH at comparable levels to live infection and did not induce IgG1 Ab, which is known to require TFH help (28). In contrast, two doses of LAIV, which uses cold-adapted influenza viruses as does the Flumist vaccine, induced TFH as well as IgG1 and IgG2 plasmablasts comparable to influenza infection. In humans, LAIV has been shown to induce broader and more long-lived Ab responses in contrast to inactivated vaccines that are provided intramuscularly and do not provide signals from infection (5).
Insights from our study of mechanisms driving robust TFH during influenza infection are also likely applicable to vaccine design for other infectious diseases. Our results suggest that an ideal universal influenza vaccine must, like natural IAV infection, provide the effector phase signals identified here at the right time and in the relevant sites, in order to drive an effective and durable anti-influenza response which is dependent on robust TFH and GC-TFH (27).
Materials and Methods
Mice.
C57BL/6J (B6), B6.CD45.1, B6.Thy1.1, B6.Nr4a1eGFP (Nur77GFP), and B6.MHC-II− were obtained from the Jackson Laboratory. Y-linked B6.OT-II mice were obtained from Dr. Linda Bradley and were originally published by Dr. Frank Carbone’s group (49); JhD mice were obtained from Dr. Mark Shlomchik; CD11cTg.H2-Ab1−/− mice were obtained from Dr. Terri Laufer; BALB/c.HNT were obtained from Dr. David Lo (50), and these strains were bred and maintained at the UMMS animal facility. Mice were at least 8 wk old prior to use.
Virus Stocks, Infections, and Immunizations.
IAVs A/Puerto Rico/8/34 (PR8), originally from St. Jude Children’s Hospital, and A/PR8-OVAII, kindly provided by Dr. Peter Doherty, were grown and maintained at the Trudeau Institute. Other viruses - LAIV, attenuated ca.A/Alaska/72/CR9 (ca.Alaska) (H3N2) and IBV, B/Ann Arbor/1/66 were originally supplied by S. Epstein (National Institutes of Health, Bethesda, MD) and grown in the Trudeau Institute. Mice were immunized i.n. with 2,500 median tissue culture infections dose (TCID50) ca.Alaska as in our previous study (7) on d 0 and both i.n. and i.v. on d 6 to ensure that Ag is supplied to the spleen. Formalin-IIV (A/PR/8/34 [H1N1]) was purchased from Charles River Laboratories (material no. 10100782) and used at a dose of 10 µg i.v. as in our previous studies (8). Mice were anesthetized with isoflurane (Piramal Healthcare) or with ketamine/xylazine (at a dose of 25/2.5 mg/kg by i.p. injection) and were infected i.n. with influenza virus corresponding to a 0.2 to 0.3 medial lethal dose (LD50) dose of IAV in 50 µL of phosphate buffered saline (PBS).
In vivo-generated 6-d CD4 T cell effectors were routinely obtained as described previously (7). An in-depth description is provided in the SI Appendix.
In Vivo APC Delivery.
To deliver Ag/APC (bone marrow derived DC [BMDC] or activated B cells), APCs were pulsed with 10 μM OVA323–339 (OVAII) peptide (New England Peptide) or no peptide as a negative control (unpulsed APCs) for 1 h at 37 °C with shaking. APCs were washed and administered either i.v. in 200 µL PBS, i.n. in 50 µL PBS, or i.s. in 10 µL PBS. A total of 0.25 × 106 to 1 × 106 BMDC or 1 × 106 B cells were transferred i.v., 0.5 × 106 to 2 × 106 BMDC or 1 × 106 to 2 × 106 B cells were transferred i.n., and 0.5 × 106 to 1 × 106 B cells were transferred i.s. Details about the i.s. APC transfer are provided in the SI Appendix.
Flow cytometry and enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (51). An in-depth description is provided in the SI Appendix.
Study Approval.
Experimental animal procedures were done in accordance with University of Massachusetts Medical School (UMMS) Animal Care and Use Committee guidelines that meet Institutional Animal Care and Use Committee (IACUC) guidelines.
Supplementary Material
Acknowledgments
We thank Dr. Richard Dutton, Dr. Kai McKinstry, and Dr. Tara Strutt for insightful discussions. We thank Dr. Jillian Richmond and Dr. Uthaman Gowthaman for manuscript feedback. We also thank Dr. Esteban Rozen, Yi Kuang, Jialing Liang, and Mike Perkins for assistance with experiments and animal husbandry. This work was supported by Grants U19 AI109858, P01 AI046530, R01 AI118820, and R21 AI128606 to S.L.S.; T32 AI007349 to A.M.V., O.K.-U., and M.C.J.; and R25 GM113686 and T32 AI132152 to O.K.-U.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2111064119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Crotty S., T follicular helper cell biology: A decade of discovery and diseases. Immunity 50, 1132–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koutsakos M., Nguyen T. H. O., Kedzierska K., With a little help from T follicular helper friends: Humoral immunity to influenza vaccination. J. Immunol. 202, 360–367 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Richards K. A., et al. , Evidence that blunted CD4 T-cell responses underlie deficient protective antibody responses to influenza vaccines in repeatedly vaccinated human subjects. J. Infect. Dis. 222, 273–277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiPiazza A. T., et al. , A novel vaccine strategy to overcome poor immunogenicity of avian influenza vaccines through mobilization of memory CD4 T cells established by seasonal influenza. J. Immunol. 203, 1502–1508 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krammer F., The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 19, 383–397 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Jelley-Gibbs D. M., et al. , Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 202, 697–706 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bautista B. L., et al. , Short-lived antigen recognition but not viral infection at a defined checkpoint programs effector CD4 T cells to become protective memory. J. Immunol. 197, 3936–3949 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia J., Kuang Y., Liang J., Jones M., Swain S. L., Influenza vaccine-induced CD4 effectors require antigen recognition at an effector checkpoint to generate CD4 lung memory and antibody production. J. Immunol. 205, 2077–2090 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eldi P., Chaudhri G., Nutt S. L., Newsome T. P., Karupiah G., Viral replicative capacity, antigen availability via hematogenous spread, and high TFH:TFR ratios drive induction of potent neutralizing antibody responses. J. Virol. 93, e01795-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allie S. R., Randall T. D., Pulmonary immunity to viruses. Clin. Sci. (Lond.) 131, 1737–1762 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Strutt T. M., et al. , Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol. Rev. 255, 149–164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinuesa C. G., Linterman M. A., Yu D., MacLennan I. C., Follicular helper T cells. Annu. Rev. Immunol. 34, 335–368 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Deenick E. K., et al. , Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33, 241–253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumjohann D., et al. , Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 38, 596–605 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Tam H. H., et al. , Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. U.S.A. 113, E6639–E6648 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merkenschlager J., et al. , Dynamic regulation of TFH selection during the germinal centre reaction. Nature 591, 458–463 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinstry K. K., et al. , Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat. Commun. 5, 5377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnaswamy J. K., Alsén S., Yrlid U., Eisenbarth S. C., Williams A., Determination of T follicular helper cell fate by dendritic cells. Front. Immunol. 9, 2169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhotra D., Fletcher A. L., Turley S. J., Stromal and hematopoietic cells in secondary lymphoid organs: Partners in immunity. Immunol. Rev. 251, 160–176 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crotty S., T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Künzli M., et al. , Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci. Immunol. 5, eaay5552 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Fazilleau N., McHeyzer-Williams L. J., Rosen H., McHeyzer-Williams M. G., The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 10, 375–384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J. Y., et al. , The transcription factor KLF2 restrains CD4+ T follicular helper cell differentiation. Immunity 42, 252–264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swarnalekha N., et al. , T resident helper cells promote humoral responses in the lung. Sci. Immunol. 6, eabb6808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulendran B., Maddur M. S., Innate immune sensing and response to influenza. Curr. Top. Microbiol. Immunol. 386, 23–71 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terajima M., Babon J. A., Co M. D., Ennis F. A., Cross-reactive human B cell and T cell epitopes between influenza A and B viruses. Virol. J. 10, 244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devarajan P., et al. , New insights into the generation of CD4 memory may shape future vaccine strategies for influenza. Front. Immunol. 7, 136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyauchi K., et al. , Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 17, 1447–1458 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Broadley I., Pera A., Morrow G., Davies K. A., Kern F., Expansions of cytotoxic CD4+CD28- T cells drive excess cardiovascular mortality in rheumatoid arthritis and other chronic inflammatory conditions and are triggered by CMV infection. Front. Immunol. 8, 195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gensous N., et al. , T follicular helper cells in autoimmune disorders. Front. Immunol. 9, 1637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Botta D., et al. , Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat. Immunol. 18, 1249–1260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitano M., et al. , Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity 34, 961–972 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Shi J., et al. , PD-1 controls follicular T helper cell positioning and function. Immunity 49, 264–274.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber J. P., et al. , ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2. J. Exp. Med. 212, 217–233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbet G., et al. , Sensing microbial viability through bacterial RNA augments T follicular helper cell and antibody responses. Immunity 48, 584–598.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballesteros-Tato A., Randall T. D., Priming of T follicular helper cells by dendritic cells. Immunol. Cell Biol. 92, 22–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deenick E. K., Ma C. S., Brink R., Tangye S. G., Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr. Opin. Immunol. 23, 111–118 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Khan T. N., Mooster J. L., Kilgore A. M., Osborn J. F., Nolz J. C., Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med. 213, 951–966 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allie S. R., et al. , The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 20, 97–108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMaster S. R., et al. , Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol. 11, 1071–1078 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takamura S., et al. , Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med. 213, 3057–3073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardi N., et al. , Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 215, 1571–1588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ndeupen S., et al. , The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience (2021). 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed]
- 44.Moyer T. J., Zmolek A. C., Irvine D. J., Beyond antigens and adjuvants: Formulating future vaccines. J. Clin. Invest. 126, 799–808 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirelli K. M., Crotty S., Germinal center enhancement by extended antigen availability. Curr. Opin. Immunol. 47, 64–69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cirelli K. M., et al. , Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell 177, 1153–1171.e28 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boopathy A. V., et al. , Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proc. Natl. Acad. Sci. U.S.A. 116, 16473–16478 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth G. A., et al. , Injectable hydrogels for sustained codelivery of subunit vaccines enhance humoral immunity. ACS Cent. Sci. 6, 1800–1812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnden M. J., Allison J., Heath W. R., Carbone F. R., Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998). [DOI] [PubMed] [Google Scholar]
- 50.Scott B., et al. , A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity 1, 73–83 (1994). [DOI] [PubMed] [Google Scholar]
- 51.Kamperschroer C., Dibble J. P., Meents D. L., Schwartzberg P. L., Swain S. L., SAP is required for Th cell function and for immunity to influenza. J. Immunol. 177, 5317–5327 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.