Abstract
Dyspnea is reported in a minority of patients affected by coronavirus disease 2019 (COVID-19). Even patients with pneumonia can present hypoxemia without any respiratory distress, a phenomenon known as “silent” or “happy hypoxemia”. During the current pandemic there were only a few studies conducted on this subject and these were quite heterogeneous. Therefore, the prevalence of “silent hypoxemia” varied substantially. While studies did not show a clear tendency of “silent hypoxemia” to poorer outcomes compared to hypoxemia presenting with dyspnea, several showed that patients with “silent hypoxemia” are not protected from poor outcomes either. There is a need for a uniform definition of “silent hypoxemia”, in order to better guide clinicians and investigators. More studies are needed to shed light on the mechanisms of “silent hypoxemia”, as well as its presentation and influence in the disease's progression and outcomes, so as to better assist physicians in the care of COVID-19 patients.
Keywords: COVID-19, “happy hypoxemia”, “silent hypoxemia”, pulse oximetry, acute respiratory failure
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified in Wuhan, China, in December 2019, 1 and quickly spread across the globe, putting unprecedented strain on healthcare systems. As of December 2021, it was responsible for over 267 million cases and over 5 million deaths. 2
According to the WHO-China joint mission report, COVID-19 presents with shortness of breath on 18.6% of cases. 3 Guan et al., 4 characterizing COVID-19 patients in China, also reported dyspnea in only 18.7% of the 1099 hospitalized COVID-19 patients included in their study, despite 41% of the study population having required supplemental oxygen therapy. This illustrates the clinical heterogeneity of COVID-19. While some hypoxemic patients present with extreme dyspnea, others show no signs of dyspnea or breathing difficulty, a phenomenon known as “silent” or “happy” hypoxemia. 5 This has generated great interest in the clinical and scientific community, as well as in the mass media. 6
The purpose of this review is to discuss the evidence regarding the prevalence, presentation and outcomes of “silent hypoxemia” in the context of COVID-19.
Material and Methods
The authors performed a literature review on PubMed until November 22th 2021, identifying the current available data on the prevalence, presentation and outcomes of “silent hypoxemia” in COVID-19. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). 7 The search terms included were “COVID-19” or “Coronavirus” and “Silent Hypoxemia” or “Happy Hypoxemia”. Prospective and retrospective studies were included. The literature search was restricted to studies published in English. The articles were initially screened by title and abstract. Those that remained unclear from the title or abstract were reviewed according to the selection criteria in a full-text review.
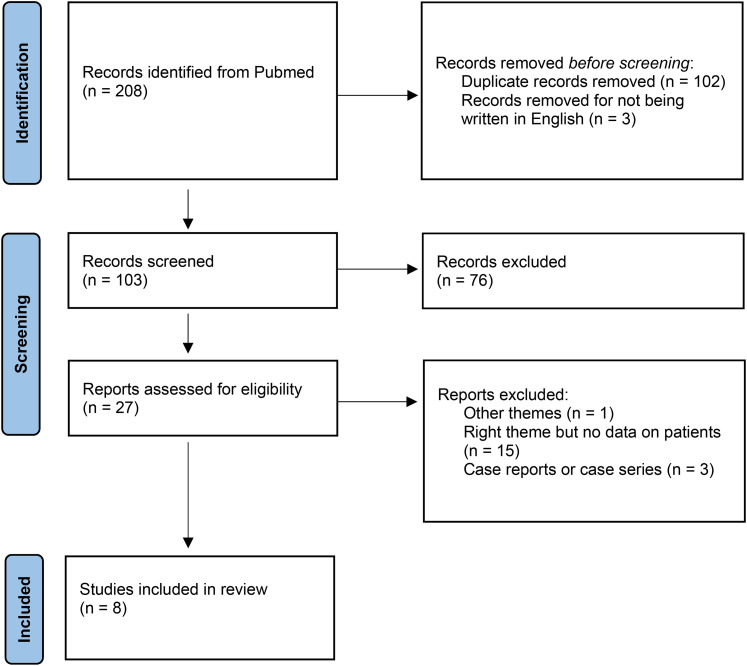
In the database search, we identified 208 articles; among these, 200 were excluded after screening the full text or abstract. A total of 8 studies were finally included in the review (Figure 1).
Figure 1.
PRISMA flowchart.
Literature Review
The reviewed studies are summarized in Table 1.
Table 1.
Summary of included studies.
| Authors | Reference | Country | Study type | Participants (n) | Prevalence of “Sylent Hypoxemia” (%) | Main results |
|---|---|---|---|---|---|---|
| Jouffroy et al. | 8 | France | Retrospective | 1201 | – | Median 2020 SpO2i/RRi value significantly higher than that of patients treated in the previous 3 years |
| Borgne et al. | 9 | France | Retrospective multicentric | 103 | 45.6 | Duration of mechanical ventilation and total length of hospitalization more prolonged in “silent hypoxemia” patients |
| Busana et al. | 10 | Italy | Retrospective multicentric | 213 | 31.9 | “Silent Hypoxemia” patients versus dyspneic patients:
Respiratory rate was an independent predictor of in-hospital mortality for the “silent hypoxemia” patients |
| Fuglebjerg et al. | 12 | Denmark | Prospective single-center | 26 | – | Half of the 26 COVID-19 patients did not complete the 6MWT due to an SpO2 inferior to 90%. Four were later found to have a pulmonary embolism. The COVID-19 subjects had less dyspnea, as measured by the Borg scale, than the IPF historical cohort. |
| García-Grimshaw et al. | 13 | Mexico | Prospective single-center | 470 | 4.9 | “Silent hypoxemia” patients arrived 2 days earlier than the dyspneic patients and had a greater prevalence of new-onset headache |
| Okuhama et al. | 14 | Japan | Retrospective single-center | 270 | 3 |
“Silent hypoxemia” patients
|
| Brouqui et al. | 15 | France | Retrospective single-center | 1712 | 56.5 | Dyspneic patients: significantly lower SpO2 and worse radiological findings. Non-dyspneic patients (with a blood gas analysis): 28.1% with a hypoxemia/hypocapnia syndrome
|
| Alhusain et al. | 16 | Saudi Arabia | Restrospective single-center | 195 | 12.8 | “Silent hypoxemia” patients versus dyspneic patients:
|
Jouffroy et al., 8 in France, retrospectively studied 1201 COVID-19 patients with acute respiratory failure who were treated by the Paris Fire Brigade's basic life-support teams in the prehospital setting. By analyzing their initial peripheral oxygen saturation (SpO2) and initial respiratory rate (RR), the authors calculated their SpO2/RR ratios, which they then compared to those of non-COVID-19 acute respiratory failure patients treated in the prehospital setting in the same period of the previous three years. They observed that median SpO2/RR value was significantly higher in the COVID-19 patients, which led them to conclude that a normal breathing rate could mask profound hypoxia in these patients.
Le Borgne et al. 9 conducted a retrospective multicentric study in the six main emergency departments (EDs) in the Greater East region of France including COVID-19 patients that were admitted to the intensive care units (ICUs). The authors defined two clinical phenotypes in the study population: Type 1 corresponding to “silent hypoxemia” without acute respiratory distress and Type 2 associating hypoxemia and clinical respiratory failure. The study included a total of 103 patients, 47 presenting with the Type 1 phenotype and 56 with the Type 2. The duration of mechanical ventilation and the total length of hospitalization were more prolonged in Type 1 patients. However, the authors found no differences in the length of ICU stay or in-hospital mortality between the two populations.
A retrospective multicentric study by Busana et al. 10 included COVID-19 patients from Luigi Sacco Hospital, Milan, and Umberto I Policlinic, Rome, with arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2) ratio less than 300 mmHg. Of the 213 patients studied, 68 (31.9%) did not have dyspnea (“silent hypoxemia”). Fever was more frequent in the dyspneic patients, while the “silent hypoxemia” patients suffered more frequently from non-respiratory symptoms such as myalgia, diarrhea and nausea. The two groups presented no differences in laboratory findings, except for higher lactate dehydrogenase (LDH) values in the dyspneic patients group. Six out of the 68 non-dyspneic patients (8.8%) and 31 out of the 145 dyspneic patients (21.3%) had a ROX index 11 below 4.88, calculated as the ratio between SaO2/FiO2 and RR. Of note, the authors found that, while the proportion of dyspneic patients increased with chest x-ray severity, a considerable fraction of them remained eupneic regardless of the severity of the radiological findings. The only independent predictor of in-hospital mortality for the “silent hypoxemia” patients was the RR.
Fuglebjerg et al. 12 conducted a study to access the degree of hypoxia and dyspnea elicited by a 6-minute walking test (6MWT) in COVID-19 patients hospitalized at the planed time of discharge from the University Hospital of Copenhagen, North Zealand, Denmark. The study included 26 COVID-19 patients without chronic pulmonary disease or cardiac failure and compared them to a previous cohort of 204 idiopathic pulmonary fibrosis (IPF) patients. The 6MWT was not completed in 13 of the 26 COVID-19 patients due to an SpO2 inferior to 90%. Four of these patients were later found to have a pulmonary embolism. The COVID-19 subjects experienced less dyspnea, as measured by the Borg scale, than the IPF patients, despite presenting a similar decrease in SpO2.
García-Grimshaw et al. 13 conducted a prospective cohort study of COVID-19 patients hospitalized at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico, who presented to the ED with an SpO2 below 80% while breathing room air. Of the 470 patients included, 447 (95.1%) presented dyspnea and 23 (4.9%) had “silent hypoxemia”. The latter arrived two days earlier than the dyspneic patients and had a greater frequency of new-onset headache. There were no differences between the two groups in terms of the severity of lung involvement measured by chest computed tomography (CT) scan or PaO2/FiO2 ratio. The authors also found no differences in the groups’ requirements of invasive mechanical ventilation and UCI admission, length of hospitalization and in-hospital mortality.
Okuhama et al. 14 conducted a retrospective study of COVID-19 patients from the National Center for Global Health and Medicine, Tokyo, Japan, who did not report dyspnea on admission despite having a SpO2 below 94%. Of the 270 COVID-19 patients hospitalized during the study period, 8 (3%) met those criteria. All of them had a moderate to high COVID-19 CT severity score. Only one patient did not require administration of supplemental oxygen. The median of the maximum FiO2 required was 55%. During the disease course, two of the non-dyspneic patients (25%) required intubation and one (12.5%) needed extracorporeal membrane oxygenation (ECMO). There were no deaths.
Brouqui et al. 15 studied COVID-19 patients diagnosed and treated in the infectious disease institute (IHU Mediterranée Infection) in Marseille, France. Of the 1712 COVID-19 patients included, 1107 (64.7%) presented without dyspnea. Of these, 757 (68.4%) had signs compatible with pneumonia on the CT scans. Of the 1107 non-dyspneic patients, 157 (14.2%) had SpO2 equal or inferior to 95%. Compared to patients without dyspnea, dyspneic patients had a significantly lower SpO2, as well as worse radiological findings. Of the 96 non-dyspneic patients who had a blood gas analysis, 27 (28.1%) presented with a hypoxemia/hypocapnia syndrome, which was associated with poor outcome. Of these patients, 33.3% required admission to the ICU and 25.9% died.
Alhusain et al. 16 conducted a retrospective cohort study based on data from the electronic information system at a tertiary hospital in Saudi Arabia. The study included all COVID-19 patients admitted through the ED with SpO2 below 90% on room air between March 24th and December 31st, 2020. Of the 195 patients, 25 (12.8%) presented with silent hypoxemia. These had significantly less cough (40%) and fever (40%) than the dyspneic group (82% and 72% respectively). The silent hypoxemia group had also a higher prevalence of chronic cardiac disease (32%) than the dyspnea group (14%). Intubation and ICU admission were higher in the dyspnea group compared to the silent hypoxemia group, with the latter achieving statistical significance. There was no difference in mortality between the two groups.
Discussion
The publications on the subject of “silent” or “happy hypoxemia” in COVID-19 are scant and quite heterogeneous. The authors use different definitions and SpO2 cutoffs for “silent hypoxemia”, as well as different study designs. Therefore, the prevalence of “silent hypoxemia” varies substantially in the different studies. Studies also did not show a clear tendency of “silent hypoxemia” to poorer outcomes compared to hypoxemia presenting with dyspnea. However, several studies showed that patients with “silent hypoxemia” are not protected from poor COVID-19 outcomes, including ICU admission, intubation and death.
Several mechanisms have been proposed to explain “silent hypoxemia” in COVID-19. Tobin et al. 17 discussed potential causes: (1) SARS-CoV-2 might have an idiosyncratic effect on the respiratory control system, by affecting the chemoreceptors in the carotid body or by entering the brain through the olfactory bulb and depressing the dyspnea response; (2) ventilatory and dyspnea responses to hypoxia are greatly influenced by partial pressure of carbon dioxide (PaCO2) and severe hypoxia leads to an effective increase in ventilation only when PaCO2 exceeds 39 mmHg; (3) the ventilatory response to hypoxia is decreased by 50% in diabetic patients and in people older than 65 years, two groups in higher risk from COVID-19; (4) the chemical drive to breathe presents as much as 300% to 600% variation between subjects.
Ottestad and Søvik 18 hypothesized that the increased minute ventilation, as a compensatory ventilatory response to hypoxemia, leads to extreme hypocapnia as carbon dioxide (CO2) diffuses through tissues 20 times quicker than oxygen (O2). Another publication, by Archer et al., 19 suggested that “silent hypoxemia” might be caused by failure of the body's O2-sensing system through the loss of hypoxic pulmonary vasoconstriction and altered carotid body function.
Anoop and Verma 20 provided another potential explanation: SARS-CoV-2 takes a neural route through the facial, glossopharyngeal and vagus nerves, leading to inflammation of the nucleus tractus solitarius. Thus, the afferent hypoxia stimuli from the carotid bodies may not be effectively relayed at this nucleus, resulting in an impaired efferent respiratory response.
González-Duarte and Norcliffe-Kaufmann 21 suggested “happy hypoxemia” in COVID-19 patients was a disorder of blood-gas interoception, possibly due to the cytokine storm or the direct effect of SARS-COV2 on mitochondria or on the nerve fibers, which might damage the afferent hypoxia-sensing neurons.
Contrastingly, Ora et al. 22 pointed out that, while there might be COVID-19 patients who do not perceive dyspnea despite severe hypoxemia, there was no evidence that this phenomenon was more prevalent in COVID-19 pneumonia than in other pneumonias or that it was caused by a neurologic pathway. The authors stated that, as hypoxemia is a weak stimulus for respiratory drive and consequently for dyspnea, in the absence of hypercapnia or a decrease in pH, there is only a physiological blunted ventilation to the hypoxic stimulus, more than “happy hypoxemia”.
Dhont et al. 23 stated that hypoxemia in SARS-CoV-2 pneumonia is caused by intrapulmonary shunting, dysregulated hypoxic pulmonary vasoconstriction, impaired lung diffusion, and formation of intravascular microthrombi. According to the authors, as in the first days of the disease the lung mechanics are well-preserved and there is no increased airway resistance or dead space ventilation, there is no dyspnea at this point. However, with disease progression, the more consolidated air spaces reduce long compliance and increases the work of breathing, inducing dyspnea.
Chauhan et al. 24 propose that, initially, only O2 exchange is compromised, not CO2. Therefore, patients feel no dyspnea and hypoxia is compensated by tachypnea while the lung injury increases, up to the point when a cytokine storm takes place, with subsequent hypercapnia, dyspnea and severe respiratory failure. Zubieta-Calleja and Zubieta-DeUrioste 25 used arterial blood gas measurements to illustrate the three theoretical pathophysiological stages of progressive hypoxemia in COVID-19: silent hypoxemia, when there is a progressive decrease in the PaO2 but minimal change in PaCo2; gasping; when PaCO2 increases and the pH decreases sharply; and death zone.
It is unclear if “silent hypoxemia” and hypoxemia with dyspnea are two separate phenotypes of COVID-19 or two phases of the disease temporal spectrum, as has been suggested by Gattinoni et al. 5 They described two COVID-19 phenotypes: Type L, characterized by low elastance (high compliance), low ventilation-to-perfusion ratio, low lung weight and low recruitability; and Type H, characterized by high elastance, high right-to left shunt, high lung weight and high recruitability. The Type L patients would present without dyspnea and, as the disease progressed, would either transition to Type H or improve. The author also advocated that these phenotypes warranted a different therapeutic approach.
Several publications highlight the value of using pulse oximetry to monitor non-dyspneic COVID-19 patients self-isolating at home as a mean to predict the need of further evaluation and treatment.26–28 Considering the availability of smartphones in the general population, several authors also proposed smartphone-based pulse oximetry as an alternative to finger oximeters in detecting “silent hypoxemia” in COVID-19 patients.29–31
Given the relevance of this entity, there is a need for the medical community to come up with a uniform definition of “silent hypoxemia”, in order to better guide clinicians and investigators. More studies are needed to shed light on the mechanisms of “silent hypoxemia”, as well as its presentation and role in the disease's progression and outcomes, so as to better assist physicians namely, but not only, in the care of COVID-19 patients.
Footnotes
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Amélia Ribeiro https://orcid.org/0000-0002-5150-8395
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, R&D Blueprint and COVID-19. Accessed December 10th 2021. https://covid19.who.int/.
- 3.Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). Accessed August 27th 2021. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitant R. The Infection That’s Silently Killing Coronavirus Patients; The New York Times Opinion (updated April 26, 2020). Accessed August 27th 2021. https://www.nytimes.com/2020/04/20/opinion/sunday/coronavirus-testing-pneumonia.html
- 7.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jouffroy R, Jost D, Prunet B. Prehospital pulse oximetry: a red flag for early detection of silent hypoxemia in COVID-19 patients. Crit Care. 2020;24(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Borgne P, Oberlin M, Bassand A, et al. Pre-hospital management of critically ill patients with SARS-CoV-2 infection: a retrospective multicenter study. J Clin Med. 2020;9(11):3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busana M, Gasperetti A, Giosa L, et al. Prevalence and outcome of silent hypoxemia in COVID-19. Minerva Anestesiol. 2021;87(3):325-333. [DOI] [PubMed] [Google Scholar]
- 11.Roca O, Messika J, Caralt B, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. 2016. Oct;35:200-205. [DOI] [PubMed] [Google Scholar]
- 12.Fuglebjerg NJU, Jensen TO, Hoyer N, et al. Silent hypoxia in patients with SARS CoV-2 infection before hospital discharge. Int J Infect Dis. 2020 Oct;99:100-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Grimshaw M, Flores-Silva FD, Chiquete E, et al. Characteristics and predictors for silent hypoxemia in a cohort of hospitalized COVID-19 patients. Auton Neurosci. 2021 Nov;235:102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuhama A, Ishikane M, Hotta M, et al. Clinical and radiological findings of silent hypoxia among COVID-19 patients. J Infect Chemother. 2021;27(10):1536-1538. Epub 2021 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouqui P, Amrane S, Million M, et al. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int J Infect Dis. 2021. Jan;102:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhusain F, Alromaih A, Alhajress G, et al. Predictors and clinical outcomes of silent hypoxia in COVID-19 patients, a single-center retrospective cohort study. J Infect Public Health. 2021;14(11):1595-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottestad W, Søvik S. COVID-19 patients with respiratory failure: what can we learn from aviation medicine? Br J Anaesth. 2020; Sep;125(3):e280-e281. [published online ahead of print, 2020 Apr 18]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications. Circulation. 2020;142(2):101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anoop UR, Verma K. Happy hypoxemia in COVID-19-A neural hypothesis. ACS Chem Neurosci. 2020;11(13):1865-1867. [DOI] [PubMed] [Google Scholar]
- 21.González-Duarte A, Norcliffe-Kaufmann L. Is “happy hypoxia” in COVID-19 a disorder of autonomic interoception? A hypothesis. Clin Auton Res. 2020;30(4):331-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ora J, Rogliani P, Dauri M, O’Donnell D. Happy hypoxemia, or blunted ventilation? Respir Res. 2021;22(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhont S, Derom E, Van Braeckel E, et al. The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res. 2020;21(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan V, Galwankar SC, Yellapu V, et al. State of the globe: the trials and tribulations of the COVID-19 pandemic: separated but together, telemedicine revolution, frontline struggle against “silent hypoxia,” the relentless search for novel therapeutics and vaccines, and the daunting prospect of “COVIFLU”. J Glob Infect Dis. 2020;12(2):39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zubieta-Calleja G, Zubieta-DeUrioste N. Pneumolysis and “silent hypoxemia” in COVID-19. Indian J Clin Biochem. 2020;36(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home. potential pitfalls and practical guidance. Ann Am Thorac Soc. 2020;17(9):1040-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020;38(10):2243. e5-2243. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med. 2020;27(8):681-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teo J. Early detection of silent hypoxia in COVID-19 pneumonia using smartphone pulse oximetry. J Med Syst. 2020;44(8):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomlinson S, Behrmann S, Cranford J, et al. Accuracy of smartphone-based pulse oximetry compared with hospital-grade pulse oximetry in healthy children. Telemed J E Health. 2018;24(7):527-535. [DOI] [PubMed] [Google Scholar]
- 31.Tayfur İ, Afacan MA. Reliability of smartphone measurements of vital parameters: a prospective study using a reference method. Am J Emerg Med. 2019;37(8):1527-1530. [DOI] [PubMed] [Google Scholar]