Abstract
Objectives
Prone positioning is widely used in mechanically ventilated patients with COVID-19; however, the specific clinical scenario in which the individual is most poised to benefit is not fully established. In patients with COVID-19 respiratory failure requiring mechanical ventilation, how effective is prone positioning in improving oxygenation and can that response be predicted?
Design
This is a retrospective observational study from two tertiary care centers including consecutive patients mechanically ventilated for COVID-19 from 3/1/2020 – 7/1/2021. The primary outcome is improvement in oxygenation as measured by PaO2/FiO2. We describe oxygenation before, during and after prone episodes with a focus on identifying patient, respiratory or ventilator variables that predict prone positioning success.
Setting
2 Tertiary Care Academic Hospitals
Patients
125 patients mechanically ventilated for COVID-19 respiratory failure.
Interventions
Prone positioning
Main Results
One hundred twenty-five patients underwent prone positioning a total of 309 times for a median duration of 23 hours IQR (14 – 49). On average, PaO2/FiO2 improved 19%: from 115 mm Hg (80 – 148) immediately before proning to 137 mm Hg (95 – 197) immediately after returning to the supine position. Prone episodes were more successful if the pre-prone PaO2/FiO2 was lower and if the patient was on inhaled epoprostenol (iEpo). For individuals with severe acute respiratory distress syndrome (ARDS) (PaO2/FiO2 < 100 prior to prone positioning) and on iEpo, the median improvement in PaO2/FiO2 was 27% in both instances.
Conclusions
Prone positioning in mechanically ventilated patients with COVID-19 is generally associated with sustained improvements in oxygenation, which is made more likely by the concomitant use of iEpo and is more impactful in those who are more severely hypoxemic prior to prone positioning.
Keywords: prone positioning, ARDS, COVID-19, oxygenation, mechanical ventilation, paO2/fiO2
Background
The COVID-19 pandemic has been marked by countless cases of respiratory failure that have inundated intensive care units (ICUs) worldwide.1 The severe pneumonia that results from COVID-19 infection requires mechanical ventilation in 10 – 20% of those hospitalized.2,3 It is apparent that respiratory failure due to COVID-19 is unique in several ways, among them: improved outcomes with immunosuppression,4–6 increased venous thromboembolism frequency,7.8 and a protracted course.9 Additionally, there appears to be a continuum of respiratory physiology, marked by differing lung compliance and imaging findings at various times in the disease course.10,11 Professional societies recommend managing COVID-1912,13 similarly to traditional ARDS,14 including: lung protective ventilation,15 conservative fluid management,16 neuromuscular blockade in select cases17 along with consideration of inhaled pulmonary vasodilators.18 Likewise, prone positioning has also been recommended and used based on previously established evidence,19 with one study demonstrating more frequent use of prone positioning in COVID-ARDS versus non-COVID-ARDS.20 However, the efficacy of prone positioning in respiratory failure due to COVID-19 remains under active investigation.21,22
In 2013, a landmark study demonstrated that early, repeated, and extensive (∼16 hours/day) prone positioning in patients with moderate to severe ARDS results in improved oxygenation, fewer days spent mechanically ventilated, and substantially improved mortality.19 The practical benefits of prone positioning are numerous: it's not a drug, there is no specific adverse effect or expense, and the timing and frequency are flexible. However, it is resource intensive and time consuming, which is particularly salient in overwhelmed ICUs. Despite that, the use of prone positioning has been widespread during the pandemic20 – including, notably, an expansion of the practice to non-intubated patients.23,24 In fact, hospital systems have established teams dedicated to navigating the logistical hurdles of proning patients on multiple organ support therapies.25
There are several reports of improved PaO2/FiO2 in COVID-19 respiratory failure immediately after assuming the prone position21,26,27, and sustained PaO2/FiO2 improvement with multiple episodes of prone positioning.22,28 Also, it has been shown that lung recruitment improves with prone positioning in COVID-19, similar to classic ARDS.26,27,29 However, currently available studies present conflicting data on the value of prone positioning's oxygenation benefit, such that there isn’t a clear conclusion about the utility of prone positioning in COVID-19, including the efficacy of prolonged and/or multiple episodes, and who specifically stands to benefit.10,21
Anecdotally, our experience is that some patients have marked prone responsiveness (as measured by improved oxygenation) while others do not. Therefore, we seek to describe the physiologic changes (in terms of oxygenation and lung compliance) associated with prone positioning of mechanically ventilated patients with COVID-19. We additionally attempt to determine if there are patient-level variables that predict if an individual will be a “prone responder,” given the importance of efficient resource utilization during the pandemic.
Methods
This is a retrospective study involving two tertiary care centers in a single heath system in Washington, DC: Medstar Georgetown University Hospital and Medstar Washington Hospital Center. All consecutive patients from 3/1/2020 to 7/1/2021 were included if diagnosed with SARS-CoV2, were mechanically ventilated for hypoxemic respiratory failure, and underwent prone positioning. The hospitals share a set of guidelines regarding the general management of patients with COVID-19; however, prone positioning was performed at the treating clinician's discretion. All instances of prone positioning for a particular individual during the hospitalization were included. Patients on extracorporeal membrane oxygenation (ECMO) were excluded.
The primary outcome is the change in oxygenation, as measured by PaO2/FiO2. We calculated PaO2/FiO2 using the arterial blood gas (ABG) at four separate time points: immediately before and after prone positioning and immediately before and after returning to the supine position, using the ABG closest in proximity to that time. We defined an individual as a “prone responder” if the PaO2/FiO2 improved 10% while prone, and a “sustained responder” if the PaO2/FiO2 improved 10% after returning to the supine position (with reference to the pre-prone value). We felt a 10% improvement was clinically meaningful, and it is a definition that has been used previously.18,30
To determine if there were features predictive of responding to the prone position, we recorded patient demographics, severity of illness as measured by the sequential organ failure support (SOFA) score31, ventilator parameters (FiO2, positive end expiratory pressure [PEEP], tidal volume [Vt], respiratory rate [RR], plateau pressure, driving pressure [plateau pressure – PEEP], static compliance [Vt / driving pressure]), fluid balance, the use of specific therapeutics (iEpo, steroids, neuromuscular blockade, therapeutic anticoagulation), and laboratory data (C-reactive protein, D-dimer, ESR). Neuromuscular blockade was defined as a continuous infusion of cisatricurium or having received vecuronium within 2 hours of prone positioning. Steroid use was defined as the administration of at least 30 mg of methylprednisolone (or equivalent) within 24 hours of prone positioning. Anticoagulation refers to therapeutic dosing of heparin or enoxaparin. Laboratory data values used were most proximate to proning.
Data were obtained directly from the health system's clinical data warehouse and supplemented with direct review of patient records when needed. Summary statistics describe the frequency of each categorical variable and either mean (for normally distributed) or median (for non-normally distributed) of continuous variables. For the purposes of predictive modelling, we considered the prone event (rather than patient) to be the level of interest in regression modelling using a change in PaO2/FiO2 as the outcome variable. Candidate predictor variables had statistically significant univariate associations or were of particular clinical interest; the models were developed in a stepwise fashion with a stopping rule based on minimum Bayesian Information Criterion (BIC). Data extraction and processing were performed in R, and statistical analysis in JMP 16 Pro (Cary, NJ). This project was approved by the Institutional Review Board (IRB) of Georgetown University.
Results
Patients
During the study period, we identified 125 mechanically ventilated patients with COVID-19 who underwent prone positioning. The mean age was 57 ± 13, 65% were male, 52% Black, and hypertension, diabetes mellitus and chronic obstructive pulmonary disorder (COPD) were the most represented comorbidities (38%, 31% and 20%, respectively) (Table 1). The timing of the first prone event was variable in reference to the initiation of mechanical ventilation: a median of 16 hours afterwards (interquartile range 6 – 49). The median time spent mechanically ventilated was 14 days (9 – 22), 19 (15%) ultimately received a tracheostomy and 75 (60%) died while hospitalized.
Table 1.
Patient Characteristics
| Total (n = 125) | |
|---|---|
| Age, mean (SD) | 57 ± 13 |
| Male, n (%) | 81 (65) |
|
BMI, median (IQR) Morbidly obese (BMI >40), n (%) |
31 (28 – 38) 24 (19) |
|
Race, n (%) African American White Other Unknown |
65 (52) 13 (10) 41 (33) 4 (3) |
|
Comorbidities, n (%) Hypertension Diabetes Mellitus CKD COPD CAD Cancer Cirrhosis Organ Transplant AIDS |
47 (38) 39 (31) 12 (10) 25 (20) 13 (10) 12 (10) 7 (6) 6 (5) 4 (3) |
The ventilator mode for most patients while undergoing prone positioning was assist control with volume cycling (AC/VC) (88%), with the remainder being on airway pressure release ventilation (APRV) (9%), or pressure control ventilation (PCV) (3%). At the time of proning, the median FiO2 was 70 (60 – 100) and PEEP was 12 cm H20 (10 – 14) (Table 2).
Table 2.
Ventilator and Respiratory Parameters during Proning
| N = 309 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| Prone Episode Duration, h | 23 (14 – 49) | |||
| I/O from Intubation, mL | 1600 (200 – 5000) | 2500 (200 – 6500) | ||
| I/O from Admission, mL | 1800 (-500 – 6000) | 2800 (-300 – 7500) | ||
| PEEP, cm H2O | 12 (10 – 14) | 12 (10 – 14) | 12 (10 – 14) | 12 (10 – 14) |
| FiO2 | 70 (60 – 100) | 70 (60 – 100) | 60 (50 – 80) | 60 (50 – 80) |
| Tidal Volume, mL | 420 (360 – 460) | 400 (360 – 450) | 400 (360 – 460) | 400 (360 – 460) |
| Respiratory Rate | 24 (20 – 28) | 24 (20 – 28) | 25 (20 – 30) | 25 (20 – 30) |
| Plateau Pressure, cm H2O | 28 (25 – 30) | 28 (25 – 30) | 27 (25 – 29) | 28 (25 – 30) |
| Static Compliance, mL/cm H2O | 26 (22 – 33) | 27 (22 – 32) | 29 (23 – 33) | 26 (21 – 32) |
| Driving Pressure, cm H2O | 15 (13 – 19) | 15 (12 – 18) | 15 (12 – 18) | 15 (13 – 19) |
| pH | 7.34 (7.29 – 7.4) | 7.35 (7.29 – 7.4) | 7.35 (7.29 – 7.4) | 7.35 (7.28 – 7.4) |
| PaO2, mm Hg | 78 (67 – 101) | 79 (67 – 101) | 83 (70 – 07) | 83 (71 – 107) |
| PaCO2, mm Hg | 47 (41 – 54) | 47 (42 – 55) | 48 (43 – 55) | 48 (42 – 55) |
| PaO2/FiO2, mm Hg | 115 (80 – 148) | 114 (86 – 156) | 140 (93 – 205) | 137 (95 – 197) |
*T1: Supine prior to prone; T2: start of prone; T3: end of prone; T4: after return to supine
Proning
There were 309 prone sessions among the 125 patients, for a median of 2 per patient (IQR [1 – 3], range [1 – 10]) lasting a median duration of 23 hours (14 – 49). For those who underwent prone positioning on multiple occasions, there was a median of 12 hours (5 – 24) between episodes. PaO2/FiO2 was calculated from the arterial blood gases (ABG) most proximal to the four following time points: before and after proning (T1 and T2) and before and after re-supination (T3 and T4) (Table 2). The median time between ABG measurement and patient position change was 4.4 hours (2.1 – 10.7).
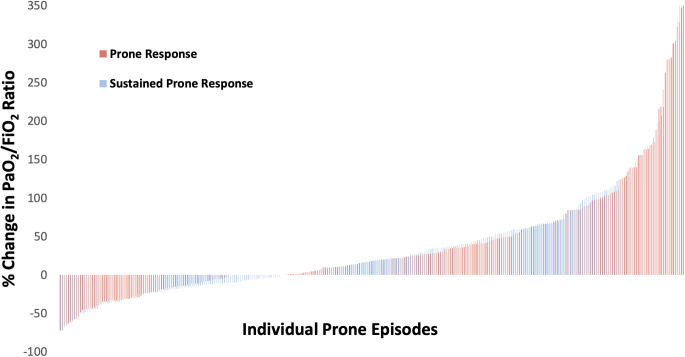
We considered “prone response” as a change in PaO2/FiO2 from T1 to T3 (that is, PaO2/FiO2 measurements before and at the end of prone positioning), and “sustained response” as a change in PaO2/FiO2 from T1 to T4. Using a binary definition of prone or sustained responsiveness as a 10% increase in PaO2/FiO218, there were 176 prone responders (57%) and 173 sustained responders (56%) (Figure 1, Table 3). On average, the sustained response was an improvement in PaO2/FiO2 of 19%: from 115 mm Hg (80 – 148) to 137 mm Hg (95 – 197) (Table 2).
Figure 1.
Graphical representation of the percent change in PaO2/FiO2 for each prone episode's response (change prior to end of prone episode) and sustained response (change after resuming supine position). The median percent change (IQR) for the prone and sustained prone response was 18% (-6 - 61) and 19% (-11 - 62), respectively.
Table 3.
Characteristics of Prone Sessions Based on Responsiveness
| N = 309 | Initial Prone Responder (n = 176) | Prone Non-responder (n = 133) | P-value | Sustained Responder (n = 173) | Sustained Non-Responder (n = 136) | P-value |
|---|---|---|---|---|---|---|
| SOFA score* | 12 (10 – 14) | 12 (9 – 14) | 0.74 | 12 (10-14) | 12 (9-14) | 0.13 |
| Fluid balance / day, mL** | 1400 (100 – 5100) | 2100 (200 – 4800) | 0.42 | 1400 (200 – 4800) | 2400 (300 – 5100) | 0.14 |
| Prone Duration, hr | 24 (16 – 54) | 17 (8 – 32) | <0.01 | 24 (16 – 60) | 18 (12 – 40) | <0.01 |
| Ventilator Days | 17 (10 – 22) | 18 (11 – 25) | 0.57 | 17 (11 – 22) | 18 (9 – 22) | 0.75 |
|
Respiratory parameters FiO2 PaO2 / FiO2, mm Hg Before proning After return to supine PaCO2 |
80 (60 – 100) 105 (76 – 139) 185 (121 – 267) 46 (41 – 53) |
70 (70 – 100) 128 (92 – 177) 109 (93 – 161) 49 (43 – 57) |
0.02 <0.01 0.01 |
80 (60 −100) 100 (72 – 135) 166 (120 – 230) 46 (41 – 53) |
70 (60 – 90) 132 (96 – 191) 108 (78 – 146) 48 (43 – 55) |
<0.01 <0.01 0.12 |
|
Ventilator parameters PEEP, cm H20 Tidal Volume, mL Respiratory Rate Plateau Pressure, cm H20 Driving Pressure, cm H20 Static Compliance, mL/cm H2O |
12 (10 – 14) 420 (370 – 460) 24 (20 – 28) 28 (25 – 30) 15 (13 – 19) 27 (22 – 35) |
12 (10 – 14) 40 (360 – 460) 24 (20 – 28) 28 (26 – 30) 16 (12 – 18) 25 (21 – 30) |
0.66 0.67 0.63 0.96 0.45 0.18 |
12 (10 – 14) 400 (360 – 460) 24 (20 – 28) 29 (26 – 31) 16 (13 – 19) 27 (21 – 32) |
12 (10 – 14) 420 (360 – 460) 24 (20 – 28) 27 (25 – 30) 15 (12 – 18) 26 (22 – 33) |
0.41 0.94 0.91 0.08 0.32 0.89 |
|
Therapeutics, n (%) Neuromuscular Blockade Anticoagulation Steroids Inhaled Epoprostenol |
94 (53) 32 (18) 97 (55) 62 (35) |
74 (56) 28 (21) 74 (56) 31 (23) |
0.69 0.53 0.93 0.02 |
96 (55) 29 (17) 97 (56) 65 (38) |
72 (53) 31 (22) 74 (54) 28 (21) |
0.65 0.25 0.77 <0.01 |
|
Laboratory Data C-reactive protein, mg/L D-dimer, mg/L ESR mm/hr |
163 (88 – 191) 3.7 (2.4 – 10.1) 86 (60 – 86) |
140 (94 – 191) 3.7 (2.6 – 8.8) 86 (72 – 86) |
0.62 0.67 0.32 |
165 (93 – 191) 3.8 (2.3 – 10.6) 86 (61 – 86) |
138 (189 – 191) 3.7 (2.3 – 8.6) 86 (71 – 86) |
0.25 0.96 0.21 |
All continuous variables are presented as medians with interquartile ranges.
Bolded P-values are statistically significant.
*Sequential Organ Failure Assessment (SOFA) score on day of Proning
**Fluid balance since initiation of mechanical ventilation
Those that were prone responders were in prone position longer (24 v. 17 hr, p < 0.01), had a lower initial PaO2/FiO2 (105 v. 128, p < 0.01) and PCO2 (46 v. 49 mm Hg, p = 0.01), and were more likely to be on inhaled epoprostenol (iEpo) (35 v. 23%, p = 0.02) (Table 3). These relationships were true for sustained responders as well, aside from initial PCO2 (Table 3). We did not find initial ventilator parameters or laboratory data to be associated with proning responsiveness (Table 3). Additionally, there were no observed changes in plateau pressure, static compliance and driving pressure while prone or afterwards (Table 2). There was no difference in total ventilator days between groups (Table 3).
Predicting Responsiveness
To explore independent predictors of proning success, we made a logistic regression model using 10% improvement in PaO2/FiO2 as the outcome, and a linear one treating PaO2/FiO2 as a continuous outcome. In both cases we focused on “sustained responders” – that is, a comparison of change in PaO2/FiO2 immediately before proning (T1) to after returning to the supine position (T4). We included variables with statistically significant univariate associations along with time between measurements, PEEP, the use of neuromuscular blockade as additional covariates given the direct relevancy to oxygenation and improvement in PaO2/FiO2 over time. In the logistic model, lower PaO2/FiO2 before proning (adjusted OR 1.15 [95% CI 1.1 – 1.2] p < 0.01 for each 10 mm Hg decrement) and use of iEpo (adjusted OR 1.9 [1.3 – 3.3] p = 0.03) remained the only independent predictors of improved oxygenation. This was true in the linear model as well: PaO2/FiO2 (coefficient 3.4 [2.2 – 4.9] p <0.01) and iEpo (coefficient 14.3 [7.1 −19.2] p = 0.01). After multivariate adjustment, prone duration (p = 0.21 when added to the logistic model, p = 0.25 in linear model) was no longer an independent predictor of PaO2/FiO2 improvement.
There were 82 patients with more than one prone episode (including 13 patients with more than 5 episodes, and 2 patients with 10). In these cases, the initial responsiveness, or lack thereof, to prone positioning did not predict a subsequent response to proning. For those whose initial prone episode was successful, subsequent episodes were successful 50% of the time versus 52% of the time for those whose initial episode was unsuccessful.
Given the association of lower PaO2/FiO2 with prone success, we examined cases of severe ARDS (PaO2/FiO2 < 100 mm Hg at T1). There were 125 such prone episodes (40% of our total sample) of which 65% and 69% had a prone and sustained response (median improvement in PaO2/FiO2 of 27% and 41%, respectively), compared to 57% and 56% response rate in the total sample (18% and 19% improvement in PaO2/FiO2, respectively).
Discussion
COVID-19 is a novel disease with distinct physiology that, provided a lack of high-quality information, clinicians are managing with interventions proven efficacious for “traditional” ARDS – including prone positioning.1,12,13 This maneuver improves ventilation-perfusion matching32, lowers risk of ventilatory-induced lung injury (VILI)33, and improves survival.19 Extrapolating its use to COVID-associated pneumonia is reasonable: there is often refractory hypoxemia, poor lung compliance, and electrical impedance tomography shows an improvement in ventral dead space and shunt while in the prone position.21 However, the specific role of prone positioning in COVID-associated respiratory failure is not fully established – in particular, identifying individuals who most stand to benefit.
Over a 15-month period across two tertiary care hospitals in an area heavily afflicted by the pandemic, we identified 125 patients mechanically ventilated for COVID-19 who were placed in the prone position a total of 309 times. In 56% of the cases there was at least a 10% increase in PaO2/FiO2 after returning to supine position. The mean PaO2/FiO2 improvement of 19% is more modest than what has been previously described26–28; however, similar to prior looks at prone positioning in COVID-1912–28, we observed a continued oxygenation benefit after returning to the supine position in the vast majority of those who responded.22,28 Additionally, there was not an improvement in static lung compliance after prone positioning, which has been observed with prone positioning in COVID-19 elsewhere.34 After multivariate adjustment, prone success was associated with a lower pre-prone PaO2/FiO2 (105 mm Hg v. 128 mm Hg) and receiving iEpo (35% v. 23%).
The mechanistic underpinnings of these findings are not completely clear. Though they may be artifactual due to multiple comparisons, the fact that they remained independent predictors of prone positioning success after adjustment lends credence to the observations. Low PaO2/FiO2 ratios represent ventilation-perfusion decoupling and greater shunt fraction, which is associated with increased dorsal lung density in the supine position, manifested as dense bilateral infiltrates on imaging.35 This offers physiologic plausibility of prone positioning's efficacy in COVID-19, as the positional change in traditional ARDS decreases dorsal lung density, thus providing more even ventilation distribution.15,21,35 Regarding iEpo, we speculate that improved ventilation in the prone position may lead to better drug distribution. It has been previously described that iEpo itself was more likely to be efficacious in mechanically ventilated patients with COVID-19 who were pronated.18 Though the average treatment effect of iEpo is modest36, there is wide variability in the individual treatment effect including those who experience marked improvements in PaO2/FiO2.18,37,38 The literature on biomarkers predicting iEpo responsiveness is sparse and inconsistent.18,38,39 As far as we are aware, augmenting the beneficial physiologic impact of prone positioning with the use of inhaled pulmonary vasodilators has not been previously described.
In contrast to other studies, we focused our analysis on discrete episodes of prone positioning rather than upfront patient features. We did this to generate information that is practical for clinical decision making. Since respiratory failure due to COVID19 is protracted, patient features change over time (eg, oxygenation, ventilator parameters and the use of therapeutics).40,41 Therefore, prone positioning success is more likely related to clinical variables at the time of prone positioning, rather than on admission to the ICU, initiation of mechanical ventilation, or during a patient's prior prone instance. Moreover, we recorded the effect of multiple sequential prone episodes along with prone duration in combination with other therapeutic interventions. While prone duration had a favorable univariate association with prone responsiveness, it did not after multivariate adjustment, and should be considered in light of the natural disease course of respiratory failure which is to gradually improve over time, regardless of interventions, in most individuals.
There are several limitations to this study. This is a retrospective observational analysis; therefore, the use of prone positioning was not protocolized. However, it encompasses two tertiary centers over the duration of the pandemic and is one of the largest individual descriptions of proning19,21,22,41 in mechanically ventilated patients. We do not have a matched cohort that did not receive prone positioning.34,40,41 We considered this; however, our institutions have a high rate of prone positioning use (50% of mechanically ventilated patients), reserved for the most hypoxemic individuals, limiting accurate identification of “control” patients with sufficiently matched physiologic variables. The 23 hour (IQR 14-49) median prone duration is longer in comparison to prior publications.21,23,26,27,41,42 This reflects an intentional clinical decision, as in many instances we elected to allow patients to remain in the prone position for extended periods of time if they were improving (with care to prevent anterior soft tissue injury). Lastly, there was a relatively low rate of steroid use; this is because our sample included individuals managed before the results of the RECOVERY trial were available.43
Conclusions
In mechanically ventilated patients with COVID-19, prone positioning was associated with a sustained increase in PaO2/FiO2 of 19%. Individuals with a lower PaO2/FiO2 and on inhaled epoprostenol respond more favorably. Notably, lack of benefit with a first prone attempt does not indicate subsequent attempts would not be successful. Further study is needed to determine whether the long-term outcomes of prone positioning in COVID-19 are as beneficial as in traditional ARDS.
Abbreviations
- ABG
arterial blood gas
- AC/VC
assist control with volume cycling
- APRV
airway pressure release ventilation
- ARDS
acute respiratory distress syndrome
- COPD
chronic obstructive pulmonary disorder
- ECMO
extracorporeal membrane oxygenation
- ESR
erythrocyte sedimentation rate
- ICU
intensive care unit
- IQR
interquartile range
- iEpo
inhaled epoprostinol
- PCV
pressure control ventilation
- PEEP
positive end expiratory pressure
- RR
respiratory rate
- SOFA
sequential organ failure support
- VILI
ventilator induced lung injury
- Vt
tidal volume
Footnotes
Author Contributions: RS, JB, and NC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CWP, CK, JB contributed substantially to data acquisition and analysis. JB and RS contributed significantly to study design, data analysis and interpretation, and writing the manuscript. The authors have no financial disclosures.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
ORCID iDs: Jacob Bell https://orcid.org/0000-0001-6689-4332
Rajiv Sonti https://orcid.org/0000-0002-3564-6720
References
- 1.Dondorp AM, Hayat M, Aryal D, Beane A, Schultz MJ. Respiratory support in COVID- 19 patients with a focus on resource limited settings. Am J Trop Med Hyg. 2020;102(6):1191‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study. Lancet. 2020 Jun 6;395(10239):1763‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayek ME, Mansour M, Ndetan H, et al. Anti-Inflammatory treatment of COVID-19 pneumonia with tofacitinib alone or in combination with dexamethasone is safe and possibly superior to dexamethasone as a single agent in a predominantly African American cohort. Mayo Clin Proc Innov Qual Outcomes. 2021;5(3):605‐613. doi: 10.1016/j.mayocpiqo.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384(9):795‐807. doi: 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384(1):20‐30. doi: 10.1056/NEJMoa2030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok FA, Kruip MJHA, van der Meer NJMet al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llitjos JF, Leclerc M, Chochois Cet al. et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743-1746. 10.1111/jth.14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto K, Yonemitsu T, Tanaka Ret al. Protracted course of coronavirus disease with severe acute respiratory distress syndrome: a case report. Acute Med Surg. 2020;7(1):e521. Published 2020 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Chiumello D, Caironi Pet al. et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Coppola S, Cressoni M, Busana M, Chiumello D. COVID-19 does not lead to a ‘typical’ acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299-1300. 10.1164/rccm.202003-0817LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasa P, Azoulay E, Khanna AK, et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a delphi method. Crit Care. 2021;25(1):106; Published 2021 Mar 16. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219‐e234. doi: 10.1097/CCM.0000000000004899 [DOI] [PubMed] [Google Scholar]
- 14.Alhazzani W, Møller MH, Arabi YMet al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID- 19). Intensive Care Med. 2020;46(5):854‐887. doi: 10.1007/s00134-020-06022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301‐1308. [DOI] [PubMed] [Google Scholar]
- 16.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GRet al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564‐2575. [DOI] [PubMed] [Google Scholar]
- 17.Papazian L, Forel JM, Gacouin Aet al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107‐1116. [DOI] [PubMed] [Google Scholar]
- 18.Sonti R, Pike CW, Cobb N. Responsiveness of inhaled epoprostenol in respiratory failure due to COVID-19. J Intensive Care Med. 2021;36(3):327-333. 10.1177/0885066620976525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guérin C, Reignier J, JC Ret al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159‐2168. [DOI] [PubMed] [Google Scholar]
- 20.Bain W, Yang H, Shah FA, et al. COVID-19 versus Non-COVID-19 acute respiratory distress syndrome: comparison of demographics, physiologic parameters, inflammatory biomarkers, and clinical outcomes. Ann Am Thorac Soc. 2021;18(7):1202‐1210. https://www.ncbi.nlm.nih.gov/pubmed/33544045. doi: 10.1513/AnnalsATS.202008-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perier F, Tuffet S, Maraffi T, et al. Effect of positive End-expiratory pressure and proning on ventilation and perfusion in COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(12):1713‐1717. doi: 10.1164/rccm.202008-3058LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelhamer MC, Wesson PD, Solari IL, et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: a cohort study and analysis of physiology. J Intensive Care Med. 2021;36(2):241‐252. doi: 10.1177/0885066620980399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raoof S, Nava S, Carpati C, Hill NS. High-Flow, noninvasive ventilation and awake (nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest. 2020;158(5):1992‐2002. doi: 10.1016/j.chest.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. Published 2020 Jan 30. doi: 10.1186/s13054-020-2738-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimmoun A, Levy B, Chenuel B, et al. Usefulness and safety of a dedicated team to prone patients with severe ARDS due to COVID-19. Crit Care. 2020;24:509. 10.1186/s13054-020-03128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560‐1564. https://www.ncbi.nlm.nih.gov/pubmed/32348678. doi: 10.1164/rccm.202004-1163LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan C, Chen L, Lu C, et al. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201(10):1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carsetti A, Damia Paciarini A, Marini B, Pantanetti S, Adrario E, Donati A. Prolonged prone position ventilation for SARS-CoV-2 patients is feasible and effective. Crit Care. 2020;24(1):225. Published 2020 May 15. doi: 10.1186/s13054-020-02956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brault C, Zerbib Y, Kontar Let al. COVID-19- versus non-COVID-19-related acute respiratory distress syndrome: differences and similarities. Am J Respir Crit Care Med. 2020 Nov 1;202(9):1301‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunkley KA, Louzon PR, Lee J, Vu S. Efficacy, safety, and medication errors associated with the use of inhaled epoprostenol for adults with acute respiratory distress syndrome: a pilot study. Ann Pharmacother. 2013;47(6):790‐796. https://www.ncbi.nlm.nih.gov/pubmed/23656748. doi: 10.1345/aph.1R540 [DOI] [PubMed] [Google Scholar]
- 31.Vincent J, de Mendonca A, Cantraine F. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units. Crit Care Med. 1998;26(11):1793‐1800. [DOI] [PubMed] [Google Scholar]
- 32.Pappert D, Rossaint R, Slama K, Grüning T, Falke KJ. Influence of positioning on ventilation-perfusion relationships in severe adult respiratory distress syndrome. Chest. 1994;106:1511‐1516. [DOI] [PubMed] [Google Scholar]
- 33.Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:S280‐S288. [DOI] [PubMed] [Google Scholar]
- 34.Park J, Lee HY, Lee J, Lee SM. Effect of prone positioning on oxygenation and static respiratory system compliance in COVID-19 ARDS vs. non-COVID ARDS. Respir Res. 2021;22(1):220. Published 2021 Aug 6. doi: 10.1186/s12931-021-01819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gattinoni L, Busana M, Giosa L, Macrì MM, Quintel M. Prone positioning in acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40(1):94‐100. doi: 10.1055/s-0039-1685180 [DOI] [PubMed] [Google Scholar]
- 36.Fuller BM, Mohr NM, Skrupky L, Fowler S, Kollef MH, Carpenter CR. The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta-analysis. Chest. 2015;147(6):1510‐1522. doi: 10.1378/chest.14-3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291(13):1603‐1609. doi: 10.1001/jama.291.13.1603 [DOI] [PubMed] [Google Scholar]
- 38.Kallet RH, Burns G, Zhuo H, et al. Severity of hypoxemia and other factors that influence the response to aerosolized prostacyclin in ARDS. Respir Care. 2017;62(8):1014‐1022. doi: 10.4187/respcare.05268 [DOI] [PubMed] [Google Scholar]
- 39.Pacheco J, Arnold H, Skrupky L, Watts P, Micek ST, Kollef MH. Predictors of outcome in 216 subjects with ARDS treated with inhaled epoprostenol. Respir Care. 2014;59(8):1178‐1185. doi: 10.4187/respcare.02939 [DOI] [PubMed] [Google Scholar]
- 40.Langer T, Brioni M, Guzzardella A, et al. Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care. 2021;25(1):128. Published 2021 Apr 6. doi: 10.1186/s13054-021-03552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chua EX, Zahir SMISM, Ng KT, et al. Effect of prone versus supine position in COVID-19 patients: a systematic review and meta-analysis. J Clin Anesth. 2021;74:110406. doi: 10.1016/j.jclinane.2021.110406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan E, Beitler JR, Brochard Let al. et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JRet al. et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2021;384(8):693-704. 10.1056/NEJMoa202143 [DOI] [PMC free article] [PubMed] [Google Scholar]