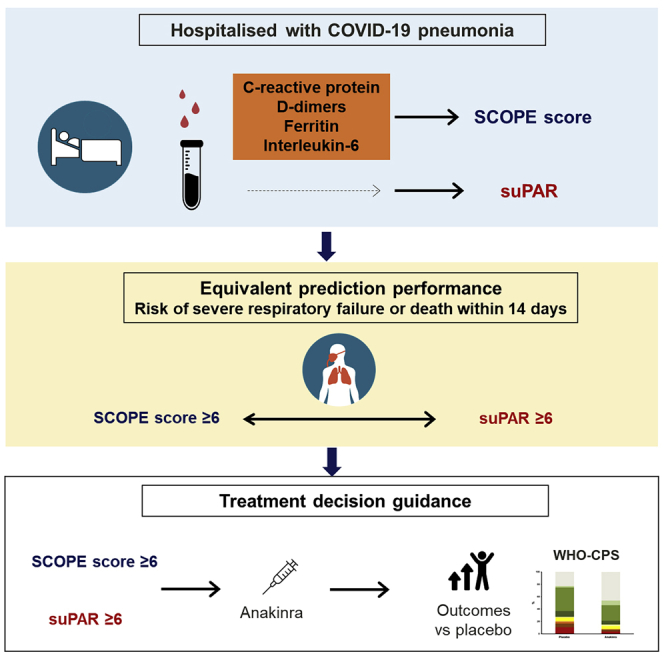
Summary
Most patients infected with SARS-CoV-2 (COVID-19) experience mild, non-specific symptoms, but many develop severe symptoms associated with an excessive inflammatory response. Elevated plasma concentrations of soluble urokinase plasminogen activator receptor (suPAR) provide early warning of progression to severe respiratory failure (SRF) or death, but access to suPAR testing may be limited. The Severe COvid Prediction Estimate (SCOPE) score, derived from circulating concentrations of C-reactive protein, D- dimers, interleukin-6, and ferritin among patients not receiving non-invasive or invasive mechanical ventilation during the SAVE-MORE study, offers predictive accuracy for progression to SRF or death within 14 days comparable to that of a suPAR concentration of ≥6 ng/mL (area under receiver operator characteristic curve 0.81 for both). The SCOPE score is validated in two similar independent cohorts. A SCOPE score of 6 or more is an alternative to suPAR for predicting progression to SRF or death within 14 days of hospital admission for pneumonia, and it can be used to guide treatment decisions.
Keywords: biomarkers, COVID-19, C-reactive protein, D dimers, disease progression, ferritin, interleukin-6, SARS-CoV-2, soluble urokinase plasminogen activator receptor
Graphical abstract
Highlights
-
•
SCOPE score is composed of C-reactive protein, D dimers, ferritin, and interleukin-6
-
•
Values of 6 or more predict 6-fold risk for severe respiratory failure or death
-
•
SCOPE score predicts risk for severe respiratory failure or death comparable to suPAR
-
•
Anakinra treatment when SCOPE is 6 or more provides lower odds of poor outcome
Giamarellos-Bourboulis et al. introduce the SCOPE score for early prognostication of the risk for severe respiratory failure or death within the next 14 days in COVID-19 pneumonia. This is composed of C-reactive protein, D dimers, ferritin, and interleukin-6 concentrations. Anakinra treatment administered when SCOPE is 6 or more provides lower odds of a poor outcome.
Introduction
Most patients infected with SARS-CoV-2 (COVID-19) experience mild, non-specific symptoms that are generally limited to malaise, fever, and a dry cough.1 However, many patients experience more severe symptoms, such as dyspnea and hypoxia, and enter into an excessive inflammatory response phase.1,2 These patients may require hospitalization for pneumonia, and a subset will progress to severe respiratory failure (SRF), which is associated with hyperinflammation characterized by excessively elevated levels of cytokines, chemokines, and other inflammatory mediators, including markers of coagulopathy such as D-dimers.2 As patients may quickly deteriorate, early identification of those at risk for progression to severe disease is crucial for timely initiation of targeted interventions that may prevent progression to SRF and reduce mortality. The SAVE-MORE trial is one such example in which biomarker-based patient stratification can lead to timely and personalized immunotherapy. Patients with COVID-19 pneumonia without signs of SRF and with plasma concentrations of the biomarker soluble urokinase plasminogen activator receptor (suPAR) of 6 ng/mL or more received early treatment with anakinra (recombinant interleukin-1 receptor antagonist) for 10 days. This strategy had lower odds of a poor outcome (odds ratio 0.36) compared with placebo. Clinical benefits were found as decreased mortality, prevention of admissions into the intensive care unit, and increase in infection resolution.3 The results of the SAVE-MORE trial were the backbone for the approval of anakinra for the therapy of COVID-19 pneumonia in adults by the European Medicines Agency.4
Elevated plasma concentrations of suPAR provide early warning of activation of the inflammatory and coagulation pathways, and of endothelial-neutrophil interaction, prior to the development of the clinical signs and symptoms of hyperinflammation.5,6 suPAR levels at the time of admission have been found to be prognostic of patients progressing to SRF after being hospitalized for COVID-19 pneumonia.3,7, 8, 9, 10, 11, 12 However, in hospital settings where rapid suPAR testing may not be routinely available,13 an alternative biomarker or combination of biomarkers based on routinely collected laboratory parameters is necessary to help readily identify patients with COVID-19 pneumonia who are at greatest risk of progressing to SRF.
As part of the randomized controlled phase 3 SAVE-MORE trial, data were collected during screening on biomarkers that reflected inflammation, coagulation, and endothelial activation.3 These biomarkers—C-reactive protein (CRP), D dimers, interleukin-6 (IL-6), and ferritin—can be readily assessed in routine clinical practice. Herein, we aimed to investigate the potential of these four biomarkers to be incorporated into a simple scoring system (the Severe COvid Prediction Estimate [SCOPE] score) that could be used as a prognostic marker for progression to SRF or death in patients hospitalized with COVID-19 pneumonia. The SCOPE score was derived using data from patients screened for enrollment in the phase 3 SAVE-MORE study and then validated using two independent cohorts: one cohort collected during the phase 2 SAVE study11 and another cohort collected in the Netherlands.
Results
Description of study cohorts
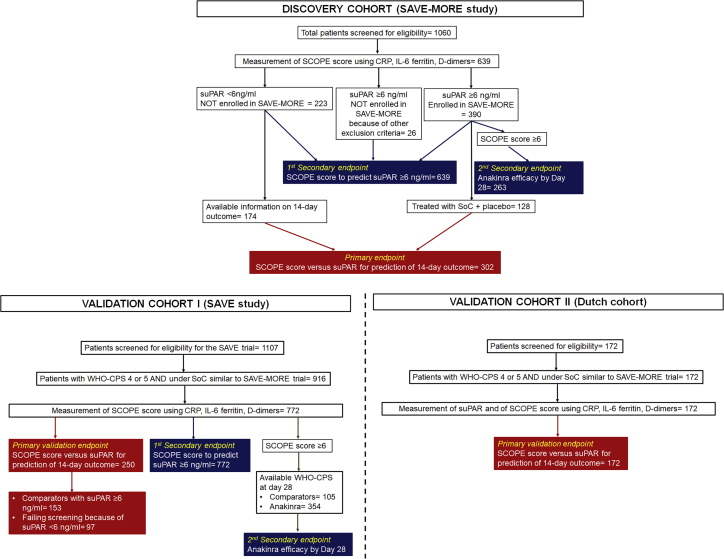
The discovery cohort was recruited from the 1,060 patients screened for the SAVE-MORE study. None of these patients were receiving non-invasive or invasive mechanical ventilation. Biomarker data were available for 639 (60.3%) patients. Of those patients, 225 had plasma suPAR concentrations of less than 6 ng/mL and were excluded from SAVE-MORE, while 390 patients with suPAR concentrations of 6 ng/mL or more were enrolled in the study and randomized 2:1 to anakinra or placebo (Figure 1 and Table S1). An additional 24 patients with suPAR levels of 6 ng/mL or more were excluded from SAVE-MORE after failing to meet other eligibility criteria. In total, of all the patients enrolled in the SCOPE score discovery cohort, 302 who were hospitalized with COVID-19 pneumonia and had both biomarker data and data on progression to SRF or death within 14 days available were analyzed for the primary analysis endpoint, i.e., the prognostic performance capacity of the SCOPE score compared with suPAR for the prediction of SRF or death within the first 14 days. Of these 302 patients, 128 had suPAR ≥6 ng/mL and participated in the SAVE-MORE trial, being treated with standard of care (SoC) and placebo, while 174 had suPAR <6 ng/mL and were not included in the randomization for the SAVE-MORE trial and were treated with SoC; 14-day outcome data were available for all these 302 patients. All patients were analyzed for the two secondary endpoints, i.e., the ability of the SCOPE score to predict suPAR of 6 ng/mL or more and the response to treatment with anakinra for patients scoring positive by the SCOPE score. Plasma samples used for the measurement of the four biomarkers were collected at the time of screening for inclusion at the SAVE-MORE trial; they were the same samples in which suPAR was measured for screening.
Figure 1.
Flowchart of the discovery and the validation cohorts
The discovery cohort was recruited from patients screened for eligibility for participation in the SAVE-MORE trial and for which the screening samples were available for the measurement of the SCOPE score. The samples of patients with suPAR of less than 6 ng/mL and of patients with suPAR of 6 ng/mL or more treated in the SAVE-MORE trial with placebo were analyzed for the primary endpoint, i.e., prediction of progression into severe respiratory failure or death within the first 14 days (302 total). Samples from all 639 patients were analyzed for the ability of the SCOPE score to predict suPAR levels; and 263 patients with SCOPE score of 6 or more were analyzed for the efficacy of anakinra treatment. The validation cohort I came from the SAVE trial and it was analyzed for the same endpoints as the discovery cohort. The validation cohort II came from the Netherlands and it was analyzed for the primary endpoint. SCOPE, Severe COvid Prediction Estimate; SoC, standard of care; suPAR, soluble urokinase plasminogen activator receptor; WHO-CPS, World Health Organization Clinical Progression Scale.
Two validation cohorts of patients with similar severity not receiving non-invasive or invasive mechanical ventilation were also studied. Validation cohort I was recruited from patients who were screened for eligibility for the SAVE study and their comparators. The SAVE study is an ongoing phase 2 open-label, non-randomized trial using the same inclusion and exclusion criteria as the SAVE-MORE trial. An interim analysis of this study was published;11 since then, 1,107 patients have been screened for eligibility, and full biomarker data allowing calculation of the SCOPE score are available for 772 patients. The primary endpoint of the analysis was validated in 153 comparators with suPAR levels of 6 ng/mL or more and in 97 patients who failed screening because of suPAR levels less than 6 ng/mL (Figure 1 and Table S1). Plasma samples used for the measurement of the four biomarkers were those collected at the time of screening for the SAVE trial and on the day of hospital admission for comparators. suPAR was measured in the same samples.
Validation cohort II was recruited from patients hospitalized at the Department of Internal Medicine of the Radboud University Medical Center in the Netherlands (Figure 1 and Table S1). Plasma samples used for the measurement of the four biomarkers and suPAR were collected on hospital admission. This cohort was studied for the validation of the primary endpoint.
Patients of the discovery cohort and of both validation cohorts were of European ancestry.
Discovery cohort: Predictive performance of SCOPE score versus suPAR
Patients of the discovery cohort with suPAR less than 6 ng/mL were younger and less severely ill than patients with suPAR of 6 ng/mL or more. Most patients were receiving SoC treatment with dexamethasone (Table S1).
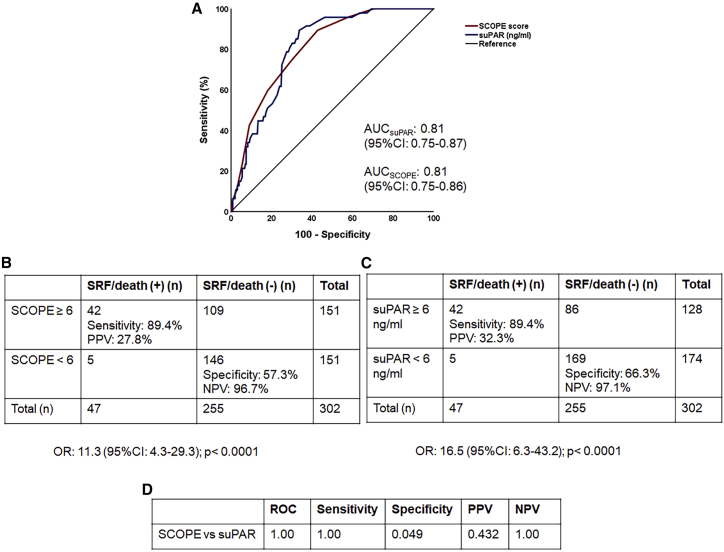
The SCOPE score was calculated by allocating CRP, ferritin, IL-6, and D-dimer concentrations a score of 0–3 points for each biomarker (total score ranging between 0 and 12 points): the specific concentrations that define the points allocated to each biomarker were defined based on the quartile within the discovery population (see Table 1, which presents the specific concentrations for each biomarker point). These four biomarkers were selected for inclusion in the score because they were also used to characterize patients included in the SAVE-MORE study following the advice of the Emergency Task Force for COVID-19 (COVID-ETF) of the European Medicines Agency (EMA).3 Area under the receiver operating characteristic curve (AUROC) for predicting progression to SRF or death within 14 days was not significantly different comparing AUROC for suPAR levels (0.81; 95% confidence interval [CI] 0.75–0.87) and the SCOPE score (0.81; 95% CI 0.75–0.86; p value of comparison 1.00) (Figure 2A). A SCOPE score of 6 or more had a sensitivity for SRF or death of 89.4% and a negative predictive value of 96.7% using the Youden index (Figure 2B). Four elements of prognostic performance (AUROC, sensitivity, positive predictive value, and negative predictive value) were similar between SCOPE score and suPAR when predicting progression to SRF or death within 14 days (Figures 2C and 2D). The prognostic performance of each biomarker separately was much poorer than that of suPAR (Figure S1). Four sub-scores were also calculated taking into consideration three of the four biomarkers; their prognostic performance was poorer than that of the SCOPE score (Figure S2). The addition of suPAR did not improve the prognostic performance of the SCOPE score (Figure S3).
Table 1.
The SCOPE score
| D-dimers (mg/L) | CRP (mg/L) | Ferritin (ng/mL) | IL-6 (pg/mL) | Points |
|---|---|---|---|---|
| 0.10–0.40 | 0.3–25.0 | 10–225.0 | 0.7–5.0 | 0 |
| >0.40–0.57 | >25.0–45.0 | >225.0–450.0 | >5.0–12.0 | 1 |
| >0.57–0.90 | >45.0–85.0 | >450.0–750.0 | >12.0–30.0 | 2 |
| >0.90 | >85 | >750 | >30 | 3 |
Each of the four biomarkers is allocated 0 to 3 points according to the concentration. The final score is the sum of the points provided by each biomarker.
Figure 2.
Discovery of the SCOPE score and comparative performance to that of suPAR for the prediction of progression into severe respiratory failure (SRF) or death within the first 14 days
(A) ROC curves of the SCOPE score and suPAR to predict progression into SRF or death within the first 14 days.
(B) Prognostic performance of SCOPE score of 6 or more to predict progression into SRF or death within the first 14 days. The odds for patients with a score of 6 or more to progress into SRF or death within the first 14 days are provided (calculation by Mantel Haenszel statistics).
(C) Prognostic performance of suPAR values of 6 ng/mL or more to predict the progression into SRF or death within the first 14 days. The odds for patients with suPAR of 6 ng/mL or more to progress into SRF or death are provided (calculation by Mantel Haenszel statistics).
(D) The p values of comparisons of the AUC of ROC, of sensitivity, of specificity, of PPV, and of NPV of the SCOPE score and of suPAR to predict progression into SRF or death within the first 14 days. AUC, area under the curve; CI, confidence intervals; NPV, negative predictive value; n, number of patients; OR, odds ratio; PPV, positive predictive value; SCOPE, Severe COvid Prediction Estimate; suPAR, soluble urokinase plasminogen activator receptor.
To better establish the correctness of the selection of these four biomarkers for the development of the SCOPE score, LASSO regression analysis was done. In this analysis, it was shown that even after correction for multiple tests, suPAR was independently and significantly correlated with each of the four biomarkers (Figures S4A–S4D). The four biomarkers did not cluster, indicating their synergism to provide predictive information on suPAR (Figure S4E). This was further proven after multivariate forward stepwise logistic regression analysis showing that the quartiles of CRP, ferritin, and IL-6 were independently associated with suPAR (Figure S4F). The results of the multivariate analysis, the lack of clustering, and the independent correlation of suPAR with each of the four biomarkers confirmed the correctness of the selection for the development of the SCOPE score.
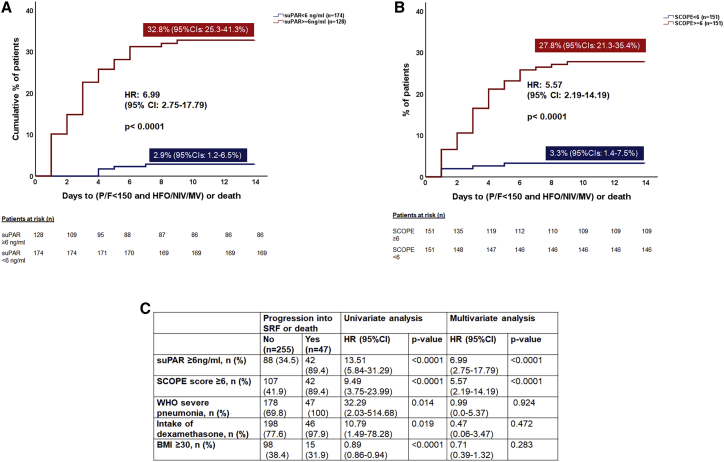
The hazard ratios (HRs) for SRF or death within 14 days for patients with suPAR ≥6 versus <6 ng/mL (32.3% versus 2.9%, HR 6.99, 95% CI 2.75–17.79, p < 0.0001) and SCOPE score ≥6 versus <6 (27.8% versus 3.3%, HR 5.57, 95% CI 2.19–14.19, p < 0.0001) were similar (Figures 3A and 3B). Univariate Cox regression analysis indicated an association between suPAR 6 ng/mL or more, SCOPE score 6 or more, World Health Organization (WHO) severe pneumonia, dexamethasone intake, and obesity (body mass index [BMI] ≥30 mg/kg2) and risk of progression to SRF or death within 14 days. However, multivariate forward stepwise Cox regression analysis indicated that only suPAR ≥6 ng/mL and SCOPE score ≥6 were predictive of SRF or death within 14 days (Figure 3C). A greater SCOPE score predicted greater risk for poor outcome (Figure S5).
Figure 3.
Estimates of SRF or death by SCOPE score and by suPAR levels within 14 days of hospitalization for COVID-19 pneumonia in the discovery cohort
The comparison includes patients enrolled in the SAVE-MORE trial and randomized to treatment with standard of care (SoC) and placebo (n = 128) and patients who were screened for enrollment in the SAVE-MORE trial and who were not enrolled because suPAR was less than 6 ng/mL (n = 174); all patients with suPAR less than 6 ng/mL received SoC treatment.
(A) Time to progression into SRF or death within the first 14 days when suPAR was 6 ng/mL or more and when suPAR was less than 6 ng/mL.
(B) Time to progression into SRF or death within the first 14 days when the SCORE score was 6 or more and when the SCOPE score was less than 6.
(C) Univariate and multivariate forward stepwise Cox regression analysis of variables associated with progression to SRF or death within 14 days. BMI, body mass index; CI, confidence intervals; HFO, high-flow oxygen; HR, hazard ratio; MV, mechanical ventilation; n, number of patients; NIV, non-invasive ventilation; P/F, respiratory failure; SCOPE, Severe COvid Prediction Estimate; suPAR, soluble urokinase plasminogen activator receptor.
Discovery cohort: Correlation between SCOPE score and suPAR values
SCOPE score and suPAR values were significantly correlated (Spearman's r = 0.387; p < 0.0001) (Figures S6A and S6B). The positive predictive value of a SCOPE score ≥6 to predict suPAR ≥6 ng/mL was 75.6% (Figure S6C and Table S2).
Discovery cohort: Efficacy of anakinra versus placebo according to SCOPE score
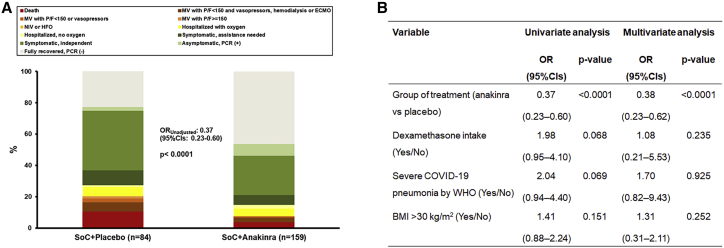
To further investigate the clinical utility of the SCOPE score, the efficacy of anakinra was studied for patients with SCOPE of 6 or more. This was done by ordinal regression analysis according to the guidance received by the COVID-ETF of the European Medicines Agency for the analysis of the SAVE-MORE trial, using, in the multivariate model, the covariates used for stratified randomization.3 Patients with a SCOPE score ≥6 who were treated with anakinra had lower odds of worse outcome as defined by the WHO Clinical Progression Scale (WHO-CPS) at day 28 (adjusted odds ratio 0.38, 95% CI 0.23–0.62, p < 0.0001) compared with patients treated with placebo (Figure 4).
Figure 4.
Response to anakinra treatment of patients enrolled in the SAVE-MORE trial with SCOPE score of 6 or more in the discovery cohort
(A) Distribution of the World Health Organization (WHO) Clinical Progression Scale (CPS) at day 28 for patients allocated to treatment with SoC and placebo and to treatment with SoC and anakinra. The OR of the unadjusted ordinal regression analysis and the 95% CIs are shown.
(B) Univariate and multivariate ordinal regression analysis of the WHO-CPS at day 28. BMI, body mass index; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; HFO, high-flow oxygen; MV, mechanical ventilation; NIV, non-invasive ventilation; OR, odds ratio; PCR, polymerase chain reaction; P/F, respiratory failure; SoC, standard of care.
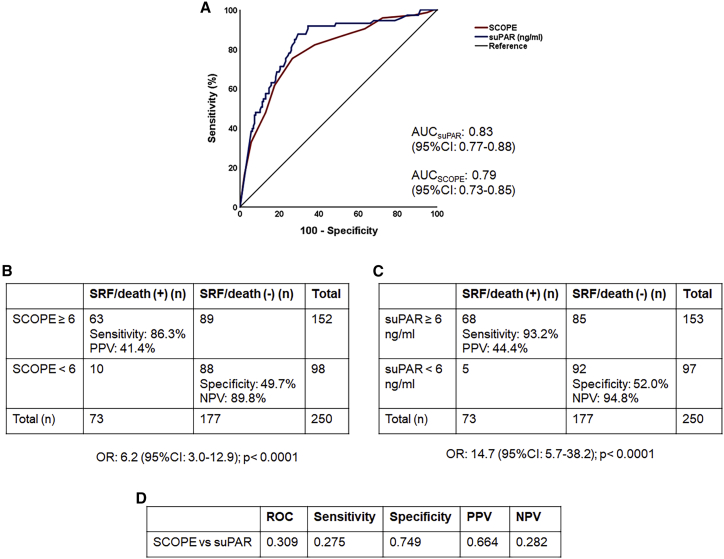
SCOPE score validation
When applied to patients included in the validation cohort I, the AUROC for a SCOPE score ≥6 (0.79, 95% CI 0.73–0.85) was similar to that of suPAR ≥6 ng/mL (0.83, 95% CI 0.77–0.88) for predicting progression to SRF or death within 14 days, as were the prognostic characteristics (Figures 5 and S7). Of the patients with a SCOPE score ≥6, 92.3% had suPAR levels ≥6 ng/mL (Table S3). Patients with a SCOPE score ≥6 who were treated with anakinra had lower odds of worse outcome as defined by the WHO-CPS at day 28 (adjusted odds ratio 0.29 [95% CI 0.19–0.43]; p < 0.0001) over comparators (Figure S8). It needs to be emphasized that SAVE was an open-label, single-arm trial in which all participants were treated with anakinra. Comparators were patients matched for age, gender, and comorbidities with suPAR of 6 ng/mL who were treated with similar SoC and hospitalized in same-level-of-care departments.11
Figure 5.
Comparative performance of the SCOPE score and suPAR to predict progression into severe respiratory failure (SRF) or death within the first 14 days in validation cohort I
(A) ROC curves of the SCOPE score and suPAR to predict progression into SRF or death within the first 14 days.
(B) Prognostic performance of SCOPE score values of 6 or more to predict progression into SRF or death within the first 14 days. The odds for patients with a score of 6 or more to progress into SRF or death are provided (calculation by Mantel Haenszel statistics).
(C) Prognostic performance of suPAR values of 6 ng/mL or more to predict progression into SRF or death within the first 14 days. The odds for patients with suPAR of 6 ng/mL or more to progress into SRF or death are provided (calculation by Mantel Haenszel statistics).
(D) The p values of comparisons of the AUC of ROC, of sensitivity, of specificity, of PPV, and of NPV of SCOPE score and of suPAR to predict progression into SRF or death within the first 14 days. AUC, area under the curve; CI, confidence interval; NPV, negative predictive value; n, number of patients; OR, odds ratio; PPV, positive predictive value; suPAR, soluble urokinase plasminogen activator receptor.
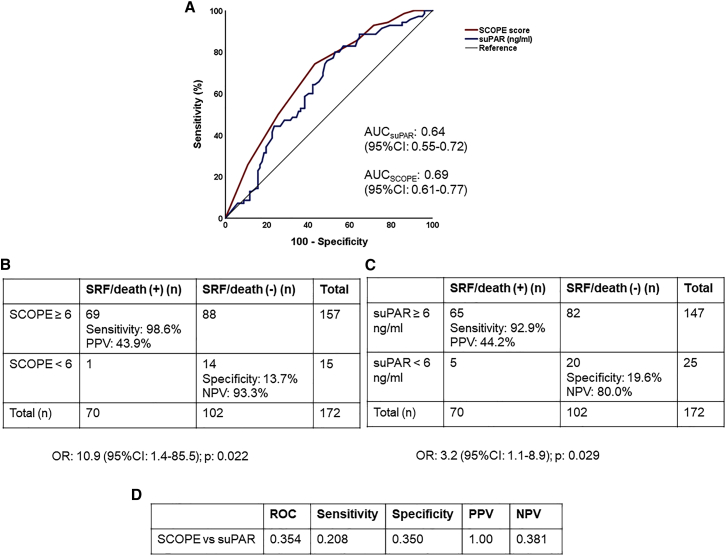
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of suPAR and of the SCOPE score for the prediction of the risk of progression to SRF or death within 14 days in validation cohort II were similar to those of the discovery cohort and the validation cohort I (Figure 6). Of the patients with a SCOPE score ≥6, 95.2% had suPAR levels ≥6 ng/mL (Table S4).
Figure 6.
Comparative performance of the SCOPE score and suPAR to predict progression into severe respiratory failure (SRF) or death within the first 14 days in validation cohort II
(A) ROC curves of the SCOPE score and suPAR to predict progression into SRF or death within the first 14 days.
(B) Prognostic performance of SCOPE score values of 6 or more to predict progression into SRF or death within the first 14 days. The odds for patients with a score of 6 or more to progress into SRF or death are provided (calculation by Mantel Haenszel statistics).
(C) Prognostic performance of suPAR values of 6 ng/mL or more to predict progression into SRF or death within the first 14 days. The odds for patients with suPAR of 6 ng/mL or more to progress into SRF or death are provided (calculation by Mantel Haenszel statistics).
(D) The p values for comparisons of the AUC of ROC, of sensitivity, of specificity, of PPV, and of NPV of SCOPE score and suPAR to predict progression into SRF or death within the first 14 days. AUC, area under the curve; CI, confidence interval; NPV, negative predictive value; n, number of patients; OR, odds ratio; PPV, positive predictive value; suPAR, soluble urokinase plasminogen activator receptor.
Discussion
A suPAR concentration of ≥6 ng/mL has been identified as predictive of progression to SRF or death in patients hospitalized with COVID-19 pneumonia.7, 8, 9, 10, 11, 12 However, its use in the clinical setting may be limited by access or low familiarity with the measure, preventing the use of anakinra in a population where the medical need has been demonstrated.13 Here, we show that a SCOPE score of 6 or more, defined as a combination of scoring based on circulating concentrations of CRP, IL-6, ferritin, and D dimers (see Table 1), offers a readily available, validated, and simple alternative to suPAR concentrations of 6 ng/mL or more, so that it can be easily applied in clinical practice. The analyses performed in this study using a discovery cohort and two independent validation cohorts demonstrated that the prognostic characteristics of suPAR levels ≥6 ng/mL and of SCOPE score ≥6 for predicting progression to SRF or death within 14 days were similar. In the SAVE and SAVE-MORE trials, suPAR was used as an indicator of patients at risk who should receive anakinra treatment. In the same sense, SCOPE is a score predicting the risk of progression into SRF, and patients scoring 6 or more have lower odds for poor outcome when receiving anakinra treatment. Since the great majority of participants in the SAVE and SAVE-MORE trials were receiving SoC including dexamethasone, the number of participants not treated with dexamethasone was too limited to be able to show an independent benefit coming from dexamethasone treatment. The odds ratios (ORs) for WHO-CPS at day 28 for dexamethasone for patients with a SCOPE score of 6 or more were similar to those described for the entire SAVE and SAVE-MORE cohorts.3,11
One major strength of the SCOPE score is the high negative predictive value, which may contribute to triage decision-making. It may be argued that IL-6 is not measured in several hospital settings, making the calculation of the SCOPE score difficult. Our sub-group analysis (provided at Figure S2) showed that by using the same concentration quartiles of CRP, D dimers, and ferritin a score with similar high negative predictive value may be derived. However, that score is lacking the high sensitivity of the SCOPE score.
The composition of the SCOPE score is consistent with previous suggestions that an effective biomarker for predicting progression among patients infected with COVID-19 needs to incorporate hematologic, inflammatory, biochemical, and immunologic parameters.14 Notably, the SCOPE score has improved prognostic performance compared with each of its single components. Results from a systematic review and meta-analysis of biomarkers suggest that single biomarkers have a relatively modest prognostic performance for predicting poor outcomes for COVID-19 pneumonia compared with a score combining several biomarkers, such as SCOPE, or a single biomarker reflecting various pathophysiological processes, such as suPAR.15 Specifically, the meta-analysis found that patients defined as higher risk based on individual biomarkers had an up to 6.33 times greater probability of poor outcomes compared with 6 to 7 times for the SCOPE score and 6 to 11 times for suPAR demonstrated here.15
The rationale of the SCOPE score is to integrate information coming from modest increases in biomarkers of activation of the inflammatory pathway, of the coagulation pathway, and of the endothelium, which can be combined into a composite measure informing early on the initiation of risk and the need to start immune intervention. The majority of patients enrolled in the CORIMUNO-ANA-1 study were under treatment necessity for oxygen supplementation by mask or nasal prongs. However, these patients were already experiencing high levels of activation of all the pathways; median ferritin was 1,479 ng/mL in the anakinra arm and 1,151 ng/mL in the placebo arm, median CRP was 120 mg/L in the anakinra arm and 121 mg/L in the placebo, and median D dimers were 0.99 mg/L in the anakinra arm and 1.28 mg/L in the placebo arm. The values of each biomarker were within the range of 3 points of the SCOPE score, indicating that the level of activation of all three pathways was already so high that it is most likely that anakinra treatment was started much too late to be able to show benefit.16
Other prognostic scores for predicting COVID-19 disease severity and mortality using a combination of biomarkers have also been proposed, but most require data to be collated from more than one source or more complex calculations.17 In one example of a biomarker combination being associated with an AUROC >0.9, the analysis was limited by the retrospective and single-center nature. Data were derived solely from the Wuhan region of China during the early stages of the COVID-19 pandemic (December 2019 to March 2020) without considering the impact of the rapid advances in the SoC during the pandemic, which may influence real prognostication.18 In contrast, the SCOPE score was developed and validated using data from prospectively enrolled cohorts receiving dexamethasone as part of the current SoC. All necessary parameters to calculate the SCOPE score can be measured using a single platform, while still offering an AUROC >0.8.
The need for a score that can predict the risk for progression into critical illness early has also been the aim of other studies during the COVID-19 pandemic. One such effort is the COVID-GRAM score, which takes into consideration 10 variables, i.e., chest X-ray abnormalities, age, hemoptysis, dyspnea, lack of consciousness, number of comorbidities, cancer history, neutrophil-to-lymphocyte ratio, lactate dehydrogenase, and direct bilirubin. The score was developed in a cohort of 1,590 patients and it was fully validated in a second cohort of 710 patients. In both cohorts, the AUROC of prediction of progression to critical illness was 0.88.19
In conclusion, suPAR circulating concentration predicts progression to SRF or death and reflects several underlying biological processes that play an important role in COVID-19 pathophysiology (inflammation, coagulation, and endothelial activation). In the absence of a point-of-care suPAR analysis being widely accessible,13 a SCOPE score of 6 or more was identified and validated as an alternative to suPAR to predict progression to SRF or death within 14 days of hospital admission. This ability to rapidly predict outcomes for patients with COVID-19 pneumonia and to guide treatment accordingly will likely offer a significant clinical benefit.
Limitations of the study
Two main limitations should be acknowledged: (1) the discovery cohort and the first validation cohort were recruited from participants screened for inclusion in interventional trials both taking place in the same country, Greece. The impact of this limitation is reduced by the recruitment of a second real-life cohort from the Netherlands. (2) The SCOPE score is based on predefined well-known and routine-measured biomarkers. However, other biomarkers may be developed in the future providing similar prognostic performance.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| suPARnostic Quick Triage kit | Virogates | T.001349A |
| Ferritin assay | Roche COBAS INTEGRA 400 plus | Ferritin Elecsys/e 411Cat #781.16.0052 |
| IL-6 assay | Roche COBAS INTEGRA 400 plus | PRECICONTROL Multimarker Elecsys 411 Cat#781.03.0055 |
| D-dimer assay | Roche COBAS INTEGRA 400 plus | D-dimer 5T Dedicio Cat #621.14.0016 |
| CRP assay | Roche COBAS INTEGRA 400 plus | CRP latex kit 100T Cat# 271.14.0008 |
| Software and algorithms | ||
| SPSS v. 26 | IBM | https://www.ibm.com/analytics/spss-statistics-software |
| Other | ||
| WHO CPS | WHO | https://www.who.int/docs/default-source/documents/emergencies/minimalcoreoutcomemeasure.pdf |
| WHO definitions of COVID disease severity | WHO | https://www.who.int/publications/i/item/clinical-management-of-covid-19 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Evangelos J. Giamarellos-Bourboulis (egiamarel@med.uoa.gr).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Human subjects
The SAVE11 and SAVE-MORE3 studies enrolled adult (age ≥18 years) male and female patients with SARS-CoV-2 infection confirmed by molecular testing who were hospitalized, had findings in chest X-rays or chest computed tomography imaging that were consistent with a lower respiratory tract infection, and had plasma suPAR levels 6 ng/mL or more. Exclusion criteria were: non-invasive or mechanical ventilation, stage IV malignancy, any do-not-resuscitate decision, respiratory ratio PaO2/FiO2 <150 mmHg, severe hepatic failure, any primary immunodeficiency, neutrophils less than 1500/mm3, oral or intravenous corticosteroids at a daily dose greater than or equal to ≥0.4 mg/kg/day of equivalent prednisone for >15 days immediately prior to hospitalization, any anti-cytokine biologic treatment (including JAK inhibitors) during the preceding month, end-stage renal failure necessitating hemofiltration or peritoneal hemodialysis, and pregnancy or lactation.
The SAVE protocol was approved by the National Ethics Committee of Greece (approval 38/20) and by the National Organization for Medicines of Greece (ISO 28/20). The SAVE was prospectively registered (EudraCT number 2020-001466-11; ClinicalTrials.gov identifier NCT04357366). The study is still on-going in 13 study sites in Greece and an interim analysis on the first 130 patients has been published.11 Participants were treated with anakinra 100 mg once daily subcutaneously once daily for 10 days. Comparators hospitalized in other study sites of the same or other tertiary hospitals and receiving the same level of standard-of-care (SoC) are also studied.
The SAVE-MORE protocol was approved by the National Ethics Committee of Greece (approval 161/20) and by the Ethics Committee of the National Institute for Infectious Diseases Lazzaro Spallanzani, IRCCS, in Rome (1 February 2021). The study was prospectively registered (EudraCT no. 2020-005828-11; ClinicalTrials.gov identifier NCT04680949). Written informed consent was provided by all patients prior to enrolment. Participants were 1:2 randomized into treatment with placebo and SoC or anakinra and SoC for 10 days. Randomization was done in a stratified manner taking into consideration pneumonia severity as defined by the need of oxygen, treatment with dexamethasone, body mass index and geographic region.
For the development of the SCOPE score, patients were divided into one discovery cohort and into two validation cohorts. Patients screened for eligibility for the SAVE-MORE study framed the discovery cohort; and patients screened for eligibility for the SAVE study framed the validation cohort I. Only patients for which samples collected during the screening visit were available for the measurement of other biomarkers participated in both cohorts.
The Dutch cohort (validation cohort II) consisted of adult patients with COVID-19 admitted to non-ICU clinical wards between March and April 2020.20 The study was carried out in the Netherlands in accordance with the applicable rules concerning the review of research ethics committees and informed consent. The study protocol was approved by the local ethics committee, CMO region Arnhem-Nijmegen, (CMO 2020 6344 and CMO 2016 2963) and performed in accordance with the latest version of the declaration of Helsinki and guidelines for good clinical practice (GCP). All patients or legal representatives were informed about the study details and could decline to participate. Ethylenediaminetetraacetic acid (EDTA) plasma samples were collected during routine blood collections for laboratory testing.
Method details
Available data of patients screened for both studies were demographics, treatment with dexamethasone, severity according to WHO, development of SRF by day 14, death until day 28 and allocation to the 11-point WHO-CPS (clinical progression scale) by day 28. suPAR levels were measured in plasma samples using the suPARnostic Quick Triage kit (Virogates) and a point-of-care reader. Plasma samples were kept refrigerated at −80°C in the central study lab. Concentrations of CRP, D-dimers, ferritin and IL-6 were measured in the Roche COBAS INTEGRA 400 plus platform.
Endpoints
The primary and secondary endpoints were common for all cohorts
The primary endpoint was the development of the SCOPE score using integrated information from CRP, D-dimers, ferritin and IL-6 to prognosticate the progression into SRF or death after 14 days. SRF was defined as PaO2/FiO2 <150 mmHg necessitating high-flow oxygen or non-invasive ventilation or mechanical ventilation. This analysis included for the discovery cohort patients who failed screening because suPAR was less than 6 ng/mL and patients who were enrolled in the SAVE-MORE study and who were allocated to treatment with SoC and placebo. This analysis included for the validation cohort I patients who failed screening because suPAR was less than 6 ng/mL and comparators with suPAR 6 ng/mL or more who were receiving SoC. This analysis included for the validation cohort II all participants.
The study has two secondary endpoints. The first secondary endpoint was the performance of the SCOPE score to predict suPAR levels 6 ng/mL or more. This analysis comprised patients of all cohorts with suPAR 6 ng/mL or more. The second secondary endpoint was the clinical efficacy of anakinra treatment for patients scoring positive for the SCOPE score as this is defined by the distribution of frequencies of the 11-point WHO-CPS. This analysis included for the discovery cohort those of patients with available SCOPE score who were allocated to treatment with SoC and placebo or with SoC and anakinra. This analysis included for the validation cohort I those of patients with available SCOPE score who were treated with SoC and anakinra and comparators who were treated with SoC. This analysis could not be done for the validation cohort II since no patient was treated with anakinra.
Statistical analysis
The analysis of the primary endpoint of the performance of suPAR and SCOPE score for predicting progression to SRF or death was done by receiver operator curve (ROC) analysis providing the area under the curve (AUCROC) and 95% confidence intervals (CIs). The best trade-off for sensitivity and specificity of the coordinate points of ROC of SCOPE was defined by applying the Youden index. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated by a 2 × 2 table. The odds ratio (OR) and 95% CIs of the selected cut-off was determined using the Mantel-Haenszel test. The AUROC of single suPAR, of single SCOPE score and of the combination of suPAR and SCOPE score were compared by the Vassar stats formula (https://www.google.com/search?client=firefox-b-d&q=comparison+of+two+roc+curves+vassar); sensitivities, specificities, PPVs and NPVs of suPAR 6 ng/mL or more and of the selected SCOPE score cut-off for the prediction of SRF or death the first 14 days were compared by the Fisher’s exact test. The odds ratios and 95% CI were calculated by Mantel Haenszel’s statistics. Cox regression analysis was done to define if SCOPE score was an independent predictor of progression to SRF or death the first 14 days. Variables also included in the step-wise Cox regression model were suPAR 6 ng/mL or more, pneumonia WHO severity, intake of dexamethasone and BMI more than 30 kg/m2; hazard ratio (HR) and 95% CI were defined.
The analysis of the first secondary endpoint was done using a 2 × 2 table and Fisher exact test. Non-parametric correlation between SCOPE score and suPAR was done according to Spearman’s rank of order. The analysis of the second secondary endpoint was done using multivariate ordinal regression analysis.
The selection of the four biomarkers for inclusion in the SCOPE score was further confirmed by LASSO regression analysis in two steps. At the first step, suPAR was correlated to each of the four biomarkers using Spearman’s rank of order and after applying correction of the p-values for multiple tests. At the second step, forward step-wise multivariate logistic regression analysis was done with suPAR 6 ng/mL or more as a binary dependent variable. The quartiles of CRP, D-dimers, ferritin and IL-6 used for the development of the SCOPE score in the discovery set entered the model as independent variables. The ORs and 95%CIs were calculated.
Any p value below 0.05 was considered statistically significant.
Acknowledgments
The study was funded in part by the Hellenic Institute for the Study of Sepsis and by Swedish Orphan Biovitrum. The Hellenic Institute for the Study of Sepsis is the sponsor of the SAVE and SAVE-MORE studies.
Author contributions
E.J.G.-B. provided substantial contributions to the conception of the work. E.J.G.-B. and M.G.N. drafted the manuscript. All authors substantially contributed to the acquisition, analysis, or interpretation of data for the manuscript. All authors participated in drafting, revising, and critically reviewing the manuscript for important intellectual content. All authors approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of interests
E.J.G.-B. has received honoraria from Abbott CH, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH, Sobi, and XBiotech, Inc.; independent educational grants from Abbott CH, AxisShield, bioMérieux, InflaRx GmbH, Johnson & Johnson, MSD, Sobi, and XBiotech, Inc.; and funding from the Horizon 2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens) and the Horizon 2020 European Grants ImmunoSep and RISCinCOVID (granted to the Hellenic Institute for the Study of Sepsis). G.P. has received independent educational grants from Pfizer, MSD, Angelini, and Bio-Rad. H.M. reports receiving honoraria, consulting fees, and non-financial support from health care companies, including Amgen, Angelini, Bayer, Mylan, MSD, Pfizer, and Servier. L.D. has received consultation honoraria from SOBI. M.B. has received funds for research grants from and/or has been an advisor/consultant and/or speaker/chairman for Angelini, Astellas, Bayer, bioMérieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, Roche, Shionogi, and Nabriva. P.P. has received honoraria from GILEAD Sciences, Janssen, and MSD. G.N.D. is an advisor or lecturer for Ipsen, Pfizer, Genkyotex, Novartis, and Sobi; received research grants from Abbvie and Gilead; and has served as PI in studies for Abbvie, Novartis, Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics, Inc., Tiziana Life Sciences, Bayer, Astellas, Pfizer, Amyndas Pharmaceuticals, CymaBay Therapeutics, Inc., Sobi, and Intercept Pharmaceuticals. M.G.N. is supported by an ERC advanced grant (833247) and a Spinoza grant of the Netherlands Organization for Scientific Research. M.G.N. is a scientific founder of TTxD and he has received independent educational grants from TTxD, GSK, Ono Pharma, and ViiV HealthCare. The other authors declare no competing interests.
Inclusion and diversity
We worked to ensure gender balance in the recruitment of human subjects. We worked to ensure ethnic or other types of diversity in the recruitment of human subjects. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
Published: February 25, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100560.
Supplemental information
Data and code availability
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request. This paper does not report original code.
References
- 1.Lippi G., Sanchis-Gomar F., Henry B.M. COVID-19: unravelling the clinical progression of nature's virtually perfect biological weapon. Ann. Transl. Med. 2020;8:693. doi: 10.21037/atm-20-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transpl. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyriazopoulou E., Poulakou G., Milionis H., Metallidis S., Adamis G., Tsiakos K., Fragkou A., Rapti A., Damoulari C., Fantoni M., et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat. Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency . 2021. EMA recommends approval for use of Kineret in adults with COVID-19.https://www.ema.europa.eu/en/news/ema-recommends-approval-use-kineret-adults-covid-19 [Google Scholar]
- 5.Eugen-Olsen J., Giamarellos-Bourboulis E.J. suPAR: the unspecific marker for disease presence, severity and prognosis. Int. J. Antimicrob. Agents. 2015;46:S33–S34. doi: 10.1016/j.ijantimicag.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Pixley R.A., Espinola R.G., Ghebrehiwet B., Joseph K., Kao A., Bdeir K., Cines D.B., Colman R.W. Interaction of high-molecular-weight kininogen with endothelial cell binding proteins suPAR, gC1qR and cytokeratin 1 determined by surface plasmon resonance (BiaCore) Thromb. Haemost. 2011;105:1053–1059. doi: 10.1160/TH10-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rovina N., Akinosoglou K., Eugen-Olsen J., Hayek S., Reiser J., Giamarellos-Bourboulis E.J. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit. Care. 2020;24:187. doi: 10.1186/s13054-020-02897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altintas I., Eugen-Olsen J., Seppälä S., Tingleff J., Stauning M.A., El Caidi N.O., Elmajdoubi S., Gamst-Jensen H., Lindstrøm M.B., Rasmussen L.J.H., et al. suPAR cut-offs for risk stratification in patients with symptoms of COVID-19. Biomark. Insights. 2021;16 doi: 10.1177/11772719211034685. 11772719211034685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oulhaj A., Alsuwaidi A.R., Suliman A., Gasmelseed H., Khan S., Alawi S., Hukan Y., George J., Alshamsi F., Sheikh F., et al. Admission levels of soluble urokinase plasminogen activator receptor (suPAR) are associated with the development of severe complications in hospitalised COVID-19 patients: a prospective cohort study. Int. J. Infect. Dis. 2021;107:188–194. doi: 10.1016/j.ijid.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G., Henry B.M., Favaloro E.J. Elevated soluble urokinase plasminogen activator receptor (suPAR) in COVID-19 patients. Clin. Chem. Lab. Med. 2021;59:e413–e415. doi: 10.1515/cclm-2021-0561. [DOI] [PubMed] [Google Scholar]
- 11.Kyriazopoulou E., Panagopoulos P., Metallidis S., Dalekos G.N., Poulakou G., Gatselis N., Karakike E., Saridaki M., Loli G., Stefos A., et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife. 2021;10:e66125. doi: 10.7554/eLife.66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold D.T., Hamilton F.W., Elvers K.T., Frankland S.W., Zahan-Evans N., Patole S., Medford A., Bhatnagar R., Maskell N.A. Pleural fluid suPAR levels predict the need for invasive management in parapneumonic effusions. Am. J. Respir. Crit. Care Med. 2020;201:1545–1553. doi: 10.1164/rccm.201911-2169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ampfel N.M. Anakinra: timely targeted anticytokine therapy for severe COVID-19. NEJM J. Watch. 2021 https://www.jwatch.org/na54236/2021/11/03/anakinra-timely-targeted-anticytokine-therapy-severe-covid [Google Scholar]
- 14.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M., Gabrilove J.L., Sacks H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid. Based Med. 2021;26:107–108. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The CORIMUNO-19 Collaborative Group Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir. Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynants L., Van Calster B., Collins G.S., Riley R.D., Heinze G., Schuit E., Bonten M.M.J., Dahly D.L., Damen J.A., Debray T.P.A., et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Cheng J., Shang J., Wang Y., Wan J., Yan Y.Q., Liu W.B., Zhang H.P., Wang J.P., Wang X.Y., et al. Clinical value of laboratory indicators for predicting disease progression and death in patients with COVID-19: a retrospective cohort study. BMJ Open. 2021;11:e043790. doi: 10.1136/bmjopen-2020-043790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., Li Y., Guan W., Sang L., Lu J., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern. Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen N.A.F., Grondman I., de Nooijer A.H., Boahen C.K., Koeken V.A.C.M., Matzaraki V., Kumar V., He X., Kox M., Koenen H.J.P.M., et al. Dysregulated innate and adaptive immune responses discriminate disease severity in COVID-19. J. Infect. Dis. 2021;223:1322–1333. doi: 10.1093/infdis/jiab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request. This paper does not report original code.