Abstract
Fibronectin binding proteins play an important role in the adherence and invasion of group A streptococci (GAS). Genotypically distinct GAS isolates were screened for the presence and expression of two streptococcal fibronectin binding protein genes, sfbI and sfbII. Of the tested strains, 64 and 36% were shown to harbor and express the sfbI and sfbII genes, respectively. All sfbII-positive strains tested were also positive for sfbI, but only 28% of the sfbII-negative strains were positive for sfbI. High levels of immunoglobulin G antibodies to both SfbI and SfbII were found in sera from 80 subjects with defined streptococcal infections.
The rates of poststreptococcal sequelae are extremely high in Aboriginal communities in tropical regions of Australia, compared to those in urban communities of Australia and in other developed countries (18). Aboriginal communities experience high rates of acute rheumatic fever (ARF) and rheumatic heart disease (RHD) (3), high rates of pyoderma and low rates of throat colonization (2), and periodic widespread epidemics of acute glomerulonephritis (AGN) and chronic renal disease linked to group A streptococcus (GAS) exposure (7, 8, 24). By examination of GAS isolates from Aboriginal communities and from patients at the Royal Darwin Hospital, we found over 100 distinct genotypes in the Northern Territory, Australia (unpublished observations). Patterns of adherence and colonization of GAS and host immune responses are likely to be important in the epidemiology and pathogenesis of streptococcal infections and their sequelae.
Fibronectin, a principal glycoprotein in plasma, in various body fluids, and in the extracellular matrix and basement membrane, can interact with both streptococci and host cells and is considered an important mediator of streptococcal adherence. The fibronectin binding protein SfbI, also called F1 (10, 25, 26), is involved in the adherence of streptococci to epithelial cells (26) via a specific binding domain (27). SfbI has a role in the invasion of nonphagocytic epithelial cells by GAS (21–23). This interaction can be blocked by anti-SfbI antibodies that prevent both bacterial attachment and internalization (20). More recently, SfbI protein has been shown to evoke a protective immune response against Streptococcus pyogenes after intranasal immunization (9).
Another fibronectin binding protein, SfbII, has been shown to possess serum opacity factor activity (15). SfbII is distinct from SfbI, except for some homology within the C-terminal repeat region. Epidemiological studies of German isolates from a region where streptococcal diseases are nonendemic revealed that sfbI is present in more than 70% of genotypically distinct GAS isolates (28), and 86% of these distinct isolates have at least one of the two sfb genes (15). We now report the distribution of sfbI and sfbII genes among 69 genotypically distinct GAS isolates and the presence of antibodies specific for SfbI and SfbII in sera of patients with defined streptococcal infections from the Northern Territory, where streptococcal diseases are endemic.
S. pyogenes strains were isolated from patients admitted to Royal Darwin Hospital from 1990 to 1997. These strains included invasive isolates from sterile sites and wound and throat isolates. Skin isolates were collected during community surveys following outbreaks of AGN. Isolates were grown in Todd-Hewitt broth (Oxoid) and were initially characterized by Vir typing (5, 6) with HaeIII and further characterized by restriction analysis with HinfI (8). Isolates were classified as invasive if grown from blood or joint fluid.
Isolation of chromosomal S. pyogenes DNA, XbaI digestion, electrophoresis, and blotting procedures were performed as previously described (28). The sfbI hybridization probe SI, representing the conserved part of the sfbI gene ranging from the start of the proline repeat region to the end of the fibronectin binding repeat region, was amplified via PCR. The primers were 5′-TATCAAAATCTTCTAAGTGCTGAG-3′ (5′ primer) and 5′-AATGGAACACTAACTT-CGGACGGG-3′ (3′ primer). The N- and C-terminal SfbII hybridization probes SII-N and SII-C were generated and labelling and hybridization were carried out as described previously (15).
Recombinant SfbI (rSfbI) and SfbII (rSfbII) fusion proteins were produced as described previously (15, 23). Generation of rabbit SfbI- and SfbII-specific antibodies was performed by a previously described immunization protocol (23). Immunoglobulin G (IgG) fractions were isolated by protein A affinity chromatography, and IgG F(ab′)2 fragments were obtained through pepsin digestion and purified by standard techniques (11).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was conducted according to standard methods (16) with a 6% stacking gel and a 15% separating gel. Briefly, 1 ml of a GAS overnight culture grown in Todd-Hewitt broth was centrifuged at 5,000 × g. Bacterial cells were resuspended in 50 μl of loading buffer, lysed by incubating the mixture for 5 min at 100°C, and then immediately put on ice before loading 5 μl of the lysate on the gel. Gels were blotted onto a nitrocellulose membrane by using a semidry blotting apparatus (Bio-Rad), blocked with 10% skim milk in phosphate-buffered saline (PBS), and incubated with the purified F(ab′)2 fragments specifically recognizing SfbI or SfbII. A second antibody—alkaline phosphatase-conjugated affinity-purified F(ab′)2 fragment, specific to goat antirabbit IgG F(ab′)2 fragment (Jackson ImmunoResearch Laboratories)—was used. Filters were developed and bands were detected with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate color reaction.
Immunofluorescence was used on a subset of isolates to confirm the data obtained by Western blotting. Overnight cultures of GAS were washed and finally diluted to 1:20 in PBS, and then 10 μl was placed in a well on a slide and allowed to dry before fixing the cells with PBS containing 3.7% formaldehyde. The sample was saturated with 20 μl of PBS–10% fetal calf serum (FCS) for 30 min at 37°C. Then 10 μl of the Sfb-specific antibody diluted to 1:20 with PBS-FCS was added, and the wells were incubated in a moist chamber for 45 min at 37°C. Slides were washed twice with 20 μl of PBS for 5 min each. Then 10 μl of the second antibody, fluorescein isothiocyanate-labelled F(ab′)2 fragment goat anti-rabbit IgG, F(ab′)2 fragment specific (Jackson Immuno Research Laboratories), was diluted to 1:50 in PBS-FCS, added to the well, and incubated in a dark, moist chamber for 45 min. Following washing and mounting, samples were examined with a fluorescent microscope.
Eighty sera from subjects ranging in age from 3 to 52 years were divided into separate categories as follows: 1, RHD (n = 16); 2, control (n = 23); 3, other non-RHD (n = 4); 4, ARF, including chorea (n = 24); 5, streptococcal bacteremia (n = 2); and 6, AGN (n = 11). Enzyme-linked immunosorbent assay (ELISA) plates (Maxisorb; Nunc) were coated with 200 ng of rSfbI or rSfbII per well. Plates were blocked with PBS containing 10% FCS for 1 h at 37°C. Patient sera were diluted to 1:100 in PBS–10% FCS, and 50 μl was added to the plates, which were then incubated for 1 h at 37°C. Plates were washed four times with PBS containing 0.05% Tween 20 before 50 μl of a 1:1,000 dilution of horseradish peroxidase-conjugated anti-human IgG (Sigma) was added. Plates were further incubated for 1 h at 37°C. Wells were again washed with PBS containing 0.05% Tween 20, tetramethylbenzidine substrate was added, and plates were developed for 30 min at room temperature before they were analyzed at 655 nm on a Bio-Rad plate reader. A positive result was an optical density value of >0.75 at 655 nm. A subset of positive and negative sera (26 samples) was tested at a 1:100 dilution for their reactivities to rSfbI and rSfbII on Western blots. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotting procedures were as described above, except for the secondary antibody, which in this case was alkaline phosphatase-conjugated anti-human IgG (Sigma). The ability of the GAS isolates to cause opacity in serum was measured by the serum opacity factor microtiter test (14).
The distribution of sfbI and sfbII characteristics of 69 strains was examined by a two-tailed Fisher exact test. Antibodies to rSfbI, rSfbII, and C-terminally truncated rSfbII were compared by clinical category for samples from RHD, ARF, and AGN patients and controls by one-way analysis of variance with Dunnett's post test. Pearson's correlation was used to determine the relationship between titers of IgG to each of the recombinant proteins. Statistical calculations were performed with GraphPad InStat.
By Southern blotting with specific probes, 44 strains (64%) were found to have sfbI and 25 strains (36%) were found to have sfbII (Table 1). Each gene probe reacted exclusively with its respective gene. The majority of the strains (64%) carried at least one of the sfb genes. All isolates that possessed sfbI or sfbII expressed SfbI or SfbII, respectively, as determined by Western blotting (data not shown). A subset of isolates was also tested for surface expression of SfbI and SfbII by immunofluorescence microscopy, and results were in accordance with the Western blot results (data not shown).
TABLE 1.
Presence of the sfbI and sfbII genes in the genomes of 69 GAS isolates, determined by Southern hybridizationa
| VT | I | II | VT | I | II | VT | I | II | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | 23 | 55 | + | |||||
| 2.1 | + | + | 26 | 57 | + | |||||
| 2.2 | + | + | 29.1 | 60 | + | |||||
| 3.1 | + | + | 29.2 | 61 | + | |||||
| 3.2 | + | + | 30 | + | + | 67 | ||||
| 3.3 | + | + | 31 | 69 | ||||||
| 4 | 32 | + | + | 71 | + | + | ||||
| 5 | 31.1 | + | 72 | |||||||
| 6 | + | + | 34 | + | + | 73 | ||||
| 7.1 | + | + | 36 | 76 | ||||||
| 7.2 | + | + | 37 | + | + | 77 | + | |||
| 8 | + | 38 | + | 78 | ||||||
| 10 | + | + | 39 | 79 | + | + | ||||
| 12.3 | 40 | + | 82 | |||||||
| 13 | + | 41 | 84 | |||||||
| 14.1 | + | + | 42 | 86 | ||||||
| 15 | 43 | 91 | ||||||||
| 16 | + | 44 | + | 96 | + | |||||
| 17.1 | + | 45 | 100 | |||||||
| 17.2 | + | 46 | 101 | |||||||
| 18 | + | 49.1 | + | + | 104 | + | + | |||
| 21 | + | + | 52 | + | + | 105 | + | + | ||
| 22 | + | + | 53 | + | 108 | + | + |
VT, Vir type; I, sfbI gene; II, sfbII gene; +, presence of the gene.
Since SfbII is known to cause opacity in serum (15), the strains in the present study were also tested for this reaction in a microtiter assay. All strains, except one, that were positive for sfbII and expressed SfbII protein were shown to be opacity factor positive. Interestingly, all of the sfbII-positive strains (i.e., class II) were found to be sfbI positive, while 43% of the sfbII-negative strains (i.e., class I) were sfbI positive and 57% were sfbI negative (P < 0.0001).
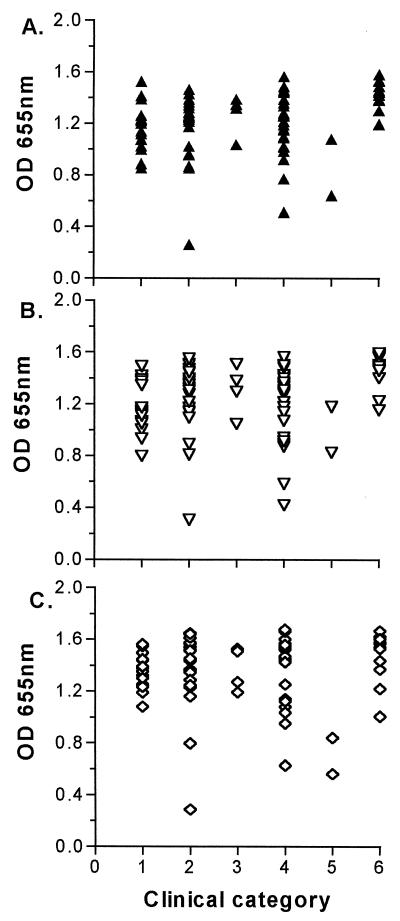
Sera from 80 subjects with defined streptococcal infections were tested by ELISA with purified rSfbI or rSfbII as the antigen (Fig. 1). With the cutoff value previously described, 77 of 80 (96%) sera tested were positive for SfbI and SfbII antibodies. Sera from patients with AGN (Fig. 1) (clinical category 6) had higher titers of antibody to SfbI and SfbII than did sera from patients with RHD or ARF and control samples (P < 0.05). No significant difference between groups was found for antibodies to the truncated rSfbII protein. Control samples from Aboriginal communities (category 2) were uniformly high, reflecting endemic exposure to GAS, whereas a control sample from the urban Darwin region (the lowest point of category 2) and non-Aboriginal sera from the two bacteremic cases (category 5) were lower. Titers for one case of ARF were low and may reflect long-term use of penicillin prophylaxis, thus lowering exposure to GAS. Overall, there was no significant difference between patients with ARF or RHD and Aboriginal controls for IgG titers of antibody to SfbI or SfbII.
FIG. 1.
Scattergram showing 80 human sera (IgG) in different clinical categories tested against three recombinant proteins by ELISA. (A) rSfbI; (B) rSfbII; (C) rSfbII lacking the C-terminal fibronectin binding repeat region. Sera were diluted to 1:100 and titers were measured by the reading for the optical density (OD) at 655 nm. Each symbol represents one serum sample.
Linear correlation analysis of the relationship between IgG antibodies to SfbI and SfbII suggests that infections with SfbI- or SfbII-positive GAS are common, that B-cell epitopes are common to both proteins, or both. High levels of IgG to SfbI were usually matched by high titers of IgG to SfbII (r = 0.8351), but when SfbI titers were compared to titers of antibody to the truncated SfbII protein (lacking the C-terminal fibronectin binding regions), the correlation was found to be lower (r = 0.772). This supports the idea that some of the antibodies to SfbII are directed towards the homologous fibronectin binding region. High titers of antibody to both SfbI and SfbII are found across all age groups (data not shown) and may be due to chronic exposure to GAS with different SfbI and SfbII proteins from a very young age.
Western blot analysis of rSfb proteins was conducted with 26 of the 80 sera previously tested by ELISA. Sera found to be positive by ELISA were also positive by Western blotting, and sera found to be negative by ELISA were also negative by Western blotting. Variations in immunodominant fragments were, however, noted for both SfbI and SfbII. Collectively, these studies show that an IgG antibody response to SfbI and SfbII is mounted among most individuals in this region.
The adherence of GAS to epithelial cells may be mediated by a variety of ligands (12). It has been suggested that given strains may utilize multiple adhesins, possibly functioning in distinct kinetic steps, in order to firmly establish such contact (1, 12). Binding to the cell matrix provides a good opportunity for interaction between host cells and the pathogen (1) and may initiate destruction or weakening of the host's defense mechanisms, allow delivery of toxins, and permit subsequent invasion (13). A number of matrix binding proteins have been described for GAS, including SfbI (25), SfbII (15), F1 (10) and FBBP (4) (which each bind to fibronectin), and VnbP, which binds to vitronectin (17).
This study allowed us to compare the distribution of sfb genes in strains from an area in which streptococcal disease is endemic to the distribution in strains from a region where the disease is nonendemic (15). The principal difference between the two sets of isolates was that 36% of the Northern Territory strains were negative for both sfbI and sfbII, whereas only 14% were double negative in the study undertaken by Kreikemeyer et al. (15). Aside from this, there was no significant difference in the distribution of the sfb genes among the two sets of isolates. A large number of strains that were negative for SfbI and SfbII interacted with another adhesive protein, vitronectin (unpublished data).
Recently, it has been demonstrated that SfbI plays an important role in the ability of GAS to invade epithelial cells (21, 23). Among strains from the Northern Territory, 64% of the strains were SfbI positive and most sera had SfbI-specific IgG. Although anti-SfbI antibodies were protective in a mouse model (9), the anti-Sfb antibodies in human sera do not appear to be protective against GAS skin infections.
The colinear antibody responses to SfbI and SfbII may be the result of cross-reactive epitopes shared between SfbI and SfbII or other streptococcal proteins, as well as concurrent exposure to both SfbI and SfbII. Since the correlation coefficient was lower than 1 when any pair of antibody titers was examined, this suggests that other unique immunodominant epitopes in both SfbI and SfbII may also be recognized in human subjects. This is supported by Western blot analysis of both and SfbI and SfbII proteins, where different fragments were found to be immunodominant in different subjects.
Furthermore, the antibody responses to SfbI proteins in patients in different clinical categories and in controls reveals that SfbI is not a marker for conditions such as ARF, RHD, or AGN where streptococcal infection is endemic. Recently, SfbI has been explored as a nontoxic mucosal adjuvant (19) and was found to increase specific IgG responses, upregulate specific CD4+ T cells, and induce specific CD8+ T cells in mice. We have demonstrated that IgG specific for fibronectin binding proteins occurs in human populations subject to intense streptococcal skin infections. The route of SfbI exposure (throat and nose mucosa versus skin), the effect of preexisting or developing blocking antibodies against GAS colonization, and the possible adjuvant effects of SfbI in regions where streptococcal diseases are endemic and nonendemic all need to be examined.
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia and the Deutsche Forschungsgemeinschaft, Germany (CH89/3-1). A.M.G. is supported by the National Heart Foundation of Australia. M.H. is the recipient of an NHMRC Aboriginal Health Training Scholarship.
We thank Sue Hutton, Jeni Wie, and Melanie Tillig, as well as the microbiology staff at Royal Darwin Hospital.
A.M.G. and M.H. contributed equally to this work.
REFERENCES
- 1.Bisno A L. Molecular aspects of bacterial colonisation. Infect Control Hosp Epidemiol. 1995;16:648–657. doi: 10.1086/647032. [DOI] [PubMed] [Google Scholar]
- 2.Carapetis J R, Connors C, Yarmirr D, Krause V, Currie B J. Success of a scabies control program in an Australian aboriginal community. Pediatr Infect Dis J. 1997;16:494–499. doi: 10.1097/00006454-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Carapetis J R, Wolff D R, Currie B J. Acute rheumatic fever and rheumatic heart disease in the top end of Australia's Northern Territory. Med J Aust. 1996;164:146–149. doi: 10.5694/j.1326-5377.1996.tb122012.x. [DOI] [PubMed] [Google Scholar]
- 4.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardiner D L, Hartas J, Hibble M, Goodfellow A M, Currie B J, Sriprakash K S. Molecular epidemiology of group A streptococcal infections in the Northern Territory of Australia. Adv Exp Med Biol. 1997;418:317–321. doi: 10.1007/978-1-4899-1825-3_76. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner D L, Hartas J, Currie B, Mathews J D, Kemp D J, Sriprakash K S. Vir typing: a long PCR typing method for group A streptococci. PCR Methods Appl. 1995;4:288–293. doi: 10.1101/gr.4.5.288. [DOI] [PubMed] [Google Scholar]
- 7.Gogna N K, Nossar V, Walker A C. Epidemic of acute poststreptococcal glomerulonephritis in Aboriginal communities. Med J Aust. 1983;1:64–66. doi: 10.5694/j.1326-5377.1983.tb136039.x. [DOI] [PubMed] [Google Scholar]
- 8.Goodfellow A M, Gardiner D L. Searching for APSGN-associated Streptococcus pyogenes in communities with endemic streptococcal skin infections. Adv Exp Med Biol. 1997;418:103–108. doi: 10.1007/978-1-4899-1825-3_26. [DOI] [PubMed] [Google Scholar]
- 9.Guzman C A, Talay S R, Molinari G, Medina E, Chhatwal G S. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with the fibronectin-binding protein SfbI. J Infect Dis. 1999;179:901–906. doi: 10.1086/314655. [DOI] [PubMed] [Google Scholar]
- 10.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 12.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoepelman A I M, Tuomanen E I. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992;60:1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson D R, Kaplan E L. Microtechnique for serum opacity factor characterization of group A streptococci adaptable to the use of human sera. J Clin Microbiol. 1988;26:2025–2030. doi: 10.1128/jcm.26.10.2025-2030.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreikemeyer B, Talay S R, Chhatwal G S. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol Microbiol. 1995;17:137–145. doi: 10.1111/j.1365-2958.1995.mmi_17010137.x. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Liang O D, Preissner K T, Chhatwal G S. The hemopexin-type repeats of human vitronectin are recognized by Streptococcus pyogenes. Biochem Biophys Res Commun. 1997;234:445–449. doi: 10.1006/bbrc.1997.6663. [DOI] [PubMed] [Google Scholar]
- 18.Martin D R, Sriprakash K S. Epidemiology of group A streptococcal disease in Australia and New Zealand. Recent Adv Microbiol. 1996;4:1–40. [Google Scholar]
- 19.Medina E, Talay S R, Chhatwal G S, Guzman C A. Fibronectin-binding protein of Streptococcus pyogenes is a promising adjuvant for antigens delivered by the mucosal route. Eur J Immunol. 1998;28:1069–1077. doi: 10.1002/(SICI)1521-4141(199803)28:03<1069::AID-IMMU1069>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Molinari G, Chhatwal G S. Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J Infect Dis. 1998;177:1600–1607. doi: 10.1086/515310. [DOI] [PubMed] [Google Scholar]
- 21.Molinari G, Chhatwal G S. Streptococcal invasion. Curr Opin Microbiol. 1999;2:56–61. doi: 10.1016/s1369-5274(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 22.Molinari G, Chhatwal G S. Role played by the fibronectin-binding protein SfbI (protein F1) of Streptococcus pyogenes in bacterial internalization by epithelial cells. J Infect Dis. 1999;179:1049–1050. doi: 10.1086/314681. [DOI] [PubMed] [Google Scholar]
- 23.Molinari G, Talay S R, Valentin-Weigand P, Rohde M, Chhatwal G S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streeton C L, Hanna J N, Messer R D, Merianos A. An epidemic of acute poststreptococcal glomerulonephritis among Aboriginal children. Paediatr Child Health. 1995;31:245–248. doi: 10.1111/j.1440-1754.1995.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 25.Talay S R, Ehrenfeld E, Chhatwal G S, Timmis K N. Expression of the fibronectin binding components of Streptococcus pyogenes in Escherichia coli demonstrates that they are proteins. Mol Microbiol. 1991;5:1724–1734. doi: 10.1111/j.1365-2958.1991.tb01921.x. [DOI] [PubMed] [Google Scholar]
- 26.Talay S R, Valentin-Weigand P, Jerlström P G, Timmis K N, Chhatwal G S. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;60:3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talay S R, Valentin-Weigand P, Chhatwal G S, Timmis K N. Domain structure and conserved epitopes of Sfb protein, the adhesin of Streptococcus pyogenes. Mol Microbiol. 1994;13:531–539. doi: 10.1111/j.1365-2958.1994.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 28.Valentin-Weigand P, Talay S R, Timmis K N, Chhatwal G S. The fibronectin-binding domain of the Sfb protein adhesin of Streptococcus pyogenes occurs in many group A streptococci and does not cross-react with heart myosin. Microb Pathog. 1994;17:111–120. doi: 10.1006/mpat.1994.1057. [DOI] [PubMed] [Google Scholar]