Abstract
Background
International guidelines recommend severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine for patients with cancer.
A substantial risk of developing vaccine-related autoimmune toxicities could be hypothesised for patients with thymic epithelial tumours (TETs) due to their high risk of autoimmune disorders (ADs). Moreover, a cross-reaction between SARS-CoV-2 spike protein antibodies and various tissue proteins has been shown, and antibodies against nucleoproteins showed overlaps in the autoimmune cross-reaction with antibodies to spike protein. Due to the rarity of TETs, no data addressing this hypothesis are available.
Methods
Patients with TETs who received SARS-CoV-2 vaccine, treated in 4 referral centres of the Italian Collaborative Group for ThYmic MalignanciEs (TYME) network between February 2021 and September 2021, were interviewed through a standardised 15-items questionnaire in order to describe the safety of SARS-CoV-2 vaccine in patients affected by TETs.
Results
Data from 245 doses of vaccine administered to 126 patients (41 = thymic carcinoma, 85 = thymoma; 38 with AD, of which 26 with active AD) were collected. Nine patients had a previous COVID-19-positive swab. No cases of AD reactivation or worsening of a pre-existing AD were seen in the study population. A new diagnosis of myasthenia gravis likely unrelated to the vaccine was made in two patients after the vaccination. Sixty-four patients (51%) experienced a total of 103 adverse events, all G1/G2, most commonly fatigue, new or worsening muscle pain and chills. None AE required patients’ hospitalisation.
Conclusions
SARS-CoV-2 mRNA vaccines appear to be safe in patients with TET, even in case of active or pre-existing AD.
Keywords: Thymic epithelial tumours, COVID vaccine, Sars-Cov-2
1. Introduction
International guidelines recommend severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine for patients with cancer. A substantial risk of developing vaccine-related autoimmune toxicities could be hypothesised for patients with thymic epithelial tumours (TETs) due to their higher risk of autoimmune disorders (ADs) and to the anecdotally reported AD flares temporally associated with SARS-CoV-2 vaccine [[1], [2], [3], [4], [5]].
Moreover, a cross-reaction between SARS-CoV-2 spike protein antibodies and various tissue proteins has been shown, and antibodies against nucleoproteins showed overlaps in the autoimmune cross-reaction with antibodies to spike protein [6].
Due to the rarity of TETs and to the exclusion of patients with active cancer from most randomised COVID-19 vaccine clinical trials [[7], [8], [9]], no data addressing this hypothesis are available.
To address this issue, we interviewed patients with TETs through a standardised 15-item questionnaire, treated in 4 referral centres of the Italian Collaborative Group for ThYmic MalignanciEs (TYME) who received SARS-CoV-2 vaccine between February 2021 and September 2021 [10].
2. Materials and methods
2.1. Study population
All patients aged 18 years old or more with pathologically confirmed diagnosis of TET (any stage) who received at least one dose of SARS-CoV-2 vaccine were identified in 4 referral centres of TYME network.
Patients who received vaccines between February 2021 and September 2021 (minimum follow-up: 10 weeks) were interviewed through a standardised 15-item questionnaire (Table 1 and Table 2 ).
Table 1.
Baseline clinical features of 126 patients affected by thymic epithelial tumours (TET) vaccinated against SARS-CoV-2.
| Thymoma | Thymic carcinomaa | ||
|---|---|---|---|
| Gender | |||
| Female | 59 | 43 | 16 |
| Male | 67 | 41 | 26 |
| Median age (range) | 61 (21–89) | 59 (21–89) | 63 (42–81) |
| Comorbidities | |||
| Hypertension | 40 | 24 | 16 |
| Diabetes | 5 | 5 | 0 |
| COPD | 5 | 4 | 1 |
| Asma | 3 | 2 | 1 |
| Vaping or smocking | 6 | 4 | 2 |
| BMI > 30 | 9 | 6 | 3 |
| Actual stageb | |||
| I | 1 | 1 | 0 |
| IIA | 2 | 2 | 0 |
| IIB | 3 | 2 | 1 |
| III | 6 | 4 | 2 |
| IVA | 18 | 13 | 5 |
| IVB | 55 | 28 | 27 |
| NED | 41 | 34 | 7 |
| Histological subtypeb | |||
| Type A | 3 | 3 | 0 |
| Type A-B | 8 | 8 | 0 |
| Type B1 | 8 | 8 | 0 |
| Type B2 | 21 | 21 | 0 |
| Type B3 | 23 | 23 | 0 |
| Thymic carcinoma | 41 | 0 | 41 |
| Other | 3 | 3 | 0 |
| Type B1/B2 | 4 | 4 | 0 |
| Type B2/B3 | 14 | 14 | 0 |
| Type B3/thymic carcinoma | 1 | 0 | 1 |
| Oncological treatment | |||
| Previous radiotherapy | 78 | 48 | 30 |
| Previous surgery on primary tumour | 95 | 71 | 24 |
| R0 | 73 | 55 | 18 |
| Not R0 | 22 | 16 | 6 |
| Received systemic treatmentc | 88 | 53 | 35 |
| Received systemic treatment within three months administration of vaccined | 36 | 19 | 17 |
Key: TET: thymic epithelial tumour; BPCO: chronic obstructive pulmonary disease; BMI: body mass index; NED: no evidence of disease.
Includes also type B3/thymic carcinoma.
Stage at the time of vaccination, according AJCC Cancer Staging, VIII ed
For any stage, independently from time of vaccination
Either before or after.
Table 2.
Characteristics of autoimmune disorders (ADs) in 126 patients affected by thymic epithelial tumours (TET) vaccinated against SARS-CoV-2.
| Total number of patients affected | Currently active AD | Immunosuppressive treatment required | Steroids (at immunosuppressive dose) | |
|---|---|---|---|---|
| Myasthenia gravis | 23 (1) | 15 (1) | 18 (1) | 7 (0) |
| Other AD | 12 (5) | 9 (4) | 6 (3) | 2 (0) |
| Myasthenia gravis + other AD | 3 (0) | 2 (0) | 2 (0) | 1 (0) |
| Total | 38 (6) | 26 (5) | 26 (4) | 10 (0) |
Key: AD: Autoimmune Disorders; TET: Thymic Epithelial Tumour. In brackets, results for patients affected by thymic carcinoma are reported.
2.2. Data collection
The institutional review board of each institution granted permission for this study (R1454/21-IEO 1527). Patient adverse events (AEs) were classified according to CTCAE v.5.0; history, activity and symptoms exacerbation of AD were defined by the treating oncologist, after alternative diagnosis had been excluded, based on current clinical guidelines. A standardised questionnaire was used to collect patients’ medical history and data about adverse events (AEs) (Table A. 1).
2.3. Statistical analysis
Primary outcomes were AE rate and incidence of new ADs or worsening of pre-existing AD in the examined population. Statistical analysis was performed using R Statistical Software (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Patient characteristics
The clinical features of 126 patients affected by TET are summarised in Table 1, Table 2. Thirty-eight (30%) were affected by AD, of which 26 (20%) were active at the time of vaccination. Namely, 23 patients (18%) were affected by myasthenia gravis (MG), of which 15 (12%) had active disease at the time of the interview.
Nine patients (7%) referred a positive swab for SARS-CoV-2 in the last 12 months (Table A. 2), of which 7 (5%) reported COVID-19 symptoms. Oxygen therapy and/or hospitalisation were required for 4 patients.
Overall, 245 doses of vaccine were administered to 126 patients. All patients received at least one dose of SARS-CoV-2 vaccine, and 118 (94%) completed the cycle with the second dose. One patient received also a third dose (booster dose). One hundred twenty-one (96%) patients received an mRNA-based vaccination, namely COMIRNATY® (Pfizer) (n = 105) or SPIVAX® (Moderna) (n = 16) (Table A. 3).
3.2. AEs to SARS-CoV-2 vaccination
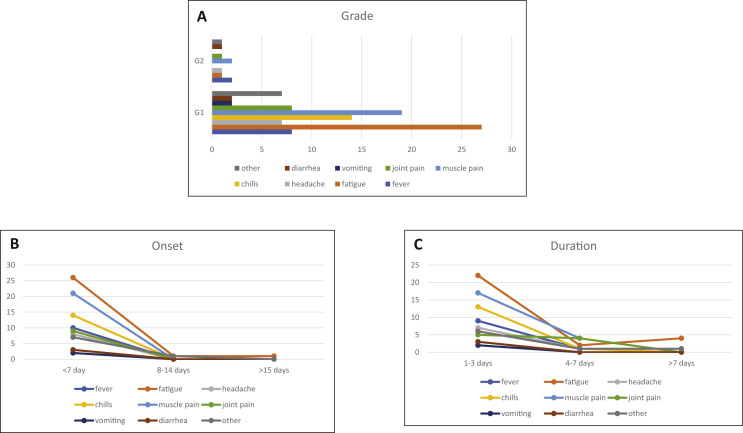
Sixty-four patients (50.7%, 95% CI 0.41%–0.59%) experienced an AE after administration of SARS-CoV-2 vaccines. A total of 103 AEs was observed after administration of SARS-CoV-2 vaccine (Table 3 and Fig. 1 ).
Table 3.
Incidence table adverse events (AE) to COVID vaccine in patients affected by thymic epithelial tumours.
| Gradea |
Onsetb |
Durationc |
Systemic pharmacological intervention required | ||||||
|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | <7 day | 8–14 days | >15 days | 1–3 days | 4–7 days | >7 days | ||
| Fever | 8 (2) | 2 (1) | 10 (3) | 0 (0) | 0 (0) | 9 (3) | 1 (0) | 0 (0) | 6 (3) |
| Fatigue | 27 (10) | 1 (0) | 26 (8) | 1 (1) | 1 (1) | 22 (8) | 2 (0) | 4 (2) | 2 (1) |
| Headache | 7 (2) | 1 (0) | 8 (2) | 0 (0) | 0 (0) | 7 (2) | 1 (0) | 0 (0) | 0 (0) |
| Chills | 14 (6) | 0 (0) | 14 (6) | 0 (0) | 0 (0) | 13 (6) | 1 (0) | 0 (0) | 2 (1) |
| Muscle pain | 19 (5) | 2 (1) | 21 (6) | 0 (0) | 0 (0) | 17 (6) | 4 (0) | 0 (0) | 3 (2) |
| Joint pain | 8 (3) | 1 (0) | 9 (3) | 0 (0) | 0 (0) | 5 (3) | 4 (0) | 0 (0) | 2 (0) |
| Vomiting | 2 (1) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 1 (0) |
| Diarrhoea | 2 (0) | 1 (0) | 3 (0) | 0 (0) | 0 (0) | 2 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 7 (0) | 1 (0) | 7 (0) | 1 (0) | 0 (0) | 6 (0) | 1 (0) | 1 (0) | 1 (0) |
Note: ‘Muscle pain’ refers to new or worsening muscle pain; ‘joint pain’ refers to new or worsening joint pain. Results for patients affected by thymic carcinoma are reported in brackets.
Keys:
Grade of AE, according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
Days from vaccination to onset of AE.
Duration of AE.
Fig. 1.
Incidence of adverse events (AEs) to COVID vaccine in patients affected by thymic epithelial tumours. A: Grade of AE, according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. B: Days from vaccination to onset of AE. C: Duration of AE. Note: ‘Muscle pain’ refers to new or worsening muscle pain; ‘joint pain’ refers to new or worsening joint pain.
All reported AEs were grade 1 (G1) or G2. No G3, G4 and G5 AEs were observed. The most common G1 AEs were fatigue (n = 28; 27.1%, 95% CI 18.8%–36.8%), new or worsening muscle pain (n = 21; 20%, 95% CI 13%–29.4%) and chills (n = 14; 13.5%, 95% CI, 7.6%–21.7%). Other G1 AE were fever (n = 8; 7%, 95% CI 3.4%–14.7%), headache (n = 7; 6.8%, 95% CI 2.7%–13.5%), vomiting (n = 2; 1.9%, 95% CI 2.4%–6.8%), diarrhoea (n = 2; 1.9%, 95% CI 2.4%–6.8%) and other (n = 7; 6.8%, 95% CI 2.7%–13.5%). The most common G2 event was fever (n = 2; 1.9%, 95% CI 2.4%–6.8%) and new or worsening muscle pain (n = 2, 1.9%, 95% CI 2.4%–6.8%). The majority (n = 100; 97%, 95% CI 91.7%–99.4%) of AEs started within 7 days from vaccination and last 1–3 days (n = 84; 81.5%, 95% CI 72.7%–88.5%). 17 AE required outpatient systemic pharmacologic intervention, namely fever (n = 6), fatigue (n = 2), chills (n = 2), muscle pain (n = 3), joint pain (n = 2), vomiting (n = 1) and other (n = 1). None of the patients were hospitalised due to AEs.
3.3. Autoimmune disease after SARS-CoV-2 vaccination
No cases of reactivation or worsening of a pre-existing AD were seen in the whole study population. Two cases of the new diagnosis of AD (MG) were observed after administration of SARS-CoV-2 vaccines (COMIRNATY®), both likely unrelated to vaccine administration.
In the first case, diagnosis osf MG was made at the same time with the first evidence of a previously unappreciated thymoma B2, three months after the second dose of vaccine.
The second patient was affected by thymic carcinoma and has had a negative testing for acetylcholine receptor antibodies (AChR-Ab) assessed in 2018. Tumour relapsed 2 years after surgery and the patient received systemic chemotherapy (last cycle on January 2021). In May 2021, just one day after the 1st vaccine administration, the patient reported the onset of asthenia and hyposthenia, and a switch-on of AChR-Ab test was found. Symptoms resolved spontaneously in less than three weeks, in the absence of specific treatments.
4. Discussion
Our results suggest that currently available SARS-CoV-2 mRNA vaccines could be safely administered in patients with TETs, even in case of active or pre-existing AD.
No G3-G4 toxicities have been captured in 245 vaccine administrations and no unexpected side effects have been observed, also in the subset of patients with a previous diagnosis of COVID-19. The rate and the spectrum of G1/G2-reported AE was consistent with those reported in the general population [[7], [8], [9]].
Two cases of newly diagnosed MG have been observed.
Several elements suggest the absence of a cause–effect relationship between vaccination and MG in both patients. The first one had a MG diagnosis in concomitance with the first evidence of a previously unappreciated thymoma; the other patient had a switch-on of AChR-Ab test just one day after the 1st vaccine administration, few months after a systemic tumour relapse.
Notably, despite the potential high risk of SARS-CoV-2 vaccines’ autoimmune toxicity in patients with TETs, no patients experienced vaccine-related severe immune AE. This observation is particularly relevant considering that 38 patients have had a diagnosis of AD, even with active symptoms at the time of vaccine administration.
These data can be helpful to more confidently support and recommend SARS-CoV-2 vaccine to patients with TET, according to the international guidelines for patients with cancer.
4.1. Limitations
The number of patients included in our analysis could be not adequate to capture infrequent immune-related vaccine AEs in patients with TETs. No data (only 5 patients) are available on non-mRNA vaccines.
5. Conclusions
SARS-CoV-2 mRNA vaccines are safe in patients with TET, even in case of active or pre-existing AD.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Disclosure: PAZ declare Consulting or Advisory Role for Astellas Pharma; AstraZeneca; Bristol-Myers Squibb; Ipsen; Janssen-Cilag; Merck Sharp & Dohme; Novartis; Pfizer; Roche; Sanofi.
MCG declares personal financial interests with the following organisations: AstraZeneca, MSD International GmbH, BMS, Boehringer Ingelheim Italia S.p.A, Celgene, Eli Lilly, Ignyta, Incyte, Inivata, MedImmune, Novartis, Pfizer, Roche, Takeda; she also declares institutional financial interests with the following organisations: Eli Lilly, MSD, Pfizer (MISP), AstraZeneca, MSD International GmbH, BMS, Boehringer Ingelheim Italia S.p.A, Celgene, Ignyta, Incyte, Inivata, MedImmune, Novartis, Pfizer, Roche, Takeda, Tiziana, Foundation Medicine; she has received research funding from the following organisations: AIRC, AIFA, Italian Moh, TRANSCAN.
GMC declares personal fees from MSD, AstraZeneca, Eli Lilly and BMS, outside the submitted work.
RB is a consultant/advisory board member for Astra Zeneca, Boehringer Ingelheim, Novartis, MSD, Otsuka, Eli-Lilly, Roche.
PQ reports advisory board relationships at Roche, Novartis, MSD, BMS, Sun Pharma, Sanofi and Pierre Fabre.
GC has received personal fees from Ellipses Pharma, Roche, Pfizer, Novartis, Eli Lilly, Foundation Medicine, Bristol–Myers Squibb, Samsung, and Daiichi Sankyo outside the submitted work.
The remaining authors declare nothing to disclose.
CRediT statement
Federica Giuglian: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Supervision; Validation; Visualization; Roles/Writing—original draft; Writing—review & editing. Paolo Andrea Zucali: Data curation; Formal analysis; Investigation, Validation; Visualization; Writing—review & editing. Giulia Galli: Data curation; Formal analysis; Investigation, Validation; Visualization; Writing—review & editing, Zelmira Ballatore: Data curation; Formal analysis; Investigation, Validation; Visualization; Writing—review & editing, Chiara Corti: Data curation; Validation; Roles/Writing—original draft; Writing—review & editing, Pamela Trillo: Aliaga: Data curation; Formal analysis; Investigation; Methodology; Writing—review & editing, Jacopo Uliano: Data curation; Formal analysis; Investigation; Methodology; Writing—review & editing, Grazia Vivanet: Data curation; Formal analysis; Investigation; Methodology; Writing—review & editing, Giuseppe Curigliano: Validation; Visualization, Writing—review & editing, Fabio Conforti: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing—original draft; Writing—review & editing, Paola Queirolo: Validation; Visualization, Writing—review & editing, Rossana Berardi: Data curation; Formal analysis; Investigation, Validation; Visualization; Roles/Writing—original draft; Writing—review & editing, Sara Manglaviti: Data curation; Formal analysis; Investigation, Validation; Visualization; Roles/Writing—original draft; Writing—review & editing, Giulia Apollonio: Data curation; Formal analysis; Investigation, Validation; Visualization; Writing—review & editing, Matteo Perrino: Data curation; Formal analysis; Investigation, Validation; Visualization; Writing—review & editing, Federica Borea: Data curation; Formal analysis; Investigation, Validation; Visualization; Writing—review & editing, Federica D'Antonio: Data curation; Formal analysis; Investigation, Validation; Visualization; Writing—review & editing, Marina Chiara Garassino: Data curation; Formal analysis; Investigation, Validation; Visualization; Writing—review & editing, Tommaso De Pas: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing—original draft; Writing—review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2022.02.011.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Engels E.A. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5(10):S260–S265. doi: 10.1097/JTO.0b013e3181f1f62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagiri T., Okumura M., Inoue M., et al. Thymoma-associated graft-versus-host disease-like erythroderma. J Thorac Oncol. 2007;2(12):1130–1132. doi: 10.1097/JTO.0b013e31815ba23a. [DOI] [PubMed] [Google Scholar]
- 3.Tagliaferri A.R., Narvaneni S., Azzam M.H., Grist W. A case of COVID-19 vaccine causing a myasthenia gravis crisis. Cureus. 2021 doi: 10.7759/cureus.15581. Published online June 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soy M., Keser G., Atagunduz P., et al. A practical approach for vaccinations including COVID-19 in autoimmune/autoinflammatory rheumatic diseases: a non-systematic review. Clin Rheumatol. 2021;40(9):3533–3545. doi: 10.1007/s10067-021-05700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capassoni M., Ketabchi S., Cassisa A., et al. AstraZeneca (AZD1222) COVID-19 vaccine-associated adverse drug event: a case report. J Med Virol. 2021;93(10):5718–5720. doi: 10.1002/jmv.27175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vojdani A., Vojdani E., Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey A.R., Sobieszczyk M.E., Hirsch I., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) covid-19 vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMoa2105290. Published online September 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas S.J., Moreira E.D., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TYME (ThyYmic MalignanciEs) network. https://urlsand.esvalabs.com/?u=http%3A/F/Fwww.tyme.eu&e=000823b1&h=a24ea583&f=y&p=n
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.