Abstract
A combination cassette format nonenzymatic rapid immunoassay for detection of Giardia and Cryptosporidium antigens was evaluated by using 556 patient stool specimens from three clinical laboratories. This assay (Genzyme Diagnostics Contrast Giardia/Cryptosporidium), which can be used with fresh or formalin-fixed specimens, had unadjusted sensitivities and specificities of 96.1 and 98.5% for Giardia and 100 and 98.7% for Cryptosporidium, respectively, in this study.
Giardiasis and cryptosporidiosis are two common causes of protozoan diarrheal diseases in humans (3, 6). Antigen detection assays for Giardia lamblia and Cryptosporidium parvum have proven utility in the diagnosis of these infections (1, 2, 4, 5, 7). These assays offer advantages in labor, time, and batching efficiency that may lead to reduced costs. Most commercially available assays utilize an enzyme immunoassay (EIA) format which involves multiple reagent additions, washing steps, and incubations. A nonenzymatic rapid immunoassay for Giardia and Cryptosporidium antigens has been developed. This test (Genzyme Diagnostics Contrast Giardia/Cryptosporidium) will be marketed commercially (Becton Dickinson ColorPAC Giardia/Cryptosporidium). The assay can be performed in approximately 10 min on formalin-fixed or unfixed stool specimens. The present study describes the results of an evaluation of this assay.
Stool specimens were obtained from the clinical parasitology laboratories of the University of California at San Francisco, the Albert Einstein College of Medicine in Bronx, N.Y., and SmithKline Beecham Clinical Laboratories in Tucker, Ga. The samples were collected in either sodium acetate-acetic acid-formalin (SAF), 10% formalin, or Cary-Blair medium. They were characterized microscopically by trichrome or iron-hematoxylin and modified acid-fast staining by each laboratory. Since the natural prevalence of Giardia- and Cryptosporidium-positive samples was low during the course of the study, a higher rate was generated by preselecting the samples by microscopy. Negative specimens were selected randomly. In some instances, more than one separately collected sample from the same patient was tested. Seventeen specimens out of a total of 556 were documented to be additional samples collected from patients included in the study. The prevalence of Giardia- or Cryptosporidium-positive stools in the study was 13.8%.
The rapid test was performed on each specimen according to the manufacturer's instructions. The Giardia detection system for the assay was comprised of immobilized avidin, a biotinylated polyclonal anti-Giardia capture antibody, and a colloidal-carbon-labelled monoclonal anti-Giardia cyst wall antigen detector antibody. For Cryptosporidium, the detection system consisted of an immobilized monoclonal capture antibody and a colloidal-carbon-labelled monoclonal detector antibody, both directed against oocyst antigens. The assay procedure involved the addition of 2 drops of sample treatment buffer to a tube, the pipeting of 60 μl of stool specimen diluted in fixative or transport medium into the tube, the addition of 2 drops of a Giardia capture antibody conjugate, and the addition of 2 drops of a colloidal-carbon-conjugated detection reagent for Giardia and Cryptosporidium. After the sample was mixed, it was immediately poured into the test device. Assay results were read after 10 min. Positive results were visualized as grey-black lines in the appropriate position in the results window (Fig. 1). Samples showing discrepancies between microscopy and the rapid assay were analyzed using microplate EIAs for Giardia and Cryptosporidium (Alexon-Trend, Ramsey, Minn.).
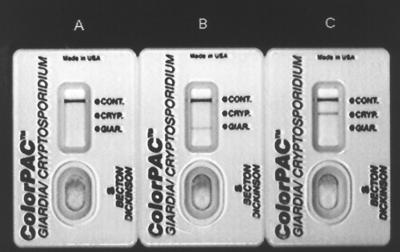
FIG. 1.
Giardia/Cryptosporidium rapid tests run with negative buffer control (A), Giardia antigen-positive control (B), and Cryptosporidium antigen-positive control (C). The positions of the flow control line (CONT.), Cryptosporidium test line (CRYP.), and Giardia test line (GIAR.) are shown.
A total of 235 specimens were microscopically characterized and tested with the rapid assay at the San Francisco site. The New York- and Atlanta-area sites characterized 242 and 79 specimens by microscopy, respectively, and sent them to Genzyme for blinded testing in the Contrast Giardia/Cryptosporidium rapid assay. Four hundred seventy-five specimens were in SAF medium, 79 were in 10% formalin, and 2 were in Cary-Blair medium. According to the manufacturer, the rapid assay is not compatible with specimens collected in polyvinyl alcohol preservative media. Due to the vastly different numbers of samples collected in each medium, it was not possible to determine if there were any significant differences in assay sensitivities or specificities among the three media tested. Summarized data from specimens collected at each site and the combined totals are presented in Table 1. The combined sensitivities and specificities for Giardia were 96.1% (74 of 77; 95% confidence interval, 89.0 to 99.2%) and 98.5% (472 of 479; 95% confidence interval, 97.0 to 99.4%), respectively. For Cryptosporidium, combined sensitivities and specificities were 100% (77 of 77; 95% confidence interval, 95.3 to 100%) and 98.7% (473 of 479; 95% confidence interval, 97.2 to 99.5%), respectively.
TABLE 1.
Giardia and Cryptosporidium antigen detection using the combination rapid immunoassay on specimens characterized by different laboratories
| Laboratory |
Giardia
|
Cryptosporidium
|
||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| University of California, San Francisco | 100 (17/17) | 96.8 (211/218) | 100 (11/11) | 100 (224/224) |
| Albert Einstein College of Medicine | 92.8 (39/42) | 100 (200/200) | 100 (5/5) | 97.5 (231/237) |
| SmithKline Beecham Clinical Laboratories | 100 (18/18) | 100 (61/61) | 100 (61/61) | 100 (18/18) |
| Combined total | 96.1 (74/77) | 98.5 (472/479) | 100 (77/77) | 98.7 (473/479) |
Analysis of the Giardia false-negative discrepant samples using the Alexon-Trend ProSpecT Giardia microplate EIA revealed all three samples to be antigen negative. Interestingly, other specimens collected from the same patients tested positive for the Giardia antigen in both the rapid assay and the microplate EIA. This suggests that the level of antigen may have temporarily dropped below the detection threshold of both immunoassays during the period of sampling.
Five of the seven Giardia false-positive discrepant samples were positive for the Giardia antigen by the microplate EIA. Of the remaining two discrepant samples, one came from a patient who submitted another sample 3 days later that was positive for Giardia by both microscopy and the rapid assay, and the second came from a patient who submitted two other specimens within a 3-day interval which were Giardia positive by the rapid assay and EIA but negative by microscopy. These results support the view that these discrepant samples may be Giardia antigen true positives. Other studies have indicated that Giardia antigen-positive specimens that are negative for Giardia by microscopy often come from patients for whom giardiasis is a likely diagnosis based on epidemiology (4, 5).
The six Cryptosporidium false-positive samples came from two patients, indicating that the positive rapid assay results were reproducible across specimens from the same individuals. Testing of these samples using the Cryptosporidium microplate EIA showed them all to be Cryptosporidium negative, as did reexamination of the microscopy slides. The results suggest either that these samples are real false positives or that the rapid assay is slightly more sensitive than the other methods.
During the course of this study, fecal specimens that tested positive microscopically for parasites other than Giardia and Cryptosporidium were analyzed. These parasites included 46 samples of Blastocystis hominis, 27 samples of Endolimax nana, 12 samples of Entamoeba hartmanni, 5 samples of Entamoeba coli, 4 samples of Dientamoeba fragilis, 3 samples of Iodamoeba buetschlii, 2 samples of Entamoeba histolytica/dispar, and one sample each of Chilomastix mesnili, Cyclospora cayetanensis, Enteromonas hominis, hookworm, microsporidia, and Taenia. No cross-reactions in the rapid test were observed. Studies conducted at Genzyme have detected no cross-reactivity to rotavirus, Clostridium difficile, Campylobacter jejuni, Escherichia coli, or Salmonella.
The ability to simultaneously detect and distinguish between Giardia and Cryptosporidium antigens in fixed or unfixed clinical specimens with a 10-min nonenzymatic immunoassay is a novel attribute of the Contrast Giardia/Cryptosporidium test. The sensitivities and specificities for this assay are in the ranges reported for many of the commercially available enzyme immunoassays for Giardia and Cryptosporidium antigen detection, which are more time-consuming and labor-intensive. The test procedure is relatively simple to perform and requires minimal training. Even though each sample requires its own tube and test device, multiple samples can be run in parallel as a batch to save time. This assay will provide diagnostic laboratories with a convenient alternative method for performing antigen detection assays for Giardia and Cryptosporidium on patients' stool samples. The suitability of this test for any given laboratory may depend on the relative prevalence of Giardia and Cryptosporidium infections, the number of specimens processed on a daily basis, and the balance between assay cost and reduced time.
Acknowledgments
This work was supported by funding from Genzyme Diagnostics.
Technical assistance from Jeffery M. White is gratefully acknowledged.
REFERENCES
- 1.Garcia L S, Shimizu R Y. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol. 1997;35:1526–1529. doi: 10.1128/jcm.35.6.1526-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kehl K S C, Cicirello H, Havens P L. Comparison of four different methods for detection of Cryptosporidium species. J Clin Microbiol. 1995;33:416–418. doi: 10.1128/jcm.33.2.416-418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall M M, Naumovitz D, Ortega Y, Sterling C R. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblatt J E, Sloan L M, Schneider S K. Evaluation of an enzyme-linked immunosorbent assay for the detection of Giardia lamblia in stool specimens. Diagn Microbiol Infect Dis. 1993;16:337–341. doi: 10.1016/0732-8893(93)90086-m. [DOI] [PubMed] [Google Scholar]
- 5.Rosoff J D, Sanders C A, Sonnad S S, De Lay P R, Hadley W K, Vincenzi F F, Yajko D M, O'Hanley P D. Stool diagnosis of giardiasis using a commercially available enzyme immunoassay to detect Giardia-specific antigen 65 (GSA 65) J Clin Microbiol. 1989;27:1997–2002. doi: 10.1128/jcm.27.9.1997-2002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe M S. Giardiasis. Clin Microbiol Rev. 1992;5:93–100. doi: 10.1128/cmr.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerman S K, Needham C A. Comparison of conventional stool concentration and preserved-smear methods with Merifluor Cryptosporidium/Giardia direct immunofluorescence assay and ProSpecT Giardia EZ microplate assay for detection of Giardia lamblia. J Clin Microbiol. 1995;33:1942–1943. doi: 10.1128/jcm.33.7.1942-1943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]