Abstract
Background
Knowledge of the exact organ manifestation is essential for a comprehensive understanding of COVID-19 infection. Here, the histopathological changes in the pituitary and adrenal glands were analyzed.
Methods
In this series, the formalin-fixed tissues of 63 pituitary glands and 50 adrenal glands were examined. We performed HE and PAS staining and examined COVID-19 nucleocapsid antibody immunohistochemically in the pituitary glands and adrenals.
Results
Histologically, there was no evidence of COVID-19-specific changes in the pituitary and adrenal glands. Large pituitary necrosis may be interpreted as a shock reaction. Independent of infection, we found one T-cell lymphoma, two adenomas, and four Rathke-type cysts in the pituitary glands, and 70% of the adrenal glands showed decreased lipid content and an increase in compact cells as a stress response. In addition, a cortical adenoma in one adrenal gland and small cortical nodules in three adrenal glands were detected independently of COVID-19.
Conclusion
Pituitary and adrenal glands do not appear histologically predominant in the course of COVID-19.
Abbreviations: SARS-CoV-2, Severe acute respiratory syndrome coronavirus type 2
Keywords: Histopathology, Pituitary, Adrenal, COVID-19 infection
1. Introduction
Understanding the underlying concept of pathomechanisms is essential for the detection, treatment and prevention of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the etiological agent of Coronavirus Disease 2019 (COVID-19). Infection with SARS-CoV-2 primarily affects the lungs, parts of the respiratory tract and often the gastrointestinal tract [10]. The infection can cause prognostically relevant thromboembolism [24] and lead to acute respiratory distress syndrome (ARDS). Organ tropism has been described [2], [4], [14], including the nervous system [14], which is partly explained by an ascending infection along the olfactory nerves [15]. Since SARS-CoV-2 infection can affect many organ systems, it appears as a systemic disease, including endocrinological pathways [8], [12].
The aim of this study was to detect specific COVID-19 histopathological changes on endocrine organs with a focus on the pituitary and the adrenal glands.
2. Material and methods
Autopsies were performed between March and December 2020 at the Institute of Legal Medicine of the University Medical Center Hamburg-Eppendorf, Germany. Institutional review board approval from the independent ethics committee of the Hamburg Chamber of Physicians was obtained for this study (reference numbers 2020-10353-BO-ff and PV7311).
All deceased patients were examined for SARS-CoV-2 virus RNA by throat swab, followed by immediate quantitative RT-PCR analysis before autopsy. At autopsy, the pituitary and adrenal glands were fixed in buffered 4% formaldehyde and examined macroscopically. A collection of 63 pituitary glands and 50 adrenal glands were subjected to further histopathologic examination. Immunostaining of tumorous lesions of the pituitary gland was performed with primary antibodies for GH, prolactin, ACTH, TSH, FSH, LH, alpha subunit, and Ki-67 (MiB-1). Lymphoma was additionally stained for LCA, CD3, CD20, and CD1a. Adrenal preparations were stained with hematoxylin-eosin (HE) and periodic acid Schiff (PAS). The pituitary (n = 10) and adrenal (n = 5) glands were examined immunohistochemically with the nucleocapsid antibody COVID-19 (clone 4A8, CoV2 HS 452 011, synaptic systems, Göttingen, Germany).
Demographic (place of death, age, sex) and medical characteristics, including cause of death, comorbidities, and post-mortem interval (PMI), were collected for the entire collective.
Statistical analysis was performed descriptively using Microsoft Excel (version 16.16, Microsoft Corporation, Redmond, USA). Variables were described as percentages, means, and standard deviations (SD).
3. Results
3.1. Pituitary
Of the 63 patients analyzed, patient characteristics were available in 51 cases (80.9%). Of these 51 patients, 50 (98%) died of fatal COVID-19 disease and one patient (2.0%) died of non-COVID-19 disease. The median age of the patients was 78.4 years (SD 11.4). The median postmortem interval (PMI) was 4.0 days (SD 3.9). Table 1 lists the patients’ characteristics and risk factors for severe disease. No preexisting endocrine diseases associated with COVID-19 were listed in the medical records. Immunostaining with the nucleocapsid antibody COVID-19 was negative in all cases.
Table 1.
Characteristics of patients, cause of death, place of death, Comorbidities (pituitaries).
| Characteristics (pituitaries) | Patients No. (%) (N = 51) | |||
|---|---|---|---|---|
| Patient characteristics | ||||
| Male | 30 (58.8) | |||
| Female | 21 (41.2) | |||
| Age mean (SD), y | 78.4 (11.4) | |||
| Postmortem interval (PMI), mean (SD), d | 4.0 (3.9) | |||
| Died of COVID-19 | 98 (50) | |||
| Place of Death | ||||
| In-patients | ||||
| Normal ward | 19 (37.3) | |||
| Intensive care unit | 14 (27.5) | |||
| Outpatients | 17 (33.3) | |||
| Unknown Place | 1 (2.0) | |||
| Comorbidities | ||||
| Chronic heart disease | 47 (92.2) | |||
| Lung | 25 (49.0) | |||
| Endocrine | 18 (35.3) | |||
| Neurologigal | 25 (49.0) | |||
| Renal | 20 (39.2) | |||
| Oncological | 10 (19.6) | |||
| Liver | 3 (5.9) | |||
| Chronic inflammation | 3 (5.9) | |||
| Pancreatic | 1 (2.0) | |||
| Immunological | 2 (3.9) | |||
| Psychatric | 1 (2.0) | |||
| Others | 4 (7.8) | |||
aMultiple inclusion of 1 patient in the various categories is possible.
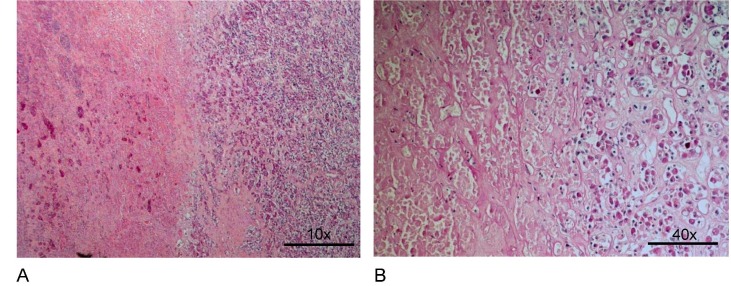
Eighty-seven percent of the pituitary glands had regular structures with normal hormone-producing cell composition and no lesions (Table 2 ). Three tumorous lesions were identified: a high-grade T-cell lymphoma infiltrated the pituitary capsule, the adenohypophysis, and the neurohypophysis (this patient's spleen, but no other organ, was also affected by the lymphoma). Two tumors were pituitary adenomas, one was a gonadotropic microadenoma, and one was a sparsely granulated prolactin microadenoma. One pituitary had large acute necrosis in the anterior pituitary lobe (Figs. 1a and 1b). Rathke-type cysts with typical respiratory epithelial lining were located in the anterior pituitary or intermediate zone in 4 cases. Very small cysts with uncharacteristic epithelial lining were found in the intermediate zone and in the adjacent anterior pituitary. In the region of the pituitary stalk, one pituitary had a large focus of Erdheim-type squamous epithelia. Staining with ß-catenin antibody did not reveal positive nuclei. Therefore, a small craniopharyngioma could be excluded.
Table 2.
Histopathology of pituitaries (N = 63).
| Lesion/Findings | Significance | Number* | Percentage |
|---|---|---|---|
| High grade T-cell lymphoma | High | 1 | 1.6% |
| Gonadotroph microadenoma | Medium | 1 | 1.6% |
| Sparsely granulated Prolactin microadenoma | Medium | 1 | 1.6% |
| Large necrosis | High | 1 | 1.6% |
| Rathke’s cyst | Low | 4 | 6.3% |
| Small cysts of intermediate zone | No | 6 | 9.5% |
| Hyperplasia of Erdheim’s squamous epithelia | No | 1 | 1.6% |
| Focal fibrosis | No | 1 | 1.6% |
| No pathological findings | 55 | 87.3% |
*) in 4 cases 2 lesions.
Fig. 1.
Necrosis of the anterior pituitary. Amorphous parenchymal tissue with bleedings (left side), preserved tissue (right side). A: Hematoxylin-eosin stain, 180x, B: PAS-reaction, 440x.
3.2. Adrenal
Of the 50 patients analyzed, patient characteristics were present in 48 cases (96.0%). Of these 48 patients, 46 (95.8%) died of fatal COVID-19 disease and 2 patients (4.2%) died of nonCOVID-19-related disease. One patient died from necrotizing fasciitis and the other from myocardial infarction with cardiac tamponade. Both causes of death were probably unrelated to COVID-19 and were defined as non-COVID-19 deaths. The mean age of the patients was 78.3 years (SD 10.6). The median postmortem interval (PMI) was 4.1 days (SD 3.1). Table 3 lists patient characteristics and risk factors for severe disease. No preexisting endocrine diseases associated with COVID-19 were listed in the medical records. Immunostaining with the nucleocapsid antibody COVID-19 was negative in all cases.
Table 3.
Characteristics of patients, cause of death, place of death, comorbidities (adrenals).
| Characteristics (adrenals) | Patients No. (N=48) (%) | |||
|---|---|---|---|---|
| Patient characteristics | ||||
| Male | 25 (52.1) | |||
| Female | 23 (47.9) | |||
| Age mean (SD), y | 78.3 (10.6) | |||
| Postmortem interval (PMI), mean (SD), d | 4.1 (3.1) | |||
| Died of COVID-19 | 46 (95.8) | |||
| Place of Death | ||||
| In-patients | ||||
| Normal ward | 20 (41.7) | |||
| Intensive care unit | 11 (22.9) | |||
| Outpatients | 16 (33.3) | |||
| Unknown Place | 1 (2.1) | |||
| Comorbidities | ||||
| Chronic heart disease | 44 (91.7) | |||
| Lung | 27 (56.3) | |||
| Endocrine | 18 (37.5) | |||
| Neurologigal | 16 (33.3) | |||
| Renal | 16 (33.3) | |||
| Oncological | 11 (22.9) | |||
| Liver | 5 (10.4) | |||
| Chronic inflammation | 2 (4.2) | |||
| Pancreatic | 1 (2.1) | |||
| Immunological | 1 (2.1) | |||
| Psychiatric | 2 (4.2) | |||
| Others | 5 (10.4) | |||
aMultiple inclusion of 1 patient in the various categories is possible.
Many adrenals exhibited reduced cytoplasmic lipid vacuoles to varying degrees, often accompanied by reduced cortical width (Table 4 ). Widened cortices were rare. A cortical adenoma with spongiocytic lipid-rich cells was found. Smaller cortical nodules composed mostly of spongiocytic cells were present in three adrenals. Small foci of fat cells and bone marrow cells (myelolipomatous metaplasia) were found in two adrenals. Small nests of lymphocytes, mostly near the adrenal medulla, were found in three adrenals. Pronounced atherosclerosis of the adrenal arteries was found in eight cases.
Table 4.
Histopathology of adrenals (N = 50).
| Lesion/Findings | Significance | Number* | Percentage |
|---|---|---|---|
| Reduced content of lipid in cortical cells | Reactive | 35 | 70.0% |
| Thin cortex | Reactive | 13 | 26.0% |
| Widened cortex | Not clear | 2 | 4.0% |
| Cortical adenoma | Medium | 1 | 2.0% |
| Micronodular hyperplasia | Medium | 3 | 6.0% |
| Myelolipomatous metaplasias | Low | 2 | 4.0% |
| Focal aggregates of lymphocytes | No | 3 | 6.0% |
| Atherosclerosis of arteries in paraadrenal adipose tissue | Not clear | 8 | 16.0% |
| No pathological findings | 12 | 24.0% |
*) in 20 cases 2 lesions/findings.
4. Discussion
The major receptor ACE2, which is required for efficient viral penetration [11], [25],is expressed in endocrine organs such as the pituitary and adrenal glands [9], [25], and viral particles have already been detected in deceased patients [5], [13]. This suggests that SARS-CoV-2 infection of endocrine tissues is possible.
In our study, the findings and lesions of the pituitary glands in our series of patients who died from COVID-19 infection were not significantly different from collections without COVID-19 infection (Table 1).
The only finding that could be associated with septic shock is the large adenohypophyseal necrosis in one case. This could be comparable to necrosis during or after birth (Sheehan’s syndrome) [23]. The anterior pituitary appears to be a typical “shock organ.” As early as 1976–1979, it was shown in an unselected autopsy series that 20.9% of all pituitary glands of patients with previous shock events showed anemic necrosis [19]. 21 years later, very similar studies showed a decrease in incidence to 7.7% [21]. This could be due to improved intensive therapy, which reduced the development of shock.
All other lesions appear to be independent of infection. The rate of adenomas in unselected routine autopsies is about 10% [3]. Most of them are sparsely granulated prolactin adenomas (39.5%), followed by gonadotrophic adenomas (7–20%) [3].
This is a good comparison with our COVID-19 collection. These microadenomas have no biological significance for the death of the patient.
In contrast, malignant lymphoma of the T-cell type (leukemia) affecting the pituitary gland and some other organs, especially the spleen, in a patient is extremely important for the patient and his predisposition to COVID-19 infection, since T-cell lymphomas are more common in patients with immunodeficiencies. The overall incidence of malignant lymphoma in surgically removed pituitary glands is 2.9% [18]. T-cell lymphomas account for 24% of these lymphomas.
Small Rathke's cysts, often indistinguishable from small intermediate zone cysts as regular findings on routine autopsies, are found in up to 30% of routine autopsies [18] and have no biological significance to the patient.
We did not obtain immunohistochemical evidence of viral protein in pituitary cells.
Staining of viral protein by immunohistochemistry in formalin-fixed tissue is less sensitive compared to diagnostic RT-qPCR because there is no amplification of the target structures.
To detect the presence of virus in tissues, many proteins must be accumulated. Thus, although we were unable to detect the presence of infectious SARS-CoV-2 particles in any of the samples examined, our results are not evidence of their absence. Apart from large acute necrosis of the anterior lobe of the pituitary gland and malignant lymphoma, no particular or specific histopathologic findings associated with COVID-19 infection were noted.
Autopsy studies of patients who had died of severe acute respiratory syndrome (SARS) in 2003 had shown degeneration and necrosis of adrenal cortex cells. The virus was found in the adrenal glands, suggesting a direct cytopathic effect of the virus [6]. Therefore, cortisol dynamics may be altered in patients with SARS and COVID-19. Cases have been reported in which COVID-19 caused adrenal hemorrhage and adrenal infarction [7], [22], further supporting our case and demonstrating that COVID-19 may affect the adrenal glands through multiple factors.
However, immunohistochemically, we could not detect any viral protein in adrenal cells. Approximately 40% of our patient group had pre-existing endocrine lesions, but none of these were associated with COVID-19 in the medical records, suggesting that a direct cytopathic effect of the virus could not be demonstrated in our cases. 70% of the adrenal glands in our series have reduced lipid content in most cortical cells as lipid-rich spongiocytes of the cortex transform into lipid-poor compact cells (Table 4). This is thought to be a response to chronic stress, as it has been shown in animal studies that increased stimulation of adrenocortical cells leads to a reduction in spongiocytes and an increase in compact cells [20]. The decreased width of the cortex may be caused by glucocorticoid therapy [16]. If the one cortical adenoma was hormonally active, this would have been significant for infection, but most of these small adenomas in autopsy series are not biologically significant [17].
Adenomas and micronodular hyperplasia as well as atherosclerosis in paraadrenal adipose tissue may be correlated with arterial hypertension in many cases [17] and are therefore important for COVID-19 infections [1].
5. Limitations
In this study, SARS-CoV-2 RNA detection was not performed. It should reveal the histopathological changes. In addition, due to the lack of clinical parameters, it is difficult to determine whether our patient had primary adrenal insufficiency due to adrenal inflammation resulting from viral infection or secondary adrenal insufficiency due to dysfunction of the hypothalamic–pituitaryadrenal axis.
6. Conclusion
Autopsy studies are important to clarify the effects of pathogens on different organ systems. In our case series, there was no evidence of COVID-19-specific changes in the pituitary and adrenal glands. However, the full spectrum of endocrine manifestations of SARS-CoV-2 infection is still unclear.
Funding
This work was supported by a grant from the Authorities for Social Welfare, Hamburg, Germany to the Institute of Legal Medicine Hamburg, Germany.
This publication was produced as part of the DEFEAT PANDEMIcs project, which was funded by the German Federal Ministry of Education and Research (BMBF) under grant number 01KX2021.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The funding for the German Pituitary Tumor Registry to WS from Novartis Pharma GmbH (Nuremberg, Germany), Novo Nordisk Pharma GmbH (Mainz, Germany), Pfizer Pharma GmbH (Berlin, Germany), and Ipsen Pharma GmbH (Berlin, Germany) is gratefully acknowledged.
References
- 1.Akbulut S., Sahin T.T. Comment on: primary hydatid cyst of the adrenal gland: a case report and a review of the literature. Int. J. Surg. Case Rep. 2021;78:340–341. doi: 10.1016/j.ijscr.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun F., Lütgehetmann M., Pfefferle S., Wong M.N., Carsten A., Lindenmeyer M.T., Nörz D., Heinrich F., Meißner K., Wichmann D., Kluge S., Gross O., Pueschel K., Schröder A.S., Edler C., Aepfelbacher M., Puelles V.G., Huber T.B. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396(10251):597–598. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.H. Buurman, W. Saeger. Subclinical adenomas in postmortem pituitaries: classification and correlation to clinical data. Exp. Clin. Endocr. Metab. 114: S 29, abstract P04-044 (2006). [DOI] [PubMed]
- 4.Casagrande M., Fitzek A., Spitzer M.S., Püschel K., Glatzel M., Krasemann S., Nörz D., Lütgehetmann M., Pfefferle S., Schultheiss M. Presence of SARS-CoV-2 RNA in the cornea of Viremic patients with COVID-19. JAMA Ophthalmol. 2021;139(4):383. doi: 10.1001/jamaophthalmol.2020.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D., Lu Y., Wu D., He L., Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkhouly M.M.N., Elazzab A.A., Moghul S.S. Bilateral adrenal hemorrhage in a man with́severe COVID-19 pneumonia. Radiol. Case Rep. 2021;16(6):1438–1442. doi: 10.1016/j.radcr.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frara S., Allora A., Castellino L., di Filippo L., Loli P., Giustina A. COVID-19 and the pituitary. Pituitary. 2021;24(3):465–481. doi: 10.1007/s11102-021-01148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu W.T., Zhou F., Xie W.Q., Wang S., Yao H., Liu Y.T., Gao L., Wu Z.B. A potential impact of SARS-CoV-2 on pituitary glands and pituitary neuroendocrine tumors. Endocrine. 2021 doi: 10.1007/s12020-021-02697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lax S.F., Skok K., Zechner P.M., Setaffy L., Kessler H.H., Kaufmann N., Vander K., Cokic N., Maierhofer U., Bargfrieder U., Trauner M. Systemic consequences and clinical aspects of SARS-CoV-2 infection. Pathologe. 2021;42:155–163. doi: 10.1007/s00292-021-00913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.M. Letko, A. Marzi, V. Munster. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 5 2020 562. [DOI] [PMC free article] [PubMed]
- 12.M.D. Lundholm, C. Poku, N. Emanuele, M.A. Emanuele, N. Lopez. SARS-CoV-2 (COVID-19) and the endocrine system 4 2020. [DOI] [PMC free article] [PubMed]
- 13.Lv Q.F., Yang Q.H., Cui Y.Q., Yang J.C., Wu G.F., Liu M., Ning Z.L., Cao S., Dong G.L., Hu J.M. Effects of taurine on ACE, ACE2 and HSP70 expression of hypothalamic-pituitary-adrenal axis in stress-induced hypertensive rats. Taurine. 2017;10(975):871–886. doi: 10.1007/978-94-024-1079-2_69. [DOI] [PubMed] [Google Scholar]
- 14.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J. Meinhardt, J. Radke, C. Dittmayer, J. Franz, C. Thomas, R. Mothes, M. Laue, J. Schneider, S. Brünink, S. Greuel, M. Lehmann, O. Hassan, T. Aschman, E. Schumann, R.L. Chua, C. Conrad, R. Eils, W. Stenzel, M. Windgassen, L. Rößler, H.-H. Goebel, H.R. Gelderblom, H. Martin, A. Nitsche, W.J. Schulz-Schaeffer, S. Hakroush, M.S. Winkler, B. Tampe, F. Scheibe, P. Körtvélyessy, D. Reinhold, B. Siegmund, A.A. Kühl, S. Elezkurtaj, D. Horst, L. Oesterhelweg, M. Tsokos, B. Ingold-Heppner, C. Stadelmann, C. Drosten, V.M. Corman, H. Radbruch, F.L. Heppner. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19, Nat. Neurosci. 24(2) 2021 168-175. [DOI] [PubMed]
- 16.Mitschke H., Saeger W. Ultrastructural pathology of the adrenal glands in Cushing’s syndrome. Curr. Topics Path. 1975;60:113–150. doi: 10.1007/978-3-642-66215-7_4. [DOI] [PubMed] [Google Scholar]
- 17.Reinhard C., Saeger W., Schubert B. Adrenocortical nodules in post-mortem series. Development, functional significance, and differentiation from adenomas. G. E. N.Diagn. Pathol. 1996;141:203–208. [PubMed] [Google Scholar]
- 18.W. Saeger, R. Buslei. 27. Pathology of non-adenomatous pituitary tumors and tumor-like lesions. In: Edited by Honegger, J., Reincke, M., Petersenn, S. Pituitary Tumors. A comprehensive and Interdisciplinary Approach, Ed.1: pp.393-404. Elsevier Academic Press, London: 2021.
- 19.Saeger W., Hanke D. Nekrosen der Hypophyse und ihre Beziehung zum Schock. Verh Dtsch Ges Path. 1978;62:301–306. [PubMed] [Google Scholar]
- 20.Saeger W., Mitschke H. Licht- und elektronenmikroskopische Untersuchungen an der Zona glomerulosa der Rattennebenniere nach Carbenoxolon. Virchows Arch. 1973;358:45–59. [PubMed] [Google Scholar]
- 21.Sandte S., Saeger W., Hanke D.K. Hypophysennekrosen: abnehmende Inzidenz durch moderne Intensivtherapie? Pathologe. 2000;21(4):292–295. doi: 10.1007/pl00006842. [DOI] [PubMed] [Google Scholar]
- 22.Sharrack N., Baxter C.T., Paddock M., Uchegbu E. Adrenal haemorrhage as a complication of COVID-19 infection. BMJ Case Rep. 2020;13(11):e239643. doi: 10.1136/bcr-2020-239643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan H.L., Stanfield J.P. The pathogenesis of post-partum necrosis of the anterior lobe of the pituitary gland. Acta Endocrin. 1961;37:479–510. [Google Scholar]
- 24.D. Wichmann, J.-P. Sperhake, M. Lütgehetmann, S. Steurer, C. Edler, A. Heinemann, F. Heinrich, H. Mushumba, I. Kniep, A.S. Schröder, C. Burdelski, G. de Heer, A. Nierhaus, D. Frings, S. Pfefferle, H. Becker, H. Bredereke-Wiedling, A. de Weerth, H.-R. Paschen, S. Sheikhzadeh-Eggers, A. Stang, S. Schmiedel, C. Bokemeyer, M.M. Addo, M. Aepfelbacher, K. Püschel, S. Kluge. Autopsy findings and venous thromboembolism in patients with COVID-19 173(4) 2020 268-277. [DOI] [PMC free article] [PubMed]
- 25.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]