Abstract
We report the first documented case of brain abscess due to the dematiaceous fungus Microascus cinereus, an organism common in soil and stored grain. M. cinereus was isolated from brain abscess material from a bone marrow transplant recipient. The patient responded well to treatment by amphotericin B lipid complex, itraconazole, and a craniotomy but later died from secondary complications caused by graft-versus-host disease.
Microascus cinereus, an ascomycetous mold in the order Microascales, family Microascaceae, is one of the most common species of the genus Microascus and has been recovered from a wide geographical range. It is relatively uncommon in humans and animals and has only recently been described as the sole pathogen of an invasive disease in humans (8). We report a case of brain abscess due to M. cinereus in a bone marrow transplant recipient, the first reported case of central nervous system disease caused by the organism, and discuss the mycology and role of M. cinereus as a cause of human disease.
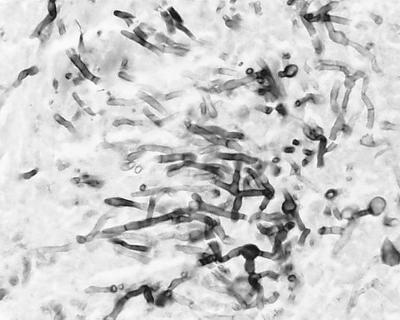
A 21-year-old female recipient of an allogeneic bone marrow transplant for aplastic anemia was admitted to the University of Alabama Hospital on day 196 posttransplant after an episode or urinary incontinence without any other neurologic symptoms. The patient had been diagnosed with graft-versus-host disease (GVHD) of the skin on day 30 posttransplant and was treated with tacrolimus and glucocorticosteroids. Mycophenolate and intravenous ganciclovir were added to her regimen on day 165 posttransplant for treatment of gastrointestinal GVHD and cytomegalovirus colitis. Because of the new finding of urinary incontinence, a magnetic resonance imaging scan (MRI) of the brain with contrast was performed, revealing a 2.5- by 2-cm enhancing lesion in the right frontal lobe. Treatment with amphotericin B (1 mg/kg of body weight per day) was initiated, and a stereotactic brain biopsy was performed, revealing septate hyphae (Fig. 1), but bacterial and fungal cultures were negative. The patient's renal function worsened, and she was placed on amphotericin B lipid complex (5 mg/kg/day), which was well tolerated. A repeat MRI 2 weeks later showed no change in the size of her lesion. Because of a lack of improvement in the size of the abscess despite 30 days of antifungal therapy, the patient underwent a right frontal craniotomy and surgical excision of the entire abscess cavity. Findings at surgery included a well-circumscribed abscess cavity that was easily resected. Cultures from the abscess material grew M. cinereus, and itraconazole (400 mg/day) was added to her antifungal regimen. The patient's overall condition improved over several months while she was on amphotericin B lipid complex and itraconazole, and follow-up MRIs of the brain demonstrated resolution of the disease. Unfortunately, her gastrointestinal GVHD worsened despite aggressive therapy, and she developed uncontrollable gastrointestinal bleeding and expired.
FIG. 1.
Gomori methenamine silver stain of the brain biopsy material.
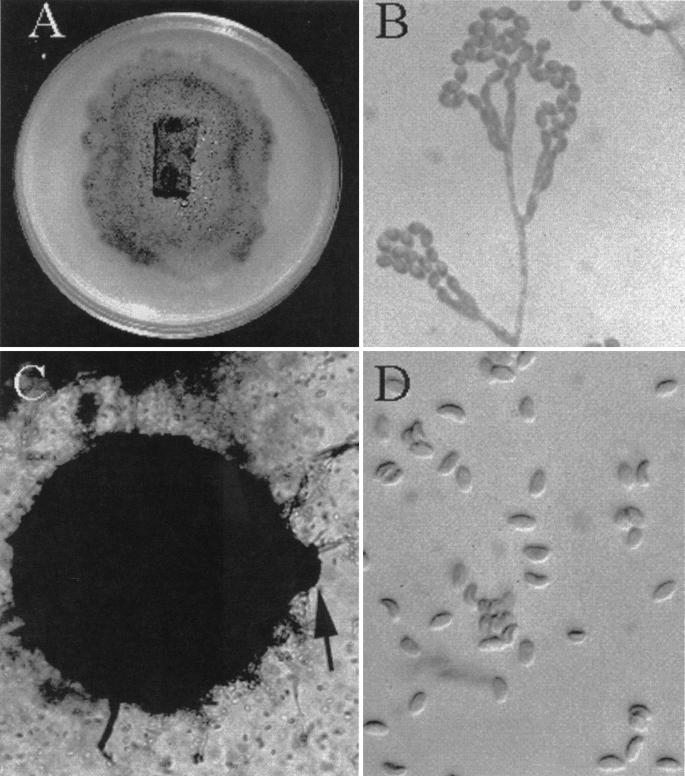
Biopsy material from the excised frontal lobe lesion was inoculated onto Sabouraud's dextrose agar (Emmons modification) and brain heart infusion agar with 10% sheep blood and gentamicin. Growth was first visible on day 5 of incubation at 30°C and developed into small mold colonies over several more days. The colonies initially were pale but developed a grey-olive color over time (Fig. 2A). Microscopic examination of potato flake agar (PFA) slide cultures revealed catenulate, dematiaceous annelloconidia (conidia formed from annellides and occurring in chains), measuring 3 to 4.5 by 2.5 to 3 μm and arising from either single or penicillate flask-shaped conidiophores attached to dematiaceous, septate hyphae. These features were consistent with a dematiaceous Scopulariopsis species (Fig. 2B). After 2 weeks of incubation, small black fruiting structures were seen growing first on the surface of PFA (Fig. 2A) and then on Sabouraud's dextrose agar. Microscopic examination of these structures revealed globose perithecia (100 by 350 μm) with a short neck (Fig. 2C). Ascospores were mature after 26 days of incubation and were pale brown to reddish and plano-convex (like orange sections) (4.5 to 5.5 by 2.5 to 3 μm) (Fig. 2D). Although other media may induce perithecial formation, the authors' experience is limited to the use of PFA. Temperature studies revealed growth of the organism at 25, 30, and 40°C. These characteristics are consistent with an identification of M. cinereus (Emilé-Weil et Gaudin) Curzi (3).
FIG. 2.
Macroscopic and microscopic features of M. cinereus. (A) Three weeks' growth on PFA (block in center) and cornmeal showing the dematiaceous character and perithecia (pinpoint black dots). (B) Dematiaceous Scopulariopsis species. Magnification, ×920. (C) A perithecium with the ostiole (arrow) borne on a very short neck. Magnification, ×230. (D) Ascospores showing typical convex shape. Magnification, ×920.
M. cinereus is one of 14 described species of Microascus. It is one of the most common species of the Microascus genus and has been recovered from a wide geographical range, from soil (10, 11) and also from stored grains such as oats and corn (7). Microascus species are ascomycetous fungi whose identification is based primarily upon the size and shape of the sexual fruiting structure (the perithecium) and its ascospores. Several Microascus species also display Scopulariopsis anamorphs (asexual forms), some of which are dematiaceous. Scopulariopsis species are quite easily identified by their truncate conidia (flattened at the base) and annellidic method of conidiogenesis, although a cursory examination may suggest a Penicillium or Paecilomyces species (phialidic conidiogenesis). Common Microascus species with dematiaceous Scopulariopsis anamorphs include M. cinereus, M. cirrosus, and M. trigonosporus. Conversely, the dematiaceous Scopulariopsis species S. brumptii does not appear to be associated with any known Microascus species. It is therefore important, when a dematiaceous Scopulariopsis species is recovered, to consider the possibility of an associated Microascus teleomorph (sexual stage) and to hold cultures for up to 6 weeks for mature ascospore formation. M. cinereus is primarily differentiated from M. cirrosus by perithecia with short necks and by ascospores resembling orange sections rather than being heart shaped (concavo-convex).
Although isolation of M. cinereus from clinical specimens has been reported, its role as a pathogen of human disease has been unclear. Recently it was reported to be the sole cause of suppurative cutaneous granulomata in an immunocompromised host with underlying chronic granulomatous disease (8). This patient was successfully treated with intravenous amphotericin B. Regarding nonimmunocompromised patients, reports include that of its isolation from an infected great toe, the report in which the organism was initially described (4). It has also been isolated from nails and identified as the presumed agent of onychomycosis (1), and it has been isolated from a dermal lesion caused by Fonsecaea pedrosoi (9). It was isolated from a mycetoma in a patient who had antibodies to Pseudallescheria boydii but not to M. cinereus (6), and it was also isolated from the maxillary sinus in conjunction with Aspergillus repens (2). Characteristic features of M. cinereus in conjunction with Aspergillus fumigatus in a section of human lung tissue have been described, but its role as a pathogen in this case could not be determined (6). Other species of Microascus have been isolated from humans, but in most cases there was no evidence for a pathogenic role. However, a recent report of disseminated M. cirrosus infection in a pediatric bone marrow transplant recipient strengthens the evidence for the role of Microascus species as potential pathogens in humans, particularly in immunocompromised patients (5).
In conclusion, we describe the first documented case of brain abscess caused by M. cinereus, an organism that is uncommon and rarely pathogenic in humans. The patient's predisposing factors for infection with this organism included severe immunosuppression, GVHD, and exposure to broad-spectrum antibiotics. Unfortunately, after successful treatment with amphotericin B, itraconazole, and a craniotomy with surgical excision, the patient succumbed to other complications of her underlying disease. The recovery of this organism from brain abscess tissue extends the list of known neurotropic dematiaceous organisms capable of causing cerebral phaeohyphomycosis.
REFERENCES
- 1.Agarwal G P, Singh S M. Microascus cinereus infection of human nail. Indian J Med Sci. 1980;34:263–265. [PubMed] [Google Scholar]
- 2.Aznar C, de Bievre C, Guigen C. Maxillary sinusitis from Microascus cinereus and Aspergillus repens. Mycopathologica. 1989;105:93–97. doi: 10.1007/BF00444031. [DOI] [PubMed] [Google Scholar]
- 3.Baron G L, Gain R F, Gilman J C. The genus Microascus. Can J Bot. 1961;39:1609–1631. [Google Scholar]
- 4.Emile-Weil P, Gaudin L. Contribution a l'etude des onychomycoses. Arch Med Exp Anat Pathol. 1919;28:452–467. [Google Scholar]
- 5.Krisher K K, Holdridge N B, Mustafa M M, Rinaldi M G, McGough D A. Disseminated Microascus cirrosus infection in pediatric bone marrow transplant recipient. J Clin Microbiol. 1995;33:735–737. doi: 10.1128/jcm.33.3.735-737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacey J. Microascus cinereus (Emile-Weil & Gaudin) Curzi—a human pathogen? Mycopathologica. 1986;105:93–97. doi: 10.1007/BF00437379. [DOI] [PubMed] [Google Scholar]
- 7.Lichtwardt R W, Barron G L, Tiffany L H. Mold flora associated with deterioration of stored corn in Iowa. Iowa State J Sci. 1958;13:1–11. [Google Scholar]
- 8.Marques A R, Kwon-Chung K J, Holland S M, Turner M L, Gallin J. Suppurative cutaneous granuloma caused by Microascus cinereus in a patient with chronic granulomatous disease. Clin Infect Dis. 1995;20:110–114. doi: 10.1093/clinids/20.1.110. [DOI] [PubMed] [Google Scholar]
- 9.Morton F J, Smith G. The genera Scopulariopsis Bainier, Microascus Zukal, and Doratomyces Corda. Mycol Pap. 1963;86:1–96. [Google Scholar]
- 10.Pandey A, Agrawal G P, Singh S M. Pathogenic fungi in soils of Jabalpur, India. Mycoses. 1990;33:116–125. doi: 10.1111/myc.1990.33.3.116. [DOI] [PubMed] [Google Scholar]
- 11.Youssef Y A, el-Din A A, Hassanein S M. Occurrence of keratinolytic fungi and related dermatophytes in soils in Cairo, Egypt. Zentbl Mikrobiol. 1992;147:80–85. doi: 10.1016/s0232-4393(11)80367-7. [DOI] [PubMed] [Google Scholar]