Abstract
Background
Whether vitamin C provides any benefit when administered in critically ill patients, including those with coronavirus disease (COVID-19), is controversial. We endeavored to estimate the effect of administration of vitamin C on clinical outcomes of critically ill patients with COVID-19 by performing an observational study and subsequent meta-analysis.
Methods
Firstly, we conducted an observational study of critically ill patients with laboratory-confirmed COVID-19 who consecutively underwent invasive mechanical ventilation in an academic intensive care unit (ICU) during the second pandemic wave. We compared all-cause mortality of patients receiving vitamin C (“vitamin C” group) or not (“control” group) on top of standard-of-care. Subsequently, we systematically searched PubMed and CENTRAL for relevant studies, which reported on all-cause mortality (primary outcome) and/or morbidity of critically ill patients with COVID-19 receiving vitamin C or not treatment. Pooled risk ratio (RR) and 95% confidence intervals (CI) were calculated using a random effects model. The meta-analysis was registered with PROSPERO.
Results
In the observational study, baseline characteristics were comparable between the two groups. Mortality was 20.0% (2/10) in the vitamin C group vs. 47.6% (49/103; p = 0.11) in the control group. Subsequently, the meta-analysis included 11 studies (6 observational; five randomized controlled trials) enrolling 1,807 critically ill patients with COVID-19. Mortality of patients receiving vitamin C on top of standard-of-care was not lower than patients receiving standard-of-care alone (25.8 vs. 34.7%; RR 0.85, 95% CI 0.57–1.26; p = 0.42).
Conclusions
After combining results of our observational cohort with those of relevant studies into a meta-analysis of data from 1,807 patients, we found that administration vitamin C as opposed to standard-of-care alone might not be associated with lower of mortality among critically ill patients with COVID-19. Additional evidence is anticipated from relevant large randomized controlled trials which are currently underway.
Systematic Review Registration
https://www.crd.york.ac.uk/prospero/, identifier: CRD42021276655.
Keywords: acute respiratory distress syndrome, acute respiratory failure, pneumonia, mechanical ventilation, intensive care unit, coronavirus
Background
In 2017, a retrospective before-and-after study showed a significant improvement in the survival of critically ill septic patients who received vitamin C (combined with hydrocortisone and thiamine) (1). Subsequently, several large randomized controlled trials explored the effect of this intervention on clinical outcomes of patients with severe sepsis, yielding contradicting results (2–6). By combining results of the above trials, a recent meta-analysis concluded that administration of vitamin C was associated with shorter duration of vasopressor use (albeit not with lower mortality) in such patients (7). Taken together, current evidence does not seem to preclude a potentially beneficial effect of administration of intravenous high-dose vitamin C on clinical outcomes of critically ill patients, at least those with sepsis.
The hypothesis that vitamin C may be beneficial in critically ill patients seems to be based on sound biological rationale. Indeed, vitamin C is a potent antioxidant, it affects inflammation and vascular integrity, while it serves as an enzyme cofactor essential for synthesis of endogenous catecholamines (8, 9). Such biological functions might justify the hypothesis that administration of vitamin C might be beneficial in patients with coronavirus disease (COVID-19) as well, at least those with critical illness (10, 11). Nevertheless, only a few studies explored the effect of this intervention on outcomes of critically ill patients with COVID-19 and those studies have not yet been systematically synthesized. Therefore, the COVID-19 Treatment Guidelines, issued by the National Institutes of Health, stated that “there is insufficient evidence to recommend either for or against the use of vitamin C for the treatment of COVID-19 in critically ill patients” (12).
Given both the interest and limited evidence on this issue, we designed a combined study. Firstly, we carried out an observational study exploring the effect of administration of vitamin C on clinical outcomes of critically ill patients with COVID-19 who consecutively underwent invasive mechanical ventilation in an academic intensive care unit (ICU). Then, we combined our results with those of relevant studies in a systematic review and meta-analysis.
Methods
The present work consisted of two components: an “observational study” and a subsequent “meta-analysis,” in which results of the observational study were combined with results of relevant studies.
Observational Study
Study Design
We conducted an observational retrospective cohort study including adult (>18 years old) patients with polymerase chain reaction (PCR)-confirmed Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection who consecutively underwent invasive mechanical ventilation in an academic ICU of a tertiary hospital (Evangelismos Hospital, Athens, Greece) during the second pandemic wave (specifically, between October 21st, 2020 and March 8th, 2021). The Institutional Review Board at Evangelismos Hospital (116/31-03-2021) approved of the data collection and waived the need of informed consent. We followed the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) statement guidelines (Supplementary Material).
Compared Groups and Data Collection
All patients underwent invasive mechanical ventilation due to hypoxemia; i.e., they met the Berlin criteria of acute respiratory distress syndrome (ARDS). All patients were administered dexamethasone (6 mg/day intravenously for at least 5 days) as part of their standard-of-care treatment of critical COVID-19 (13). Based on clinical judgment of their treating ICU clinicians and following national standard operating procedures for the administration of “off-label” medications, several patients received vitamin C on top of standard-of-care treatment and those patients comprised the “vitamin C” group. Specifically, within the first 24 h from their intubation, those patients received intravenously 1 g vitamin C plus thiamine 500 mg every 8 h for 4 days; then, intravenously 500 mg vitamin C plus thiamine 250 mg every 8 h for 3 days; and, finally, intravenously 500 mg vitamin C plus thiamine 250 mg every 12 h for 3 days. Therefore, they received intravenous high-dose vitamin C (Pabrinex®, Kyowa Kirin Limited, United Kingdom) for a total of 10 days or less (in case of ICU discharge or death). On the other hand, patients who received only standard-of-care treatment comprised the “control” group. This observational retrospective study took advantage of the fact that attending ICU clinicians gave vitamin C in some (“vitamin C” group) but not all (“control” group) patients.
In addition to data on administration or not of vitamin C, we gathered data on demographics, comorbidities, respiratory support (including high-flow nasal oxygen) prior to intubation along with ventilator settings, lung mechanics and Sequential Organ Failure Assessment (SOFA) score on day of intubation. The respiratory component of SOFA was calculated after the intubation, while the remaining SOFA components (namely, coagulation, hepatic, cardiovascular, neurologic and renal) were calculated prior to intubation.
Outcomes
We considered all-cause ICU-mortality as the primary outcome of the observational study. Vasopressor-free days, continuous renal replacement-free days, ventilator-free days and ICU-free days were the secondary outcomes. As previously (14, 15), we calculated vasopressor-free days, continuous renal replacement therapy-free days, ventilator-free days and ICU-free days by the number of days in the first 28 days following intubation that a patient was alive and not receiving vasopressors, not receiving continuous renal replacement therapy, not on a ventilator or not in the ICU, respectively. We censored outcomes at day 28 following intubation.
Meta-Analysis
Subsequently, we carried out a systematic review and meta-analysis of relevant studies in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (16). We prespecified search strategy, data extraction and outcomes in a protocol registered with PROSPERO (CRD42021276655) and available online.
Search Strategy
Two authors (EX and NAX) independently conducted the literature search. In addition to PubMed and CENTRAL, we systematically searched preprint servers (namely, medRxiv and Research Square) to capture rapidly accumulated evidence, as previously done (17). We used Boolean logic to create the search phrase: (“ascorbic” OR “vitamin C” OR “vit C”) AND (“coronavirus” OR “COVID” OR “COVID 19” OR “SARS-CoV2”). We retrieved relevant literature up to December 18th, 2021, with no language restrictions. We considered for inclusion observational studies and randomized controlled trials, which compared administration of vitamin C on top of standard-of-care vs. standard-of-care alone in critically ill patients with COVID-19 and reported data on all-cause mortality and/or morbidity. Case reports and case series involving less than 5 patients were excluded.
Data Extraction and Risk of Bias Assessment
Two authors (EX and NAX) independently extracted data in a prespecified worksheet and cross-checked their findings. For each included study, we collected data on author, country, study design, number of critically ill patients with COVID-19 receiving or not vitamin C, administered regimen of vitamin C, patient characteristics (i.e., demographics and comorbidities, such as diabetes mellitus, hypertension, ischemic cardiac disease) and outcomes.
Two authors (EX and NAX) independently assessed the risk of bias of included studies. Any disagreements were discussed with the corresponding author (IIS). For assessment of observational studies, we used the Tool to Assess Risk of Bias in Cohort Studies, developed by the CLARITY Group at McMaster University (18). For assessment of randomized controlled trials, we used the Risk of Bias 2 (RoB 2) tool (19). We provided details on the risk of bias assessment in the Supplementary Material.
Outcomes
We considered all-cause mortality as the primary outcome of the meta-analysis. Length of ICU stay, duration of mechanical ventilation, need for renal replacement therapy and adverse events related to vitamin C were the secondary outcomes.
Statistical Analyses
For the observational study, we used SPSS software 22.0 (IBM, Armonk, NY, USA). We presented continuous variables as median and interquartile range (IQR) and compared them using Mann-Whitney rank sum test. We presented categorical variables as number of patients (percentage) and compared them using chi-squared or Fisher's exact test. We performed a complete case analysis because missing data on outcomes were below 3% and completely at random according to Little's MCAR test (20). All statistical tests were 2-tailed.
For the meta-analysis, we used Review Manager 5.4 (RevMan 5.4.1, Cochrane Collaboration) (21). We expressed pooled dichotomous effect measures and pooled continuous effect measures as risk ratio (RR) with 95% confidence intervals (CI) and mean difference (MD) with 95% CI, respectively. We transformed continuous values presented as median to mean (22). We conservatively utilized a random effects model. We assessed the presence of statistical heterogeneity with I2, interpreted according to the Cochrane Handbook recommendations; 0–40%: might not be important; 30–60%: may represent moderate heterogeneity; 50–90%: substantial heterogeneity; 75–100%: considerable heterogeneity (22). We carried out two pre-specified sensitivity analyses by including only (a) studies with low risk of bias; and (b) randomized controlled trials. We considered a p < 0.05 to denote statistical significance.
Results
Observational Study
During the study period, 113 patients [24.8% female, median age 69.0 (IQR 57.0–76.5) years] consecutively underwent invasive mechanical ventilation in the ICU and were therefore included in the observational study. Ten patients (8.8% of the cohort) received intravenous high-dose vitamin C on top of standard-of-care (vitamin C group), while the rest received only standard-of-care (control group). Table 1 shows that baseline characteristics of included patients, such as demographics, comorbidities, SOFA score and lung mechanics, were comparable between the two groups. At baseline, partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2:FiO2) was lower in the vitamin C than control group [95.4 (68.3–145.8) vs. 142.5 (113.3–182.5); p = 0.012].
Table 1.
Baseline characteristics of patients included in the observational study.
| Characteristic | Vitamin C group (n = 10) | Control group (n = 103) | p-value |
|---|---|---|---|
| Age, years | 70.5 (58.0–75.0) | 69.0 (55.0–77.0) | 0.927 |
| Female sex | 3 (30.0) | 25 (24.3) | 0.709 |
| Race | 1.000 | ||
| Caucasian | 10 (100.0) | 100 (97.1) | |
| Asian/Middle Eastern | 0 (0.0) | 2 (1.9) | |
| African | 0 (0.0) | 1 (1.0) | |
| Comorbidity | 7 (70.0) | 77 (74.8) | 0.715 |
| Chronic kidney disease | 2 (20.0) | 13 (12.6) | 0.620 |
| Chronic lung disease | 1 (10.0) | 16 (15.5) | 1.000 |
| Heart condition | 3 (30.0) | 25 (24.3) | 0.707 |
| Hypertension | 6 (60.0) | 55 (53.4) | 0.751 |
| Liver disease | 0 (0.0) | 0 (0.0) | - |
| Diabetes mellitus | 3 (30.0) | 23 (22.3) | 0.694 |
| Malignancy | 0 (0.0) | 9 (8.7) | 1.000 |
| SOFA score on the day of intubation | 4.0 (4.0–5.3) | 4.0 (4.0–5.0) | 0.743 |
| Respiratory | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 0.139 |
| Coagulation | 0.0 (0.0–0.3) | 0.0 (0.0–0.0) | 0.708 |
| Hepatic | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.363 |
| Cardiovascular | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.363 |
| Neurologic | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.397 |
| Renal | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.587 |
| Days from symptom onset to intubation | 5.5 (3.8–9.3) | 7.0 (4.0–11.0) | 0.314 |
| Usage of high-flow nasal oxygen | 8 (80.0) | 69 (67.0) | 0.499 |
| Duration of high-flow nasal oxygen, days | 1.5 (1.0–5.8) | 2.0 (1.0–4.5) | 0.930 |
| Usage of non-rebreather mask | 0.0 (0.0) | 22 (21.4) | 0.205 |
| Duration of non-rebreather mask, days | NA | 2.0 (1.0–3.0) | NA |
| Lung mechanics on the day of intubation | |||
| Ventilation mode | 0.013 | ||
| Volume Control | 10 (100.0) | 61 (59.2) | |
| Pressure Control | 0.0 (0.0) | 42 (40.8) | |
| Respiratory rate, bpm | 25.0 (22.0–30.0) | 25.0 (22.0–28.0) | 0.722 |
| Tidal volume, mL | 475.0 (442.5–500.0) | 480.0 (440.0–490.0) | 0.775 |
| PEEPext, cmH2O | 10.5 (10.0–14.0) | 12.0 (10.0–13.0) | 0.857 |
| PEEPtotal, cmH2O | 10.5 (10.0–14.0) | 13.0 (10.0–15.0) | 0.237 |
| Pplateau, cmH2O | 24.5 (22.3–27.8) | 25.0 (23.0–27.8) | 0.760 |
| Pdriving, cmH2O | 13.0 (13.0–14.8) | 12.0 (10.0–14.3) | 0.223 |
| FiO2 | 1.0 (0.9–1.0) | 0.9 (0.7–1.0) | 0.052 |
| PaO2, mmHg | 76.5 (68.3–145.8) | 110.0 (95.0–140.0) | 0.040 |
| PaO2:FiO2 | 95.4 (68.3–145.8) | 142.5 (113.3–182.5) | 0.012 |
| PaCO2, mmHg | 50.5 (42.9–58.5) | 47.0 (41.0–56.0) | 0.511 |
n, number; NA, not applicable; SOFA, sequential organ failure assessment; bpm, breaths per minute; PEEP, positive end expiratory pressure; Pplateau, plateau pressure; Pdriving, driving pressure; PaO2, partial pressure of arterial oxygen; FiO2, fraction of inspired oxygen; PaCO2, partial pressure of arterial carbon dioxide. Data are presented as median (interquartile range) or number (%).
Heart condition included congestive heart failure, coronary artery disease, and cardiomyopathies.
Table 2 summarizes outcomes of included patients. All-cause ICU-mortality was 20.0% (2/10) in the vitamin C group vs. 47.6% (49/103; p = 0.110) in the control group. There was no difference between the vitamin C and control group in terms of vasopressor-free days (9.0 vs. 0.0, p = 0.271), continuous renal replacement therapy-free days (26.0 vs. 19.0; p = 0.644), ventilator-free days (0.0 vs. 0.0; p = 0.832) or ICU-free days (0.0 vs. 0.0; p = 0.667).
Table 2.
Outcomes of patients included in the observational study.
| Outcome | Vitamin C group (n = 10) | Control group (n = 103) | p-value |
|---|---|---|---|
| Vasopressor-free days, days | 9.0 (0.0–24.0) | 0.0 (0.0–14.0) | 0.271 |
| Continuous renal replacement therapy-free days, days | 26.0 (6.8–28.0) | 19.0 (5.8–28.0) | 0.644 |
| Ventilator-free days, days | 0.0 (0.0–18.5) | 0.0 (0.0–15.0) | 0.832 |
| ICU-free days, days | 0.0 (0.0–3.3) | 0.0 (0.0–8.0) | 0.667 |
| ICU-mortality | 2 (20.0) | 49 (47.6) | 0.110 |
n, number; ICU, intensive care unit.
Data are presented as median (interquartile range) or number (%).
Vasopressor-free days, continuous renal replacement therapy-free days, ventilator-free days and ICU-free days were calculated by the number of days in the first 28 days following intubation that a patient was alive and not receiving vasopressors, not receiving continuous renal replacement therapy, not on a ventilator or not in the ICU, respectively. All outcomes were censored at day 28 following intubation.
Meta-Analysis
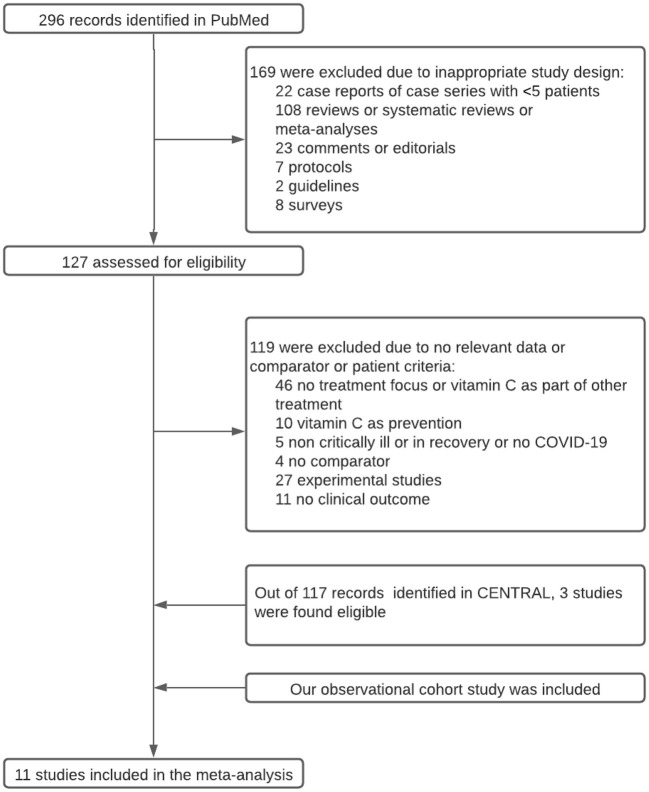
Figure 1 shows the flow diagram for study selection. Out of the 413 initially retrieved articles, 11 studies [i.e., 10 studies (23–32) from the literature plus our observational study], involving a total of 1,807 critically ill patients (515 received vitamin C) with COVID-19, were incorporated in the meta-analysis. Table 3 summarizes characteristics of the included studies. Six of them were retrospective observational studies (23, 24, 26, 28, 30) and five were randomized controlled trials (25, 27, 29, 31, 32). Supplementary Table 1 summarizes risk of bias assessment of the included studies. Six (23, 25, 28–30, 32) of them were considered to have low risk of bias.
Figure 1.
Study flow diagram.
Table 3.
Characteristics of individual studies included in the meta-analysis comparing vitamin C vs. control.
| Author/Country | Study design | Administered vitamin C regimen | Number of critically ill patients (n) | Female sex (%) | Age (years) | Baseline severity | Hypertension (%) | Diabetes Mellitus (%) | Coronary heart disease (%) |
|---|---|---|---|---|---|---|---|---|---|
| Al Sulaiman/Saudi Arabia (23) | Observational retrospective, multi-center | Enterally 1 g q24h | 739 | 15.2 vs. 30.0 |
60.5 ± 15.1 vs. 60.7 ± 14.8 |
4.0 (2.0–6.0) vs. 5.0 (3.0–8.0) |
56.4 vs. 56.9 |
60.3 vs. 61.2 |
8.2 vs. 8.6 |
| Beigmohammadi/Iran (32) | Randomized controlled trial, single-center | Enterally 2 g q24h | 60 | 50.0 vs. 46.7 |
51.0 ± 17.3 vs. 53.0 ± 7.0 |
7.0 ± 2.3 vs. 7.0 ± 3.0 |
NA | NA | NA |
| Darban/Iran (31) | Observational retrospective, single-center | IV 2 g q6h | 20 | NA | NA | NA | NA | NA | NA |
| Gao*/China (24) | Observational retrospective, single-center | IV 6 g q12h on 1st day; then, IV 6 g q24h for the next 4 days | 76 | 54.3 vs. 53.3 |
63.0 (54.0–71.0) vs. 57.0 (49.0–67.0) |
NA | 34.8 vs. 20.0 |
23.9 vs. 13.3 |
6.5 vs. 6.7 |
| Gavrielatou/Greece | Observational retrospective, single-center | IV 1 g q8h for 4 days; then, IV 500 mg q8h for 3 days; and, finally, IV 500 mg q12h for 3 days | 113 | 30.0 vs. 24.3 |
70.5 (58.0–75.0) vs. 69.0 (55.0–77.0) |
4.0 (4.0–5.3) vs. 4.0 (4.0–5.0) |
60.0 vs. 53.4 |
30.0 vs. 22.3 |
30.0 vs. 24.3** |
| JamaliMoghadamSiahkali/ Iran (25) |
Randomized controlled trial, single-center | IV 1.5 g q6h for 5 days | 60 | 50.0 vs. 50.0 |
57.5 ± 18.2 vs. 61 ± 15.9 |
3.6 ± 1.4 vs. 3.4 ± 1.5 |
50.0 vs. 33.3 |
40.0 vs. 36.7 |
13.3 vs. 23.3 |
| Krishnan/United States (26) | Observational retrospective, multi-center | NA | 152 | NA | NA | NA | NA | NA | NA |
| Kumari/Pakistan (27) | Randomized controlled trial, single-center | IV 50 mg/kg q24h | 150 | NA | 53 ± 11 vs. 53 ± 12 |
NA | NA | NA | NA |
| Li/United States (28) | Observational retrospective, single-center | IV 1.5 g q6h for up to 4 days | 32 | 63.0 vs. 63.0 |
64.1 ± 8.3 vs. 64.9 ± 11.8 |
6.6 ± 3.5 vs. 9.4 ± 3.2 |
75.0 vs. 54.2 |
50.0 vs. 45.8 |
12.5 vs. 4.2 |
| Zhang/China (29) | Randomized controlled trial, multi-center | IV 12 g q12h for 7 days | 56 | 44.4 vs. 24.1 |
66.3 ± 11.2 vs. 67 ± 14.3 |
14.0 (11.0–16.0) vs. 13.0 (9.5–15.0) |
37.0 vs. 51.7 |
29.6 vs. 32.1 |
14.8 vs. 27.6 |
| Zheng/China (30) | Observational retrospective, single-center | IV 2–4 g q24 24h after admission or during follow up before discharge | 397 | 40.0 vs. 49.5 |
67.5 (58.0–74.8) vs. 67.0 (62.0–74.0) |
NA | 18.6 vs. 21.4 |
15.7 vs. 15.6 |
4.3 vs. 6.7 |
n, number; IV, intravenous; h, hours; NA, not applicable.
Data are presented as mean ± standard deviation or median (interquartile range).
Baseline severity is presented as Sequential Organ Failure Assessment Score (SOFA) (23, 28) as Acute Physiology And Chronic Health Evaluation (APACHE II) (29) or other (25, 32).
Data from the entire cohort of patients (not only critically ill) are presented.
Numbers include coronary heart disease along with congestive heart failure and cardiomyopathies.
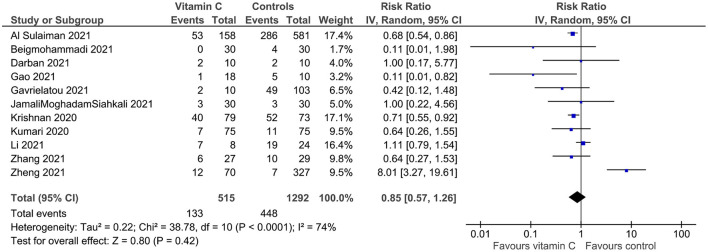
All 11 (23–32) studies provided data on all-cause mortality. Statistical heterogeneity was important (I2 = 74%). Mortality was not lower in the vitamin C than control group (25.8 vs. 34.7%; RR 0.85, 95% CI 0.57–1.26; p = 0.42; 11 studies; 1,807 patients; 561 deaths; Figure 2). In the sensitivity analysis of studies with low risk of bias (23, 25, 28–30, 32), mortality was 25.1% in the vitamin C group and 32.2% in the control group (RR 1.13, 95% CI 0.59–2.16; p = 0.72; six studies; 1,344 patients; 410 deaths; Supplementary Figure 1); while, in the sensitivity analysis of randomized controlled trials (25, 27, 29, 31, 32), the relevant numbers were 10.5 and 17.2%, respectively (RR 0.66, 95% CI 0.39–1.14; p = 0.14; five studies; 346 patients; 48 deaths; Supplementary Figure 2).
Figure 2.
All-cause mortality of critically ill patients with COVID-19 receiving vitamin C on top of standard-of-care (vitamin C group) vs. standard-of-care alone (control group). Pooled risk ratio (RR) and 95% confidence intervals (CI) were calculated using a random effects model.
Regarding secondary outcomes of the meta-analysis, ICU length of stay was longer in the vitamin C than control group (MD 1.56 days, 95% CI 0.63–2.49 days; p = 0.001; four studies; 887 patients; Supplementary Figure 3). There was no difference between the vitamin C group and control group in terms of duration of mechanical ventilation (MD 0.40 days, 95% CI −1.81–2.60 days, p = 0.73; 2 studies; 852 patients; Supplementary Figure 4) or need for renal replacement therapy (21.6 vs. 34.0%; RR 1.27, 95% CI 0.68–2.39; p = 0.45; two studies; 169 patients; 53 events; Supplementary Figure 5). Data on adverse events related to vitamin C were not consistently reported in the included studies.
Discussion
We carried out a combined study (observational cohort and meta-analysis) to elucidate the effect of vitamin C on clinical outcomes of critically ill patients with COVID-19. In the observational study, we found that mortality of patients with COVID-19 who consecutively underwent invasive mechanical ventilation in an academic ICU was 20.0% in the vitamin C group vs. 47.6% in the control group. In the subsequent meta-analysis of data from 1,807 critically ill patients with COVID-19 enrolled in 11 studies (six observational; five randomized controlled trials), including our observational study, we found that mortality was not lower in the vitamin C than control group (25.8 vs. 34.7%; RR 0.85).
The main finding of our observational study was an association between administration of vitamin C on top of standard-of-care, as opposed to standard-of-care alone, and lower (albeit statistically non-significant) mortality (20.0 vs. 47.6%) in critical COVID-19. Consistently, important clinical outcomes other than mortality, namely vasopressor-free days (9.0 vs. 0.0) and continuous renal replacement therapy-free days (26.0 vs. 19.0), were also in favor (albeit statistically non-significant) of the administration of vitamin C. In our study, vitamin C was administered in high dose, intravenously, initiating as early as 24 h following intubation and for a total of 10 days. All these parameters, namely high (vs. low) dose, intravenous (vs. enteral) administration, early (vs. delayed) initiation, and longer (vs. shorter) duration may reportedly increase the likelihood of a benefit of vitamin C when administered in critically ill patients (27–29). We thought that the fact that favorable differences in outcomes (including mortality) did not reach statistical significance might be due to the small sample size of the observational study (involving 113 patients), which made results prone to statistical error type II. The latter could be addressed by performing a meta-analysis.
We indeed performed a meta-analysis. The main finding of our subsequent meta-analysis (involving 1,807 patients) was again a lack of association between administration of vitamin C on top of standard-of-care, as opposed to standard-of-care alone, and mortality (25.8 vs. 34.7%) in critical COVID-19. This was also the case for the sensitivity analysis of 6 studies with low risk of bias (23, 25, 28–30, 32) (25.1 vs. 32.2%; RR 1.13) and for the sensitivity analysis of 5 randomized controlled trials (25, 27, 29, 31, 32) (10.5 vs. 17.2%; RR 0.66). Another finding of the meta-analysis, as depicted in Table 3, was the variability among the included studies in terms of dose, route and duration of administration along with the lack of information regarding timing of initiation of vitamin C. Given that these parameters may influence the effect of this intervention on clinical outcomes (27–29), standardization of the administration of vitamin C may be desirable.
The main finding of our study was in line with that of a recent relevant meta-analysis, which concluded that “no significant benefit was noted with administration of vitamin C in COVID-19” (33). The latter meta-analysis took into consideration only randomized controlled trials involving both patients with severe and patients with non-severe COVID-19 (33). In contrast, our endeavor may be more comprehensive (by taking into consideration both randomized controlled trials and observational studies) and focused (by taking into consideration only critically ill patients). On the other hand, the authors of another recent relevant pragmatic review of the literature concluded that “intravenous vitamin C intervention may improve oxygenation parameters and reduce inflammatory markers” (34). The latter review, albeit detailed, lacked a meta-analytic approach (34). Taken together, our and previous (33, 34) contributions may provide the readers with the whole picture of the potential role of vitamin C in patients with COVID-19.
Our combined study (observational study and subsequent meta-analysis) has limitations. Firstly, our observational study, due to its design, could not rule out the effect of confounders on the examined association between vitamin C and mortality. However, there was no difference between the compared groups (vitamin C vs. control) in terms of known predictors of mortality in COVID-19 (and therefore potential confounders), such as age, sex, comorbidities, SOFA score and lung mechanics at baseline (35). If anything, baseline oxygenation of mechanically ventilated patients was worse in the vitamin C than control group (PaO2:FiO2 95.4 vs. 142.5), which could attenuate a potentially beneficial effect of vitamin C on mortality. Secondly, our observational study was relatively small and therefore it could not lead to a definitive answer by itself. Nevertheless, it contributed valuable data for synthesis in a subsequent meta-analysis. Thirdly, our meta-analysis may be limited by its size (enrolling 1,807 critically ill patients) and the fact that included 6 observational studies, which are probably prone to confounding. However, we attempted to address this limitation by performing a sensitivity analysis of the 5 randomized controlled trials. Lastly, the included studies (23–32) in the meta-analysis did not consistently report on adverse events potentially related to administration of vitamin C, such as oxalate nephropathy, hypernatremia and glucometer error. Nevertheless, relevant evidence before the pandemic indicated that high-dose vitamin C may be relatively safe (36).
Conclusions
After combining results of our observational cohort with those of relevant studies into a meta-analysis of data from 1,807 patients, we found that administration of vitamin C as opposed to standard-of-care alone might not be associated with lower mortality among critically ill patients with COVID-19. Our combined study (observational cohort and meta-analysis) may constitute the most comprehensive effort to-date to clarify the effect of vitamin C on outcomes of critically ill patients with COVID-19. Additional evidence is anticipated from relevant large randomized controlled trials which are currently underway.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
EG contributed to study design, collected data, and interpreted data. EX and NX contributed to study design and the execution of the meta-analysis and they wrote the first draft of the manuscript. EX also undertook statistical analyses. AM, EI, and AKa contributed to data collection. DZ contributed to study design and data interpretation. CR and AKo contributed to data interpretation and critically revised the manuscript. IS conceived of the study, designed the study, supervised the data collection and statistical analyses, and is the guarantor, and final responsibility for the decision to submit for publication. All authors read and approved the final manuscript.
Funding
This study was supported by grants to IS from the Hellenic Thoracic Society (2019) and the Hellenic Foundation for Research and Innovation (HFRI) under the 2nd Call for HFRI Research Projects to support Post-Doctoral Researchers (Project number: 80-1/15.10.2020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully thank Drs. B. Bagheri and M. T. Beigmohammadi for showing praise-worthy academic attitude and generously providing us with additional data and/or clarifications regarding their study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.814587/full#supplementary-material
References
- 1.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. (2017) 151:1229–38. 10.1016/j.chest.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 2.Fowler AA, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the cITRIS-ALI randomized clinical trial. JAMA. (2019) 322:1261–70. 10.1001/jama.2019.11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. (2020) 323:423–31. 10.1001/jama.2019.22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sevransky JE, Rothman RE, Hager DN, Bernard GR, Brown SM, Buchman TG, et al. Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. JAMA. (2021) 325:742–50. 10.1001/jama.2020.24505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iglesias J, Vassallo AV, Patel VV, Sullivan JB, Cavanaugh J, Elbaga Y. Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: the ORANGES trial. Chest. (2020) 158:164–73. 10.1016/j.chest.2020.02.049 [DOI] [PubMed] [Google Scholar]
- 6.Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, et al. Combined treatment with hydrocortisone, vitamin c, and thiamine for sepsis and septic shock: a randomized controlled trial. Chest. (2020) 158:174–82. 10.1016/j.chest.2020.02.065 [DOI] [PubMed] [Google Scholar]
- 7.Sato R, Hasegawa D, Prasitlumkum N, Ueoka M, Nishida K, Takahashi K, et al. Effect of IV high-dose vitamin c on mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. (2021) 49:2121–30. 10.1097/CCM.0000000000005263 [DOI] [PubMed] [Google Scholar]
- 8.Hooper MH, Hager DN. Understanding vitamin c in critical illness: a focus on dose, route, and disease. Crit Care Med. (2019) 47:867–9. 10.1097/CCM.0000000000003718 [DOI] [PubMed] [Google Scholar]
- 9.Carr AC. Vitamin C in pneumonia and sepsis. In: Chen Q, Vissers MCM. editors. Vitamin C: New Biochemical and Functional Insights [Internet]. Boca Raton, FL: CRC Press; (2020). [PubMed] [Google Scholar]
- 10.Milani GP, Macchi M, Guz-Mark A. Vitamin C in the treatment of COVID-19. Nutrients. (2021) 13:1172. 10.3390/nu13041172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr AC, Rowe S. The emerging role of vitamin c in the prevention and treatment of COVID-19. Nutrients. (2020) 12:E3286. 10.3390/nu12113286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Information on COVID-19 Treatment, Prevention and Research [Internet] . COVID-19 Treatment Guidelines. (2021). Available online at: https://www.covid19treatmentguidelines.nih.gov/.
- 13.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. (2021) 384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siempos II, Xourgia E, Ntaidou TK, Zervakis D, Magira EE, Kotanidou A, et al. Effect of Early vs. Delayed or no intubation on clinical outcomes of patients with COVID-19: an observational study. Front Med. (2020) 7:614152. 10.3389/fmed.2020.614152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenck EJ, Oromendia C, Torres LK, Berlin DA, Choi AMK, Siempos II. Rapidly improving ARDS in therapeutic randomized controlled trials. Chest. (2019) 155:474–82. 10.1016/j.chest.2018.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. (2021) 25:121. 10.1186/s13054-021-03540-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tool to Assess Risk of Bias in Cohort Studies . Contributed by the CLARITY Group at McMaster University. (2021). Available online at: https://www.evidencepartners.com/wp-content/uploads/2021/03/Tool-to-Assess-Risk-of-Bias-in-Case-Control-Studies-DistillerSR.pdf.
- 19.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 20.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. (2017) 17:162. 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Review Manager (RevMan) [Computer program] . Version 5.4.1, The Cochrane Collaboration; (2020). [Google Scholar]
- 22.Higgins J, Thomas J, Chandler J, Cumpston M, Li M, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). (2021). Available online at: www.training.cochrane.org/handbook.
- 23.Al Sulaiman K, Aljuhani O, Saleh KB, Badreldin HA, Al Harthi A, Alenazi M, et al. Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: a propensity score matched study. Sci Rep. (2021) 11:17648. 10.1038/s41598-021-96703-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao D, Xu M, Wang G, Lv J, Ma X, Guo Y, et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study. Aging. (2021) 13:7020–34. 10.18632/aging.202557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.JamaliMoghadamSiahkali S, Zarezade B, Koolaji S, SeyedAlinaghi S, Zendehdel A, Tabarestani M, et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. (2021) 26:20. 10.1186/s40001-021-00490-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan S, Patel K, Desai R, Sule A, Paik P, Miller A, et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. (2020) 67:110005. 10.1016/j.jclinane.2020.110005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumari P, Dembra S, Dembra P, Bhawna F, Gul A, Ali B, et al. The role of vitamin c as adjuvant therapy in COVID-19. Cureus. (2020) 12:e11779. 10.7759/cureus.11779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Ching TH, Hipple C, Lopez R, Sahibzada A, Rahman H. Use of intravenous vitamin c in critically ill patients with covid-19 infection. J Pharm Pract. (2021) 8:8971900211015052. 10.1177/08971900211015052 [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. (2021) 11:5. 10.1186/s13613-020-00792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng S, Chen Q, Jiang H, Guo C, Luo J, Li S, et al. No significant benefit of moderate-dose vitamin C on severe COVID-19 cases. Open Med Wars Pol. (2021) 16:1403–14. 10.1515/med-2021-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darban M, Malek F, Memarian M, Gohari A, Kiani A, Emadi A, et al. Efficacy of High dose vitamin C, melatonin and zinc in iranian patients with acute respiratory syndrome due to coronavirus infection: a pilot randomized trial. J Cell Mol Anesth. (2021) 6:164–7. 10.22037/jcma.v6i2.32182 [DOI] [Google Scholar]
- 32.Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Soltani D. The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial. Trials. (2021) 22:802. 10.1186/s13063-021-05795-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawat D, Roy A, Maitra S, Gulati A, Khanna P, Baidya DK. Vitamin C and COVID-19 treatment: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. (2021) 15:102324. 10.1016/j.dsx.2021.102324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holford P, Carr AC, Zawari M, Vizcaychipi MP. Vitamin C intervention for critical COVID-19: a pragmatic review of the current level of evidence. Life. (2021) 11:1166. 10.3390/life11111166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel BV, Haar S, Handslip R, Auepanwiriyakul C, Lee TM, Patel S, et al. Natural history, trajectory, and management of mechanically ventilated COVID-19 patients in the United Kingdom. Intensive Care Med. (2021) 47:549–65. 10.1007/s00134-021-06389-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanase F, Fujii T, Naorungroj T, Belletti A, Luethi N, Carr AC, et al. Harm of IV high-dose vitamin C therapy in adult patients: a scoping review. Crit Care Med. (2020) 48:e620–8. 10.1097/CCM.0000000000004396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.