Structured Abstract
Objectives:
The objectives were to measure the effect of stimulus rate and vowel-change direction on the acoustic change complex (ACC) latencies and amplitudes and compare ACC metrics to behavioral measures of vowel-contrast detection for infants tested under the age of 1 year. We tested the hypothesis that the direction of spectral energy shift from a vowel change would result in differences in the ACC, owing to the sensitivity of cortical neurons to the direction of frequency change. We evaluated the effect of the stimulus rate (1/s vs. 2/s) on the infants’ ACC. We evaluated the ACC amplitude ratio’s sensitivity (proportion of ACCs present for each change trial) and compared it to perceptual responses obtained using a visually-reinforced infant speech discrimination paradigm (VRISD). This report provides normative data from infants for the ACC towards the ultimate goal of developing a clinically useful index of neural capacity for vowel discrimination.
Design:
Twenty-nine infants, nine females, aged 4.0–11.8 months, participated. All participants were born at full-term and passed their newborn hearing screens. None had risk factors for hearing or neurologic impairment. Cortical auditory evoked potentials were obtained in response to synthesized vowel tokens /a/, /i/, /o/ and /u/ presented at a rate of 1- or 2/s in an oddball stimulus paradigm with a 25% probability of the deviant stimulus. All combinations of vowel tokens were tested at the two rates. The ACC was obtained in response to the deviant stimulus. The infants were also tested for vowel-contrast detection using a VRISD paradigm with the same combinations of vowel tokens used for the ACC. The mean age at the time of the ACC test was 5.4 months, while the mean age at the behavioral test was 6.8 months.
Results:
Variations in ACC amplitude and latency occurred as a function of the initial vowel token and the contrast token. However, the hypothesis that the direction of vowel (spectral) change would result in significantly larger change responses for high-to-low spectral changes was not supported. The contrasts with /a/ as the leading vowel of the contrast pair resulted in the largest ACC amplitudes than other conditions. Significant differences in the ACC presence and amplitude were observed as a function of rate, with 2/s resulting in ACCs with the largest amplitude ratios. Latency effects of vowel contrast and rate were present, but not systematic. The ACC amplitude ratio’s sensitivity for detecting a vowel contrast was greater for the 2/s rate than the 1/s rate. For an amplitude ratio criterion of ≥ 1.5, the sensitivity was 93% for ACC component P2-N2 at 2/s, whereas at 1/s sensitivity was 70%. VRISD tests of vowel-contrast detection had a 71% hit and a 21% false-positive rate. Many infants who could not reach performance criteria for VRISD had ACC amplitude ratios ≥ 2.0.
Discussion:
The ACC for vowel contrasts presented at a rate of 2/s is a robust index of vowel-contrast detection when obtained in typically developing infants under the age of 1 year. The ACC is present in over 90% of infants tested at this rate when an amplitude ratio criterion of ≥1.5 is used to define a response. The amplitude ratio appears to be a sensitive metric for the difference between a control and contrast condition. The ACC can be obtained in infants who do not yet exhibit valid behavioral responses for vowel change contrasts and may be useful for estimating neural capacity for discriminating these sounds.
Introduction
Infants with hearing loss may be significantly delayed in developing perceptual abilities to distinguish between phonemes, even in their native language (Eisenberg et al., 2007; Moeller et al., 2007), ultimately leading to significant receptive and expressive oral language delays (Tomblin et al., 2015). Measures of speech perception ability are critical as an outcome measure after fitting hearing aids (Pediatric Amplification Guidelines, 2013; Uhler et al., 2017). Despite the importance of speech feature perception and discrimination abilities for developing oral language (Eimas et al., 1971; Jusczyk et al., 1998; Kuhl, 2004; Werker &Tees, 1984), there are no standardized clinical methods for assessing this ability in infants/toddlers who have a receptive oral language age below 2.4 years, such as that required by the Northwestern University Children’s Perception of Speech (NU-CHIPS, Elliot and Katz, 1980). Establishing reliable techniques for evaluating speech-feature discrimination would be of tremendous benefit for clinicians who need to measure auditory abilities of infants and children with hearing loss and to determine the effects of treatment.
Measuring speech-feature discrimination in infants is essential because clinicians need to know whether their interventions provide the capacity for these building blocks of oral language development. Behavioral methods used in classic investigations of speech perception abilities in infants, such as preferential looking or high-amplitude sucking (Werker et al., 1998) do not have carry-over to the clinic as they require extensive sampling from groups of infants to obtain a significant result. Eisenberg et al. (2004, 2007) developed a test known as Visual Reinforcement Assessment of the Perception of Speech Pattern Contrasts that used a visually-reinforced infant speech discrimination (VRISD) paradigm to test the abilities of infants to discriminate between vowel types and consonant features of voicing, manner, and place. Nevertheless, the clinical translation of this approach was limited because even infants with normal hearing had difficulty learning the conditioned-head-turn task (Martinez et al., 2008). This finding is consistent with other lab-based studies of infant speech perception or psychoacoustics requiring a discriminative response wherein as many as 40% of infants with normal hearing do not meet the criteria for providing valid data.
Uhler and Gifford (2014) surveyed pediatric audiologists in the USA to determine conventional methods of speech perception assessment of infants and children with hearing loss. None used VRISD methods. They found that the Northwestern University-Children’s Perception of Speech (Elliot & Katz, 1980) was used by only 30% of audiologists to assess speech perception abilities in toddlers (25–35 months). This low usage is likely because the test requires a receptive language age of 2.4 years; and attaining this level of auditory receptive language ability is often quite delayed in infants born with hearing loss.
Cortical Auditory Evoked Potentials as an indicator of infant speech-feature perception
Rance et al. (2002) measured cortical auditory evoked potentials (CAEPs) in a group of infants and young children diagnosed with an auditory neuropathy. They demonstrated a strong, statistically significant, positive correlation between CAEP presence and the child’s speech perception abilities. These findings suggested that CAEP could be used as a prognostic measure of speech perception in infants and young children with auditory neuropathy (Cone, 2008). Other lab groups have replicated these findings (Golding et al., 2007; Sharma et al., 2011; He et al., 2015). Additionally, it is now recognized that CAEP component P1 latency may be used to indicate cortical plasticity attributed to the use of cochlear implants. Decreasing P1 latency during the first months of implant use can be used as a “biomarker” of auditory maturation or plasticity following electrical stimulation of the auditory nerve (Sharma et al., 2002; 2005).
Cone & Whitaker (2013) postulated that CAEP onset response components, P1-N1-P2, evoked by tonal and speech tokens could be used to estimate speech detection abilities in infants (4–12 months of age). They established CAEP amplitude input-output functions as a function of level for tone tokens and “Ling sound” tokens (Ling, 1988) commonly used in a simple, functional, trouble-shooting test of hearing-aids. They found that CAEP threshold estimates and amplitude input-output functions mirrored the maturity of cochlear compressive amplitude growth. They also found that perceptual estimates of threshold were elevated with respect to CAEP threshold in the same way as for auditory brainstem response (ABR) and perceptual threshold comparisons (Werner et al., 1993).
Acoustic Change Complex
Cone (2015) then used a quasi-steady-state stimulus and an odd-ball paradigm for obtaining CAEPs in response to vowel contrasts (/a/, /i/, /o/, /u/). These CAEPs for acoustic change are known as Acoustic Change Complexes (ACCs). The ACC was defined as the difference between the waveform obtained in response to the vowel change, and the waveform obtained in the control condition when there was no vowel change. The largest ACC amplitudes derived in this way were in response to contrasts presented as a quasi-steady-state train with a 2/s rate. Infants had robust ACCs for vowel contrasts when presented at a 2/s rate, although these were not always evident at a slower stimulus rate (1/s). ACCs were present for over 90% of the contrasts presented at a 2/s rate. Conversely, perceptual performance for detection of vowel contrasts, obtained from VRISD tests, averaged 68% correct and a d-prime (d’) of 1.13. Even so, there were CAEP latency and amplitude measures that showed moderate to strong correlation (r = 0.5 to 0.81) with the behavioral performance.
The relationship between ACC and perceptual performance appears to be strong when tested in adults. He et al. (2012) demonstrated an excellent correspondence between perceptual and ACC measures of frequency and intensity discrimination and gap detection when measured in adults with normal hearing. Likewise, Kirby & Brown (2015) showed that the ACC was sensitive to frequency compression parameters in listeners tested while using a hearing aid. Cheek and Cone (2020) further evaluated the correspondence between an individual’s ability to discriminate between vowel types and their ACC response and showed a 99% sensitivity of ACC for perceptual detection of vowel change. These results in adults give impetus to developing the ACC methods for measuring speech feature discrimination in infants.
In Cone (2015), ACCs were obtained when /a/ was contrasted with either /i/, /o/ or /u/. ACC latencies and amplitudes varied with the rate of stimulation (1/ vs. 2/s), and the vowel contrasted with /a/. The derived waveform method for defining the ACC in that study presented some issues in interpretation because latency (phase) differences between the response in the control condition and the response to the contrast stimulus can diminish the amplitude of the ACC in the derived waveform. Further, the difference waveform may contain the mismatch negativity (MMN) response in some listeners. In contrast, Cheek and Cone (2020) defined the ACC as a ratio of the ACC amplitude for the change condition, divided by the amplitude of the ACC in the control condition. The ACC amplitude ratio is unaffected by latency differences that may be present between control and contrast responses.
This report extends the findings of Cone (2015) by including results for vowel contrasts /i/, /o/ and /u/ with each other and also with /a/. We tested the hypothesis that the direction of spectral energy shift from a vowel change may result in differences in the amplitude of the ACC, owing to the sensitivity of cortical neurons to the direction of frequency change. For example, /u/-/i/ represents a change from a low-front vowel to a high front vowel, whereas /i/-/u/ is a high-to-low change. As in Cone (2015), we also evaluate the differences in ACC amplitude and latency when obtained at a stimulus rate of 1/s vs. 2/s, as rate also has a significant effect upon the cortical evoked response, particularly during development (Gilley et al., 2005; Wunderlich et al., 2006), including significant reductions in amplitude or absence of CAEP components (e.g., N1) at younger ages and faster stimulus rates. We calculated the ACC amplitude ratio metric of Cheek and Cone (2020), applied it to responses from infants, and evaluated the sensitivity of this metric. Furthermore, we compare the ACC to perceptual responses from infants. The over-arching goal is to expand the evidence-base for using the ACC as a clinical tool to index neural capacity for vowel discrimination in infants.
Methods
The research was approved by the University of Arizona Human Subjects Protection Program.
Subjects.
Twenty-nine infants, aged 4.0–11.8 months, participated in the experiment. The group included nine females. The mean age at the time of the CAEP test was 5.8 (s.d.=1.3) months, while the mean age at the time of the behavioral test was 7.8 (s.d. = 1.9) months. All infant participants were born at full-term and had passed their newborn hearing screens. None had risk factors for hearing or neurologic impairment. All infants had normal otoscopic and tympanometric test findings and passed a distortion product otoacoustic emission screening test at the time of admission to the study. Otoscopy and tympanometry were repeated if a parent reported that the infant had experienced an upper respiratory or ear infection before the visit. Data were obtained only when otoscopy and tympanometry findings were normal. Infants were allowed to participate in up to five test sessions to obtain electrophysiologic and perceptual data.
Stimuli.
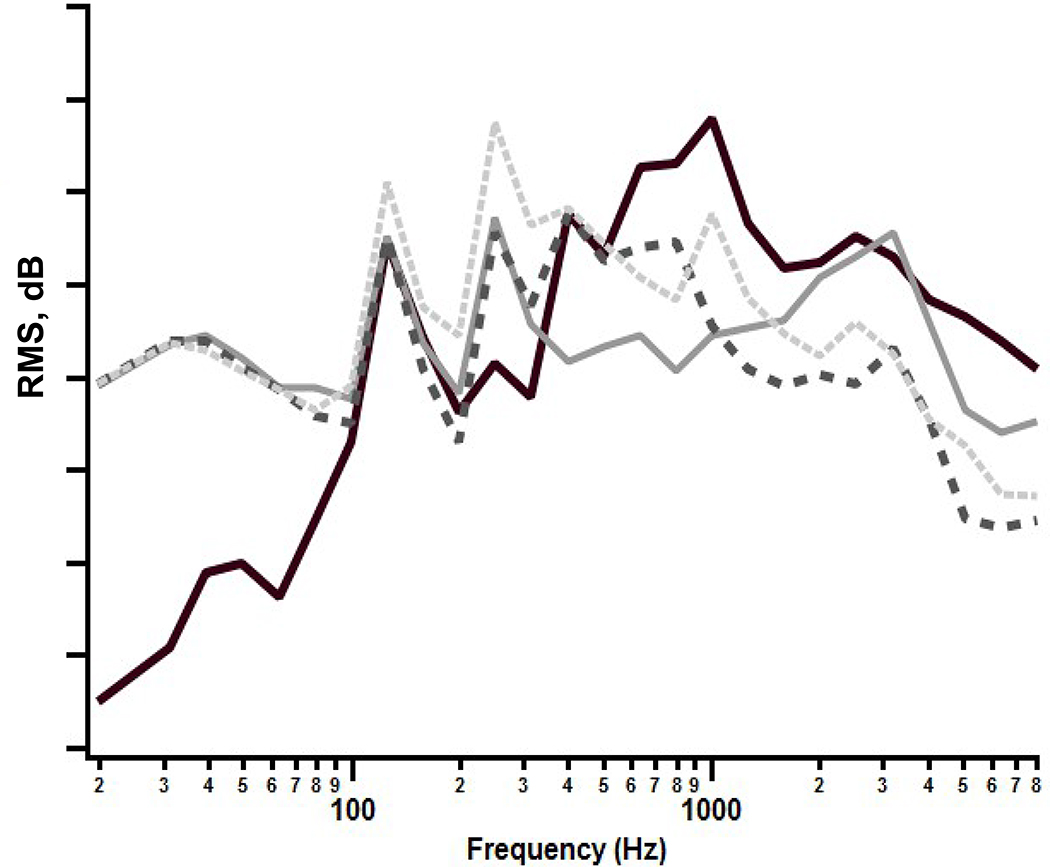
The stimuli were synthesized (Story, 2011) vowel tokens: /a/, /i/, /o/, /u/, each with a 500 ms total duration, and shaped with a 10 ms linear onset-offset ramp. The spectra of these tokens are shown in Figure 1. Synthesized, rather than natural speech tokens were used so that the fundamental and formant frequencies and their levels could be precisely controlled. The stimuli were presented at 70 dBA in the sound field, via a JBL Control 1x model speaker with a Crown D-75A amplifier. The loudspeaker was at 0 degrees azimuth and 1.2 m distance relative to the position of the infant for both CAEP and VRISD tests. These stimuli were calibrated in the sound field using a Larsen Davis Model 824 sound pressure level meter with a half-inch microphone suspended from the ceiling in the position approximating the position of the infant’s head during testing. During calibration, the samples were presented in a steady-state fashion with an inter-stimulus interval of < 1.0 ms.
Figure 1:
Vowel Spectra: Spectral energy by third octave band (20–8000 Hz) for synthesized vowel stimuli. (Cheek and Cone, 2020, with permission).
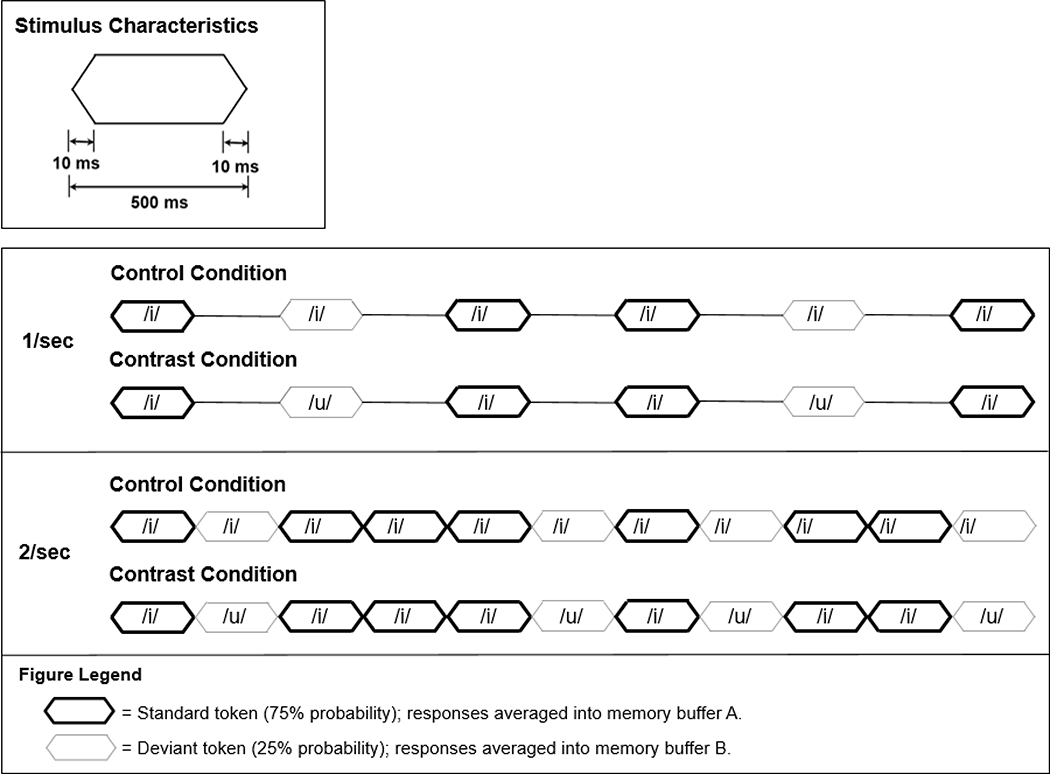
The vowel tokens were presented using an oddball stimulus paradigm, with a deviant probability of 25%. For example, for an /i/-/u/ contrast, 75% of the tokens were /i/ (standard) and 25% were /u/ (deviant). The computer averaging system stored the averaged responses to standard and deviant tokens in separate buffers in the oddball paradigm mode. The ACC is the response evoked by the deviant token, in this case, /u/. A control condition was obtained for each vowel token in which the “standard” and “deviant” tokens were the same, in this example, /i/. Again, the averaging system separates the responses into two buffers, one for the “standard” and one for the “deviant”. The control condition, in which there is no vowel change, provided a means for comparison of the ACC response to the /i/ token (at 25% probability) to the test condition in which a contrast token, /u/, also had a 25% probability. A schematic of the stimulus train for the contrast and control conditions is presented in Fig. 2. The ACC tests were presented at both a 1 Hz (offset to onset interval = 500 ms) and 2 Hz (offset to onset interval ≤ 1 ms) stimulus token rate. The 2 Hz rate resulted in a quasi-steady-state stimulus train, as the duration of each token was 500 ms. The decision to use this paradigm was based upon pilot data from our lab as well as previously published research on mismatch negativity to which ACC is sometimes compared.
Figure 2:
Vowel token envelope characteristics and stimulus paradigm. The vowel-token envelope had 10 ms linear rise-fall times and 480 ms plateau duration for a total duration of 500 ms. The oddball stimulus paradigm for the control and contrast test conditions for the /i/-/u/) contrasts is illustrated. The MMN/P300 module allows for separate averaging of responses to standard tokens presented with 75% probability into one buffer (A) and responses to the deviant tokens presented at 25% probability into another buffer (B). In the control condition, the “deviant” token is the same as the standard (/i/), but sampled and averaged over 25% of the stimulus presentations into memory buffer B. In the contrast condition, the deviant vowel token, /u/, is different from the standard (/i) occurs with a 25% probability and the response is averaged into memory buffer B.
For the perceptual discrimination test, two vowel tokens were concatenated for a total stimulus token duration of 1000 ms. A “change” or “contrast” stimulus consisted of two tokens in which the second token was different from the first (e.g., /i/+/u/). “The “no change” stimulus consisted of two identical 500 ms tokens (e.g., /i/+/i/). The rate of token presentation for the vowel-change discrimination test was 0.4 Hz.
CAEP Recordings
CAEPs were recorded using silver-silver chloride single-use disposable electrodes placed at Cz (vertex, non-inverting), A2 (right mastoid, inverting), and A1 (left mastoid, ground) using electrode paste and paper tape, after cleansing each site with Nu-Prep. Electrode impedances were maintained at <10 kΩ and with < 3 kΩ inter-electrode impedance. If an electrode became displaced during testing, it was replaced, and electrode impedances checked before resuming recording. The EEG was amplified by a factor of 94 dB and filtered at 1–30 Hz and digitized at 0.5 kHz. Amplitude-based artifact rejection levels were set at between 50–90 μV, levels that have proven efficacious in past research (Wunderlich et al., 2006; Cone & Whitaker, 2013; Cone, 2015). If needed, the artifact rejection levels could be adjusted during the test session.
All recordings were made using the Intelligent Hearing Systems Smart-EP™ (Miami, FL) system. The MMN/P300 software module was used to present the stimuli in an oddball paradigm and obtain separate waveforms in response to the standard (75% probability) and deviant (25% probability) tokens. At least 50 artifact-free samples were obtained in response to the “deviant” stimulus, the ACC, for each contrast tested. The samples were averaged over a 500 ms epoch following the onset of each token.
Infants were tested while awake and seated in a high chair or on the parent’s lap. A test assistant manipulated toys to keep the infants in a calm but alert state conducive for CAEP tests.
Vowel-discrimination Test
Infants were trained and tested using visually-reinforced operant procedures with an observer-based psychophysical method as described previously (Cone & Garinis; 2009; Cone & Whitaker, 2013; Cone, 2015). The Intelligent Hearing Systems VRISD software module was used to present the stimuli and record the response.
Infants were brought into the test booth and seated in a high chair while the “control” stimulus was presented continuously; the stimulus used for this habituation procedure was the 1000 ms token created by concatenating two samples of the same token, e.g., /i/-/i/, as described above. Infants were trained to respond to a change in the speech stimulus. The training consisted of pairing the presentation of a change token (e.g., /i/-/a/) with visual reinforcement, which was an animated cartoon played on a video screen, placed at 45 degrees with respect to the infant’s head position. The response behavior could be a head turn, an eye movement towards the reinforcer, a change or cessation of facial expression, or an increase in body movement. Training consisted of 5 pairings of the change trial with the reinforcer. Between change trials, the control stimulus was played at a rate of 0.4 Hz. After five pairings, a probe trial was presented: a stimulus change token with the reinforcer withheld until the infant emitted a response. If the infant responded, the reinforcer was introduced. The infant had to respond correctly to 2/3 probe trials before testing began. If the infant did not reach this criterion, five more training trials were given, followed by three probe trials. Up to 15 training trials were given per test session, but testing was discontinued if the infant did not meet the criterion.
The testing phase consisted of 12 trials for a given vowel contrast. The infant was tested for the vowel contrast used in training. During testing, the observer controlled when a trial was initiated, that is, when the infant was in a quiet, alert state. All auditory monitors and feed-back were turned off, so the observer was masked as to whether the trial contained a vowel change (contrast) token or a control (no change) token. Up to six trials per a 12-trial test were control trials. The randomization of test and control trials was done automatically by computer software. The observer voted on each trial by pressing a response button; that is, the observer had to determine whether the trial contained a vowel change based solely on observation of the infant’s behavior. When the observer made a correct detection, the infant was shown the visual reinforcer. When the observer made a correct rejection (no response on a control trial), there was no reinforcer. Likewise, there was no reinforcer for a miss (failure to detect a change trial) or a false alarm (voting that a change token had occurred on a control trial). The percentage of correct responses for both change and control trials was measured.
During all training and testing, a test assistant manipulated toys at mid-line to keep the infants in an appropriate response state for the vowel discrimination test.
Procedure
Infants could participate in the CAEP vowel-change test and the perceptual vowel-discrimination test on the same day. The order of contrasts tested was /a/, /i/, /o/, and /u/ for all subjects. For example, a subject would undergo testing with /a/ vs. /i/, then /a/ vs. /o/ and /a/ vs. /u/, before proceeding with the next set of contrasts, /i/ vs. /a/, /i/ vs. /o/ and /i/ vs. /o/. The order of the test (CAEP or perceptual) was not controlled. Typically, the test session started with a perceptual test for one vowel contrast; those methods are described above. Then, CAEPs for the same contrast were tested with the order for presentation rate (1 Hz vs. 2 Hz) randomized. It was the case that infants yielded CAEP results at younger ages before they were able to pass training criteria for the VRISD, although VRISD training and testing was attempted on all infants at the time of their first visit to the lab, which could be as young as 4 months of age.
CAEP and VRISD tests were performed by the principal investigator (Cone) and by two graduate research assistants who had been trained by the principal investigator.
Data Analyses
CAEP Data Analyses
CAEP waveforms were analyzed using rule-based visual detection methods by the principal investigator (BC) and research assistants who had been trained by the principal investigator. The rules for visual detection of CAEP components included latency and amplitude criteria for each component, based upon values derived from the published literature on infant CAEP (Cone & Whitaker, 2013; Wunderlich & Cone-Wesson, 2006). CAEP component peaks had to fall within their respective latency range criteria, and peak-to-trough amplitude differences were 1.0 μV or greater to be considered a peak. The latency ranges for P1, N1, P2 and N2 were 80–220, 160–300, 240–380 and 300–490 ms, respectively, with a peak-to-trough latency difference of at least 50 ms. Peaks and troughs were marked with a cursor to determine peak latency, and amplitudes were calculated as the difference between the peak and succeeding trough, and trough-to peak,i.e., P1-N1 or N1-P2. Amplitudes of greater than 22 μV were excluded from the analyses as these indicated possible contaminations with movement artifact. The ACC amplitude ratio was calculated as the amplitude of the ACC (response to deviant) in the contrast condition divided by the amplitude of the ACC in the control condition. In the case of absent components in the control condition, a value of 1.0 was used as the denominator. Amplitude ratios that are ≤ 1.0 indicate that the response to the contrast was of smaller amplitude than the response to the control, and therefore, do not indicate detection of the vowel change. The amplitude ratio is a measure that normalizes for individual differences in response amplitude.
Vowel Discrimination Test Analyses
The IHS Smart VRA software recorded observer responses for each trial. From the total number of trials, the percentage of hits (correct detection of stimulus change), misses (failure to detect stimulus change), false alarms (detection in the absence of stimulus change), and correct rejections (correct judgment of a control trial) were calculated.
Descriptive and inferential statistical analyses (analyses of variance) were completed using Statview (v5.0.2) software. Linear mixed-model regression analyses were completed in R (https://www.r-project.org/).
Results
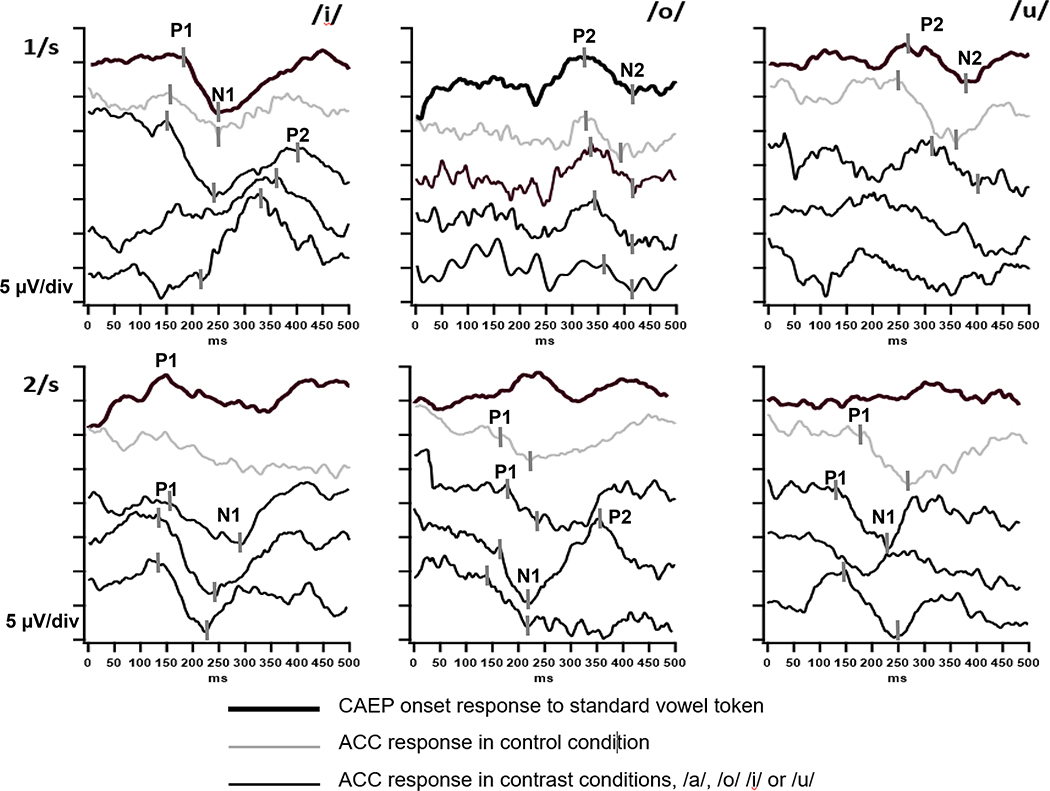
Representative responses are shown in Fig. 3 for vowel contrasts at the 1/s and 2/s rates. The waveforms were averaged over 500 ms from vowel onset in the case of standards (presented at a probability of 75%), or from the vowel change onset of deviant tokens (presented at 25% probability). At 1/s, the typical waveform has two positive peaks, each with a succeeding negative trough, and these components are labeled P1-N1-P2-N2, sequentially. Variations in CAEP amplitude and latency occurred as a function of the initial vowel token and the contrast token, and these are evaluated in subsequent sections of results. Dramatic differences in the presence and amplitude of components are observed as a function of rate, with CAEPs to the standard vowel and control vowel tokens largely absent at 2/s because of neural adaptation.
Figure 3:
Examples of CAEP onset and ACC responses from individual infants for contrasts /i/, /o/ and /u/. Waveform key:
 CAEP onset response to standard vowel token
CAEP onset response to standard vowel token
 ACC response in control condition
ACC response in control condition
 ACC response in contrast conditions, /a/, /o/ /i/ or /u/
ACC response in contrast conditions, /a/, /o/ /i/ or /u/
ACC waveforms shown in alphabetical order for each condition: for /i/, the contrasts shown are /a/, /o/ and /u/. For /o/, the contrast are /a/, /i/ and /u/. For /u/, the contrasts are /a/, /i/, /o/. Markers have been provided for response components in some waveforms as a guide for identifying the components in the subsequent waveforms. ACCs are identified as differences in amplitude between the control (thin gray trace) and contrast (thin black trace) conditions. In the 1/s example, ACCs for the /u/ condition are questionable for the /i/ and /o/ (bottom two) traces. As described in the text, CAEP onset responses to the standard vowel token are frequetly absent at the 2/s rate and this is the case in the examples found for /o/ and /u/. Examples of waveforms for /a/ standards, controls and /i/, /o/ and /u/ contrasts are found in Cone (2015).
Results for 1/s
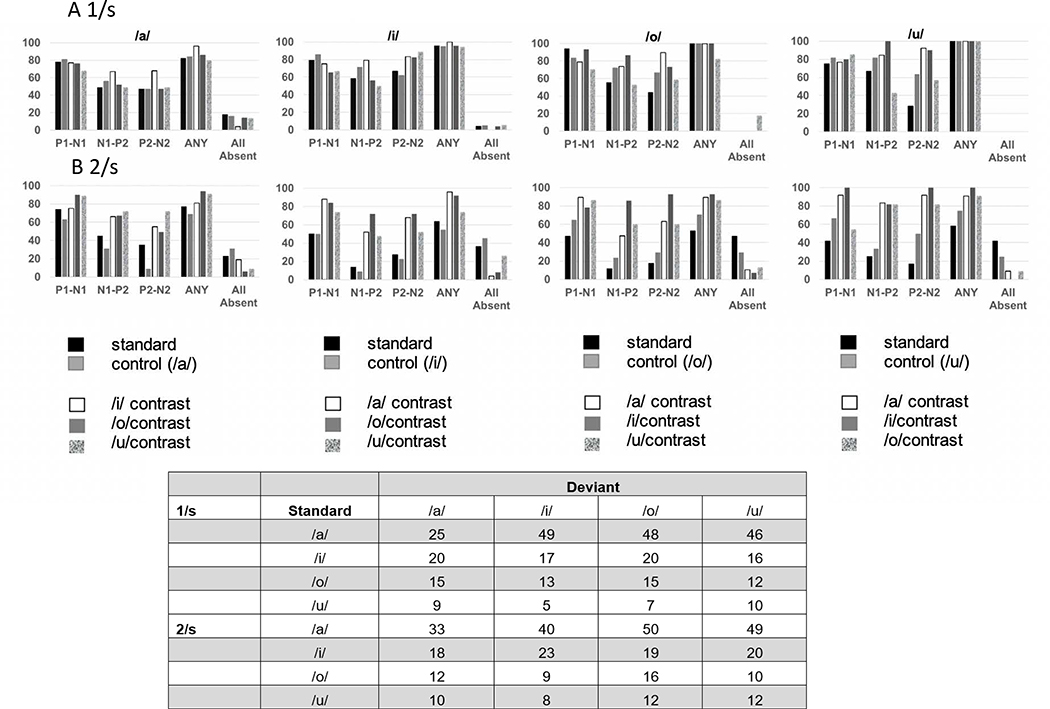
Presence of CAEP Components
The distributions of detected CAEP components for the 1/s rate are displayed in Figure 4A. The percentage of CAEP components detected was calculated for each test condition, control and contrast The P1-N1 component was the most prevalent CAEP component present across all test conditions. These distributions suggested some differences in the percentage of components present as a function of CAEP component and the deviant vowel contrast. To determine if the deviant vowel type affected the percentage of response components present a one-way analyses of variance was conducted for each component (P1-N1, N1-P2 and P2-N2) These analyses yielded a significant result for N1-P2 only (F3,8 = 11.27, p =.003), with post-hoc paired comparisons with Bonferroni corrections indicating fewer responses present for /o/ and /u/ deviants compared to /a/ and /i/ deviants.
Figure 4:
Percent detected components for P1-N1, N1-P2 and P2-N2 for 1) onset responses to the standard token; and 2) ACC responses in control and contrast conditions. ANY refers the percent of trials in which any CAEP or ACC component was present, and all absent refers to the percent of trials in which no identifiable components were present. The embedded table indicates the number of test trials for each vowel pair, at each rate.
Amplitude
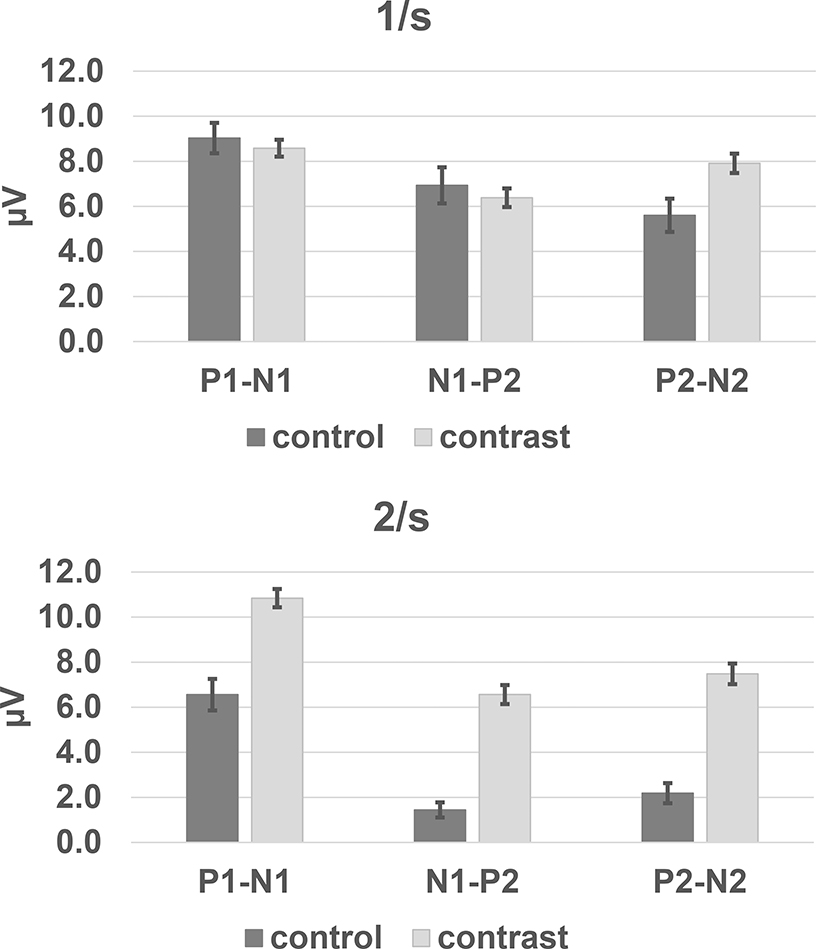
The peak-to-trough amplitudes were measured for CAEP components P1-N1, N1-P2, and P2-N2. These analyses focus on the responses to the deviant token as this is the way ACC is determined using the oddball stimulus paradigm. A value of 0 μV was entered when a component was absent. The mean amplitudes are shown for the control and the test (contrast) conditions (Fig 5). The effect of test condition (control vs. contrast) was evaluated for each component using analyses of variance. An amplitude difference between the control and contrast response indicates an effect of the acoustic change. The results of these analyses of variance are summarized in Table 1. A statistically significant difference between the control and contrast conditions was found only for the P2-N2 component. Amplitudes for P2-N2 were significantly larger in response to deviants when presented in test conditions compared to control conditions. This finding suggests that the P2-N2 component indexed the acoustic change between the standard and the deviant token.
Figure 5:
Mean peak-to-trough amplitudes of ACC responses in control and contrast conditions, A= 1/s rate and B = 2/s rate. Error bars indicate ± 1 standard error.
Table 1:
Summary of analyses of variance and effect size (Cohen’s d) for amplitudes of ACC response components as a function of test condition, control (no vowel change) vs. contrast (vowel change). Statistical results considered significant are indicated by an asterisk.
| Control vs. Contrast Amplitudes | |||
|---|---|---|---|
| A | F | p | Cohen’s d |
| 1/s | |||
| P1-N1 | F1,325 = 0.30 | p = 0.58 | n.a. |
| N1-P2 | F1,325 = 0.37 | p = 0.54 | n.a. |
| P2-N2 | F1,325 =5.72 | p = 0.02* | 0.34 |
| B | |||
| 2/s | |||
| P1-N1 | F1,337 = 27.92 | p < 0.0001* | 0.67 |
| N1-P2 | F1,337 = 45.26 | p < 0.0001* | 0.98 |
| P2-N2 | F1,337 = 40.97 | p < 0.0001* | 0.90 |
| C | |||
| 2/s (>0) | |||
| P1-N1 | F1,259 = 8.63 | p = 0.004* | 0.46 |
| N1-P2 | F1,189 = 21.42 | p < 0.0001* | 1.10 |
| P2-N2 | F1,174 = 13.40 | p =0 .003* | 0.94 |
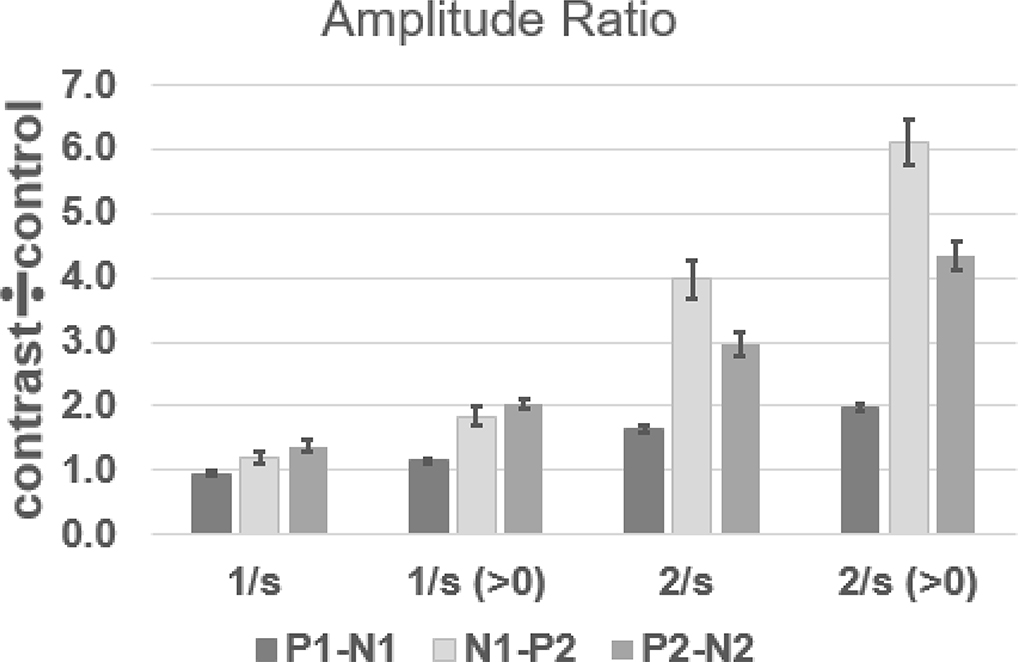
Another way to consider the difference between the responses to the control (no vowel token change) and contrast (token change) conditions is to calculate an amplitude ratio, using the control condition amplitude as the denominator and the contrast condition as the numerator (Cheek & Cone, 2020). This maneuver normalizes for amplitude differences that occur between subjects. If a response component was missing for the control condition, its amplitude value was changed to 1.0 from 0 μV. The mean amplitude for each vowel as measured in the control condition was used as the denominator to calculate the amplitude ratio. The mean amplitude ratios for the 1/s condition are shown in Figure 6. The largest amplitude ratios were found for P2-N2, and this is consistent with the findings from the analysis of peak-to-trough amplitudes that demonstrated a statistically significant difference in amplitude between the control and contrast conditions. Because the P2-N2 control vs. contrast amplitude difference was statistically significant, and resulted in the largest amplitude ratios, the P2-N2 amplitude ratios were also evaluated for any differences as a function of vowel contrast. For this analysis, those with absent responses in the test condition were eliminated (87 out of 260 cases). The vowel pairs in which the /a/-token was first were larger than those for other leading vowel tokens. T-tests with an alpha level of p < .05, were used to determine if there was “directionality” for the vowel change. Amplitude ratios were larger for conditions in which /a/ was the standard (e.g., /a/-/o/) compared to cases in which /a/ was deviant (e.g., /o/-/a/). These comparisons and the non-significant differences for other vowel-pairs are shown in Table 2.
Figure 6:
Mean ACC amplitude ratios as a function of rate. When means are calculated with values >0 μV for the contrast condition, the mean amplitude ratios increase for each rate. Mean amplitude ratios at 2/s are greater than amplitude ratios at 1/s for all calculations. Error bars indicate ± 1 standard error.
Table 2:
Summary of t-tests for directionality of vowel contrasts as evidenced by amplitude ratio differences. Statistical results considered significant are indicated by an asterisk.
| Directionality of Contrast | ||||
|---|---|---|---|---|
| t | p | |||
| 1/s | ||||
| P2-N2 | /a-i/ vs. /i-a/ | t49 = 2.28 | p = 0.03* | |
| /a-o/ vs. /o-a/ | t33 = 4.19 | p = 0.0002* | ||
| /a-u/ vs. /u-a/ | t25 = 3.93 | p = 0.0006* | ||
| /i-o/ vs. /o-i/ | t25 = -0.06 | p = 0.95 | ||
| /i-u/ vs. /u-i/ | t18 = -0.68 | p = 0.50 | ||
| /o-u/ vs. /u-o/ | t11 = -0.88 | p = 0.40 | ||
| 2/s | ||||
| P1-N1 | /a-i/ vs. /i-a/ | t60 = 1.72 | p = 0.09 | |
| /a-o/ vs. /o-a/ | t70 = 1.01 | p = 0.32 | ||
| /a-u/ vs. /u-a/ | t60 = 1.17 | p = 0.025* | ||
| /i-o/ vs. /o-i/ | t31 =1.85 | p = 0.07 | ||
| /i-u/ vs. /u-i/ | t26 = 2.20 | p = 0.04* | ||
| /o-u/ vs. /u-o/ | t21 =1.16 | p = 0.26 | ||
| N1-P2 | /a-i/ vs. /i-a/ | t49 = 3.30 | p = 0.002* | |
| /a-o/ vs. /o-a/ | t51 = 2.96 | p = 0.005* | ||
| /a-u/ vs. /u-a/ | t51 = 3.97 | p = 0.0002* | ||
| /i-o/ vs. /o-i/ | t28 =1.48 | p = 0.15 | ||
| /i-u/ vs. /u-i/ | t22 = 2.59 | p = 0.02* | ||
| /o-u/ vs. /u-o/ | t19 =1.86 | p = 0.08 | ||
| P2-N2 | /a-i/ vs. /i-a/ | t44 = 1.88 | p = 0.07 | |
| /a-o/ vs. /o-a/ | t40 = 1.95 | p = 0.06 | ||
| /a-u/ vs. /u-a/ | t39 = 4.55 | p < 0.0000* | ||
| /i-o/ vs. /o-i/ | t329 =0.44 | p = 0.67 | ||
| /i-u/ vs. /u-i/ | t22 =2.00 | p = 0.06 | ||
| /o-u/ vs. /u-o/ | t19 =1.85 | p = 0.08 | ||
Latency
Latencies for ACC components P1, N1, P2, and N2 were evaluated for the contrast conditions. The mean latencies and standard deviations as a function of the leading vowel of the contrast pair are shown in Table 3. Analyses of variance indicate that latency differences as a function of a leading vowel are significant only for P2 and N2 (Table 4a). Contrasts with the leading /a/ in the vowel pair have longer latencies than those with a leading /i/ or leading /u/ in the vowel pair (Table 4b). No other conditions demonstrated latency differences as a function of a leading vowel.
Table 3:
Summary of mean latencies and standard deviations for ACC components shown by vowel token in the leading position. N=number of peaks` contributing to the calculation of the mean
| Summary of mean, standard deviation of ACC component latencies | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1/s | ||||||||
| /a/ | N | /i/ | N | /o/ | N | /u/ | N | |
| P1 | 160, 27 | 104 | 163, 25 | 48 | 160, 35 | 35 | 162, 26 | 19 |
| N1 | 243, 41 | 109 | 232, 34 | 50 | 234, 44 | 36 | 229, 31 | 20 |
| P2 | 326, 34 | 78 | 301, 37 | 50 | 315, 34 | 34 | 292, 32 | 17 |
| N2 | 408, 38 | 73 | 388, 40 | 50 | 395, 44 | 34 | 370, 48 | 18 |
| 2/s | ||||||||
| /a/ | N | /i/ | N | /o/ | N | /o/ | N | |
| P1 | 160, 24 | 117 | 152, 28 | 47 | 147, 30 | 24 | 147, 28 | 26 |
| N1 | 234, 31 | 117 | 234, 31 | 48 | 226, 31 | 25 | 228, 28 | 26 |
| P2 | 324, 37 | 80 | 322, 32 | 33 | 330, 28 | 20 | 322, 30 | 26 |
| N2 | 408, 42 | 72 | 416, 33 | 33 | 433, 32 | 20 | 409, 38 | 26 |
Table 4:
A. Summary of analyses of variance for ACC component latency differences as a function of the leading vowel; B: Summary of post-hoc (Bonferroni-Dunn) tests for ACC component latency differences as a function of leading vowel (1/s only); C. Summary of analyses of variance for ACC component latency differences as a function of stimulus rate. Statistical results considered significant are indicated by an asterisk.
|
A
Analyses of Variance for Effect of Vowel Token on ACC Component Latency | |||||
| F | p | F | p | ||
| 1/s | 2/s | ||||
| P1 | F3,144 = 1.20 | p = 0.31 | P1 | F3,138 = 1.4 | p = 0.25 |
| N1 | F3,144 = 0.37 | p = 0.54 | N1 | F3,138 = 0.120 | p = 0.95 |
| P2 | F3,144 = 8.13 | p <0.000* | P2 | F3,138 = 0.47 | p = 0.72 |
| N2 | F3,144 = 5.09 | p = 0.002* | N2 | F3,138 = 1.92 | p = 0.13 |
|
B
Bonferroni-Dunn Post-hoc tests for analyses of variance | |||||
| Mean Diff | Crit. Diff | p | |||
| P2 | /a/, /i/ | 25.42 | 17.45 | 0.0001* | |
| /a/, /u/ | 37.85 | 25.12 | 0.0001* | ||
| N2 | /a/, /i/ | 24.74 | 21.78 | 0.003* | |
| /a/, /u/ | 38.02 | 31.35 | 0.002* | ||
|
C
Analyses of variance for ACC component latency | |||||
| 1/s vs. 2/sec | |||||
| F | p | Cohen’s d | |||
| P1 | F1,288 = 4.48 | = 0.035* | 0.025 | ||
| N1 | F1,288 = 0.51 | = 0.47 | n.a. | ||
| P2 | F1,288 = 7.99 | = 0.005* | 0.33 | ||
| N2 | F1,288 = 11.13 | = 0.001* | 0.39 | ||
Results for 2/s
Presence of CAEP components
When contrasts were presented at 2/s, the distribution of response components differed from those at 1/s rate (Fig 4b). The most striking differences were that responses to the standard vowel token and the control deviant (no vowel change) are frequently absent. However, at least one ACC component was present for 90% of the 12 vowel contrast types. Across all contrasts, the P1-N1 the most frequent component present, at 83%. The N1-P2 and P2-N2 components were less frequently evident at the 2/s rate, at 68 and 71.5%.
Amplitude
The mean ACC component amplitudes, P1-N1, N1-P2, and P2-N2, are shown in Figure 5b. (Missing components were assigned a value 0 μV.) There were statistically significant differences in component amplitude as a function of test condition (control vs. contrast) for all components, with contrast amplitudes larger than control amplitudes (Table 1b). Because responses were so frequently absent for the control condition (see Fig 4), we also evaluated the control vs. contrast condition using only amplitude values > 0. These analyses also revealed statistically significant differences components N1-P2 and P2-N2 for control vs. contrast conditions (Table 1c). The higher mean amplitudes in the contrast condition are consistent with the prevalence of ACCs in the group data.
Amplitude ratios were also calculated in the same manner as for the 1/s rate conditions. The mean amplitude ratios are shown in Figure 6. The amplitude ratios were largest for the N1-P2 component, and indicate that the mean amplitudes for the contrasts were nearly four times larger than the amplitudes for the control tokens. T-tests were used to test the hypotheses of directionality in the amplitude of the ACC (Table 2). For N1-P2, the /a/-contrast conditions had larger amplitude ratios compared to the contrast-/a/ condition, e.g.,the /a/-/i/ amplitude ratio 5.16 whereas the /i/-/a/ ratio was 2.71. The /i/-/u/ vs./u/-/i/ contrasts also exhibited some directionality for the P1-N1, and N1-P2 components. Considering the spectra of the vowel tokens (Fig 1), /a/ had considerably less low frequency energy than the other tokens, so that all contrast pairs in which /a/ was the standard would result in a greater intensity for the contrast token and thus increase the ACC amplitude (Martin & Boothroyd, 2000).
Latency
There were no differences in ACC component latencies as a function of vowel-contrast, as evaluated using analysis of variance (Table 4a). Each ACC component’s latencies are summarized as a function of the leading vowel in the contrast pair (Table 3). The effect of rate on latency for each component was also evaluated using univariate analysis of variance. The rate effects are significant only for ACC components P1, P2 and N2 (Table 4c), but the direction of latency difference was not consistent across components. ACC component latencies are shorter for P1 at 2/s than 1/s, on average, for but longer for P2 and N2 (Table 3).
Sensitivity of the Amplitude Ratio Metric
It may be assumed that when the response amplitude to a contrast exceeds that to a control token, this difference indexes a discriminative response at the level of the auditory cortex. Thus, the amplitude ratio that is calculated by dividing the response amplitude to contrast by that of the control may be a metric for vowel discrimination. Amplitude ratios that exceed 1.0 could be considered a “hit” for discrimination of vowel differences. Recall that for 1/s, only the P2-N2 component was found to have statistically significant differences between contrast and control trials. 85% of the amplitude ratios for the P2-N2 component, when present, met the criterion of having an amplitude ratio of ≥1.0. However, when an amplitude ratio of ≥1.5 is the criterion, 70.0% of responses at 1/s meet this criterion, and only 59% of amplitude ratios are greater than 2.0 at this rate. The same criteria applied to the P2-N2 amplitude ratio distribution for the 2/s rate yields “hit” or sensitivity rates of 99.5%, 95%, and 87% for the 1.0, 1.5, and 2.0 criteria, respectively. Because P1-N1 and N1-P2 components also had significantly larger amplitudes for the contrast compared to the control conditions at 2/s, it is possible to determine sensitivity rates for their amplitude ratios as well. These were 90%, 72%, and 51% using criteria of 1.0, 1.5, and 2.0 for P1-N1 amplitude ratios, respectively, and 98%, 92%, and 89% for N1-P2 amplitude ratios for the same criteria.
Perceptual Detection of Vowel Contrasts
Across all vowel contrasts and all infants, there was, on average, a 71% hit-rate for detecting a vowel change, and a 21% false alarm rate. The distribution of hit rates obtained for all vowel contrast tests (N = 237) reveals that the hit rate was less than 50% for 24% of the tests.
Eilers et al. (1977) used the dual criteria of hit and correct rejection rates of >66% to determine the validity of their VRISD method. That is, an infant had to meet both the hit and correct rejection rate of 66% for a response to be considered valid. Only 125 out of 237 (53%) tests in our data set met these criteria, and for those trials, the average hit rate was 84%, and the average correct rejection rate was 86%. 72% of the sample met the hit rate criterion alone, and 82% met the correct rejection criterion alone. In their study employing VRISD, Uhler et al. (2015) used a criterion of P(c) = 0.75, where P(c) = (hit rate +correct rejection rate) ÷ 2. In the current data set, 57% (137/237) of the tests met this criterion.
Hit and false alarm rates were calculated as a function of age using three age groups: 4-to-6 months (mean = 5.8 months, n = 78), 7-to-9 months (mean = 8.1 months, n = 115) and 10–12 months (mean = 10.8 months, n = 44). The mean hit rates were 74%, 71% and 67% and the false alarm rates were 24%, 21% and 16% for the three age groups, respectively. It should be noted that these infant performance data were available for only those who met the training criteria for the observer-based psychophysical VRISD test.
Differences in hit and false alarm rate as a function of age were evaluated using a linear mixed model to predict these rates. There were fixed effects for age group, and because an infant could undergo repeated testing, a fixed effect was also assigned to whether the data were obtained from a first, second or third test session. There were no interactive effects for these fixed effects. The results of these analyses show a significant effect for age group on hit rate with a significant decrease in hit rate in the 10–12 month group compared to the 4–6 month age group. There was also an effect for test number, with a significant increase in hit rate for a “third” test session compared to a “first” test session. The mean hit rate as a function of test number was 71% for a first and second test, and 74% for a third test.
The false alarm rates were analyzed in the same way as the hit rates. False alarm rates decreased with age, and these decrements were statistically significant. False alarm rates for a “second” test were 23%, higher than for a first (21%) or third test (15%), and this difference for a second test was statistically significant. Summaries of the Linear Mixed Model analyses are found in Table 5.
Table 5:
Summary of Hit and False Alarm Rates as a function of age and test session.
| Age Group and Test Number | Estimate | SE | CI | p | |||
|---|---|---|---|---|---|---|---|
| N subjects | N Tests | Hit Rate | |||||
| 4–6 months, first | 20 | 78 | Intercept* | 0.73 | 0.04 | 0.65 – 0.8 | <0.001 |
| 7–9 months, first | 8 | 30 | age group 7–9 mo | −0.11 | 0.07 | − 0.24 – 0.03 | 0.112 |
| 7–9 months, second | 18 | 84 | age group 10–12 mo | −0.25 | 0.09 | −0.43 – − .07 | 0.007* |
| 7–9 months, third | 1 | 1 | Test number, 2nd | 0.11 | 0.06 | −0.02 – 0.24 | 0.10 |
| 10–12 months, first | 1 | 1 | Test number, 3rd | 0.21 | 0.1 | 0.01 – 0.42 | 0.037* |
| 10–12 months, second | 8 | 18 | |||||
| 10–12 months, third | 7 | 25 | |||||
| Hit Rate, % | FA Rate, % | False Alarm Rate | |||||
| 4–6 months, first | 74 | 24 | Intercept* | 0.24 | 0.02 | 0.19 – 0.29 | <0.001 |
| 7–9 months, first | 62 | 14 | age group 7–9 mo | −0.11 | 0.04 | − 0.20 – −0.02 | 0.022* |
| 7–9 months, second | 74 | 24 | age group 10–12 mo | −0.16 | 0.07 | − 0.29 – −0.02 | 0.021* |
| 7–9 months, third | 75 | 0 | Test number, 2nd | 0.10 | 0.04 | 0.01 – 0.19 | 0.024* |
| 10–12 months, first | 57 | 0 | Test number, 3rd | 0.07 | 0.07 | − 0.08 – 0.21 | 0.368 |
| 10–12 months, second | 58 | 18 | |||||
| 10–12 months, third | 74 | 16 | |||||
Part A summarizes the number of subjects and number of tests completed in each age group and the hit and false alarm rates for each grouping. Part B summarizes the results of the linear mixed model analyses of hit and false alarm rates as a function of age and test session. The regression model used age group and test session as fixed effects, with age group = 4–6 months, test session = first, and hit or false alarm rate = 0, with subject identifier as a random effect. S.E. = stanard error, CI = confidence interval. Significant effects are indicated with an asterisk.
It is not possible to directly compare the vowel-contrast perception results to the ACC test results, given only 52% of the VRISD tests met the Eilers et al. (1977) or Uhler et al. (2015) response validity criteria. Many infants who could not meet the training nor the validity criteria for the perceptual test had ACC tests (at 2/s) yielding amplitude ratios of greater than 1.5 for which hit rates were 72%, 93%, and 93% for P1-N1, N1-P2, and P2-N2, respectively. There was a 2-to-1 ratio of ACC tests (for the 2/s rate) meeting the 1.0 amplitude ratio criteria, compared to the number of VRISD tests that met Eilers and colleagues’ (1977) validity criteria. Furthermore, there were 515 ACC vowel contrast tests for which data could be obtained, compared to only 237 tests from which VRISD data could be obtained.
Discussion
Auditory evoked potentials, particularly the ABR, and to a lesser extent, the ASSR, have ubiquitous use in pediatric audiology, specifically for estimating hearing thresholds in infants who are too young to give adequate behavioral responses. Pediatric audiology practice guidelines mandate the use of electrophysiologic tests to verify hearing integrity (JCIH 2019). Now, pediatric audiology working groups are calling for standardized assessment of speech discrimination measures as part of any habilitation/rehabilitation planning for infants and young children with hearing loss (Uhler et al., 2017).
There is substantial evidence that CAEPs provide useful information about how infants detect and discriminate sound. Evidence that CAEPs can provide an estimate of audibility in infants has come from studies of infants with normal hearing (Cone & Whitaker, 2013; Purdy et al., 2013) and in those with hearing loss (Ching et al., 2016; Gardner-Berry et al., 2016; Golding et al., 2007). The practice of testing for CAEPs in infants with hearing loss appears to facilitate treatment decisions (Punch, 2016; Mehta et al., 2017). Furthermore, CAEPs have been used to indicate the positive effects of amplification on neuroplasticity for infants with sensorineural and auditory neuropathy type hearing losses (Sharma & Cardon, 2015).
The use of CAEP to indicate discrimination of speech sounds in infants has most often relied upon the mismatch negativity (MMN) paradigm, in which an oddball stimulation paradigm is used, with the prevalence of the rare (oddball) stimulus being at 20% or less, and a stimulus offset asynchrony of 400–1000 ms. Although research employing the MMN has demonstrated that newborns have MMN evoked by contrasting speech sounds (Cheour et al., 2001; Gilley et al., 2017; Uhler et al., 2018), the magnitude and variability of the response do not make it suitable as a sensitive within-subject measure. The exact mechanisms of MMN and its development are also controversial, with some claiming early maturation and others questioning this tenet with empirical data (Morr et al., 2002).
In contrast, the ACC appears to have sufficient sensitivity as an index of discrimination at the level of the individual. It is sensitive to stimulus level differences as small as 3 dB and to vowel sound contrasts (Martin & Boothroyd, 2000). Recently, Kumar et al. (2020) have demonstrated the utility of ACC as an objective tool for evaluating intensity difference limens in participants with cochlear or auditory neuropathy type hearing losses. Small & Werker (2012) showed that ACC is present for some, but not all, consonant-vowel contrasts that were used to study a group of 4-month old infants. Cone (2015) used vowel contrasts /a/-/i/, /a/-/o/ and /a/-/u/ to evoke ACCs in infants less than a year of age. ACCs were present in over 89 percent of all trials utilizing a 2/s stimulus rate.
The results of the present study confirm and extend the findings of Cone (2015) to nine more vowel contrasts. As in the previous study, all contrasts were tested at two rates: 1/s and 2/s. The 1/s rate is similar to that used to evoke MMN, and the 2/s rate, to produce a quasi-steady-state stimulus to evoke ACCs. An innovation of this study is the use of an oddball paradigm to evoke an ACC using this quasi-steady-state stimulus. Martin et al. (2010) have shown that ACC can be obtained from stimulus paradigms using a 50% ratio of standard and contrast tokens, yet, this finding was in older children (mean age seven years). We reasoned that because the CAEP is so sensitive to stimulus rate (Gilley et al., 2005), it may be more advantageous to use a constant ratio of standard to contrast (75%:25%), to enhance the response to deviant at both rates. The prevalence of onset CAEPs for standards was >90% at the 1/s rate, but lower (~60%) for the 2/s rate. However, the prevalence of responses to oddball contrasts was much higher at the 2/s rate than the 1/s rate. At the 1/s rate, only the P2/N2 component showed a consistent response to the stimulus contrast, whereas, at 2/s, the stimulus contrast evoked P1-N1, N1-P2, and P2-N2 ACC response components.
The rate and or probability of the acoustic change tokens appears to have a profound effect on the ACC, as exemplified by the results of Uhler et al. (2018). Their vowel tokens had a duration of 0.5 s and were presented in a block: /i/-/a/-/i/, with a block offset-to-onset interval of 1.5 s. Cortical responses were present for each token in the block, but there were no differences in response amplitude for individual tokens that signaled the detection of acoustic change. In contrast, their mismatch response (MMR) paradigm utilized the same tokens, /i/ and /a/, but each token was presented with a stimulus onset asynchrony of 1.5 s, with a probability of a contrast being 15%. In this condition, MMRs indicating vowel acoustic differences were present.
Amplitude and Amplitude Ratio
The amplitude data provide additional information about how the infant auditory cortex responds to vowel contrasts. The amplitude and amplitude ratio data have some variability (Fig 5), owing primarily to the background myogenic noise that is present when testing babies who are awake, as well as the relatively low number of artifact free samples (N=50) that were used to define the control and contrast ACC tests. Although the focus of this study was on the ACC, some observations of the CAEP amplitudes in response to the standard tokens offer some insight into underlying mechanisms of rate on the ACC. CAEP amplitudes decrease with stimulus rate, owing to the effects of adaptation (Gilley et al., 2005, Wunderlich et al., 2006). In the present study, when CAEPs were present for standard tokens at 2/s, the CAEP component amplitudes were less than half the amplitude of the responses obtained at 1/s. Also, at both stimulus rates, responses to the standard token in the contrast condition were larger than those obtained in the control condition. This likely represents a release from adaptation (Cheek & Cone, 2020), as the effective stimulus rate for the standard token in the contrast condition is 0.75 Hz at 1/s, and 1.5 Hz at 2/s. The CAEP components in response to the contrast deviant tokens (Fig. 5) are of the same amplitude across both rates, with effective stimulus rates of 0.25 Hz and 0.5 Hz, respectively.
ACC amplitude normalization was achieved by dividing the contrast deviant response amplitude by the control deviant amplitude response amplitude. The advantage of this maneuver over a subtractive technique (i.e., subtracting the deviant response from the control response), as is common for MMN (e.g., Cone, 2015), is that it does not increase the amplitude of the background noise in the derived recording. The amplitude ratios obtained in the 2/s condition were 2–4 times greater than those obtained in the 1/s condition.
These differences in amplitude ratio as a function of stimulus rate raise the issue of mechanisms underlying the response to the contrast deviant. The MMN is highly dependent upon interstimulus interval and the probability of the deviant, with greater magnitude responses being found for interstimulus intervals that are less than 2 s and deviant probabilities that are 20% or less. The effective inter-stimulus interval (or stimulus offset asynchrony) was 500 ms for the 1/s rate. There is no consensus regarding the role that adaptation or habituation plays in the MMN. Some posit that it has a significant role, and others suggest that the response to deviant is something other than the cortex registering acoustic differences and may be associated with a “memory trace” being established for the standard stimulus and the deviant stimulus triggering brain mechanisms of novelty detection (Naantanen et al., 2005). One way to investigate these mechanisms would be to vary both inter-stimulus interval and stimulus rate systematically and determine the “trading ratios” for each parameter. A limitation of the present study is that only a limited number of averages (N =50) were obtained in response to the deviant token, which clearly may not be enough to resolve the small magnitude of the MMN for typical recording conditions. Nevertheless, we sought to determine the magnitude of the discriminative response provided by the ACC under conditions that might be applied in a clinical evaluation rather than in a laboratory study during which longer test sessions could provide a greater number of samples and include replicability data.
There is less controversy surrounding the mechanism of the ACC. The neural generators and the mechanisms of the ACC are thought to be the same for the P1-N1-P2 CAEP onset complex (Martin et al. 2008). Specifically, the neural generators for P1 are primary auditory cortex neurons of Heschl’s gyrus sensitive to stimulus onset; N1 generators are also in Heschl’s gyrus, although anterior to those of P1, whereas P2 generators involve parabelt regions (Picton, 2011). An ACC is evoked by the change in stimulus parameters from the preceding stimulus. The ACC amplitude is systematically related to the magnitude of the difference in acoustic parameters, just as the CAEP onset complex amplitude is tied to the acoustic features of stimuli. Because of these mechanisms, the ACC may have an advantage for documenting speech feature discrimination. The ACC is not dependent upon a “memory trace” but rather may document whether and how the acoustic differences in the stimuli are encoded by the ascending auditory nervous system and processed by the primary auditory cortex.
Latency
The stimulus variables of type (standard vs. deviant) and condition (control vs. contrast) did not produce any statistically significant latency differences for the ACC. No consistent latency differences were found as a function of vowel type, either, although previous research has shown that the infant P1-N1-P2 latency may be sensitive to spectral-temporal differences in speech sounds (Digeser et al., 2009; Kurtzberg et al., 1984). The stimulus rate had a significant effect on latency for ACC components (Table 3), with longer latencies for the 2/s rate. These longer latencies with increased rate may have been anticipated because of differences observed in older children when the rate is manipulated (Gilley et al., 2005). The developmental trajectory for latency has been established for the CAEP onset complex P1 (Ponton et al., 1996), and to a lesser extent, for N1 and P2 components (Wunderlich et al. 2006). Latencies for CAEP components mature into the later teen years (Ponton et al.), although rate dependencies for latency have not been established.
Directionality of Acoustic Change
Cortical neurons are sensitive to the rate and direction of acoustic change. ACCs also exhibit directional sensitivity, with ACCs being larger for a change from lower amplitude to a higher amplitude stimulus (Martin et al., 2000) or a frequency change from a higher frequency to lower frequency stimulus (Vonck et a.l, 2019, Pratt et al., 2009). Thus, we tested the hypothesis that ACCs to vowel changes would demonstrate directionality, with larger ACCs for vowel change from high- to low-frequency vowel contrasts, e.g., /i-u/) compared to the contrast in the opposite direction, /u-i/. Although there were some statistically significant differences in amplitude found between vowel pairs, we did not find any consistent patterns of directionality in the ACCs of infants in this study. McCarthy et al. (2019), however, had robust ACC directionality for vowel contrasts moving from higher to lower first formant frequencies in their developmental study of neural responses to vowels during the first year of life. McCarthy et al. used vowel tokens that were produced from natural speech. These tokens likely had greater spectral complexity (and salience) than the synthesized vowel tokens that we used. Also, their research protocol allowed for many more samples to be averaged for each response, reducing variability due to noise.
Behavioral Responses
The 71% hit rate and a 21% false alarm rate for the vowel discrimination task is typical of that found in laboratory-based studies of infant perceptual responses using visually-reinforced operant procedures (Casey & Small, 2014; Hulecki & Small, 2011; Parry et al., 2003). These lab-based studies of hearing thresholds for low-risk, typically developing infants have provided valuable age-specific norms for infant thresholds for tones, and also data that speak to the limitations of the method. For example, in Hulecki and Small (2011), 11/48 infants were excluded because they could not do the task. In Parry et al., only 19% of infants provided minimum response level data for all four stimuli (500, 1000, 2000, and 4000 Hz) in one test session, whereas 63% could only provide 1 or 2 minimum response levels.
Visually-reinforced operant methods have had widespread adoption in clinical audiology to determine sound (tone, noise, or speech token) detection abilities in infants (Widen et al., 1993, 2000, 2005). However, they have not been adopted for speech discrimination applications (Uhler & Gifford, 2014), despite its existence in research since the report of Eilers et al. (1977). In Eilers et al. seminal work, the research team demonstrated the feasibility of using conditioned, visually reinforced head-turn responses to evaluate speech feature discrimination development in infants aged 6–14 months of age.
Others have studied infant discrimination using similar methods (Nozza, 1987; Nozza et al., 1991). As in the lab-based threshold studies cited above, there appear to be 15–30% of typically developing infants who cannot meet the training criteria for a discrimination task. The difficulties seem to increase with age (Eisenberg et al., 2007). In the present study the hit rate decreased from 74% to 67%, but the false alarm rate improved from 24% to 16% in infants tested at 4–6 months of age compared to those tested at 10–12 months Using Uhler et al.’s (2015)5 performance calculation ( P(c) = (hit rate +correct rejection rate) ÷ 2) yields a P(c) of 75% and 75.5% at these two age points. Despite these limitations, a consensus report (Uhler et al., 2017) has now endorsed the use of VRISD in assessing infants who have hearing loss and in the fitting of hearing devices. Uhler et al. (2015) demonstrated the clinical feasibility of visually-reinforced infant speech discrimination (VRISD) test. While 20/21 normally hearing infants met the 75% correct criterion for detection of a vowel change (/a/-/i/), only 15/21 could meet this criterion for a /ba/ vs. /da/ (place-of-articulation) discrimination.
Behavioral vs. Electrophysiologic Indices of Discrimination
One of the stated purposes of Eilers et al. (1977) was that a VRISD procedure could be used to assess discrimination of speech features in individual infants. Applying the criteria of Eilers et al., only 53% of the behavioral tests met that criteria even after those infants had met training criteria. Furthermore, there were many more cases in which behavioral performance did not meet the Eilers et al. criterion. In contrast, the ACC amplitude ratios greater than 1.0 indicated a difference in neural activation between control and contrast conditions that could be a marker for discrimination.
This type of discrepancy also exists at the level of the brainstem, in which it is possible to observe adult-like ABR thresholds for clicks and tone-bursts in infants, whereas infant behavioral responses are elevated compared to those found in adults (Werner et al., 1993). Such discrepancies are also observed in infant CAEP thresholds for tones and speech sounds (Cone & Whitaker, 2013). The age at which there is an adult-like correspondence between ABR and behavioral thresholds, or CAEP and behavioral thresholds, has not yet been determined. It could be hypothesized that ABR or CAEP latencies reaching adult-like values may be a pre-requisite for having a consistent relationship between behavioral and electrophysiologic indices of perception. In clinical practice, however, ABR thresholds are often used as the basis for estimating the perceptual threshold for purposes of fitting amplification devices or determining that hearing acuity is within normal limits. Similarly, it may be possible to use an ACC index of discrimination, the amplitude ratio, to evaluate whether or not there is the neural capacity for differentiation of speech sounds.
Conclusions
The ACC for vowel contrasts presented at a rate of 2/s indexes vowel discrimination at the level of the cortex when obtained in typically developing infants under the age of 1 year. ACCs at 2/s were present in over 90% of infants tested. The amplitude ratio appears to be a sensitive metric for the difference between a control and contrast condition. Amplitude ratios of 1.5 will yield a hit rate of at least 90% for detection of the N1-P2 and P2-N2 ACC components for the 2/s oddball paradigm of this study. The 2/s ACC can be obtained in infants who do not yet exhibit valid behavioral responses for vowel change contrasts and may be useful for estimating neural capacity for discriminating these sounds. The ACC responses could, in turn, be used to fine-tune aids-to-hearing as clinicians would want to be able to determine if a modification to the hearing aid results in improved discrimination capacity. Tests of ACC in adults with hearing loss (Kumar et al., 2020) and in those who use amplification (Kirby & Brown, 2015) have provided evidence that this could be possible. Further investigation of ACC in infants and children with hearing loss is warranted, in particular focusing on the feasibility and effectiveness in clinical settings.
Acknowledgments
This research was supported in part by NIH-NIDCD K24 DC 008826, Electrophysiology of Infant Speech Perception, to BK Cone, principal investigator, and by K01DC017192, Investigating Relationships between Objective Measures of Binaural Hearing and Speech-in-Noise Performance in Middle-Aged Listeners, to Spencer Smith.
Footnotes
We have no known conflicts of interests to disclose
LITERATURE CITED
- Casey KA, Small SA (2014) Comparisons of auditory steady-state response and behavioral air conduction and bone conduction thresholds for infants and adults with normal hearing. Ear and Hearing 35(4): 423–439. [DOI] [PubMed] [Google Scholar]
- Cheek D, Cone B (2020) Evidence of vowel discrimination provided by the acoustic change complex. Ear and Hearing 41(4) 855–867. [DOI] [PubMed] [Google Scholar]
- Ching TY, Zhang VW, Hou S, Van Buynder P (2016) Cortical evoked potentials reveal changes in audibility with nonlinear frequency compression in hearing aids for children: clinical implications. Seminars in Hearing. 37(1):25–35, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M, Lepannen PH, Kraus N (2000) Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology 111 (1), 4–16. [DOI] [PubMed] [Google Scholar]
- Cone BK (2015) Infant cortical electrophysiology and perception of vowel contrasts. International Journal of Psychophysiology 95: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone B (2008) The electrophysiology of auditory neuropathy spectrum disorder. In Northern J (Ed). Guidelines for Identification and Management of Infants and Young Children with Auditory Neuropathy Spectrum Disorder. Denver, Colorado: The Bill Daniel’s Center for Children’s Hearing. [Google Scholar]
- Cone B, and Whitaker R (2013) Dynamics of Infant Cortical Auditory Evoked Responses for Tones and Speech. International Journal of Pediatric Otorhinolaryngology 77 (7) 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone B and Garinis (2009) Infant ASSR and speech feature discrimination. Journal of the American Academy of Audiology, 20 (10) 629–643. [DOI] [PubMed] [Google Scholar]
- Digeser FM, Wohlberedt T, Hoppe U (2009) Contribution of spectrotemporal features on auditory event-relate potentials elicited by consonant-vowel syllables. Ear and Hearing 30 (6) 704–712. [DOI] [PubMed] [Google Scholar]
- Eilers RE, Wilson WR, Moore JM (1977) Developmental changes in speech discrimination in infants. Journal of Speech and Hearing Research 20, 766–779. [DOI] [PubMed] [Google Scholar]
- Eimas PD. Siqueland ER. Jusczyk P. Vigorito J. (1971) Speech perception in infants. Science. 171(968):303–6. [DOI] [PubMed] [Google Scholar]
- Eisenberg LS, Martinez AS & Boothroyd A (2004) Perception of phonetic contrasts in infants: Development of the VRASPAC. In Miyamoto RT (Ed.) Cochlear Implants. International Congress Series 1273 (pp. 364–367). Amsterdam: Elsevier. [Google Scholar]
- Eisenberg LS, Martinez AS & Boothroyd A (2007) Assessing auditory capabilities in young children. International Journal of Pediatric Otorhinolaryngology 71, 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott LL, Katz D (1980). Development of a New Children’s Test of Speech Discrimination (Technical Manual). Auditec, St. Louis. [Google Scholar]
- Gardner-Berry K, Chang H, Ching TY, Hou S (2016) Detection rates of cortical auditory evoked potentials at different sensation levels in infants with sensory/neural hearing loss and auditory neuropathy spectrum disorder. Seminars in Hearing 37(1), 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Martin K (2005) Developmental changes in refractoriness of the cortical auditory evoke potential. Clin. Neurophysiol. 116, 648–657. [DOI] [PubMed] [Google Scholar]
- Golding M, Pearce W, Seymour Cooper J, King A, Ching T, Dillon H (2007) The relationship between obligatory cortical auditory evoked potentials (CAEPs) and functional measures in young infants.Journal of the American Academy of Audiology. 18(2):117–25. [DOI] [PubMed] [Google Scholar]
- He S, Grose JH, Buchman CA (2012) Auditory discrimination: the relataionship between psychophysical and electrophysiologic measures. International Journal of Audiology 51, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S; Grose JH; Teagle HF; Woodard J; Park LR; Hatch DR; Roush P; Buchman CA. (2015) Acoustically evoked auditory change complex in children with auditory neuropathy spectrum disorder: a potential objective tool for identifying cochlear implant candidates. Ear & Hearing. 36(3):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulecki LR, Small SA (2011) Behavioral bone-conduction thresholds for infants with normal hearing. Journal of the American Academy of Audiology 22(2): 81–92. [DOI] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing (2019). Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. Journal of Early Hearing Detection and Intervention, 4(2), 1–44. [PubMed] [Google Scholar]
- Jusczyk PW, Houston D, Goodman M. (1998). Speech perception during the first year. In Slater A (Ed). Perceptual Development: visual auditory and speech perception in infancy. Psychology Press, East Sussex, U.K. pp.357–388. [Google Scholar]
- Kirby BJ, Brown CJ (2015) Effects of nonlinear frequency compression on ACC amplitude and listener performance. Ear and Hearing 36 (5), 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK (2004) Early language acquisition: Cracking the speech code. Nature Rev Neurosci 5, 831–843. [DOI] [PubMed] [Google Scholar]
- Kumar P, Sanju HK, Huusain RO, Ganapathy MK, Sing NK (2020) American Journal of Audiology, 1–9, 10.1044/2020_AJA-19-00084 [DOI] [Google Scholar]
- Kurtzberg D,Hilpert PL, Kreuzer JA (1984) Differential maturation of cortical auditory evoke potentials to speech sounds in normal full-term and very-low-birthweight infants. Developmental Medicine and children Neurology 26, 466–475. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A (1999) Cortical auditory event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear and Hearing 20 (1), 33–44. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A (2000) Cortical auditory evoked potentials in response to changes of spectrum and amplitude. J. Acoust. Soc. Amer, 107 (4), 278–287. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A, Ali D, Leach-Berth T (2010) Stimulus presentation strategies for eliciting the acoustic change complex: increasing efficiency. Ear and Hearing 31(3), 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BA, Tremblay KL, Korczak P(2008) Speech evoked potentials: from the laboratory to the clinic. Ear and Hearing 29(3) 285–313. [DOI] [PubMed] [Google Scholar]
- Martinez AS, Eisenberg LS, Boothroyd A (2013) The acoustic change complex in young children with hearing loss: a preliminary study. Seminars in Hearing 34(4) 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Skoruppa K, Iverson P (2019) Development of neural perceptual vowel spaces during the first yeara of life. Scientific Reports 9: 19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K, Mahon M, Van Dun B, Marriage J, Vickers D (2020) International Journal of Audiology 59(2), 81–89. [DOI] [PubMed] [Google Scholar]
- Mehta K, Watkin P, Baldwin M, Marriage J, Mahon M, Vickers D (2017) Role of cortical auditory evoked potentials in reducing the age at hearing aid fitting in children with hearing loss identified by newborn hearing screening. Trends in Hearing 21, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller MP, Stelmachowicz PG, Hoover B et al. (2007) Vocalizations of infants with hearing loss compared to infants with normal hearing. Part I—Phonetic development. Ear and Hearing 28, 605–627. [DOI] [PubMed] [Google Scholar]
- Moog J & Geers A (1990) Early Speech Perception Test. St. Louis, MO: Central Institute of the Deaf. [Google Scholar]
- Morr ML, Schafer VL, Kreuzer JA, Kurtzberg D (2002) Maturation of mismatch negativity in typically developing infants and pre-school children. Ear and Hearing 23 (2) 118–136. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Jacobsen T, Winkler I. (2005) Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology (42): 25–32. [DOI] [PubMed] [Google Scholar]
- Nozza RJ (1987) Infant speech-sound discrimination testing: effects of stimulus intensity and procedural model on measures of performance. Journal of the Acoustical Society of America 81 (6): 1928–1939. [DOI] [PubMed] [Google Scholar]
- Nozza RJ, Rossman RN, Bond LC (1991) Infant-adult differences in unmasked thresholds for the discrimination of consonant-vowel syllable pairs. Audiology 30(2) 102–112. [DOI] [PubMed] [Google Scholar]
- Parry G, Hacking C, Bamford J, Day J (2003) Minimal response levels for visual reinforcement audiometry in infants. International Journal of Audiology 42(7), 413–417. [DOI] [PubMed] [Google Scholar]
- Pediatric Amplification Clinical Practice Guidelines (2013) The American Academy of Audiology. https://www.audiology.org/publications-resources/document-library/pediatric-rehabilitation-hearing-aids.
- Picton TW (2011) Human Auditory Evoked Potentials. Plural Publishing, San Diego. [Google Scholar]
- Ponton CW, on M, Eggermont JJ, Waring MD, Masuda A (1996) Maturation of human cortical auditory function: differences between normal-hearing children and children with cochlear implants. Ear and Hearing 17(5) 430–437. [DOI] [PubMed] [Google Scholar]
- Pratt H, Starr A, Michaelewski HJ, Dimitrijevic A, Bleich N, Mittleman N (2009) Auditory-evoke potentials to frequency increase and decrease of high- and low frequency tones. Clinical Neurophysiology, 120, 360–373. [DOI] [PubMed] [Google Scholar]
- Punch S, Van Dun B, King A, Carter L, Pearce W (2016) Clinical experience of using cortical auditory evoked potentials in the treatment of infant hearing loss in Australia. Seminars in Hearing 37:36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy SC, Sharma M, Munro KJ, Morgan CL (2013) Stimulus level effects on speech-evoked obligatory cortical auditory evokekd potentials in infants with normal hearing. Clinical Neurophysiology 124(3), 474–480. [DOI] [PubMed] [Google Scholar]
- Rance G, Cone-Wesson B, Wunderlich J, Dowell RC (2002) Speech perception and cortical event related potentials in children with auditory neuropathy. Ear and Hearing 23 (3) 239–253. [DOI] [PubMed] [Google Scholar]
- Sharma A, Cardon G (2015) Cortical development and neuroplasticity in auditory neuropathy spectrum disorder. Hearing Research 330 (Pt. B), 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A Cardon G Henion K Roland P (2011) Cortical maturation and behavioral outcomes in children with auditory neuropathy spectrum disorder. International Journal of Audiology. 50(2):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, & Kral A (2005). The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res, 203, 134–143. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, & Spahr AJ (2002). A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear, 23, 532–539. [DOI] [PubMed] [Google Scholar]
- Small SA, Werker JF (2012) Does the ACC have potential as an index of early speech discrimination ability? A preliminary study in 4-month-old infants with normal heariang. Ear and Hearing 33 (6) e59–e69. [DOI] [PubMed] [Google Scholar]
- Story B (2011) TubeTalker: An airway modulation model of human sound production. Proceedings of the First Annual Workshop on Performative Speech and Singing Synthesis. (Edited by Fels Sidney and d’Allessandro Nicolas) Vancouver, BC, Canada, March 14–15. [Google Scholar]
- Tomblin et al. (2015) Language outcomes in young children with mild-to-severe hearing loss. Ear Hear 36, Suppl 1, 76S–91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler K & Gifford RH (2014) Current trends in pediatric cochlear implant candidate selection and postoperative follow-up. American Journal of Audiology 23, 30–325. [DOI] [PubMed] [Google Scholar]
- Uhler KM, Hunger SK, Tierney E, Gilley PM (2018). The relationship between mismatch response and the acoustic change complex in normal hearing infanats. Clinical Neurophysiology 129, 1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler K, Warner-Czyz A, Gifford R, PMSTB Working Group (2017) Pediatric minimum speech test battery. Journal of the American Academy of Audiology 28, 232–247. [DOI] [PubMed] [Google Scholar]
- Van Dun B; Carter L; and Dillon H. (2012) Sensitivity of cortical auditory evoked potential detection for hearing-impaired infants in response to short speech sounds. Audiology research. 2(1):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonck BMD, Lammers MJW, van der Walls M, van Zanten GA, Versnel H (2019) Cortical auditory evoked potentials in response to frequency changes with varied magnitude, rate, and direction. Journal of the Association for Researach in Otolaryngology 20, 489–498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF & Tees (1984) Cross-language speech perception: Evidence for perceptual re-organization during the first year of life. Infant Behav. Dev (7), 49–63. [Google Scholar]
- Werker JF, Shi R, Desjardins R, Pegg JE, Polka L & Patterson M (1998) Three methods for testing infant speech perception. In Slater A (Ed) Perceptual Development: Visual, Auditory and Speech Perception in Infancy. Psychology Press Ltd, East Sussex. [Google Scholar]
- Werner LA, Folsom RC, Mancl LR (1993) The relationship between auditory brainstem response and behavioral thresholds in normal hearing infants and adults. Hearing Research 68, 131–141. [DOI] [PubMed] [Google Scholar]
- Widen JE (1993) Adding objectivity to infant behavioral audiometry. Ear and Hearing 14(1): 49–57. [DOI] [PubMed] [Google Scholar]
- Widen JE, Folsom RC, Cone-Wesson B, Carty L, Dunnell JJ, Koebsell K, Levi A, Mancl L, Ohlrich B, Trouba S, Gorga MP, Sininger YS, Vohr BR, Norton SJ. (2000) Identification of neonatal hearing impairment: hearing status at 8 to 12 months correcte age using a visual reinforcement audiometry protocol. Ear and Hearing 21(5); 471–487. [DOI] [PubMed] [Google Scholar]
- Widen JE, Johnson JL, White KR, Gravel JS, Vohr BR, Kennalley JM, Maxon AB, Spivak L, Sullivan-Mahoney M, Weirather Y, Meyer S. (2005) A multi-site study to examine the efficacy of the otoatcoustic emissions/automated auditory brainstem response newborn hearing screening protocol: results of visual reinforcement audiometry. American Journal of Audiology 14(2): S200–216. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK (2006) Maturation of CAEP in infants and children: a review. Hearing Research 212, 212–223. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK, Shepherd R (2006) Maturation of the cortical auditory evoked potential in infants and young children. Hearing Research 212 (1–2) 185–202. [DOI] [PubMed] [Google Scholar]