Abstract
Objectives
This study evaluated the 1-year treatment outcomes of bevacizumab for diabetic macular oedema (DMO) in routine clinical practice.
Methods
A retrospective analysis was performed on 298 eyes of 220 patients with DMO that received intra-vitreal bevacizumab between 1 September 2013 and 31 August 2018 that were tracked by a prospectively designed, web-based observational registry—the Fight Retinal Blindness! Registry.
Results
The mean visual acuity (95% confidence interval [CI]) at 1-year was 3 (2, 5) letters better than a mean (SD) of 68 (15) letters at study entry. Nearly a quarter of eyes achieved ≥20/40. Eyes presenting with better vision (≥20/40) tended to maintain that vision during the period of observation, whereas those presenting with worse vision (<20/40) gained a mean (95% CI) of 9 (5, 13) letters. A mean reduction in the macular thickness was observed over the study period with the central subfield improving by 29 µm (95% CI 17, 40) from a mean (SD) of 402 (109) µm at study entry. Eyes that completed 1 year of follow-up received a median (Q1, Q3) of 7 (4, 9) bevacizumab injections. Sixty-two eyes, ~20%, that started with bevacizumab changed to either another VEGF inhibitor or steroid (triamcinolone) during the period of observation. This did not lead to functional improvement for eyes changed to either ranibizumab or aflibercept despite a further reduction in macular thickness. An improvement in vision and reduction in macular thickness was noted in the 13 eyes that subsequently received triamcinolone. Approximately 10% of eyes dropped out over 12 months, even though their mean visual acuity had improved by seven letters from the initial visit.
Conclusions
Bevacizumab is an effective treatment for DMO in unselected populations.
Subject terms: Retinal diseases, Outcomes research
Introduction
The vascular endothelial growth factor (VEGF) inhibitor bevacizumab has been extensively used for the treatment of chorioretinal vascular conditions particularly in those jurisdictions where ranibizumab and aflibercept were not readily available or where their use was restricted [1–5]. The Diabetic Retinopathy Clinical Research (DRCR) Network Protocol T studied outcomes of treatment of bevacizumab, which was used off-label, ranibizumab and aflibercept for diabetic macular oedema (DMO) [6, 7]. Bevacizumab was similar to aflibercept and ranibizumab in improving vision at 2 years in eyes presenting with better visual acuity (VA), ≥69 letters (Snellen equivalent 20/40), while it was inferior to those on aflibercept in eyes with VA ≤68 letters (20/50) [7]. The Cochrane meta-analysis of twenty-four clinical trials of the three VEGF inhibitors for DMO found ‘high-certainty’ evidence that bevacizumab prevented visual loss and improved vision in eyes with DMO [8].
The use of bevacizumab, thus far the cheapest of the three VEGF inhibitors, for retinal diseases varies among countries according to drug regulatory processes, reimbursement policies and its availability. It was the most widely used VEGF inhibitor in the U.S from 2006 to 2015 [9]. Studies evaluating outcomes of bevacizumab for DMO in routine clinical practice provide data to assess whether these are consistent with the promising results of pivotal clinical trials and may identify why they do not. This study was designed to assess the 12-month functional and anatomical outcomes of bevacizumab for DMO in routine clinical practice.
Methods
Design, data sources and measurements
This was a retrospective analysis of data recorded in the prospectively designed web-based registry—The Fight Retinal Blindness! Registry. The registry adapted its age-related macular degeneration treatment outcomes module to collect data on outcomes of treatment of DMO [10]. This DMO module, first implemented in Australia, New Zealand and Switzerland in April 2015, has expanded to other countries in Asia and Europe. The present analysis included eyes from clinical practices in Australia and New Zealand where a sufficient number of eyes started bevacizumab for the treatment of DMO.
The data recorded at each clinical visit include the number of letters read on a logarithm of the minimum angle of resolution VA Chart, treatment given, the central subfield thickness (CST [µm]) measured using spectral domain optical coherence tomography (OCT), the activity of DMO (centre-involving, non-centre-involving or no DMO), procedures and ocular adverse events [11]. Duration and type of diabetes, grading of diabetic retinopathy (DR) and previous treatment for DMO were recorded at study entry. All treatment decisions, including choice of treatment and frequency of visits, were based on VA and OCT at the discretion of the practitioner in consultation with the patient, thereby reflecting real-world clinical practice.
Institutional approval was obtained from the Royal Australian and New Zealand College of Ophthalmologists Human Research Ethics Committee who approved the use of ‘opt-out’ patient consent. This study adhered to the tenets of the Declaration of Helsinki.
Patient selection
Inclusion criteria required a diagnosis of DMO and at least two injections of bevacizumab (1.25-mg Avastin; Genentech, Inc., CA, USA/Roche, Basel, Switzerland) between 1 September 2013 and 31 August 2018. Eyes that were followed for at least 12 months and underwent exclusive treatment with bevacizumab were termed ‘completers’. Some eyes that received the minimal number of treatments with bevacizumab, switched to either a steroid or another VEGF inhibitor during the 12 months of follow-up (‘Switchers’). Their outcomes were censored from the time of their last bevacizumab treatment. Data were also censored from the time of the last visit for eyes that did not complete 12 months of observation (‘non-completers’).
Outcomes
The primary outcome was the mean change in VA 12 months after starting treatment with bevacizumab. Secondary outcomes were mean change in CST, the number of treatments and number of visits, the proportion of eyes with VA ≥69 (20/40) letters and ≤35 letters (20/200) and the proportion of eyes that gained ≥10 letters and those that lost ≥10 letters at 12 months. In addition, these outcomes were analysed in eyes stratified by VA at study entry into two groups, ≥69 and ≤68 letters (20/50), to study the relationship between acuity measured at study entry and 12 months later. Other outcomes of interest were the proportion of switchers and those of non-completers.
Statistical analysis
Descriptive data included the mean (standard deviation), median (first and third quartiles) and percentages where appropriate. Eyes were considered to have been observed from the first treatment visit up to their 12-month (365 ± 30 days) visit. t-tests, Wilcoxon rank sum tests, Chi-square tests and Fisher’s exact tests were used as appropriate to compare study entry characteristics. Paired t-tests were used to determine whether a change in VA and CST from study entry were significant.
We used a generalized additive model to display VA and CST over 12 months. We compared the number of injections and visits in eyes stratified by initial VA using Quasi-Poisson regression models adjusted for age, VA, CST and DMO activity at study entry, and nesting of outcomes within practice with an offset for log days of follow-up. Kaplan–Meier survival analysis was used to plot survival curves for time to non-completion.
All analyses were conducted using R version 4.0.2 (http://www.R-project.org/) with the lme4 package (V1.1–21) for mixed-effects regression analysis, mgcv package (V1.8–31) for generalized additive (mixed) model computation and survival package (V 2.38) for switching and dropout analysis [12–14].
Results
Study participants
The electronic registry contained data from 298 eyes of 220 patients who had DMO and undergone two or more injections of bevacizumab in the interval between September 2013 and August 2018. Data from both eyes of 78 patients were included in the analysis. Table 1 summarizes the characteristics of these eyes at study entry. Approximately half of the eyes in this study had undergone treatment for DMO prior to the study entry. One hundred eyes (34%) had received macular laser, 30 eyes (10%) had a history of intra-vitreal injection of a VEGF inhibitor and another 10 eyes (3%) had received a steroid injection. Eyes that were treatment-naïve (n = 175) and those that had received prior treatment (n = 128) had similar characteristics at study entry (Table 1).
Table1.
Demographic characteristics.
| All eyes | Treatment-naïve eyes | Pre-treated eyes | p value | |
|---|---|---|---|---|
| Eyes, n | 298 | 175 | 123 | |
| Patients, na | 220b | 136 | 91 | |
| Female, na (%) | 100 (46) | 53 (39) | 48 (53) | |
| Right eye, n (%) | 153 (51) | 91 (52) | 62 (50) | |
| Age years, mean (SD) | 62 (11) | 61 (12) | 63 (9) | 0.10 |
| Diabetes duration years, mean (SD) | 16 (9) | 16 (10) | 15 (7) | 0.70 |
| Diabetes type % | ||||
| Type I | 10 | 12 | 6 | 0.10 |
| Type II | 90 | 88 | 94 | |
| Diabetic retinopathy % | ||||
| Mild | 17 | 20 | 12 | <0.01 |
| Moderate | 39 | 34 | 46 | |
| Severe NPDR | 20 | 17 | 24 | |
| PDR non-high risk | 23 | 28 | 16 | |
| PDR high risk | 1 | 1 | 2 | |
| Baseline VA letters, mean (SD) | 67.7 (14.7) | 67.8 (15.4) | 67.6 (13.8) | 0.94 |
| VA ≥ 69 letters % | 61 | 62 | 59 | 0.70 |
| VA ≤ 35 less % | 5 | 5 | 5 | 1 |
| CST µm, mean (SD) | 402 (109) | 401(110) | 403(108) | 0.92 |
| DMO grades % | ||||
| Centre-involving | 90 | 89 | 92 | |
| Non-centre-involving | 4 | 4 | 4 | 0.48 |
| No DMO | 6 | 7 | 4 | |
n number, SD standard deviation, NPDR non-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, VA visual acuity, CST central subfield thickness, DMO diabetic macular oedema.
aPatients with each eye in two groups are repeated.
bData from both eyes of 78 patients were included in the analysis.
Visual outcomes at 12 months
Figure 1 illustrates the mean VA over 12 months for all 298 eyes, including switchers and non-completers. The mean VA improved within first few months and was maintained over the 12 months. The mean (95% confidence interval) VA change at 12 months, using last observation carried forward for switchers and non-completers, was 3.1 (1.6, 4.9) letters (p = 0.004, Table 2). The proportion of eyes with VA ≥ 69 letters increased (71% versus 61% at study entry; p < 0.001) while those with VA ≤ 35 letters decreased (2% versus 5% at study entry; p < 0.01). Eyes that were treatment-naïve and those that had received prior treatment had similar mean (95% CI) VA change, 2.7 (0.8, 4.6) versus 3.6 (1.4, 5.9) letters for prior treatment group (p = 0.54).
Fig. 1. Line graph depicting mean visual acuity (solid red line) in logMAR letters (y-axis) and central subfield thickness (CST, red dashed line) in microns (z-axis) for all 298 eyes entered in the study.
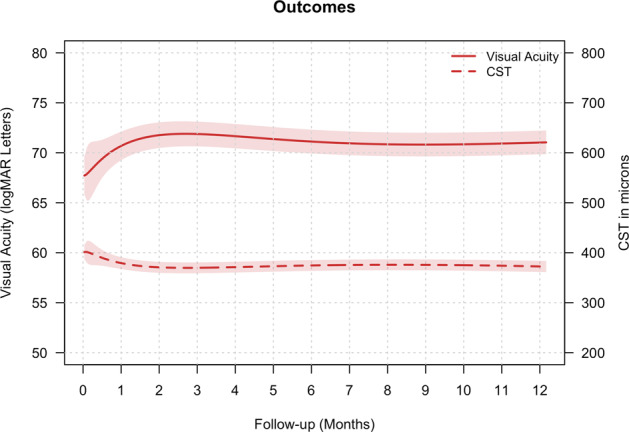
The red shaded area represents the 95% confidence interval.
Table 2.
Outcomes at 12 months.
| All eyesa | Completers | Switchers | Non-completers | ||
|---|---|---|---|---|---|
| VEGF inhibitors | Triamcinolone | ||||
| Eyes, n | 298 | 209 | 49 | 13 | 27 |
| Patients, nb | 220c | 158 | 37 | 10 | 22 |
| Baseline VA letters, mean (SD) | 67.7 (14.7) | 67.9 (14.5) | 68.7 (14) | 66.2 (8.9) | 65.3 (19.8) |
| Final VA letters, mean (SD) | 70.8 (11.2) | 71.1 (11.1) | 71 (11.2) | 62.9 (9.6) | 72.1 (11.2) |
| Change VA letters, mean (95% CI) | 3.1 (1.6, 4.6) | 3.2 (1.4, 4.9) | 2.4 (−1.1, 5.9) | −3.2 (−9.4, 3) | 6.9 (0.6, 13.1) |
| Gain ≥ 10 letters % | 21 | 22 | 14 | 8 | 30 |
| Loss ≥ 10 letters % | 10 | 9 | 12 | 23 | 4 |
| VA ≥ 69 letters %, baseline/final | 61/71 | 62/72 | 63/74 | 46/31 | 56/70 |
| VA ≤ 35 letters %, baseline/final | 5/2 | 4/2 | 6/4 | 0/0 | 11/4 |
| Baseline CST µm, mean (SD) | 402 (109) | 405 (106) | 384 (108) | 449 (158) | 385 (110) |
| Final CST µm, mean (SD) | 372 (105) | 369 (100) | 368 (99) | 493 (182) | 347 (78) |
| Change CST µm, mean (95% CI) | −29 (−40, −17) | −35 (−50, −21) | −16 (−37, 6) | 44 (−31, 120) | −33 (−69, 3) |
| Injectionsd, median (Q1, Q3) | 6 (4, 8) | 7 (4, 9) | 5 (4, 7) | 4 (3, 5) | 3 (2, 5) |
| Additional laser, n | 25 | 21 | 0 | 2 | 2 |
| Additional triamcinolone, n | 13 | 0 | 0 | 13 | 0 |
| Additional Ozurdex®, n | 0 | 0 | 0 | 0 | 0 |
| Visitsα, median (Q1, Q3) | 8 (5, 10) | 8 (6, 12) | 5 (5, 8) | 6 (5, 7) | 4 (3, 7) |
All eyes—includes completers, switchers and non-completers. ‘Completers’—eyes with 12 months of observation from the start of treatment, ‘switchers’—eyes receiving other treatment drug prior to completion of 12 months from the start of treatment. Only the observations from the visit before the switch occurred were included in the analysis. ‘Non-completers’—eyes not completing 12 months of observations from the start of treatment.
n number, VA visual acuity, SD standard deviation, CI confidence interval, CST central subfield thickness, Q1 first quantile, Q3 third quantile.
aLast observation carried forward for switchers and non-completers.
bPatients with each eye in two groups are repeated.
cData from both eyes of 78 patients were included in the analysis.
dNumber of bevacizumab injections.
Two-hundred and nine (70%) eyes completed 1 year of bevacizumab monotherapy. The mean (95% CI) VA change in these eyes was 3.2 (1.4, 4.9) letters (p = 0.01, Table 2). Eyes with good initial vision (VA ≥ 69 letters; 130 eyes [62%]) had a minimal mean (95% CI) VA loss of 0.2 letters (p = 0.78) at 12 months while those with initial vision ≤68 letters (79 eyes [38%]) had a greater gain of 8.7 letters (p < 0.001). However, the final vision in the initial VA ≤ 68 letters group, despite the higher gain, was lower than in the good initial vision group (Table 3).
Table 3.
Outcomes in completers stratified by visual acuity at presentation.
| Visual acuity ≥69 letters (20/40 or better) | Visual acuity ≤68 letters (20/50 or worse) | |
|---|---|---|
| Eyes, n | 130 | 79 |
| Patients, na | 110 | 63 |
| Baseline VA letters, mean (SD) | 75.8 (5.1) | 54.9 (15.5) |
| Final VA letters, mean (SD) | 75.6 (5.8) | 63.6 (13.5) |
| Change VA letters, mean (95% CI) | −0.2 (−1.3, 0.9) | 8.7 (4.7, 12.6) |
| Gain ≥ 10 letters % | 9 | 43 |
| Loss ≥ 10 letters % | 10 | 8 |
| VA ≥ 69 letters %, baseline/final | 100/89 | 0/44 |
| VA ≤ 35 letters %, baseline/final | 0/0 | 11/5 |
| Baseline CST µm, mean (SD) | 384 (83) | 441 (129) |
| Final CST µm, mean (SD) | 358 (86) | 386 (119) |
| Change CST µm, mean (95% CI) | −25 (−38, −13) | −52 (−84, −21) |
| Injectionsb, median (Q1, Q3) | 6 (4, 9) | 7 (4, 9) |
| Additional laser, n | 18 | 3 |
| Visits, median (Q1, Q3) | 8 (6, 12) | 9 (6, 10) |
‘Completers’—eyes with 12 months of observation from the start of treatment
n number, VA visual acuity, SD standard deviation, CI confidence interval, CST central subfield thickness, Q1 first quantile, Q3 third quantile.
aPatients with each eye in two groups are repeated.
bNumber of bevacizumab injections.
Macular thickness
Bevacizumab significantly reduced the mean CST in all eyes with DMO (Fig. 1). The mean (95% CI) change in CST at 12 months was −29 (−40, −17) µm from a mean of 402 (109) µm at study entry (p < 0.001). The mean [95% CI] change in CST in the previously treated group and those that were treatment-naïve was similar (−24 [−38, −9] versus −36 [−54, −18] µm, respectively, [p = 0.07]). Eyes in both VA groups had significant reduction in the mean CST after 12 months of treatment (Table 3).
Treatments and visits
The median (Q1, Q3) number of injections in eyes completing 1 year on bevacizumab monotherapy was 7 (4, 9) from a median (Q1, Q3) of 8 (6, 12) visits. Around one-tenth of these required additional treatment with macular laser during the 12 months (Table 2). The median number of bevacizumab injections in treatment-naïve and previously treated eyes were (6 [4,9]) versus (7 [5,10], p = 0.33). There was no difference in the median number of bevacizumab injections when eyes were stratified based on the initial VA; however, more eyes in the good initial vision group received additional macular laser (Table 3).
Treatment switch
Almost one-fifth (62 eyes) switched treatment, most often to the other VEGF inhibitors (49 eyes) although a few (13 eyes) switched to triamcinolone, before completing 12 months. The median (Q1, Q3) time to switch was 176 (128, 203) days and 167 (140, 267) days for those switching to the other VEGF inhibitors and steroid, respectively. Eyes that switched treatment had similar mean VA and CST at study entry compared to those that completed 12 months on bevacizumab treatment (Table 2). The mean change in VA and CST at the time of switch in eyes switching to other VEGF inhibitors was similar to those that completed 12 months on bevacizumab: 2.4 versus 3.2 letters (p = 0.69) and −16 versus −35 µm (p = 0.13) but they were different in eyes that switched to steroid, −3.2 versus 3.2 letters (p = 0.04) and 44 versus −35 µm (p = 0.04, Table 2).
Most of the participants who had treatment switches were receiving bevacizumab for DMO in clinical practices in Australia where reimbursement for ranibizumab for this indication started from July 2014 and for aflibercept from October 2015 [15, 16]. More eyes were switched to aflibercept than ranibizumab when both agents were available (Fig. 2A). The mean (95% CI) VA and CST change from the treatment switch to the 12-month visit in eyes that switched to other VEGF inhibitors were −0.8 (−3.7, 2) letters (p = 0.56) and −31 (−49, −14) µm (p < 0.001) after a median (Q1, Q3) of 3 (2, 5) injections while for those switching to steroid was 6 (1.2, 10.8) letters (p = 0.01) and −90 (−180, 0.1) µm (p = 0.05) after a median (Q1, Q3) of 1 (1, 1) injection. The mean (SD) VA and CST at the 12-month visit in eyes that switched to steroid was similar to eyes that completed 12 months on bevacizumab, 69.9 (14.3) versus 71.1 (11.1) letters (p = 0.56) and 346 (97) versus 369 (100) µm in completers (p = 0.11).
Fig. 2. Switchers and non-completers.
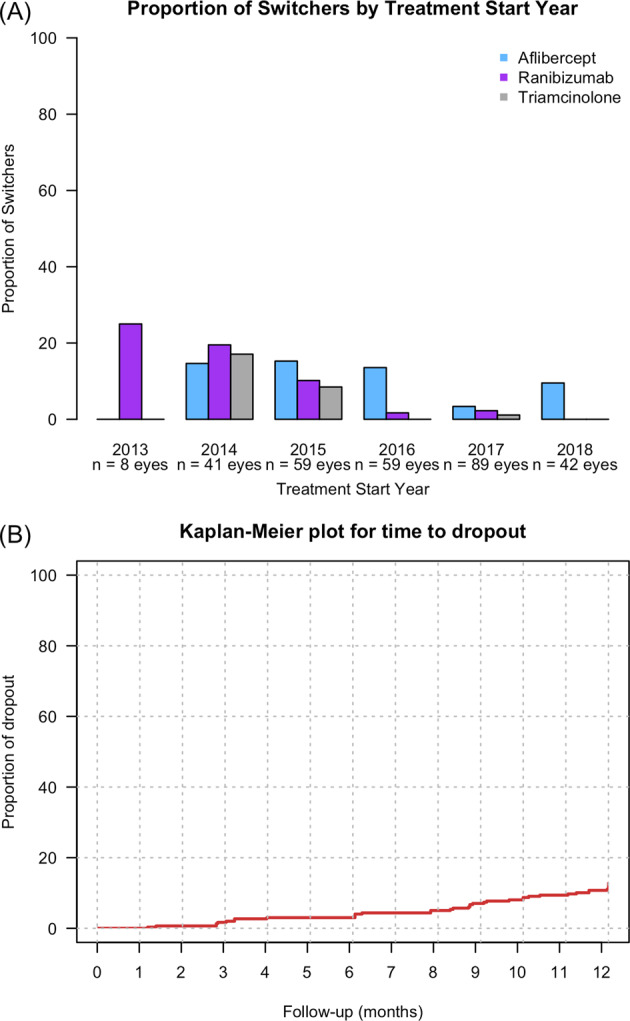
A Proportion of eyes that switched treatment from bevacizumab to ranibizumab (purple), aflibercept (blue) and triamcinolone (grey) stratified by the year of starting bevacizumab treatment (n = number of eyes starting bevacizumab treatment). B Kaplan–Meier plots for the time from starting treatment to dropout in eyes treated with bevacizumab over 12 months.
Non-completion rate at 12 months
Twenty-seven eyes (9%) dropped out before completing 12 months of observations (Fig. 2B). The median (Q1, Q3) time to dropout was 238 (98, 271) days. The mean (95% CI) VA change from the start of treatment to their last visit was 6.9 (−0.6, 13.1) letters while the CST reduced by a mean (95% CI) of 33 (−69, 3) µm. These eyes received a median (Q1, Q3) of 3 (2, 3) of bevacizumab injections from 3 (2, 5) visits.
Adverse events
A total of 1905 bevacizumab injections were administered in the 298 eyes over the 12 months. Endophthalmitis, the most serious adverse event associated with intraocular injections, was not observed in the study cohort.
Discussion
This study demonstrates the efficacy of bevacizumab as a treatment for DMO in routine clinical practice outside the constraints of clinical trials. We found with 12 months of prospectively collected data that eyes with good vision, VA ≥ 69 letters at study entry, tended to maintain it while those with VA ≤ 68 letters experienced a mean gain of nine letters. Eyes received a median of seven bevacizumab injections over the 12 months. Not all the eyes continued with bevacizumab treatment. Treatment switches, observed in one-fifth of eyes, were more often to VEGF inhibitors though steroid appeared to have been preferred in eyes that had worse vision and thicker maculae. Aflibercept was preferred over ranibizumab when both were available. Very few eyes (9%) dropped out before completion of 1 year of treatment. These eyes had gained a mean of seven letters from a median of three bevacizumab injections before they dropped out. These data indicate that bevacizumab is an effective treatment for DMO in real-world clinical practice at least for 12 months.
The visual and anatomical improvements in eyes with DMO treated with bevacizumab, which we observed, were also reported in a Cochrane meta-analysis and the DRCR.net Protocol T study [6, 8]. We found these improvements irrespective of whether the eyes had been previously treated for DMO or not. Mean visual gains were higher in eyes with initial VA ≤ 68 letters than in eyes with initial VA ≥ 69 letters, although eyes in each group received a median of seven bevacizumab injections. We believe that eyes with better presenting vision experienced a ceiling effect on the further gains they can make.
Several observational studies have found that the visual gains in eyes treated with VEGF inhibitors for DMO were inferior to those reported in the clinical trials [17–23]. Outcomes may be inferior in real-world practice because the participants are different to the population selected for the clinical trials. They often receive fewer treatments. The mean VA gain in the present study (+3.3 letters from a mean VA of 67.9 letters at study entry) after 1 year of treatment was lower than in the bevacizumab group in the DRCR.net Protocol T study (+9.7 from 64.8 letters) [6]. Eyes in the present study received fewer bevacizumab injections over 1 year than the DRCR.net Protocol T study cohort (median of seven versus ten injections). However, the mean VA at 1 year in the present study (71.2 letters) was similar to that of the bevacizumab group in the DRCR.net Protocol T study (74.2 letters) suggesting that the higher VA at study entry in the present study may account partly for the lower gains. Approximately 71% of eyes in the present study had VA ≥ 69 letters at 1 year, which was similar to those that received ranibizumab in the DRCR.net Protocol I Study [24].
Studies that have evaluated treatment outcomes of the VEGF inhibitors ranibizumab and aflibercept for DMO in routine clinical practice have reported a mean VA gain ranging from +3.3 to +9.9 letters after 1 year of treatment from a mean of 68–51 letters at study entry [17–23]. The mean VA at study entry of 67.9 letters in the present study was better than reported in those studies. The mean VA 1 year after the start of treatment, which is of the most important concern to the patient, in these studies ranged from 54 to 71 letters. The VA gain of +3.5 letters in the present study was one of the lower gains reported by observational studies of other VEGF inhibitors; however, the mean VA of 71 letters at 1 year was one of the better 12-month outcomes [17–23]. Our findings therefore provide good support that bevacizumab is beneficial for DMO at least when vision is good. Our sample does not provide good evidence that bevacizumab may achieve similar outcomes as the more expensive medications when starting vision is poor.
The DRCR.net study reported that eyes on bevacizumab treatment for DMO had the least reduction in the mean macular thickness at 1 year [6]. The mean reduction in macular thickness we observed, was much lower than in any of the groups in the DRCR.net Protocol T study. This was probably because our patients received fewer treatments than were given in the clinical trial. The drop in the macular thickness we observed was lower than those reported in other observational studies from a similar CST at baseline after similar median number of injections of ranibizumab or aflibercept [19–23]. This suggests that bevacizumab may be less effective than either aflibercept or ranibizumab in reducing macular thickness in eyes with DMO.
VEGF inhibitors may be switched in the hope of a better outcome. Eyes that switched treatment from bevacizumab to either ranibizumab or aflibercept in the present study had similar VA and CST when treatment started compared to those that completed 1 year on monotherapy. Nor were there differences in visual gain or reduction in macular thickness between completers at 1 year and at the time of treatment switch in switchers that switched to the other VEGF inhibitors. Eyes that switched treatment to the licensed VEGF inhibitors had similar 12-month VA outcomes as eyes that did not switch, although they had an additional reduction in the macular thickness after the switch, suggesting that variables other than the macular thickness affect vision in eyes with DMO [25]. The similarity of the mean VA of switchers to the completers and the fact that VA did not improve further after the treatment switch suggests that the switch may have occurred despite an initial visual improvement with bevacizumab, for example because another drug became reimbursed.
Macular oedema can persist is some DMO eyes despite treatment with VEGF inhibitors [26]. Clinicians appeared to prefer to switch treatment to steroid in eyes that had suboptimal response to VEGF inhibitors, since the eyes that switched to steroid had worse VA and thicker maculas than those that switched to the VEGF inhibitors. The mean VA and CST at 12 months in eyes that switched to steroid were similar to those that completed 12 months on bevacizumab monotherapy. This suggests that steroid is beneficial in eyes with DMO when the response to Avastin is suboptimal. We are unable to say whether switching to the other VEGF inhibitors in eyes with suboptimal response to bevacizumab is beneficial or not.
Observational studies on treatment outcomes may be biased by patients that switch treatment or dropout, which may be due to poor outcomes. Very few eyes (9%) in the present study dropped out before completing 12-month observation. These eyes had a higher VA gain and CST reduction from fewer treatments than those that completed 1 year on monotherapy suggesting that non-compliance to treatment could be related to reasons other than poor outcomes [27].
This study has a few limitations that are inherent in observational data. Treatment decisions in routine clinical practice, in contrast to those in the clinical trials, are made without reference from a reading centre or guided by study protocols. Case selection and treatment regimen may also differ among physicians and from clinical trials. The reasons for the choice of bevacizumab for DMO in the Australian cohort, where ranibizumab and aflibercept are registered for DMO, for treatment switch and those that failed to complete 12-month study period despite the visual gain cannot be deduced from the data presented. Nevertheless, this study reports the treatment outcomes of bevacizumab as it was used in our patients in routine clinical practice irrespective of selection criteria such as VA at presentation, glycaemic control and prior treatment with VEGF inhibitors, which would make them ineligible in the clinical trial. Our results are more applicable to the general population with the disease as opposed to the selective cohort in randomised trials that make up a minority of patients that were treated. There is evidence that a well-designed observational study, such as the present study, is unlikely to overestimate the therapeutic effectiveness of an agent [28].
To conclude, we found that bevacizumab treatment in eyes with DMO in routine clinical practice yielded visual outcomes that were similar to those reported in observational studies of ranibizumab and aflibercept. Its effect in reducing macular thickness was, however, inferior to those reported for ranibizumab and aflibercept. Observational studies evaluating long-term outcomes may determine if patients on bevacizumab continue to benefit with ongoing treatment. Factors such as cost and availability, in addition to the relative efficacy of the drugs, may be more helpful in determining treatment for DMO in routine clinical practice.
Summary
What was known before
Studies reported improved outcomes in eyes that received ranibizumab or aflibercept for DMO in routine clinical practice.
Eyes with DMO in routine clinical practice received fewer treatments than those in the clinical trials.
What this study adds
Treatment outcomes of eyes with DMO receiving bevacizumab, the cheapest and the most commonly used VEGF inhibitor, in routine clinical practice were good.
Eyes on bevacizumab for DMO that switched to the licensed VEGF inhibitors, despite an additional reduction in macular thickness after the treatment switch, had similar visual gains as those that completed bevacizumab monotherapy.
Bevacizumab is a reasonable option for the treatment of DMO when cost, convenience and availability pose an issue.
Acknowledgements
Fight Retinal Blindness! Investigators: Auckland District Health Board, New Zealand (DS); Armadale Eye Clinic, Victoria (Dr A Cohn); Associate Professor Fred Chen’s Clinic, Western Australia (Professor F Chen); Australian Eye Specialists (Bacchus Marsh), Victoria (Dr N Jaross); Australian Eye Specialists (Wyndham), Victoria (Dr N Jaross); Bunbury and Busselton Eye Doctors, Western Australia (RB); Canberra Hospital, Australian Capital Territory (Dr R Essex, JMW); Central Coast Eye Specialist, New South Wales (Dr S Young); Eye Associates, New South Wales (MG); Eye Wide Bay, Queensland (Dr Z Louw); Gladesville Eye Specialists, New South Wales (Dr S Young); Marsden Eye Specialists, New South Wales (Dr J Arnold, Dr D Chan, TT); North Queensland Retina, Queensland (Dr I Reddie); Retina Associates, New South Wales (Dr S Fraser-Bell); Retina Specialists, New Zealand (RB); Specialist Eye Group, Victoria (Dr A Cohn); Sydney Eye Hospital, New South Wales (Dr S Fraser-Bell); Victorian Eye Surgeons, Victoria (Dr A Cohn).
Author contributions
Conception and design—SB, DS, VN, DB, MG. Analysis and Interpretation—SB, DS, VN, MG. Data collection—SB, DS, NW, JMW, TT, R Barnes, R Barry, MG. Overall responsibility—SB, DS, VN, NW, JMW, TT, R Barnes, R Barry, DB, MG.
Funding
The Fight Retinal Blindness! Project was supported by a grant from the Royal Australian and New Zealand College of Ophthalmologists (RANZCO) Eye Foundation (2007–2009); a grant from the National Health and Medical Research Council, Australia (NHMRC 2010-2012); and a grant from the Macular Disease Foundation, Australia. MG is a Sydney Medical Foundation Fellow and is supported by an NHMRC practitioner fellowship. DB was supported by Walter Gertud Siegenthaler Foundation, Zurich, Switzerland and the Swiss National Foundation.
Compliance with ethical standards
Conflict of interest
MG: grants from NHMRC, grants form RANZCO Eye Foundation, grants and others from Novartis, grants and other from Bayer and is an inventor of the software used to track real-world outcomes in this study. DB: received research grants from Novartis and Bayer and is an inventor of the software used to track real-world outcomes in this study. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–50. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–47. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72.e5. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2018;6:Cd007419. doi: 10.1002/14651858.CD007419.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh R, Ross JS, Sangaralingham LR, Adelman RA, Shah ND, Barkmeier AJ. Trends of anti-vascular endothelial growth factor use in ophthalmology among privately insured and medicare advantage patients. Ophthalmology. 2017;124:352–8. doi: 10.1016/j.ophtha.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Gillies MC, Walton R, Liong J, Arnold JJ, McAllister I, Morlet N, et al. Efficient capture of high-quality data on outcomes of treatment for macular diseases: the fight retinal blindness! Project. Retina. 2014;34:188–95. doi: 10.1097/IAE.0b013e318296b271. [DOI] [PubMed] [Google Scholar]
- 11.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. doi: 10.1001/archopht.1985.01050120030015. [DOI] [PubMed] [Google Scholar]
- 12.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 13.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc (B) 2011;73:3–36. doi: 10.1111/j.1467-9868.2010.00749.x. [DOI] [Google Scholar]
- 14.Therneau Terry M. A package for survival analysis in S, version 2.38. https://CRAN.R-project.org/package=survival. Accessed 07 Mar 2020.
- 15.Australian Government Department of Health Therapeutic Goods Administration. Australian public assessment report for Ranibizumab. https://www.tga.gov.au/sites/default/files/auspar-ranibizumab-141014.doc. Accessed 20 Apr 2020.
- 16.Australian Government Department of Health Therapeutic Goods Administration. Australian public assessment report for Aflibercept. https://www.tga.gov.au/sites/default/files/auspar-aflibercept-150721.pdf. Accessed 20 Apr 2020.
- 17.Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti–vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retin. 2018;2:1179–87. doi: 10.1016/j.oret.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Holekamp NM, Campbell J, Almony A, Ingraham H, Marks S, Chandwani H, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83–91. doi: 10.1016/j.ajo.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Bhandari S, Nguyen V, Fraser-Bell S, Mehta H, Viola F, Baudin F, et al. Ranibizumab or aflibercept for diabetic macular edema: comparison of 1-year outcomes from the Fight Retinal Blindness! Registry. Ophthalmology. 2020;127:608–15. doi: 10.1016/j.ophtha.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Biechl AC, Bhandari S, Nguyen V, Arnold JJ, Young S, Fraser-Bell S, et al. Changes in real-world treatment patterns for diabetic macular oedema from 2009–2019 and five-year outcomes: data from the Fight Retinal Blindness! Registry. Clin Exp Ophthalmol. 2020;48:802–12. doi: 10.1111/ceo.13781. [DOI] [PubMed] [Google Scholar]
- 21.Egan C, Zhu H, Lee A, Sim D, Mitry D, Bailey C, et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101:75–80. doi: 10.1136/bjophthalmol-2016-309313. [DOI] [PubMed] [Google Scholar]
- 22.Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, et al. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. Am J Ophthalmol. 2016;172:51–7. doi: 10.1016/j.ajo.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Lukic M, Williams G, Shalchi Z, Sim D, Patel PJ, Keane PA, et al. Intravitreal aflibercept for diabetic macular oedema: Moorfields’ real-world 12-month visual acuity and anatomical outcomes. Eur J Ophthalmol. 2020;30:557–62. doi: 10.1177/1120672119833270. [DOI] [PubMed] [Google Scholar]
- 24.Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018;136:257–69. doi: 10.1001/jamaophthalmol.2017.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss M, Sim DA, Herold T, Schumann RG, Liegl R, Kern C, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38:2293–300. doi: 10.1097/IAE.0000000000001892. [DOI] [PubMed] [Google Scholar]
- 28.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–92. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]


