Abstract
Introduction
J-DISCOVER is a prospective, observational cohort study that aimed to understand characteristics, glycaemic control, comorbidities and real-world management of patients with early-stage type 2 diabetes mellitus (T2DM) in Japan, by enrolling patients initiating second-line treatment from both diabetes specialist and non-specialist care settings.
Methods
As part of the global DISCOVER programme, J-DISCOVER enrolled 1798 patients with T2DM aged at least 20 years old from 142 sites across Japan, from September 2014 to December 2015, and followed these patients for 3 years. Glycaemic control, body mass index (BMI), blood pressure, lipid profiles, treatment patterns, and prevalence of CKD and retinopathy were examined from baseline to 6, 12, 24 and 36 months, stratified by class of second-line treatment.
Results
At baseline, the median time after T2DM diagnosis was 3.1 years and mean glycated haemoglobin (HbA1c) was 7.7%. The mean individualized HbA1c target was 6.7 ± 0.5%, and 55.3% of patients were set the target of < 7.0%. HbA1c reductions were noted from 6 months and mean HbA1c was 7.1% at 36 months. The proportion of patients with HbA1c < 7.0% increased from 28.8% at baseline to 53.3% at 36 months, and the achievement rate of individualized HbA1c targets increased from 6.1% to 30.3%. Only two cases of severe hypoglycaemia occurred during the study. No major changes in BMI, blood pressure, lipid profile or prescription of antihypertensive or dyslipidaemia medications were observed. The frequencies of screening to detect retinopathy and chronic kidney disease (CKD) were 17.0–21.0% and 14.5–16.0%, respectively, during the follow-up period. The prevalence of CKD, but not retinopathy, increased over the follow-up period.
Conclusions
This study provided an overview of the 3-year management of early-stage T2DM in patients initiating second-line treatment. Contemporary management improved glycaemic control with an acceptable risk–benefit balance, although hurdles remain to sufficient implementation of guideline-recommended treatments in current clinical practice.
Trial Registration
ClinicalTrials.gov identifier, NCT02226822.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01192-x.
Keywords: Antidiabetic drugs, Diabetes complications, Glycaemic control, Second-line treatment, Type 2 diabetes mellitus
Key Summary Points
| Why carry out this study? |
| Multifactorial interventions for the improvement of glycated haemoglobin (HbA1c), blood pressure and dyslipidaemia have demonstrated reductions in the risk of microvascular and macrovascular complications in patients with type 2 diabetes mellitus (T2DM). |
| In Japan, previous studies reporting on the real-world management of patients with T2DM are limited to information up to 2011 and to patients with a long T2DM duration who were managed mainly by diabetologists. |
| This study aims to describe real-world T2DM management and outcome in patients with a short duration of T2DM (median 3.1 years) who were initiating second-line treatment for a duration of 3 years until 2018. |
| What was learned from the study? |
| The achievement rates of an HbA1c target of < 7.0% and individualized HbA1c targets were 53.3% and 30.3% at 36 months, respectively, with only two cases of severe hypoglycaemia during the study period. The frequency of screening to detect retinopathy and chronic kidney disease (CKD) remained relatively low (14.5–21.0%) and the prevalence of CKD increased over the follow-up period. |
| The study results highlight achievements of absolute and individualized HbA1c targets with few hypoglycaemia events, and room for improvement with respect to routinely screening patients for T2DM complications, and implementation of guideline-recommended treatment protocols. |
Introduction
According to estimates by the International Diabetes Federation (IDF) in 2019, the prevalence of diabetes is 9.3% among the global adult population (463 million individuals aged 20–79 years), with type 2 diabetes mellitus (T2DM) accounting for approximately 90.0% of cases [1]. The IDF also estimates that 7.4 million adults in Japan have diabetes, while local survey data suggest that the number is closer to 9.5 million [2]. Indeed, the burden of diabetes in Japan is substantial; approximately 71,000 diabetes-related deaths occurred in 2019, and the estimated healthcare costs were approximately $23.5 billion [1].
Strict glycaemic control early in the course of T2DM has been shown to reduce the risk of microvascular complications compared with less intensive treatment [3–7]. The United Kingdom Prospective Diabetes Study (UKPDS) reported that the incidence of microvascular complications was reduced when glycated haemoglobin (HbA1c) was controlled to approximately 7.0%, and the American Diabetes Association (ADA) and the Japan Diabetes Society (JDS) have set an HbA1c control target of ‘less than 7.0%’ [6, 8, 9]. At the same time, treatment guidelines developed by the JDS and the ADA/European Association for the Study of Diabetes (EASD) recommend that the general treatment target for HbA1c should be individualized according to treatment risks and patient characteristics [8, 9]. In particular, the JDS guidelines recommend that HbA1c targets > 7.0% may be appropriate for older patients (including those at risk of cognitive decline), patients at higher risk of hypoglycaemia or with severe complications, and patients who may not have an effective social support system [8]. When aiming for euglycaemia, the JDS guidelines also recommend an HbA1c target of < 6.0% for patients who can achieve the target with diet and exercise therapy, or with pharmacotherapy without developing hypoglycaemia. However, information on individual HbA1c targets and their achievement rates is limited.
The DISCOVER study programme and J-DISCOVER in Japan [10, 11] were set up to address the knowledge gaps in the management and clinical outcomes of patients with T2DM. The overarching aim of the programme was to describe patient characteristics, evolution of disease management patterns and clinical outcomes over 3 years in patients with T2DM who were initiating a second-line glucose-lowering therapy in a real-world setting. These patients were chosen as the focus of the study because of the diversity of treatment options recommended at this stage of the disease. A better understanding of practice variations across and within different countries, and their determinants and associated patient outcomes, is key for providing effective treatment decisions [10].
Unlike ADA/EASD guidelines, the JDS guidelines do not specify metformin as the first-line glucose-lowering agent [3]. Instead, first-line treatments are to be chosen in light of their pharmacological and adverse effect profiles, and depending on the individual patient’s disease condition [8]. In patients for whom first-line treatment does not achieve HbA1c targets, consideration may be given to increasing the dose of the first-line treatment, switching to a more potent glucose-lowering agent, or combining the first-line treatment with another glucose-lowering agent with a different mechanism of action [8]. Observational data on the treatment of patients with T2DM in Japan are available from large cohort studies [12, 13]. However, these studies are becoming outdated owing to the clinical availability of newer oral antidiabetic drugs, including sodium–glucose cotransporter 2 inhibitors (SGLT2is). In addition, the previous studies enrolled patients exclusively from diabetes specialist settings and, as a result, many patients included had mid- to late-stage diabetes with a long disease duration. Furthermore, most of these studies focused on first-line treatment, and there has not been sufficient clinical data to describe real-world second-line treatment in Japan. Therefore, to obtain a holistic, up-to-date understanding of real-world T2DM treatment patterns in Japan, data from patients in the early stages of T2DM managed by both diabetes specialists and non-specialists (e.g. cardiologists, primary care physicians) are needed.
Here we report the results of the J-DISCOVER study, which examined real-world treatment and long-term glucose control, including individualized HbA1c targets, of patients with T2DM who were initiating second-line diabetes treatment, at both diabetes specialist and non-specialist sites across Japan, to understand clinical practice in the early disease stage.
Methods
Study Design
J-DISCOVER (ClinicalTrials.gov identifier NCT02226822) was a 3-year, multicentre, prospective, longitudinal cohort study that enrolled patients with T2DM, whose diabetes was inadequately controlled with first-line treatment and who were initiating second-line treatment, from 142 sites in Japan. Second-line treatment was defined as adding or switching to a second oral or parenteral antidiabetic medication after first-line oral monotherapy. Definitions and diagnostic criteria for T2DM, objectives of J-DISCOVER, and the full inclusion and exclusion criteria for the study have been reported in detail elsewhere [11, 14]. Standard treatment targets for HbA1c, body mass index (BMI), systolic blood pressure (SBP)/diastolic blood pressure (DBP), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides were based on the JDS guidelines [8].
The study was conducted in accordance with the International Conference on Harmonization of Good Clinical Practice, the Ethical Guidelines for Epidemiological Research of Japan, the ethical standards of the responsible committee on human experimentation (institutional and national), and the Helsinki Declaration of 1964, as revised in 2013. This study was approved by the institutional review committee of each participating institution (D1692R0001); a complete list of those review committees can be found in Table S3 in the supplementary material. All participants provided written informed consent.
Data Collection
The methods of data collection have been published previously [11]. Briefly, data on demographics (e.g. sex, age, BMI and duration of T2DM), risk factors (e.g. smoking), vital signs (e.g. SBP and DBP), T2DM medical and treatment history, complications and concomitant (non-diabetic/diabetic) medications, and clinical variables (HbA1c and lipid profile) were collected at baseline (initiation of second-line treatment) and 6, 12, 24 and 36 months. Individualized HbA1c targets were determined on the basis of individual patient characteristics (age, disease duration, risk of hypoglycaemia, support system, comorbidities, etc.) as shared decisions between physicians and patients at baseline, as recommended in the Japanese Clinical Practice guideline [8]. Data on the prevalence of chronic kidney disease (CKD) and retinopathy were also collected if available. Severe hypoglycaemia was defined as an event requiring external assistance for recovery, such as check-in with healthcare professionals, emergency room visit or hospitalization. Mild hypoglycaemia was defined as when patients were aware of symptoms such as hand tremors, palpitations, tachycardia, sweating, and anxiety, and recovered after glucose supplementation. Data were collected using a standardized electronic case report form which was completed by the investigators. All forms were checked to ensure that there were no missing data or outliers.
Statistical Analyses
The statistical analyses performed have been described elsewhere [11]. Demographic variables (e.g. age, sex), clinical characteristics (e.g. complications, concomitant medications) and T2DM treatment patterns were summarized using standard descriptive statistics. Discrete (yes/no) variables were calculated as patient numbers and percentages. For continuous variables, means [standard deviations (SDs)] and medians [interquartile ranges (IQRs)] were calculated. Cumulative incidence estimates were calculated by Kaplan–Meier analysis to examine the trends in discontinuation rates during the study. In a post hoc analysis, HbA1c levels of 6%, 7% and 8% were used on the basis of the treatment goals stated in the Japanese Clinical Practice guideline [8]. We also adopted HbA1c 6.5%, which is used as a diagnostic criterion for diabetes mellitus [15].
Results
Participant Flow and Data Availability
From September 2014 to December 2015, 1914 patients were enrolled from 142 sites across Japan. After the exclusion of 116 patients from the analysis, owing to the withdrawal of their consent, meeting the exclusion criteria or the discretion of the study investigator, 1798 eligible patients were evaluated in this study. The follow-up rates at 6, 12, 24 and 36 months were 96.1%, 91.7%, 84.5% and 81.5%, respectively (Supplemental Fig. S1). The majority of patients were enrolled from clinics (80.4%), with the remainder enrolled from hospitals (19.6%). Most patients received care from endocrinologists or diabetologists (69.2%), followed by cardiologists (16.2%), primary care physicians (13.2%) and nephrologists (0.3%).
Measurements of HbA1c, body weight and blood pressure were available for more than 90.0% of patients during the 3-year follow-up period. There were fewer data available on lipid profiles (HDL and LDL cholesterol, more than 70.0% of patients; total cholesterol, more than 50.0%; triglycerides, more than 40.0%) and BMI (more than 60.0%) (Supplemental Fig. S2).
Baseline Patient Characteristics
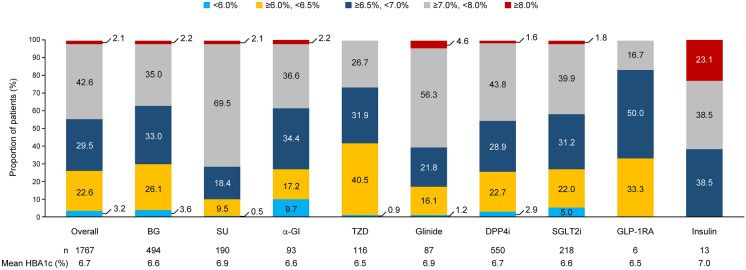
Baseline data for the patients who were followed for the full 3-year study period were not significantly different from previously published data from a patient population with a 2-year follow-up [14]. The mean age of patients at baseline was 61.6 ± 12.8 years and 61.8% of patients were male (Supplemental Table S1). The median duration after diagnosis of T2DM and after initiation of first-line diabetic treatment was 3.1 (IQR 0.8–7.2) years and 11.0 (IQR 4.0–32.0) months, respectively. The mean HbA1c value in the overall population was 7.7 ± 1.3%, and 71.2% of patients had an HbA1c value of ≥ 7.0% (Supplemental Table S1). The mean individualized HbA1c target was 6.7 ± 0.5%, and 55.3% had a target of < 7.0% (Fig. 1). Approximately half of patients (47.8%) were in the target BMI range (≥ 18.5 and < 25 kg/m2; Supplemental Table S1). Mean blood pressure values were 131.5 ± 15.7 mmHg for SBP and 76.8 ± 11.4 mmHg for DBP. Mean serum lipid values were 113.9 ± 31.2 mg/dL for LDL cholesterol, 53.7 ± 17.3 mg/dL for HDL cholesterol and 160.5 ± 157.0 mg/dL for triglycerides.
Fig. 1.
Distribution of individualized HbA1c targets at baseline. n denotes the number of patients who have relevant data available. α-GI alpha-glucosidase inhibitor, BG biguanide, DPP4i dipeptidyl peptidase 4 inhibitor, GLP-1RA glucagon-like peptide 1 receptor agonist, HbA1c glycated haemoglobin, SGLT2i sodium–glucose cotransporter 2 inhibitor, SU sulfonylurea, TZD thiazolidinedione
The most frequently prescribed classes of first-line treatment were dipeptidyl peptidase 4 inhibitors (DPP4is; 53.8% of patients) and biguanides (21.4%) (Supplemental Table S2). These were also the most frequently prescribed second-line treatments, being prescribed to 31.0% and 28.0% of patients, respectively. In patients with individualized HbA1c targets of ≥ 7.0%, sulfonylureas (SUs), glinides and insulin were the most frequently prescribed of the medication classes available for second-line treatment (Fig. 1).
Glycaemic Control
HbA1c Change over 36 Months After Second-Line Initiation
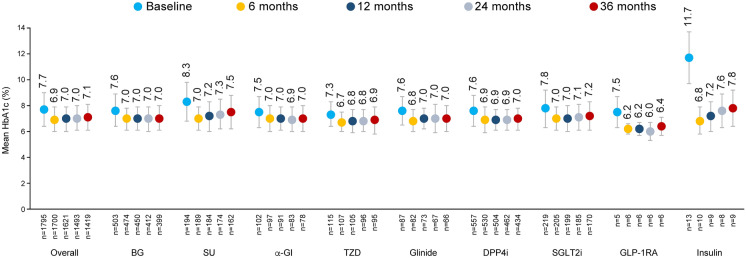
In total, 48.2% of patients had changed medications at least once in the 36 months after starting second-line treatment (Supplemental Fig. S3). The rate of second-line treatment discontinuation at 36 months was lowest for DPP4is (40.6%), followed by SGLT2is (45.2%) and biguanides (47.9%). Mean HbA1c values in the overall population had decreased from 7.7 ± 1.3% at baseline to 6.9 ± 0.9% at 6 months, and were sustained at that level thereafter (7.0 ± 0.9% at 12 months, 7.0 ± 1.0% at 24 months and 7.1 ± 1.0% at 36 months) (Fig. 2). Similar patterns of mean HbA1c change over time were observed for most classes of second-line treatment except insulin and SUs. Mean HbA1c values for insulin and SUs decreased from 11.7 ± 2.0% and 8.3 ± 1.5% at baseline to 6.8 ± 1.0% and 7.0 ± 0.9% at 6 months, respectively, and increased to 7.8 ± 1.4% and 7.5 ± 1.3% at 36 months, respectively.
Fig. 2.
Mean HbA1c by second-line treatment. Whiskers represent standard deviations. n denotes the number of patients who have relevant data available. α-GI alpha-glucosidase inhibitor, BG biguanide, DPP4i dipeptidyl peptidase 4 inhibitor, GLP-1RA glucagon-like peptide 1 receptor agonist, HbA1c glycated haemoglobin, SGLT2i sodium–glucose cotransporter 2 inhibitor, SU sulfonylurea, TZD thiazolidinedione
Achievement Rate of HbA1c Targets
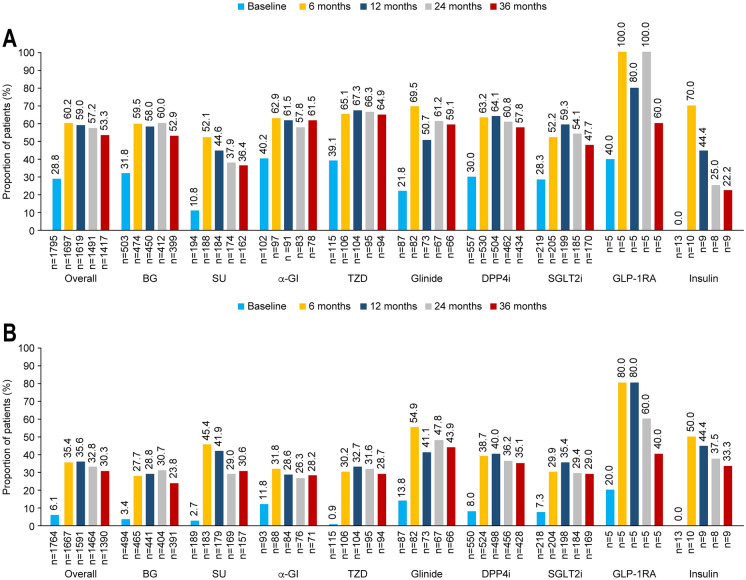
The proportion of patients in whom an HbA1c value of < 7.0% was achieved increased from 28.8% at baseline to 53.3% at 36 months (Fig. 3A); the highest achievement rate over this period was observed for thiazolidinedione (64.9%), followed by alpha-glucosidase inhibitor (61.5%), and the lowest achievement rate was observed for insulin (22.2%), followed by SUs (36.4%). The mean baseline HbA1c value for patients prescribed second-line insulin or SUs was considerably higher (11.7 ± 2.0% or 8.3 ± 1.5%, respectively) than for patients prescribed other classes of second-line treatment (7.3–7.8%; Fig. 2).
Fig. 3.
Proportions of patients who achieved A HbA1c < 7.0% and B individualized targets. n denotes the number of patients who have relevant data available. α-GI alpha-glucosidase inhibitor, BG biguanide, DPP4i dipeptidyl peptidase 4 inhibitor, GLP-1RA glucagon-like peptide 1 receptor agonist, HbA1c glycated haemoglobin, SGLT2i sodium–glucose cotransporter 2 inhibitor, SU sulfonylurea, TZD thiazolidinedione
The overall proportion of patients achieving individualized HbA1c targets increased from 6.1% at baseline to 30.3% at 36 months, with the highest achievement rate observed in patients who initiated second-line glinides (43.9%), followed by glucagon-like peptide 1 receptor agonists (GLP-1RAs; 40.0%; Fig. 3B). Mild hypoglycaemic events were reported in 1.2%, 0.6%, 0.5% and 0.3% of the patients at 6, 12, 24 and 36 months, respectively (Table 1). There were two cases of severe hypoglycaemia during the study period.
Table 1.
Occurrence of hypoglycaemic events and prevalence of CKD and retinopathy during the follow-up period
| Baseline | 6 months | 12 months | 24 months | 36 months | |
|---|---|---|---|---|---|
| Number of patients who have relevant data available | 1798 | 1713 | 1629 | 1502 | 1450 |
| Severe hypoglycaemic events since last visit, n (%) | N/A | 1 (0.1) | 0 (0.0) | 1 (0.1) | 0 (0.0) |
| Mild hypoglycaemic events in the past 4 weeks, n (%) | 18 (1.0) | 20 (1.2) | 9 (0.6) | 7 (0.5) | 4 (0.3) |
| Number of patients who have relevant data available | 1798 | 1713 | 1629 | 1503 | 1450 |
| CKD | |||||
| Present, n (%) | 251 (14.0) | 252 (14.7) | 271 (16.6) | 279 (18.6) | 312 (21.5) |
| > 60 mL/min/1.73 m2, n (%) | 90 (5.0) | 64 (3.7) | 58 (3.6) | 47 (3.1) | 68 (4.7) |
| 30–60 mL/min/1.73 m2, n (%) | 70 (3.9) | 59 (3.4) | 54 (3.3) | 61 (4.1) | 77 (5.3) |
| 15–29 mL/min/1.73 m2, n (%) | 24 (1.3) | 8 (0.5) | 4 (0.2) | 4 (0.3) | 6 (0.4) |
| < 15 mL/min/1.73 m2, n (%) | 14 (0.8) | 5 (0.3) | 1 (0.1) | 4 (0.3) | 3 (0.2) |
| Unknown, n (%) | 53 (2.9) | 116 (6.8) | 154 (9.5) | 163 (10.8) | 158 (10.9) |
| Retinopathy present, n (%) | 137 (7.6) | 121 (7.1) | 120 (7.4) | 116 (7.7) | 116 (8.0) |
CKD chronic kidney disease, N/A not applicable
Control of BMI, Blood Pressure and Lipids
The mean BMI was 25.5 ± 4.6 kg/m2 at baseline and had not changed at the 36-month follow-up (Supplemental Fig. S4). Mean SBP (131.5 ± 15.7 mmHg) also did not change from baseline (Supplemental Fig. S5). Small numerical decreases from baseline in DBP, LDL cholesterol and triglycerides, and a small increase from baseline in HDL cholesterol were observed over the 36-month study period (Supplemental Figs. S6–S9). The proportion of patients for whom BMI, blood pressure and lipid profile targets were achieved did not vary substantially during the study (Supplemental Fig. S10). There were no major changes in the percentages of patients prescribed antihypertensive and dyslipidaemia medications during the 36-month follow-up (Supplemental Fig. S11). At the end of the study period, 53.2% of patients were taking an antihypertensive medication, most frequently angiotensin II receptor blockers and calcium channel blockers. Lipid-lowering agents (mainly statins) were taken by 48.3% of patients.
Prevalence of CKD and Retinopathy
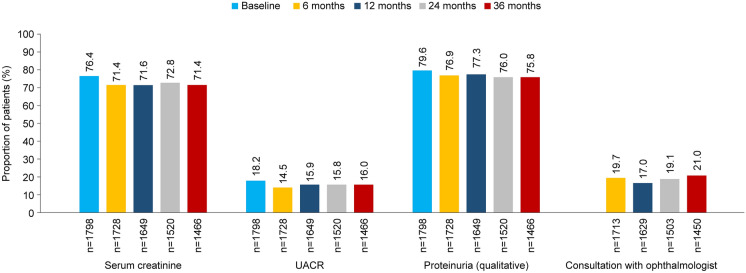
The proportions of patients who had available screening data for CKD and retinopathy were almost constant during the study period. Qualitative proteinuria data and serum creatinine were reported for 75.8–77.3% and 71.4–72.8% of patients, respectively, while urine albumin-to-creatinine ratio (UACR) data were reported for only 14.5–16.0% of patients during the follow-up period (Fig. 4). The proportion of patients with an ophthalmologist consultation for retinopathy was 17.0–21.0% (Fig. 4). The changes in prevalence of CKD and retinopathy during the study period are shown in Table 1. The prevalence of CKD was 14.0% at baseline and increased to 21.5% at 36 months, while the prevalence of retinopathy did not change substantially during the follow-up period (7.6% at baseline and 8.0% at 36 months). The distribution of patient proportions across CKD stages changed minimally over 36 months.
Fig. 4.
Proportions of patients with available screening data for CKD and retinopathy. n denotes the total number of patients, except for consultation by ophthalmologist (for which n denotes the number of patients for whom microvascular complications were investigated)
Discussion
The aim of J-DISCOVER was to obtain a comprehensive and up-to-date picture of T2DM care in Japan. In contrast to previous observational studies, J-DISCOVER (and its global counterpart) included patients with relatively early-stage T2DM who were initiating second-line treatment, and recruited patients receiving care from both diabetologists and other specialists, such as cardiologists and primary care physicians. In J-DISCOVER, the majority of patients (80.4%) were enrolled at clinics and the median duration of time post-diagnosis with T2DM was 3.1 (IQR 0.8–7.2) years, which is shorter than that of previous large-scale diabetes clinical studies that enrolled patients treated by diabetes specialists in Japan: 11 years in the Japan Diabetes Complications Study (JDCS) [16], 10.8 years in the Japan Diabetes Complication and its Prevention prospective (JDCP) study [17] and 8.5 years in the Japan Diabetes Outcome Intervention Trial 3 (J-DOIT3) [18]. Additionally, J-DISCOVER reported not only the proportion of patients who achieved an HbA1c target of < 7.0% but also the proportion who achieved individualized HbA1c goals, together with control of BMI, blood pressure and lipids.
Compared with the global DISCOVER population, patients in J-DISCOVER were older (61.6 vs 57.7 years), had a shorter disease duration (3.1 vs 4.1 years) and had a lower mean HbA1c level (7.7% vs 8.3%) at baseline [19]. The frequency of use of non-biguanide DPP4i as first-line treatment was much higher in Japan (53.8%) than in the global DISCOVER population (9.5%; Supplemental Table S2), which could be because the JDS guidelines do not specifically recommend any class of medications including biguanides as first-line treatment [19]. Conversely, use of metformin as first-line treatment was less common in Japan compared with global data (21.4% vs 58.5%) [19].
Three large-scale clinical studies—Action to Control Cardiovascular Risk in Diabetes (ACCORD), Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) and Veterans Affairs Diabetes Trial (VADT)—have aimed to demonstrate the effect of strict blood glucose control (HbA1c 6.0–6.5%) on macrovascular outcomes [3, 20, 21]. However, intensive therapy did not result in a significant reduction in macrovascular outcomes and mortality unexpectedly increased in the ACCORD study [20]. It was suggested that the increased mortality was associated with an increase in iatrogenic unaware hypoglycaemia in the intensive therapy group, and results from the study are now reflected in guidelines; recommendations are to set individualized HbA1c targets according to treatment risks and patient characteristics. In J-DISCOVER, individualized targets for HbA1c were frequently set at < 7.0% at baseline, with an average value of 6.7%. Individualized HbA1c targets of ≥ 7.0% were documented more frequently in patients initiating second-line SUs and insulin compared with other drugs, which may either be a result of higher mean HbA1c values at baseline or concerns over hypoglycaemic events. At 36 months, 30.3% of patients achieved individualized HbA1c targets, while 53.3% of patients achieved an HbA1c value of < 7.0%. As described previously [22], this may be explained by the fact that patients in J-DISCOVER had a relatively shorter T2DM duration than patients in previous observational studies, and that physicians might have set more stringent targets for these patients. Moreover, the introduction of new drug classes after 2009, such as DPP4is and SGLT2is, which are less likely to cause hypoglycaemia, may have contributed to physicians setting strict individualized HbA1c targets. This is supported by the fact that, although the achievement rate of < 7.0% at 36 months was relatively low for SUs and glinide, the achievement rates of individualized targets were similar to those of other drugs, implying that higher individualized targets were set for these patients. The frequency of hypoglycaemic events in this study was lower than that in the global DISCOVER study, even with the stringent individualized HbA1c targets [23]. This may suggest that physicians in Japan are managing hypoglycaemic risk relatively well by choosing glucose-lowering agents that are appropriate for each patient’s condition, and that patients are relatively well educated on how to take antidiabetes agents.
For the management of microvascular complications of diabetes, the JDS guidelines recommend the measurement of albuminuria once every 3–6 months, and consultation with an ophthalmologist once every 6–12 months for patients without retinopathy [8]. Using a national database, Sugiyama et al. reported that although 96.7% of patients underwent HbA1c or glycated albumin examination in 2015–2016, only 46.5% underwent retinopathic examinations and only 19.4% underwent urinary quantitative albumin or protein examination [24]. A similar result was also reported in a study by Tanaka et al., in which 38.7% of patients with diabetes underwent retinopathy screening and 24.2% underwent urine protein or urine albumin excretion tests [25]. In J-DISCOVER, UACR was measured in 14.5–16.0% of patients and ophthalmologist consultation was conducted in 17.0–21.0% of patients; these rates are lower than rates reported in the previous studies [24, 25] and did not increase considerably during the follow-up period. This may be because of incomplete data collection or input by physicians in J-DISCOVER, especially with respect to ophthalmologist consultation, which occurs at a different department or institution. Even with this potential limitation, J-DISCOVER data indicate that there is room for improvement with respect to the frequency of screening for CKD and retinopathy complications.
Birkeland et al. reported that CKD and/or heart failure is most frequently the first disease manifestation in patients with T2DM without a history of cardiovascular or renal disease, using a large number of multinational healthcare records, including those from Japan [26]. In J-DISCOVER, patients had a relatively short duration of disease, with a median time since diabetes diagnosis of 3.1 years at baseline, and the prevalence of CKD increased over the follow-up period from 14.0% at baseline to 21.5% at 36 months. The results of these studies indicate that comprehensive management, including appropriate screening for CKD, should be considered even in the early stages of T2DM. It is well known that improving control of blood glucose alone cannot lead to meaningful reductions in macrovascular outcomes [3–6]. Improving macrovascular outcomes in patients with T2DM requires a multifactorial treatment approach, as demonstrated in the Steno-2 study [27, 28]. In the study, a regimen of behavioural modification and pharmacotherapy for HbA1c, total cholesterol, triglycerides and blood pressure was found to reduce the risk of cardiovascular complications and death (cardiovascular-related and all-cause) compared with less stringent treatment targets [27, 28]. A similar effect was demonstrated in the J-DOIT3 study, an open-label, randomized trial that enrolled Japanese patients with T2DM who were treated by diabetologists between 2006 and 2009 [18]. The mean duration of T2DM was longer (8.5 years), and HbA1c, SBP, DBP and LDL values at baseline were also higher (HbA1c 8.0%, SBP/DBP 134/80 mmHg, LDL 126 mg/dL) than those of patients in J-DISCOVER. In J-DOIT3, conventional treatment targets for hyperglycaemia, hypertension and lipid profiles based on JDS guidelines [3] were compared with more stringent targets; HbA1c < 6.2%, SBP/DBP < 120/75 mmHg, LDL cholesterol < 80 mg/dL (< 70 mg/dL if coronary artery disease is present), BMI ≤ 22 kg/m2 and triglycerides < 120 mg/dL [18]. Although the primary cardiovascular endpoint (a composite of myocardial infarction, stroke and cardiovascular interventions) was not significantly reduced in incidence, the risk of cerebrovascular events was significantly lower in the intensive therapy group than in the conventional therapy group. Moreover, the risk of onset or progression of nephropathy and retinopathy was significantly reduced in the intensive therapy group compared with the conventional therapy group [18]. Rates of target achievement with respect to SBP, DBP and LDL cholesterol in the J-DOIT3 conventional arm after a median follow-up of 8 years were similar to those seen in J-DISCOVER, with most gains observed in the first year of intervention and maintained at approximately the same level throughout the study period [18]. Given the lower risk of complication events in the J-DOIT3 trial for the intensive treatment group compared with the conventional treatment group, more intensive multifactorial intervention strategies could be applied, even in patients with early-stage T2DM like those in the J-DISCOVER study, to prevent macrovascular complications.
The management of T2DM has been progressing with the accumulation of evidence on comprehensive strategies to reduce the burden of comorbidities, and with the availability of newer hypoglycaemic agents offering more treatment options to healthcare professionals and patients. Guidelines provide up-to-date management recommendations and algorithms; however, their implementation could be challenging in real-world practice. A study to capture data on diabetes daily clinical practice, like J-DISCOVER, is useful to identify treatment gaps and potential hurdles in the implementation of guidelines, and to ultimately increase the quality of disease management and patient care.
Limitations of this study include the relatively short duration of follow-up and smaller sample size compared with other observational studies of T2DM treatment conducted in Japan. In addition, the study relied on data collection and input by physicians and availability of data was lower for some variables (e.g. lipid profiles), which may result in information bias.
Conclusions
J-DISCOVER provides 3 years of real-world treatment data from patients with early-stage T2DM who initiated second-line treatment. Contemporary clinical management of T2DM has improved glycaemic control with an acceptable risk of hypoglycaemic events; however, there remains room for improvement towards sufficient implementation of guideline-recommended treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the investigators and patients participating in the J-DISCOVER study.
Funding
The J-DISCOVER study is funded by AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd., Osaka, Japan. The Rapid Service Fee for this manuscript was funded by AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd., Osaka, Japan.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
The general content of the manuscript was agreed upon by all authors. The first draft of the manuscript was developed by Mitsuyoshi Takahara and Fumitaka Wada, and all authors contributed to its development. All authors approved the final version of the manuscript before its submission. An AstraZeneca team reviewed the manuscript during its development and was allowed to make suggestions. However, the final content was determined by the authors.
Medical Writing and Editorial Assistance
The authors also thank Masaru Kawashima, Suguru Okami and Piao Yi for study management support and intellectual input, and Kim Hyosung for statistical support. The data analysis was performed by IQVIA Services Japan Co., Ltd. Medical writing support was provided by Dr Michael Molloy-Bland and Dr Svetha Sankar of Oxford PharmaGenesis Pty Ltd, and was funded by AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd.
Disclosures
Mitsuyoshi Takahara has received subsidies from the Japan Diabetes Society, and has endowed departments with commercial entities from AstraZeneca, Keiseikai Hospital, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme, Nippon Boehringer Ingelheim, Novo Nordisk, Ono Pharmaceutical and Taisho Toyama Pharmaceutical. Tomoya Mita has received research funds from Kagaku Kenkyusho, Kowa, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme, Ono Pharmaceutical, Sanwa Nippon Boehringer Ingelheim and Takeda Pharmaceutical, and lecture fees from AstraZeneca, Eli Lilly, Kowa, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme, Nippon Boehringer Ingelheim, Ono Pharmaceutical and Takeda Pharmaceutical. Naoto Katakami has received research funds from Merck Sharp & Dohme and lecture fees from Arkray, Astellas, AstraZeneca, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Kowa, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme, Nippon Boehringer Ingelheim, Novartis, Novo Nordisk, Ono Pharmaceutical, Otsuka, Sanofi-Aventis, Shionogi, Taisho Toyama Pharmaceutical and Takeda Pharmaceutical. Iichiro Shimomura has received research funding from the Japan Agency for Medical Research and Development, Kowa, Mochida Pharmaceutical and Rohto Pharmaceutical, and has received subsidies or donations from Astellas, AstraZeneca, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Gokeikai Osaka Kaisei Hospital, the Japan Diabetes Foundation, the Japan Diabetes Society, the Japan Foundation for Applied Enzymology, Kissei Pharma, Kowa Life Science Foundation, Kyowa Hakko Kirin, Midori Health Care Foundation, Mitsubishi Tanabe Pharma, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Mochida Pharmaceutical, Merck Sharp & Dohme, Merck Sharp & Dohme Life Science Foundation, Novartis, Novo Nordisk, Ono Pharmaceutical, Sanofi, Shionogi, Suzuken Memorial Foundation, Takeda Pharmaceutical and Teijin Pharma. Hirotaka Watada has acted as an advisory board member for Astellas, AstraZeneca, Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Kowa, Kyowa Hakko Kirin, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho and Takeda Pharmaceutical, and as a speaker for Astellas, AstraZeneca, Dainippon Sumitomo Pharma, Eli Lilly, Kowa, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho and Takeda Pharmaceutical; and has received research support from Astellas, Daiichi Sankyo, Dainippon Sumitomo Pharma, Kissei Pharma, Kowa, Kyowa Hakko Kirin, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novartis, Novo Nordisk, Ono Pharmaceutical, Pfizer, Sanofi, Sanwa Kagaku Kenkyusho, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical, Teijin Pharma and Terumo. FW, NM, YK and TY are employees of AstraZeneca.
Compliance with Ethics Guidelines
The study was conducted in accordance with the International Conference on Harmonization of Good Clinical Practice, the Ethical Guidelines for Epidemiological Research of Japan, the ethical standards of the responsible committee on human experimentation (institutional and national), and the Helsinki Declaration of 1964, as revised in 2013. This study was approved by the institutional review committee of each participating institution (D1692R0001); a complete list of those review committees can be found in Table S3 in the supplementary material. All participants provided written informed consent.
Data Availability
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas (9th Edition 2019). 2019. https://www.diabetesatlas.org/en/. Accessed 11 May 2020.
- 2.Ministry of Health Labour and Welfare . The National Health and Nutrition Survey in Japan. Tokyo: Ministry of Health Labour and Welfare; 2014. [Google Scholar]
- 3.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 5.Advance Collaborative Group. Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.UKPDS Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 8.Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020;11(4):1020–1076. doi: 10.1111/jdi.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 10.Ji L, Bonnet F, Charbonnel B, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Compl. 2017;31(7):1188–1196. doi: 10.1016/j.jdiacomp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Katakami N, Mita T, Takahara M, et al. Rationale and design for the J-DISCOVER study: discovering the treatment reality of type 2 diabetes in a real-world setting in Japan a protocol. Diabetes Ther. 2018;9(1):165–175. doi: 10.1007/s13300-017-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujihara K, Hanyu O, Heianza Y, et al. Comparison of clinical characteristics in patients with type 2 diabetes among whom different antihyperglycemic agents were prescribed as monotherapy or combination therapy by diabetes specialists. J Diabetes Investig. 2016;7(2):260–269. doi: 10.1111/jdi.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashino Y, Izumi K, Okamura S, Nishimura R, Origasa H, Tajima N. Duration of diabetes and types of diabetes therapy in Japanese patients with type 2 diabetes: The Japan Diabetes Complication and its Prevention prospective study 3 (JDCP study 3) J Diabetes Investig. 2017;8(2):243–249. doi: 10.1111/jdi.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katakami N, Mita T, Takahara M, et al. Baseline characteristics of patients with type 2 diabetes initiating second-line treatment in Japan: findings from the J-DISCOVER study. Diabetes Ther. 2020;11(7):1563–1578. doi: 10.1007/s13300-020-00846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasaki T, Hirose A, Azuma T, et al. Association between eating behavior and poor glycemic control in Japanese adults. Sci Rep. 2019;9(1):3418. doi: 10.1038/s41598-019-39001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajima N, Nishimura R, Izumi K, et al. A large-scale, observational study to investigate the current status of diabetes complications and their prevention in Japan: research outline and baseline data for type 2 diabetes—JDCP study 1. Diabetol Int. 2015;6(4):243–251. doi: 10.1007/s13340-015-0223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diabetes Database Construction Committee. Tajima N, Nishimura N, et al. Large-scale observational study on the actual condition of diabetic complications and their suppression-research plan and baseline data of type 2 diabetes: JDCP study 1 [Japanese] Diabetes. 2015;58(5):346–357. [Google Scholar]
- 18.Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):951–964. doi: 10.1016/S2213-8587(17)30327-3. [DOI] [PubMed] [Google Scholar]
- 19.Khunti K, Chen H, Cid-Ruzafa J, et al. Glycaemic control in patients with type 2 diabetes initiating second-line therapy: results from the global DISCOVER study programme. Diabetes Obes Metab. 2020;22(1):66–78. doi: 10.1111/dom.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The ADVANCE Collaborative Group. Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 22.Schmieder RE, Tschöpe D, Koch C, Ouarrak T, Gitt AK. Individualised treatment targets in patients with type-2 diabetes and hypertension. Cardiovasc Diabetol. 2018;17(1):18. doi: 10.1186/s12933-018-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathmann W, Charbonnel B, Gomes MB, et al. Socioeconomic factors associated with hypoglycaemia in patients starting second-line glucose-lowering therapy: the DISCOVER study. Diabetes Res Clin Pract. 2020;165:108250. doi: 10.1016/j.diabres.2020.108250. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Imai K, Ihana-Sugiyama N, et al. Variation in process quality measures of diabetes care by region and institution in Japan during 2015–2016: an observational study of nationwide claims data. Diabetes Res Clin Pract. 2019;155:750. doi: 10.1016/j.diabres.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Sugiyama T, Ihana-Sugiyama N, Ueki K, Kobayashi Y, Ohsugi M. Changes in the quality of diabetes care in Japan between 2007 and 2015: a repeated cross-sectional study using claims data. Diabetes Res Clin Pract. 2019;149:188–199. doi: 10.1016/j.diabres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607–1618. doi: 10.1111/dom.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 28.Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353(9153):617–622. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request.