Abstract
Background/objectives
The objective of this study is to investigate and compare changes in orbital volume, eyelid parameters, and eyeball position after inferomedial and balanced (medial + deep lateral walls) orbital decompression (OD) in patients with Graves’ orbitopathy (GO).
Subjects/methods
Prospective interventional trial. Forty-two patients with inactive GO and clinical indication for OD were randomly assigned to inferomedial or balanced OD. Preoperative and postoperative Hertel exophthalmometry, standardized photography, and computed tomography were used to evaluate upper and lower eyelid margin reflex distances (MRD1 and MRD2), orbital expansion, and changes in eyeball position.
Results
Clinical and radiological exophthalmometry improved significantly after OD with both surgical techniques (p < 0.001), but more so with balanced OD (p = 0.02). Concurrent eyeball descent (p = 0.01) and orbital volume expansion (p < 0.001) were observed with both techniques. The mean decompression volume was similar for the medial wall and the lateral wall but significantly smaller for the inferior wall (p < 0.05). Significant correlation coefficients were found for Hertel reduction vs. total decompression volume (p < 0.05). In the multivariate linear analysis, lateral wall decompression volume (LWDV) was predictive of exophthalmos reduction (p < 0.05). The two techniques produced a similar reduction in MRD1 and MRD2. A significant correlation was also found between Hertel reduction and lower lid elevation (p < 0.05).
Conclusions
Both inferomedial and balanced OD successfully expanded orbit capacity, but the latter was more efficient at reducing exophthalmos probably due to the inclusion of the lateral wall. Upper and lower eyelid retraction improved after OD, but only lower eyelid elevation was correlated with exophthalmos reduction.
Subject terms: Outcomes research, Eyelid diseases, Thyroid diseases
Introduction
Clinical manifestations of Graves’ orbitopathy (GO) arise from the expansion of orbital fat, soft tissue swelling, and extraocular muscle enlargement leading to a number of sequelae, including disfiguring proptosis [1, 2].
Surgical treatment of exophthalmos is one of the mainstay of patient rehabilitation through orbital decompression (OD) [3, 4] and recent refinements in surgical techniques may help improve the predictability of axial proptosis reduction, customize approach, and combine exophthalmos reduction with eyelid and eyeball repositioning along the vertical and horizontal plane [5].
Quantitative measurements of eyelid and eyeball position are essential to evaluate the success of customized OD approaches, the accuracy of which has been significantly improved with the advent of digital image analysis of eyelid retraction and contour [6–9]. Also, digitized computed tomography (CT) can generate important information on changes in orbital volume and eyeball position following OD [10–13]. Nevertheless, to our knowledge, no previous study has prospectively compared the outcome of the two most commonly used OD techniques, based on standardized quantitative methods.
Thus, we designed this randomized prospective study to investigate and compare changes in eyelid and eyeball position in GO patients submitted to inferomedial OD (IMOD) vs. balanced OD (BOD). We also performed a CT-based orbital volume expansion analysis to evaluate how exophthalmos reduction was affected by the removal of each orbital wall.
Methods
After institutional review board approval and obtained informed consent from subjects, a prospective, randomized, interventional trial was conducted from 2016 to 2019, according to the principles of the Declaration of Helsinki. Fifty patients with inactive GO and indication for OD were assessed for eligibility. Inactive GO was defined based on disease duration >2 years and a clinical activity score ≤ 4 [14]. After excluding eight patients (three not meeting the inclusion criteria, five declined to participate), 42 patients were studied. Patients with ocular or orbital abnormalities, myasthenia gravis, strabismus, or eyelid surgery were excluded.
Preoperative and follow-up complete ophthalmic examinations at 1, 3, and 6 months were performed. Hertel exophthalmometry (HE) was performed preoperatively and the 6-month follow-up visit by a single senior surgeon using a double mirror-fitted exophthalmometer (Oculus Inc.)
Randomization
Eligible patients were randomly assigned to either IMOD or BOD surgery. Assignment was made by lot for the first patient, then alternately for the remaining subjects. Preoperative and postoperative consultations were performed by a researcher blinded to surgical technique.
Surgical techniques
In the IMOD group, a C-shaped incision was made just behind the caruncle, dissecting posteriorly through the subconjunctival tissue and medially in the preseptal plane to the posterior lacrimal crest. The medial wall was fractured, and ethmoid bone and sinus mucosa were debrided or removed, respecting the upper and posterior limits and preserving the inferomedial orbital strut at the junction with the maxillary bone [15, 16]. The orbital floor was accessed through a fornix transconjunctival incision [17, 18]. Inferior wall removal was limited laterally by the infraorbital groove. Periorbital opening was performed and no orbital fat was removed.
In the BOD group, the above-described approach was used to remove the medial wall. Subsequently, rim-sparing lateral wall OD was performed, as proposed by Goldberg et al. [19]. The superolateral orbital rim was exposed through a lateral, upper eyelid sulcus incision. Subsequently, three areas of thick bone (lacrimal keyhole, doorjamb, and basin) were sculpted and thinned with a diamond surgical drill. Periorbital incisions were made, enabling orbital tissues herniation. No orbital fat was removed.
Radiological evaluation
Within 2 weeks of the preoperative consultation, just before surgery and at the 6-month postoperative visit the patients underwent multi-detector CT scanning without contrast (Brilliance 16, Philips). Images were acquired in continuous axial sections instructing the patients to keep their eyes closed in the primary position of gaze. The acquisition parameters were: 120 Kv, 200 mA, detector 16 × 0.75 mm, 1.5-mm slice thickness, and 0.7-mm increment.
The images were sent to IntelliSpace PACS Radiology (Philips Medical System) and analyzed on a dedicated workstation by a head-and-neck radiologist. The following measurements were obtained:
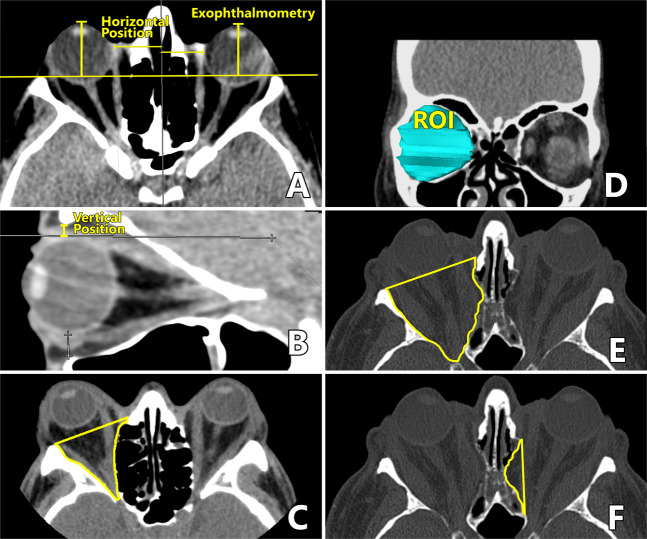
CT exophthalmometry (CTE): on adjusted axial sections, with the optical canals aligned, as the distance from the corneal apex to the interzygomatic line (Fig. 1A).
Horizontal eyeball displacement: the difference between preoperative and postoperative distance from the most medial point of the eyeball and the nasal septum (Fig. 1A).
Vertical eyeball displacement: the difference between the preoperative and postoperative distance (in the adjusted parasagittal plane allowing full view of the optic nerve) from a line tangent to the uppermost limit of eyeball to a parallel line tangent to the upper orbital rim (Fig. 1B).
Orbit volume (OV) and total OD volume (ODV): after segmenting the orbits into consecutive axial slices, we carefully traced a line along the contour separating the bony orbit from the orbital contents. To define the anterior boundary of the orbit, a straight line was drawn connecting the lateral and the medial orbital rim (Fig. 1C). The region of interest (ROI) included the entire orbit, from the topmost axial slice and downwards (Fig. 1D). After surgery, the limits included herniated tissues in the newly created space (Fig. 1E), with the help of simultaneous coronal views to define the inferior limits of the herniated tissue. OV was calculated automatically by multiplying the area in each slice by the thickness of the cut. The difference between the preoperative and postoperative volume capacity corresponded to the total ODV.
Medial wall decompression volume (MWDV): determined from axial scans, as described above, but limiting the ROI to the area expanded by the MWDV (Fig. 1F).
LWDV: in the BOD group, calculated by subtracting MWDV from total ODV.
Inferior wall decompression volume (IWDV): in the IMOD group, calculated by subtracting MWDV from total ODV.
Fig. 1. Radiological measuring methods.
A Radiological exophthalmometry and distance from the eyeball to the nasal septum. B Distance from the eyeball to the upper orbital edge. C Axial segmentation of orbit prior to volume assessment. Anterior boundary of the orbit: straight line connecting the lateral orbital and the medial orbital rim. D Region of interest (ROI). E Limits including herniated tissues. F expanded area after OD.
Photographic evaluation
Standardized frontal photographs were taken at each visit by a single trained ophthalmologist using a Sony DSC-H300 digital camera (20.1 MP, zoom ×35) at a distance of 1 m. The patient was seated in front of a solid background and instructed to face the camera directly with the eyelids open, brows relaxed, and head levelled. The camera was aligned with the patient’s gaze on the horizontal axis. A 12-mm diameter circular sticker was positioned on the forehead for digital calibration.
Images were analyzed by a single examiner using the software Contour [20]. Following calibration (pixels and mm), and after defining the pupillary center, the software automatically drew multiple radial lines, including a vertical line (90°). The lengths of the vertical lines intersecting the eyelid margin at the 12 o’clock and 6 o’clock position were designated, respectively, as the midpupil upper eyelid distance (MRD1) and the midpupil lower eyelid distance (MRD2) (Fig. 2).
Fig. 2. Photographic evaluation.
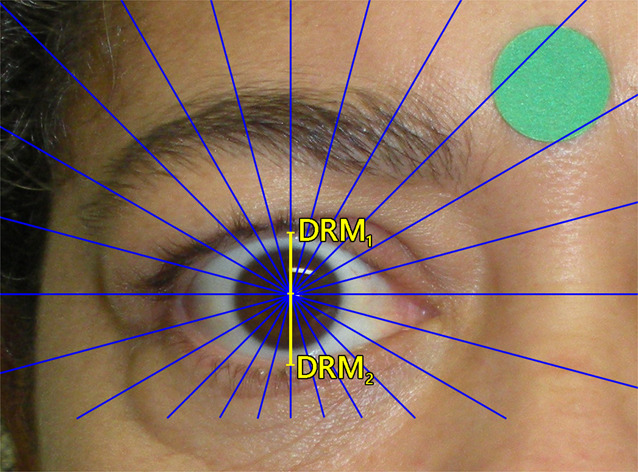
Measurement of midpupil upper eyelid distance (MRD1) and midpupil lower eyelid distance (MRD2) using the software Contour.
Statistical analysis
The sample size was calculated based on the main outcome variable (proptosis reduction). The mean standard deviation (SD) found in the literature is 2.1 mm and the intended effect size, based on clinical judgment, is 1.5 mm. This required a sample size of 24 or more eyes per group.
Demographic and clinical variables were assessed using the chi-squared or Fisher’s exact test. Group comparisons were performed using variance analysis (followed by the Tukey-HSD test) or the Friedman test. Mean differences in each group were compared using Student’s t test. Pearson’s correlation evaluated possible associations between variables, while multivariate linear regression analysis was used to identify factors influencing exophthalmos reduction. p values < 0.05 were considered significant.
Results
Forty-two patients were included. The demographic and clinical characteristics were similar for the two groups (see Supplementary table). No significant complications occurred in either group.
The preoperative HE and CTE values were similar and displayed a significant reduction after OD in both groups. After surgery, the mean ± SD HE and CTE measures were significantly smaller for BOD than for IMOD. Eyeball descent occurred significantly in both groups. No significant horizontal eyeball displacement was observed in either group. OD significantly increased total OV in both groups, with no significant difference between techniques. MWDV was also statistically similar in the two groups. Mean ± SD was significantly greater for LWDV and MWDV than for IWDV (Table 1).
Table 1.
Hertel exophthalmometry (HE) values and computerized tomography parameters in the two treatment groups (42 orbits each) before and after orbital decompression.
| Surgical technique | Preoperative (mm) | Postoperative (mm) | Change (mm) | ||
|---|---|---|---|---|---|
| Mean ± SD (range) | Mean ± SD (range) | p value | Mean ± SD (range) | ||
| Clinical parameter | |||||
| HEa (mm) | Inferomedial | 23.9 ± 2.8 (20–30) | 21.4 ± 2.9 (14–28) | <0.001 | −2.4 ± 1.9 (−8.0; 0.0) |
| Balanced | 23.5 ± 2.6 (20–34) | 19.6 ± 2.2 (14–24) | <0.001 | −3.8 ± 3.1 (−9.0; −1.0) | |
| (p = 0.899) | (p = 0.010) | ||||
| Radiologic parameters | |||||
| CT exophthalmometryb (mm) | Inferomedial | 25.2 ± 2.8 | 23.6 ± 3.5 | <0.001 | −1.6 ± 1.7 (−4.1; 0.7) |
| Balanced | 24.1 ± 2.4 | 21.8 ± 1.8 | <0.001 | −2.3 ± 1.7 (−6.7; 0.0) | |
| (p = 0.301) | (p = 0.017) | ||||
| Eyeball to upper wall distance (vertical displacement, mm)a | Inferomedial | 3.0 ± 1.1 | 3.6 ± 1.7 | 0.011 | 0.6 ± 1.2 (−1.4; 5.5) |
| Balanced | 2.6 ± 1.2 | 3.1 ± 1.6 | 0.016 | 0.6 ± 1.0 (−1.0; 3.1) | |
| (p = 0.540) | (p = 0.471) | ||||
| Eyeball to nose septum distance (horizontal displacement, mm)a | Inferomedial | 19.9 ± 4.7 | 19.6 ± 1.9 | 0.934 | −0.4 ± 4.9 (−4.9; 1.9) |
| Balanced | 20.7 ± 2.0 | 19.4 ± 1.9 | 0.113 | −1.3 ± 1.1 (−4.1; 1.6) | |
| (p = 0.598) | (p = 0.998) | ||||
| Total orbit volume (cm3)b | Inferomedial | 24.2 ± 1.7 | 26.1 ± 2.0 | <0.001 | 1.9 ± 0.8 (0.7; 3.7) |
| Balanced | 23.7 ± 3.2 | 26.2 ± 3.2 | <0.001 | 2.5 ± 0.9 (1.4; 6.0) | |
| (p = 0.872) | (p = 0.999) |
| Decompressed volume after surgery (cm3) | Mean ± SD | ||||
|---|---|---|---|---|---|
| Total orbit decompressed volume, n = 84 | 2.2 ± 0.9 | ||||
| Medial wall decompressed volume, n = 84 | 1.3 ± 0.7 | ||||
| Lateral wall decompressed volume, n = 42 | 1.2 ± 0.7 | ||||
| Inferior wall decompressed volume, n = 42 | 0.6 ± 0.5 |
| Correlations | r | p value | |||
|---|---|---|---|---|---|
| Total orbit decompressed volume (cm3) vs. HE (mm), n = 84 | 0.234 | 0.045 | |||
| Medial wall decompressed volume (cm3) vs. HE (mm), n = 84 | 0.058 | 0.622 | |||
| Lateral wall decompressed volume (cm3) vs. HE (mm), n = 42 | 0.253 | 0.029 | |||
| Inferior wall decompressed volume (cm3) vs. HE (mm), n = 42 | −0.048 | 0.780 |
Total and individual wall decompression volumes and correlation coefficients (r) between decompression volume and exophthalmos reduction. Significant values are in bold. r = Pearson’s correlation coefficients.
aRepeated measures ANOVA/Tukey-HSD test.
bFriedman test.
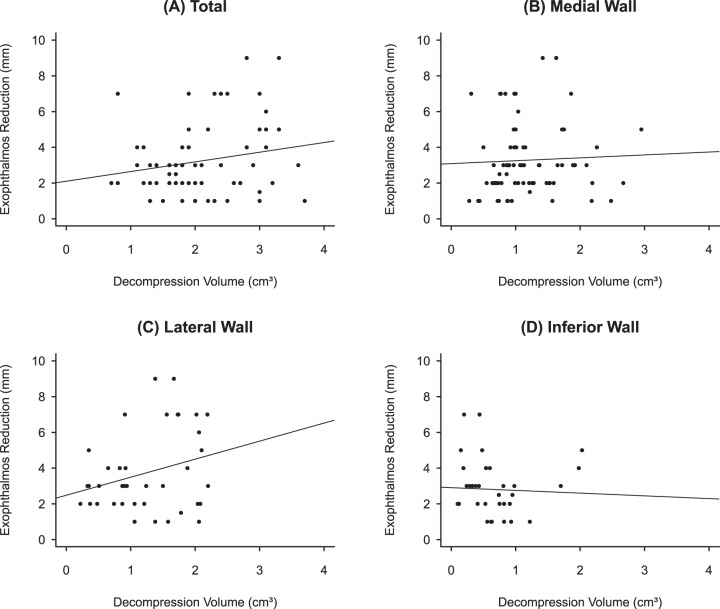
Table 1 also shows the correlation between HE reduction and estimated ODV. Statistically significant relationship was found between HE reduction and both total ODV and LWDV. Figure 3 shows scatterplots of exophthalmos reduction vs. decompressive volume (total and per wall). In multivariate linear regressions using decompression volume as independent variable, LWDV remained the only significant predictor of postoperative of HE reduction (p = 0.033). The amount of reduction per unit of ODV was greatest for LWDV (0.92 ± 0.36 mm/cm3), followed by MWDV (0.34 ± 0.32 mm/cm3) and IWDV (0.07 ± 0.57 mm/cm3).
Fig. 3. Scatterplots of exophthalmos reduction vs. decompressive volume.
A Total decompressive volume (r = 0.234, p < 0.05). B Medial wall (r = 0.058, p > 0.05). C Lateral wall (r = 0.253, p < 0.05). D Inferior wall (r = −0.048, p > 0.05).
In the two groups, both MRD1 and MRD2 decreased after OD. The groups did not differ significantly. Significant correlations were found between exophthalmos reduction and lower lid elevation (MRD2 reduction) with both techniques (Table 2).
Table 2.
Eyelid displacement before and after orbital decompression in the two groups.
| Eyelid position on photographic evaluation | ||||
|---|---|---|---|---|
| Technique | Before (mm) Mean ± SD |
After (mm) Mean ± SD |
p value | Postoperative change (mm) Mean ± SD (range) |
| MRD1a | ||||
| Inferomedial | 4.8 ± 1.2 | 3.9 ± 0.9 | <0.001 | −0.9 ± 1.3 (−1.5; 3.4) |
| Balanced | 5.7 ± 1.8 | 4.6 ± 1.6 | <0.001 | −1.1 ± 1.6 (−1.8; 4.3) |
| MRD2a | ||||
| Inferomedial | 6.3 ± 1.2 | 5.7 ± 1.3 | <0.001 | −0.6 ± 0.8 (−1.6; 2.2) |
| Balanced | 6.8 ± 1.1 | 5.9 ± 1.1 | <0.001 | −0.9 ± 1.0 (−1.0; 4.1) |
| Correlations | r | p value | ||
|---|---|---|---|---|
| Exophthalmometry (Hertel) vs. MRD2 reduction (inferomedial technique) | 0.36 | 0.01 | ||
| Exophthalmometry (Hertel) vs. MRD2 reduction (balanced technique) | 0.43 | 0.01 | ||
| Exophthalmometry (Hertel) vs. MRD1 reduction (inferomedial technique) | 0.16 | 0.31 | ||
| Exophthalmometry (Hertel) vs. MRD1 reduction (balanced technique) | 0.24 | 0.13 |
Measurement of midpupil upper eyelid distance (MRD1) and midpupil lower eyelid distance (MRD2), and correlations with exophthalmometry changes. Significant values are in bold. r = Pearson correlation coefficient.
aFriedman test.
Discussion
In this study, we found both the IMOD and the BOD techniques to be effective at reducing exophthalmos, but proptosis reduction was greater with the BOD than the IMOD technique. Despite the difference in proptosis reduction, the two techniques had similar effects on globe positioning. A previous study on lateral wall OD showed a correlation between exophthalmos reduction and eyeball descent, especially when the approach included the zygomatic basin [6]. In the current study, we also found a significant downward eyeball displacement with the balanced technique, but a similar effect was observed in the IMOD group.
As for horizontal globe movement, one previous study found a significant CT-measured horizontal nasal shift in eyeball position following BOD [11], while another [4] reported an increase in interpupillary distance after lateral wall OD. Our study, however, found no significant horizontal globe displacement, regardless of surgical technique, suggesting that horizontal globe position may be influenced by factors not evaluated in current analysis.
We also investigated the efficiency, predictability, and role of each orbital wall in OD using volumetric CT analysis [21]. The total ODV expansion was similar for the two techniques and both were significantly correlated with HE reduction. LWDV and MWDV were similar and both were greater than IWDV (Table 1). Our results for LWDV were comparable to those of previous studies [10–12]. On the other hand, our MWDV results were >2 [10, 12] and smaller than 1 previous study [11]. Finally, IWDV was greater in our study than in Kim et al. [10].
Although the MWDV was similar to that of LWDV, it appeared to be less efficient at axial eyeball retroplacement since HE reduction correlated with LWDV but not with MWDV. This finding reinforces the notion that OD efficiency depends not only on volume expansion, but also on soft tissue compliance, bone shape, and location. Others have also suggested that eyeball retrodisplacement is greater after deep lateral than medial or inferior wall expansion [10, 19, 22, 23].
As for the effect of OD on eyelid position, in our sample of patients, both upper and lower eyelid retraction improved significantly after OD, regardless of technique. In addition, a significant correlation was observed between proptosis reduction and lower eyelid elevation, suggesting this is a predictable effect, as reported elsewhere [4, 6, 8, 24]. Our patients also experienced a lowering of the upper eyelid regardless of technique, matching the results of studies using digital photography [8, 9].
In order to minimize selection and confounding biases, we conducted a randomized trial in which all patients could be evaluated carefully and prospectively. Nevertheless, our study may have been limited by sample size and inherent measurement errors. Moreover, although digital photography and CT procedures were standardized and consistent, all measurements were performed by only one examiner. The quantification of OV required manual definition of the ROI, which in theory could compromise the accuracy of the measurements. Finally, two-dimensional photography cannot fully depict the three-dimensional relationship between eyelid contour and eyeball.
In conclusion, our study indicates that BOD and IMOD can both provide moderate exophthalmos reduction and expanded orbit capacity. BOD is more efficient at reducing proptosis, probably due to the decompressive effect on the lateral wall. Upper and lower eyelid retraction improved with both techniques, but only lower eyelid elevation was significantly correlated with exophthalmos reduction. Our results confirm the usefulness of CT and photography in the evaluation of the effects and surgical planning of OD.
Summary
What was known before
Technical advances have expanded the indications of OD to include cosmetic and functional rehabilitation. The choice of technique should take into account an array of factors not limited to diplopia and axial proptosis in order to improve surgical predictability and efficiency.
There is still controversy on the effects of orbital decompression on changes of eyeball position and eyelid parameters. Most studies published were not comparative or prospective in order to evaluate these changes.
What this study adds
Both inferomedial and balanced orbital decompression successfully expands orbit capacity, but the latter was more efficient at reducing exophthalmos probably due to the inclusion of the lateral wall. In the multivariate linear analysis, lateral wall decompression volume was predictive of exophthalmos reduction.
Eyeball retrodisplacement is more easily achieved by deep lateral wall expansion than by expansion of the medial and inferior walls.
Upper and lower eyelid retraction and contour improve after orbital decompression, but only lower eyelid elevation was correlated with exophthalmos reduction. This is the first prospective randomized study to describe such changes combining clinical, radiological, and photographic data.
Supplementary information
Funding
This study is supported by grants from CAPES (Coordenação de Aperfeiçoamento de Nível Superior, Brasília, Brazil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasília, Brazil, grant #308172/2018-3). The funding organizations had no role in the design or conduct of the study.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-021-01480-7.
References
- 1.Mimura LY, Villares SM, Monteiro ML, Guazzelli IC, Bloise W. Peroxisome proliferator-activated receptor-gamma gene expression in orbital adipose/connective tissues is increased during the active stage of Graves’ ophthalmopathy. Thyroid. 2003;13:845–50. doi: 10.1089/105072503322401032. [DOI] [PubMed] [Google Scholar]
- 2.Smith TJ. Pathogenesis of Graves’ orbitopathy: a 2010 update. J Endocrinol Investig. 2010;33:414–21. doi: 10.1007/BF03346614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mourits MP, Bijl H, Altea MA, Baldeschi L, Boboridis K, Curro N, et al. Outcome of orbital decompression for disfiguring proptosis in patients with Graves’ orbitopathy using various surgical procedures. Br J Ophthalmol. 2009;93:1518–23. doi: 10.1136/bjo.2008.149302. [DOI] [PubMed] [Google Scholar]
- 4.Fichter N, Krentz H, Guthoff RF. Functional and esthetic outcome after bony lateral wall decompression with orbital rim removal and additional fat resection in graves’ orbitopathy with regard to the configuration of the lateral canthal region. Orbit. 2013;32:239–46. doi: 10.3109/01676830.2013.788662. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Briceno CA, Douglas RS. Customized minimally invasive orbital decompression for thyroid eye disease. Expert Rev Ophthalmol. 2013;8:255–66. doi: 10.1586/eop.13.10. [DOI] [Google Scholar]
- 6.Pieroni Goncalves AC, Gupta S, Monteiro MLR, Douglas RS. Customized minimally invasive orbital decompression surgery improves lower eyelid retraction and contour in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2017;33:446–51. doi: 10.1097/IOP.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 7.Miot HA, Fernandes LP, Jorge EN, Pivotto DR, Nogueira CR, Mazeto GM. Comparative evaluation of oculometric variables in Graves’ ophthalmopathy. Clinics. 2009;64:885–9. doi: 10.1590/S1807-59322009000900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HH, Chun YS, Moon NJ, Kim JT, Park SJ, Lee JK. Change in eyelid parameters after orbital decompression in thyroid-associated orbitopathy. Eye. 2018;32:1036–41. doi: 10.1038/s41433-018-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang EL, Bernardino CR, Rubin PA. Normalization of upper eyelid height and contour after bony decompression in thyroid-related ophthalmopathy: a digital image analysis. Arch Ophthalmol. 2004;122:1882–5. doi: 10.1001/archopht.122.12.1882. [DOI] [PubMed] [Google Scholar]
- 10.Kim KW, Byun JS, Lee JK. Surgical effects of various orbital decompression methods in thyroid-associated orbitopathy: computed tomography-based comparative analysis. J Craniomaxillofac Surg. 2014;42:1286–91. doi: 10.1016/j.jcms.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Alsuhaibani AH, Carter KD, Policeni B, Nerad JA. Orbital volume and eye position changes after balanced orbital decompression. Ophthal Plast Reconstr Surg. 2011;27:158–63. doi: 10.1097/IOP.0b013e3181ef72b3. [DOI] [PubMed] [Google Scholar]
- 12.Choi SU, Kim KW, Lee JK. Surgical outcomes of balanced deep lateral and medial orbital wall decompression in korean population: clinical and computed tomography-based analysis. Korean J Ophthalmol. 2016;30:85–91. doi: 10.3341/kjo.2016.30.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitaguchi Y, Takahashi Y, Kakizaki H. Computed tomography-based prediction of exophthalmos reduction after deep lateral orbital wall decompression for Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2019;257:2759–67. doi: 10.1007/s00417-019-04500-1. [DOI] [PubMed] [Google Scholar]
- 14.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol. 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RA, Perry JD, Hortaleza V, Tong JT. Strabismus after balanced medial plus lateral wall versus lateral wall only orbital decompression for dysthyroid orbitopathy. Ophthalmic Plast Reconstr Surg. 2000;16:271–7. doi: 10.1097/00002341-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kim JW, Goldberg RA, Shorr N. The inferomedial orbital strut: an anatomic and radiographic study. Ophthalmic Plast Reconstr Surg. 2002;18:355–64. doi: 10.1097/00002341-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 17.McCord CD., Jr Orbital decompression for Graves’ disease. Exposure through lateral canthal and inferior fornix incision. Ophthalmology. 1981;88:533–41. doi: 10.1016/S0161-6420(81)34995-1. [DOI] [PubMed] [Google Scholar]
- 18.Goncalves AC, Moura F, Moura J, Bloise W, Monteiro ML. Comparação entre os resultados da descompressão orbitária antro-etmoidal isolada e associada à remoção de tecido adiposo. Arq Bras Oftalmol. 2005;68:445–9. doi: 10.1590/S0004-27492005000400006. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg RA, Kim AJ, Kerivan KM. The lacrimal keyhole, orbital door jamb, and basin of the inferior orbital fissure. Three areas of deep bone in the lateral orbit. Arch Ophthalmol. 1998;116:1618–24. doi: 10.1001/archopht.116.12.1618. [DOI] [PubMed] [Google Scholar]
- 20.Milbratz GH, Garcia DM, Guimarães FC, Cruz AA. Multiple radial midpupil lid distances: a simple method for lid contour analysis. Ophthalmology. 2012;119:625–8. doi: 10.1016/j.ophtha.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 21.Rha EY, Kim JM, Yoo G. Volume measurement of various tissues using the Image J Software. J Craniofac Surg. 2015;26:e505–6. doi: 10.1097/SCS.0000000000002022. [DOI] [PubMed] [Google Scholar]
- 22.Garrity JA, Fatourechi V, Bergstralh EJ, Bartley GB, Beatty CW, DeSanto LW, et al. Results of transantral orbital decompression in 428 patients with severe Graves’ ophthalmopathy. Am J Ophthalmol. 1993;116:533–47. doi: 10.1016/S0002-9394(14)73194-0. [DOI] [PubMed] [Google Scholar]
- 23.Sellari-Franceschini S, Lenzi R, Santoro A, Muscatello L, Rocchi R, Altea MA, et al. Lateral wall orbital decompression in Graves’ orbitopathy. Int J Oral Maxillofac Surg. 2010;39:16–20. doi: 10.1016/j.ijom.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Cho RI, Elner VM, Nelson CC, Frueh BR. The effect of orbital decompression surgery on lid retraction in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2011;27:436–8. doi: 10.1097/IOP.0b013e3182232465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.