Abstract
Introduction
Perineural corticosteroid injection is an extensively used and accepted treatment for carpal tunnel syndrome (CTS). However, to this date, there is no guideline as to which corticosteroid has to be used as the standard treatment for CTS. Triamcinolone acetonide is a commonly used particulate steroid that can cause permanent nerve injury if it is accidentally injected into the nerve. Conversely, dexamethasone sodium phosphate is a nonparticulate steroid that would not cause permanent nerve damage following accidental injection.
Methods
Mild to moderate cases of CTS, confirmed by nerve conduction studies (NCS), with symptoms greater than three months were recruited. The participants received one session of ultrasound-guided perineural injection by the in-plane axial ulnar-sided approach with 4 mL of either dexamethasone (dexamethasone sodium phosphate 8 mg (2 mL) + 2 mL 0.5% bupivacaine) or triamcinolone (triamcinolone acetonide 40 mg/mL (1 mL) + 2 mL 0.5% bupivacaine + 1 mL normal saline) solution. The parameters assessed were Phalen’s test time (in seconds), visual analog scale (VAS), and Boston carpal tunnel questionnaire (BCTQ) scores at baseline and two and four months, and NCS changes in sensory nerve conduction velocity (SNCV) and distal motor latency (DML) of the median nerve at baseline and four months. Statistical analysis was conducted using the software SPSS version 26.0 (IBM Corporation, Armonk, NY, USA). Independent samples t-test was used for comparison between groups and the paired t-test for improvement within each group. P values < 0.05 were considered statistically significant.
Results
The mean age was 42.64 ± 10.99 in the dexamethasone and 45.22 ± 10.602 in the triamcinolone group cases (P = 0.324).There were 58 females (84.06%) and 11 males (15.94%). Each of Phalen’s test time, VAS, and BCTQ scores significantly improved within both dexamethasone and triamcinolone groups at the second and fourth months after injection (P < 0.05). The NCS parameters (SNCV and DML) also significantly improved in both groups at the fourth month after the injection (P < 0.05). However, there were no significant differences in the improvement of Phalen’s test time between the two groups (P = 0.745), VAS score (P = 0.319), BCTQ score (P = 0.137), SNCV (P = 0.511), or DML (P = 0.753). Postprocedural pain lasted significantly longer in the triamcinolone group (P < 0.05). No major complications were noted in either of the two groups.
Conclusion
Dexamethasone is as effective as triamcinolone in improving the symptoms of CTS and can be used as a safer and more effective alternative in the treatment of mild to moderate CTS cases.
Keywords: nonparticulate steroid, ultrasound-guided perineural injection, vas, bctq, particulate steroid, triamcinolone, dexamethasone, local corticosteroid injection, cts, carpal tunnel syndrome
Introduction
Carpal tunnel syndrome (CTS) is a symptomatic compressive neuropathy of the median nerve at the level of the wrist and accounts for 90% of all peripheral entrapment neuropathies [1]. It is mostly a cumulative trauma disorder (caused by repetitive tasks, forceful exertions, vibrations, mechanical compression, and sustained postures) along with a multitude of other causes such as trauma, inflammatory arthropathy, hypothyroidism, obesity, and diabetes. It affects the day-to-day activities of the affected population, and the associated healthcare costs impose a considerable socioeconomic burden on society, both in terms of productivity loss and treatment costs. The incidence of CTS has been reported to be approximately 276 per 100,000, which is markedly more common in females than in males, and is mostly bilateral with a peak incidence in the age group of 40-60 years [2]. The overall prevalence in the general population was estimated to be approximately 3.8% [3].
CTS is characterized by hand pain, numbness, and tingling sensation in the sensory distribution of the median nerve (lateral 3½ fingers) along with a reduction in grip strength and hand function. Patients commonly complain of a nocturnal worsening of symptoms, worsening of symptoms while driving, inability to hold a handset, gripping issues, and hand clumsiness during daytime with activities requiring wrist flexion. The numbness is aggravated by activities such as typing, driving, or knitting; additionally, nocturnal dysesthesia interrupts sleep and is relieved by shaking or flicking the hand, referred to as the “flick sign” [4].
Diagnosis requires a thorough history of symptom onset, provocative factors, work activity, pain localization and radiation, maneuvers that alleviate the symptoms (Phalen’s test, Tinel’s sign, etc.), and the presence of predisposing factors, such as diabetes, obesity, chronic polyarthritis, myxedema, acromegaly, pregnancy, and sports activities [5]. Phalen’s test has a sensitivity of 85% and a specificity of 90% [6], and Tinel’s test has a sensitivity of 62% and a specificity of 93% [7]. Other tools used for severity assessment include the visual analog scale (VAS), positive Phalen’s test time (in seconds), Boston carpal tunnel questionnaire (BCTQ) developed by Levine et al. in 1993 [8], and others. Nerve conduction studies (NCS) are considered the gold standard for the diagnosis of CTS [9] and require prolongation of motor and sensory latencies in conjunction with reduced sensory and motor conduction velocities of the median nerve [10].
Corticosteroid injection is an extensively used and accepted treatment in mild to moderate CTS according to the guidelines of the American Academy of Orthopedic Surgeons [11] as corticosteroids reduce the inflammation and edema associated with CTS. However, there is no guideline as to which corticosteroid has to be used as the standard treatment in CTS. Triamcinolone acetonide, a commonly used steroid for this indication, is a particulate steroid, which can cause permanent nerve injury if accidentally injected into the nerve [12]. Dexamethasone sodium phosphate is another steroid with a better safety profile that has also been shown to be effective in CTS [12-14]. It is a nonparticulate steroid that would not cause permanent nerve damage even if it is accidentally injected into the nerve [12]. In this study, we compare the efficacy of these two steroids in CTS, which has not yet been studied to date in the Indian population.
Materials and methods
Aim
In this study, we aim to compare the efficacy of dexamethasone and triamcinolone acetonide injection in patients with CTS.
Objectives
Our primary objective is to compare the positive Phalen’s test time (in seconds) in each group at baseline and two and four months. Our secondary objectives are to compare the pain relief obtained in each group using the VAS at baseline and two and four months, to compare the improvement in the BCTQ score in each group at baseline and two and four months, and to observe the changes in NCS at baseline and four months.
Inclusion criteria
The inclusion criteria included the following: ages between 20 and 80 years and mild to moderate cases of CTS, confirmed by electrophysiological tests, with symptoms lasting for a minimum of three months.
Exclusion criteria
The exclusion criteria were malignancies, cervical radiculopathy, brachial plexopathy, thoracic outlet syndrome, infections, inflammatory joint and connective tissue disorders, uncontrolled diabetes, burns/any local tissue contractures, history of wrist trauma/surgery, and pregnancy.
Sampling and sample size
Dilokhuttakarn et al. (2018) reported an overall positive Phalen’s test time at the two-month follow-up with a mean value of 52.00 with a standard deviation (SD) of 11.42 in the dexamethasone group and a mean value of 42.33 with a standard deviation of 16.95 in the triamcinolone group [15]. Considering this for sample size calculation, we estimated a sample size of 36 in each group with a 95% confidence interval and power of 80%. Thus, a total of 72 patients with CTS were recruited and randomized into two groups.
Methodology and data collection
The study was conducted at the outpatient clinic in the Department of Physical Medicine and Rehabilitation at All India Institute of Medical Sciences (AIIMS), Jodhpur, Rajasthan, India. It was an open-label parallel design randomized control trial conducted from January 2020 to December 2021. Approval was obtained from the scientific committee and Institute Ethics Committee (IEC) of AIIMS Jodhpur prior to the commencement of the study (certificate reference number: AIIMS/IEC/2019-20/968). The entire study was conducted in accordance with the principles of the Declaration of Helsinki, and written informed consent was taken from all the participants prior to the procedure. All patients satisfying the inclusion and exclusion criteria during the study period were considered eligible for participation. The participants were randomized into the dexamethasone and triamcinolone groups using the sequentially numbered opaque sealed envelope technique for allocation concealment, followed by block randomization using block sizes equal to four and five. This was an open-label trial where patients were not blinded to their allocated treatment. All patients clinically diagnosed with CTS and confirmed by electrophysiological studies [10] (using criteria and cutoffs mentioned in Table 1 and Table 2, respectively) received the basic conservative management with hand splint; medications such as gabapentin (300-600 mg), NSAIDs, and paracetamol (up to maximum 4 g/day); and nerve and tendon gliding exercises prior to recruitment for the procedure.
Table 1. AANEM grading of CTS based on NCS findings.
AANEM: American Association of Neuromuscular and Electrodiagnostic Medicine
CTS: carpal tunnel syndrome
NCS: nerve conduction study
SNCV: sensory nerve conduction velocity
DML: distal motor latency
| CTS grade | NCS finding |
| Minimal | Abnormal segmental or comparative tests only |
| Mild | Abnormal SNCV only with normal DML |
| Moderate | Abnormal SNCV and abnormal DML |
| Severe | Absent sensory response and abnormal DML |
| Extreme | Absence of motor and sensory responses |
Table 2. Abnormal nerve conduction study cutoff values.
NCS: nerve conduction study
SNCV: sensory nerve conduction velocity
DML: distal motor latency
DSL: distal sensory latency
| Nerve conduction study parameters | Abnormal cutoff value |
| SNCV | <50 m/s |
| DML | >4.3 ms |
| DSL | >3.6 ms |
| Amplitude (sensory) | <10 uV |
| Amplitude (motor) | <5 mV |
The participants in the dexamethasone group received one session of ultrasound-guided perineural injection with 4 mL of dexamethasone solution (dexamethasone sodium phosphate 8 mg/2 mL + 2 mL 0.5% bupivacaine), and those in the triamcinolone group received one session of ultrasound-guided perineural injection with 4 mL of triamcinolone solution (triamcinolone acetonide 40 mg/1 mL + 2 mL 0.5% bupivacaine + 1 mL normal saline). This ensured that nearly equivalent doses of steroids were used in both groups [16]. Then, 1 mL of normal saline was added to the triamcinolone solution to equalize the injectate volumes in both groups while keeping the bupivacaine volume constant. The demographic, clinical, and procedural details of all patients who underwent the procedure, including any adverse reactions, were noted. Ultrasonography was conducted using the Sonosite SII ultrasound machine (FUJIFILM Sonosite, Inc., WA, USA), and NCS was assessed using the Nihon Kohden EMG/EP measuring system (Nihon Kohden Corporation, Shinjuku-ku, Tokyo, Japan). The participants were instructed to refrain from all other CTS treatments throughout the study period.
The entire procedure was conducted under ultrasound guidance following all aseptic precautions in accordance with the standard perineural injection protocol as follows.
Injection Technique
The participants received one session of ultrasound-guided perineural injection by the in-plane axial ulnar-sided approach.
Patient Positioning
The patients were asked to sit with the affected arm resting comfortably on the table. A rolled towel was placed underneath the wrist to create a mild extension.
Probe Position
The transducer was placed along the short axis (transverse) to the median nerve at the wrist. The scan was done proximally and distally until the nerve was clearly identified under the transverse carpal ligament, at approximately the level of the pisiform.
Markings
Because of the shallow needle plane angle, the ulnar nerve and artery were identified, and the needle was inserted just radially or along the depth direction in these structures.
Needle Position
The 1-inch 23 G needle was inserted on the ulnar side of the wrist crease parallel to the transducer for optimal needle visualization using an in-plane approach.
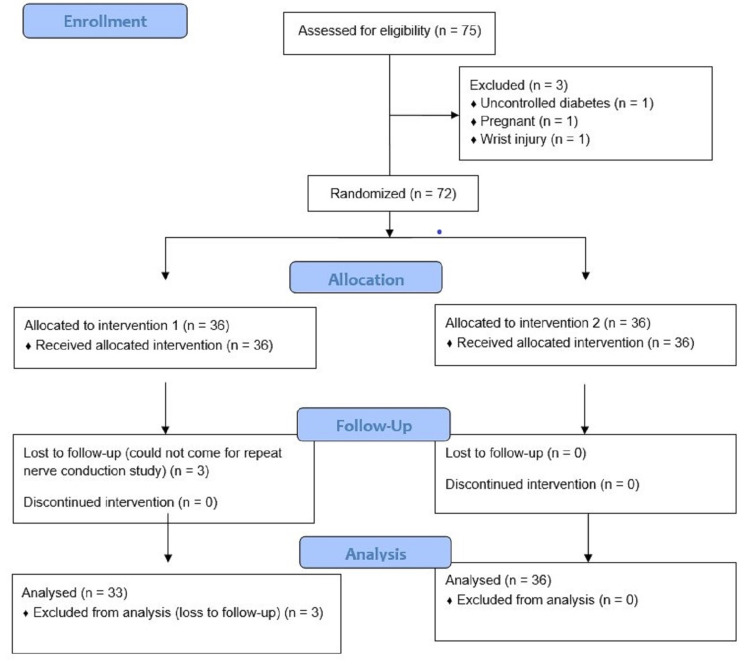
The solution, depending on the randomized grouping of the patient, was injected around the median nerve in the carpal tunnel. After the injection, the participants were advised to apply cold packs at the injection site for three days and to take no other medications for pain apart from paracetamol (up to 4 g/day). Among those who completed the study, 33 patients were in the dexamethasone group and 36 patients were in the triamcinolone group. Figure 1 shows the analysis flowchart of the recruited participants.
Figure 1. Analysis of the recruited participants.
The primary outcome was positive Phalen’s test time, and the secondary outcomes included the VAS scored from 0 (no pain) to 10 (unbearable pain), BCTQ score, and NCS changes of the median nerve. The positive Phalen’s test time, VAS, and BCTQ score were evaluated before the treatment (0) and at two and four months after the injection. NCS was evaluated at baseline and four months after the injection.
Statistical analysis
Data were entered in Microsoft Excel (Microsoft, Redmond, WA, USA), and analysis was conducted using SPSS for Windows version 26.0 (IBM Corporation, Armonk, NY, USA). Quantitative variables such as age, positive Phalen’s test time, and VAS score were described using means and standard deviations. Independent samples t-test was used for comparison between groups and paired t-test for improvement within each group. A P < 0.05 was considered statistically significant.
Results
A total of 72 patients who were clinically diagnosed with CTS were evaluated during the study period. Electrophysiological studies confirmed who met the inclusion or exclusion criteria. Out of the 72 recruited patients, three patients did not attend the follow-up meeting, while the remaining 69 patients were monitored up to four months after the procedure. Table 3 lists the demographic characteristics of the recruited participants, which were comparable in both groups with no significant differences noted between the two groups.
Table 3. Demographic data of the patients recruited in this study.
CTS: carpal tunnel syndrome
| Label | Triamcinolone (n = 36) | Dexamethasone (n = 33) |
| Age (years) | ||
| Mean ± standard deviation (SD) | 45.22 ± 10.6 | 42.64 ± 10.99 |
| Gender | ||
| Male, n (%) | 7 (19.4) | 4 (12.9) |
| Female, n (%) | 29 (80.6) | 29 (87.1) |
| Occupation, n (%) | ||
| Housewife | 19 (52.8) | 21 (63.7) |
| Manual worker | 11 (30.5) | 8 (24.2) |
| Others | 6 (16.7) | 4 (12.1) |
| Dominant hand, n (%) | ||
| Right | 33 (91.7) | 31 (93.9) |
| Left | 3 (8.3) | 2 (6.1) |
| Side of symptoms, n (%) | ||
| Right | 19 (52.8) | 19 (57.6) |
| Left | 17 (47.2) | 14 (42.4) |
| Dominant hand | 18 (50) | 19 (57.6) |
| Nondominant hand | 18 (50) | 14 (42.4) |
| Severity of CTS, n (%) | ||
| Mild | 22 (61.1) | 15 (45.5) |
| Moderate | 14 (38.9) | 18 (54.5) |
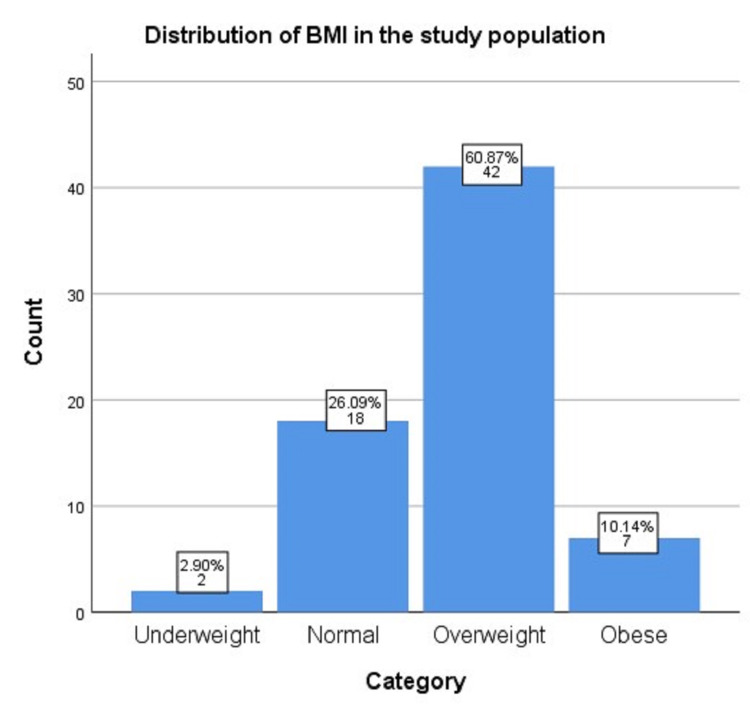
Figure 2 shows the distribution of the body mass index (BMI) for the studied population according to the World Health Organization cutoff categories. There were two (2.9%) underweight, 18 (26.09%) normal, 42 (60.87%) overweight, and seven (10.14%) obese women in this population. The striking majority of patients were in the overweight category.
Figure 2. Categorization of the study population according to BMI.
BMI: body mass index
Table 4 lists the improvement observed in each group for each of the assessed parameters. As is evident from the table, the P values associated with improvements at the second and fourth months after injection each for Phalen’s test time, VAS score, BCTQ score, and the NCS parameters (SNCV and DML) were <0.05 in both groups. Thus, it can be inferred that there were significant improvements in both groups for each of the assessed parameters.
Table 4. Intragroup comparison.
VAS: visual analog scale
BCTQ: Boston carpal tunnel questionnaire
SNCV: sensory nerve conduction velocity
DML: distal motor latency
| Parameter | Dexamethasone group (n = 33) | Triamcinolone group (n = 36) | ||||
| Phalen’s test time | Mean | Standard deviation (SD) | P value | Mean | SD | P value |
| At baseline | 33.73 | 8.304 | 35.5 | 8.687 | ||
| After two months | 51.45 | 5.154 | <0.05 | 52.81 | 4.845 | <0.05 |
| After four months | 42.88 | 3.806 | <0.05 | 43.22 | 4.817 | <0.05 |
| VAS score | ||||||
| At baseline | 6.36 | 0.994 | 6.17 | 0.910 | ||
| After two months | 1.85 | 0.870 | <0.05 | 1.39 | 1.225 | <0.05 |
| After four months | 2.06 | 1.116 | <0.05 | 1.75 | 1.422 | <0.05 |
| BCTQ score | ||||||
| At baseline | 49.18 | 17.74 | 51.81 | 17.139 | ||
| After two months | 21.15 | 3.043 | <0.05 | 22.78 | 6.551 | <0.05 |
| After four months | 21.61 | 4.723 | <0.05 | 23.83 | 7.193 | <0.05 |
| SNCV | ||||||
| At baseline | 37.38 | 6.2455 | 39.217 | 6.2764 | ||
| After four months | 44.394 | 6.7027 | <0.05 | 45.428 | 6.2912 | <0.05 |
| DML | ||||||
| At baseline | 4.242 | 0.7150 | 4.244 | 1.1816 | ||
| After four months | 3.367 | 0.5605 | <0.05 | 3.300 | 1.0871 | <0.05 |
Table 5 shows the intergroup comparison of the mean Phalen’s test time at baseline and after two and four months. The mean baseline Phalen’s test time in the dexamethasone group was 33.73 ± 8.304 seconds and that of the triamcinolone group was 35.5 ± 8.687 seconds. The P value was >0.05. Hence, there is no significant difference between the two groups at baseline with respect to Phalen’s test time. The mean Phalen’s test time at the second month in the dexamethasone group was 51.45 ± 5.154 seconds and that of the triamcinolone group was 52.81 ± 4.845 seconds. The P value was >0.05. Hence, there is no significant difference between the two groups in the follow-up at the second month. The mean Phalen’s test time at the fourth month in the case of the dexamethasone group was 42.88 ± 3.806 seconds and that of the triamcinolone group was 43.22 ± 4.817 seconds. The P value was >0.05. Hence, there is no significant difference between the two groups even four months later.
Table 5. Intergroup comparison of Phalen’s test time.
*Independent samples t-test
| Phalen’s test time | Treatment | Mean | Standard deviation (SD) | P value* |
| At baseline | Dexamethasone | 33.73 | 8.304 | |
| Triamcinolone | 35.50 | 8.687 | 0.390 | |
| After two months | Dexamethasone | 51.45 | 5.154 | |
| Triamcinolone | 52.81 | 4.845 | 0.266 | |
| After four months | Dexamethasone | 42.88 | 3.806 | |
| Triamcinolone | 43.22 | 4.817 | 0.745 |
Table 6 lists the intergroup comparison of the mean VAS score at baseline (VAS0), after two months (VAS2), and after four months (VAS4). As is evident from the table, the P value is >0.05 at baseline and at the second and fourth months, which implies that there was no significant difference between the two groups in terms of the VAS score.
Table 6. Intergroup comparison of VAS score.
VAS: visual analog scale
| VAS score | Treatment | Mean | Standard deviation (SD) | P value |
| At baseline | Dexamethasone | 6.36 | 0.994 | |
| Triamcinolone | 6.17 | 0.910 | 0.393 | |
| After two months | Dexamethasone | 1.85 | 0.870 | |
| Triamcinolone | 1.39 | 1.225 | 0.079 | |
| After four months | Dexamethasone | 2.06 | 1.116 | |
| Triamcinolone | 1.75 | 1.422 | 0.319 |
Table 7 shows the intergroup comparison of the mean BCTQ scores at baseline (BCTQ0) and after two months (BCTQ2) and four months (BCTQ4). As is evident from the table, the P value is >0.05 at baseline and at the second and fourth months, which implies that there was no significant difference between the two groups in terms of the BCTQ score.
Table 7. Intergroup comparison of BCTQ score.
BCTQ: Boston carpal tunnel questionnaire
| BCTQ score | Treatment | Mean | Standard deviation (SD) | P value |
| At baseline | Dexamethasone | 49.18 | 17.747 | |
| Triamcinolone | 51.81 | 17.139 | 0.534 | |
| After two months | Dexamethasone | 21.15 | 3.043 | |
| Triamcinolone | 22.78 | 6.551 | 0.197 | |
| After four months | Dexamethasone | 21.61 | 4.723 | |
| Triamcinolone | 23.83 | 7.193 | 0.137 |
Table 8 shows the intergroup comparison of mean NCS parameters, namely, the SNCV and DML, at baseline and after two and four months. As is evident from the table, the P value is >0.05 at baseline and after the second and fourth months, which implies that there was no significant difference between the two groups in terms of the NCS parameters as well.
Table 8. Intergroup comparison of NCS parameters.
NCS: nerve conduction study
SNCV: sensory nerve conduction velocity
DML: distal motor latency
| NCS parameter | Treatment | Mean | Standard deviation (SD) | P value |
| SNCV at baseline | Dexamethasone | 37.380 | 6.2455 | |
| Triamcinolone | 39.217 | 6.2764 | 0.228 | |
| SNCV after four months | Dexamethasone | 44.394 | 6.7027 | |
| Triamcinolone | 45.428 | 6.2912 | 0.511 | |
| DML at baseline | Dexamethasone | 4.242 | 0.7150 | |
| Triamcinolone | 4.244 | 1.1816 | 0.995 | |
| DML after four months | Dexamethasone | 3.367 | 0.5605 | |
| Triamcinolone | 3.300 | 1.0871 | 0.753 |
Table 9 shows the intergroup comparison of postprocedural pain duration, which was significantly more pronounced in the triamcinolone group (mean duration: 5.31 ± 1.037 days) compared with the dexamethasone group (mean duration: 2.67 ± 0.692 days) with a P value < 0.05.
Table 9. Intergroup comparison of postprocedural pain duration.
| Label | Mean (days) | Standard deviation (SD) | P value |
| Dexamethasone | 2.67 | 0.692 | |
| Triamcinolone | 5.31 | 1.037 | 0.048 |
Discussion
CTS is a very commonly encountered condition in our clinical setups. If left untreated, the initial sensory symptoms can progress to motor weakness of the hand that will cause difficulties in holding and gripping objects and will thus increase the patient’s dependence on others for their daily activities. Many patients report increased symptoms during the night hours that disturb their sleep patterns and result in daytime sleepiness, difficulties, and decreased efficiency in executing their daily living activities, thereby decreasing the quality of life.
Similar to the disease prevalence, we found that CTS was much more common in females than in males (84% versus 16%), as also reported by Mondelli et al. [2]. Obesity is a well-recognized risk factor for the development of CTS as mentioned by Genova et al. [17]. In our study, we found that 60% of the patients were found to be in the overweight category.
Our study was conducted in the western state of Rajasthan where milking of cows is a very common daily task in many households. Accordingly, this activity causes repetitive trauma to the wrist, which is a primary factor predisposing the population to the development of CTS.
Local corticosteroid injections have been successfully used for the treatment of CTS for more than half a century and have been found to be effective [11,18,19]. Different authors, such as Marshall et al. [19], Piazzini et al. [20], Peters-Veluthamaningal et al. [21], Ertem et al. [22], and others, had previously shown that local corticosteroid injections in CTS had good short-term efficacy in reducing the symptoms of CTS. In our study, we obtained similar results, which imply that steroids are indeed effective in reducing the symptoms of CTS.
To this date, there has been no specification in the literature regarding any particular corticosteroid to be used as the standard treatment in CTS. It has been noted that triamcinolone acetonide is currently one of the most commonly used steroid injections for the treatment of CTS. However, similar to the findings by Mackinnon et al. that owing to its characteristics of being water-insoluble, due to its potential to form white sediments, and property of crystallization at the injection site, triamcinolone is more prone to develop more adverse events after local injection [12]. Additionally, we made similar observations that confirmed our findings in our study as postinjection flare and injection site pain were significantly more pronounced in the triamcinolone group compared with the dexamethasone group. Mackinnon et al. had also reported that triamcinolone caused widespread axonal and myelin degeneration, and if a physician accidentally injects triamcinolone acetonide directly into the nerves, it could cause permanent nerve injury [12]. However, given that we performed all the injections under ultrasonic guidance, we did not encounter any nerve injury events in either group. Thus, the propensity of more nerve damage with triamcinolone cannot be commented on based on our study.
Furthermore, as reported by Habib et al. and Wang et al., the use of local dexamethasone injections did not have any systemic side effects, such as changing the blood sugar level of the patients, which was also confirmed in our study as the blood sugar values of the patients who received dexamethasone in our study remained in the normal range after the procedure [23,24].
Only a few studies have been conducted on the efficacy of dexamethasone for the treatment of CTS. Niempoog et al. studied the efficacy of dexamethasone injection for the treatment of CTS in pregnancy and showed that it was an effective treatment option for controlling the symptoms of CTS in pregnant women [13]. Moghtaderi et al. also reported significant improvement in pain intensity and electrophysiological parameters after dexamethasone injection in pregnant women with CTS [14]. However, owing to ethical concerns, we have not included pregnant women in our study; accordingly, they were managed conservatively without any injections.
Dilokhuttakarn et al. in a prospective, randomized, double-blind, controlled, clinical trial compared the efficacy of dexamethasone and triamcinolone injections in CTS and found that dexamethasone injections were effective and significantly improved the positive Phalen’s test time compared with triamcinolone acetonide, but there was no significant difference between the two groups [15]. Our study also yielded similar results with significant improvements in all the assessed parameters in both groups, but there were not any significant differences between the two groups in any of the assessed parameters.
Strengths
Prior to the recruitment for the study, all the clinically diagnosed cases were confirmed by electrophysiological tests.
Limitations
One limitation of the current study is the reduced sample size. In our study, the recruitment period was 18 months, during which we could recruit only 69 patients despite the fact that we had initially expected more. However, the coronavirus disease pandemic and the subsequent lockdown drastically affected the patient inflow and led to a reduction in the number of recruited patients. This was an open-label study, and no blinding was done. We excluded pregnant women with CTS from our study owing to ethical concerns. The follow-up duration was only four months after the injection. Accordingly, the long-term efficacy of these steroids could not be assessed.
Conclusions
Our study concludes that both dexamethasone and triamcinolone injections significantly improve the symptoms and electrophysiological parameters of carpal tunnel syndrome up to four months after the injection without any significant difference between the two groups. Postprocedural pain, however, was found to be significantly more pronounced in the triamcinolone group compared with the dexamethasone group. Our findings show that it is likely that the injection of dexamethasone sodium phosphate can be used as a safe and equally effective alternative to the conventionally used injection of triamcinolone acetonide in cases of mild to moderate CTS.
This is relevant in the current practice of physiatrists and pain physicians who regularly inject steroids to relieve symptoms of carpal tunnel syndrome. Using nonparticulate steroids can make the procedure less painful for the patient. The use of ultrasound guidance is recommended as it can avoid the potential complications caused by blind injection into the nerve.
Our results may be further established with future bigger study designs. Comparison of different doses of dexamethasone and triamcinolone in CTS may be an area of future research.
Acknowledgments
We would like to thank Enago (www.enago.com) for the English language review.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. The Institutional Review Board of All India Institute of Medical Sciences issued approval AIIMS/IEC/2019-20/968
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Carpal tunnel syndrome. Aroori S, Spence RA. Ulster Med J. 2008;77:6–17. [PMC free article] [PubMed] [Google Scholar]
- 2.Carpal tunnel syndrome incidence in a general population. Mondelli M, Giannini F, Giacchi M. Neurology. 2002;58:289–294. doi: 10.1212/wnl.58.2.289. [DOI] [PubMed] [Google Scholar]
- 3.Prevalence of carpal tunnel syndrome in a general population. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. JAMA. 1999;282:153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 4.The flick sign in carpal tunnel syndrome. Krendel DA, Jöbsis M, Gaskell PC Jr, Sanders DB. J Neurol Neurosurg Psychiatry. 1986;49:220–221. doi: 10.1136/jnnp.49.2.220-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diagnosis, treatment and follow-up of the carpal tunnel syndrome: a review. Alfonso C, Jann S, Massa R, Torreggiani A. Neurol Sci. 2010;31:243–252. doi: 10.1007/s10072-009-0213-9. [DOI] [PubMed] [Google Scholar]
- 6.The carpal-tunnel syndrome. Seventeen years’ experience in diagnosis and treatment of six hundred fifty-four hands. Phalen GS. https://pubmed.ncbi.nlm.nih.gov/5934271/ J Bone Joint Surg Am. 1966;48:211–228. [PubMed] [Google Scholar]
- 7.Sensitivity and specificity of clinical testing for carpal tunnel syndrome. Wiesman IM, Novak CB, Mackinnon SE, Winograd JM. Can J Plast Surg. 2003;11:70–72. doi: 10.1177/229255030301100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN. J Bone Joint Surg Am. 1993;75:1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 9.The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. Graham B. J Bone Joint Surg Am. 2008;90:2587–2593. doi: 10.2106/JBJS.G.01362. [DOI] [PubMed] [Google Scholar]
- 10.Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Acta Neurol Scand. 1997;96:211–217. doi: 10.1111/j.1600-0404.1997.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 11.Carpal Tunnel Syndrome - Clinical Practice Guideline (CPG) | American Academy of Orthopaedic Surgeons. https://www.aaos.org/quality/quality-programs/upper-extremity-programs/carpal-tunnel-syndrome/ 2021
- 12.Peripheral nerve injection injury with steroid agents. Mackinnon SE, Hudson AR, Gentili F, Kline DG, Hunter D. Plast Reconstr Surg. 1982;69:482–490. doi: 10.1097/00006534-198203000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Local injection of dexamethasone for the treatment of carpal tunnel syndrome in pregnancy. Niempoog S, Sanguanjit P, Waitayawinyu T, Angthong C. J Med Assoc Thai. 2007;90:2669–2676. [PubMed] [Google Scholar]
- 14.Evaluating the effectiveness of local dexamethasone injection in pregnant women with carpal tunnel syndrome. Moghtaderi AR, Moghtaderi N, Loghmani A. J Res Med Sci. 2011;16:687–690. [PMC free article] [PubMed] [Google Scholar]
- 15.The efficacy of dexamethasone sodium phosphate compared to triamcinolone acetonide in the treatment of carpal tunnel syndrome: a randomized double-blind controlled trial. Dilokhuttakarn T, Lertnantapanya S, Vechmamontien S, Suwanchatchai C. http://www.jmatonline.com/index.php/jmat/article/view/8776 J Med Assoc Thai. 20181;101:1634–1639. [Google Scholar]
- 16.A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Liu D, Ahmet A, Ward L, et al. Allergy Asthma Clin Immunol. 2013;9:30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpal tunnel syndrome: a review of literature. Genova A, Dix O, Saefan A, Thakur M, Hassan A. https://www.cureus.com/articles/29112-carpal-tunnel-syndrome-a-review-of-literature. Cureus. 2020;12:0. doi: 10.7759/cureus.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpal tunnel syndrome. Middleton SD, Anakwe RE. BMJ. 2014;349:0. doi: 10.1136/bmj.g6437. [DOI] [PubMed] [Google Scholar]
- 19.Local corticosteroid injection for carpal tunnel syndrome. Marshall S, Tardif G, Ashworth N. Cochrane Database Syst Rev. 2002:0. doi: 10.1002/14651858.CD001554. [DOI] [PubMed] [Google Scholar]
- 20.A systematic review of conservative treatment of carpal tunnel syndrome. Piazzini DB, Aprile I, Ferrara PE, et al. Clin Rehabil. 2007;21:299–314. doi: 10.1177/0269215507077294. [DOI] [PubMed] [Google Scholar]
- 21.Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. Peters-Veluthamaningal C, Winters JC, Groenier KH, Meyboom-de Jong B. BMC Fam Pract. 2010;11:54. doi: 10.1186/1471-2296-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Electrophysiological responsiveness and clinical outcomes of local corticosteroid injection in the treatment of carpal tunnel syndrome. Ertem DH, Sirin TC, Yilmaz I. Arq Neuropsiquiatr. 2019;77:638–645. doi: 10.1590/0004-282X20190106. [DOI] [PubMed] [Google Scholar]
- 23.Lack of effect of corticosteroid injection at the shoulder joint on blood glucose levels in diabetic patients. Habib GS, Abu-Ahmad R. Clin Rheumatol. 2007;26:566–568. doi: 10.1007/s10067-006-0353-8. [DOI] [PubMed] [Google Scholar]
- 24.The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. Wang AA, Hutchinson DT. J Hand Surg Am. 2006;31:979–981. doi: 10.1016/j.jhsa.2006.03.022. [DOI] [PubMed] [Google Scholar]