There is a lot of excitement surrounding the novel sustained anti-vascular endothelial growth factor (anti-VEGF) drug delivery system named Port Delivery System with ranibizumab (PDS, Roche/ Genentech Inc., San Francisco, CA) that could address the major unmet need of reducing injection burden in various retinal diseases such as neovascular age-related macular degeneration, diabetic macular edema and diabetic retinopathy (DR) [1]. The United States—food and drug administration has recently (October 22, 2021) approved PDS with ranibizumab for clinical use [2]. The PDS has gone through 3 clinical trial phases (Phase 1, Phase 2 LADDER, and Phase 3 ARCHWAY) [3, 4]. One of the major roadblocks during the initial stages of the trial was the occurrence of vitreous hemorrhage in a significant number of cases. This manuscript will highlight the journey of mitigation of vitreous hemorrhage (VH) from Phase 1 to Phase 3.
To understand this, it is important to understand the structure of the PDS system.
PDS—structure
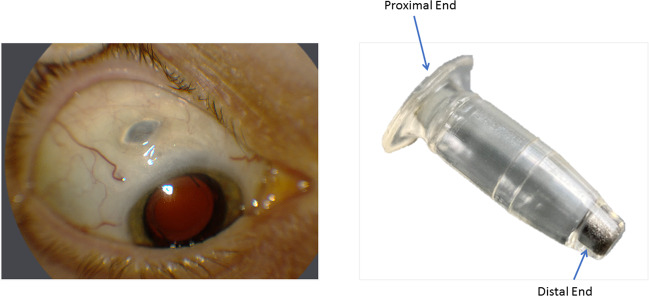
PDS is a novel drug delivery device consisting of a nonbiodegradable, refillable implant that provides a continuous release of ranibizumab into the vitreous. The device is approximately the size of a grain of rice at 2.6 mm in width and 8.4 mm in length. It is composed of polysulfone and the presence of a self-sealing septum in the center of the implant flange (proximal end) remains accessible through the conjunctiva and allows access to the implant reservoir for drug replenishment with a special needle without the need to remove the implant from the eye. At the distal end, there is a semipermeable titanium membrane that permits continuous passive diffusion of the drug from the higher concentration in the reservoir into the vitreous, a process that follows Fick’s law of diffusion [3]. (Fig. 1).
Fig. 1. Port Delivery System (PDS) with Ranibizumab.
The left figure shows the external appearance of PDS after placement. The right figure shows the structure of PDS.
Phase 1
The Phase 1 trial was a single center study with 20 patients. Vitreous hemorrhage was noticed in 5 of 20 (25%) cases. It was related to the surgical procedure for PDS implant insertion, this VH led to a transient, but in some cases prolonged, decrease in vision. The source of intraocular bleeding leading to VH was not known [3].
Phase 2 (LADDER study)
There was an unanticipated high rate (50%) of post-surgical vitreous hemorrhage in 11/22 patients who received the implant during the initial few months [3].
Surgical technique used in Phase 1 and Early Phase 2 (LADDER study)
Limbal based conjunctival and Tenons flap is created at the superotemporal quadrant followed by diathermy to control the scleral bleed. A Stab incision is created in the pars plana 4 mm posterior to limbus and the prefilled implant is inserted. Conjunctiva and Tenons are closed by suturing without any scleral suturing.
Animal study to understand surgical factors causing vitreous hemorrhage
A minipig animal model was used to test the surgical factors causing vitreous hemorrhage due to PDS insertion. Factors like prophylactic pars plana hemostasis, scleral incision length, scleral cauterization, surgical blade type/size, and viscoelastic usage were evaluated. The only surgical parameter that was consistently associated with alleviating the incidence and severity of vitreous hemorrhage after PDS implant insertion was the use of pars plana hemostasis before pars plana incision. Prophylactic laser ablation of the pars plana was performed with overlapping 1000-ms 532-nm laser spots after scleral dissection that exposed the full length of the intended pars plana incision. A long laser duration (1000 ms) was vital in reducing intraocular bleeding from the pars plana. Shorter duration (200–500 ms) laser spots were not as effective at mitigating vitreous hemorrhage, presumably because they created only a partial-thickness ablation [5].
Optimized surgical implantation technique
The initial procedure is done in an operating room under aseptic precautions with local anesthesia and sedation. 27-gauge cannula is inserted and an infusion line is attached but not turned on, then dissection of the conjunctival flap (superotemporal) is done under the Tenon’s capsule so that it remains adhered to conjunctiva. End points of scleral dissection are marked followed by scleral dissection with a length of exactly 3.5 mm. After scleral dissections, laser photocoagulation (532 nm, 1000 ms) is performed overlapping edge-to-edge at the pars plana.
Implant filled with 20 μL of ranibizumab formulation (100 mg/ml) is placed in the wound with the internal mouth facing the vitreous cavity and the external sides just outside the sclera. The conjunctival flap with attached Tenon’s capsule is closed and sutured. The limbal edge of the conjunctiva should actually overlap the peripheral edge of the cornea so that as it retracts, it settles down right at the limbus. Refill can be done through the self-sealing septum on an outpatient basis with a specially designed syringe.
Virtual reality training for trial investigators
To minimize the risk of surgical errors, Genentech’s team collaborated with VRmagic to train the study investigators on the PDS implant procedure. Genentech and VRmagic rolled out two completely new, state-of-the-art virtual reality simulator platforms enabling its users to practice implant insertion and refill-exchange of the PDS [6].
Phase 2 (LADDER) after implementing the improved techniques as described above
The incidence of vitreous hemorrhage reduced from 50% (11/22) to 4.5% (7/157) after implementing the changes recommended based on experiments conducted on the minipig.
Phase 3 (ARCHWAY study)
The overall incidence of vitreous hemorrhage is reported to be 5.2% [4]. Detailed results are yet to be known.
To summarize, vitreous hemorrhage which was the major obstacle in PDS insertion has been effectively overcome with a modification of the surgical technique. However, implementation of the standardized technique in the real world would be the key factor in deciding the incidence of vitreous hemorrhage associated with implant insertion. We have seen disconnect between the trial and real world in the recent past with brolucizumab [7]. However, here, the success depends on the technique rather than the drug, hence the chances of surprises could be minimal. With the evidences available till date, if the standard surgical protocols are followed, vitreous hemorrhage can be minimized and PDS can be a game changer in reducing the need of multiple injections.
Acknowledgements
BDK acknowledges an unrestricted grant from Research to Prevent Blindness to the Gavin Herbert Eye Institute at the University of California, Irvine.
Author contributions
AS: conception, analysis, drafting, integrity check, final approval. NP, NK, BDK, FB: drafting, analysis, integrity check.
Competing interests
AS: CONSULTANT: for Novartis, Allergan, Bayer and Intas. BDK: CLINICAL RESEARCH: Alcon, Alimera, Allegro, Allergan, Apellis, Clearside, Genentech, GSK, Ionis, jCyte, Novartis, Regeneron, ThromboGenics; CONSULTANT: Alimera, Allegro, Allergan, Cell Care, Dose, Eyedaptic, Galimedix, Genentech, Glaukos, Interface Biologics, jCyte, Novartis, Ophthotech, Regeneron, Revana, Theravance Biopharma. FB: CONSULTANT: Allergan, Bayer, Boehringer- Ingelheim, FidiaSooft, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Thrombogenics, Zeiss. NK: None. NP: None.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sharma A, Kumar N, Parachuri N, Kuppermann BD, Bandello F, Regillo CD. Ranibizumab port delivery system (RPDS): realising long awaited dream of prolonged VEGF suppression. Eye. 2020;34:422–3. doi: 10.1038/s41433-019-0479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA Approves Genentech’s Susvimo, a First-of-Its-Kind Therapeutic Approach for Wet Age-Related Macular Degeneration (AMD). https://www.gene.com/media/press-releases/14935/2021-10-22/fda-approves-genentechs-susvimo-a-first-. Accessed Oct 2021.
- 3.Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology. 2019;126:1141–54. doi: 10.1016/j.ophtha.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 4.https://www.retinasociety.org/content/meetingarchive/2020/awh-carl-primary-analysis-results-of-the-phase-3-archway-trial-of-the-port-delivery-system-with-ranibizumab-(pds)-for-patients-with-neovascular-amd-(namd).pdf. Accessed Sep 2021.
- 5.Bantseev V, Schuetz C, Booler HS, Bantseev V, Schuetz C, Booler HS, et al. Evaluation of surgical factors affecting vitreous hemorrhage following port delivery system with ranibizumab implant insertion in a minipig model. Retina. 2020;40:1520–8. doi: 10.1097/IAE.0000000000002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heimann F, Barteselli G, Brand A, Dingeldey A, Godard L, Hochstetter H, et al. A custom virtual reality training solution for ophthalmologic surgical clinical trials. Adv Simul. 2021;6:12. doi: 10.1186/s41077-021-00167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma A, Kumar N, Parachuri N, Singh S, Bandello F, Kuppermann BD, et al. Brolucizumab-related retinal vasculitis: emerging disconnect between clinical trials and real world. Eye. 2021;35:1292–94. doi: 10.1038/s41433-020-01227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]