Abstract
Objective
To evaluate visual acuity (VA) and factors influencing VA using new multimodal imaging-based classification of central serous chorioretinopathy (CSCR).
Methods
Retrospective, observational and cross-sectional study on 229 naïve eyes diagnosed as CSCR with available baseline data and multimodal imaging. Each case was classified into (i) simple/complex/atypical; (ii) primary/recurrent/resolved; (iii) persistent or not; (iv) outer retinal atrophy(ORA) present/absent; (v) foveal involvement present/absent; and (vi) macular neovascularization(MNV) present/absent. Best corrected visual acuity (BCVA) was correlated to the classification as well as every parameter of the classification.
Results
Median BCVA was 0.18 logMAR [95% Confidence Interval (CI)0.16–0.18] with median duration of complaints of one month (95% CI,6.14–13.0 months). Age of the patient (r = −0.24, p = 0.002) and duration of the disease (r = −0.32, p < 0.001) correlated significantly with BCVA. Logistic regression model showed that older age [odds ratio (OR) = 0.96, p = 0.05], female gender (OR = 2.45, p = 0.046), presence of ORA(OR = 0.34, p = 0.012),and foveal involvement(OR = 0.18, p = 0.007) were statistically significantly associated with poorer BCVA. Eyes classified as complex, persistent CSCR, with ORA or foveal involvement demonstrated lower BCVA compared to those with simple, non-persistent CSCR, without ORA or without foveal involvement (p < 0.05). Eyes with complex CSCR (p < 0.001), atypical CSCR(p = 0.025), persistent subretinal fluid (SRF) (p = 0.001) and those with ORA (p < 0.001) demonstrated a trend towards severe visual loss. Prevalence of persistent SRF, recurrent episodes and ORA was significantly higher among eyes with complex CSCR (p < 0.001) while there was no difference in prevalence of resolved cases (p = 0.07), foveal involvement (p = 0.28) and MNV (p = 0.45) between simple and complex cases.
Conclusion
There is a strong correlation between VA and foveal involvement and ORA using the new classification. Thus, the objective parameters of the classification can be incorporated in establishing the treatment guidelines for CSCR.
Subject terms: Predictive markers, Retinal diseases
Introduction
Central serous chorioretinopathy (CSCR) is characterized by one or more serous retinal detachment with or without serous pigment epithelial detachment (PED) due to retinal pigment epithelium (RPE) leak, frequently associated with choroidal hyperpermeability [1, 2]. Although, it is a common chorioretinal disease frequently seen by retina specialists, the consensus on its classification and terminology is still poor [2–4]. Recently, Chhablani and Behar-Cohen et al. [5] proposed a newer classification system for CSCR based on multimodal imaging. According to this classification, each case is classified as i) simple versus complex [< or > 2-disc diameters (DD) of retinal pigment epithelium (RPE) abnormality]; (ii) primary versus recurrent versus resolved CSCR; (iii) Persistent (> 6 months) or not; (iv) outer retinal atrophy (ORA) present or absent; (v) foveal involvement present or absent; and (vi) macular neovascularization (MNV) present or absent (Table 1).
Table 1.
New multimodal imaging-based classification of central serous chorioretinopathy.
|
Simple Total area of RPE alteration ≤ 2 DA |
Primary First known episode of SRF |
± Persistent SRF > 6 months |
± with outer retinal atrophy ONL thinning and/ or ELM disruption and/ or EZ attenuation |
± with CNV |
|
Recurrent Presence of SRF with history or signs of resolved episode(s) | ||||
|
Resolved Absence of SRF | ||||
|
Complex Total area of RPE alteration > 2 DA or multifocal |
Primary First known episode of SRF |
± Persistent SRF > 6 months |
± with outer retinal atrophy ONL thinning and/ or ELM disruption and/ or EZ attenuation ± with intraretinal fluid |
|
|
Recurrent Presence of SRF with history or signs of resolved episode(s) | ||||
|
Resolved Absence of SRF |
||||
| Atypical | Bullous variant, RPE tear, association with other retinal diseases | |||
RPE retinal pigment epithelium, DA disc areas, SRF subretinal fluid, ONL outer nuclear layer, ELM external limiting membrane, EZ ellipsoid zone, CNV choroidal neovascularization.
Various associations have been described between visual acuity (VA) (at presentation and final outcome) and factors such as: persistence of subretinal fluid (SRF) or PED [6, 7], recurrences [6], ORA at fovea [ellipsoid zone (EZ) disruption [8–10], external limiting membrane (ELM) disruption [8, 9], outer nuclear layer (ONL) thinning [11]], RPE atrophic changes at fovea [6, 8, 12, 13] and MNV [6]. Poor baseline VA has also been shown to be a predictor of poor final visual outcome [7, 13]. However, there are several deficiencies in how this knowledge is applied clinically to plan treatment of CSCR cases. Firstly, an arbitrary duration threshold between acute and chronic CSCR and the dilemma of treatment in cases with duration of disease between 3 to 6 months. Secondly, there is an unclear distinction in terminologies such as chronic and non-resolving and ill-defined terms such as acute on chronic, acute recurrent, chronic persistent and subclinical disease [2, 3]. Using the newer multimodal imaging-based classification, study of relationship of VA with various parameters would be an important step towards establishing standardized treatment guidelines.
The purpose of this study is to evaluate the correlation of visual acuity (VA) at the baseline with the new CSCR classification and various parameters of the classification.
Materials and methods
This was a cross-sectional and observational study in which the retrospective data of cases diagnosed with CSCR at multiple centres (USA, Italy, Russia, India) was evaluated. The study adhered to the tenets of the Declaration of Helsinki and ethical clearance was obtained by the institutional review board. Informed consent was obtained from all the patients. The eyes with available baseline data of age, sex, best corrected visual acuity (BCVA), duration of complaints, reliable history of any previous such episodes, retinal treatment or steroid use and availability of good quality multimodal imaging including fundus autofluorescence (FAF), Spectral Domain optical coherence tomography (SD OCT) (B scan) and optical coherence tomography angiography (OCTA) or fundus fluorescein angiography (FFA) with indocyanine angiography (ICGA) were included. FAF, fundus photographs, FFA and ICGA were obtained from Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany) or F-10 scanning laser ophthalmoscope (NIDEK, Gamagori, Japan). OCTA examinations were performed with the RTVue-XR Avanti (Optovue, Fremont, CA) or Spectralis HRA + OCT. For each eye, horizontal raster pattern scan through the centre of the macula was obtained. OCTA examination including a 6 × 6-mm (2 orthogonal volumes with 400 × 400 A scans) pattern centred in the centre of the fovea was performed with RTVue-XR Avanti.
Exclusion criteria included evidence of any other retinal disease or any intraocular surgery other than an uncomplicated cataract surgery. Inclusion and exclusion criteria were satisfied by 229 eyes of 213 patients. Double blind classification was performed by two retinal experts [SA and DM] as per the new multimodal imaging-based classification system of CSCR proposed recently. In cases of non-consensus, senior investigator (JC) was consulted. All images were available for all graders.
Definitions
Classification of CSCR into simple and complex subtypes was based on area of RPE alterations identified on FAF imaging. Eyes with total area (cumulative) of RPE alteration > 2 DD or multifocal area of involvement were classified as complex CSCR, while eyes with ≤ 2 DD of RPE abnormality were classified as simple CSCR. Atypical CSCR was documented if it was a bullous variant or there was a RPE tear or any other retinal disease was associated. For categorizing the cases into primary/ recurrent/ resolved; an eye was noted to have a primary episode of CSCR if there was no history or signs of a previous episode; recurrent CSCR was noted if there was a history or signs of a previous episode and CSCR was noted to be resolved if there was no SRF on SD OCT. Eyes were categorized to have persistent SRF if SRF was noted on SD OCT along with a history of current episode for > 6 months. ORA was documented on SD OCT if there was outer nuclear layer (ONL) thinning or EZ and ELM disruption. MNV was documented if a complex was visualized on FFA or OCTA. Fovea was noted to be involved (Fovea + ) if there was SRF or PED or ORA at fovea on the SD OCT. Minimal visual loss was defined as a VA ≥ 20/25 Snellen equivalent, mild visual loss as VA ≥ 20/40 and <20/25, moderate visual loss as VA ≥ 20/100 and < 20/40 and severe visual loss as VA < 20/100.
Correlation of VA with baseline demographic factors (age, sex, steroid use), duration of complaints, classification as well as each parameter of the classification was evaluated. A study of the trend of visual loss in each category of the classification was performed. Comparison was also made between simple CSCR and complex CSCR cases in terms of other parameters of the classification (type of episode/ persistence/ ORA/ foveal involvement/ MNV).
Statistical analysis
Statistical analysis was performed using MedCalc 18.4.1 (MedCalc Software, Ostend, Belgium). BCVA measured with a standard Snellen chart was converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis. Normality of distribution of continuous variables was checked using the Kolmogorov–Smirnov test. Since normality was rejected, median value and 95% confidence interval (CI) were used for descriptive statistics. Correlation coefficient was calculated between BCVA and age and duration of the disease. Mann-Whitney test was used to compare the difference in BCVA derived from each classification variable. Multiple logistic regression analysis was performed to find the variables that were significantly associated with poorer BCVA. Odds ratios (ORs) with 95% CI were reported. Chi-square test was used to compare the prevalence of persistent, recurrent, and resolved cases, ORA, MNV and foveal involvement between simple and complex CSCR. Additionally, Chi-square test was used to evaluate statistical significance of the trends in the prevalence of eyes with minimal, mild, moderate, or severe visual loss for different CSCR categories as per the newer classification. All P values of < 0.05 were considered statistically significant.
Results
The dataset included 229 eyes of 213 patients consisting of 175 males (82.2%), 38 females (17.8%), and bilateral involvement in 16 patients (7.5%). Median age of patients was 43 years (range 23 to 77 years). There was a history of steroid use in 19 patients (8.9%). For ruling out MNV, OCTA was available for 120 eyes and FFA with ICGA was available for 109 eyes.
Out of 229 eyes, 110 eyes (48%) were diagnosed as simple CSCR, 114 eyes (49.8%) had a complex CSCR and 5 eyes (2.2%) had an atypical CSCR (Supplementary Fig. 1A). One hundred seventy-two eyes (75.1%) had a primary episode of CSCR, 46 eyes (28.1%) had a recurrent disease, and 11 eyes (4.8%) had a resolved CSCR. Amongst the eyes diagnosed as simple CSCR, 89.1% eyes had a primary episode of CSCR, 9.1% eyes had a recurrent episode of CSCR and 1.8% eyes had a resolved CSCR. Amongst the eyes diagnosed as complex CSCR, 63.2% had a primary episode of CSCR, 29% had a recurrent episode of CSCR and 7.9% had a resolved CSCR. In the simple CSCR cases, only 4.5% eyes had a persistent SRF while 95.5% eyes had SRF for < 6 months duration (sporadic). In the complex CSCR cases, 38.6% eyes had persistent SRF and 61.4% eyes had a sporadic CSCR. In 11.8% of simple CSCR cases, ORA was present while it was present in 70.2% of complex CSCR cases. Foveal involvement was documented in 96.4% eyes of simple CSCR and 92.1% eyes of complex CSCR eyes. MNV was detected in 4.5% of simple CSCR cases and 7.9% of complex CSCR cases. Distribution of eyes in each category have been tabulated in Table 2. Supplementary Fig. 1B to 1F shows the graphical distribution of eyes within simple, complex and atypical CSCR.
Table 2.
Comparison between simple central serous chorioretinopathy (CSCR) and complex CSCR cases in terms of various parameters of the classification.
| Classification parameters | Simple | Complex | P value | |
|---|---|---|---|---|
| Total number of eyes | 110 (48% of all study eyes) | 114 (49.8% of all study eyes) | ||
| Episode of CSCR | Primary | 98 (89.09% of simple CSCR) (57% of all primary cases) | 72 (63.16% of complex CSCR) (41.9% of all primary cases) | ≤0.001 |
| Recurrent | 10 (9.09% of simple CSCR) (21.7% of all recurrent cases) | 33 (28.95% of complex CSCR) (71.7% of all recurrent cases) | 0.0003 | |
| Resolved | 2 (1.81% of simple CSCR) (18.2% of all resolved cases) | 9 (7.89% of complex CSCR) (81.8% of all resolved cases) | 0.07 | |
| Persistent subretinal fluid | Present | 5 (4.5% of simple CSCR) (9.6% of all persistent CSCR) | 44 (38.6% of complex CSCR) (84.6% of all persistent CSCR) | <0.001 |
| Outer retinal atrophy (ORA) | Present | 13 (11.8% of simple CSCR) (13.4% of all cases with ORA) | 80 (70.2% of complex CSCR) (82.5% of all cases with ORA) | <0.001 |
| Foveal involvement | Present | 106 (96.4% of simple CSCR) (49.1% cases with foveal involvement) | 105 (92.1% of complex CSCR) (48.6% cases with foveal involvement) | 0.28 |
| Macular neovascularization (MNV) | Present | 5 (4.5% of simple CSCR) (31.2% of cases with MNV) | 9 (7.9% of cases with complex CSCR) (56.2% of cases with MNV) | 0.45 |
Prevalence of persistent SRF, recurrent cases and ORA was statistically significantly higher among eyes with complex CSCR (p < 0.001) while there was no difference in prevalence of resolved cases (p = 0.07), MNV (p = 0.45) and foveal involvement (p = 0.28) between simple and complex cases. Primary episode of CSCR was significantly more prevalent among simple CSCR cases (p < 0.001). Supplementary Fig. 2 demonstrates the distribution of eyes as per the parameters of the classification (recurrence/ persistence/ ORA/ foveal involvement/MNV).
Median visual acuity was 0.18 logMAR [95% Confidence Interval (CI) 0.16 to 0.18] (Snellen equivalent 20/30) and median duration of complaints was one month (95% CI 6.14 to 13.0) (range 1 day to 19 years). Age of the patient (r = −0.24, p = 0.002) and duration of the disease (r = −0.32, p < 0.001) correlated significantly with BCVA. Correlation between patient’s age and disease duration was also statistically significant (r = 0.32, p < 0.001). Eyes classified as complex CSCR, persistent SRF, with ORA or foveal involvement demonstrated lower BCVA compared to those with simple CSCR, non-persistent SRF, without ORA, or without foveal involvement (P < 0.05) (Table 3). In univariate analysis, other factors including the presence of MNV and steroid use showed no significant association with BCVA (Table 3). Logistic regression model showed that only age (OR = 0.96, p = 0.05) (older age was associated with poorer BCVA), female gender (OR = 2.45, p = 0.046), presence of ORA (OR = 0.34, p = 0.012), and foveal involvement (OR = 0.18, P = 0.007) were statistically significant factors associated with poorer BCVA. Trend of visual loss (minimal/ mild/ moderate/ severe) was correlated with various categories of the classification such as (A) simple versus complex CSCR; (B) primary versus recurrent versus resolved CSCR; (C) persistent SRF versus sporadic episode; (D) presence or absence of ORA; (E) presence or absence of foveal involvement; and (F) presence or absence of MNV. (Supplementary Fig. 3) It was observed that there was a statistically significant trend towards severe visual loss in eyes with complex (p < 0.001), atypical (p = 0.025), persistent CSCR (p = 0.001) as well as those with ORA (p < 0.001). Eyes with simple CSCR demonstrated a trend towards minimal visual loss (p < 0.001). (Table 4)
Table 3.
Association of best corrected visual acuity (BCVA) with the classification and every parameter of the classification.
| Category | Median BCVA, logMAR (95% CI) | Median decimal BCVA | P-value | |
|---|---|---|---|---|
| Classification | Simple | 0.1 (0.05 to 0.18) | 0.80 | <0.0001 |
| Complex | 0.2 (0.18 to 0.30) | 0.64 | ||
| Episode | Primary | 0.18 (0.1 to 0.18) | 0.66 | 0.23 |
| Resolved | 0.48 (0.0 to 0.72) | 0.33 | ||
| Recurrent | 0.22 (0.17 to 0.30) | 0.61 | 0.11a | |
| Persistence | Non-persistent | 0.17 (0.1 to 0.18) | 0.66 | 0.0006 |
| Persistent | 0.3 (0.18 to 0.48) | 0.50 | ||
| Presence of ORA | ORA absent | 0.1 (0.05 to 0.16) | 0.80 | <0.0001 |
| ORA present | 0.3 (0.19 to 0.45) | 0.50 | ||
| Presence of foveal involvement | No foveal involvement | 0.0 (−0.08 to 0.34) | 1.0 | 0.04 |
| Foveal involvement | 0.18 (0.16 to 0.18) | 0.66 | ||
| Presence of MNV | MNV absent | 0.18 (0.13 to 0.18) | 0.66 | 0.12 |
| MNV present | 0.26 (0.12 to 0.55) | 0.55 | ||
| Steroids use | No steroids use | 0.18 (0.16 to 0.18) | 0.66 | 0.34 |
| Steroids use | 0.16 (0.0 to 0.18) | 0.70 |
CI confidence interval, logMAR logarithm of the minimum angle of resolution, ORA outer retinal atrophy, MNV macular neovascularization.
aprimary versus recurrent cases.
Table 4.
Distribution of eyes according to various parameters of the classification and the visual loss.
| Minimal visual loss | Mild visual loss | Moderate visual loss | Severe visual loss | P value | |
|---|---|---|---|---|---|
| Total number of eyes | 95 | 71 | 62 | 20 | |
| Simple CSCR | 58 (61.1%) | 37 (52.1%) | 15 (24.2%) | 2 (10%) | <0.001a |
| Complex CSCR | 37 (38.9%) | 33 (46.5%) | 44 (71%) | 17 (85%) | <0.001b |
| Atypical CSCR | 0 | 1 (1.4%) | 3 (4.8%) | 1 (5%) | 0.025b |
| Primary CSCR | 75 (78.9%) | 55 (77.5%) | 41 (66.1%) | 15 (75%) | 0.16 |
| Recurrent CSCR | 16 (16.8%) | 15 (21.1%) | 15 (24.2%) | 3 (15%) | 0.55 |
| Resolved CSCR | 4 (4.2%) | 1 (1.4%) | 6 (9.7%) | 2 (10%) | 0.1 |
| Persistent SRF | 15 (15.8%) | 12 (17%) | 25 (40.3%) | 9 (45%) | 0.001b |
| ORA + | 25 (26.3%) | 28 (39.4%) | 44 (71%) | 15 (75%) | <0.001b |
| Fovea+ | 86 (90.5%) | 70 (98.6%) | 59 (95.2%) | 18 (90%) | 0.5 |
| MNV + | 4 (4.2%) | 6 (8.5%) | 6 (9.7%) | 3 (15%) | 0.07 |
asignificant trend towards minimal visual loss.
bsignificant trend towards severe visual loss.
Minimal visual loss: visual acuity ≥ 20/25 Snellen equivalent; Mild visual loss: Visual acuity ≥ 20/40 and <20/25; Moderate visual loss: visual acuity ≥ 20/100 and < 20/40; Severe visual loss: visual acuity < 20/100; CSCR: central serous chorioretinopathy; SRF: subretinal fluid; ORA + : outer retinal atrophy present; Fovea + : foveal involvement present for subretinal fluid/ pigment epithelial detachment/ outer retinal atrophy; MNV + : macular neovascularization present.
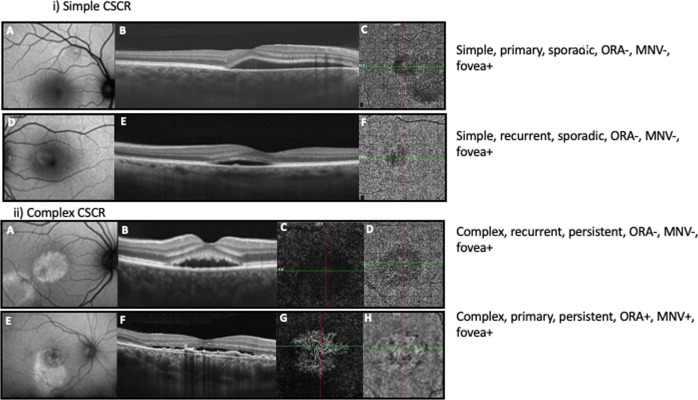
Representative cases, classified as per the newer classification, have been demonstrated in Fig. 1.
Fig. 1.
i) Representative cases showing simple central serous chorioretinopathy (CSCR).First panel: A 38-year-old patient presented with metamorphopsia for 3 weeks in their right eye, best corrected visual acuity (BCVA) of 20/20 (Snellen) and no history of recurrence. A Fundus autofluorescence (FAF) image demonstrating < 2-disc diameters (DD) of retinal pigment epithelium (RPE) alteration thereby classifying the case as simple CSCR. B Optical coherence tomography (OCT) B scan passing in the horizontal direction through the fovea demonstrating subretinal fluid (SRF) and no evidence of outer retinal atrophy (ORA). C OCT angiography (OCTA) at the level of choriocapillaris is unremarkable. The eye is classified as Simple CSCR, primary episode, sporadic (<6 months duration), absent ORA (ORA-), with foveal involvement (fovea + ) and absent macular neovascularization (MNV-). Second panel: A 44-year-old patient presented with metamorphopsia for 2 months in their left eye, BCVA of 20/15 (Snellen) and a history of previous such episode. D FAF imaging demonstrates < 2 DD of RPE alteration, E OCT B scan passing in the horizontal direction through the fovea shows SRF, a tiny pigment epithelial detachment (PED) and no evidence of ORA (ORA-). F OCTA at the level of choriocapillaris is unremarkable. The eye is classified as Simple CSCR, recurrent episode, sporadic, ORA-, fovea + , MNV-. 1 ii). Representative cases showing complex central serous chorioretinopathy (CSCR). Third panel: A 38-year-old patient presented with complaints of blurred vision for 6.5 months in their right eye with a history of previous such episode. Best corrected visual acuity (BCVA) was 20/20. A Fundus autofluorescence (FAF) image demonstrating > 2-disc diameters (DD) of retinal pigment epithelium (RPE) alteration (multifocal) thereby classifying the case as complex CSCR. B Optical coherence tomography (OCT) B scan passing in the horizontal direction through the fovea demonstrating subretinal fluid (SRF) and no evidence of outer retinal atrophy (ORA -). OCT angiography (OCTA) at the level of outer retina (C) and choriocapillaris (D) is unremarkable. The eye is classified as complex CSCR, recurrent episode, persistent (>6 months duration), ORA-, with foveal involvement (fovea + ) and absent macular neovascularization (MNV-). Fourth panel: A 49-year-old patient complained of decreased vision in right eye for 2 years. BCVA was 20/200 with no history of previous such episode. E FAF imaging shows > 2 DD of RPE alteration thereby classifying the case as complex CSCR. F OCT B scan passing in the horizontal direction through the fovea demonstrating SRF and flat irregular pigment epithelial detachment (FIPED). OCTA at the level of outer retina (G) and choriocapillaris (H) shows MNV (MNV + ). Thus, the eye was classified as complex CSCR, primary episode, persistent SRF, ORA + , fovea + , MNV + .
Discussion
Our study reports older age of patients, foveal involvement and ORA as factors associated with a poor VA at presentation. These results are in accordance with previous studies in CSCR which have shown older age to be associated with poor VA at presentation [14], worse visual outcome [8], more RPE decompensation and secondary MNV [12, 15]. Studies have shown ORA at fovea to be associated with poor VA at presentation as well as a poor visual outcome on follow up [8, 9, 16, 17]. Duration of symptoms, which was significant in univariate analysis, was not an independent risk factor in multivariate analysis. This could be because duration of disease as reported by the patient could be fallacious, especially in a setting where patient is asymptomatic due to non-involvement of fovea initially and becomes symptomatic as disease progresses to involve the fovea. Therefore, conventional classification system primarily based on duration of disease reported by the patient (acute versus chronic) is bound to have deficiencies. Use of multimodal imaging, as in new classification, helps mitigate many of such weaknesses.
In this study, eyes with complex CSCR (RPE abnormalities > 2 DD on FAF) had a significantly lower BCVA as compared to eyes with simple CSCR. On analysis of trend of visual loss, simple CSCR showed a significant trend towards minimal visual loss while complex CSCR and atypical CSCR showed a significant trend towards severe visual loss. Mrejen et al. classified RPE alterations on FAF as < 2 DD, 2 to 4 DD and > 4 DD and demonstrated FAF change at central fovea to be significantly associated with poorer BCVA outcome [8]. In another study by Mohabati et al., poor final visual outcome was strongly associated with surface of diffuse atrophic RPE alterations (DARA) in DD (assessed on FFA) and presence of these alterations within 1 DD of fovea [13]. Based on their study, they proposed a definition of severity as > 5 DD of DARA. Thus, extent of damage on autofluorescence correlates with VA and may play an important role as its predictor.
Notably, the prevalence of recurrent episode was significantly higher in complex CSCR cases while primary episode was more prevalent among simple CSCR cases. Prevalence of persistent SRF and ORA was also significantly higher in complex CSCR cases as compared to simple CSCR cases (p < 0.001), while there was no difference in prevalence of MNV and foveal involvement between simple and complex CSCR cases, as per the classification (Table 2). During recurrent episodes of CSCR, recurrence may occur at the same site or a different site [18]. When it occurs at a different site, it would lead to multifocal RPE alteration which classifies as complex CSCR as per this classification. Diffuse RPE atrophy is also known to be an indicator of prior episodes [6, 19]. This may explain higher prevalence of recurrent cases in complex CSCR. Wider area of RPE abnormality (complex CSCR) may lead to inefficient pumping out of SRF and leading to persistent SRF. Prolonged separation of photoreceptors from RPE and choroid may lead to its hypoxic injury. Higher prevalence of ORA in complex cases is supported by previous literature demonstrating FAF changes correlating with ORA [20]. Significantly higher prevalence of ORA in complex cases also explains poor VA in complex cases as compared to simple CSCR. Moreover, since persistence of SRF and recurrences have been associated with a poor VA [6], higher prevalence of persistent and recurrent cases in complex CSCR further explains poor VA in complex CSCR cases. No significant difference in the prevalence of MNV between simple and complex CSCR is very interesting and needs to be studied in a bigger cohort of patients. A recent study revealed that inner choroidal attenuation, outer nuclear layer thinning and dome shaped PED were risk factors for RPE atrophy developing in cases of resolved CSCR while Type 1 choroidal neovascularization may serve to nourish the outer retina and RPE [21]. This is interesting, as studies on neovascular age related macular degeneration have also shown SRF to be associated with better visual outcome [22, 23].
Thus, we found that the majority of variables included in the new classification system for CSCR have a significant association with functional status of eyes with CSCR and are important predictors of visual prognosis. However, the presence of MNV showed no significant association with BCVA. Although, in our study, the eyes with MNV showed numerically lower BCVA, the difference with eyes without MNV was not statistically significant (p = 0.13). This was probably because we had only 7% of study eyes with MNV. Larger cohort with presence of MNV may help further to understand the association.
Although persistent SRF, wider area of RPE abnormality, recurrences, ORA and foveal involvement are known to be associated with worse VA [6, 8, 13, 16], but currently, treatment guidelines are not inclusive of these objective criteria. This study was performed to evaluate the features included in the new classification and correlate it with VA at the time of classification. Incorporation of objective criteria in classification and treatment protocols in this complex disease will be a great aid in clinical practice.
There are certain limitations in the new classification such as non-inclusion of pachychoroid features in the classification which needs to be addressed further as we understand further about pachychoroid. Limitations of this study include non-availability of follow up data including therapeutic interventions. Therefore, we are unable to comment about visual and anatomical outcome using this classification, which needs future studies. In this study, as in the proposed classification [5], ONL thinning was assessed qualitatively. Although ONL thickness measurements can be performed in some devices, it needs further validation and is still not applicable in clinical practice. Another limitation of the study was that majority of the patients had a short duration of the disease. Some studies have shown multicolour imaging to be superior to FAF in delineation of RPE abnormalities [24], however, multicolour imaging is not widely available. Although the proposed classification [5] includes dye-based angiography, we included OCTA in more than half of our patients. Recent studies have shown that OCTA appears to be superior in detection of choroidal neovascularization compared to dye based angiographies in eyes with CSCR [25–28]. Thus, it is prudent to replace the dye based angiographies with this non-invasive imaging [29].
To conclude, there is a strong correlation of VA to the classification (simple versus complex CSCR) as well as various parameters of the classification [episode (primary versus recurrent), persistence of SRF, ORA and foveal involvement]. Our study attests that the new multimodal classification is an informative instrument, reflecting not only morphological but also functional status of eyes with CSCR. Thus, incorporation of new classification in the clinical practice could be useful for management of patients with CSCR more effectively. Future studies are warranted to study the long term follow up data and establish treatment guidelines.
Summary table
What was known before
Discrepancies in the classification and terminology of central serous chorioretinopathy (CSCR) are well known. The current treatment protocols for CSCR are designed around a flawed classification and do not employ the structural changes on multimodal imaging.
Recently a new multimodal imaging-based classification of CSCR was proposed by CSCR international group to overcome the existing pitfalls.
What this study adds
Eyes with complex CSCR [ > 2-disc diameters (DD) of retinal pigment epithelium (RPE) abnormality] had a lower visual acuity as compared to eyes with simple CSCR [ < 2 DD of RPE abnormality]. Recurrent episodes, persistent subretinal fluid and outer retinal atrophy was significantly more in eyes with complex CSCR.
Majority of variables included in the new classification system for CSCR have a significant association with functional status of eyes with CSCR and are important predictors of visual prognosis. Thus, incorporation of these objective criteria in establishing the treatment guidelines will greatly aid in clinical practice.
Supplementary information
Distribution of number of eyes among various categories and parameters.
Distribution of eyes as per parameters of classification.
Graphs demonstrating trend analysis of various parameters of classification versus visual loss.
Author contributions
SA contributed to concept and design of the work, analysis and interpretation of the data, writing the draft, revising it and finally approving it. DM contributed to acquisition of the data, analysis and interpretation of the data, writing the draft and approving it. NS contributed to acquisition of the data and its analysis, revising the draft and approving it. DP contributed to acquisition of the data and its analysis, revising the draft and approving it. CI contributed to acquisition of the data and its analysis and approving the final draft. TA contributed in analysis and interpretation of the data, writing the draft and approving it. AK helped in acquisition of the data and approving the final draft. FT was responsible for acquisition of the data and approving the final draft. RV contributed to acquisition of the data and approving the final draft. NR contributed to acquisition of the data and approving the final draft. RP contributed to acquisition of the data and approving the final draft. SS contributed to acquisition of the data and approving the final draft. EP contributed to acquisition of the data and approving the final draft. JC designed and conceptualized the study, was responsible for acquisition of the data, analysis and interpretation of the data, writing the manuscript, revising it and finally approving it. Supplementary information is available at Eye’s website.
Funding
No grants/ support was received for this study.
Financial disclosures
Dr Jay Chhablani is a consultant for Allergan, OD-OS, Novartis and Biogen. The remaining authors have no proprietary or financial interest in any aspect of this report. No grants/ support was received for this study.
Competing interests
Dr Jay Chhablani is a consultant for Allergan, OD-OS, Novartis and Biogen. The remaining authors have no proprietary or financial interest in any aspect of this report. There is no conflict of interest.
Footnotes
The original online version of this article was revised: The name of the author Ramesh Venkatesh was corrected.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/8/2021
A Correction to this paper has been published: 10.1038/s41433-021-01837-y
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-021-01788-4.
References
- 1.Kaye R, Chandra S, Sheth J, Boon CJF, Sivaprasad S, Lotery A. Central serous chorioretinopathy: An update on risk factors, pathophysiology and imaging modalities. Prog Retin Eye Res. 2020:100865. [DOI] [PubMed]
- 2.Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Singh SR, Matet A, van Dijk EHC, Daruich A, Fauser S, Yzer S, et al. Discrepancy in current central serous chorioretinopathy classification. Br J Ophthalmol. 2019;103:737–42. doi: 10.1136/bjophthalmol-2018-312435. [DOI] [PubMed] [Google Scholar]
- 4.van Rijssen TJ, van Dijk EHC, Yzer S, Ohno-Matsui K, Keunen JEE, Schlingemann RO, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019;73:100770. doi: 10.1016/j.preteyeres.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Chhablani J, Cohen FB. Multimodal imaging-based central serous chorioretinopathy classification. Ophthalmol Retin. 2020;4:1043–6. doi: 10.1016/j.oret.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Loo RH, Scott IU, Flynn HW, Jr, Gass JD, Murray TG, Lewis ML, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22:19–24. doi: 10.1097/00006982-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Aggio FB, Roisman L, Melo GB, Lavinsky D, Cardillo JA, Farah ME. Clinical factors related to visual outcome in central serous chorioretinopathy. Retina. 2010;30:1128–34. doi: 10.1097/IAE.0b013e3181cdf381. [DOI] [PubMed] [Google Scholar]
- 8.Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126:576–88. doi: 10.1016/j.ophtha.2018.12.048. [DOI] [PubMed] [Google Scholar]
- 9.Suwal B, Khadka D, Shrestha A, Shrestha S, Shrestha N, Khatri B. Baseline predictive factors of visual outcome and persistence of subretinal fluid based on morphologic changes in spectral domain optical coherence tomography in patients with idiopathic central serous chorioretinopathy. Clin Ophthalmol. 2019;13:2439–44. doi: 10.2147/OPTH.S233273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SR, Iovino C, Zur D, Masarwa D, Iglicki M, Gujar R, et al. Central serous chorioretinopathy imaging biomarkers. Br J Ophthalmol. 2020. [DOI] [PubMed]
- 11.Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009;148:105–10.e1. doi: 10.1016/j.ajo.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Imamura Y, Fujiwara T, Spaide RF. Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology. 2011;118:700–5. doi: 10.1016/j.ophtha.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Mohabati D, van Rijssen TJ, van Dijk EH, Luyten GP, Missotten TO, Hoyng CB, et al. Clinical characteristics and long-term visual outcome of severe phenotypes of chronic central serous chorioretinopathy. Clin Ophthalmol. 2018;12:1061–70. doi: 10.2147/OPTH.S160956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson BP, Ali Idris AM, Bakri SJ. Central Serous Chorioretinopathy: Clinical Characteristics Associated with Visual Outcomes. Semin Ophthalmol. 2018;33:804–7. doi: 10.1080/08820538.2018.1503690. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070–9. doi: 10.1016/S0161-6420(96)30386-2. [DOI] [PubMed] [Google Scholar]
- 16.Piccolino FC, de la Longrais RR, Ravera G, Eandi CM, Ventre L, Abdollahi A, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139:87–99. doi: 10.1016/j.ajo.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Yalcinbayir O, Gelisken O, Akova-Budak B, Ozkaya G, Gorkem Cevik S, Yucel AA. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina. 2014;34:705–12. doi: 10.1097/IAE.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815–20. doi: 10.1136/bjo.68.11.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91:1554–72. doi: 10.1016/S0161-6420(84)34117-3. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Cho NS, Kim K, Kim ES, Kim DG, Kim JM, et al. Fundus autofluorescence patterns in central serous chorioretinopathy. Retina. 2020;40:1387–94. doi: 10.1097/IAE.0000000000002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrelli E, Battista M, Sacconi R, Gelormini F, Querques L, Grosso D, et al. OCT risk factors for 3-year development of macular complications in eyes with “resolved” chronic central serous chorioretinopathy. Am J Ophthalmol. 2021;223:129–39. doi: 10.1016/j.ajo.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Siedlecki J, Fischer C, Schworm B, Kreutzer TC, Luft N, Kortuem KU, et al. Impact of sub-retinal fluid on the long-term incidence of macular atrophy in neovascular age-related macular degeneration under treat & extend anti-vascular endothelial growth factor inhibitors. Sci Rep. 2020;10:8036. doi: 10.1038/s41598-020-64901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianniou C, Dirani A, Jang L, Mantel I. Refractory intraretinal or subretinal fluid in neovascular age-related macular degeneration treated with intravitreal ranizubimab: functional and structural outcome. Retina. 2015;35:1195–201. doi: 10.1097/IAE.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 24.Govindahari V, Singh SR, Rajesh B, Gallego-Pinazo R, Marco RD, Nair DV, et al. Multicolor imaging in central serous chorioretinopathy - a quantitative and qualitative comparison with fundus autofluorescence. Sci Rep. 2019;9:11728. doi: 10.1038/s41598-019-48040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bousquet E, Bonnin S, Mrejen S, Krivosic V, Tadayoni R, Gaudric A. Optical coherence tomography angiography of flat irregular pigment epithelium detachment in chronic central serous chorioretinopathy. Retina. 2018;38:629–38. doi: 10.1097/IAE.0000000000001580. [DOI] [PubMed] [Google Scholar]
- 26.Bonini Filho MA, de Carlo TE, Ferrara D, Adhi M, Baumal CR, Witkin AJ, et al. Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:899–906. doi: 10.1001/jamaophthalmol.2015.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quaranta-El Maftouhi M, El Maftouhi A, Eandi CM. Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. Am J Ophthalmol. 2015;160:581–7.e1. doi: 10.1016/j.ajo.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Dansingani KK, Balaratnasingam C, Klufas MA, Sarraf D, Freund KB. Optical coherence tomography angiography of shallow irregular pigment epithelial detachments in pachychoroid spectrum disease. Am J Ophthalmol. 2015;160:1243–54.e2. doi: 10.1016/j.ajo.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Arora S, Kulikov AN, Maltsev DS. Implementation of the new multimodal imaging-based classification of central serous chorioretinopathy. Eur J Ophthalmol. 2021:11206721211013651. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of number of eyes among various categories and parameters.
Distribution of eyes as per parameters of classification.
Graphs demonstrating trend analysis of various parameters of classification versus visual loss.